Published online Jun 21, 2024. doi: 10.3748/wjg.v30.i23.2981
Revised: May 2, 2024
Accepted: May 20, 2024
Published online: June 21, 2024
Processing time: 161 Days and 10.2 Hours
Lymph node metastasis is a specific type of metastasis in hepatic alveolar echinococcosis (AE). Currently, there is a scarcity of describing the clinical characteristics and lymph node metastasis rules of patients with hepatic AE combined with lymph node metastasis and its mechanism and management are still controversial. Radical hepatectomy combined with regional lymph node dissection is a better treatment.
To analyse the clinical features of hepatic AE combined with lymph node metastasis to explore its treatment and efficacy.
A total of 623 patients with hepatic AE admitted to the First Affiliated Hospital of Xinjiang Medical University from 1 January 2012 to 1 January 2022 were retro
A lymph node metastasis rate of 8.8% (55/623) was reported in patients with hepatic AE, with a female predi
Lymph node metastasis is a rare form of metastasis in hepatic AE, which is more frequent in women. Para-hepatoduodenal ligament lymph nodes are commonly observed. Radical hepatectomy combined with regional lymph node dissection is a safe, effective, and feasible treatment for liver AE combined with lymph node metastasis.
Core Tip: We retrospectively summarized and analyzed the clinical data, diagnosis and treatment, follow-up efficacy and characteristics of lymph node metastasis in 55 patients combined with lymph node metastasis among 623 patients with hepatic alveolar echinococcosis (AE) admitted to our hospital from January 2012 to January 2022. This study is the first and largest retrospective study specifically describing the management of hepatic AE combined with lymph node metastases. We present a radical hepatectomy combined with regional lymph node dissection strategy for patients with hepatic AE combined with lymph node metastasis based on the outcomes of this study and our experience.
- Citation: Aimaitijiang Y, Jiang TM, Shao YM, Aji T. Fifty-five cases of hepatic alveolar echinococcosis combined with lymph node metastasis: A retrospective study. World J Gastroenterol 2024; 30(23): 2981-2990
- URL: https://www.wjgnet.com/1007-9327/full/v30/i23/2981.htm
- DOI: https://dx.doi.org/10.3748/wjg.v30.i23.2981
Echinococcosis multilocularis infection causes hepatic alveolar echinococcosis (AE). It is known as a “malignant parasitic disease” owing to its chronic, progressive, and infiltrative growth[1-3]. Distant metastases to the lungs, brain, kidneys, and surrounding tissues via the infiltrative, haematogenous, and lymphatic routes occur in hepatic AE. Reports and related studies on lymph node metastasis are scarce, and conclusive evidence supporting the regional lymphatic metastasis pathway is lacking[4,5]. Lymph node might metastases occur as a result of AE draining via the deep and superficial multidirectional lymphatic reflux pathways in the liver to regional lymph nodes[6]. Despite the reports on multiple related cases, more comprehensive literature reports proposing diagnostic and treatment norms for lymph node metastases do not exist[7-10]. Metastatic lymph node management is controversial. Additionally, the World Health Organization (WHO) treatment guidelines do not specify whether regional lymph node dissection should be performed as part of the radical treatment protocol for hepatic AE. Therefore, analysing the clinical characteristics and treating patients with hepatic AE combined with lymph node metastasis is crucial. Herein, we analysed the clinical characteristics of 55 patients with hepatic AE combined with lymph node metastasis at our centre and summarised its diagnosis and treatment experience for the first time.
Study subject: A total of 623 patients (295 females and 328 males) with hepatic AE were admitted to our hospital from 1 January 2012 to 1 January 2022. Fifty-five patients (17 males and 38 females) (8.8%), aged 35.5 ± 11.55 years (range: 15-66 years), who were diagnosed with combined lymph node metastasis, were selected. The liver lesions were in the right lobe in 25 cases, the left lobe in eight cases, and both the right and left lobes of the liver in 22 cases. The mean maximum diameter of the lesions was 12.93 cm, with blood vessel invasion in 43 cases (78.2%) and bile duct invasion in 37 cases (67.3%).
Symptoms: Twenty-five patients did not have any obvious symptoms. Initial symptoms of abdominal pain and bloating occurred in 21 patients. Seven patients had yellow discolouration of the skin and the sclera. One patient had a headache, dizziness, and convulsions. One other patient had chest distress, cough, and sputum production.
Medical history: Previous palliative partial hepatectomy, percutaneous transhepatic cholangial drainage, portal vein embolisation, and perforated drainage for echinococcosis-caused liquefaction and necrosis within the cavity of liver peritoneal worms were performed in ten, six, one, and two patients, respectively. History of exposure to infected areas and canines was recorded in all patients. A preoperative diagnosis was established in 14 patients, and they received irregular intermittent oral albendazole.
Staging characteristics: According to the parasite lesion, neighbouring organ invasion, and metastasis (PNM) staging suggested by the WHO, there were 12 cases of P2NIM0, three cases of P2N1M1, eight cases of P3N1M0, six cases of P3N1M1, 16 cases of P4N1M0, and 10 cases of P4N1M1. All patients belonged to the intermediate and advanced clinical stages.
Comorbidity: Extrahepatic metastases occurred in 17 cases, including 10 cases of pulmonary metastases, one case of brain metastasis, one case of pancreatic metastasis, two cases of pulmonary-cerebral metastases, and three cases of metastases to three or more organs (lung, brain, kidney, etc.). In addition, one patient had combined portal vein cavernous degene
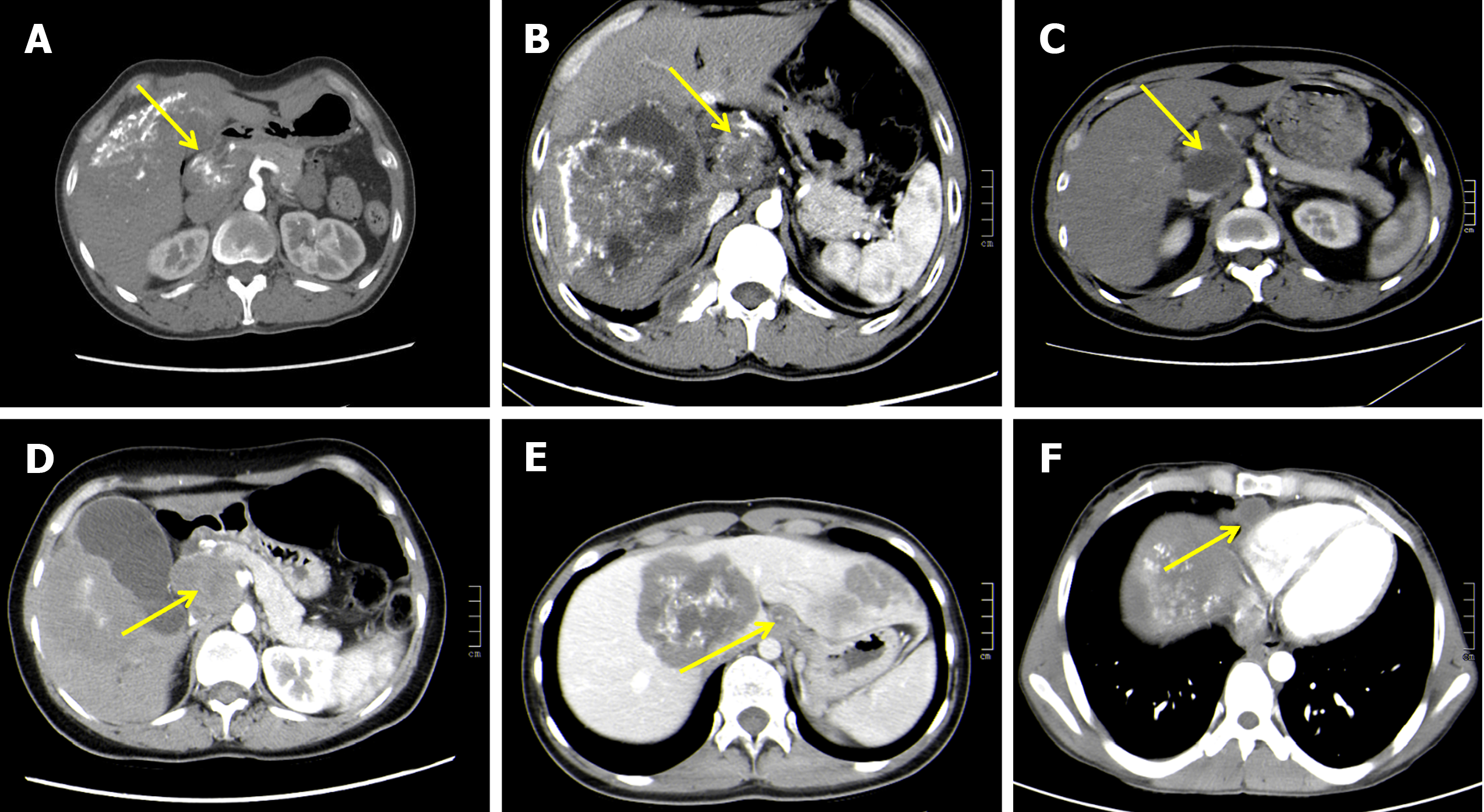
Adjunctive examinations: Preoperative routine blood examination, liver function tests, abdominal ultrasonography (USG), and abdominal computed tomography (CT) were performed on 55 patients. Among them, abdominal magnetic resonance imaging (MRI) was performed on 33 patients. The CT manifestations of lymph node metastasis are presented in Figure 1.
Indications: The indications for surgical treatment were as follows: (1) Patients without serious cardiopulmonary disease who could tolerate surgery; (2) Patients with Child-Pugh class A or B liver function; (3) Patients who were willing to undergo radical surgery; (4) Patients with imaging findings suggestive of lymph node metastasis or suspicious enlarged lymph nodes detected intraoperatively; (5) Patients with lung metastasis who could tolerate extended radical surgery; and (6) Patients with brain metastasis and stable brain lesions.
Contraindications: The contraindications were as follows: (1) Patients with a poor cardiopulmonary function who could not tolerate radical treatment; (2) Patients with Child-Pugh class C liver function; and (3) Patients who could not undergo radical surgical treatment due to other reasons.
Hepatic surgery: Radical hepatectomy was performed considering the location, severity, and individualised treatment of the lesion[11]. In addition, right trisegmentectomy, right hemihepatectomy, left trisegmentectomy, left hemihepatectomy, left lateral hepatic lobectomy, and ex vivo liver resection with autologous liver transplantation were performed in nine, fourteen, six, three, one, and twenty-two patients, respectively.
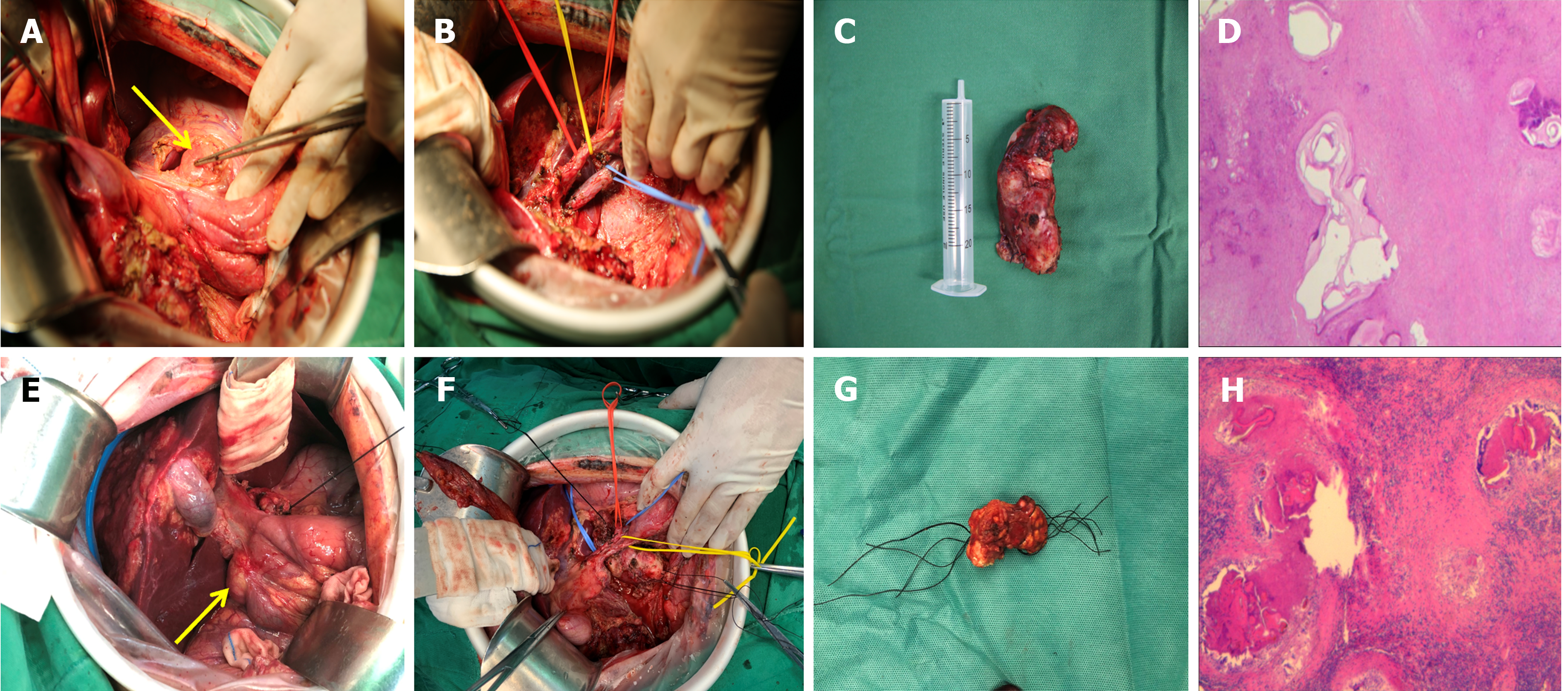
Treatment of the lymph nodes: Regional lymph nodes, including the para-cystic duct, para-common bile duct, porta hepatis, para-portal vein, para-pancreatic, celiac trunk, and superior mesenteric lymph nodes, were routinely explored intraoperatively. The detected lymph nodes that were enlarged or suspected of metastases were dissected based on the skeletal criteria (Figure 2). The hepatoduodenal ligament and the portacaval space were intraoperatively explored to avoid leakage. In addition, we prophylactically dissected the remaining suspicious lymph nodes in the lymphatic reflux pathway. The supradiaphragmatic and subdiaphragmatic lymph nodes were explored in patients who required intraoperative resection and reconstruction of the diaphragm. A routine pathological examination was performed on the dissected lymph nodes.
Treatment of intrahepatic and extrahepatic invaded tissues: Liver resection combined with organ and tissue resection should be performed according to the principles of radical tumour-free treatment when the lesion invades multiple organs or surrounding tissues. Combined resection, repair, and reconstruction should be performed to achieve radical treatment when the intrahepatic and extrahepatic vessel and biliary system invasion are severe. Herein, hepatectomy combined with total left nephrectomy, pulmonary wedge resection, pancreaticoduodenectomy, diaphragmatic repair, portal or hepatic vein reconstruction, portal vein thrombosis removal, posthepatic inferior vena cava thrombosis removal, portal or inferior vena cava vascular grafting (six cases with autologous vessels and one case with artificial vessels), bile duct repair and formation, and bilioenteric anastomosis were performed in one, two, one, six, seven, one, one, seven, fifteen, and seven patients, respectively.
Postoperatively, the patients were dynamically monitored for changes in vital signs and liver function. Postoperative complications were graded using the Clavien-Dindo classification[12]. After discharge, all patients were instructed to wait until normalisation of the liver function to receive regular oral albendazole (10 mg/kg) according to the guidelines. They also underwent abdominal USG or CT to evaluate regular liver and kidney function. The patients were followed up at the outpatient clinic and telephonically to check their postoperative medication status. In addition, routine blood, liver function, abdominal USG, and CT re-examinations were performed to understand recurrence and recovery.
Descriptive and correlation analyses of the data were performed using SPSS 26.0. Categorical variables are expressed as frequencies and percentages, and the analysis of difference was performed using the χ2 test. The Bonferroni method was used for pairwise comparisons when statistical differences existed between multiple categorical variables. Statistical significance was set at P < 0.05.
This study was approved by the Ethics Committee of First Affiliated Hospital of Xinjiang Medical University and conducted in accordance with the Helsinki Declaration. Written and signed informed consent was obtained from all patients or their legal custodians.
A total of 623 patients, comprising 295 (47.4%) females and 328 (52.6%) males, with hepatic AE, were included in this study. No statistically significant difference by sex was observed (χ2 = 1.748, P = 0.186). Of the 55 patients with combined lymph node metastasis, 38 (69.1%) were females, and 17 (30.9%) were males. The incidence of lymph node metastasis had a female predilection (χ2 = 8.018, P = 0.005).
USG, CT, and MRI are accurate, non-invasive, and effective methods for diagnosing hepatic AE. Herein, all these methods had a 100% diagnostic rate for hepatic AE; however, a lower diagnostic rate was observed for lymph node metastasis (Table 1). CT had better diagnostic significance for metastatic lymph nodes with typical morphology and large diameter. On CT, the lymph nodes had a round or ovoid appearance with mixed density or hypodense foci, which were surrounded by sand-like, ring-like, and sheet-like calcifications.
| Diagnosis method | Number of examined cases (n) | Diagnosis of hepatic AE | Diagnosis of lymph node metastasis | ||
| n | % | n | % | ||
| USG | 55 | 55 | 100 | 9 | 16.4 |
| CT | 55 | 55 | 100 | 17 | 30.9 |
| MRI | 33 | 33 | 100 | 6 | 18.2 |
The surgery was performed successfully in all patients, and no serious intraoperative complications occurred. All patients had a transient elevation in their liver function postoperatively, which gradually decreased 3-5 d after surgery and returned to normal before discharge. Clavien-Dindo I complications (hypoproteinaemia in nine cases, bile leakage in two cases, and pancreatic fistula in one case) occurred in 10 cases during postoperative hospitalisation. These patients recovered well after symptomatic treatment with electrolyte supplementation, albumin supplementation, and adequate irrigation and drainage. Clavien-Dindo class IIIa complications (pleural effusion in ten cases, seroperitoneum in seven cases, and pneumothorax in one case) occurred in 14 patients, which improved after symptomatic treatment with puncture tube placement and drainage and closed chest drainage.
Pathological examination revealed 209 lymph nodes, 106 multilocular echinococcus cyst protoscolexes and 103 inflammatory enlarged lymph nodes. Six groups of lymph node metastases at different sites existed, including 39 cases of para-hepatoduodenal ligament lymph nodes, nine cases of posterior pancreatic head lymph nodes, four cases of para-common hepatic artery lymph nodes, five cases of para-aorta abdominalis lymph nodes, one case of celiac trunk lymph nodes, and two cases of para-diaphragmatic lymph nodes. Single-group metastasis occurred in 51 cases, and multi-group metastasis (including two cases of para-hepatoduodenal ligament + para-aorta abdominalis, one case of para-hepatoduodenal ligament + posterior pancreatic head, and one case of para-hepatoduodenal ligament + para-diaphragmatic + para-common hepatic artery metastases) occurred in four cases. The incidence of metastasis at different lymph node sites was subjected to differential analysis using the χ2 test, yielding χ2 = 128.089 and P = 0.000 < 0.05. Therefore, a significant difference was observed in the rate of metastasis among the different lymph node sites. The Bonferroni method was used to perform a further pairwise comparison. The rate of para-hepatoduodenal ligament metastasis was significantly higher than the five other groups (P = 0 < 0.01), while no statistical difference was observed between the five other groups (Table 2).
| Sites | Para-hepatoduodenal ligament lymph nodes | Posterior pancreatic head lymph nodes | Para-common hepatic artery lymph nodes | Para-aorta abdominalis lymph nodes | Para-diaphragmatic lymph nodes | Celiac trunk lymph nodes |
| Number of metastasis cases | 39a | 9b | 4b | 5b | 2b | 1b |
| Number of non-metastasis cases | 16a | 46b | 51b | 50b | 53b | 54b |
| Metastasis rate (%) | 70.9 | 16.4 | 7.3 | 9.1 | 3.6 | 1.8 |
| χ2 | 128.089a | |||||
| P value | 0 |
AE is the third most dreadful foodborne parasitic disease worldwide[13]. It is projected that 91% of new cases of AE worldwide occur in China, mainly in agricultural and pastoral areas, such as Xinjiang and Tibet, seriously endangering the people’s physical and mental health[14-16]. AE almost originates in the liver and is often diagnosed in the intermediate or advanced stage due to early insidious clinical symptoms. The growth characteristics of hepatic AE are aggressive and infiltrative, similar to malignant tumours, and can metastasise to surrounding tissues or distant organs via haematological and lymphatic pathways[17-19]. The incidence of lymph node metastasis is low (8.8% in this study), and its mechanism is unclear. The predominant assumption is that AE drains to regional lymph nodes via the lymphatic fluid from the intrahepatic lymphatics[4]. Radical hepatectomy is the preferred treatment for hepatic AE. Patients treated with liver transplantation for end-stage hepatic AE experience re-infection of the graft several years later[20]. This finding supports the previous hypothesis that lymph node metastasis might be a potential risk for persistent infection. In addition, serious complications, such as obstructive jaundice, portal hypertension, acute pancreatitis, and Budd-Chiari syndrome, could occur through infiltration and compression as a result of lymph node metastases in different locations. Therefore, radical treatment of the primary hepatic AE site, along with the dissection of regional lymph nodes, is crucial. However, no distinct specification exists on the scope and indications of lymph node dissection. There is a close association between the mode of lymph node metastasis and the lymphatic fluid drainage pathway. Therefore, understanding the lymphatic return pathways in the liver is essential.
The lymphatic system of the liver can be divided into deep and superficial lymphatic systems. The deep lymphatic vessels are distributed along the portal vein and the hepatic veins. The superficial lymphatic vessels are on the liver surface, comprising the visceral and diaphragmatic surfaces. A total of 80% or more of the hepatic lymph flows along the deep lymphatic system around the portal vein into the para-hepatoduodenal ligament lymph nodes and posterior pancreatic head lymph nodes[21]. Herein, six groups of different lymph node metastases sites existed. The most common site of lymph node metastasis was para-hepatoduodenal ligament (70.9%), which significantly differed from each group (P < 0.01). It might be the first station of lymphatic fluid flow from AE to posterior pancreatic head and para-common hepatic artery or more distant lymph nodes. In this study, patients with lymph node metastasis had more primary foci concentrated in the left interior lobe of the liver (segment IV) or the right anterior lobe of the liver (segments V and VIII), and their first hepatic invasion of the porta hepatis was more severe (69.7%) with larger lesion diameters. In addition, a high metastatic rate was observed with respect to the posterior pancreatic head lymph nodes (nine cases) and para-common hepatic artery lymph nodes (four cases), indicating that these three sites are important regional nodes. Para-aorta abdominalis lymph nodes, a distant lymph node pathway, mainly comprise three pathways converging from the porta hepatis, posterior pancreatic head, and deep lymphatic system around the hepatic vein. Herein, we observed five cases of para-aorta abdominalis lymph node metastases. In addition, we observed two cases of para-diaphragmatic lymph node metastases attributed to the superficial lymphatic system on the liver’s diaphragmatic surface that was distributed along the bilateral coronary ligaments, bilateral deltoid ligaments, and sickle ligaments. Hepatic AE lesions superior to the liver, breaching the hepatic tegument, can pass through the hepatic lymph directly through the diaphragm into the distal lymphatic system of the thoracic cavity, including the pericardial, diaphragmatic, and paraoesophageal lymphatic systems. In addition, AE lesions might also get dislodged and colonise the parietal, anterior, and posterior mediastinal lymph nodes with the thoracic lymphatic reflux when hepatic lesions invade the diaphragm and protrude into the thoracic cavity. Moreover, the posterior pancreatic head, para-common hepatic artery, para-celiac trunk, and para-aorta abdominalis lymph node metastases occurred separately in six, three, one, and three patients, respectively, indicating that AE can metastasize directly to the next-station or distant lymph nodes without passing through the first-station lymph nodes. This is because AE was not effectively terminated and eliminated at the first station lymph node, following which it spread and metastasised to the next station lymph node via the lymphatic flow. Multiple groups of lymph node metastasis were observed in four cases. The AE drained both ways through the deep and superficial hepatic lymphatic return system to different sites causing lymph node metastasis when there were large-diameter AE lesions that spanned the left and right lobes of the liver, from the top of the liver down to the porta hepatis and caudate lobes. Single-group, multiple-group or even jumping lymph node metastasis from deep and superficial lymphatic reflux pathways of the liver can occur, depending on the location, size, and vascular invasion of the lesion.
Lymph node metastases from hepatic AE resemble a special type of extrahepatic metastases. Intrahepatic lesions invading the right and left lobes of the liver are observed more frequently in patients with extrahepatic metastases. When the primary lesions are larger in diameter, they invade the intrahepatic and extrahepatic vessels, bile ducts, or other adjacent tissues and organs more aggressively[22]. Herein, 55 patients with a PNM stage of P3N1M0 or higher accounted for 72.7% of the cases, with most patients belonging to the intermediate or advanced stages of the disease. The mean maximum lesion diameter was 12.93 cm, and severe intrahepatic and extrahepatic vascular and biliary invasions were observed. No statistical difference was observed in terms of the sex of the 623 patients with hepatic AE in this study (χ2 = 1.748, P = 0.186). The incidence of hepatic AE combined with lymph node metastases among the 55 patients was significantly higher among women (69.1%) than in men (χ2 = 8.018, P = 0.005). Oestrogen receptors are present in the nucleus and envelope of some hormone-sensitive malignancies, and oestrogen binding to this receptor can directly participate in the gene transcriptional regulation of tumour cell survival and proliferation[23-24], stimulating cell proliferation, thereby enhancing their lymph node metastasis[25]. An association might exist between the high morbidity of female patients who developed lymph node metastasis in this study and the high oestrogen expression. Further clinical studies are warranted to confirm this conjecture.
Diagnosing lymph node metastases in hepatic AE based on preoperative imaging is challenging despite advances in imaging evaluation. Only large-diameter lymph node metastases and typical morphologies can be identified. Therefore, intraoperative exploration and postoperative histopathological examination are often required[7,26]. Kantarci et al[27] demonstrated that the USG, CT, and MRI assist in the radiological diagnosis of liver AE. Herein, a diagnostic rate of 100% for diagnosing hepatic AE using preoperative abdominal USG, MRI, and CT was reported, while the detection rate of lymph node metastasis was low (16.4%, 18.2%, and 30.9%, respectively). This might be attributed to the deeper location of the lesion, smaller diameter, or the lack of distinct boundaries between multiple lymph nodes and surrounding tissues after fusion. CT has a better diagnostic significance for lymph node metastasis compared with USG and MRI, as it can more accurately and qualitatively demonstrate lymph node metastasis. A round or ovoid shape, mixed density or hypointense foci, non-enhancement on an enhanced scan, and necrosis, nodules, and calcification in lymph nodes are the characteristic features of lymph nodes on a CT scan. Our centre believes that preoperative imaging focusing on the first porta hepatis region (regions surrounding the neck of gallbladder, para-common bile duct, and periportal vein), the second porta hepatis region (para-inferior vena cava), and retroperitoneal lymph nodes (para-aorta abdominalis) has diagnostic significance and can avoid missed diagnoses. Therefore, this step should be mandatory for preoperative evaluation.
Histopathological examination is the gold standard for diagnosing lymph node metastasis in hepatic AE. Identification of structures, such as multilocular echinococcus cyst, protoscolexes, or head hooks in lymph node histopathology sections or identifying the nucleotide sequence of Echinococcus multilocularis by polymerase chain reaction help in establishing a positive diagnosis[28]. Herein, 209 enlarged lymph nodes, 106 positive lymph nodes, and 103 inflammatory lymph nodes were identified postoperatively. Not all lymph nodes demonstrated positive results and inflamed enlarged lymph nodes were also present. This particular metastatic pattern might be associated with the body’s strong and weak immune response stimulated when AE spreads via the lymphatic drainage to the lymph nodes in a region[29], resulting in local fibrosis and chronic inflammation. Chronic inflammation can weaken lymphatic vessel contraction, impede lymphatic flow to the subsequent station lymph nodes, and cause local lymphoedema. In addition, increased infiltration of macrophages, multinucleated giant cells, and lymphocytes can result in typical granulomatous changes[30,31]. Herein, the postoperative pathogenic diagnosis of patients highly suspected of intraoperative lymph node metastasis resulted in inflammatory enlargement. False-negative results could not be ruled out due to the diagnostic positivity, sensitivity, and specificity of unconfirmed conventional haematoxylin and eosin (H&E) staining. Therefore, immunohistochemistry is a crucial adjunctive diagnostic tool to support conventional histopathological examination when AE invasion is highly suspected in a biopsy tissue sample and when the H&E staining result is negative. Monoclonal antibody (mAb) Em2G11 and mAb EmG3 are the two main antibodies that exist against multilocular echinococcus antigens[26,32]. Grimm et al[33] reported the presence of small particles of Echinococcus multilocularis (SPEMS) in the lymph node germ layer of patients with hepatic AE, and their immunohistochemical staining was strongly positive, along with the specificity of mAb. In addition, Hillenbrand et al[20] also detected SPEMS by immunohistochemical methods with mAb Em2G11 in patients with negative H&E staining on histopathological examination. All histopathological examinations of the lymph node specimens in this group were performed under conventional H&E staining at high magnification. Some limitations existed in the pathogenic diagnosis owing to the single examination modality. Therefore, histopathological examination methods need to be selected reasonably according to the actual situation to improve the lymph node metastasis diagnostic rate in patients with hepatic AE when intraoperative lymph node metastasis is highly suspected.
Radical liver resection combined with systemic regional lymph node dissection was performed on all 55 patients. The surgical feasibility of the combined procedure depends largely on the precise preoperative evaluation. The optimal surgical plan should be selected based on the size and location of the primary focus, the vascular and biliary invasion characteristics, the resectability of distant metastases, the location and number of lymph node metastases, their adjacency to important vasculature, and the patient’s tolerance to the procedure. A better understanding of the multidirectional lymphatic reflux pathways in the liver is needed for targeted or prophylactic complete dissection of suspected lymph nodes when combining any surgical approach with systemic or regional lymph node dissection. Since positive and inflammatory enlarged lymph nodes cannot be accurately distinguished by intraoperative visual examination, pathologically evaluating the suspected lymph nodes together is essential. Lymph node dissection is performed by scraping and suction dissection, along with alternating electrocoagulation and pushing and peeling, to complete the skeletal dissection quickly and easily, with less bleeding and clear boundaries. In particular, the vascular sheath should be opened first and dissected close to the vessels to dissect the lymph nodes from the root and protect the vessels when the porta hepatis lymph nodes are in close proximity to blood vessels. In addition, all sections and/or margins should be treated with electrocoagulation to eliminate any residual AE, and the vessel wall is susceptible to localised effusion or secondary infection after debulking due to the loss of protection by the surrounding connective tissue, increasing the risk of late postoperative bleeding. Therefore, the porta hepatis should be routinely drained after surgery. End-stage hepatic AE changes occurred in 22 patients, making conventional surgery incurable[34]. Therefore, we performed ex vivo liver resection with autologous liver transplantation combined with regional lymph node dissection. Mastering organ transplantation procedures will also be a must as the foundation for combined surgical treatment when patients with lymph node metastases from hepatic AE have complex lesions that make conventional surgery inapplicable.
Serious intraoperative complications did not occur in any of the patients in this study. Postoperatively, the liver and other organs recovered well without any technique-related complications. After recovery, all patients were discharged. Clavien-Dindo grade IIIa complication occurred in 14 patients owing to high surgical trauma, preoperative jaundice, abnormal function of multiple organs, and intraoperative combined organ resection. The operation time was relatively prolonged due to lymph node dissection, while there was no association between postoperative complications and lymphatic dissection, consistent with the findings of previous studies[20]. In addition, lymph node metastasis generally occurs in para-hepatoduodenal ligament, and it can further metastasise to the posterior pancreatic head, para-common hepatic artery, or the celiac trunk, and subsequently to the para-inferior vena cava and para-aorta abdominalis lymph nodes. Pancreatic head invasion and biliary obstruction occur as a result of rapid lymph node growth that metastasises to the posterior pancreatic head[17], thereby increasing the surgical difficulty and the risk of bleeding, biliary leakage, and pancreatic fistula. Therefore, the lymph nodes are cautiously stripped by the surgeon while clearing them in strict accordance with the anatomical approach. In addition, large-diameter metastatic lymph nodes in the first hepatic porta hepatis that cause fusion can severely invade the porta hepatic structures and cause serious complications, namely portal vein spongiform degeneration and portal hypertension. Wang et al[35] proposed such patients can be treated safely and effectively by radical hepatectomy combined with revascularisation. Multidisciplinary collaboration can be employed to perform combined multiorgan radical resection while diagnosing and treating patients with multiorgan complex hepatic AE combined with lymph node metastasis[36]. Suturing or repair techniques should be strictly controlled to prevent postoperative stenosis or thrombosis in patients with severe vascular and biliary tract invasion requiring repair or reconstruction. Herein, portal or hepatic vein reconstruction, portal or inferior vena cava vascular grafting, bile duct repair, and formation (27.3%), and bilioenteric anastomosis (12.7%) were performed in seven (12.7%), seven (12.7%) (six cases with autologous vessels and one case with artificial vessels), 15 (27.3%), and seven (12.7%) patients, respectively. Applying the enhanced recovery after surgery protocol for postoperative patient management can also lower the occurrence of complications, accelerate liver functional recovery, and achieve rapid recovery[37].
The recurrence rate in this study was 1.8% (recurrence in one patient). In situ liver recurrence occurred without reinfection at the site of lymph node dissection or in the extrahepatic organs. The “infiltrative zone” where the lesion was actively proliferating was not eliminated by radical hepatectomy, resulting in recurrence at the hepatic margin. Clinical cure without disease recurrence was achieved in the remaining patients with combined regular oral albendazole. Patients who underwent revascularisation or replacement took long-term oral anticoagulants postoperatively, and complications such as thrombosis did not occur. Herein, one patient reported a 9-year history of recurrence 8 years after the previous partial hepatectomy with severe complications, such as lymph node metastasis and portal vein spongiform degeneration, and a clinical cure was achieved after this treatment. Therefore, radical hepatectomy combined with regional lymph node dissection effectively lowers the risk of disease recurrence, progression, and adjacent organ invasion.
In summary, lymph node metastasis is a specific type of metastasis in hepatic AE, which has a female predilection and has a higher incidence in intermediate to advanced hepatic AE. AE can involve single or multiple groups of lymph nodes through the unique lymphatic reflux of the liver and has a jumping metastatic characteristic. Metastases to para-hepatoduodenal ligament lymph nodes occur frequently. Therefore, intraoperative clearance of these lymph nodes should be routinely performed. Postoperative histopathological examination is the gold standard for diagnosis. Basic principles of individualised treatment should be followed while treating hepatic AE with lymph node metastasis. A comprehensive preoperative evaluation should be performed. In addition, the most appropriate surgical approach should be selected based on rigorous planning and multidisciplinary collaboration. Radical hepatectomy combined with regional lymph node dissection is safe, feasible, and effective for treating hepatic AE combined with lymph node metastasis and is worthy of widespread clinical application.
| 1. | Wen H, Vuitton L, Tuxun T, Li J, Vuitton DA, Zhang W, McManus DP. Echinococcosis: Advances in the 21st Century. Clin Microbiol Rev. 2019;32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 421] [Cited by in RCA: 637] [Article Influence: 106.2] [Reference Citation Analysis (0)] |
| 2. | Gharbi HA, Hassine W, Brauner MW, Dupuch K. Ultrasound examination of the hydatic liver. Radiology. 1981;139:459-463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 528] [Cited by in RCA: 487] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 3. | McManus DP, Zhang W, Li J, Bartley PB. Echinococcosis. Lancet. 2003;362:1295-1304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 691] [Cited by in RCA: 717] [Article Influence: 32.6] [Reference Citation Analysis (0)] |
| 4. | Eckert J, Thompson RC, Mehlhorn H. Proliferation and metastases formation of larval Echinococcus multilocularis. I. Animal model, macroscopical and histological findings. Z Parasitenkd. 1983;69:737-748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 61] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 5. | Ali-Khan Z, Siboo R, Gomersall M, Faucher M. Cystolytic events and the possible role of germinal cells in metastasis in chronic alveolar hydatidosis. Ann Trop Med Parasitol. 1983;77:497-512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 6. | Buttenschoen K, Kern P, Reuter S, Barth TF. Hepatic infestation of Echinococcus multilocularis with extension to regional lymph nodes. Langenbecks Arch Surg. 2009;394:699-704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 7. | Buttenschoen K, Gruener B, Carli Buttenschoen D, Reuter S, Henne-Bruns D, Kern P. Palliative operation for the treatment of alveolar echinococcosis. Langenbecks Arch Surg. 2009;394:199-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 28] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 8. | Moriichi K, Fujiya M, Goto T, Okumura T. Echinococcosis infection diagnosed based on the histological findings of a lymph node involvement obtained by EUS-FNA. Endosc Ultrasound. 2018;7:210-211. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 9. | Wang Q, Cui Y, Ren L, Wang H, Wang Z, Fan H. Suspected Regional Lymph Node Metastasis in Hepatic Alveolar Echinococcosis: A Case Report. Iran J Parasitol. 2020;15:138-141. [PubMed] |
| 10. | Amano T, Hayashi S, Nishida T, Matsubara T, Takahashi K, Nakamatsu D, Tomimaru Y, Yamamoto M, Nakajima S, Fukui K, Tamura H, Adachi S, Dono K, Inada M. Alveolar Echinococcosis Mimicking a Hepatic Neoplasm with Lymph Node Metastasis: A Case Report. Case Rep Gastroenterol. 2018;12:587-596. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 11. | Brunetti E, Kern P, Vuitton DA; Writing Panel for the WHO-IWGE. Expert consensus for the diagnosis and treatment of cystic and alveolar echinococcosis in humans. Acta Trop. 2010;114:1-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1108] [Cited by in RCA: 1333] [Article Influence: 88.9] [Reference Citation Analysis (0)] |
| 12. | Clavien PA, Barkun J, de Oliveira ML, Vauthey JN, Dindo D, Schulick RD, de Santibañes E, Pekolj J, Slankamenac K, Bassi C, Graf R, Vonlanthen R, Padbury R, Cameron JL, Makuuchi M. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg. 2009;250:187-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6210] [Cited by in RCA: 8620] [Article Influence: 538.8] [Reference Citation Analysis (0)] |
| 13. | Craig P. Echinococcus multilocularis. Curr Opin Infect Dis. 2003;16:437-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 111] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 14. | Torgerson PR, Keller K, Magnotta M, Ragland N. The global burden of alveolar echinococcosis. PLoS Negl Trop Dis. 2010;4:e722. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 395] [Cited by in RCA: 364] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 15. | Deplazes P, Rinaldi L, Alvarez Rojas CA, Torgerson PR, Harandi MF, Romig T, Antolova D, Schurer JM, Lahmar S, Cringoli G, Magambo J, Thompson RC, Jenkins EJ. Global Distribution of Alveolar and Cystic Echinococcosis. Adv Parasitol. 2017;95:315-493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 432] [Cited by in RCA: 638] [Article Influence: 79.8] [Reference Citation Analysis (0)] |
| 16. | Wang S, Ma Y, Wang W, Dai Y, Sun H, Li J, Wang S, Li F. Status and prospect of novel treatment options toward alveolar and cystic echinococcosis. Acta Trop. 2022;226:106252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 17] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 17. | Xu X, Gao C, Qian X, Liu H, Wang Z, Zhou H, Zhou Y, Wang H, Hou L, He S, Feng X, Fan H. Treatment of Complicated Hepatic Alveolar Echinococcosis Disease With Suspicious Lymph Node Remote Metastasis Near the Inferior Vena Cava-Abdominal Aorta: A Case Report and Literature Review. Front Oncol. 2022;12:849047. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 18. | Gottstein B, Wunderlin E, Tanner I. Echinococcus multilocularis: parasite-specific humoral and cellular immune response subsets in mouse strains susceptible (AKR, C57B1/6J) or 'resistant' (C57B1/10) to secondary alveolar echinococcosis. Clin Exp Immunol. 1994;96:245-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 52] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 19. | Kern P, Wen H, Sato N, Vuitton DA, Gruener B, Shao Y, Delabrousse E, Kratzer W, Bresson-Hadni S. WHO classification of alveolar echinococcosis: principles and application. Parasitol Int. 2006;55 Suppl:S283-S287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 214] [Article Influence: 10.7] [Reference Citation Analysis (1)] |
| 20. | Hillenbrand A, Beck A, Kratzer W, Graeter T, Barth TFE, Schmidberger J, Möller P, Henne-Bruns D, Gruener B. Impact of affected lymph nodes on long-term outcome after surgical therapy of alveolar echinococcosis. Langenbecks Arch Surg. 2018;403:655-662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 21. | Morine Y, Shimada M. The value of systematic lymph node dissection for intrahepatic cholangiocarcinoma from the viewpoint of liver lymphatics. J Gastroenterol. 2015;50:913-927. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 64] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 22. | Rubini-Campagna A, Kermarrec E, Laurent V, Régent D. [Hepatic and extrahepatic alveolar echinococcosis: CT and MR imaging features]. J Radiol. 2008;89:765-774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 23. | Raoul F, Hegglin D, Giraudoux P. Trophic ecology, behaviour and host population dynamics in Echinococcus multilocularis transmission. Vet Parasitol. 2015;213:162-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 41] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 24. | Frasor J, Danes JM, Komm B, Chang KC, Lyttle CR, Katzenellenbogen BS. Profiling of estrogen up- and down-regulated gene expression in human breast cancer cells: insights into gene networks and pathways underlying estrogenic control of proliferation and cell phenotype. Endocrinology. 2003;144:4562-4574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 589] [Cited by in RCA: 598] [Article Influence: 27.2] [Reference Citation Analysis (0)] |
| 25. | Hershberger PA, Vasquez AC, Kanterewicz B, Land S, Siegfried JM, Nichols M. Regulation of endogenous gene expression in human non-small cell lung cancer cells by estrogen receptor ligands. Cancer Res. 2005;65:1598-1605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 131] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 26. | Barth TF, Herrmann TS, Tappe D, Stark L, Grüner B, Buttenschoen K, Hillenbrand A, Juchems M, Henne-Bruns D, Kern P, Seitz HM, Möller P, Rausch RL, Deplazes P. Sensitive and specific immunohistochemical diagnosis of human alveolar echinococcosis with the monoclonal antibody Em2G11. PLoS Negl Trop Dis. 2012;6:e1877. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 58] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 27. | Kantarci M, Aydin S, Eren S, Ogul H, Akhan O. Imaging Aspects of Hepatic Alveolar Echinococcosis: Retrospective Findings of a Surgical Center in Turkey. Pathogens. 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 28. | Li T, Ito A, Nakaya K, Qiu J, Nakao M, Zhen R, Xiao N, Chen X, Giraudoux P, Craig PS. Species identification of human echinococcosis using histopathology and genotyping in northwestern China. Trans R Soc Trop Med Hyg. 2008;102:585-590. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 50] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 29. | Jebbawi F, Bellanger AP, Lunström-Stadelmann B, Rufener R, Dosch M, Goepfert C, Gottstein B, Millon L, Grandgirard D, Leib SL, Beldi G, Wang J. Innate and adaptive immune responses following PD-L1 blockade in treating chronic murine alveolar echinococcosis. Parasite Immunol. 2021;43:e12834. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 30. | Kataru RP, Baik JE, Park HJ, Wiser I, Rehal S, Shin JY, Mehrara BJ. Regulation of Immune Function by the Lymphatic System in Lymphedema. Front Immunol. 2019;10:470. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 68] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 31. | Ghanta S, Cuzzone DA, Torrisi JS, Albano NJ, Joseph WJ, Savetsky IL, Gardenier JC, Chang D, Zampell JC, Mehrara BJ. Regulation of inflammation and fibrosis by macrophages in lymphedema. Am J Physiol Heart Circ Physiol. 2015;308:H1065-H1077. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 126] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 32. | Reinehr M, Micheloud C, Grimm F, Kronenberg PA, Grimm J, Beck A, Nell J, Meyer Zu Schwabedissen C, Furrer E, Müllhaupt B, Barth TFE, Deplazes P, Weber A. Pathology of Echinococcosis: A Morphologic and Immunohistochemical Study on 138 Specimens With Focus on the Differential Diagnosis Between Cystic and Alveolar Echinococcosis. Am J Surg Pathol. 2020;44:43-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 45] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 33. | Grimm J, Nell J, Hillenbrand A, Henne-Bruns D, Schmidberger J, Kratzer W, Gruener B, Graeter T, Reinehr M, Weber A, Deplazes P, Möller P, Beck A, Barth TFE. Immunohistological detection of small particles of Echinococcus multilocularis and Echinococcus granulosus in lymph nodes is associated with enlarged lymph nodes in alveolar and cystic echinococcosis. PLoS Negl Trop Dis. 2020;14:e0008921. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 34. | Aji T, Dong JH, Shao YM, Zhao JM, Li T, Tuxun T, Shalayiadang P, Ran B, Jiang TM, Zhang RQ, He YB, Huang JF, Wen H. Ex vivo liver resection and autotransplantation as alternative to allotransplantation for end-stage hepatic alveolar echinococcosis. J Hepatol. 2018;69:1037-1046. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 77] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 35. | Wang ML, Ran B, Yasen A, Jiang TM, Aini A, Zhang RQ, Guo Q, Aji T, Wen H, Shao YM. [Hepatectomy for hepatic alveolar echinococcosis complicating cavernous transformation of the portal vein]. Zhongguo Putong Waike Zazhi. 2021;36:595-599. |
| 36. | Cicerone O, Lissandrin R, Brunetti E, Maestri M. Simultaneous Echinococcal superinfection in a patient with polycystic liver disease. Clin Case Rep. 2023;11:e8083. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 37. | Yang M, Su W, Deng X, Deng J, Li P, Li X. Enhanced recovery after surgery program in patients from Tibet Plateau undergoing surgeries for hepatic alveolar echinococcosis. J Surg Res. 2017;219:188-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |