Published online Jun 21, 2024. doi: 10.3748/wjg.v30.i23.2964
Revised: May 8, 2024
Accepted: May 24, 2024
Published online: June 21, 2024
Processing time: 112 Days and 8 Hours
Metabolic dysfunction-associated fatty liver disease (MAFLD) is a hepatic mani
Core Tip: Dysbiosis of gut microbiota leading to gut-liver axis disruption is a significant contributor to the development of metabolic dysfunction-associated fatty liver disease (MAFLD). However, the precise role of enteric bacteria in the pathogenesis of MAFLD remains unclear. Glucagon-like peptide-1 receptor agonists (GLP-1 RAs) may modulate MAFLD progression mechanisms to alleviate dysfunction, partly by modifying gut microbiota. The association between MAFLD pathogenesis and gut microbiota dysbiosis needs to be understood, as well as how GLP-1 RAs can regulate these impaired mechanisms and improve patient outcomes.
- Citation: Rochoń J, Kalinowski P, Szymanek-Majchrzak K, Grąt M. Role of gut-liver axis and glucagon-like peptide-1 receptor agonists in the treatment of metabolic dysfunction-associated fatty liver disease. World J Gastroenterol 2024; 30(23): 2964-2980
- URL: https://www.wjgnet.com/1007-9327/full/v30/i23/2964.htm
- DOI: https://dx.doi.org/10.3748/wjg.v30.i23.2964
Metabolic dysfunction-associated fatty liver disease (MAFLD) is a complex metabolic disorder with hepatic manifestation that was formerly known as non-alcoholic fatty liver disease (NAFLD). In this review, we will retain the former name NAFLD for older literature data due to the gradually changing nomenclature for hepatic steatosis coexisting with the metabolic syndrome. MAFLD affects approximately 25%-30% of adult patients, making it the most commonly observed liver disease in the world[1]. There has been an increase in the prevalence of this condition in rapidly growing countries[2]. The elevated prevalence of this disease is due to a sedentary lifestyle, reduced physical activity, and an unhealthy diet with a significantly higher calorie intake compared to energy expenditure[3]. MAFLD pathogenesis is closely related to mechanisms regulating occurrence of the metabolic syndrome and obesity[4]. MAFLD features an accumulation of excess lipids in the liver, causing lipotoxicity that may progress to metabolic-associated steatohepatitis (MASH). The mech
Microbiota is a complex ecological community of microorganisms, including bacteria, archaea, fungi, and viruses which live in human organism as commensals, that through their collective metabolic activities and host interactions, influence both normal physiology and disease susceptibilities[16]. The gut is the primary location of human microbiota, as well as the microbiome that refers to the collection of genomes of the resident microorganisms[17]. Healthy gut microbiomes, as assessed by 16S rRNA operon sequencing, comprise over 50 phyla, are consistently dominated by bacterial phyla, and predominantly by the bacterial genera Bacteroidetes and Firmicutes[18] that belong to the bacterial phyla Bacteroidota and Bacillota, respectively, as determined by ribosomal multilocus sequence typing[19]. Gut microbiota are represented by over 1000 species of microorganisms (defined also as species-level phylotypes i.e. clusters of sequences with about as much diversity in their small subunit rRNA genes as in validly named species) that are estimated at over 1 × 1014 bacterial cells per mL of faeces. Many studies have shown bidirectional crosstalk among the gut microbiota and the liver along the so-called ‘gut-liver’ axis. This special communication controls gastrointestinal health and disease and exploits environmental and host mediators. The reciprocal interaction between the gut and liver is facilitated by the portal vein that transports gut-derived products directly to the liver. In turn, the liver secretes bile and antibodies that travel to the intestine. The intestinal barrier is a crucial anatomical and functional structure that facilitates interactions between the gut and the liver. It limits the systemic dissemination of microbes and toxins while allowing nutrients to access the circulation and reach the liver. Maintaining homeostasis of the gut-liver axis is dependent on controlling microbial communities. The liver plays a key role in shaping these communities through bidirectional communication[20]. Sig
| Increase | Decrease |
| Genus/species (phylum) | Genus/species (phylum) |
| Anaerobacter (Firmicutes) | Akkermansia muciniphila (Verrucomicrobiota) |
| Clostridium (Firmicutes) | Alistipes (Bacteroidetes) |
| Desulfovibrio (Thermodesulfobacteriota) | Bifidobacterium (Actinomycetota) |
| Enterobacter (Proteobacteria) | Faecalibacterium prausnitzii (Firmicutes) |
| Escherichia (Proteobacteria) | Flavonifractor (Firmicutes) |
| Fusobacterium (Fusobacteriota) | Lactobacillus (Firmicutes) |
| Mucispirillum (Deferribacterota) | Odoribacter (Bacteroidetes) |
| Peptoniphilus (Firmicutes) | Oscillibacter (Firmicutes) |
| Ruminococcus (Firmicutes) | Prevotella (Bacteroidetes) |
| Streptococcus (Firmicutes) | Rikenellaceae (Bacteroidetes) |
The emergence of novel therapeutic approaches based on or directed at mechanisms related to gastrointestinal hormones makes them an interesting topic for the analysis of interactions with other mechanisms of NAFLD patho
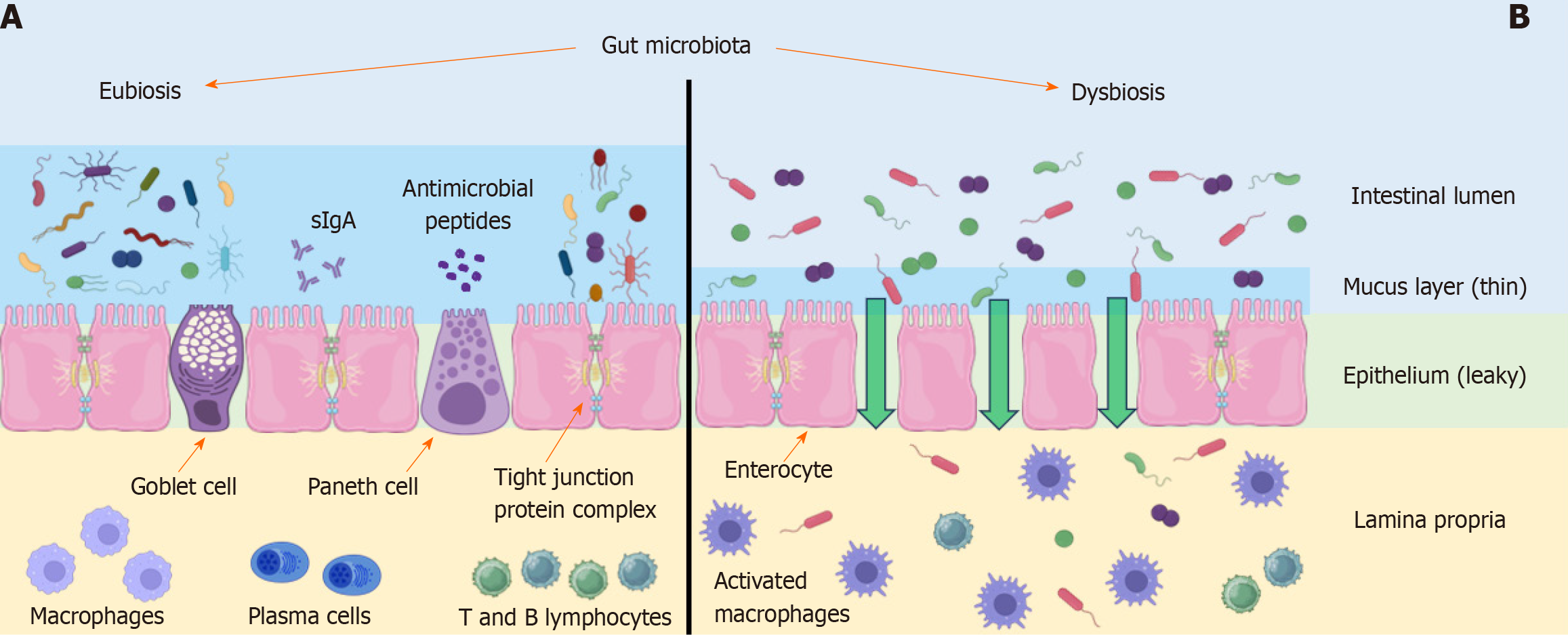
The intestinal barrier permits the selective transportation of nutrients from the intestinal lumen while restricting the transportation of pathogens and toxic metabolites. When the intestinal barrier becomes more permeable, bacterial endotoxins and digestion products can pass through non-selectively into the portal vein lumen, ultimately reaching the liver[28]. The permeability of the intestinal barrier depends on several factors, including the protective mucosal layer, antimicrobial peptides, tight junction proteins, and immune cells[29]. The mechanisms involved in the disruption of this barrier are shown in Figure 1. In patients with NAFLD, a change in the structure of gut microbiota, together with an increase in intestinal permeability, increases hepatic exposure to bacterial products from the gut, leading to the induction of metabolic endotoxemia and changes in the functionality of the ‘gut-liver axis’[30].
The role of the mucus layer is to protect intestinal cells from external agents and to facilitate nutrient absorption. It consists of heavily glycosylated proteins released by the gut goblet cells[31]. The mucus layer not only represents an important bacterial niche but also displays antimicrobial properties derived from the antimicrobial peptides secreted by Paneth cells, such as defensins and IgA. The thickness and composition of the mucus layer influence the properties of this bacterial niche, while the bacteria can also impact the properties of the mucus layer[32]. Mucispirillum sp. bacteria present in MAFLD patients cause disruptions of the intestinal mucosal surface[33]. Intestinal dysbiosis inhibits mucin 2 gene expression, reducing the production of the main intestinal mucin and primary component of the mucus layer[32]. As the symptoms of liver damage associated with MAFLD worsen, an increase in the level of bacterial proteins in blood can be observed that is caused by increased permeability of the intestinal barrier[34].
Tight junction proteins, normally seal the junction between intestinal endothelial cells and have a vital role in preventing translocation of harmful substances from the gut into the portal system. MAFLD syndrome is characterized by a decrease in the levels of these proteins. Dysbiosis leads to an increase in endotoxin levels in the gut lumen. Lipopolysaccharide (LPS), an endotoxin of gram-negative bacteria, plays a key role in MAFLD progression through activation of toll-like receptor 4 (TLR4) in the small intestine and liver[35]. In the intestine, TLR4 forms an aggregated receptor complex with the membrane-bound protein CD14 and myeloid differentiation protein 2, as well as other adaptor proteins. CD14 plays a specific role in this complex as it is responsible for the binding of the LPS/LPS binding protein complex[36]. In the study by Nighot et al[37], it was shown that stimulation of the TLR4 receptor complex in the gut by LPS leads to acti
Immune cells of the intestinal barrier are located in the lamina propria and Peyer's patches. These cells provide protection against harmful substances but also induce tolerance to harmless substances and commensal bacteria[43]. In people with MAFLD, the number of T lymphocytes in the lamina propria is reduced and levels of pro-inflammatory cytokines such as tumour necrosis factor (TNF)-α, interleukin 6, and interferon gamma are increased compared to healthy people. Interestingly, levels of pro-inflammatory bacteria such as Escherichia coli and Streptococcus sp. are increased in NAFLD[23]. These factors are associated with impaired immune function and the subsequent increase in intestinal permeability further mediates the pathogenesis of NAFLD via the gut-liver axis.
The above examples suggest that multiple factors influence gut barrier integrity in MAFLD, and that gut microbiota are critical in this process, highlighting the importance of maintaining gut homeostasis.
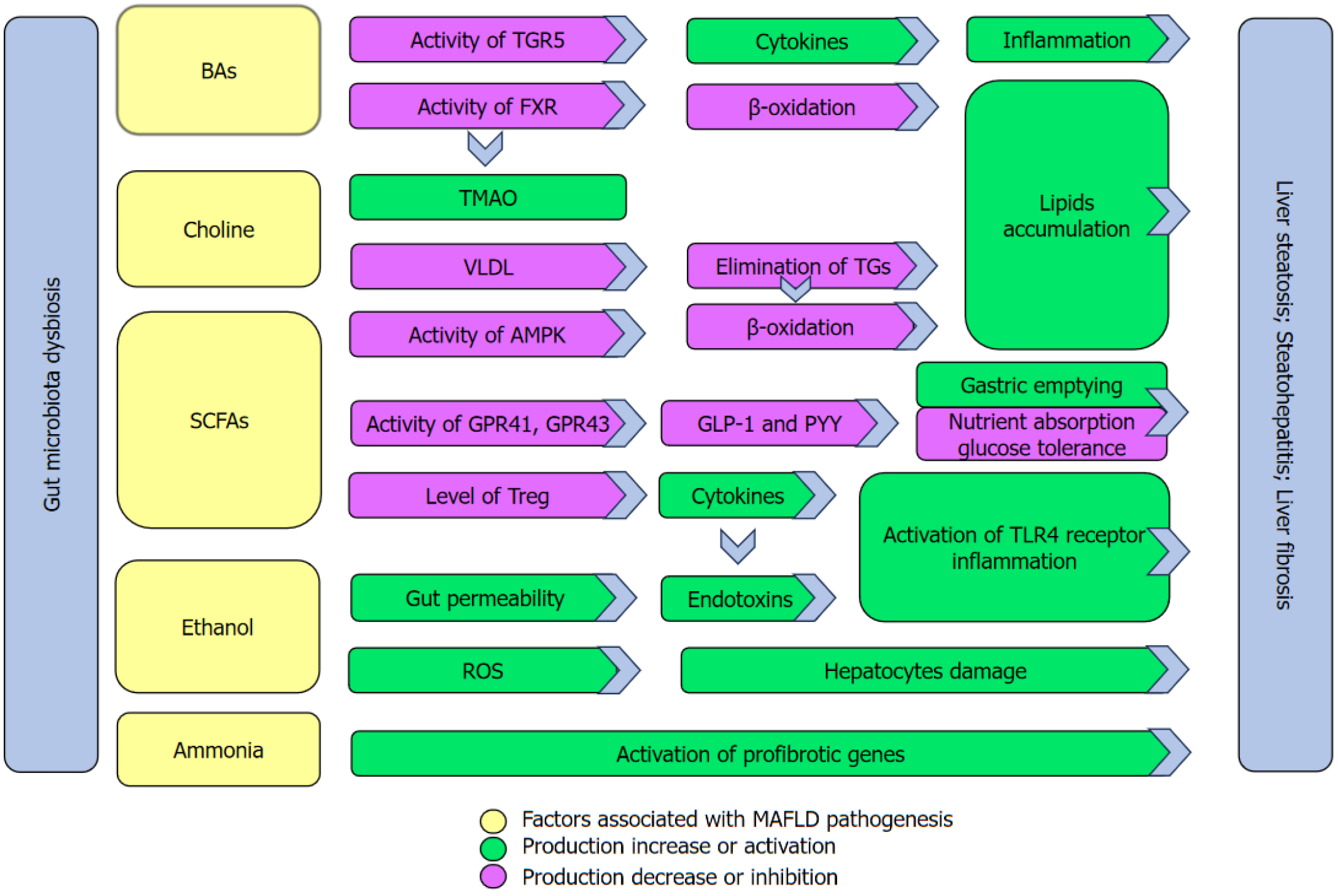
The pathogenesis of MAFLD is mediated by numerous metabolites, such as bile acids (BAs), choline, trimethylated N-oxide (TMAO), short-chain fatty acids (SCFAs), branched-chain amino acids (BCAAs), ammonia and endogenous ethanol[44]. The mechanisms associated with metabolite disruption in MAFLD progression are presented in Figure 2.
BAs: BAs are synthesized in the liver through complex cholesterol metabolism. They are divided into primary BAs, such as cholic acid (CA) and chenodeoxy CA (CDCA), and secondary BAs, such as deoxy CA (DCA) and litho CA (LCA). Primary BAs are conjugated with either glycine or taurine and subsequently stored in the gallbladder until they are released into the intestine after a meal. Once in the intestine, BAs facilitate the absorption of fats, cholesterol, and fat-soluble vitamins. Primary BAs undergo deconjugation, dehydroxylation, oxidation, and desulfation by gut microbiota, leading to the formation of more hydrophobic secondary BAs. The secondary BAs are reabsorbed in the distal ileum and transported back to the liver via the portal vein[45]. The deconjugation process involves bacteria representing the following phyla: Firmicutes, Proteobacteria, and Bacteroidetes, and the species representing the following genera: Bacillus, Staphylococcus, Bacteroides, Lactobacillus, Clostridium, and Enterococcus. They produce bile salt hydrolases that deconjugate the taurine and glycine groups in the primary BAs produced in the liver[46]. Firmicutes bacteria, particularly strains belonging to the genus Clostridium, play an important role in the dehydroxylation process. They produce BA 7α-dehydroxylase that converts primary BAs (CA and CDCA) into secondary BAs (DCA, LCA)[47,48]. The main bacteria involved in BA oxidation are from the genera: Bacteroides, Clostridium, Eubacterium, Escherichia, Eggerthella, Peptostreptococcus, and Ruminococcus. They produce BA hydroxysteroid dehydrogenase that converts toxic BAs into urodeoxycholic acid that is less toxic to human cells and more water-soluble[49]. Finally, several intestinal bacteria, such as Clostridium sp. strain S2, produce sulfatases that can increase the desulfation of BAs. Desulfation of BAs by intestinal bacteria helps with the reabsorption of BAs and is crucial for maintaining homeostasis in the BAs pool[48,50]. Disruption of BA metabolism occurs during the development of NAFLD, leading to a characteristic pattern of BAs in patients with NASH. Patients with MAFLD, and particularly with MASH, exhibit increased hepatic primary and secondary BAs production, resulting in higher levels of total BAs in the blood. The results of a recent meta-analysis show that different geographic locations or disease severity influence the diversity in BA profiles. In particular, the elevated levels of taurocholic acid (TCA), taurodeoxycholic acid, taurolithocholic acid, and glycolithocholic acids were observed in patients with MASH[51]. The increase in the concentration of glycocholic acid and TCA in serum was observed to be a relevant factor of severe liver fibrosis (> F2)[52]. As signaling molecules that regulate glucose, lipids, and the immune system, BAs contribute to host cell metabolism mainly through the farnesoid X receptor (FXR) and Takeda G protein-coupled receptor 5 (TGR5)[53]. CDCA is the most potent FXR agonist of all of the major BAs, followed by CA > LCA > DCA[54]. The most potent BA ligands for TGR5 are ranked as follows: LCA > DCA > CDCA > CA[55]. Activation of the FXR leads to improved glucose uptake by adipocytes through the induction of fibroblast growth factor 19. However, FXR also prevents the expression of sterol regulatory element-binding protein 1c and stimulates peroxisome proliferator-activated receptor alpha that inhibits lipid accumulation in the liver and increases the efficiency of β-oxidation[56,57]. Activation of TGR5 increases glucagon-like peptide-1 (GLP-1) secretion, leading to increased glucose-dependent insulin secretion and reduced appetite, by inhibition of neuropeptide Y (NPY) and agouti-related neuropeptide neurons in the arcuate area[58,59]. Furthermore, BA-induced activation of the TGR5 receptor on Kupffer cells has been shown to inhibit LPS-induced cytokine expression, suggesting its potential role in the immune response[60]. Both receptors play a role in reducing BA secretion through a negative feedback loop by inhibiting the expression of CYP7A1[61]. Patients with NAFLD have a reduced expression of FXR and increased levels of triglycerides (TGs) in serum[62]. In addition, the level of DCA, is elevated compared to CDCA[63]. The observed alterations in BA levels and compositions may result in a reduction in the capacity of the FXR and TGR5 receptors that in turn may contribute to the development of insulin resistance and an increase in lipid accumulation[52].
Choline: Choline is a crucial nutrient serving as the primary donor of methyl groups. It is obtained from diet, although choline synthesis can occur de novo in the liver[64]. In the liver, choline plays an important role in the synthesis of very low-density lipoprotein that facilitates the elimination of excess TGs. Therefore, dietary choline deficiency is an important factor in the development of MAFLD[65]. Choline is partly metabolized by intestinal microbiota in the gut, forming trimethylamine (TMA), which then travels to the liver through the portal circulation. In the liver, TMA is oxidized, leading to the formation of TMAO. The bacteria primarily engaged in choline metabolism in the gut belong to the phylum Proteobacteria (specifically the species Proteus penneri and Providencia rettgeri) and Firmicutes (Anaerococcus hydrogenalis, Clostridium asparagiforme, Clostridium hathewayi, and Clostridium sporogenes)[66]. A choline-poor diet and the presence of intestinal dysbiosis leads to increased TMAO accumulation in the liver that may result in the development of MAFLD due to decreased choline bioavailability[67]. Furthermore, TMAO may aggravate liver steatosis by suppressing BA-mediated hepatic FXR signaling[68]. TMAO levels show a robust correlation with hypertension and cardiovascular disease, suggesting its potential use as a cardiovascular biomarker in individuals with MAFLD[69].
SCFAs: SCFAs consist of molecules with 1 to 8 carbon (C) atoms and are produced primarily by the fermentation of dietary fiber in the gut. Acetate (C2), propionate (C3), and butyrate (C4) constitute the largest proportion of SCFAs in the human body[70]. Bacteroides spp., Anaerostipes spp., and other gut bacteria are primarily responsible for the production of SCFAs[71]. People with MAFLD have reduced levels of total SCFAs, particularly acetate, propionate, and butyrate, compared to healthy people[72]. SCFAs, especially butyrate, are involved in the nourishment of intestinal epithelial cells, and a decrease in SCFA levels is associated with an increase in intestinal barrier permeability. In the liver, propionate (C3) acts as a substrate for gluconeogenesis and inhibits cholesterol synthesis. Meanwhile, acetate is used as a substrate for the synthesis of long-chain fatty acids, glutamine, glutamate, and beta-hydroxybutyric acid. Butyrate is oxidized directly by hepatocytes, thereby preventing potentially harmful systemic concentrations[70]. Three G-protein receptors (GPRs) are known to interact with SCFAs: GPR41, GPR43, and GPR109A. Butyrate interacts predominantly with GPR41 and GPR109A receptors, whereas acetate and propionate have an affinity for GPR43[73]. It is noteworthy that GPR41 and GPR43 are expressed in human white adipose tissue, skeletal muscle, and liver, suggesting that SCFAs may have a direct effect on substrate metabolism in peripheral tissues[74]. Interaction with these receptors determines several physiological functions, such as the generation of ROS, promotion of neutrophil chemotaxis, and regulation of T-regulatory cells. Experimental studies have demonstrated the role of butyrate in regulating the transcription of forkhead box protein 3 factor that acts as a mediator in Treg cell development and activity whilst also suppressing the release of pro-inflammatory cytokines[75]. Acetate and butyrate may also inhibit LPS-induced TNF-α release and nuclear factor-kappa B (NF-κB) activation, leading to reduced inflammation in the liver[76] A reduction in SCFAs levels is the cause of disruption of these processes that leads to liver steatohepatitis and defective gut integrity[77]. SCFAs inhibit the growth of micro-organisms by lowering the pH in the intestinal lumen. It has been observed that acetate generated by Bifidobacterium spp. can restrain the growth of enteropathogenic bacteria[78]. Moreover, both in vitro and in vivo studies have indicated that elevated concentrations of butyrate are linked to an augmented production of mucin and a decrease in bacterial atta
BCAAs: BCAAs, leucine, isoleucine, and valine, form a group of proteinogenic essential amino acids possessing a branched aliphatic side chain structure. They act as signaling molecules regulating the metabolism of glucose, lipids, protein synthesis, intestinal health, and immunity via a special signaling network, especially PI3K/AKT/mTOR signal pathway[84]. Previous reports have shown that the expression and activity of BCAA catabolic enzymes are altered in metabolic disorders. In patients with T2DM, these enzymes are downregulated[85]. Clinical studies have shown that elevated plasma BCAA levels correlate with insulin resistance and increased risk of T2DM[86]. The mechanism for this is not well understood. One potential cause of insulin resistance is the persistent activation of mTOR that may uncouple the insulin receptor from the insulin signalling mediator, insulin receptor substrate 1. Another potential mechanism is the abnormal metabolism of BCAAs in obesity that results in the accumulation of toxic BCAA metabolites. These metabolites can trigger mitochondrial dysfunction and stress signaling that are associated with insulin resistance and T2DM[87]. Interestingly, bariatric surgery was observed to cause a decrease in BCAA levels in obese individuals[88]. The bacteria Prevotella copri and Bacteroides vulgatus that correlate strongly with the amount of BCAAs, appear to be closely involved in the development of insulin resistance[89]. However, oral supplementation of BCAAs resulted in increased levels of beneficial gut microbiota, including Ruminococcus flavefaciens and Bifidobacterium sp. and induced a reduction in hepatic fat accumulation[90]. Additionally, the elevated level of Bifidobacterium sp. resulted in an increase in GLP-1 secretion through the increase in SCFAs, particularly acetate[91]. The contradictory role of BCAAs may be due to the varied composition of gut microbiota. Further research is necessary to better understand the mechanism between gut microbiota composition and the role of BCAAs in MAFLD pathogenesis.
Ammonia:In vitro and in vivo studies have shown that hepatic steatosis results in a reduction in the efficiency of urea cycle enzymes. In the study by De Chiara et al[92] reduced levels of ornithine transcarbamylase and carbamoyl phosphate synthetase, both urea cycle proteins, were observed in patients with NAFLD. This leads to an accumulation of ammonia in the liver that activates the profibrotic genes in hematopoietic stem cells (HSCs)[93]. Fibrosis is the major cause of the increased mortality observed in patients with NAFLD, and the prevention and reduction of fibrosis should be the main aims of treatment[94]. Ammonia is produced from amino acids by gut microbiota. Therefore, the composition of gut microbiota plays an essential role in the levels of circulating ammonia. In vivo studies have shown that absolute anaerobic gram-positive bacteria (members of the genus Clostridium) and gram-negative bacilli of the family Enterobacteriaceae are the major contributors to ammonia production[95]. Furthermore, elevated levels of Escherichia coli belonging to Enterobacteriaceae have been consistently linked to the occurrence of NAFLD[96]. Ammonia is a neurotoxic compound that travels easily across the blood-brain barrier. Inflammation that increases as MAFLD progresses also contributes significantly to the development of hepatic encephalopathy via the gut-liver-brain axis and appropriate modulation of gut microbiota dysbiosis appears to be critically important in therapeutic strategies[97,98].
Endogenous ethanol: Endogenous ethanol synthesis by gut microbiota is another contributor to the development of MAFLD. Blood alcohol levels are increased after intake of alcohol-free food. However, these endogenous alcohol levels are much higher in people with NAFLD than in healthy individuals[99]. Ethanol produced in the gut stimulates NF-κB signaling molecules that can provoke tissue damage. This damage can increase intestinal permeability, leading to higher levels of LPS in the hepatic portal system. The increased LPS levels can then cause inflammation in the liver by activating TLR4 and the inflammasome[100]. In addition, alcohol dehydrogenase (ADH) levels are significantly lower in people with NAFLD than in healthy people[101]. This impaired detoxification process can lead to an increase in the production of ROS, resulting in oxidative damage to hepatocytes[102]. Bacteria from class Gammaproteobacteria (particularly Escherichia coli, Klebsiella pneumoniae, and other members of the Enterobacteriaceae family) are involved in the process of endogenous alcohol synthesis[100]. Impairments in insulin signaling may alter ADH activity in the liver, subsequently leading to impaired ethanol metabolism and elevated blood ethanol levels in patients with NAFLD[103]. Therefore, appropriate modification of gut microbiota with prevention of insulin resistance may be key to preventing the progr
GLP-1 is derived from the proglucagon gene that is also the genetic origin of glucagon-like peptide-2 (GLP-2) and glucagon. In alpha cells of the pancreas, the proglucagon gene is processed to produce glucagon whose expression is stimulated by fasting and inhibited by insulin. In the enteroendocrine L-cells of the intestine, proglucagon gene expression decreases in a fasting state and increases during food consumption. Proglucagon is processed to produce GLP-1, GLP-2, and glicentin[104,105].
GLP-1 is secreted into the portal circulation from intestinal L cells that are located with increasing abundance from the duodenum to the colon. The L-cells are stimulated by nutrients, neural, and endocrine mechanisms. Different types of nutrients, such as sugars, fatty acids, amino acids, and dietary fiber, stimulate GLP-1 secretion. Secretion of GLP-1 is also stimulated by gut microbiota and their metabolites, such as SCFAs[106].
GLP-1 is an incretin, and this implies that it enhances the secretion of insulin in a glucose-dependent manner leading to a decrease in glycemia. It has also been shown to inhibit glucagon secretion when glycemia increases above fasting levels. The other peptide belonging to the incretin family is the gastric inhibitory polypeptide (GIP) secreted from K cells present in the upper intestine that also promotes postprandial insulin secretion. Besides its insulinotropic effects, GLP-1 has also been associated with various regulatory and protective effects. Unlike GIP, the action of GLP-1 is preserved in patients with T2DM[107]. GLP-1 inhibits gastric emptying and reduces acid secretion and motility, and this promotes satiety. Food intake is also reduced as a result of GLP-1 action in the hypothalamus and brainstem. GLP-1 exerts protective and regulatory effects in different tissues, including the liver, brain, heart, adipose tissue, muscles, bones, kidneys, and lungs[108].
A reduction in body weight and insulin resistance are the main mechanisms induced by GLP-1 that contribute to a decrease in hepatic steatosis, inflammation, and fibrosis observed in NASH. The anti-inflammatory effects of GLP-1 may also be mediated by modulating Kupffer cell activity in the liver and via anti-fibrotic effects involving inhibition of HSC activation[109].
There is limited evidence supporting the expression and function of GLP-1 receptors in hepatic tissue and their potential to reduce steatosis[110]. An RNA sequencing study by Boland et al[111] did not find GLP-1 receptor transcripts in bulk human liver and isolated non-parenchymal cells and this is a strong argument against GLP-1 receptor expression in human liver tissue. Therefore, currently, the beneficial effects of GLP-1 are attributed to indirect mechanisms.
GLP-2 is derived from the same gene as GLP-1 and is co-secreted, but its role is to repair the intestinal lining. It improves nutrient absorption, reduces gut permeability, and stimulates cell proliferation in the gut[112]. In a murine model, GLP-2 also showed a beneficial hepatic influence. Fuchs et al[113] have reported that GLP-2 treatment attenuated the activation of HSCs. GLP-2 promoted intestinal FXR-Fgf15/19 signaling resulting in reduced CYP7A1 and increased CYP2C70 expression in the liver, contributing to hepatoprotective and antifibrotic effects of GLP-2 in the Mdr2-/-mouse model.
Endogenous GLP-1 undergoes rapid degradation by the enzyme dipeptidyl peptidase-4 (DPP-4). Two different strategies were employed to increase the effects of GLP-1: Inhibition of degradation or exogenous administration of GLP-1 RAs resistant to degradation. Endogenous GLP-1 is degraded by DPP-4 at various sites including the intestine (75%) and the liver (further 50%) that leaves only 10%-15 % of intact GLP-1 in circulation[114]. DPP-4 inhibitors have been developed to increase GLP-1 activity and are commonly used in the treatment of T2DM. Their action leads to an increase in incretin hormones, GLP-1 and GIP, and a decrease in glucose and glucagon levels. Circulating GLP-1 increases by about 2-4 fold[115]. GLP-1 RAs lead to a 10-fold increase in GLP-1 and their effect is persistent. This difference in the resulting GLP-1 activity translates into almost no weight loss with DPP-4 inhibitors.
GLP-1 RAs in obesity: GLP-1 RAs originally introduced as pharmacotherapy for T2DM improved glycemic control and also promoted weight loss that was uncommon among hypoglycemic agents. GLP-1 RAs became the preferred treatment in patients with T2DM and obesity and then obesity alone. Among GLP-1 RAs, liraglutide 3.0 mg once daily was the first medication approved by the United States Food and Drug Administration and European Medicines Agency for the treatment of obesity. The results of the SCALE trial including more than 3700 patients with obesity [body mass index (BMI) > 30] or being overweight (BMI > 27) with comorbidities confirmed its effectiveness in weight reduction. However, the benefit of 5.4% body weight loss compared to placebo observed during treatment gradually decreased after treatment cessation[116]. The STEP 5 trial assessed the efficacy and safety of a once-weekly subcutaneous dose of 2.4 mg sema
GLP-1 RAs in MAFLD: The American Association for the Study of Liver Diseases guidelines published in 2018 and joint guidelines of the European Association for the Study of the Liver, European Association for the Study of Diabetes, and European Association for the Study of Obesity published in 2016 do not consider GLP-1 RAs for the treatment of liver disease in patients with NAFLD or NASH[120,121]. Similarly, a recent review of published guidelines presents current management of NAFLD that is based on lifestyle changes that promote an energy deficit leading to weight loss. In patients with obesity and NAFLD, weight loss medications may be considered, particularly GLP-1 RAs and bariatric surgery for the morbidly obese. In patients with T2DM and NAFLD, diabetes medications could be used, such as pioglitazone and GLP-1 RAs[122]. Pharmacological therapies designed specifically for NAFLD are lacking[123]. Lifestyle modification based on a low-calorie diet and physical activity remain the mainstay of the therapy for patients with fatty liver[124]. Weight loss of 7%-10% is an effective treatment for NAFLD that results in improvement of histological features of steatosis and fibrosis[121]. The lifestyle interventions and drugs suggested in NAFLD therapy are frequently commonly used in the treatment of T2DM, metabolic syndrome, or obesity. These pharmacological treatments include pioglitazone, metformin, SGLT2 inhibitors, and GLP-1 RAs. Minimally invasive approaches, including metabolic surgery and endoscopic bariatric procedures, are also effective in patients with metabolic syndrome, fatty liver, and morbid obesity[125,126].
GLP-1 RAs are effective in T2DM and promote weight loss, ameliorate liver enzyme level perturbations and liver steatosis in patients with NAFLD/NASH[127]. In a phase 2 trial of semaglutide, 40% of NASH resolution was reported in patients with or without T2DM[128].
As GLP-1 RAs can reduce body weight, several studies have investigated the potential for GLP-1 RAs in the treatment of NAFLD and NASH. A meta-analysis published in 2016 that included 136 patients with NAFLD and T2DM treated with either GLP-1 RAs (exenatide twice daily or liraglutide) or DPP4 inhibitors, concluded that incretin-based treatment was effective in reducing biochemical biomarkers of NAFLD and significantly reduced signs of inflammation, steatosis and fibrosis in biopsy samples and observed via imaging[129]. In the LEAN study that included 52 patients with NASH, a dose of 1.8 mg liraglutide once daily resulted in biopsy-confirmed resolution of NASH in 39% of patients, compared with 9% in the placebo group[130]. Furthermore, in a study comparing 26 weeks of treatment with 3.0 mg liraglutide once daily to lifestyle intervention (the currently recommended treatment), patients in both groups achieved similar reductions in levels of alanine aminotransferase, liver fat fraction, liver stiffness, and body weight[131]. In a murine study of streptozotocin- and high-fat diet (HFD)-induced T2DM and NASH, liraglutide significantly ameliorated steatosis, inflammation, hepatocyte ballooning, and suppressed hepatocarcinogenesis[132].
Semaglutide treatment resulted in contradictory results. In a recent study by Loomba et al[133] in patients with NASH and compensated cirrhosis, semaglutide did not significantly improve fibrosis or achieve resolution of NASH vs placebo. In a study by Newsome et al[128], the percentage of patients in whom NASH resolution was achieved with no worsening of fibrosis was dose-dependent and reached 59% in the 0.4-mg group vs 17% in the placebo group (P < 0.001). Scavo et al[134] described a mechanism by which GLP-1 RAs may improve NASH. In patients responding to therapy with semaglutide, the authors suggested a mechanism for reducing activation of HSCs and downregulation of extracellular matrix components such as vimentin, collagen, and fibronectin. Further, recent large retrospective cohort studies with GLP-1 RAs application revealed its potential beneficial impact in patients with chronic liver diseases and T2DM to decrease the risk of major adverse liver outcomes, including hepatic decompensation, portal hypertension, HCC, and liver transplantation. These findings suggest that treatment with GLP-1 RAs may be a promising option for reducing the risk of chronic liver disease progression[135,136].
GLP-1 RAs are known to affect the intestinal environment and, changes in the gut microbiota have been linked to GLP-1 RAs[137]. In general, GLP-1 is thought to have two effects, one exerted via the central nervous system and the other exerted via local receptors in the periphery. In a pioneer study investigating the mechanisms of GLP-1 RA-related changes in gut microbiota, Kato et al[138] reported a release of norepinephrine into the intestinal lumen in vitro and an activation of the sympathetic nervous system by acute administration of GLP-1 RAs with a concomitant rapid increase in E. coli in vivo. At the phylum level, liraglutide administration significantly decreased Bacteroidetes and tended to increase Actinobacteria. However, Firmicutes and Proteobacteria were not changed. At the genus level, liraglutide administration significantly reduced Ruminococcus spp. and did not increase Akkermansia spp. The gene expression levels of bacterial proteins that could affect the host metabolism were analysed. Expression of formate-tetrahydrofolate ligase that is related to acetic acid synthesis, was significantly increased and, conversely, the expression of butyryl-CoA: Acetate CoA-transferase that is related to butyrate synthesis, was significantly decreased. The increase in E. coli may help to promote bacterial translocation by attenuating intestinal tight junction gene levels under stress conditions such as colitis.
Chaudhari et al[139] described a GLP-1 activation pathway involving TGR5 agonist-CA-7-sulfate (CA7S). A microbial metabolite, LCA, is increased in murine portal veins after sleeve gastrectomy (SG) and by activating the vitamin D receptor, induces hepatic SULT2A enzyme expression to drive CA7S production. An SG-induced shift in the microbiome increases gut expression of the BA transporters Asbt and Ostα that in turn facilitate selective transport of LCA across the gut epithelium. Activation of this gut-liver pathway leads to CA7S synthesis and GLP-1 secretion, causally connecting a microbial metabolite with glucoregulatory benefits of SG in human patients.
Several novel agents acting as dual and triple incretin agonists have been developed.
GLP-1R/GcgR (glucagon receptor): The anti-obesity effects of dual GLP-1R/glucagon receptor agonists were first demonstrated in diet-induced obese mice, where dual agonism reduced body weight, hyperglycemia, and hepatic lipid compared with that resulting from GLP-1 RA treatment alone[140]. Ambery et al[141] studied a dual-agonist Cotadutide (MEDI0382) in overweight patients with T2DM and observed a significant improvement in post-prandial glucose excursions and reductions in body weight and liver fat. Others have shown that a related dual agonist can reduce liver fat and slow the development of hepatic fibrosis in HFD and HFD/carbon tetrachloride mouse models[142]. Furthermore, in methionine- and choline-deficient diet-fed mice with partial hepatectomy, treatment with a similar dual agonist reduced inflammation, cell death, and improved hepatic regeneration[143]. These data are suggestive of GLP-1/Gcg RAs as potential therapeutics. However, the studies were prophylactic, rather than interventional, and not conducted in a pathophysiologically relevant model of NASH[144]. Cotadutide has been engineered to balance GLP-1 and Gcg RAs (with an approximately 5:1 bias towards GLP-1 receptor affinity) to optimize beneficial outcomes for metabolic disease.
GLP-1/GLP-2: Interesting effects are expected from a combined intervention targeting both GLP-1 and GLP-2 receptors that will impact glucose homeostasis and intestine integrity. In a rodent study, GLP-1/GLP-2 co-agonists revealed effects on gut morphometry, showing a marked increase in intestinal volume and mucosal surface area. Furthermore, effects on glucose tolerance and long-term glycemic control were evident as well as a decrease in body weight and delayed gastric emptying[145]. Madsen et al[146] reported similar gut bacterial compositional changes following liraglutide and dual GLP-1/GLP-2 RA treatment, characterized by discrete shifts in low-abundance species and related bacterial metabolic pathways. Both compounds suppressed caloric intake, promoted a marked weight loss, improved glucose tolerance, and reduced plasma cholesterol levels. These microbiome alterations may be associated with the converging biological actions of GLP-1 and GLP-2 receptor signaling on caloric intake, glucose metabolism, and lipid handling. In another pre-clinical study, a GLP-1/GLP-2 receptor dual agonist improved BMI, glucose homeostasis, liver TGs, liver fibrosis, and intestinal barrier permeability in a murine model of NASH. SCFA-producing Bifidobacterium spp. increased, as well as bacteria associated with a healthy phenotype such as Prevotella spp., Lactobacillus spp., and Akkermansia spp. Firmicutes, associated with obesity, were decreased[147].
The metabolic effects of bariatric surgery may be attributed to a positive influence on the secretion of multiple gut hormones that results in an early improvement in glycemic control in T2DM patients[148]. These include insulin, GLP-1, GLP-2, GIP, PYY, CCK, OXY, and ghrelin. The profile of gastrointestinal hormone secretion after bariatric surgery depends on the surgical technique applied. The model technique in metabolic surgery is Roux-en-Y Gastric Bypass (RYGB). After RYGB, postprandial GLP-1 levels increase, while fasting levels remain unchanged. In a less malabsorptive version with a shorter Roux limb length, the increase in postprandial GLP-1 is more pronounced[149]. Other common procedures, such as SG and mini-gastric bypass, are based on different anatomical principles but also lead to an improvement in the hormone profile[150].
The idea to pharmacologically replicate the effects of RYGB with multiple peptide receptor agonists is particularly attractive. In a study by Behary et al[151], obese patients with prediabetes or diabetes were randomized to receive an infusion of GLP-1, oxyntomodulin, and PYY (GOP) that replicates post-surgical post-prandial gut hormone levels vs saline for 4 weeks. The two groups of RYGB patients and very-low-calorie diet (VLCD) patients served as comparators. The glucose tolerance and variability were better with GOP, but weight loss was inferior to both RYGB and VLCD.
Both RYGB and SG lead to improvement in liver disease in patients with obesity and NAFLD or NASH. RYGB results in a significant reduction of steatohepatitis and fibrosis while SG improves steatohepatitis but does not reverse fibrosis. Both procedures significantly improve the NAFLD Activity Score and biochemical indices[152].
After bariatric surgery, gut microbiota have been considered as a factor associated with metabolic improvements and weight loss. The observed alterations in microbiota vary between procedures and individual patients. In a recent systematic review of 18 trials, Coimbra et al[153] reported greater amount of Bacteroidetes, Proteobacteria, and diversity after BS. Firmicutes, Bacteroidetes, and the Firmicutes to Bacteroidetes ratio was inconsistent, increasing or decreasing after RYGB and SG. There was a reduction in the relative proportion of Firmicutes. Moreover, a higher proportion of Actinobacteria was observed after RYGB that were not observed after SG.
Changes in GLP-1, GLP-2, and the gut microbiome were studied after RYGB. After surgery, zonulin decreased and an increase in area under the curve (AUC) after the meal tolerance test was observed for GLP-1 and GLP-2. Species belonging to Streptococcaceae, Akkermansiaceae, Rickenellaceae, Sutterellaceae, Enterobacteriaceae, Oscillospiraceae, Veillonellaceae, and Fusobacteriaceae families increased after intervention and correlated positively with AUC of GLP-1 and GLP-2, and negatively with glucose, hemoglobin A1c, TGs, and adiposity markers. Clostridium perfringens and Roseburia sp. 40_7 behaved similarly. In contrast, some species belonging to Lachnospiraceae, Erysipelotrichaceae, and Oscillospiraceae families decreased and showed opposite correlations[154]. Table 2 presents the changes in gut microbiota following various types of treatment.
| Bacteria levels | GLP-1 RAs | GLP-1/GLP-2 RA | Roux-en-Y gastric bypass surgery |
| Increase | Bifidobacteriaceae | Bifidobacteriaceae | Bacteroidetes |
| Actinobacteria | Prevotellaceae | Actinobacteria | |
| Akkermansiaceae | Lactobacillaceae | Proteobacteria | |
| Akkermansiaceae | Akkermansiaceae | ||
| Decrease | Firmicutes | Firmicutes | Firmicutes |
| Oscillospiraceae | Oscillospiraceae | ||
| Bacteroidetes | |||
| Microbiota diversity | Increase | Increase | Increase |
Gut microbiota are strongly associated with the progression of MAFLD. The mechanisms involved in these effects are complex and depend on the functionality of the gut-liver axis with any alteration of gut permeability leading to bacterial translocation. There is also a significant role played by metabolites such as BAs, SCFAs, BCAAs, choline, ammonia, and ethanol. The composition of gut microbiota is altered in patients with obesity, metabolic syndrome, and MAFLD. It is affected by the above-mentioned pathogenic mechanisms but also influences all components of the gut-liver axis that results in a constant crosstalk. As the disease progresses from simple steatosis and early stages of MAFLD to advanced fibrosis and cirrhosis there is a loss of bacterial diversity and depletion of beneficial bacterial content. However, it is challenging to form any conclusive claims about gut microbiota profiles in MAFLD patients due to the variation in gut microbiota composition between MAFLD and non-MAFLD patients and also among different stages of MAFLD. Further insights into mechanisms and new therapeutic strategies might be offered by studies of the gut metabolome in patients with MAFLD. Gastrointestinal hormones such as insulin, glucagon, GLP-1, GLP-2, and others, are involved in the pathogenesis of MAFLD but also play an important role in maintaining gut-liver axis functionality through a complex network of reciprocal communication with gut microbiota. Novel therapeutics from a family of GLP-1 RAs effective in the treatment of T2DM and obesity have been proposed as a promising solution for patients with MAFLD. Not surprisingly, these agents affect many other physiological mechanisms and pathways including the gut-liver axis and the gut microbiome. Some insights into potential mechanisms of this interaction may be drawn from previous experience with bariatric surgery patients. Metabolic surgical procedures such as RYGB, SG, or one-anastomosis gastric bypass represent models of alterations in the gut microbiome with concomitant changes in the secretion of multiple gastrointestinal hormones in patients with MAFLD. Most of the mechanisms are not fully elucidated and further well-designed studies are needed to support therapeutic decision making as more complex therapies are being developed that involve multiple peptide agonists.
| 1. | Younossi Z, Tacke F, Arrese M, Chander Sharma B, Mostafa I, Bugianesi E, Wai-Sun Wong V, Yilmaz Y, George J, Fan J, Vos MB. Global Perspectives on Nonalcoholic Fatty Liver Disease and Nonalcoholic Steatohepatitis. Hepatology. 2019;69:2672-2682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1415] [Cited by in RCA: 1306] [Article Influence: 217.7] [Reference Citation Analysis (0)] |
| 2. | Inoue Y, Qin B, Poti J, Sokol R, Gordon-Larsen P. Epidemiology of Obesity in Adults: Latest Trends. Curr Obes Rep. 2018;7:276-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 205] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 3. | Guo W, Ge X, Lu J, Xu X, Gao J, Wang Q, Song C, Zhang Q, Yu C. Diet and Risk of Non-Alcoholic Fatty Liver Disease, Cirrhosis, and Liver Cancer: A Large Prospective Cohort Study in UK Biobank. Nutrients. 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 44] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 4. | Yki-Järvinen H. Non-alcoholic fatty liver disease as a cause and a consequence of metabolic syndrome. Lancet Diabetes Endocrinol. 2014;2:901-910. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 764] [Cited by in RCA: 934] [Article Influence: 84.9] [Reference Citation Analysis (0)] |
| 5. | Tilg H, Adolph TE, Moschen AR. Multiple Parallel Hits Hypothesis in Nonalcoholic Fatty Liver Disease: Revisited After a Decade. Hepatology. 2021;73:833-842. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 113] [Cited by in RCA: 248] [Article Influence: 62.0] [Reference Citation Analysis (0)] |
| 6. | Krawczyk M, Rau M, Schattenberg JM, Bantel H, Pathil A, Demir M, Kluwe J, Boettler T, Lammert F, Geier A; NAFLD Clinical Study Group. Combined effects of the PNPLA3 rs738409, TM6SF2 rs58542926, and MBOAT7 rs641738 variants on NAFLD severity: a multicenter biopsy-based study. J Lipid Res. 2017;58:247-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 149] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 7. | Alvares D, Hoffman S, Stankovic B, Adeli K. Gut peptide and neuroendocrine regulation of hepatic lipid and lipoprotein metabolism in health and disease. Biochim Biophys Acta Mol Cell Biol Lipids. 2019;1864:326-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 8. | Grabherr F, Grander C, Effenberger M, Adolph TE, Tilg H. Gut Dysfunction and Non-alcoholic Fatty Liver Disease. Front Endocrinol (Lausanne). 2019;10:611. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 68] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 9. | Alisi A, Carpino G, Oliveira FL, Panera N, Nobili V, Gaudio E. The Role of Tissue Macrophage-Mediated Inflammation on NAFLD Pathogenesis and Its Clinical Implications. Mediators Inflamm. 2017;2017:8162421. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 97] [Cited by in RCA: 131] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 10. | Eslam M, Newsome PN, Sarin SK, Anstee QM, Targher G, Romero-Gomez M, Zelber-Sagi S, Wai-Sun Wong V, Dufour JF, Schattenberg JM, Kawaguchi T, Arrese M, Valenti L, Shiha G, Tiribelli C, Yki-Järvinen H, Fan JG, Grønbæk H, Yilmaz Y, Cortez-Pinto H, Oliveira CP, Bedossa P, Adams LA, Zheng MH, Fouad Y, Chan WK, Mendez-Sanchez N, Ahn SH, Castera L, Bugianesi E, Ratziu V, George J. A new definition for metabolic dysfunction-associated fatty liver disease: An international expert consensus statement. J Hepatol. 2020;73:202-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2883] [Cited by in RCA: 2798] [Article Influence: 559.6] [Reference Citation Analysis (1)] |
| 11. | Younossi ZM, Golabi P, de Avila L, Paik JM, Srishord M, Fukui N, Qiu Y, Burns L, Afendy A, Nader F. The global epidemiology of NAFLD and NASH in patients with type 2 diabetes: A systematic review and meta-analysis. J Hepatol. 2019;71:793-801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 773] [Cited by in RCA: 1485] [Article Influence: 247.5] [Reference Citation Analysis (0)] |
| 12. | Younossi ZM, Otgonsuren M, Henry L, Venkatesan C, Mishra A, Erario M, Hunt S. Association of nonalcoholic fatty liver disease (NAFLD) with hepatocellular carcinoma (HCC) in the United States from 2004 to 2009. Hepatology. 2015;62:1723-1730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 495] [Cited by in RCA: 615] [Article Influence: 61.5] [Reference Citation Analysis (0)] |
| 13. | Sberna AL, Bouillet B, Rouland A, Brindisi MC, Nguyen A, Mouillot T, Duvillard L, Denimal D, Loffroy R, Vergès B, Hillon P, Petit JM. European Association for the Study of the Liver (EASL), European Association for the Study of Diabetes (EASD) and European Association for the Study of Obesity (EASO) clinical practice recommendations for the management of non-alcoholic fatty liver disease: evaluation of their application in people with Type 2 diabetes. Diabet Med. 2018;35:368-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 89] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 14. | Buchwald H, Estok R, Fahrbach K, Banel D, Jensen MD, Pories WJ, Bantle JP, Sledge I. Weight and type 2 diabetes after bariatric surgery: systematic review and meta-analysis. Am J Med. 2009;122:248-256.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1816] [Cited by in RCA: 1732] [Article Influence: 108.3] [Reference Citation Analysis (0)] |
| 15. | Huang Z, Zhuang X, Huang R, Liu M, Xu X, Fan Z, Dai R, Li H, Xiong Z, Guo Y, Liang Q, Liao X. Physical Activity and Weight Loss Among Adults With Type 2 Diabetes and Overweight or Obesity: A Post Hoc Analysis of the Look AHEAD Trial. JAMA Netw Open. 2024;7:e240219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Reference Citation Analysis (0)] |
| 16. | Fan Y, Pedersen O. Gut microbiota in human metabolic health and disease. Nat Rev Microbiol. 2021;19:55-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 729] [Cited by in RCA: 2611] [Article Influence: 522.2] [Reference Citation Analysis (1)] |
| 17. | Marchesi JR, Ravel J. The vocabulary of microbiome research: a proposal. Microbiome. 2015;3:31. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 621] [Cited by in RCA: 665] [Article Influence: 66.5] [Reference Citation Analysis (0)] |
| 18. | Human Microbiome Project Consortium. Structure, function and diversity of the healthy human microbiome. Nature. 2012;486:207-214. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9292] [Cited by in RCA: 8038] [Article Influence: 618.3] [Reference Citation Analysis (2)] |
| 19. | Jolley KA, Bliss CM, Bennett JS, Bratcher HB, Brehony C, Colles FM, Wimalarathna H, Harrison OB, Sheppard SK, Cody AJ, Maiden MCJ. Ribosomal multilocus sequence typing: universal characterization of bacteria from domain to strain. Microbiology (Reading). 2012;158:1005-1015. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 349] [Cited by in RCA: 432] [Article Influence: 33.2] [Reference Citation Analysis (0)] |
| 20. | Albillos A, de Gottardi A, Rescigno M. The gut-liver axis in liver disease: Pathophysiological basis for therapy. J Hepatol. 2020;72:558-577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 542] [Cited by in RCA: 1240] [Article Influence: 248.0] [Reference Citation Analysis (1)] |
| 21. | Yang C, Xu J, Xu X, Xu W, Tong B, Wang S, Ji R, Tan Y, Zhu Y. Characteristics of gut microbiota in patients with metabolic associated fatty liver disease. Sci Rep. 2023;13:9988. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 21] [Reference Citation Analysis (0)] |
| 22. | Rizzatti G, Lopetuso LR, Gibiino G, Binda C, Gasbarrini A. Proteobacteria: A Common Factor in Human Diseases. Biomed Res Int. 2017;2017:9351507. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 388] [Cited by in RCA: 781] [Article Influence: 97.6] [Reference Citation Analysis (0)] |
| 23. | Jiang W, Wu N, Wang X, Chi Y, Zhang Y, Qiu X, Hu Y, Li J, Liu Y. Dysbiosis gut microbiota associated with inflammation and impaired mucosal immune function in intestine of humans with non-alcoholic fatty liver disease. Sci Rep. 2015;5:8096. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 473] [Cited by in RCA: 452] [Article Influence: 45.2] [Reference Citation Analysis (0)] |
| 24. | Lin YC, Lin HF, Wu CC, Chen CL, Ni YH. Pathogenic effects of Desulfovibrio in the gut on fatty liver in diet-induced obese mice and children with obesity. J Gastroenterol. 2022;57:913-925. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 41] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 25. | Song Q, Zhang X. The Role of Gut-Liver Axis in Gut Microbiome Dysbiosis Associated NAFLD and NAFLD-HCC. Biomedicines. 2022;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 80] [Article Influence: 26.7] [Reference Citation Analysis (0)] |
| 26. | Dai Y, He H, Li S, Yang L, Wang X, Liu Z, An Z. Comparison of the Efficacy of Glucagon-Like Peptide-1 Receptor Agonists in Patients With Metabolic Associated Fatty Liver Disease: Updated Systematic Review and Meta-Analysis. Front Endocrinol (Lausanne). 2020;11:622589. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 27. | Maestri M, Santopaolo F, Pompili M, Gasbarrini A, Ponziani FR. Gut microbiota modulation in patients with non-alcoholic fatty liver disease: Effects of current treatments and future strategies. Front Nutr. 2023;10:1110536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 42] [Reference Citation Analysis (0)] |
| 28. | Liu L, Yin M, Gao J, Yu C, Lin J, Wu A, Zhu J, Xu C, Liu X. Intestinal Barrier Function in the Pathogenesis of Nonalcoholic Fatty Liver Disease. J Clin Transl Hepatol. 2023;11:452-458. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 16] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 29. | Safari Z, Gérard P. The links between the gut microbiome and non-alcoholic fatty liver disease (NAFLD). Cell Mol Life Sci. 2019;76:1541-1558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 317] [Cited by in RCA: 339] [Article Influence: 56.5] [Reference Citation Analysis (0)] |
| 30. | Wiest R, Albillos A, Trauner M, Bajaj JS, Jalan R. Targeting the gut-liver axis in liver disease. J Hepatol. 2017;67:1084-1103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 304] [Article Influence: 38.0] [Reference Citation Analysis (0)] |
| 31. | Pelaseyed T, Bergström JH, Gustafsson JK, Ermund A, Birchenough GM, Schütte A, van der Post S, Svensson F, Rodríguez-Piñeiro AM, Nyström EE, Wising C, Johansson ME, Hansson GC. The mucus and mucins of the goblet cells and enterocytes provide the first defense line of the gastrointestinal tract and interact with the immune system. Immunol Rev. 2014;260:8-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 646] [Cited by in RCA: 916] [Article Influence: 91.6] [Reference Citation Analysis (0)] |
| 32. | Bergstrom KS, Kissoon-Singh V, Gibson DL, Ma C, Montero M, Sham HP, Ryz N, Huang T, Velcich A, Finlay BB, Chadee K, Vallance BA. Muc2 protects against lethal infectious colitis by disassociating pathogenic and commensal bacteria from the colonic mucosa. PLoS Pathog. 2010;6:e1000902. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 397] [Cited by in RCA: 471] [Article Influence: 31.4] [Reference Citation Analysis (0)] |
| 33. | Belzer C, Gerber GK, Roeselers G, Delaney M, DuBois A, Liu Q, Belavusava V, Yeliseyev V, Houseman A, Onderdonk A, Cavanaugh C, Bry L. Dynamics of the microbiota in response to host infection. PLoS One. 2014;9:e95534. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 50] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 34. | Verdam FJ, Rensen SS, Driessen A, Greve JW, Buurman WA. Novel evidence for chronic exposure to endotoxin in human nonalcoholic steatohepatitis. J Clin Gastroenterol. 2011;45:149-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 80] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 35. | Guo S, Nighot M, Al-Sadi R, Alhmoud T, Nighot P, Ma TY. Lipopolysaccharide Regulation of Intestinal Tight Junction Permeability Is Mediated by TLR4 Signal Transduction Pathway Activation of FAK and MyD88. J Immunol. 2015;195:4999-5010. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 305] [Article Influence: 30.5] [Reference Citation Analysis (1)] |
| 36. | Abreu MT. Toll-like receptor signalling in the intestinal epithelium: how bacterial recognition shapes intestinal function. Nat Rev Immunol. 2010;10:131-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 856] [Cited by in RCA: 913] [Article Influence: 60.9] [Reference Citation Analysis (0)] |
| 37. | Nighot M, Al-Sadi R, Guo S, Rawat M, Nighot P, Watterson MD, Ma TY. Lipopolysaccharide-Induced Increase in Intestinal Epithelial Tight Permeability Is Mediated by Toll-Like Receptor 4/Myeloid Differentiation Primary Response 88 (MyD88) Activation of Myosin Light Chain Kinase Expression. Am J Pathol. 2017;187:2698-2710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 164] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 38. | Sheth P, Delos Santos N, Seth A, LaRusso NF, Rao RK. Lipopolysaccharide disrupts tight junctions in cholangiocyte monolayers by a c-Src-, TLR4-, and LBP-dependent mechanism. Am J Physiol Gastrointest Liver Physiol. 2007;293:G308-G318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 102] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 39. | Miele L, Valenza V, La Torre G, Montalto M, Cammarota G, Ricci R, Mascianà R, Forgione A, Gabrieli ML, Perotti G, Vecchio FM, Rapaccini G, Gasbarrini G, Day CP, Grieco A. Increased intestinal permeability and tight junction alterations in nonalcoholic fatty liver disease. Hepatology. 2009;49:1877-1887. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1133] [Cited by in RCA: 1099] [Article Influence: 68.7] [Reference Citation Analysis (1)] |
| 40. | Han H, Jiang Y, Wang M, Melaku M, Liu L, Zhao Y, Everaert N, Yi B, Zhang H. Intestinal dysbiosis in nonalcoholic fatty liver disease (NAFLD): focusing on the gut-liver axis. Crit Rev Food Sci Nutr. 2023;63:1689-1706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 79] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 41. | Muccioli GG, Naslain D, Bäckhed F, Reigstad CS, Lambert DM, Delzenne NM, Cani PD. The endocannabinoid system links gut microbiota to adipogenesis. Mol Syst Biol. 2010;6:392. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 447] [Cited by in RCA: 506] [Article Influence: 33.7] [Reference Citation Analysis (0)] |
| 42. | Lam YY, Ha CW, Campbell CR, Mitchell AJ, Dinudom A, Oscarsson J, Cook DI, Hunt NH, Caterson ID, Holmes AJ, Storlien LH. Increased gut permeability and microbiota change associate with mesenteric fat inflammation and metabolic dysfunction in diet-induced obese mice. PLoS One. 2012;7:e34233. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 377] [Cited by in RCA: 466] [Article Influence: 35.8] [Reference Citation Analysis (0)] |
| 43. | Mowat AM, Agace WW. Regional specialization within the intestinal immune system. Nat Rev Immunol. 2014;14:667-685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 828] [Cited by in RCA: 1163] [Article Influence: 105.7] [Reference Citation Analysis (0)] |
| 44. | Vallianou N, Christodoulatos GS, Karampela I, Tsilingiris D, Magkos F, Stratigou T, Kounatidis D, Dalamaga M. Understanding the Role of the Gut Microbiome and Microbial Metabolites in Non-Alcoholic Fatty Liver Disease: Current Evidence and Perspectives. Biomolecules. 2021;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 170] [Article Influence: 42.5] [Reference Citation Analysis (0)] |
| 45. | Dawson PA, Karpen SJ. Intestinal transport and metabolism of bile acids. J Lipid Res. 2015;56:1085-1099. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 285] [Cited by in RCA: 407] [Article Influence: 37.0] [Reference Citation Analysis (0)] |
| 46. | Song Z, Cai Y, Lao X, Wang X, Lin X, Cui Y, Kalavagunta PK, Liao J, Jin L, Shang J, Li J. Taxonomic profiling and populational patterns of bacterial bile salt hydrolase (BSH) genes based on worldwide human gut microbiome. Microbiome. 2019;7:9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 185] [Cited by in RCA: 318] [Article Influence: 53.0] [Reference Citation Analysis (0)] |
| 47. | Stellwag EJ, Hylemon PB. 7alpha-Dehydroxylation of cholic acid and chenodeoxycholic acid by Clostridium leptum. J Lipid Res. 1979;20:325-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 115] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 48. | Li T, Chiang JYL. Bile acids as metabolic regulators: an update. Curr Opin Gastroenterol. 2023;39:249-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 49. | Lee JY, Arai H, Nakamura Y, Fukiya S, Wada M, Yokota A. Contribution of the 7β-hydroxysteroid dehydrogenase from Ruminococcus gnavus N53 to ursodeoxycholic acid formation in the human colon. J Lipid Res. 2013;54:3062-3069. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 118] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 50. | Robben J, Parmentier G, Eyssen H. Isolation of a rat intestinal Clostridium strain producing 5 alpha- and 5 beta-bile salt 3 alpha-sulfatase activity. Appl Environ Microbiol. 1986;51:32-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 27] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 51. | Lai J, Luo L, Zhou T, Feng X, Ye J, Zhong B. Alterations in Circulating Bile Acids in Metabolic Dysfunction-Associated Steatotic Liver Disease: A Systematic Review and Meta-Analysis. Biomolecules. 2023;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 22] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 52. | Puri P, Daita K, Joyce A, Mirshahi F, Santhekadur PK, Cazanave S, Luketic VA, Siddiqui MS, Boyett S, Min HK, Kumar DP, Kohli R, Zhou H, Hylemon PB, Contos MJ, Idowu M, Sanyal AJ. The presence and severity of nonalcoholic steatohepatitis is associated with specific changes in circulating bile acids. Hepatology. 2018;67:534-548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 302] [Article Influence: 43.1] [Reference Citation Analysis (0)] |
| 53. | Xia Y, Ren M, Yang J, Cai C, Cheng W, Zhou X, Lu D, Ji F. Gut microbiome and microbial metabolites in NAFLD and after bariatric surgery: Correlation and causality. Front Microbiol. 2022;13:1003755. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 21] [Reference Citation Analysis (0)] |
| 54. | Lew JL, Zhao A, Yu J, Huang L, De Pedro N, Peláez F, Wright SD, Cui J. The farnesoid X receptor controls gene expression in a ligand- and promoter-selective fashion. J Biol Chem. 2004;279:8856-8861. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 167] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 55. | Chiang JY. Bile acid metabolism and signaling. Compr Physiol. 2013;3:1191-1212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 596] [Cited by in RCA: 1024] [Article Influence: 85.3] [Reference Citation Analysis (1)] |
| 56. | Watanabe M, Houten SM, Wang L, Moschetta A, Mangelsdorf DJ, Heyman RA, Moore DD, Auwerx J. Bile acids lower triglyceride levels via a pathway involving FXR, SHP, and SREBP-1c. J Clin Invest. 2004;113:1408-1418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 865] [Cited by in RCA: 1040] [Article Influence: 49.5] [Reference Citation Analysis (0)] |
| 57. | Carr RM, Reid AE. FXR agonists as therapeutic agents for non-alcoholic fatty liver disease. Curr Atheroscler Rep. 2015;17:500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 95] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 58. | Thomas C, Gioiello A, Noriega L, Strehle A, Oury J, Rizzo G, Macchiarulo A, Yamamoto H, Mataki C, Pruzanski M, Pellicciari R, Auwerx J, Schoonjans K. TGR5-mediated bile acid sensing controls glucose homeostasis. Cell Metab. 2009;10:167-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1223] [Cited by in RCA: 1390] [Article Influence: 86.9] [Reference Citation Analysis (0)] |
| 59. | Lun W, Yan Q, Guo X, Zhou M, Bai Y, He J, Cao H, Che Q, Guo J, Su Z. Mechanism of action of the bile acid receptor TGR5 in obesity. Acta Pharm Sin B. 2024;14:468-491. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 27] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 60. | Keitel V, Donner M, Winandy S, Kubitz R, Häussinger D. Expression and function of the bile acid receptor TGR5 in Kupffer cells. Biochem Biophys Res Commun. 2008;372:78-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 281] [Cited by in RCA: 317] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 61. | Goodwin B, Jones SA, Price RR, Watson MA, McKee DD, Moore LB, Galardi C, Wilson JG, Lewis MC, Roth ME, Maloney PR, Willson TM, Kliewer SA. A regulatory cascade of the nuclear receptors FXR, SHP-1, and LRH-1 represses bile acid biosynthesis. Mol Cell. 2000;6:517-526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1411] [Cited by in RCA: 1514] [Article Influence: 60.6] [Reference Citation Analysis (0)] |
| 62. | Yang ZX, Shen W, Sun H. Effects of nuclear receptor FXR on the regulation of liver lipid metabolism in patients with non-alcoholic fatty liver disease. Hepatol Int. 2010;4:741-748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 170] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 63. | Jiao N, Baker SS, Chapa-Rodriguez A, Liu W, Nugent CA, Tsompana M, Mastrandrea L, Buck MJ, Baker RD, Genco RJ, Zhu R, Zhu L. Suppressed hepatic bile acid signalling despite elevated production of primary and secondary bile acids in NAFLD. Gut. 2018;67:1881-1891. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 389] [Cited by in RCA: 505] [Article Influence: 72.1] [Reference Citation Analysis (1)] |
| 64. | Wang Z, Klipfell E, Bennett BJ, Koeth R, Levison BS, Dugar B, Feldstein AE, Britt EB, Fu X, Chung YM, Wu Y, Schauer P, Smith JD, Allayee H, Tang WH, DiDonato JA, Lusis AJ, Hazen SL. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature. 2011;472:57-63. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4469] [Cited by in RCA: 4029] [Article Influence: 287.8] [Reference Citation Analysis (0)] |
| 65. | Stephenson K, Kennedy L, Hargrove L, Demieville J, Thomson J, Alpini G, Francis H. Updates on Dietary Models of Nonalcoholic Fatty Liver Disease: Current Studies and Insights. Gene Expr. 2018;18:5-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 97] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 66. | He M, Tan CP, Xu YJ, Liu Y. Gut microbiota-derived trimethylamine-N-oxide: A bridge between dietary fatty acid and cardiovascular disease? Food Res Int. 2020;138:109812. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 67. | Chen YM, Liu Y, Zhou RF, Chen XL, Wang C, Tan XY, Wang LJ, Zheng RD, Zhang HW, Ling WH, Zhu HL. Associations of gut-flora-dependent metabolite trimethylamine-N-oxide, betaine and choline with non-alcoholic fatty liver disease in adults. Sci Rep. 2016;6:19076. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 161] [Cited by in RCA: 251] [Article Influence: 27.9] [Reference Citation Analysis (0)] |
| 68. | Tan X, Liu Y, Long J, Chen S, Liao G, Wu S, Li C, Wang L, Ling W, Zhu H. Trimethylamine N-Oxide Aggravates Liver Steatosis through Modulation of Bile Acid Metabolism and Inhibition of Farnesoid X Receptor Signaling in Nonalcoholic Fatty Liver Disease. Mol Nutr Food Res. 2019;63:e1900257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 151] [Article Influence: 25.2] [Reference Citation Analysis (0)] |
| 69. | Farhangi MA, Vajdi M, Asghari-Jafarabadi M. Gut microbiota-associated metabolite trimethylamine N-Oxide and the risk of stroke: a systematic review and dose-response meta-analysis. Nutr J. 2020;19:76. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 52] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 70. | Martin-Gallausiaux C, Marinelli L, Blottière HM, Larraufie P, Lapaque N. SCFA: mechanisms and functional importance in the gut. Proc Nutr Soc. 2021;80:37-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 794] [Article Influence: 158.8] [Reference Citation Analysis (0)] |
| 71. | Morrison DJ, Preston T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes. 2016;7:189-200. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1566] [Cited by in RCA: 2351] [Article Influence: 261.2] [Reference Citation Analysis (0)] |
| 72. | Cao X, Zolnikova O, Maslennikov R, Reshetova M, Poluektova E, Bogacheva A, Zharkova M, Ivashkin V. Differences in Fecal Short-Chain Fatty Acids between Alcoholic Fatty Liver-Induced Cirrhosis and Non-alcoholic (Metabolic-Associated) Fatty Liver-Induced Cirrhosis. Metabolites. 2023;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 73. | Tan J, McKenzie C, Potamitis M, Thorburn AN, Mackay CR, Macia L. The role of short-chain fatty acids in health and disease. Adv Immunol. 2014;121:91-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1076] [Cited by in RCA: 1608] [Article Influence: 146.2] [Reference Citation Analysis (0)] |
| 74. | Brown AJ, Goldsworthy SM, Barnes AA, Eilert MM, Tcheang L, Daniels D, Muir AI, Wigglesworth MJ, Kinghorn I, Fraser NJ, Pike NB, Strum JC, Steplewski KM, Murdock PR, Holder JC, Marshall FH, Szekeres PG, Wilson S, Ignar DM, Foord SM, Wise A, Dowell SJ. The Orphan G protein-coupled receptors GPR41 and GPR43 are activated by propionate and other short chain carboxylic acids. J Biol Chem. 2003;278:11312-11319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1583] [Cited by in RCA: 1739] [Article Influence: 79.0] [Reference Citation Analysis (0)] |
| 75. | Zeng H, Chi H. Metabolic control of regulatory T cell development and function. Trends Immunol. 2015;36:3-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 220] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 76. | Liu T, Li J, Liu Y, Xiao N, Suo H, Xie K, Yang C, Wu C. Short-chain fatty acids suppress lipopolysaccharide-induced production of nitric oxide and proinflammatory cytokines through inhibition of NF-κB pathway in RAW264.7 cells. Inflammation. 2012;35:1676-1684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 178] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 77. | Li M, van Esch BCAM, Wagenaar GTM, Garssen J, Folkerts G, Henricks PAJ. Pro- and anti-inflammatory effects of short chain fatty acids on immune and endothelial cells. Eur J Pharmacol. 2018;831:52-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 397] [Article Influence: 56.7] [Reference Citation Analysis (0)] |
| 78. | Fukuda S, Toh H, Hase K, Oshima K, Nakanishi Y, Yoshimura K, Tobe T, Clarke JM, Topping DL, Suzuki T, Taylor TD, Itoh K, Kikuchi J, Morita H, Hattori M, Ohno H. Bifidobacteria can protect from enteropathogenic infection through production of acetate. Nature. 2011;469:543-547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1444] [Cited by in RCA: 1681] [Article Influence: 120.1] [Reference Citation Analysis (0)] |
| 79. | Jung TH, Park JH, Jeon WM, Han KS. Butyrate modulates bacterial adherence on LS174T human colorectal cells by stimulating mucin secretion and MAPK signaling pathway. Nutr Res Pract. 2015;9:343-349. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 128] [Cited by in RCA: 173] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 80. | Tolhurst G, Heffron H, Lam YS, Parker HE, Habib AM, Diakogiannaki E, Cameron J, Grosse J, Reimann F, Gribble FM. Short-chain fatty acids stimulate glucagon-like peptide-1 secretion via the G-protein-coupled receptor FFAR2. Diabetes. 2012;61:364-371. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1524] [Cited by in RCA: 1629] [Article Influence: 125.3] [Reference Citation Analysis (0)] |
| 81. | Psichas A, Sleeth ML, Murphy KG, Brooks L, Bewick GA, Hanyaloglu AC, Ghatei MA, Bloom SR, Frost G. The short chain fatty acid propionate stimulates GLP-1 and PYY secretion via free fatty acid receptor 2 in rodents. Int J Obes (Lond). 2015;39:424-429. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 395] [Cited by in RCA: 570] [Article Influence: 51.8] [Reference Citation Analysis (0)] |
| 82. | Iannucci LF, Sun J, Singh BK, Zhou J, Kaddai VA, Lanni A, Yen PM, Sinha RA. Short chain fatty acids induce UCP2-mediated autophagy in hepatic cells. Biochem Biophys Res Commun. 2016;480:461-467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 39] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 83. | Gallage S, Kotsiliti E, Heikenwalder M. When Soluble Fibers Meet Hepatocellular Carcinoma: The Dark Side of Fermentation. Cell Metab. 2018;28:673-675. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 84. | Nie C, He T, Zhang W, Zhang G, Ma X. Branched Chain Amino Acids: Beyond Nutrition Metabolism. Int J Mol Sci. 2018;19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 370] [Cited by in RCA: 478] [Article Influence: 68.3] [Reference Citation Analysis (0)] |
| 85. | Sjögren RJO, Rizo-Roca D, Chibalin AV, Chorell E, Furrer R, Katayama S, Harada J, Karlsson HKR, Handschin C, Moritz T, Krook A, Näslund E, Zierath JR. Branched-chain amino acid metabolism is regulated by ERRα in primary human myotubes and is further impaired by glucose loading in type 2 diabetes. Diabetologia. 2021;64:2077-2091. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 86. | Ruiz-Canela M, Guasch-Ferré M, Toledo E, Clish CB, Razquin C, Liang L, Wang DD, Corella D, Estruch R, Hernáez Á, Yu E, Gómez-Gracia E, Zheng Y, Arós F, Romaguera D, Dennis C, Ros E, Lapetra J, Serra-Majem L, Papandreou C, Portoles O, Fitó M, Salas-Salvadó J, Hu FB, Martínez-González MA. Plasma branched chain/aromatic amino acids, enriched Mediterranean diet and risk of type 2 diabetes: case-cohort study within the PREDIMED Trial. Diabetologia. 2018;61:1560-1571. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 91] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 87. | Lynch CJ, Adams SH. Branched-chain amino acids in metabolic signalling and insulin resistance. Nat Rev Endocrinol. 2014;10:723-736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 809] [Cited by in RCA: 1017] [Article Influence: 92.5] [Reference Citation Analysis (0)] |
| 88. | Laferrère B, Reilly D, Arias S, Swerdlow N, Gorroochurn P, Bawa B, Bose M, Teixeira J, Stevens RD, Wenner BR, Bain JR, Muehlbauer MJ, Haqq A, Lien L, Shah SH, Svetkey LP, Newgard CB. Differential metabolic impact of gastric bypass surgery versus dietary intervention in obese diabetic subjects despite identical weight loss. Sci Transl Med. 2011;3:80re2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 277] [Cited by in RCA: 291] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 89. | Pedersen HK, Gudmundsdottir V, Nielsen HB, Hyotylainen T, Nielsen T, Jensen BA, Forslund K, Hildebrand F, Prifti E, Falony G, Le Chatelier E, Levenez F, Doré J, Mattila I, Plichta DR, Pöhö P, Hellgren LI, Arumugam M, Sunagawa S, Vieira-Silva S, Jørgensen T, Holm JB, Trošt K; MetaHIT Consortium, Kristiansen K, Brix S, Raes J, Wang J, Hansen T, Bork P, Brunak S, Oresic M, Ehrlich SD, Pedersen O. Human gut microbes impact host serum metabolome and insulin sensitivity. Nature. 2016;535:376-381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1072] [Cited by in RCA: 1449] [Article Influence: 161.0] [Reference Citation Analysis (0)] |
| 90. | Iwao M, Gotoh K, Arakawa M, Endo M, Honda K, Seike M, Murakami K, Shibata H. Supplementation of branched-chain amino acids decreases fat accumulation in the liver through intestinal microbiota-mediated production of acetic acid. Sci Rep. 2020;10:18768. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 42] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 91. | Aoki R, Kamikado K, Suda W, Takii H, Mikami Y, Suganuma N, Hattori M, Koga Y. A proliferative probiotic Bifidobacterium strain in the gut ameliorates progression of metabolic disorders via microbiota modulation and acetate elevation. Sci Rep. 2017;7:43522. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 177] [Cited by in RCA: 157] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 92. | De Chiara F, Heebøll S, Marrone G, Montoliu C, Hamilton-Dutoit S, Ferrandez A, Andreola F, Rombouts K, Grønbæk H, Felipo V, Gracia-Sancho J, Mookerjee RP, Vilstrup H, Jalan R, Thomsen KL. Urea cycle dysregulation in non-alcoholic fatty liver disease. J Hepatol. 2018;69:905-915. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 137] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 93. | De Chiara F, Thomsen KL, Habtesion A, Jones H, Davies N, Gracia-Sancho J, Manicardi N, Hall A, Andreola F, Paish HL, Reed LH, Watson AA, Leslie J, Oakley F, Rombouts K, Mookerjee RP, Mann J, Jalan R. Ammonia Scavenging Prevents Progression of Fibrosis in Experimental Nonalcoholic Fatty Liver Disease. Hepatology. 2020;71:874-892. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 69] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 94. | Ekstedt M, Hagström H, Nasr P, Fredrikson M, Stål P, Kechagias S, Hultcrantz R. Fibrosis stage is the strongest predictor for disease-specific mortality in NAFLD after up to 33 years of follow-up. Hepatology. 2015;61:1547-1554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1353] [Cited by in RCA: 1697] [Article Influence: 169.7] [Reference Citation Analysis (1)] |
| 95. | Richardson AJ, McKain N, Wallace RJ. Ammonia production by human faecal bacteria, and the enumeration, isolation and characterization of bacteria capable of growth on peptides and amino acids. BMC Microbiol. 2013;13:6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 84] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 96. | Aron-Wisnewsky J, Vigliotti C, Witjes J, Le P, Holleboom AG, Verheij J, Nieuwdorp M, Clément K. Gut microbiota and human NAFLD: disentangling microbial signatures from metabolic disorders. Nat Rev Gastroenterol Hepatol. 2020;17:279-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 290] [Cited by in RCA: 680] [Article Influence: 136.0] [Reference Citation Analysis (0)] |
| 97. | Wright G, Jalan R. Ammonia and inflammation in the pathogenesis of hepatic encephalopathy: Pandora's box? Hepatology. 2007;46:291-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 57] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 98. | Luo M, Xin RJ, Hu FR, Yao L, Hu SJ, Bai FH. Role of gut microbiota in the pathogenesis and therapeutics of minimal hepatic encephalopathy via the gut-liver-brain axis. World J Gastroenterol. 2023;29:144-156. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 2] [Cited by in RCA: 19] [Article Influence: 9.5] [Reference Citation Analysis (2)] |
| 99. | Meijnikman AS, Davids M, Herrema H, Aydin O, Tremaroli V, Rios-Morales M, Levels H, Bruin S, de Brauw M, Verheij J, Kemper M, Holleboom AG, Tushuizen ME, Schwartz TW, Nielsen J, Brandjes D, Dirinck E, Weyler J, Verrijken A, De Block CEM, Vonghia L, Francque S, Beuers U, Gerdes VEA, Bäckhed F, Groen AK, Nieuwdorp M. Microbiome-derived ethanol in nonalcoholic fatty liver disease. Nat Med. 2022;28:2100-2106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 118] [Article Influence: 39.3] [Reference Citation Analysis (0)] |
| 100. | Zhu L, Baker SS, Gill C, Liu W, Alkhouri R, Baker RD, Gill SR. Characterization of gut microbiomes in nonalcoholic steatohepatitis (NASH) patients: a connection between endogenous alcohol and NASH. Hepatology. 2013;57:601-609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1015] [Cited by in RCA: 1275] [Article Influence: 106.3] [Reference Citation Analysis (1)] |
| 101. | Li H, Toth E, Cherrington NJ. Alcohol Metabolism in the Progression of Human Nonalcoholic Steatohepatitis. Toxicol Sci. 2018;164:428-438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 42] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 102. | Chen X, Zhang Z, Li H, Zhao J, Wei X, Lin W, Zhao X, Jiang A, Yuan J. Endogenous ethanol produced by intestinal bacteria induces mitochondrial dysfunction in non-alcoholic fatty liver disease. J Gastroenterol Hepatol. 2020;35:2009-2019. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 62] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 103. | Engstler AJ, Aumiller T, Degen C, Dürr M, Weiss E, Maier IB, Schattenberg JM, Jin CJ, Sellmann C, Bergheim I. Insulin resistance alters hepatic ethanol metabolism: studies in mice and children with non-alcoholic fatty liver disease. Gut. 2016;65:1564-1571. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 93] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 104. | Drucker DJ. The role of gut hormones in glucose homeostasis. J Clin Invest. 2007;117:24-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 439] [Cited by in RCA: 444] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 105. | Baggio LL, Drucker DJ. Biology of incretins: GLP-1 and GIP. Gastroenterology. 2007;132:2131-2157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2461] [Cited by in RCA: 2652] [Article Influence: 147.3] [Reference Citation Analysis (0)] |
| 106. | Greiner TU, Bäckhed F. Microbial regulation of GLP-1 and L-cell biology. Mol Metab. 2016;5:753-758. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 94] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 107. | Seino Y, Fukushima M, Yabe D. GIP and GLP-1, the two incretin hormones: Similarities and differences. J Diabetes Investig. 2010;1:8-23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 350] [Cited by in RCA: 493] [Article Influence: 44.8] [Reference Citation Analysis (0)] |
| 108. | Nauck MA, Meier JJ. The incretin effect in healthy individuals and those with type 2 diabetes: physiology, pathophysiology, and response to therapeutic interventions. Lancet Diabetes Endocrinol. 2016;4:525-536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 310] [Article Influence: 34.4] [Reference Citation Analysis (0)] |
| 109. | Wu LK, Liu YC, Shi LL, Lu KD. Glucagon-like peptide-1 receptor agonists inhibit hepatic stellate cell activation by blocking the p38 MAPK signaling pathway. Genet Mol Res. 2015;14:19087-19093. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 110. | Gupta NA, Mells J, Dunham RM, Grakoui A, Handy J, Saxena NK, Anania FA. Glucagon-like peptide-1 receptor is present on human hepatocytes and has a direct role in decreasing hepatic steatosis in vitro by modulating elements of the insulin signaling pathway. Hepatology. 2010;51:1584-1592. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 412] [Cited by in RCA: 406] [Article Influence: 27.1] [Reference Citation Analysis (0)] |
| 111. | Boland ML, Laker RC, Mather K, Nawrocki A, Oldham S, Boland BB, Lewis H, Conway J, Naylor J, Guionaud S, Feigh M, Veidal SS, Lantier L, McGuinness OP, Grimsby J, Rondinone CM, Jermutus L, Larsen MR, Trevaskis JL, Rhodes CJ. Resolution of NASH and hepatic fibrosis by the GLP-1R/GcgR dual-agonist Cotadutide via modulating mitochondrial function and lipogenesis. Nat Metab. 2020;2:413-431. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 185] [Article Influence: 37.0] [Reference Citation Analysis (0)] |
| 112. | Guan X, Karpen HE, Stephens J, Bukowski JT, Niu S, Zhang G, Stoll B, Finegold MJ, Holst JJ, Hadsell D, Nichols BL, Burrin DG. GLP-2 receptor localizes to enteric neurons and endocrine cells expressing vasoactive peptides and mediates increased blood flow. Gastroenterology. 2006;130:150-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 213] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 113. | Fuchs CD, Claudel T, Mlitz V, Riva A, Menz M, Brusilovskaya K, Haller F, Baumgartner M, Königshofer P, Unger LW, Sjöland W, Scharnagl H, Stojakovic T, Busslinger G, Reiberger T, Marschall HU, Trauner M. GLP-2 Improves Hepatic Inflammation and Fibrosis in Mdr2(-/-) Mice Via Activation of NR4a1/Nur77 in Hepatic Stellate Cells and Intestinal FXR Signaling. Cell Mol Gastroenterol Hepatol. 2023;16:847-856. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 114. | Holst JJ. The physiology of glucagon-like peptide 1. Physiol Rev. 2007;87:1409-1439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2055] [Cited by in RCA: 2335] [Article Influence: 129.7] [Reference Citation Analysis (1)] |
| 115. | He YL, Serra D, Wang Y, Campestrini J, Riviere GJ, Deacon CF, Holst JJ, Schwartz S, Nielsen JC, Ligueros-Saylan M. Pharmacokinetics and pharmacodynamics of vildagliptin in patients with type 2 diabetes mellitus. Clin Pharmacokinet. 2007;46:577-588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 77] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 116. | Pi-Sunyer X, Astrup A, Fujioka K, Greenway F, Halpern A, Krempf M, Lau DC, le Roux CW, Violante Ortiz R, Jensen CB, Wilding JP; SCALE Obesity and Prediabetes NN8022-1839 Study Group. A Randomized, Controlled Trial of 3.0 mg of Liraglutide in Weight Management. N Engl J Med. 2015;373:11-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1103] [Cited by in RCA: 1559] [Article Influence: 155.9] [Reference Citation Analysis (0)] |
| 117. | Garvey WT, Batterham RL, Bhatta M, Buscemi S, Christensen LN, Frias JP, Jódar E, Kandler K, Rigas G, Wadden TA, Wharton S; STEP 5 Study Group. Two-year effects of semaglutide in adults with overweight or obesity: the STEP 5 trial. Nat Med. 2022;28:2083-2091. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 137] [Cited by in RCA: 355] [Article Influence: 118.3] [Reference Citation Analysis (0)] |
| 118. | Rubino DM, Greenway FL, Khalid U, O'Neil PM, Rosenstock J, Sørrig R, Wadden TA, Wizert A, Garvey WT; STEP 8 Investigators. Effect of Weekly Subcutaneous Semaglutide vs Daily Liraglutide on Body Weight in Adults With Overweight or Obesity Without Diabetes: The STEP 8 Randomized Clinical Trial. JAMA. 2022;327:138-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 457] [Cited by in RCA: 495] [Article Influence: 165.0] [Reference Citation Analysis (0)] |
| 119. | Wilding JPH, Batterham RL, Davies M, Van Gaal LF, Kandler K, Konakli K, Lingvay I, McGowan BM, Oral TK, Rosenstock J, Wadden TA, Wharton S, Yokote K, Kushner RF; STEP 1 Study Group. Weight regain and cardiometabolic effects after withdrawal of semaglutide: The STEP 1 trial extension. Diabetes Obes Metab. 2022;24:1553-1564. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 440] [Article Influence: 146.7] [Reference Citation Analysis (0)] |
| 120. | Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, Harrison SA, Brunt EM, Sanyal AJ. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67:328-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3544] [Cited by in RCA: 4919] [Article Influence: 702.7] [Reference Citation Analysis (9)] |
| 121. | European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J Hepatol. 2016;64:1388-1402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2290] [Cited by in RCA: 3171] [Article Influence: 352.3] [Reference Citation Analysis (4)] |
| 122. | Cusi K, Isaacs S, Barb D, Basu R, Caprio S, Garvey WT, Kashyap S, Mechanick JI, Mouzaki M, Nadolsky K, Rinella ME, Vos MB, Younossi Z. American Association of Clinical Endocrinology Clinical Practice Guideline for the Diagnosis and Management of Nonalcoholic Fatty Liver Disease in Primary Care and Endocrinology Clinical Settings: Co-Sponsored by the American Association for the Study of Liver Diseases (AASLD). Endocr Pract. 2022;28:528-562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 365] [Cited by in RCA: 563] [Article Influence: 187.7] [Reference Citation Analysis (1)] |
| 123. | Roeb E, Geier A. Nonalcoholic steatohepatitis (NASH) - current treatment recommendations and future developments. Z Gastroenterol. 2019;57:508-517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 61] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 124. | Romero-Gómez M, Zelber-Sagi S, Trenell M. Treatment of NAFLD with diet, physical activity and exercise. J Hepatol. 2017;67:829-846. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 844] [Cited by in RCA: 920] [Article Influence: 115.0] [Reference Citation Analysis (0)] |
| 125. | von Schönfels W, Beckmann JH, Ahrens M, Hendricks A, Röcken C, Szymczak S, Hampe J, Schafmayer C. Histologic improvement of NAFLD in patients with obesity after bariatric surgery based on standardized NAS (NAFLD activity score). Surg Obes Relat Dis. 2018;14:1607-1616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 52] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 126. | Lee YM, Low HC, Lim LG, Dan YY, Aung MO, Cheng CL, Wee A, Lim SG, Ho KY. Intragastric balloon significantly improves nonalcoholic fatty liver disease activity score in obese patients with nonalcoholic steatohepatitis: a pilot study. Gastrointest Endosc. 2012;76:756-760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 102] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 127. | Armstrong MJ, Hull D, Guo K, Barton D, Hazlehurst JM, Gathercole LL, Nasiri M, Yu J, Gough SC, Newsome PN, Tomlinson JW. Glucagon-like peptide 1 decreases lipotoxicity in non-alcoholic steatohepatitis. J Hepatol. 2016;64:399-408. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 232] [Cited by in RCA: 318] [Article Influence: 35.3] [Reference Citation Analysis (0)] |
| 128. | Newsome PN, Buchholtz K, Cusi K, Linder M, Okanoue T, Ratziu V, Sanyal AJ, Sejling AS, Harrison SA; NN9931-4296 Investigators. A Placebo-Controlled Trial of Subcutaneous Semaglutide in Nonalcoholic Steatohepatitis. N Engl J Med. 2021;384:1113-1124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 513] [Cited by in RCA: 1177] [Article Influence: 294.3] [Reference Citation Analysis (0)] |
| 129. | Carbone LJ, Angus PW, Yeomans ND. Incretin-based therapies for the treatment of non-alcoholic fatty liver disease: A systematic review and meta-analysis. J Gastroenterol Hepatol. 2016;31:23-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 74] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 130. | Armstrong MJ, Gaunt P, Aithal GP, Barton D, Hull D, Parker R, Hazlehurst JM, Guo K; LEAN trial team, Abouda G, Aldersley MA, Stocken D, Gough SC, Tomlinson JW, Brown RM, Hübscher SG, Newsome PN. Liraglutide safety and efficacy in patients with non-alcoholic steatohepatitis (LEAN): a multicentre, double-blind, randomised, placebo-controlled phase 2 study. Lancet. 2016;387:679-690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1100] [Cited by in RCA: 1449] [Article Influence: 161.0] [Reference Citation Analysis (1)] |
| 131. | Khoo J, Hsiang J, Taneja R, Law NM, Ang TL. Comparative effects of liraglutide 3 mg vs structured lifestyle modification on body weight, liver fat and liver function in obese patients with non-alcoholic fatty liver disease: A pilot randomized trial. Diabetes Obes Metab. 2017;19:1814-1817. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 72] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 132. | Kojima M, Takahashi H, Kuwashiro T, Tanaka K, Mori H, Ozaki I, Kitajima Y, Matsuda Y, Ashida K, Eguchi Y, Anzai K. Glucagon-Like Peptide-1 Receptor Agonist Prevented the Progression of Hepatocellular Carcinoma in a Mouse Model of Nonalcoholic Steatohepatitis. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 39] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 133. | Loomba R, Abdelmalek MF, Armstrong MJ, Jara M, Kjær MS, Krarup N, Lawitz E, Ratziu V, Sanyal AJ, Schattenberg JM, Newsome PN; NN9931-4492 investigators. Semaglutide 2·4 mg once weekly in patients with non-alcoholic steatohepatitis-related cirrhosis: a randomised, placebo-controlled phase 2 trial. Lancet Gastroenterol Hepatol. 2023;8:511-522. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 141] [Cited by in RCA: 229] [Article Influence: 114.5] [Reference Citation Analysis (0)] |
| 134. | Scavo MP, Lisco G, Depalo N, Rizzi F, Volpe S, Arrè V, Carrieri L, Notarnicola M, De Nunzio V, Curri ML, De Pergola G, Piazzolla G, Giannelli G. Semaglutide Modulates Extracellular Matrix Production of LX-2 Cells via Exosomes and Improves Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD). Int J Mol Sci. 2024;25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 135. | Wester A, Shang Y, Toresson Grip E, Matthews AA, Hagström H. Glucagon-like peptide-1 receptor agonists and risk of major adverse liver outcomes in patients with chronic liver disease and type 2 diabetes. Gut. 2024;73:835-843. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 27] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 136. | Elsaid MI, Li N, Firkins SA, Rustgi VK, Paskett ED, Acharya C, Reddy KR, Chiang CW, Mumtaz K. Impacts of glucagon-like peptide-1 receptor agonists on the risk of adverse liver outcomes in patients with metabolic dysfunction-associated steatotic liver disease cirrhosis and type 2 diabetes. Aliment Pharmacol Ther. 2024;59:1096-1110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 24] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 137. | Wang L, Li P, Tang Z, Yan X, Feng B. Structural modulation of the gut microbiota and the relationship with body weight: compared evaluation of liraglutide and saxagliptin treatment. Sci Rep. 2016;6:33251. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 111] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 138. | Kato S, Sato T, Fujita H, Kawatani M, Yamada Y. Effects of GLP-1 receptor agonist on changes in the gut bacterium and the underlying mechanisms. Sci Rep. 2021;11:9167. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 139. | Chaudhari SN, Luo JN, Harris DA, Aliakbarian H, Yao L, Paik D, Subramaniam R, Adhikari AA, Vernon AH, Kiliç A, Weiss ST, Huh JR, Sheu EG, Devlin AS. A microbial metabolite remodels the gut-liver axis following bariatric surgery. Cell Host Microbe. 2021;29:408-424.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 97] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 140. | Pocai A, Carrington PE, Adams JR, Wright M, Eiermann G, Zhu L, Du X, Petrov A, Lassman ME, Jiang G, Liu F, Miller C, Tota LM, Zhou G, Zhang X, Sountis MM, Santoprete A, Capito' E, Chicchi GG, Thornberry N, Bianchi E, Pessi A, Marsh DJ, SinhaRoy R. Glucagon-like peptide 1/glucagon receptor dual agonism reverses obesity in mice. Diabetes. 2009;58:2258-2266. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 296] [Cited by in RCA: 325] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 141. | Ambery P, Parker VE, Stumvoll M, Posch MG, Heise T, Plum-Moerschel L, Tsai LF, Robertson D, Jain M, Petrone M, Rondinone C, Hirshberg B, Jermutus L. MEDI0382, a GLP-1 and glucagon receptor dual agonist, in obese or overweight patients with type 2 diabetes: a randomised, controlled, double-blind, ascending dose and phase 2a study. Lancet. 2018;391:2607-2618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 223] [Article Influence: 31.9] [Reference Citation Analysis (0)] |
| 142. | Patel V, Joharapurkar A, Kshirsagar S, Sutariya B, Patel M, Patel H, Pandey D, Patel D, Ranvir R, Kadam S, Bahekar R, Jain M. Coagonist of GLP-1 and Glucagon Receptor Ameliorates Development of Non-Alcoholic Fatty Liver Disease. Cardiovasc Hematol Agents Med Chem. 2018;16:35-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 35] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 143. | Valdecantos MP, Pardo V, Ruiz L, Castro-Sánchez L, Lanzón B, Fernández-Millán E, García-Monzón C, Arroba AI, González-Rodríguez Á, Escrivá F, Álvarez C, Rupérez FJ, Barbas C, Konkar A, Naylor J, Hornigold D, Santos AD, Bednarek M, Grimsby J, Rondinone CM, Valverde ÁM. A novel glucagon-like peptide 1/glucagon receptor dual agonist improves steatohepatitis and liver regeneration in mice. Hepatology. 2017;65:950-968. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 79] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 144. | Hansen HH, Feigh M, Veidal SS, Rigbolt KT, Vrang N, Fosgerau K. Mouse models of nonalcoholic steatohepatitis in preclinical drug development. Drug Discov Today. 2017;22:1707-1718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 176] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 145. | Wismann P, Pedersen SL, Hansen G, Mannerstedt K, Pedersen PJ, Jeppesen PB, Vrang N, Fosgerau K, Jelsing J. Novel GLP-1/GLP-2 co-agonists display marked effects on gut volume and improves glycemic control in mice. Physiol Behav. 2018;192:72-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 33] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 146. | Madsen MSA, Holm JB, Pallejà A, Wismann P, Fabricius K, Rigbolt K, Mikkelsen M, Sommer M, Jelsing J, Nielsen HB, Vrang N, Hansen HH. Metabolic and gut microbiome changes following GLP-1 or dual GLP-1/GLP-2 receptor agonist treatment in diet-induced obese mice. Sci Rep. 2019;9:15582. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 74] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 147. | Kim ER, Park JS, Kim JH, Oh JY, Oh IJ, Choi DH, Lee YS, Park IS, Kim S, Lee DH, Cheon JH, Bae JW, Lee M, Cho JW, An IB, Nam EJ, Yang SI, Lee MS, Bae SH, Lee YH. A GLP-1/GLP-2 receptor dual agonist to treat NASH: Targeting the gut-liver axis and microbiome. Hepatology. 2022;75:1523-1538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 47] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 148. | Hutch CR, Sandoval D. The Role of GLP-1 in the Metabolic Success of Bariatric Surgery. Endocrinology. 2017;158:4139-4151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 163] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 149. | Jirapinyo P, Jin DX, Qazi T, Mishra N, Thompson CC. A Meta-Analysis of GLP-1 After Roux-En-Y Gastric Bypass: Impact of Surgical Technique and Measurement Strategy. Obes Surg. 2018;28:615-626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 66] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 150. | De Bandt D, Rives-Lange C, Frigout Y, Bergerot D, Blanchard A, Le Gall M, Lacorte JM, Chevallier JM, Czernichow S, Poghosyan T, Carette C, Le Beyec J. Similar Gut Hormone Secretions Two Years After One Anastomosis Gastric Bypass and Roux-en-Y Gastric Bypass: a Pilot Study. Obes Surg. 2022;32:757-762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 151. | Behary P, Tharakan G, Alexiadou K, Johnson N, Wewer Albrechtsen NJ, Kenkre J, Cuenco J, Hope D, Anyiam O, Choudhury S, Alessimii H, Poddar A, Minnion J, Doyle C, Frost G, Le Roux C, Purkayastha S, Moorthy K, Dhillo W, Holst JJ, Ahmed AR, Prevost AT, Bloom SR, Tan TM. Combined GLP-1, Oxyntomodulin, and Peptide YY Improves Body Weight and Glycemia in Obesity and Prediabetes/Type 2 Diabetes: A Randomized, Single-Blinded, Placebo-Controlled Study. Diabetes Care. 2019;42:1446-1453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 85] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 152. | de Brito E Silva MB, Tustumi F, de Miranda Neto AA, Dantas ACB, Santo MA, Cecconello I. Gastric Bypass Compared with Sleeve Gastrectomy for Nonalcoholic Fatty Liver Disease: a Systematic Review and Meta-analysis. Obes Surg. 2021;31:2762-2772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 45] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 153. | Coimbra VOR, Crovesy L, Ribeiro-Alves M, Faller ALK, Mattos F, Rosado EL. Gut Microbiota Profile in Adults Undergoing Bariatric Surgery: A Systematic Review. Nutrients. 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 154. | Hernández-Montoliu L, Rodríguez-Peña MM, Puig R, Astiarraga B, Guerrero-Pérez F, Virgili N, López-Urdiales R, Osorio J, Monseny R, Lazzara C, Sobrino L, Pérez-Maraver M, Pérez-Prieto M, Pellitero S, Fernández-Veledo S, Vendrell J, Vilarrasa N. A specific gut microbiota signature is associated with an enhanced GLP-1 and GLP-2 secretion and improved metabolic control in patients with type 2 diabetes after metabolic Roux-en-Y gastric bypass. Front Endocrinol (Lausanne). 2023;14:1181744. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |