Published online Jun 14, 2024. doi: 10.3748/wjg.v30.i22.2881
Revised: April 26, 2024
Accepted: May 20, 2024
Published online: June 14, 2024
Processing time: 92 Days and 20.3 Hours
Posthepatectomy liver failure (PHLF) is one of the most important causes of death following liver resection. Heparin, an established anticoagulant, can protect liver function through a number of mechanisms, and thus, prevent liver failure.
To look at the safety and efficacy of heparin in preventing hepatic dysfunction after hepatectomy.
The data was extracted from Multiparameter Intelligent Monitoring in Intensive Care III (MIMIC-III) v1. 4 pinpointed patients who had undergone hepatectomy for liver cancer, subdividing them into two cohorts: Those who were injected with heparin and those who were not. The statistical evaluations used were unpaired t-tests, Mann-Whitney U tests, chi-square tests, and Fisher’s exact tests to assess the effect of heparin administration on PHLF, duration of intensive care unit (ICU) stay, need for mechanical ventilation, use of continuous renal replacement the
In this study, 1388 patients who underwent liver cancer hepatectomy were analyzed. PSM yielded 213 matched pairs from the heparin-treated and control groups. Initial univariate analyses indicated that heparin potentially reduces the risk of PHLF in both matched and unmatched samples. Further analysis in the matched cohorts confirmed a significant association, with heparin reducing the risk of PHLF (odds ratio: 0.518; 95% confidence interval: 0.295-0.910; P = 0.022). Additionally, heparin treatment correlated with improved short-term post
Liver failure is an important hazard following hepatic surgery. During ICU care heparin administration has been proved to decrease the occurrence of hepatectomy induced liver failure. This indicates that heparin may provide a hopeful option for controlling PHLF.
Core Tip: This study emphasizes that heparin, which is commonly identified with its anticoagulant characteristics, also offers benefits in prevention of posthepatectomy liver failure (PHLF). Application of the Multiparameter Intelligent Monitoring in Intensive Care III database shows that the administration of heparin in the postoperative intensive care unit (ICU) setting is linked to a decreased occurrence of PHLF, shortened ICU stays, and lesser need for mechanical ventilation and renal support. These outcomes underscore heparin’s potential as a valuable therapeutic option to enhance short-term postoperative results for patients undergoing liver surgery.
- Citation: Xu ZY, Peng M, Fan MM, Zou QF, Li YR, Jiang D. Heparin is an effective treatment for preventing liver failure after hepatectomy. World J Gastroenterol 2024; 30(22): 2881-2892
- URL: https://www.wjgnet.com/1007-9327/full/v30/i22/2881.htm
- DOI: https://dx.doi.org/10.3748/wjg.v30.i22.2881
Posthepatectomy liver failure (PHLF) stands as one of the most critical complications after liver surgery, marked by significant rates of morbidity and mortality[1,2]. Studies recently published indicate that the occurrence of PHLF fluctuates between 4.9% and 9.0%[3]. The International Study Group of Liver Surgery defines PHLF with a grading system: Grade A involves an elevation in international normalized ratio (INR) or total bilirubin (TBIL) without altering the clinical pathway; Grade B includes clinical deviations that are managed non-invasively; and Grade C encompasses deviations requiring invasive interventions[4]. An alternative diagnostic measure, the 50-50 criterion, is applied on the fifth postoperative day, characterizing PHLF by serum total bilirubin levels exceeding 50 μmol/L and a prothrombin time (PT) index below 50%[4]. The onset of PHLF indicates a decline in the liver’s ability to synthesize, excrete, and detoxify, evidenced by heightened levels of INR and bilirubin shortly after surgery[5]. The regeneration of the liver remnant is crucial for a patient’s prognosis following hepatectomy. Factors such as hepatic hemodynamic disturbances, immune-inflammatory responses, and metabolic dysfunctions can exacerbate hepatocyte death, thereby impairing the function of the liver remnant and precipitating liver failure[6]. In the context of liver resection, predominant risk factors encompass pre-existing liver conditions, the extent of resection, and the specifics of the intraoperative procedures. Mitigating the risk of PHLF hinges on thorough preoperative assessment and preparation, choosing the optimal surgical techniques, and implementing rigorous postoperative surveillance and management[7]. Despite these measures, comprehensive strategies to address PHLF remain incomplete, necessitating further investigations to bridge these gaps and enhance the understanding of contributory factors to PHLF.
Coagulation abnormalities are widely acknowledged as strong indicators predicting adverse outcomes in liver diseases. Heparin, a prevalent anticoagulant, not only shields endothelial cells and prevents the thrombosis of hepatic vessels but also mitigates hepatic hemodynamic abnormalities[8]. It further serves as a hepatoprotective agent by modulating cellular metabolism and the inflammatory response, which in turn reduces hepatocyte damage. Dr. Silva’s recent in vivo research highlighted that heparin significantly reduces hepatic cell apoptosis during hemorrhagic shock and reperfusion injuries[9]. Additionally, another investigation illustrated that heparin influences lipoprotein processing within the liver[10]. Despite these findings, the use of heparin as a prophylactic treatment in liver surgery is scarcely documented. The clinical consensus on the early administration of heparin post-liver surgery is still under debate. Consequently, this retrospective study was designed to assess heparin’s efficacy in preventing PHLF.
Data for this study were sourced from version 1.4 of the Multiparameter Intelligent Monitoring in Intensive Care III (MIMIC-III) database. MIMIC-III, which is freely accessible, contains records for over 50000 critical care patients who were treated at Beth Israel Deaconess Medical Center between 2001 and 2012. Prior to accessing the database, completion of the “Protecting Human Research Participants” course offered by the National Institutes of Health was mandatory (record ID: 11186516). Both the Massachusetts Institute of Technology and Beth Israel Deaconess Medical Center’s Institutional Review Boards approved the use and creation of this database. The need for informed consent was waived due to the de-identification of all data.
To be included in the study, patients were required to meet several criteria: They needed to be between 18 and 79 years old, undergoing their initial admission to the intensive care unit (ICU), with a postoperative stay of more than two days following a hepatectomy. We established exclusion criteria to refine the study population further: patients diagnosed with additional cancer types, those undergoing treatment with warfarin or other anticoagulants, individuals with pre-existing liver failure or dysfunction in other organs, a history of embolic or thrombotic events, or other significant hematological disorders, and any cases where more than 10% of the essential data were missing.
For the study, comprehensive baseline characteristics and pre-surgical laboratory values were meticulously recorded. These included demographic details such as gender, age, height, and weight, along with clinical data encompassing the presence of malignant tumors, the use of laparoscopic techniques, smoking status, ethnic background, and medical history of conditions like hypertension and portal vein tumor thrombosis (PVTT). Preoperative liver conditions noted were cirrhosis and portal hypertension, the latter defined by a hepatic venous pressure gradient exceeding 6 mmHg[11]. Additionally, patient histories of chronic obstructive pulmonary disease, chronic kidney disease, and essential hematological indices such as red blood cell (RBC), plasma, and laboratory diagnostics including TBIL, aspartate transaminase (AST), alanine transaminase (ALT), lactate dehydrogenase, albumin (ALB), serum creatinine (Cr), blood urea nitrogen, glomerular filtration rate, white blood cell, platelets (PLT), INR, PT, and activated partial thromboplastin time (APTT) were also systematically collected.
Study participants were divided into two cohorts: The heparin group consisted of patients who were administered heparin either subcutaneously or via continuous infusion in preventive or therapeutic doses (either 0.1 U/mL or 0.2 U/mL or higher) for a duration of over five consecutive days; the control group included patients who did not receive anticoagulant therapy or received it for fewer than five days[12]. The principal measure of the study was the rate of PHLF. Liver failure was identified by a TBIL level of 5 mg/dL or higher, or a PT of less than 40%, indicative of a worsening underlying liver condition. Secondary outcomes measured were the duration of ICU stay, the need for respiratory support, continuous renal replacement therapy (CRRT), occurrences of hypoxemia, incidents of acute kidney injury, and deaths in the ICU.
Due to notable differences in baseline characteristics between the two groups, propensity score matching (PSM) was utilized to mitigate the influence of confounding variables. The propensity score was developed considering all the variables listed in Table 1. Then, the caliper 0.05 was used where patients of both cohorts were matched one to one through nearest neighbor matching.
| Before matching | After matching | |||||
| Heparin-free group (n = 421) | Heparin group (n = 967) | P value | Heparin-free group (n = 213) | Heparin group (n = 213) | P value | |
| Gender (male/female) | 243/178 | 507/409 | 0.069 | 133/80 | 122/91 | 0.277 |
| Age (yr) | 56.70 ± 14.88 | 58.10 ± 14.46 | 0.100 | 56.00 ± 14.90 | 57.58 ± 15.58 | 0.274 |
| Height (cm) | 169.20 ± 8.82 | 168.30 ± 8.57 | 0.080 | 170.00 ± 9.09 | 169.50 ± 9.10 | 0.551 |
| Weight (kg) | 68.10 ± 14.55 | 66.30 ± 13.28 | 0.820 | 69.00 ± 14.36 | 68.81 ± 14.03 | 0.870 |
| Malignant tumor (Yes/No) | 119 (28.3%) | 421 (43.5%) | 0.000 | 55 (25.8%) | 74 (34.7%) | 0.045 |
| Laparoscopic (Yes/No) | 379 (90.0%) | 802 (82.9%) | 0.001 | 206 (96.7%) | 198 (93%) | 0.080 |
| Smoking (Yes/No) | 50 (11.9%) | 100 (10.3%) | 0.397 | 38 (17.8%) | 39 (18.3%) | 0.900 |
| Ethnicity (white/not white) | 296 (70.3%) | 625 (64.6%) | 0.040 | 146 (68.5%) | 161 (75.6%) | 0.105 |
| Hypertension (Yes/No) | 190 (45.1%) | 231 (54.9%) | 0.787 | 94 (44.1%) | 97 (45.5%) | 0.770 |
| PVTT (Yes/No) | 21 (5.0%) | 37 (3.8%) | 0.320 | 12 (5.6%) | 14 (6.6%) | 0.686 |
| Diabetes (Yes/No) | 5 (1.2%) | 61 (6.3%) | 0.000 | 1 (0.5%) | 2 (0.9%) | 0.562 |
| Cirrhosis (Yes/No) | 81 (19.2%) | 137 (14.2%) | 0.017 | 56 (26.3%) | 50 (23.5%) | 0.501 |
| Portal hypertension (Yes/No) | 61 (14.5%) | 75 (7.8%) | 0.000 | 40 (18.8%) | 25 (11.7%) | 0.043 |
| COPD (Yes/No) | 25 (5.9%) | 45 (4.7%) | 0.315 | 14 (6.6%) | 10 (4.7%) | 0.401 |
| CKD (Yes/No) | 39 (9.3%) | 40 (4.1%) | 0.000 | 23 (10.8%) | 19 (8.9%) | 0.516 |
| Transfusion (Yes/No) | 83 (19.7%) | 92 (9.5%) | 0.000 | 48 (22.5%) | 44 (20.7%) | 0.638 |
| Laboratory tests | ||||||
| TBIL (mg/mL) | 1.1 [0.5, 3.9] | 0.9 [0.5, 1.8] | 0.002 | 1.3 [0.5, 4.7] | 1.1 [0.6, 3.8] | 0.808 |
| AST (U/L) | 65 [33, 158] | 116 [53, 277] | 0.000 | 68.0 [35.0, 186.5] | 89.0 [44.0, 240.0] | 0.704 |
| ALT (U/L) | 54 [27, 158] | 101 [43, 237] | 0.000 | 55.0 [28.0, 168.5] | 76.0 [30.0, 210.5] | 0.847 |
| LDH (U/L) | 263.0 [192.0, 405.0] | 272.0 [198.8, 403.5] | 0.882 | 274.0 [199.5, 437.5] | 288.0 [201.0, 440.5] | 0.487 |
| ALB (g/dL) | 3.4 [2.8, 3.8] | 3.3 [2.9, 3.7] | 0.724 | 3.4 [2.8, 3.8] | 3.2 [2.8, 3.7] | 0.639 |
| Cr (mg/dL) | 0.9 [0.7, 1.3] | 1 [1.0, 1.0] | 0.000 | 0.9 [0.7, 1.4] | 1.0 [0.7, 1.4] | 0.901 |
| BUN (mg/dL) | 16 [11, 28] | 14 [11, 20] | 0.000 | 18 [12, 31] | 16 [11, 26] | 0.629 |
| GFR (mg/dL) | 16 [11, 28] | 14 [11, 20] | 0.000 | 18 [12, 31] | 16 [11, 26] | 0.629 |
| WBC (K/μL) | 7.3 [5.0, 10.0] | 10.0 [7.0, 14.0] | 0.000 | 6.9 [4.7, 9.7] | 8.1 [5.0, 12.3] | 0.241 |
| PLT (K/μL) | 177 [113, 261] | 205 [145, 266] | 0.001 | 160.0 [96.0, 235.5] | 187.0 [117.5, 245.0] | 0.054 |
| INR | 1.2 [1.1, 1.6] | 1.2 [1.1, 1.4] | 0.000 | 1.2 [1.1, 1.6] | 1.3 [1.1, 1.6] | 0.408 |
| PT (s) | 13.6 [12.1, 18.1] | 13.4 [12.2, 15.3] | 0.000 | 13.5 [11.9, 17.8] | 14.2 [12.4, 17.3] | 0.436 |
| APTT (s) | 32 [28.1, 38.7] | 29.9 [27.2, 33.9] | 0.004 | 31.6 [28.2, 37.3] | 31.0 [27.6, 37.0] | 0.202 |
The representation of continuous variables in the study was adjusted to their distribution, which were either presented as means plus or minus standard deviations (SD) or as medians with interquartile ranges (IQR). Categorical variables were shown by means of their counts and corresponding percentages. Analysis of covariates and interactions across these variables was performed using various statistical tests such as unpaired Student’s t test, Mann-Whitney test, Two-way ANOVA for group comparisons, chi-square and Fisher’s exact tests for nominal data, chosen based on appropriateness to data type. Logistic regression analyses were performed before and after introduction of the PSM to look deeper into the association between different factors and PHLF. At first, all the variables underwent a univariate logistic regression to identify the variables mainly associated with PHLF, where the variables with P value less than 0.1 were evaluated using multivariable with a backward stepwise selection strategy. The investigation extended to examining the influence of heparin on other adverse clinical outcomes employing a bivariate logistic regression framework. Detailed subgroup analyses were also performed to evaluate the differential effects of heparin on PHLF across various stratified groups. Statistical significance for all tests was determined at a P value of less than 0.05, using a two-tailed hypothesis test. These analyses were carried out utilizing SPSS software, version 25.0 (IBM SPSS, Chicago, IL, United States), and R software, version 4.2.1 (Institute for Statistics and Mathematics, Vienna, Austria; http://www.r-project.org/).
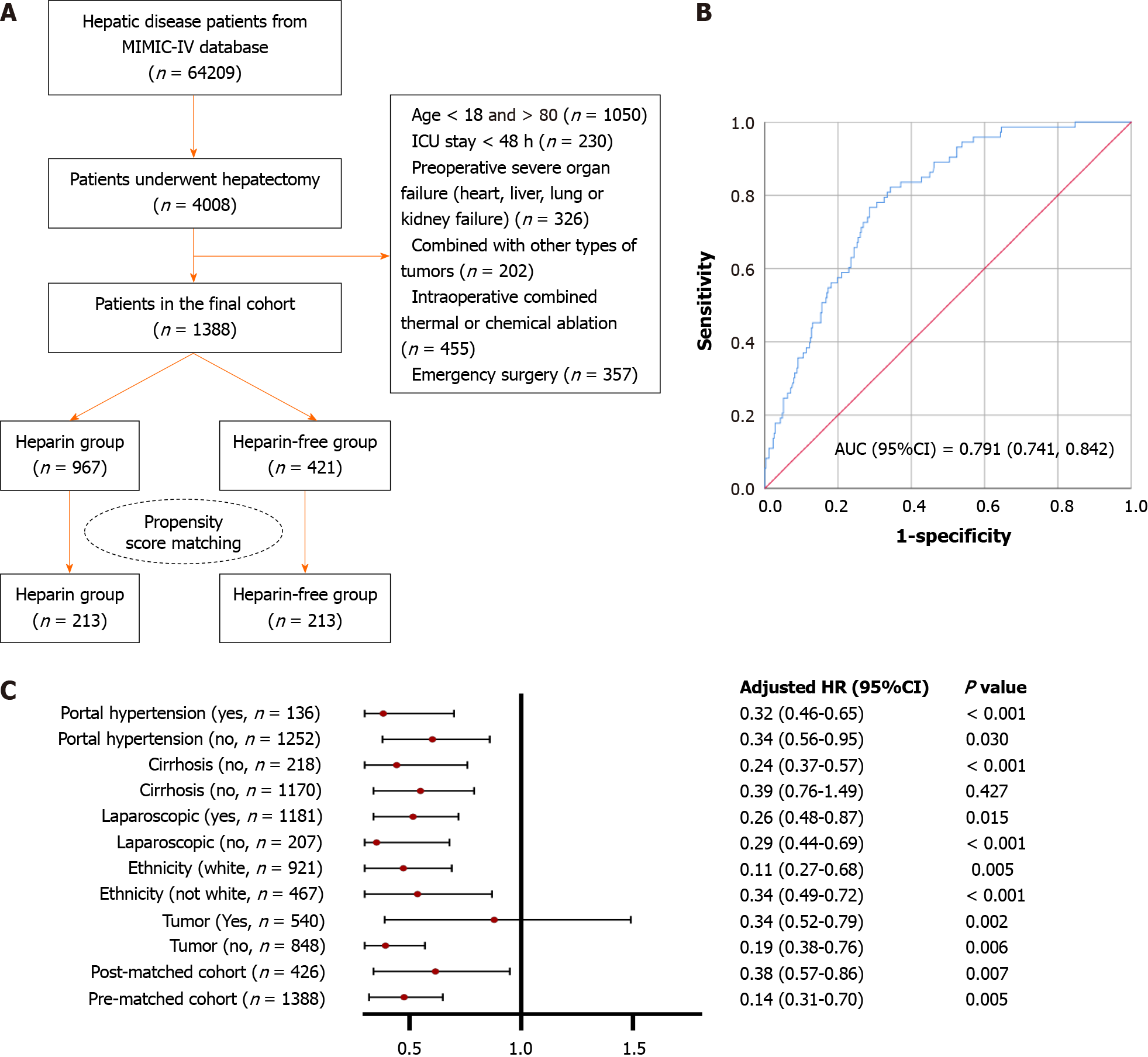
A review of 1388 patients who underwent liver resection identified the same number for inclusion in the final study cohort. Of these, 967 were treated with heparin while the remaining 421 constituted the heparin-free group, as depicted in Figure 1A. Baseline characteristics and laboratory findings are detailed in Table 1. Prior to PSM, significant imbalances in several factors were noted between the groups. For example, the heparin group showed a higher incidence of malignant tumors and a reduced use of laparoscopic procedures. Additionally, notable differences were observed across most laboratory parameters. These disparities suggest that those in the heparin group were typically more severely ill. To address these imbalances, PSM was employed, resulting in 213 matched pairs. Post-PSM analysis showed that most variables were well-balanced between the two groups, with portal hypertension being the primary exception.
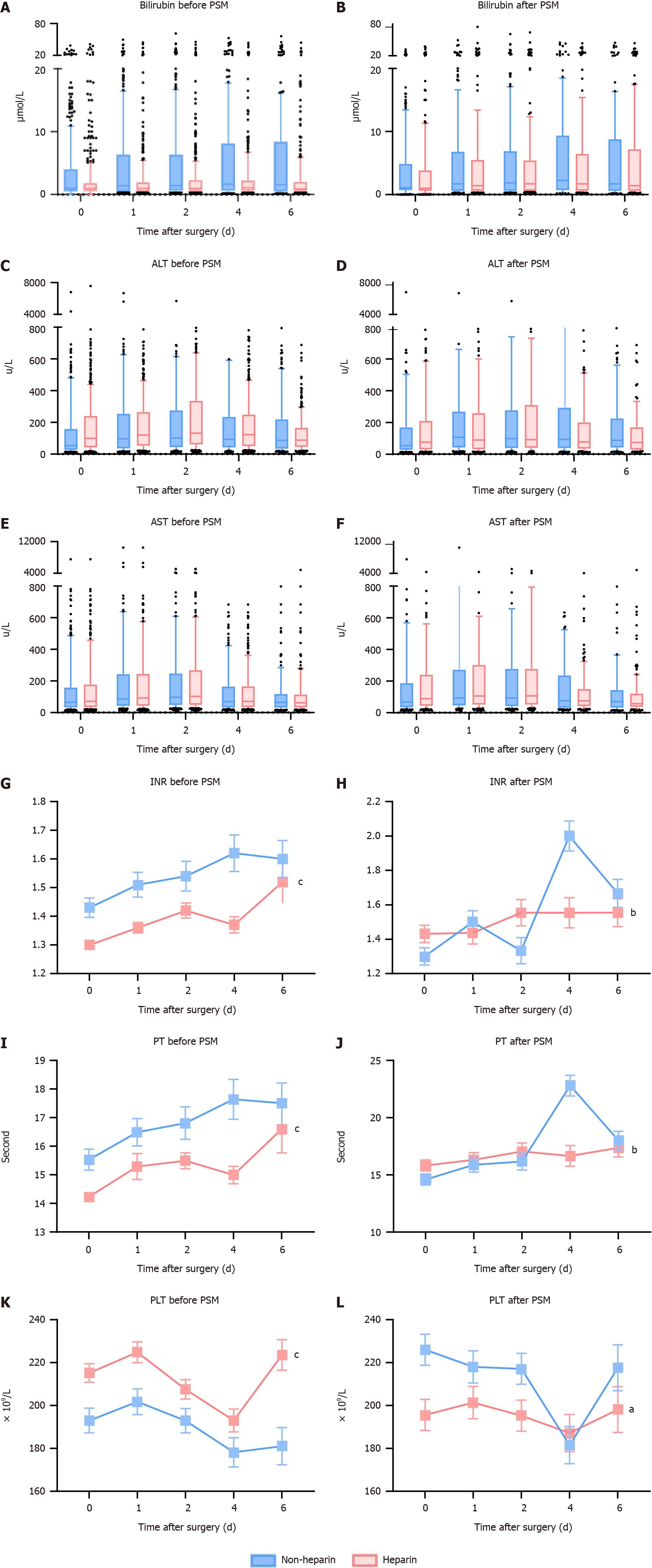
The association between the use of heparin and the subsequent clinical outcomes is systematically detailed in Table 2. In the broader patient sample, 142 individuals experienced liver failure following surgery. Notably, the incidence of PHLF was less prevalent among patients who did not receive heparin, with figures reported at 15.7% compared to 7.9% in the non-heparin group, resulting in an odds ratio (OR) of 2.180 and a 95% confidence interval (CI) ranging from 1.533 to 3.099, with a significant P value of less than 0.001. Following the application of PSM, the trend persisted, though with a narrower margin (21.1% vs 13.1%, OR: 0.530; 95%CI: 0.303-0.928; P = 0.026). For secondary outcomes, unadjusted logistic regression analysis of the entire cohort demonstrated that patients not treated with heparin experienced longer durations in the ICU [hazard ratio (HR): 1.501; 95%CI: 1.104-2.040; P = 0.01]. Additionally, this group required more extensive respiratory support (HR: 2.479; 95%CI: 1.745, 3.523; P < 0.001) and were more likely to undergo CRRT (HR: 5.044; 95%CI: 2.160, 11.782; P < 0.001). They also faced higher risks of developing hypoxemia (HR: 1.260; 95%CI: 1.955-3.032; P = 0.003) and increased chances of ICU mortality (HR: 2.354; 95%CI: 1.543-3.593; P < 0.001). Post-operative blood tests aimed at evaluating liver function and the coagulation system revealed no significant variations in total bilirubin, ALT, and AST levels as shown in Figure 2A-F. However, the INR values significantly improved in the heparin group, both before and after PSM (Figure 2G and H, P < 0.001). The results related to other coagulation indicators such as PT and PLT initially showed poorer outcomes in patients treated with heparin pre-PSM, but these markers improved post-PSM, as depicted in Figure 2I-L, likely indicating the effectiveness of the PSM in balancing these groups.
| Heparin-free group, n (%) | Heparin group, n (%) | OR (95%CI) | P value | |
| Before PSM | n = 421 | n = 967 | ||
| PHLF | 66 (15.7) | 76 (7.9) | 2.180 (1.533, 3.099) | < 0.001 |
| ICU stay | 79 (18.8) | 129 (13.3) | 1.501 (1.104, 2.040) | 0.010 |
| Respiratory support | 70 (16.6) | 72 (7.4) | 2.479 (1.745, 3.523) | 0.000 |
| CRRT | 17 (4) | 8 (0.8) | 5.044 (2.160, 11.782) | < 0.001 |
| Hypoxemia | 39 (9.3) | 48 (5.0) | 1.955 (1.260, 3.032) | 0.003 |
| AKI | 40 (9.5) | 66 (6.8) | 1.433 (0.951, 2.161) | 0.086 |
| ICU death | 43 (10.2) | 43 (4.4) | 2.354 (1.543, 3.593) | < 0.001 |
| After PSM | n = 213 | n = 213 | ||
| PHFL | 45 (21.1) | 28 (13.1) | 0.530 (0.303, 0.928) | 0.026 |
| ICU stay | 49 (23.0) | 51 (23.9) | 1.722 (0.933, 3.177) | 0.082 |
| Respiratory support | 43 (20.2) | 30 (14.1) | 0.502 (0.262, 0.960) | 0.037 |
| CRRT | 5 (2.3) | 4 (1.9) | 1.059 (0.249, 4.496) | 0.939 |
| Hypoxemia | 23 (10.8) | 23 (10.8) | 1.181 (0.598, 2.334) | 0.631 |
| AKI | 22 (10.3) | 21 (9.9) | 1.017 (0.525, 1.971) | 0.960 |
| ICU death | 27 (12.7) | 24 (11.3) | 0.956 (0.469, 1.949) | 0.901 |
In the univariate analysis conducted post-PSM, 13 variables emerged as potential risk factors for PHLF, each with an unadjusted P value below 0.1. These variables were subsequently incorporated into a multivariate model, from which five factors were identified as significantly correlated with PHLF. These include treatment with heparin, which demonstrated a protective effect (OR: 0.518; 95%CI: 0.295-0.910, P = 0.022), diagnosis of PVTT (OR: 3.825; 95%CI: 1.486-9.844; P = 0.005), blood transfusion (OR: 3.316; 95%CI: 1.851-5.940; P < 0.001), total bilirubin (TBIL) (OR: 1.050; 95%CI: 1.011-1.089; P = 0.010), and ALB levels (OR: 0.473; 95%CI: 0.296, 0.755; P = 0.002) as detailed in Table 3. The receiver operating characteristic curves for this refined model are displayed in Figure 1B, highlighting its promising predictive capacity for PHLF. Conversely, the regression analysis conducted on patients before PSM identified 17 variables with an unadjusted P value below 0.1, as detailed in Supplementary Table 1. Following the multivariate selection process, only four factors were retained in the final model, including PVTT, transfusion, PLT, and ALB levels. Notably, in this pre-PSM analysis, heparin did not emerge as a significant prognostic factor for PHLF.
| Factors | B | SE | Wald | OR (95%CI) | P value |
| Univariate | |||||
| Heparin | -0.571 | 0.263 | 4.703 | 0.565 [0.337, 0.947] | 0.030 |
| Gender | -0.231 | 0.267 | 0.749 | 0.794 [0.470, 1.340] | 0.387 |
| Age | -0.009 | 0.008 | 1.099 | 0.991 [0.975, 1.008] | 0.294 |
| Height | 0.021 | 0.014 | 2.055 | 1.021 [0.992, 1.050] | 0.152 |
| Weight | 0.026 | 0.008 | 9.392 | 1.026 [1.009, 1.043] | 0.002 |
| Malignant tumor | -0.169 | 0.287 | 0.347 | 0.845 [0.482, 1.481] | 0.556 |
| Laparoscopic | -0.802 | 0.468 | 2.929 | 0.449 [0.179, 1.123] | 0.087 |
| Smoking | 0.032 | 0.252 | 0.016 | 1.032 [0.630, 1.691] | 0.900 |
| Ethnicity | -0.361 | 0.274 | 1.732 | 0.697 [0.407, 1.193] | 0.188 |
| Hypertension | 0.085 | 0.258 | 0.108 | 1.088 [0.656, 1.804] | 0.743 |
| PVTT | 1.207 | 0.426 | 8.003 | 3.343 [1.451, 7.703] | 0.005 |
| Diabetes | 0.891 | 1.232 | 0.523 | 2.437 [0.218, 27.243] | 0.469 |
| Cirrhosis | 0.635 | 0.276 | 5.319 | 1.888 [1.100, 3.239] | 0.021 |
| Portal hypertension | 0.854 | 0.310 | 7.579 | 2.348 [1.279, 4.312] | 0.006 |
| COPD | 0.389 | 0.631 | 0.380 | 0.678 [0.197, 2.334] | 0.537 |
| CKD | 0.465 | 0.388 | 1.440 | 1.592 [0.745, 3.404] | 0.230 |
| Transfusion | 1.565 | 0.275 | 32.311 | 4.783 [2.788, 8.205] | 0.000 |
| Laboratory tests | |||||
| TBIL | 0.068 | 0.017 | 15.985 | 1.070 [1.035, 1.107] | 0.000 |
| AST | 0.000 | 0.000 | 2.462 | 1.000 [1.000, 1.001] | 0.117 |
| ALT | 0.000 | 0.000 | 0.308 | 1.000 [1.000, 1.001] | 0.579 |
| LDH | 0.000 | 0.000 | 3.279 | 1.000 [1.000, 1.000] | 0.070 |
| ALB | -1.020 | 0.218 | 21.989 | 0.360 [0.235, 0.552] | 0.000 |
| Cr | 0.054 | 0.105 | 0.264 | 1.056 [0.859, 1.298] | 0.608 |
| BUN | -0.005 | 0.008 | 0.457 | 0.995 [0.979, 1.010] | 0.499 |
| GFR | -0.005 | 0.008 | 0.457 | 0.995 [0.979, 1.010] | 0.499 |
| WBC | 0.025 | 0.016 | 2.278 | 1.025 [0.993, 1.058] | 0.131 |
| PLT | -0.006 | 0.002 | 13.876 | 0.994 [0.991, 0.997] | 0.000 |
| INR | 0.529 | 0.158 | 11.266 | 1.697 [1.246, 2.312] | 0.001 |
| PT | 0.049 | 0.014 | 11.643 | 1.050 [1.021, 1.081] | 0.001 |
| APTT | 0.010 | 0.007 | 1.797 | 1.010 [0.996, 1.024] | 0.180 |
| Multivariate | |||||
| Heparin | -0.657 | 0.287 | 5.236 | 0.518 [0.295, 0.910] | 0.022 |
| PVTT | 1.342 | 0.482 | 7.734 | 3.825 [1.486, 9.844] | 0.005 |
| Transfusion | 1.199 | 0.297 | 16.248 | 3.316 [1.851, 5.940] | < 0.000 |
| TBIL | 0.048 | 0.019 | 6.558 | 1.050 [1.011, 1.089] | 0.010 |
| ALB | -0.749 | 0.239 | 9.815 | 0.473 [0.296, 0.755] | 0.002 |
In the detailed subgroup analysis conducted, regardless of the patients’ cirrhosis status, the type of surgical approach (laparoscopic or not), their ethnicity, or whether they had portal hypertension, those who were administered heparin showed consistently lower rates of PHLF across all categories compared to their counterparts who did not receive heparin treatment. This was statistically significant, as indicated in Figure 1C, where all P values were below 0.05. However, a deeper examination of the data stratified by tumor presence revealed a more nuanced relationship. Specifically, the protective effects of heparin were predominantly observed in patients undergoing liver surgeries for benign diseases, with these patients showing a significantly reduced risk of developing PHLF (OR: 0.19; 95%C: 0.38-0.76; P = 0.006).
Coagulation disturbances, commonly observed following liver surgery, are often linked to liver failure and a negative prognosis due to microvascular thrombosis[13]. Therefore, moderating the excessively activated coagulation cascade post-hepatectomy could serve as an effective strategy to mitigate the risk of PHLF. This retrospective study, utilizing clinical data from a publicly accessible database, illustrates that short-term heparin administration post-liver surgery or during ICU stays can decrease the incidence of PHLF and enhance overall clinical outcomes, including organ func
Heparin, a heterogeneous mixture of heparan sulfate glycosaminoglycans isolated from porcine intestines, exerts a potent anticoagulant effect through selective interactions with numerous proteins. Among these proteins is the serine protease inhibitor antithrombin-III (AT III), which influences thrombin, factors Xa, IXa, XIa, XIIa, and tissue plasminogen activator. Extensive clinical research has confirmed the efficacy and safety of heparin for treating patients at high risk of coagulation disorders. Notably, a retrospective analysis by Peng et al[15] demonstrated that un-fractioned heparin could enhance outcomes for patients with sepsis-induced coagulopathy. However, the benefits of heparin therapy following surgery are still under debate, with some studies indicating reduced hospital mortality[16], while others report no impact on short-term surgical outcomes[17]. A primary limitation of these studies is the lack of a predefined target population for heparin use and the absence of a universally recognized clinical biomarker for its application. Moreover, the selection of anticoagulation or hemostasis strategies after liver surgery remains complicated due to the heterogeneity of patient conditions and the complexity of surgical techniques[18]. Our findings suggest that heparin therapy serves as a valuable organ protection strategy in major surgeries, reducing dysfunction in both respiratory and urinary systems, and even decreasing ICU mortality rates. Initially, our multivariable model before PSM showed no statistically significant association between heparin treatment and PHLF, likely due to confounding factors. This aligns with findings from other randomized clinical trials suggesting that less critically ill patients might not benefit from anticoagulant therapy[19]. Following PSM, two matched cohorts were established, featuring patients with lower severity and reduced PHLF rates.
The evidence gathered supports the premise that initiating anticoagulation with heparin immediately following hepatic surgery can significantly prevent the onset of liver failure, likely attributed to the elevated risk of micro
Several significant limitations are inherent to this study and merit discussion. Given the retrospective nature of this analysis, there is a potential for both selection and ascertainment biases. Table 1 illustrates marked discrepancies across numerous variables between the groups, with those receiving heparin typically exhibiting more severe medical conditions. In response, we applied both multivariate regression analysis and PSM to mitigate these confounding influences effectively. Furthermore, the database from which this study draws its data lacks essential peri-operative variables, including intra-operative blood loss records, and fails to analyze methodologies related to ICU treatments or interventions. Another critical gap is the absence of data on tumor stage and the impact of preoperative treatments such as radiotherapy or chemotherapy, which significantly influence the residual liver volume: An essential factor in determining the likelihood of PHLF. This gap highlights a significant limitation of the MINIC database, which is primarily geared towards gathering data from patients within ICU settings. As such, interpretations of our findings should be approached with caution due to these dataset constraints.
In summarizing the findings from the MIMIC-3 database analysis, it was determined that administration of heparin not only diminishes the rate of post-hepatectomy liver failure but may also contribute to improved clinical outcomes overall. Interestingly, heparin application was associated with enhanced INR values within the treatment group and did not elevate bleeding risks. To substantiate these observations and elucidate the underlying mechanisms, future prospective clinical trials are warranted.
| 1. | Calthorpe L, Rashidian N, Cacciaguerra AB, Conroy PC, Hibi T, Hilal MA, Hoffman D, Park KM, Wang J, Adam MA, Alseidi A; International Post-Hepatectomy Liver Failure Study Group. Using the Comprehensive Complication Index to Rethink the ISGLS Criteria for Post-hepatectomy Liver Failure in an International Cohort of Major Hepatectomies. Ann Surg. 2023;277:e592-e596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 14] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 2. | Chen HS, Joo DJ, Shaheen M, Li Y, Wang Y, Yang J, Nicolas CT, Predmore K, Amiot B, Michalak G, Mounajjed T, Fidler J, Kremers WK, Nyberg SL. Randomized Trial of Spheroid Reservoir Bioartificial Liver in Porcine Model of Posthepatectomy Liver Failure. Hepatology. 2019;69:329-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 52] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 3. | Sparrelid E, Gilg S, van Gulik TM. Systematic review of MARS treatment in post-hepatectomy liver failure. HPB (Oxford). 2020;22:950-960. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 4. | Pang Q, Zhou S, Liu S, Liu H, Lu Z. Prognostic role of preoperative albumin-bilirubin score in posthepatectomy liver failure and mortality: a systematic review and meta-analysis. Updates Surg. 2022;74:821-831. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 5. | Rahbari NN, Garden OJ, Padbury R, Brooke-Smith M, Crawford M, Adam R, Koch M, Makuuchi M, Dematteo RP, Christophi C, Banting S, Usatoff V, Nagino M, Maddern G, Hugh TJ, Vauthey JN, Greig P, Rees M, Yokoyama Y, Fan ST, Nimura Y, Figueras J, Capussotti L, Büchler MW, Weitz J. Posthepatectomy liver failure: a definition and grading by the International Study Group of Liver Surgery (ISGLS). Surgery. 2011;149:713-724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1224] [Cited by in RCA: 1711] [Article Influence: 122.2] [Reference Citation Analysis (0)] |
| 6. | Ocak İ, Topaloğlu S, Acarli K. Posthepatectomy liver failure. Turk J Med Sci. 2020;50:1491-1503. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 7. | Søreide JA, Deshpande R. Post hepatectomy liver failure (PHLF) - Recent advances in prevention and clinical management. Eur J Surg Oncol. 2021;47:216-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 131] [Article Influence: 26.2] [Reference Citation Analysis (1)] |
| 8. | Hussein KH, Park KM, Kang KS, Woo HM. Heparin-gelatin mixture improves vascular reconstruction efficiency and hepatic function in bioengineered livers. Acta Biomater. 2016;38:82-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 74] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 9. | de Jesus-Silva SG, de Moraes Silva MA, Carbonel AAF, Grillo Filho GFR, Grigório TS, Simões MJ, Cardoso RS, Fagundes DJ. Heparin Attenuates Visceral Apoptosis in a Swine Model of Hemorrhagic Shock and Reperfusion Injury. Ann Vasc Surg. 2020;67:449-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 10. | Blessing F, Wang Y, Walli AK, Seidel D. Heparin-mediated extracorporeal low-density lipoprotein precipitation: rationale for a specific adjuvant therapy in cardiovascular disease. Transfus Apher Sci. 2004;30:255-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 11. | Leung JC, Loong TC, Pang J, Wei JL, Wong VW. Invasive and non-invasive assessment of portal hypertension. Hepatol Int. 2018;12:44-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 32] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 12. | Oduah EI, Linhardt RJ, Sharfstein ST. Heparin: Past, Present, and Future. Pharmaceuticals (Basel). 2016;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 175] [Cited by in RCA: 181] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 13. | Bagante F, Ruzzenente A, Beal EW, Campagnaro T, Merath K, Conci S, Akgül O, Alexandrescu S, Marques HP, Lam V, Shen F, Poultsides GA, Soubrane O, Martel G, Iacono C, Guglielmi A, Pawlik TM. Complications after liver surgery: a benchmark analysis. HPB (Oxford). 2019;21:1139-1149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 59] [Article Influence: 9.8] [Reference Citation Analysis (37)] |
| 14. | Gray E, Hogwood J, Mulloy B. The anticoagulant and antithrombotic mechanisms of heparin. Handb Exp Pharmacol. 2012;43-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 103] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 15. | Peng JC, Nie F, Li YJ, Xu QY, Xing SP, Li W, Gao Y. Favorable Outcomes of Anticoagulation With Unfractioned Heparin in Sepsis-Induced Coagulopathy: A Retrospective Analysis of MIMIC-III Database. Front Med (Lausanne). 2021;8:773339. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 16. | Di Castelnuovo A, Costanzo S, Antinori A, Berselli N, Blandi L, Bonaccio M, Cauda R, Guaraldi G, Menicanti L, Mennuni M, Parruti G, Patti G, Santilli F, Signorelli C, Vergori A, Abete P, Ageno W, Agodi A, Agostoni P, Aiello L, Al Moghazi S, Arboretti R, Astuto M, Aucella F, Barbieri G, Bartoloni A, Bonfanti P, Cacciatore F, Caiano L, Carrozzi L, Cascio A, Ciccullo A, Cingolani A, Cipollone F, Colomba C, Colombo C, Crosta F, Danzi GB, D’Ardes D, de Gaetano Donati K, Di Gennaro F, Di Tano G, D’Offizi G, Fantoni M, Fusco FM, Gentile I, Gianfagna F, Grandone E, Graziani E, Grisafi L, Guarnieri G, Larizza G, Leone A, Maccagni G, Madaro F, Maitan S, Mancarella S, Mapelli M, Maragna R, Marcucci R, Maresca G, Marongiu S, Marotta C, Marra L, Mastroianni F, Mazzitelli M, Mengozzi A, Menichetti F, Meschiari M, Milic J, Minutolo F, Molena B, Montineri A, Mussini C, Musso M, Niola D, Odone A, Olivieri M, Palimodde A, Parisi R, Pasi E, Pesavento R, Petri F, Pinchera B, Poletti V, Ravaglia C, Rognoni A, Rossato M, Rossi M, Sangiovanni V, Sanrocco C, Scorzolini L, Sgariglia R, Simeone PG, Taddei E, Torti C, Vettor R, Vianello A, Vinceti M, Virano A, Vocciante L, De Caterina R, Iacoviello L. Heparin in COVID-19 Patients Is Associated with Reduced In-Hospital Mortality: The Multicenter Italian CORIST Study. Thromb Haemost. 2021;121:1054-1065. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 74] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 17. | Junqueira DR, Zorzela LM, Perini E. Unfractionated heparin versus low molecular weight heparins for avoiding heparin-induced thrombocytopenia in postoperative patients. Cochrane Database Syst Rev. 2017;4:CD007557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 18. | Cross AJ, Connor SJ. Hemostasis and thrombosis in major liver resection. Semin Thromb Hemost. 2015;41:99-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 19. | Umemura Y, Yamakawa K. Efficacy and safety of anticoagulant therapy in three specific populations with sepsis: a meta-analysis of randomized controlled trials: reply. J Thromb Haemost. 2016;14:2310-2311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 20. | Jia C, Ge K, Xu S, Liu L, Weng J, Chen Y. Selective occlusion of the hepatic artery and portal vein improves liver hypertrophy for staged hepatectomy. World J Surg Oncol. 2019;17:167. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 21. | Ainoa E, Uutela A, Nordin A, Mäkisalo H, Sallinen V. Pre- vs. postoperative initiation of thromboprophylaxis in liver surgery. HPB (Oxford). 2021;23:1016-1024. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 22. | Gard AP, Sayles BD, Robbins JW, Thorell WE, Surdell DL. Hemorrhage Rate After External Ventricular Drain Placement in Subarachnoid Hemorrhage: Time to Heparin Administration. Neurocrit Care. 2017;27:350-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 23. | Beattie GW, Jeffrey RR. Is there evidence that fresh frozen plasma is superior to antithrombin administration to treat heparin resistance in cardiac surgery? Interact Cardiovasc Thorac Surg. 2014;18:117-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 24. | Zarychanski R, Abou-Setta AM, Kanji S, Turgeon AF, Kumar A, Houston DS, Rimmer E, Houston BL, McIntyre L, Fox-Robichaud AE, Hébert P, Cook DJ, Fergusson DA; Canadian Critical Care Trials Group. The efficacy and safety of heparin in patients with sepsis: a systematic review and metaanalysis. Crit Care Med. 2015;43:511-518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 125] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 25. | Messmore HL. Clinical efficacy of heparin fractions: issues and answers. Crit Rev Clin Lab Sci. 1986;23:77-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.2] [Reference Citation Analysis (0)] |