Published online Jun 14, 2024. doi: 10.3748/wjg.v30.i22.2852
Revised: May 4, 2024
Accepted: May 21, 2024
Published online: June 14, 2024
Processing time: 136 Days and 1 Hours
Diabetes, commonly known for its metabolic effects, also critically affects the enteric nervous system (ENS), which is essential in regulating gastrointestinal (GI) motility, secretion, and absorption. The development of diabetes-induced enteric neuropathy can lead to various GI dysfunctions, such as gastroparesis and irregular bowel habits, primarily due to disruptions in the function of neuronal and glial cells within the ENS, as well as oxidative stress and inflammation. This editorial explores the pathophysiological mechanisms underlying the develop
Core Tip: Diabetic enteric neuropathy, an often-overlooked complication of diabetes, significantly impacts gastrointestinal (GI) functions and impairs patients’ quality of life. This editorial examines the link between diabetes and enteric neuropathy, emphasizing its impact on essential GI functions. It discusses how diabetes-induced neuropathy leads to GI issues like gastroparesis and altered bowel habits and highlights recent advances in early diagnostic methods and management. The editorial reviews various treatment strategies, both current and emerging, addressing associated challenges and future directions. It stresses the importance of a multidisciplinary approach in managing this complication.
- Citation: Abdalla MMI. Enteric neuropathy in diabetes: Implications for gastrointestinal function. World J Gastroenterol 2024; 30(22): 2852-2865
- URL: https://www.wjgnet.com/1007-9327/full/v30/i22/2852.htm
- DOI: https://dx.doi.org/10.3748/wjg.v30.i22.2852
Diabetes, recognized as a global health challenge, affects millions and is increasingly prevalent. Among its myriad complications, enteric neuropathy emerges as a critical, yet often overlooked, condition[1]. Diabetic enteric neuropathy is a pathology impairing the enteric nervous system (ENS), essential for regulating gastrointestinal (GI) functions. This complication leads to various GI disturbances in diabetic patients, significantly impacting their quality of life[2-4].
The incidence of diabetic neuropathy, including enteric neuropathy, is rising in parallel with the escalating prevalence of diabetes. Epidemiological data indicate that a significant proportion of individuals with diabetes are likely to experience some form of neuropathy during their disease trajectory, with enteric neuropathy being a particularly concerning manifestation[5]. This form of neuropathy is characterized by diverse GI symptoms, adversely affecting patient health, and placing substantial strain on healthcare systems[6]. Notably, the International Diabetes Federation reports approximately 537 million adults globally diagnosed with diabetes, a number expected to reach 643 million by 2030[7]. This rising trend in diabetes prevalence correlates with an increased incidence of complications like gastroparesis, a common manifestation of enteric neuropathy[8]. Research indicates varying prevalence and incidence rates, with one study in Minnesota (1996-2000) reporting 9.8 cases per 100000 females and 2.5 in males[9]. In contrast, a 2020 United States survey found gastroparesis to be more prevalent in males across all age groups[10].
The pathophysiology of diabetic enteric neuropathy involves a complex interplay of hyperglycemic damage, autoimmune responses, oxidative stress, and vascular insufficiency, leading to ENS dysfunction. This dysfunction is not merely a peripheral complication but reflects systemic pathological changes in diabetic patients, necessitating an in-depth understanding of its broader implications[11,12].
Additionally, the socioeconomic and psychological impacts of enteric neuropathy on patients are considerable. The condition often leads to decreased work productivity, social withdrawal, and increased healthcare utilization, underscoring the need for urgent and precise management of this complication[13].
This editorial aims to bring into focus enteric neuropathy as a crucial aspect of diabetic complications, especially its impact on GI functions. It will examine the intricate pathophysiology underlying this condition and how diabetes-induced changes in the ENS modify GI processes. Additionally, it will explore the clinical manifestations, emphasizing the importance of accurate diagnosis and effective management strategies.
In integrating this information, the editorial seeks to bridge knowledge gaps, illuminate diagnostic challenges, and review the effectiveness of current treatment approaches. The goal is to enhance awareness and understanding of diabetic enteric neuropathy, guiding future research and informing clinical practice for improved patient outcomes.
The ENS, often hailed as the body’s “second brain”, is a key component of the autonomic nervous system (ANS), playing a crucial role in regulating GI functions. This complex neural network, embedded within the GI tract (GIT) walls and spanning from the esophagus to the anus, comprises two main plexuses: The myenteric (Auerbach’s) plexus, positioned between the muscle layers, and the submucosal (Meissner’s) plexus, situated in the submucosa[14-16].
The myenteric plexus primarily governs GI motility by regulating the rhythm and force of muscle contractions along the tract, thus ensuring the efficient movement of contents[17]. In contrast, the submucosal plexus plays an integral role in managing GI secretion, blood flow, and nutrient absorption[18]. The ENS, composed of diverse neuron types including sensory, interneurons, and motor neurons, forms complex circuits capable of autonomously mediating reflexes[19].
Functionally, the ENS utilizes a variety of neurotransmitters, such as acetylcholine, calcitonin gene-related peptide, tachykinin, serotonin, and nitric oxide, to modulate GI physiology[19,20]. It operates both independently and in concert with the central nervous system (CNS), responding to local environmental cues and coordinating with central inputs to maintain digestive homeostasis. Sensory neurons in the ENS detect changes in the gut’s chemical composition and physical state, initiating appropriate reflexive responses[19].
Enteric glial cells, akin to astrocytes in the CNS, support the ENS, contributing to neuronal function maintenance, mucosal barrier integrity, and response to injury or inflammation[21]. This network’s integrity is critical not only for normal digestive functioning but also in pathophysiological conditions, where its dysfunction can lead to various GI disorders[22].
In diabetes, both type 1 and type 2, enteric neuropathy manifests as a complication marked by ENS neuronal damage. While hyperglycemia-induced damage and microvascular complications are common pathological processes in both diabetes types, specific manifestations and underlying mechanisms differ[23]. In type 1 diabetes, enteric neuropathy often associates with prolonged disease duration and suboptimal glycemic control, where hyperglycemia and autoimmune-related inflammation are primary contributors[24-27]. Type 2 diabetes, often linked with metabolic syndrome, brings additional factors like insulin resistance, obesity, and dyslipidemia, exacerbating enteric neuropathy[28]. This condition in type 2 diabetes forms part of broader metabolic dysfunction, including changes in gut microbiota, increased intestinal permeability, and chronic low-grade inflammation[24,29-31]. Moreover, lifestyle factors, notably dietary habits and physical activity, play a significant role in type 2 diabetes, influencing the severity and progression of enteric neuropathy[32]. Modifiable risk factors such as diet and exercise are thus crucial in managing GI complications in diabetic patients[28,33,34].
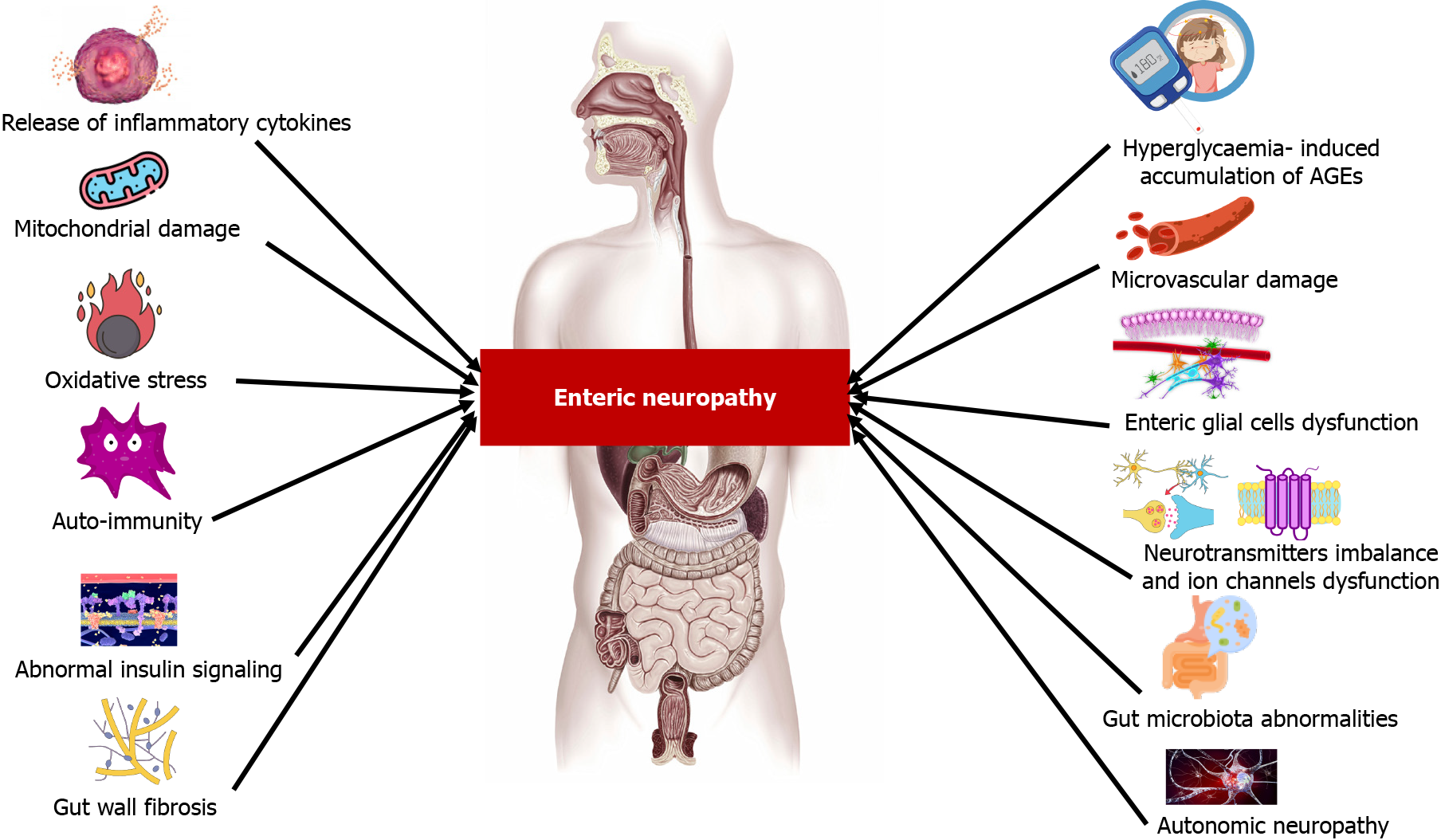
The pathophysiology of diabetic enteric neuropathy is multifaceted, involving metabolic disturbances, vascular and autonomic dysfunction, immune responses, mitochondrial and neurotransmitter alterations, connective tissue remodeling, and shifts in gut microbiota[12,35,36]. Figure 1 summarizes the pathophysiology of diabetic enteric neuropathy. A comprehensive understanding of these mechanisms is vital for developing targeted therapies and improving management strategies for diabetic patients with GI complications.
Chronic hyperglycemia serves as a principal initiator in the pathophysiology of diabetic enteric neuropathy, primarily by inducing biochemical alterations within neurons. A key feature of this alteration is the accumulation of advanced glycation end-products (AGEs)[37]. These AGEs bind to their specific receptors (RAGE) on neuronal cells, leading to a cascade of oxidative stress and inflammatory responses[38,39]. This chain of reactions impairs neuronal function and promotes apoptosis, predominantly affecting neurons that regulate GI motility and secretion[40].
Furthermore, hyperglycemia-induced mitochondrial stress plays a significant role in this pathology. In enteric neurons, such stress leads to impaired energy production and an elevated generation of reactive oxygen species (ROS), which contribute to neuronal damage[41-43]. This mitochondrial dysfunction is a critical factor in the degeneration of neuronal health under diabetic conditions.
Oxidative stress, coupled with an imbalance in antioxidant defenses, constitutes another major factor in enteric neuronal damage. Chronic hyperglycemia exacerbates ROS production, overwhelming the body’s inherent antioxidant systems. This suggests that enhancing these defenses could be a viable strategy to protect enteric neurons from oxidative damage[41,43]. Strengthening these antioxidant defenses could be a therapeutic approach to protect enteric neurons from oxidative damage.
Moreover, autophagy, a process essential for cellular maintenance and stress response, may be altered in diabetes, leading to impaired maintenance and increased vulnerability of enteric neurons[41,43]. This alteration in cellular processes underscores the complexity of diabetic enteric neuropathy’s pathophysiology.
The immune response is also a key player in the progression of diabetic enteric neuropathy. Chronic hyperglycemia can trigger autoimmune responses, resulting in inflammation and subsequent damage to the ENS[44,45]. This immune-mediated aspect of neuropathy’s pathophysiology is an area of growing research interest, offering potential avenues for therapeutic intervention.
Moreover, the integrity of the GI barrier is compromised in chronic diabetes, partly due to the elevated levels of pro-inflammatory cytokines. These cytokines induce stress and apoptosis in neuronal cells, further exacerbating neuropathic conditions[46,47]. The resulting inflammation, along with compromised GI mucosal barrier integrity, increases gut permeability, allowing more harmful substances to directly affect the ENS and aggravate neuropathic symptoms[48-50].
Diabetes-induced microvascular complications extend to the blood vessels supplying the ENS. Resultant ischemia impairs essential nutrient and oxygen delivery to enteric neurons and glial cells, exacerbating neuronal damage and dysfunction. This ischemic state furthers the degeneration of neural networks in the GIT, compounding neuropathy’s impact[36,44,51,52].
Diabetic autonomic neuropathy involves significant impairment of the ANS and is a key factor in the development of enteric neuropathy. The ANS, especially the vagus nerve, plays an essential role in the regulation of GI functions. In diabetes, damage to these autonomic nerves compromises their ability to effectively regulate the GIT. This disruption, particularly in the vagus nerve, leads to a breakdown in the coordination between the ANS and ENS. As a result, normal GI motility patterns are altered, manifesting in conditions such as gastroparesis. The altered motility patterns stemming from this neuropathy highlight the interconnectedness and dependency of the ENS on proper ANS functioning for maintaining GI homeostasis[53].
In diabetes, enteric glial cell dysfunction emerges as a pivotal factor in the development of enteric neuropathy, leading to a progressive decline in the functionality of the ENS[21]. Chronic hyperglycemia, a hallmark of diabetes, inflicts stress and damage on these cells, triggering pathways such as oxidative stress, inflammation, and impaired cellular signaling[54]. This impairment compromises the glial cells’ support for enteric neurons, resulting in a range of detrimental effects on the ENS. These include impaired neurotransmitter handling, disrupted cellular communication, and an increased vulnerability of neurons to damage and apoptosis[55].
Additionally, diabetic-induced dysfunction of enteric glial cells contributes to the breakdown of the mucosal barrier, enhancing gut permeability. This change exacerbates the inflammatory state within the GIT, further affecting neuronal function and potentially disrupting the crucial interaction between the gut microbiota and the ENS[56]. This interaction is essential for maintaining GI motility and overall gut health. The significance of glial cell dysfunction in the progression of diabetic enteric neuropathy underscores their role in GI health and highlights the need for therapeutic strategies targeting the preservation or restoration of glial cell function[57].
Alterations in neurotransmitter function are also evident in diabetes. Changes in the levels and functions of key neurotransmitters, such as nitric oxide, vasoactive intestinal peptide, and serotonin, which regulate GI motility and secretion, disrupt the necessary balance for coordinated GI function, leading to symptoms like altered bowel habits and dysmotility[58]. Furthermore, diabetes can lead to dysfunctions in ion channels within enteric neurons. These changes, particularly in calcium and potassium signaling, disrupt neuronal excitability and neurotransmitter release in the ENS, contributing to GI motility disorders[59-61].
Furthermore, diabetes can induce changes in the gut wall’s connective tissue, leading to fibrosis. This fibrosis disrupts the structure of the ENS and impairs its functionality, contributing to motility disorders[62,63].
The interaction of the ENS with gut microbiota represents a burgeoning field of research, particularly in the context of diabetic enteric neuropathy. Dysbiosis, or the imbalance in gut microbiome composition, has been identified as a key factor influencing both gut motility and neuronal function. This dysregulation presents novel therapeutic targets, offering significant potential for the management of diabetic enteric neuropathy[64,65].
The relationship between diabetic enteropathy and gut microbiota is intricate and complex. Diabetes-induced alterations in gut motility lead to changes in the composition of the gut microbiota[66]. These alterations have substantial implications for neurotransmission within GIT. This complex interaction is partly moderated by the brain-gut axis, a crucial communication pathway that may involve the vagus nerve. In the diabetic state, where vagal function is often impaired, this communication pathway can be disrupted, exacerbating the symptoms of enteropathy. The gut microbiota exerts its influence on the ENS through the production of neurotransmitter-like molecules, such as Gamma-aminobutyric acid, serotonin, melatonin, histamine, and acetylcholine. These molecules play pivotal roles in regulating gut motility and, by extension, influence the overall function of the GIT[67]. The emerging understanding of this dynamic interaction underscores the importance of gut microbiota in the pathophysiology of diabetic enteropathy. This insight not only advances our comprehension of the disease mechanism but also opens up new avenues for therapeutic intervention, particularly those targeting the microbiota to modulate gut motility and ENS function.
In diabetes, especially type 2, altered insulin signaling pathways in enteric neurons can contribute to neuropathic changes, highlighting the potential of restoring insulin sensitivity in the ENS as a therapeutic strategy[44].
Neurotrophic factors, such as nerve growth factor and glial cell line-derived neurotrophic factor (GDNF), are essential for the health and maintenance of enteric neurons. Diabetes-induced alterations in these factors contribute to the pathology of enteric neuropathy[68-71]. Additionally, the ability of neurons to adapt or undergo neuroplastic changes is also impacted in diabetes, further contributing to ENS dysfunction[72-74].
Epigenetic changes, including DNA methylation and histone acetylation, influence gene expression in diabetic patients. These epigenetic modifications may affect genes crucial for neuronal health and function, thereby playing a significant role in the development and progression of enteric neuropathy[75].
Metabolic products, hormones, and other signaling molecules in diabetes might have direct or indirect effects on enteric neuronal functions, influencing the overall health of the ENS[76]. The interaction between systemic metabolic dysregulation in diabetes and local gut metabolism is another area of interest.
Furthermore, the regulatory pathways controlling gut motility, including the functionality of the interstitial cells of Cajal (ICCs), are also affected in diabetes. Disruption in these pathways contributes to the development of enteric neuropathy, as diabetes can impact the function or survival of these cells, leading to disorders in gut motility[61,77,78].
In addition to the mentioned mechanisms and research areas, another important aspect that could be further explored in diabetic enteric neuropathy is the role of extracellular matrix (ECM) remodeling. Diabetes can lead to alterations in the ECM of the GIT, which may affect the structural and functional integrity of the ENS. These ECM changes can impact cell adhesion, tissue architecture, and possibly interfere with nerve signal transmission, contributing to neuropathic complications[27,79].
Another area of interest is the exploration of circadian rhythm disruptions in diabetic patients and their impact on the ENS. Circadian rhythms play a crucial role in regulating various physiological processes, including GI functions. Disruptions in these rhythms, which are common in diabetes due to factors like irregular eating patterns and sleep disturbances, could exacerbate the symptoms of enteric neuropathy[80].
The potential role of exosomes and microRNAs (miRNAs) in diabetic enteric neuropathy also presents a promising research avenue. Exosomes are small vesicles released by cells that can carry miRNAs, proteins, and other molecules, influencing cellular communication and processes. Investigating how diabetes alters exosome production and content, and how these changes affect the ENS, could provide insights into novel mechanisms of disease progression and potential therapeutic targets. miRNAs, in particular, are known to regulate gene expression and could play a role in the pathophysiology of diabetic enteric neuropathy by modulating neuronal survival, inflammation, and cellular stress responses[81].
Additionally, the interaction between vascular health and the ENS is a critical area needing further exploration. Vascular dysregulation, a common occurrence in diabetes, may not only lead to direct ischemic damage to enteric neurons but also induce secondary effects due to impaired nutrient and oxygen delivery. Understanding how vascular changes intertwine with neuropathic processes could open up new strategies for preserving ENS function in diabetic patients.
Finally, exploring the impact of diabetes on the sensory function of the ENS presents another valuable research direction. Sensory neurons in the ENS are crucial for detecting mechanical and chemical changes in the gut. Diabetes may alter the sensitivity or response of these neurons, leading to dysregulated GI reflexes and symptoms. Studies focusing on sensory neuron dysfunction could reveal new aspects of diabetic enteric neuropathy’s pathogenesis and potential interventions to restore normal sensory function[82].
Each of these identified mechanisms and pathways offers a potential avenue for future research, and they collectively highlight the complex nature of diabetic enteric neuropathy. Understanding the full spectrum of pathophysiological changes and their intricate interplay remains a critical area of investigation. This comprehensive approach is essential for developing targeted therapies and improving management strategies for diabetic patients who suffer from GI complications.
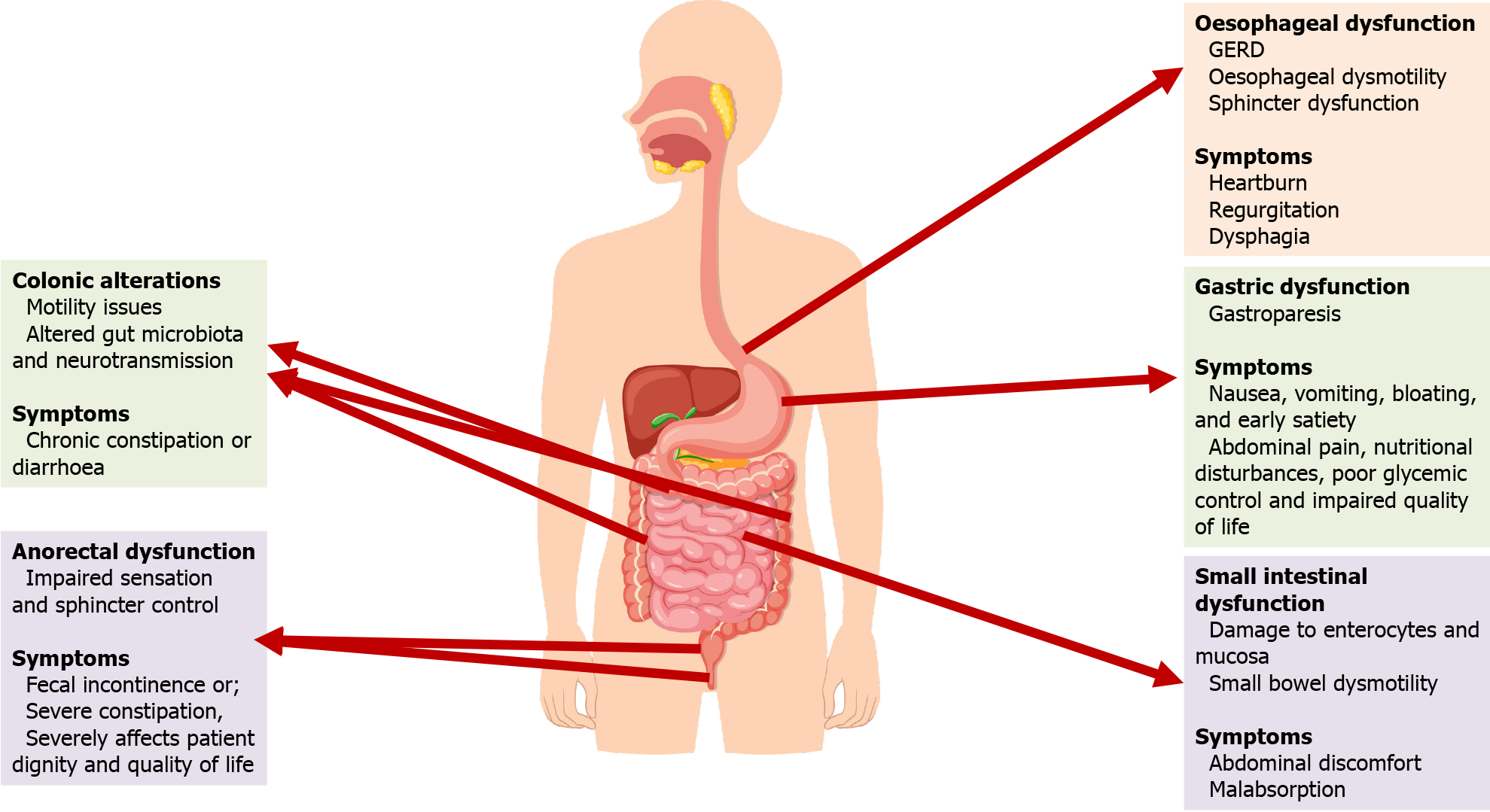
Diabetic enteric neuropathy presents a multifaceted challenge across the entire GIT, with each segment exhibiting distinct yet interrelated dysfunctions due to neuropathic damage[53].
In the esophagus, this neuropathy primarily disrupts motility, often leading to gastroesophageal reflux disease and esophageal dysmotility[83]. These motility issues are typically characterized by disrupted peristaltic waves and sphincter dysfunction, frequently linked to prolonged hyperglycemia which intensifies oxidative stress on esophageal neurons[84,85]. Such findings have been elucidated through advanced imaging and manometry techniques, prompting the exploration of novel therapeutic strategies, including targeted neuromodulation, to mitigate these dysfunctions[86,87]. Symptoms commonly experienced by patients include heartburn, regurgitation, and dysphagia, further aggravated by the oxidative stress on esophageal neurons due to hyperglycemia[88].
In the stomach, diabetic neuropathy frequently culminates in gastroparesis, marked by delayed gastric emptying in the absence of mechanical obstruction[8,89]. This disorder manifests as nausea, vomiting, bloating, and early satiety, severely impacting both nutritional status and glycemic control in diabetic patients. Gastroparesis significantly diminishes the quality of life, leading to poor glycemic control, which is linked with various complications, abdominal discomfort, poor nutrition, and increased hospitalizations. The resulting psychological distress further complicates the patient’s overall health[90]. Research has highlighted the crucial role of the ICC in gastric motility disorders in diabetes, with emerging therapies focused on restoring their function[77,78]. The pathophysiology of gastroparesis involves dysfunction in both the gastric myenteric plexus and the ICC, leading to delayed gastric emptying and significant health impacts[8].
Diabetic enteric neuropathy causes significant dysfunction in the small intestine, manifesting as a spectrum of symptoms from abdominal discomfort to severe malabsorption. This condition is exacerbated by oxidative stress due to persistent hyperglycemia, leading to damage in enterocytes and neuronal cells[91-93]. Further complicating the scenario is hyperglycemia’s impairment of mucosal healing[94] and its impact on insulin growth factors, contributing to accelerated apoptosis[95]. Recent studies have shifted focus from autonomic neuropathy to the loss of nitrergic neurons and ICC, crucial in GI motility[78]. Damage to ICCs by ROS disrupts their function in coordinating gut contractions, altering motility[96]. Additionally, the role of neuronal nitric oxide synthase (nNOS) in intestinal motility is recognized, with hyperglycemia-induced changes in nNOS contributing to small bowel dysmotility[97].
Colonic function is similarly impacted, primarily through weakened muscular contractions and oxidative stress induced by persistent hyperglycemia[41,98]. This condition disrupts the normal functioning of ICC and neuronal cells, leading to colonic motility issues like chronic constipation or diarrhea[30,99]. Contributing factors include altered gut microbiota and dysregulation of neurotransmitters and inflammatory mediators, further characterized by abnormalities in neurotransmission and an imbalance between excitatory and inhibitory signals[64,65,76]. Research is focused on developing pharmacological treatments targeting these neurotransmitter systems to improve colonic function in diabetic patients.
Lastly, diabetic enteric neuropathy leads to anorectal dysfunction, including impaired sensation and sphincter control, leads to fecal incontinence or severe constipation, severely affecting patient dignity and quality of life[100,101]. Advanced diagnostic techniques such as anorectal manometry have enhanced our understanding of these neuromuscular impairments[102]. Research indicates a potential link between anorectal dysfunction and systemic diabetic complications, emphasizing the need for integrated management approaches[100,103].
Overall, diabetic enteric neuropathy affects the GIT from the esophagus to the anorectum, with each part exhibiting specific dysfunctions and associated symptoms as presented in Figure 2. This complex condition necessitates a comprehensive approach to clinical management, integrating advanced diagnostics, targeted treatments, and ongoing research to improve patient outcomes. The need for continued research into the molecular and cellular underpinnings of this condition is crucial for developing effective interventions.
Diagnosing diabetic enteric neuropathy is a complex and nuanced process that demands a comprehensive approach. It starts with a thorough clinical assessment, where key indicators such as altered bowel habits, GI pain, bloating, and signs of gastric emptying disorders like gastroparesis are evaluated. In this stage, a detailed patient history is crucial to differentiate neuropathy from other GI disorders.
Central to the diagnostic process are GI motility studies. These include esophageal manometry and gastric emptying scintigraphy, which provide quantitative data on the motility of different segments of the GIT. Gastric emptying scintigraphy is particularly significant as it’s considered the gold standard for diagnosing gastroparesis, a common manifestation of enteric neuropathy[8,104]. Complementing these are endoscopic and radiologic examinations, such as magnetic resonance imaging or computed tomography scans. While these do not directly diagnose neuropathy, they are instrumental in ruling out mechanical obstructions or other structural abnormalities that could present neuropathic symptoms[2,105].
The field of diagnostics is also witnessing the emergence of innovative techniques like capsule endoscopy and smart pills. These technologies, capable of measuring pH, pressure, and temperature, offer a less invasive method to directly visualize and measure GI function[106,107]. Additionally, the identification of specific biomarkers in blood or stool samples, including inflammatory markers and gut peptides, is an area of ongoing research, promising non-invasive diagnostic options[108-110]. Autonomic testing, such as heart rate variability, provides indirect indicators of enteric neuropathy by assessing the integrity of the ANS[111].
However, the diagnostic process is fraught with challenges. Non-specific GI symptoms, the lack of standardized diagnostic criteria, the limited availability of specialized tests, and challenges in interpreting test results all add layers of complexity. Furthermore, the variability in patient presentations, the overlap with other diabetic complications like peripheral neuropathy and autonomic dysfunction, and the potential for psychological factors and functional GI disorders like irritable bowel syndrome to mimic neuropathy symptoms, complicate the diagnosis[111-113].
An interdisciplinary approach enhances the accuracy and comprehensiveness of the diagnosis. Collaborative care models involving gastroenterologists, endocrinologists, primary care physicians, dietitians, and mental health professionals can provide a holistic assessment of the patient’s condition[5,35]. The importance of patient history cannot be overstated, as it offers invaluable insights for distinguishing neuropathy from other GI disorders[2,114]. Symptom diaries and quality of life assessments help understand the impact of symptoms on the patient’s daily life and psychological well-being[115]. Yet, challenges persist in patient-centered diagnosis. Variability in symptom perception and reporting, cultural and language barriers, and the need for culturally sensitive and linguistically appropriate patient care present additional hurdles[116].
Overall, the diagnosis of diabetic enteric neuropathy requires a concerted, interdisciplinary effort that combines clinical assessment with specialized diagnostic tests. Overcoming the challenges of non-specific symptoms, lack of standardized criteria, and complexity in test interpretation is crucial. Emphasizing patient-centered care and considering individual differences and cultural factors are key to the effective diagnosis and management of this complex condition.
Diabetic enteric neuropathy presents with a range of GI symptoms. Effective management of these symptoms is crucial for enhancing patient quality of life and overall disease outcomes.
Pharmacological approaches include prokinetic agents like metoclopramide and domperidone for gastroparesis and other motility disorders[117]. These agents enhance GI motility and facilitate gastric emptying, but their long-term use is limited by potential side effects, such as tardive dyskinesia with metoclopramide[118]. Antiemetics like ondansetron offer relief from nausea and vomiting[119], while antidiarrheal and laxative agents address diarrhea and constipation, respectively[12,120]. However, these treatments mainly provide symptomatic relief.
Clonidine, an alpha-2 adrenergic agonist, has demonstrated significant efficacy in the management of diabetic diarrhea, a debilitating condition often refractory to conventional treatments. This medication exerts its therapeutic effects by reducing GI motility and enhancing fluid absorption in the intestines, mechanisms that are crucial for controlling the symptoms of diabetic diarrhea[121]. Additionally, somatostatin analogs, such as octreotide, have been utilized due to their ability to inhibit the secretion of various GI hormones and slow gastric emptying, thus improving diarrheal symptoms in diabetic patients[122,123]. Selective serotonin 5-hydroxytryptamine type 3 inhibitors also play a critical role in this context by blocking serotonin receptors, which are involved in enhancing gut motility and secretion, thereby reducing the frequency and urgency of diarrhea[124,125]. Collectively, these medications address the complex pathophysiology of diabetes-related enteropathic diarrhea and provide a comprehensive approach to treatment, offering symptomatic relief and improving quality of life for affected individuals.
Dietary management is another cornerstone, with recommendations for small, frequent meals that are low in fat and fiber, particularly beneficial for gastroparesis[126,127]. Adhering to these dietary guidelines is critical, though ensuring adequate nutrition remains a challenge[128].
Tight glycemic control is essential in managing diabetic enteric neuropathy. Improved blood glucose levels can alleviate symptoms and prevent further progression of neuropathy[90,129]. However, this strategy requires careful monitoring to avoid hypoglycemia, especially in patients with gastroparesis where absorption is unpredictable[130].
Emerging therapies such as gastric electrical stimulation show promise, especially in gastroparesis cases unresponsive to conventional treatments[131,132]. In severe cases, endoscopic and surgical interventions, like pyloric botulinum toxin injections or gastric per-oral endoscopic myotomy, are considered but are generally reserved for refractory cases due to their invasive nature[133,134].
Alternative and complementary medicines, including acupuncture and herbal supplements, have been explored, though their efficacy is not fully established[135]. These methods should complement, not replace, conventional treatments.
Educating patients about diabetic enteric neuropathy and its impact on GI function is vital. Encouraging self-management practices, such as dietary adjustments and blood glucose monitoring, plays a crucial role in managing symptoms and improving quality of life[136].
The primary challenge in current treatments is that they offer symptomatic relief without reversing the underlying neuropathic changes. Adherence to treatment protocols can be influenced by lifestyle, economic factors, and individual patient preferences. Emerging therapies, including neuromodulation and personalized medicine, face challenges in efficacy, safety, and accessibility. Ensuring equitable access and integrating these therapies into standard care practices are essential steps for their successful implementation[137].
Incorporating new therapies into clinical practice requires continuous medical education and updates in clinical protocols. Regular monitoring and adjustments to treatment plans are vital for effective management. Patient education and engagement, including the use of mobile health applications, are crucial for empowering patients in self-management. Healthcare systems need to adapt and support a multidisciplinary approach, ensuring accessible resources for comprehensive care. Policies should facilitate the integration of various specialties and support necessary infra
One of the significant gaps in current research is the incomplete understanding of the mechanistic pathways through which diabetes leads to enteric neuropathy. Although the link between hyperglycemia and neuronal damage is established, the precise molecular and cellular processes remain unclear. Additionally, the field lacks robust biomarkers for the early detection of enteric neuropathy in diabetic patients. The development of such biomarkers could revolutionize early intervention strategies, potentially preventing the progression of GI complications.
Another area that requires further exploration is the long-term efficacy and safety of existing treatments, including pharmacological agents and neuromodulation therapies. Longitudinal studies assessing the risks and benefits over extended periods are scarce, leaving a gap in our understanding of the long-term management of this condition. Furthermore, the role of the gut microbiota in the context of diabetic enteric neuropathy is not fully explored. Investigating how alterations in gut microbiome composition and function influence the development and progression of neuropathy could unveil new therapeutic targets.
Looking to the future, research should focus on novel therapeutic targets within the pathophysiological pathways of diabetic enteric neuropathy. This includes a deeper investigation into neuroinflammation, oxidative stress, and mitochondrial dysfunction. The development of non-invasive, reliable diagnostic tools is also essential. Advanced imaging techniques, biomarker assays, and smart technology-based monitoring systems could offer new ways to detect and monitor enteric neuropathy more effectively and non-invasively.
Personalized medicine approaches are another promising direction. Tailoring treatment strategies to individual patient profiles, considering genetics, lifestyle, and specific disease characteristics, could lead to more effective management of enteric neuropathy. Additionally, the impact of dietary and lifestyle interventions warrants more comprehensive investigation. Specific dietary components, the role of probiotics, and the influence of physical activity regimens on the management of neuropathy are areas ripe for exploration.
Lastly, the role of psychosocial factors in managing diabetic enteric neuropathy is an area that needs more attention. Investigating how psychological support and behavioral therapies can complement traditional medical treatments could provide a more holistic approach to improving patient outcomes.
In conclusion, diabetic enteric neuropathy is a complex and significant complication of diabetes, affecting GI function and patient quality of life. Effective management requires a comprehensive approach, integrating pharmacological treatments, dietary modifications, and glycemic control, supported by patient education. The role of personalized medicine, technological advancements, and mental health care integration are emerging as crucial aspects in treatment strategies. Challenges such as treatment adherence, long-term efficacy of therapies, and access to care need addressing. Future research should focus on novel therapies, improved diagnostics, and understanding the diabetes-gut microbiota relationship. A multidisciplinary approach and updated healthcare policies are essential for optimizing patient care and enhancing outcomes for those with diabetic enteric neuropathy.
| 1. | Hansen CS, Määttä LL, Andersen ST, Charles MH. The Epidemiology of Diabetic Neuropathy. In: Tesfaye S, Gibbons CH, Malik RA, Veves A, editors. Diabetic Neuropathy. Contemporary Diabetes. Humana, Cham, 2023: 5-36. [DOI] [Full Text] |
| 2. | Jones KL, Marathe CS, Wu T, Rayner CK, Horowitz M. Gastrointestinal Neuropathy. In: Tesfaye S, Gibbons CH, Malik RA, Veves A, editors. Diabetic Neuropathy. Contemporary Diabetes. Humana, Cham, 2023: 471-490. [DOI] [Full Text] |
| 3. | Bassi A, Bossa M, d’Alba L, Greco C, Casini A, Pellicano C, Simonelli M, Zampatti S. Gastrointestinal Autonomic Disorders. In: Micieli G, Hilz M, Cortelli P, editors. Autonomic Disorders in Clinical Practice. Springer, Cham, 2023: 133-177. [DOI] [Full Text] |
| 4. | Ghafoor A, Sundar S, Karunaratne T, Rao SS, Sharma A. Neurology and the gut: Autonomic neuropathy and dysautonomia. In: Rao SSC, Parkman HP, McCallum RW, editors. Handbook of Gastrointestinal Motility and Disorders of Gut-Brain Interactions. 2nd ed. Academic Press, 2023: 345-359. [DOI] [Full Text] |
| 5. | Quiroz-Aldave J, Durand-Vásquez M, Gamarra-Osorio E, Suarez-Rojas J, Jantine Roseboom P, Alcalá-Mendoza R, Coronado-Arroyo J, Zavaleta-Gutiérrez F, Concepción-Urteaga L, Concepción-Zavaleta M. Diabetic neuropathy: Past, present, and future. Caspian J Intern Med. 2023;14:153-169. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (1)] |
| 6. | Du YT, Rayner CK, Jones KL, Talley NJ, Horowitz M. Gastrointestinal Symptoms in Diabetes: Prevalence, Assessment, Pathogenesis, and Management. Diabetes Care. 2018;41:627-637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 111] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 7. | Sun H, Saeedi P, Karuranga S, Pinkepank M, Ogurtsova K, Duncan BB, Stein C, Basit A, Chan JCN, Mbanya JC, Pavkov ME, Ramachandaran A, Wild SH, James S, Herman WH, Zhang P, Bommer C, Kuo S, Boyko EJ, Magliano DJ. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res Clin Pract. 2022;183:109119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3033] [Cited by in RCA: 4798] [Article Influence: 1599.3] [Reference Citation Analysis (36)] |
| 8. | Zahid SA, Tated R, Mathew M, Rajkumar D, Karnik SB, Pramod Roy A, Jacob FP, Baskara Salian R, Razzaq W, Shivakumar D, Khawaja UA. Diabetic Gastroparesis and its Emerging Therapeutic Options: A Narrative Review of the Literature. Cureus. 2023;15:e44870. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 9. | Jung HK, Choung RS, Locke GR 3rd, Schleck CD, Zinsmeister AR, Szarka LA, Mullan B, Talley NJ. The incidence, prevalence, and outcomes of patients with gastroparesis in Olmsted County, Minnesota, from 1996 to 2006. Gastroenterology. 2009;136:1225-1233. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 474] [Cited by in RCA: 404] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 10. | Syed AR, Wolfe MM, Calles-Escandon J. Epidemiology and Diagnosis of Gastroparesis in the United States: A Population-based Study. J Clin Gastroenterol. 2020;54:50-54. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 58] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 11. | Bodi JM, Nsibu CN, Longenge RL, Aloni MN, Akilimali PZ, Kayembe PK, Omar AH, Verhaegen J, Tshibassu PM, Lukusa PT, Lumaka A, Hirayama K. Exploring association between MBL2 gene polymorphisms and the occurrence of clinical blackwater fever through a case-control study in Congolese children. Malar J. 2020;19:25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 12. | Meldgaard T, Keller J, Olesen AE, Olesen SS, Krogh K, Borre M, Farmer A, Brock B, Brock C, Drewes AM. Pathophysiology and management of diabetic gastroenteropathy. Therap Adv Gastroenterol. 2019;12:1756284819852047. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 13. | Abdu Seid M, Diress M, Mohammed A, Sinamaw D. Chronic constipation and its associated factors in patients with type-2 diabetes: A multicenter cross-sectional study. Diabetes Res Clin Pract. 2023;204:110905. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 14. | Bayliss WM, Starling EH. The movements and innervation of the small intestine. J Physiol. 1899;24:99-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 636] [Cited by in RCA: 596] [Article Influence: 33.1] [Reference Citation Analysis (0)] |
| 15. | Schofield G. Anatomy of muscular and neural tissues in the alimentary canal. Handb phy. 1968;4:1579-1627. |
| 16. | Waxenbaum JA, Reddy V, Varacallo M. Anatomy, Autonomic Nervous System. 2023 Jul 24. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-. [PubMed] |
| 17. | Kunze WA, Furness JB. The enteric nervous system and regulation of intestinal motility. Annu Rev Physiol. 1999;61:117-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 282] [Cited by in RCA: 265] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 18. | Timmermans JP, Hens J, Adriaensen D. Outer submucous plexus: an intrinsic nerve network involved in both secretory and motility processes in the intestine of large mammals and humans. Anat Rec. 2001;262:71-78. [PubMed] [DOI] [Full Text] |
| 19. | Spencer NJ, Hu H. Enteric nervous system: sensory transduction, neural circuits and gastrointestinal motility. Nat Rev Gastroenterol Hepatol. 2020;17:338-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 320] [Cited by in RCA: 366] [Article Influence: 73.2] [Reference Citation Analysis (0)] |
| 20. | Fleming MA 2nd, Ehsan L, Moore SR, Levin DE. The Enteric Nervous System and Its Emerging Role as a Therapeutic Target. Gastroenterol Res Pract. 2020;2020:8024171. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 71] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 21. | Pawolski V, Schmidt MHH. Neuron-Glia Interaction in the Developing and Adult Enteric Nervous System. Cells. 2020;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 37] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 22. | Holland AM, Bon-Frauches AC, Keszthelyi D, Melotte V, Boesmans W. The enteric nervous system in gastrointestinal disease etiology. Cell Mol Life Sci. 2021;78:4713-4733. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 88] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 23. | Choung RS, Locke GR 3rd, Schleck CD, Zinsmeister AR, Melton LJ 3rd, Talley NJ. Risk of gastroparesis in subjects with type 1 and 2 diabetes in the general population. Am J Gastroenterol. 2012;107:82-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 175] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 24. | Zhao X, Zhang Y, Guo R, Yu W, Zhang F, Wu F, Shang J. The Alteration in Composition and Function of Gut Microbiome in Patients with Type 2 Diabetes. J Diabetes Res. 2020;2020:8842651. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 38] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 25. | Farmer AD, Pedersen AG, Brock B, Jakobsen PE, Karmisholt J, Mohammed SD, Scott SM, Drewes AM, Brock C. Type 1 diabetic patients with peripheral neuropathy have pan-enteric prolongation of gastrointestinal transit times and an altered caecal pH profile. Diabetologia. 2017;60:709-718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 52] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 26. | Jackson MW, Gordon TP, Waterman SA. Disruption of intestinal motility by a calcium channel-stimulating autoantibody in type 1 diabetes. Gastroenterology. 2004;126:819-828. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 47] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 27. | Bagyánszki M, Bódi N. Key elements determining the intestinal region-specific environment of enteric neurons in type 1 diabetes. World J Gastroenterol. 2023;29:2704-2716. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 28. | Krishnan B, Babu S, Walker J, Walker AB, Pappachan JM. Gastrointestinal complications of diabetes mellitus. World J Diabetes. 2013;4:51-63. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 120] [Cited by in RCA: 125] [Article Influence: 10.4] [Reference Citation Analysis (2)] |
| 29. | Wang Y, Ye X, Ding D, Lu Y. Characteristics of the intestinal flora in patients with peripheral neuropathy associated with type 2 diabetes. J Int Med Res. 2020;48:300060520936806. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 44] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 30. | Chandrasekharan B, Anitha M, Blatt R, Shahnavaz N, Kooby D, Staley C, Mwangi S, Jones DP, Sitaraman SV, Srinivasan S. Colonic motor dysfunction in human diabetes is associated with enteric neuronal loss and increased oxidative stress. Neurogastroenterol Motil. 2011;23:131-138, e26. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 140] [Cited by in RCA: 141] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 31. | Iatcu CO, Steen A, Covasa M. Gut Microbiota and Complications of Type-2 Diabetes. Nutrients. 2021;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 207] [Article Influence: 51.8] [Reference Citation Analysis (0)] |
| 32. | Guan W, Li S, Sun W, Kang Y, Li X. Endocrine characteristics and risk factors of type 2 diabetes complicated with gastrointestinal autonomic neuropathy: A single-center retrospective study. Medicine (Baltimore). 2023;102:e33467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 33. | Mirmiran P, Bahadoran Z, Azizi F. Functional foods-based diet as a novel dietary approach for management of type 2 diabetes and its complications: A review. World J Diabetes. 2014;5:267-281. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 135] [Cited by in RCA: 130] [Article Influence: 11.8] [Reference Citation Analysis (1)] |
| 34. | Petroni ML, Brodosi L, Marchignoli F, Sasdelli AS, Caraceni P, Marchesini G, Ravaioli F. Nutrition in Patients with Type 2 Diabetes: Present Knowledge and Remaining Challenges. Nutrients. 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 41] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 35. | Concepción Zavaleta MJ, Gonzáles Yovera JG, Moreno Marreros DM, Rafael Robles LDP, Palomino Taype KR, Soto Gálvez KN, Arriola Torres LF, Coronado Arroyo JC, Concepción Urteaga LA. Diabetic gastroenteropathy: An underdiagnosed complication. World J Diabetes. 2021;12:794-809. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 33] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (4)] |
| 36. | Meldgaard T, Olesen SS, Farmer AD, Krogh K, Wendel AA, Brock B, Drewes AM, Brock C. Diabetic Enteropathy: From Molecule to Mechanism-Based Treatment. J Diabetes Res. 2018;2018:3827301. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 35] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 37. | Friedman EA. Advanced glycosylated end products and hyperglycemia in the pathogenesis of diabetic complications. Diabetes Care. 1999;22 Suppl 2:B65-B71. [PubMed] |
| 38. | Soulis T, Thallas V, Youssef S, Gilbert RE, McWilliam BG, Murray-McIntosh RP, Cooper ME. Advanced glycation end products and their receptors co-localise in rat organs susceptible to diabetic microvascular injury. Diabetologia. 1997;40:619-628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 142] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 39. | Indyk D, Bronowicka-Szydełko A, Gamian A, Kuzan A. Advanced glycation end products and their receptors in serum of patients with type 2 diabetes. Sci Rep. 2021;11:13264. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 67] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 40. | Phuong-Nguyen K, McNeill BA, Aston-Mourney K, Rivera LR. Advanced Glycation End-Products and Their Effects on Gut Health. Nutrients. 2023;15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 38] [Reference Citation Analysis (0)] |
| 41. | Stavely R, Ott LC, Rashidi N, Sakkal S, Nurgali K. The Oxidative Stress and Nervous Distress Connection in Gastrointestinal Disorders. Biomolecules. 2023;13. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 42. | Viader A, Wright-Jin EC, Vohra BP, Heuckeroth RO, Milbrandt J. Differential regional and subtype-specific vulnerability of enteric neurons to mitochondrial dysfunction. PLoS One. 2011;6:e27727. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 43. | Sifuentes-Franco S, Pacheco-Moisés FP, Rodríguez-Carrizalez AD, Miranda-Díaz AG. The Role of Oxidative Stress, Mitochondrial Function, and Autophagy in Diabetic Polyneuropathy. J Diabetes Res. 2017;2017:1673081. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 95] [Cited by in RCA: 111] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 44. | Bagyánszki M, Bódi N. Diabetes-related alterations in the enteric nervous system and its microenvironment. World J Diabetes. 2012;3:80-93. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 38] [Cited by in RCA: 44] [Article Influence: 3.4] [Reference Citation Analysis (1)] |
| 45. | Anitha M, Gondha C, Sutliff R, Parsadanian A, Mwangi S, Sitaraman SV, Srinivasan S. GDNF rescues hyperglycemia-induced diabetic enteric neuropathy through activation of the PI3K/Akt pathway. J Clin Invest. 2006;116:344-356. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 191] [Cited by in RCA: 198] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 46. | De Giorgio R, Guerrini S, Barbara G, Stanghellini V, De Ponti F, Corinaldesi R, Moses PL, Sharkey KA, Mawe GM. Inflammatory neuropathies of the enteric nervous system. Gastroenterology. 2004;126:1872-1883. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 197] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 47. | Okdahl T, Wegeberg AM, Jensen ABH, Jensen ST, Andersen HRP, Størling J, Brock B, Brock C. Systemic Cytokine Expression in Diabetes Is Associated with Prolonged Gastrointestinal Transit Times and Cardinal Gastroparesis Symptoms. Biomedicines. 2023;11. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (1)] |
| 48. | Shen L, Ao L, Xu H, Shi J, You D, Yu X, Xu W, Sun J, Wang F. Poor short-term glycemic control in patients with type 2 diabetes impairs the intestinal mucosal barrier: a prospective, single-center, observational study. BMC Endocr Disord. 2019;19:29. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 49. | Thaiss CA, Levy M, Grosheva I, Zheng D, Soffer E, Blacher E, Braverman S, Tengeler AC, Barak O, Elazar M, Ben-Zeev R, Lehavi-Regev D, Katz MN, Pevsner-Fischer M, Gertler A, Halpern Z, Harmelin A, Aamar S, Serradas P, Grosfeld A, Shapiro H, Geiger B, Elinav E. Hyperglycemia drives intestinal barrier dysfunction and risk for enteric infection. Science. 2018;359:1376-1383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 416] [Cited by in RCA: 620] [Article Influence: 88.6] [Reference Citation Analysis (0)] |
| 50. | Yuan JH, Xie QS, Chen GC, Huang CL, Yu T, Chen QK, Li JY. Impaired intestinal barrier function in type 2 diabetic patients measured by serum LPS, Zonulin, and IFABP. J Diabetes Complications. 2021;35:107766. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 44] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 51. | Tentolouris A, Tentolouris N. Diabetic Autonomic Neuropathy. In: Chokroverty S, Cortelli P, editors. Autonomic Nervous System and Sleep. Springer, Cham, 2021: 307-315. [DOI] [Full Text] |
| 52. | Zimmerman MA, Flores SC. Autoimmune-mediated oxidative stress and endothelial dysfunction: implications of accelerated vascular injury in type I diabetes. J Surg Res. 2009;155:173-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 53. | Kuźnik E, Dudkowiak R, Adamiec R, Poniewierka E. Diabetic autonomic neuropathy of the gastrointestinal tract. Prz Gastroenterol. 2020;15:89-93. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 54. | Sampath C, Raju AV, Freeman ML, Srinivasan S, Gangula PR. Nrf2 attenuates hyperglycemia-induced nNOS impairment in adult mouse primary enteric neuronal crest cells and normalizes stomach function. Am J Physiol Gastrointest Liver Physiol. 2022;322:G368-G382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 55. | Bassotti G, Villanacci V, Fisogni S, Rossi E, Baronio P, Clerici C, Maurer CA, Cathomas G, Antonelli E. Enteric glial cells and their role in gastrointestinal motor abnormalities: introducing the neuro-gliopathies. World J Gastroenterol. 2007;13:4035-4041. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 59] [Cited by in RCA: 55] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 56. | Baghdadi MB, Kim TH. The multiple roles of enteric glial cells in intestinal homeostasis and regeneration. Semin Cell Dev Biol. 2023;150-151:43-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 57. | López-Gómez L, Abalo R. Modulation of enteric glial cells by nutraceuticals during pathological processes. In: de Oliveira MR. Natural Molecules in Neuroprotection and Neurotoxicity. Academic Press, 2024: 229-257. [DOI] [Full Text] |
| 58. | Chandrasekharan B, Srinivasan S. Diabetes and the enteric nervous system. Neurogastroenterol Motil. 2007;19:951-960. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 172] [Cited by in RCA: 112] [Article Influence: 6.2] [Reference Citation Analysis (1)] |
| 59. | Proks P, Lippiat JD. Membrane ion channels and diabetes. Curr Pharm Des. 2006;12:485-501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 60. | Field M. Intestinal ion transport and the pathophysiology of diarrhea. J Clin Invest. 2003;111:931-943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 181] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 61. | Sanders KM, Ordög T, Ward SM. Physiology and pathophysiology of the interstitial cells of Cajal: from bench to bedside. IV. Genetic and animal models of GI motility disorders caused by loss of interstitial cells of Cajal. Am J Physiol Gastrointest Liver Physiol. 2002;282:G747-G756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 103] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 62. | Grover M, Farrugia G, Lurken MS, Bernard CE, Faussone-Pellegrini MS, Smyrk TC, Parkman HP, Abell TL, Snape WJ, Hasler WL, Ünalp-Arida A, Nguyen L, Koch KL, Calles J, Lee L, Tonascia J, Hamilton FA, Pasricha PJ; NIDDK Gastroparesis Clinical Research Consortium. Cellular changes in diabetic and idiopathic gastroparesis. Gastroenterology. 2011;140:1575-85.e8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 375] [Cited by in RCA: 331] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 63. | Ban CR, Twigg SM. Fibrosis in diabetes complications: pathogenic mechanisms and circulating and urinary markers. Vasc Health Risk Manag. 2008;4:575-596. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 147] [Cited by in RCA: 202] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 64. | Singh R, Zogg H, Wei L, Bartlett A, Ghoshal UC, Rajender S, Ro S. Gut Microbial Dysbiosis in the Pathogenesis of Gastrointestinal Dysmotility and Metabolic Disorders. J Neurogastroenterol Motil. 2021;27:19-34. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 166] [Cited by in RCA: 141] [Article Influence: 35.3] [Reference Citation Analysis (0)] |
| 65. | Heiss CN, Olofsson LE. The role of the gut microbiota in development, function and disorders of the central nervous system and the enteric nervous system. J Neuroendocrinol. 2019;31:e12684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 188] [Article Influence: 31.3] [Reference Citation Analysis (0)] |
| 66. | Barbara G, Stanghellini V, Brandi G, Cremon C, Di Nardo G, De Giorgio R, Corinaldesi R. Interactions between commensal bacteria and gut sensorimotor function in health and disease. Am J Gastroenterol. 2005;100:2560-2568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 241] [Cited by in RCA: 218] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 67. | Asano Y, Hiramoto T, Nishino R, Aiba Y, Kimura T, Yoshihara K, Koga Y, Sudo N. Critical role of gut microbiota in the production of biologically active, free catecholamines in the gut lumen of mice. Am J Physiol Gastrointest Liver Physiol. 2012;303:G1288-G1295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 494] [Cited by in RCA: 437] [Article Influence: 33.6] [Reference Citation Analysis (0)] |
| 68. | Liu S. Neurotrophic factors in enteric physiology and pathophysiology. Neurogastroenterol Motil. 2018;30:e13446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 42] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 69. | Apfel SC. Neurotrophic factors and diabetic peripheral neuropathy. Eur Neurol. 1999;41 Suppl 1:27-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 68] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 70. | Moosaie F, Mohammadi S, Saghazadeh A, Dehghani Firouzabadi F, Rezaei N. Brain-derived neurotrophic factor in diabetes mellitus: A systematic review and meta-analysis. PLoS One. 2023;18:e0268816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 19] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 71. | Wei L, Ji L, Miao Y, Han X, Li Y, Wang Z, Fu J, Guo L, Su Y, Zhang Y. Constipation in DM are associated with both poor glycemic control and diabetic complications: Current status and future directions. Biomed Pharmacother. 2023;165:115202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 72. | Lomax AE, Fernández E, Sharkey KA. Plasticity of the enteric nervous system during intestinal inflammation. Neurogastroenterol Motil. 2005;17:4-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 142] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 73. | Longo S, Rizza S, Federici M. Microbiota-gut-brain axis: relationships among the vagus nerve, gut microbiota, obesity, and diabetes. Acta Diabetol. 2023;60:1007-1017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 69] [Reference Citation Analysis (1)] |
| 74. | Stenkamp-Strahm CM, Nyavor YE, Kappmeyer AJ, Horton S, Gericke M, Balemba OB. Prolonged high fat diet ingestion, obesity, and type 2 diabetes symptoms correlate with phenotypic plasticity in myenteric neurons and nerve damage in the mouse duodenum. Cell Tissue Res. 2015;361:411-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 39] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 75. | D’Addio F, La Rosa S, Maestroni A, Jung P, Orsenigo E, Ben Nasr M, Tezza S, Bassi R, Finzi G, Marando A, Vergani A, Frego R, Albarello L, Andolfo A, Manuguerra R, Viale E, Staudacher C, Corradi D, Batlle E, Breault D, Secchi A, Folli F, Fiorina P. Circulating IGF-I and IGFBP3 Levels Control Human Colonic Stem Cell Function and Are Disrupted in Diabetic Enteropathy. Cell Stem Cell. 2015;17:486-498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 66] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 76. | Chakaroun RM, Massier L, Kovacs P. Gut Microbiome, Intestinal Permeability, and Tissue Bacteria in Metabolic Disease: Perpetrators or Bystanders? Nutrients. 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 144] [Cited by in RCA: 178] [Article Influence: 35.6] [Reference Citation Analysis (0)] |
| 77. | Veličkov A, Radenković G, Petrović V, Veličkov A. Diabetic Alterations Of Interstitial Cells of Cajal. Acta Medica Medianae. 2017;56:100-107. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 78. | He CL, Soffer EE, Ferris CD, Walsh RM, Szurszewski JH, Farrugia G. Loss of interstitial cells of cajal and inhibitory innervation in insulin-dependent diabetes. Gastroenterology. 2001;121:427-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 235] [Cited by in RCA: 237] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 79. | Pompili S, Latella G, Gaudio E, Sferra R, Vetuschi A. The Charming World of the Extracellular Matrix: A Dynamic and Protective Network of the Intestinal Wall. Front Med (Lausanne). 2021;8:610189. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 80] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 80. | Jensen MM, Wegeberg AL, Jensen SL, Sørensen PS, Wigh IMN, Zaugg VS, Færch K, Quist JS, Brock C. The day-night pattern of colonic contractility is not impaired in type 1 diabetes and distal symmetric polyneuropathy. Chronobiol Int. 2021;38:801-806. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 81. | Ashrafizadeh M, Kumar AP, Aref AR, Zarrabi A, Mostafavi E. Exosomes as Promising Nanostructures in Diabetes Mellitus: From Insulin Sensitivity to Ameliorating Diabetic Complications. Int J Nanomedicine. 2022;17:1229-1253. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 52] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 82. | Bulc M, Palus K, Całka J, Zielonka Ł. Changes in Immunoreactivity of Sensory Substances within the Enteric Nervous System of the Porcine Stomach during Experimentally Induced Diabetes. J Diabetes Res. 2018;2018:4735659. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 83. | Chedid V, Brandler J, Vijayvargiya P, Park SY, Szarka LA, Camilleri M. Characterization of Upper Gastrointestinal Symptoms, Gastric Motor Functions, and Associations in Patients with Diabetes at a Referral Center. Am J Gastroenterol. 2019;114:143-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 41] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 84. | Kurniawan AH, Suwandi BH, Kholili U. Diabetic Gastroenteropathy: A Complication of Diabetes Mellitus. Acta Med Indones. 2019;51:263-271. [PubMed] |
| 85. | Lin J, Liu G, Duan Z. The mechanism of esophagus dysmotility in diabetes and research progress of relating treatments. Expert Rev Gastroenterol Hepatol. 2021;15:919-927. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 86. | Savarino E, Marabotto E, Bodini G, Furnari M, Della Coletta M, Ghisa M, Barberio B, Frazzoni M, De Bortoli N, Zentilin P, Pellegatta G, Tolone S, Ottonello A, Savarino V. Advancements in the use of manometry and impedance testing for esophageal functional disorders. Expert Rev Gastroenterol Hepatol. 2019;13:425-435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 87. | Rude MK, Gyawali CP. High-Resolution Manometry and Assessment of Esophageal Reflux. In: Jonnalagadda S, editors. Gastrointestinal Endoscopy. New York: Springer, 2015: 107-126. [DOI] [Full Text] |
| 88. | Zhao J, Gregersen H. Diabetes-induced mechanophysiological changes in the esophagus. Ann N Y Acad Sci. 2016;1380:139-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 89. | Bharucha AE, Kudva YC, Prichard DO. Diabetic Gastroparesis. Endocr Rev. 2019;40:1318-1352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 134] [Article Influence: 22.3] [Reference Citation Analysis (3)] |
| 90. | Krishnasamy S, Abell TL. Diabetic Gastroparesis: Principles and Current Trends in Management. Diabetes Ther. 2018;9:1-42. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 92] [Cited by in RCA: 95] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 91. | D’Addio F, Fiorina P. Type 1 Diabetes and Dysfunctional Intestinal Homeostasis. Trends Endocrinol Metab. 2016;27:493-503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 92. | Bhor VM, Raghuram N, Sivakami S. Oxidative damage and altered antioxidant enzyme activities in the small intestine of streptozotocin-induced diabetic rats. Int J Biochem Cell Biol. 2004;36:89-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 126] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 93. | Pereira EC, Ferderbar S, Bertolami MC, Faludi AA, Monte O, Xavier HT, Pereira TV, Abdalla DS. Biomarkers of oxidative stress and endothelial dysfunction in glucose intolerance and diabetes mellitus. Clin Biochem. 2008;41:1454-1460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 44] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 94. | Qing Q, Zhang S, Chen Y, Li R, Mao H, Chen Q. High glucose-induced intestinal epithelial barrier damage is aggravated by syndecan-1 destruction and heparanase overexpression. J Cell Mol Med. 2015;19:1366-1374. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 33] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 95. | Gotfried J, Priest S, Schey R. Diabetes and the Small Intestine. Curr Treat Options Gastroenterol. 2017;15:490-507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 96. | Klein S, Seidler B, Kettenberger A, Sibaev A, Rohn M, Feil R, Allescher HD, Vanderwinden JM, Hofmann F, Schemann M, Rad R, Storr MA, Schmid RM, Schneider G, Saur D. Interstitial cells of Cajal integrate excitatory and inhibitory neurotransmission with intestinal slow-wave activity. Nat Commun. 2013;4:1630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 174] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 97. | Korenaga K, Micci MA, Taglialatela G, Pasricha PJ. Suppression of nNOS expression in rat enteric neurones by the receptor for advanced glycation end-products. Neurogastroenterol Motil. 2006;18:392-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 98. | Horváth VJ, Putz Z, Izbéki F, Körei AE, Gerő L, Lengyel C, Kempler P, Várkonyi T. Diabetes-related dysfunction of the small intestine and the colon: focus on motility. Curr Diab Rep. 2015;15:94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 39] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 99. | Blair PJ, Rhee PL, Sanders KM, Ward SM. The significance of interstitial cells in neurogastroenterology. J Neurogastroenterol Motil. 2014;20:294-317. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 106] [Cited by in RCA: 98] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 100. | Reszczyńska M, Kempiński R. The Prevalence of Enteropathy Symptoms from the Lower Gastrointestinal Tract and the Evaluation of Anorectal Function in Diabetes Mellitus Patients. J Clin Med. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 101. | Bharucha AE, Dunivan G, Goode PS, Lukacz ES, Markland AD, Matthews CA, Mott L, Rogers RG, Zinsmeister AR, Whitehead WE, Rao SS, Hamilton FA. Epidemiology, pathophysiology, and classification of fecal incontinence: state of the science summary for the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) workshop. Am J Gastroenterol. 2015;110:127-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 201] [Article Influence: 20.1] [Reference Citation Analysis (1)] |
| 102. | Oblizajek NR, Gandhi S, Sharma M, Chakraborty S, Muthyala A, Prichard D, Feuerhak K, Bharucha AE. Anorectal pressures measured with high-resolution manometry in healthy people-Normal values and asymptomatic pelvic floor dysfunction. Neurogastroenterol Motil. 2019;31:e13597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 61] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 103. | Eldesoky A, Bahgat M, El-shreif M, El-gamal S, Taha K. Anorectal dysfunctions in diabetic autonomic neuropathy and microangiopathy. Arab J Gastroenterol. 2010;11:79-82. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 104. | Ziessman HA. Gastrointestinal Transit Assessment: Role of Scintigraphy: Where Are We Now? Curr Treat Options Gastroenterol. 2016;14:452-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 105. | Gong EJ. Analysis of Whole Gut Motility for the Evaluation of Diabetic Gastroenteropathy: Still the Road Untraveled? J Neurogastroenterol Motil. 2021;27:307-309. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 106. | Sangnes DA. Diabetic gastroenteropathy examined with wireless motility capsule. 2022. [cited 1 May 2024]. Available from: https://bora.uib.no/bora-xmlui/bitstream/handle/11250/2981948/archive.pdf?sequence=1. |
| 107. | Rondonotti E, Spada C, Adler S, May A, Despott EJ, Koulaouzidis A, Panter S, Domagk D, Fernandez-Urien I, Rahmi G, Riccioni ME, van Hooft JE, Hassan C, Pennazio M. Small-bowel capsule endoscopy and device-assisted enteroscopy for diagnosis and treatment of small-bowel disorders: European Society of Gastrointestinal Endoscopy (ESGE) Technical Review. Endoscopy. 2018;50:423-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 333] [Cited by in RCA: 288] [Article Influence: 41.1] [Reference Citation Analysis (0)] |
| 108. | Hoffmanová I, Sánchez D, Hábová V, Anděl M, Tučková L, Tlaskalová-Hogenová H. Serological markers of enterocyte damage and apoptosis in patients with celiac disease, autoimmune diabetes mellitus and diabetes mellitus type 2. Physiol Res. 2015;64:537-546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 35] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 109. | Demir E, Ozkan H, Seckin KD, Sahtiyancı B, Demir B, Tabak O, Kumbasar A, Uzun H. Plasma Zonulin Levels as a Non-Invasive Biomarker of Intestinal Permeability in Women with Gestational Diabetes Mellitus. Biomolecules. 2019;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 34] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 110. | Mostafa I, Hasan SMT, Gazi MA, Alam MA, Fahim SM, Saqeeb KN, Ahmed T. Alteration of stool pH and its association with biomarkers of gut enteropathy among slum-dwelling women of reproductive age in Bangladesh. BMC Womens Health. 2023;23:661. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 111. | Kornum DS, Terkelsen AJ, Bertoli D, Klinge MW, Høyer KL, Kufaishi HHA, Borghammer P, Drewes AM, Brock C, Krogh K. Assessment of Gastrointestinal Autonomic Dysfunction: Present and Future Perspectives. J Clin Med. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 18] [Reference Citation Analysis (0)] |
| 112. | Pabolu S, Dudekula A, Pitchumoni CS. Gastrointestinal Manifestations of Non-GI Disorders. In: Pitchumoni CS, Dharmarajan T, editors. Geriatric Gastroenterology. Springer, Cham, 2021: 2117-2166. [DOI] [Full Text] |
| 113. | Lindberg G. Pseudo-obstruction, enteric dysmotility and irritable bowel syndrome. Best Pract Res Clin Gastroenterol. 2019;40-41:101635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 114. | Keller J, Bassotti G, Clarke J, Dinning P, Fox M, Grover M, Hellström PM, Ke M, Layer P, Malagelada C, Parkman HP, Scott SM, Tack J, Simren M, Törnblom H, Camilleri M; International Working Group for Disorders of Gastrointestinal Motility and Function. Expert consensus document: Advances in the diagnosis and classification of gastric and intestinal motility disorders. Nat Rev Gastroenterol Hepatol. 2018;15:291-308. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 198] [Cited by in RCA: 171] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 115. | Naraev BG, Mailman J, Halfdanarson TR, Soares HP, Mittra ES, Hallet J. Consideration of quality of life in the treatment decision-making for patients with advanced gastroenteropancreatic neuroendocrine tumors. Expert Rev Anticancer Ther. 2023;23:601-615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 116. | Shaw SJ, Huebner C, Armin J, Orzech K, Vivian J. The role of culture in health literacy and chronic disease screening and management. J Immigr Minor Health. 2009;11:460-467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 131] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 117. | Camilleri M, Atieh J. New Developments in Prokinetic Therapy for Gastric Motility Disorders. Front Pharmacol. 2021;12:711500. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 59] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 118. | Al-Saffar A, Lennernäs H, Hellström PM. Gastroparesis, metoclopramide, and tardive dyskinesia: Risk revisited. Neurogastroenterol Motil. 2019;31:e13617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 51] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 119. | Camejo M, Gaddis ML, Dinh P. Rethinking Ondansetron as a First Line Agent for Nausea and Vomiting in the Emergency Department. J Emerg Med. 2023;64:414-415. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 120. | Meling S, Bertoli D, Sangnes DA, Brock C, Drewes A, Ejskjaer N, Dimcevski G, Søfteland E. Diabetic Gastroenteropathy: Soothe the Symptoms or Unravel a Cure? Curr Diabetes Rev. 2022;18:e220321192412. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 121. | Kahawita T, Leong LJP, Mcconaghy R. The use of clonidine to manage chronic refractory diarrhoea in a palliative patient: A case report. Prog Palliat Care. 2024;32:93-97. [DOI] [Full Text] |
| 122. | Mourad FH, Gorard D, Thillainayagam AV, Colin-Jones D, Farthing MJ. Effective treatment of diabetic diarrhoea with somatostatin analogue, octreotide. Gut. 1992;33:1578-1580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 33] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 123. | Gomes-Porras M, Cárdenas-Salas J, Álvarez-Escolá C. Somatostatin Analogs in Clinical Practice: a Review. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 146] [Article Influence: 29.2] [Reference Citation Analysis (0)] |
| 124. | Merecz K, Hirsa M, Biniszewska O, Fichna J, Tarasiuk A. An overview of 5-HT(3) receptor antagonists as a treatment option for irritable bowel syndrome with diarrhea. Expert Opin Pharmacother. 2023;24:1189-1198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 125. | Guzel T, Mirowska-Guzel D. The Role of Serotonin Neurotransmission in Gastrointestinal Tract and Pharmacotherapy. Molecules. 2022;27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 72] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 126. | Bridges M. Dietary therapy for gastroparesis. In: McCallum RW, Parkman HP, editors. Gastroparesis. Academic Press, 2021: 311-321. [DOI] [Full Text] |
| 127. | Limketkai BN, LeBrett W, Lin L, Shah ND. Nutritional approaches for gastroparesis. Lancet Gastroenterol Hepatol. 2020;5:1017-1026. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 128. | Aguilar A, Malagelada C, Serra J. Nutritional challenges in patients with gastroparesis. Curr Opin Clin Nutr Metab Care. 2022;25:360-363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 129. | Callaghan BC, Little AA, Feldman EL, Hughes RA. Enhanced glucose control for preventing and treating diabetic neuropathy. Cochrane Database Syst Rev. 2012;6:CD007543. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 204] [Cited by in RCA: 268] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 130. | Abdelsayed N, Juarez A, Carter M. Severe Gastroparesis Leading to Hypoglycemia and Subsequent Seizures. Cureus. 2022;14:e30527. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 131. | Gourcerol G, Gonzalez JM, Bonaz B, Fontaine S, Zerbib F, Mion F, Basile P, Gillibert A, Labonde A, Soliman H, Vitton V, Coffin B, Jacques J. Gastric electrical stimulation vs per-oral pyloromyotomy for the treatment of nausea and vomiting associated with gastroparesis: An observational study of two cohorts. Neurogastroenterol Motil. 2023;35:e14565. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 132. | Saeed S, Kamran M, Bhagwani K, Shaikh N, Ekhator C, Farahat M, Abdelaziz AM, Shehryar A. Gastric Electrical Stimulation for Refractory Gastroparesis: A Promising Treatment Modality for Symptom Control and Gastric Emptying. Cureus. 2023;15:e41630. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 133. | Tran D, Leeds SG, Fair L, Fang J, Rubarth C, McGowan T, Ramakrishnan S, Ogola G, Aladegbami B, Ward MA. Gastric per-oral endoscopic myotomy vs pyloric injection of botulinum toxin for the treatment of gastroparesis: our institutional experience and a systematic review of the literature. Surg Endosc. 2023;37:7280-7287. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 134. | Karna R, Kumar A, Kalra T, Rana T, Butt MA, Muzammil TS, Mohan B, Dhawan M, Adler DG. S1887 Efficacy of Endoscopic Intrapyloric Injection of Botulinum Toxin in the Treatment of Gastroparesis: A Systematic Review and Meta-Analysis. Am J Gastroenterol. 2023;118:S1403-S1403. [DOI] [Full Text] |
| 135. | Zhang YX, Zhang YJ, Miao RY, Fang XY, Wei JH, Wei Y, Lin JR, Tian JX. Effectiveness and safety of traditional Chinese medicine decoction for diabetic gastroparesis: A network meta-analysis. World J Diabetes. 2023;14:313-342. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 4] [Cited by in RCA: 5] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 136. | Tao Y, Wang Y. Effect of empowerment theory health education on disease control level and compliance of elderly T2DM. Pak J Pharm Sci. 2023;36:643-648. [PubMed] |
| 137. | Ansari MA, Chauhan W, Shoaib S, Alyahya SA, Ali M, Ashraf H, Alomary MN, Al-Suhaimi EA. Emerging therapeutic options in the management of diabetes: recent trends, challenges and future directions. Int J Obes (Lond). 2023;47:1179-1199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 14] [Article Influence: 7.0] [Reference Citation Analysis (0)] |