Published online Jun 7, 2024. doi: 10.3748/wjg.v30.i21.2817
Revised: April 17, 2024
Accepted: April 23, 2024
Published online: June 7, 2024
Processing time: 94 Days and 16.5 Hours
The association between the intestinal microbiota and psychiatric disorders is becoming increasingly apparent. The gut microbiota contributes to colorectal carcinogenesis (CRC), as demonstrated with colibactin-producing Escherichia coli (CoPEC).
To evaluate the association between CoPEC prevalence and anxiety- and depressive-like behaviors with both preclinical and clinical approaches.
Patients followed after a CRC surgery and for whom the prevalence of CoPEC has been investigated underwent a psychiatric interview. Results were compared according to the CoPEC colonization. In parallel C57BL6/J wild type mice and mice with a CRC susceptibility were chronically infected with a CoPEC strain. Their behavior was assessed using the Elevated Plus Maze test, the Forced Swimming Test and the Behavior recognition system PhenoTyper®.
In a limited cohort, all patients with CoPEC colonization presented with psychiatric disorders several years before cancer diagnosis, whereas only one patient (17%) without CoPEC did. This result was confirmed in C57BL6/J wild-type mice and in a CRC susceptibility mouse model (adenomatous polyposis colimultiple intestinal neoplasia/+). Mice exhibited a significant increase in anxiety- and depressive-like behaviors after chronic infection with a CoPEC strain.
This finding provides the first evidence that CoPEC infection can induce microbiota-gut-brain axis disturbances in addition to its procarcinogenic properties.
Core Tip: Colibactin-producing Escherichia coli (CoPEC) have been associated to colorectal carcinogenesis. We demonstrated that all colorectal cancer patients colonized by CoPEC had psychiatric disorders that occurred several years before colorectal cancer diagnosis. Mice chronically infected with a CoPEC strain exhibited a significant increase of anxiety-/depressive-like behaviors in several tests. These first results suggest that CoPEC infection could induce microbiota-gut-brain axis disturbances in addition to their pro-carcinogenic properties.
- Citation: Rondepierre F, Meynier M, Gagniere J, Deneuvy V, Deneuvy A, Roche G, Baudu E, Pereira B, Bonnet R, Barnich N, Carvalho FA, Pezet D, Bonnet M, Jalenques I. Preclinical and clinical evidence of the association of colibactin-producing Escherichia coli with anxiety and depression in colon cancer. World J Gastroenterol 2024; 30(21): 2817-2826
- URL: https://www.wjgnet.com/1007-9327/full/v30/i21/2817.htm
- DOI: https://dx.doi.org/10.3748/wjg.v30.i21.2817
The gastrointestinal tract and the nervous system are closely connected by a complex bidirectional system in which the gut and the central and enteric nervous systems interact with each other, engaging neuronal, endocrine and immune activities. The intestinal microbiota has been described recently as a third component of the brain–gut axis[1]. The gut microbiome is composed of a wide variety of microbial species and contributes to the maintenance of digestive tract integrity[2]. Disturbances in the gut microbiome (dysbiosis) may play a key role in the pathogenesis of gastrointestinal diseases (inflammatory bowel disease, irritable bowel syndrome, colorectal cancer, etc.) as well as extradigestive disorders (hypertension, autism spectrum disorders, anxiety, depression and Parkinson’s disease). Clinical studies were first performed on Parkinson’s disease given the high prevalence of gut dysfunction in these patients. A decrease in the abundance of the anti-inflammatory genus Faecalibacterium is associated with an increase in the abundance of the proinflammatory genus Proteobacteria[3,4]. Interestingly, Enterobacteriaceae levels correlated with postural instability and gait issues[4]. Modifications in the microbiome can induce depressive-like behaviors, which are comorbidities of neurodegenerative disorders. Similar dysbiosis has also been described in anxiety and depressive disorders[5-7]. These clinical observations and experiments in animal models support the suggested role of the intestinal microbiome in the development and progression of anxiety and depression[8-11].
Associations between gut microbiota disturbances and colorectal carcinogenesis (CRC) have also been demonstrated[12,13]. Metagenomic studies have shown that the gut microbiota has a greater abundance of procarcinogenic taxa, such as Escherichia, Bacteroides, and Fusobacterium, in CRC patients than in healthy individuals[13]. Colibactin-producing Escherichia coli (CoPEC) are pathobiont bacteria that have been shown to be more prevalent in the biopsies of CRC patients than in those of control patients[14-16]. Colibactin is almost exclusively closed to Escherichia coli (E. coli) phylogroup B2 that includes the extraintestinal pathogenic E. coli which are the commonest causative agent of urinary tract infections and highly frequent in neonatal meningitis and sepsis[17]. In vitro and in vivo studies have also demonstrated the role of CoPEC and the colibactin toxin in CRC [15,18-21].
Severe depressive symptoms have been associated with CRC. Because microbiota–gut–brain axis disturbances and Enterobacteria imbalance have been implicated in both depression and CRC pathologies, the aim of this study was to evaluate psychiatric disorders in CRC patients colonized by CoPEC. Our clinical observations were confirmed through the study of anxiety- and depressive-like behaviors in animal models chronically infected with CoPEC.
Patients: The study was recommended to all nondemented adult patients who were treated at the digestive surgery department of Clermont-Ferrand University Hospital after CRC surgery and tested for the presence of CoPEC via PCR analysis of CRC samples[18]. Informed consent was obtained from all subjects involved in the study.
Psychiatric evaluation: Patients were evaluated by a psychiatrist for current and lifetime Axis I psychiatric disorders as defined by the DSM-IV using the Mini International Neuropsychiatric Interview (MINI 5.0.0)[22], a standardized, structured psychiatric interview that has been validated in French[23,24]. The severity of depression and anxiety was determined with the Hamilton Depression Rating Scale (HDRS)[25] and Hamilton Anxiety Rating Scale (HAMA)[26], respectively. These two scales were completed by the psychiatrist after an interview, and higher scores reflected greater severity. For the HAMA, psychic and somatic anxiety subscores were used.
Data collection: Sociodemographic (age, sex, educational level and way of life) and medical (tumor location, TNM CRC stage, laparoscopy or laparotomy, presence of CoPEC) data were collected from both surveys and medical records via the Research Electronic Data Capture (REDCap) tool hosted at Clermont-Ferrand University Hospital[27]. REDCap is a secure, web-based application designed to support data capture for research studies.
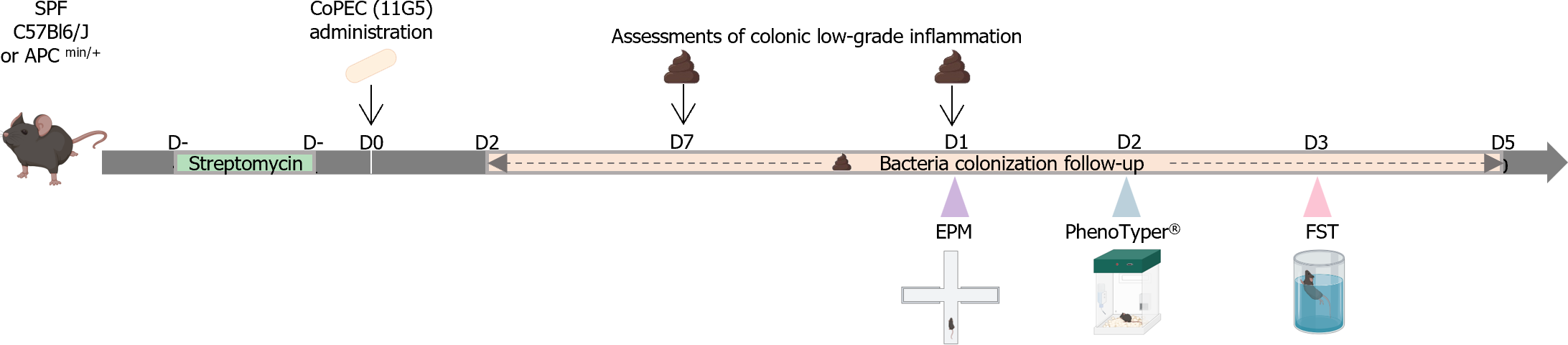
Animals: All animals were housed at animal biosafety level 2 conditions (21-22°C, 12:12 h light-dark cycle) with access to food and water ad libitum. Studies were performed using five-week-old wild-type (WT) C57BL/6 male mice (n = 12 mice/group, 24 in total) purchased from Janvier Lab (Le Genest-Saint-Isle, France) and five- to six-week-old C57BL/6J-adenomatous polyposis coli (Apc)multiple intestinal neoplasia (Min)/+ mice purchased from The Jackson Laboratory (Bar Harbor, Maine, United States; n = 4 control and 5 infected mice, 9 in total).
To enhance bacterial colonization, streptomycin (2.5 g/L) was administered for 3 days prior to oral inoculation with 109 bacteria (Figure 1). The representative strain of CoPEC used was the 11G5 strain, which was isolated from the colonic tissue of a patient with colon cancer as previously described[28]. Control mice were inoculated with 200 µL of sterile 1X phosphate-buffered saline (PBS). Fresh fecal pellets were collected every week (Figure 1) and resuspended in sterile 1X PBS. After serial dilution, the bacteria were enumerated by plating on LB agar medium supplemented with 50 µg/mL ampicillin and 50 µg/mL kanamycin and incubated at 37°C overnight. Colony forming units were enumerated and normalized to the weight of the feces.
Colonic low-grade inflammation: Fecal samples were collected at 7 d postinfection (DPI) and 15 DPI (Figure 1), weighed and homogenized in sterile 1X PBS. The vials were centrifuged (10000 × g, 10 min), and the supernatants were stored at -20°C until analysis. The supernatants were subsequently diluted in kit-recommended reagent (1% bovine serum albumin in 1X PBS), and the concentration of lipocalin-2 was estimated using the Mouse Lipocalin-2/NGAL Duoset ELISA kit (R&D Systems, Minneapolis, MN) according to the manufacturer’s instructions. Concentrations were normalized to the weight of the feces.
Anxiety-like behavior: Anxiety-like behavior was assessed using the elevated plus maze (EPM) test (ViewPoint Behavior Technology, Lissieu, France) at 15 DPI (Figure 1), as previously described[29]. Mice were acclimated to the room for at least 45 min before the test. Individual animals were placed in the central zone and allowed to explore the maze for 5 min. Mice were recorded with a camera, and the data were automatically scored by EthoVision XT® software (version 14; Noldus Information Technology, Wageningen, Netherlands). Anxiety was characterized according to the number of entries in each arm (defined as all four paws located within the arm) and the time spent in the open arms.
Behavior recognition system PhenoTyper®: PhenoTyper® (Noldus Information Technology, Wageningen, The Netherlands) is an automated infrared (IR) video-tracking system for measuring animal behavior[29]. Briefly, this device is composed of 8 plexiglass cages (45 cm × 45 cm), each containing an opaque plastic square shelter accessible by two entrances and delimited areas for feeding and drinking. The cages are surrounded by a top unit with IR lighting and an IR camera for video recording during light and dark cycles. At 20 DPI (Figure 1), the mice were transferred into PhenoTyper® cages (1 per cage), and animal activities were recorded with Mediarecorder® (Noldus Information Technology, Wageningen, Netherlands) for 24 h, including a 12 h dark period and a 12 h light period. During the test, the mice had ad libitum access to food, water and shelter. The raw data were analyzed with EthoVision XT® software (version 14; Noldus Information Technology, Wageningen, Netherlands), and various specific behaviors were compared between the infected and control animals.
Forced Swimming Test (FST): At 35 DPI (Figure 1), after acclimatization for 45 min, each mouse was placed in a glass cylinder (17 cm in diameter and 30 cm high) containing water at 22 ± 2 °C. The immobility time of the mice within a 6 min period was recorded. Immobility was defined as when a mouse stopped swimming and floated, making only those movements necessary to keep its head above water. Each experiment was filmed and recorded so the videos could be analyzed blindly to avoid experimenter bias.
For clinical data, quantitative data are presented as medians (interquartile ranges) and were compared between samples with and without CoPEC with the Mann-Whitney test, while categorical data are presented as numbers (percentages) and were compared with Fisher’s exact test. Absolute differences with 95%CIs are presented for relevant factors. Factor analysis of the mixed data was performed using the dudi.mix function of the ade4 package in R (http://cran.r-project.org/web/packages/ade4/index.html) to determine which parameters could be used to discriminate the presence of CoPEC (yes/no). The parameters considered in this analysis were determined based on the univariate analysis results and clinical relevance among sociodemographic, surgical, tumoral and psychiatric data. Quantitative data were centered and scaled.
For animal studies, the study sample is described by numbers and percentages for categorical data and by means ± SDs [or medians (interquartile ranges) when the data were not normally distributed] for continuous data. Normality was assessed graphically and using the Shapiro-Wilk test. Student’s t test (or the Mann–Whitney test when the assumptions to apply the t test were not met) was used to compare continuous data between two groups, and the chi-square test (or Fisher’s exact test when appropriate) was used to compare categorical data between two groups. Comparisons between groups were conducted using analysis of variance (ANOVA) or the Kruskal-Wallis test when the assumptions for ANOVA were not met. Post hoc tests for two-by-two multiple comparisons were applied: Tukey-Kramer after ANOVA and Dunn after the Kruskal-Wallis test. Pearson’s test was performed to determine correlations between two independent variables.
Statistical analyses were performed with GraphPad Prism 7 software (GraphPad, La Jolla, CA, United States) and STATA V15 (StataCorp, College Station, Texas, United States). A P value < 0.05 was considered to indicate statistical significance, without corrections other than those mentioned above.
Of the 64 eligible patients, only 12 agreed to participate. These participants did not differ from the nonparticipants in terms of age, sex, CoPEC status, TNM stage or tumor location. All clinical data are summarized in Table 1. The sex ratio was 1:1, and the median age was 67 years (63-80.5). CoPEC was detected in the colonic tissue of 6 patients (CoPEC+), and the other 6 patients were negative for CoPEC (CoPEC-). CoPEC+ patients were younger than CoPEC- patients (P = 0.013). No differences were observed for colon cancer clinical data.
| Total, n = 12 | CoPEC+, n = 6 | CoPEC-, n = 6 | P value | |
| Sociodemographic | ||||
| Age (yr) | 67 (63-85) | 63 (57-65) | 80.5 (79-82) | 0.013 |
| Sex, female | 6 (50) | 2 (33) | 4 (67) | 0.567 |
| Educational level, more than 12 yr | 2 (17) | 1 (17) | 1 (17) | 1 |
| Cohabiting couple | 10 (83) | 6 (100) | 4 (67) | 0.455 |
| CRC | ||||
| Follow-up since CRC surgery (yr) | 4.9 (4.2-8.2) | 6.5 (4.3-8.4) | 4.7 (4.0-5.3) | 0.513 |
| Laparotomy | 8 (67) | 3 (50) | 5 (83) | 0.545 |
| Left colon cancer | 6 (50) | 3 (50) | 3 (50) | 1 |
| TNM CRC stage | 0.545 | |||
| 1 | 2 (17) | 2 (33) | - | |
| 2 | 6 (50) | 3 (50) | 3 (50) | |
| 3 | 3 (25) | 1 (17) | 2 (33) | |
| 4 | 1 (8) | - | 1 (17) | |
| Psychiatry | ||||
| Personal past anxiety/major depressive disorders | 7 (58) | 6 (100) | 1 (17) | 0.015 |
| Family psychiatric disorders | 4 (33) | 2 (33) | 2 (33) | 1 |
| HDRS total score | 5.5 (3.0-7.5) | 6.5 (5.0-8.0) | 4.5 (2.0-7.0) | 0.422 |
| HAMA total score | 6.5 (3.5-10.5) | 7.5 (6.0-10.0) | 4.5 (3.0-11.0) | 0.52 |
| HAMA somatic score | 0.5 (0.0-2.0) | 1.0 (0.0-2.0) | 0.0 (0.0-2.0) | 0.388 |
| HAMA psychic score | 5 (2.5-8.5) | 5.5 (4.0-8.0) | 4.5 (1.0-11.0) | 0.936 |
The presence of current psychiatric disorders and HAMA and HDRS scores were comparable between CoPEC+ and CoPEC- patients. However, their psychiatric history profiles were different. Only one CoPEC- patient had psychiatric disorders (agoraphobia and specific phobia), but all CoPEC+ patients had psychiatric disorders [P = 0.015, absolute difference = 0.83 (0.54;1.13)]: One patient presented with a generalized anxiety disorder; one patient had a specific phobia; and four patients, including one with a specific phobia, had one or more past major depressive disorders. For all included patients, independent of CoPEC presence, the first psychiatric disorder occurred several years before colon cancer diagnosis.
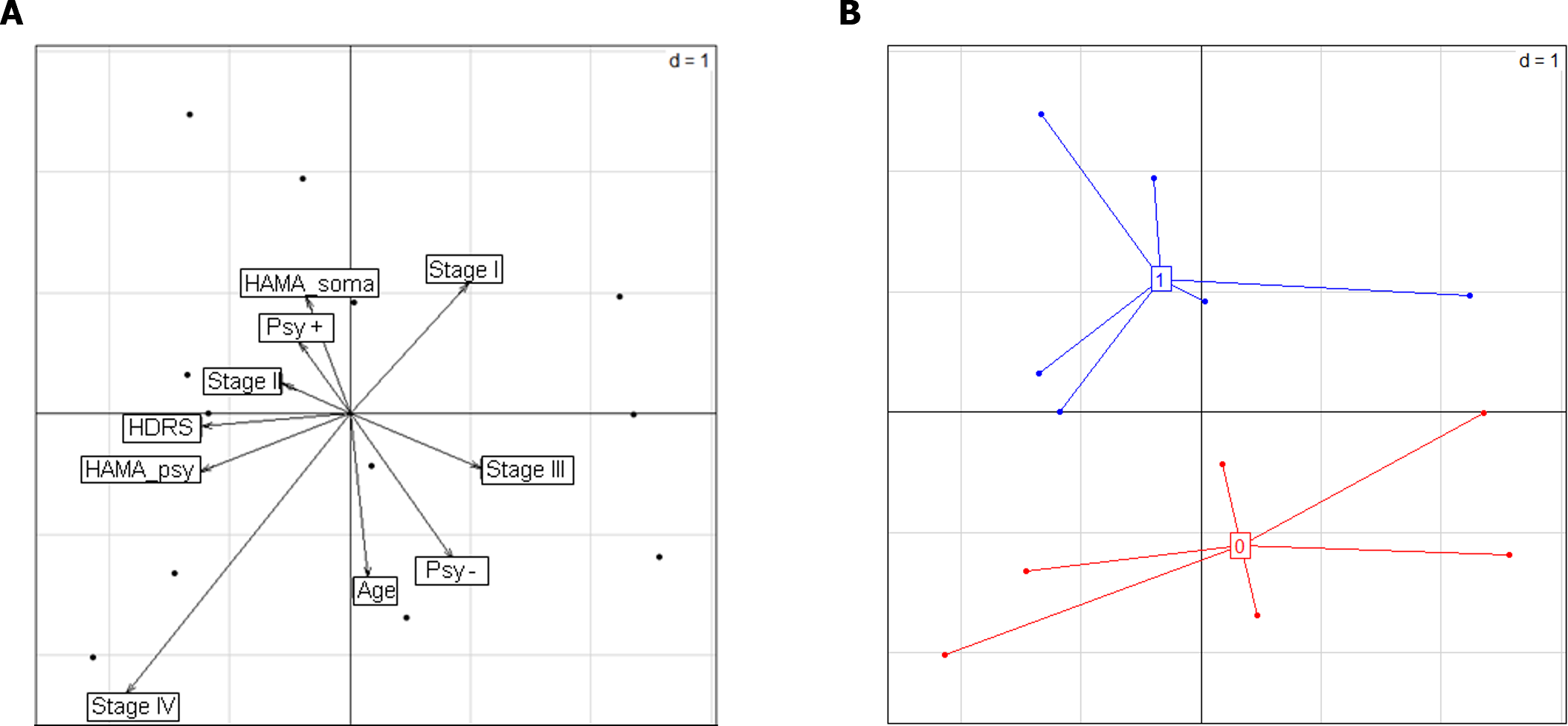
Factor analysis of mixed data, including age, TNM CRC stage, psychiatric disorders, and HDRS and HAMA scores, revealed different clinical profiles according to the presence of CoPEC, and psychiatric disorders were a major factor contributing to these differences (Figure 2). Importantly, these results were confirmed after excluding age from the analysis (data not shown).
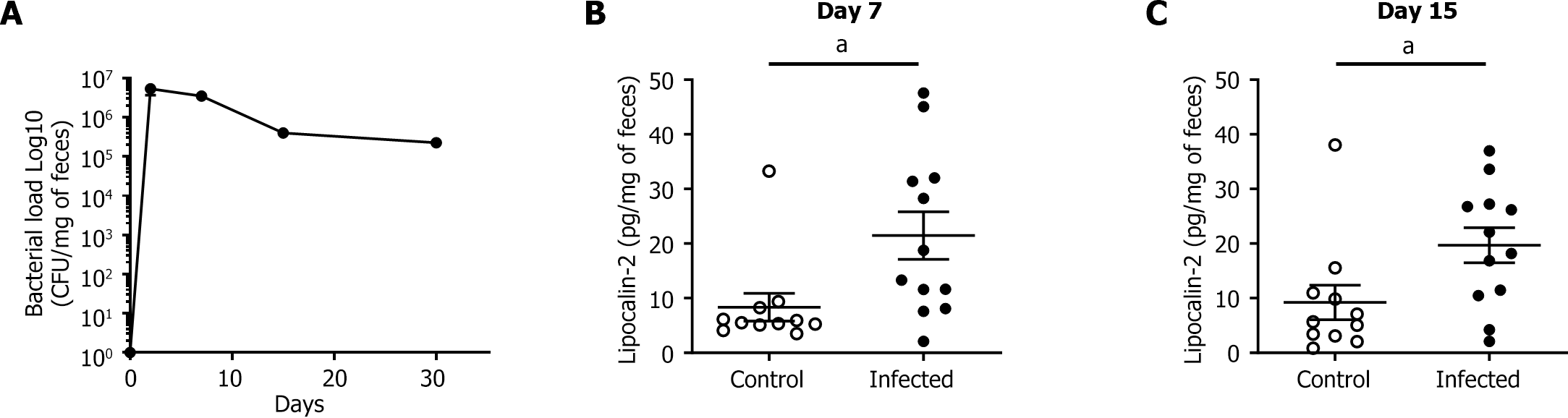
At 4 DPI, all the infected WT mice were colonized by the 11G5 CoPEC strain (Figure 3A). The bacterial load remained high throughout the experiment until 30 DPI, indicating persistent and chronic infection in the mice. Low-grade inflammation in the colon was assessed by measuring fecal lipocalin-2 levels via ELISA. At 7 DPI, the fecal level of lipocalin-2 was significantly greater in the infected group than in the control group (P < 0.05; Figure 3B and C). This difference persisted up to 15 DPI (P < 0.05).
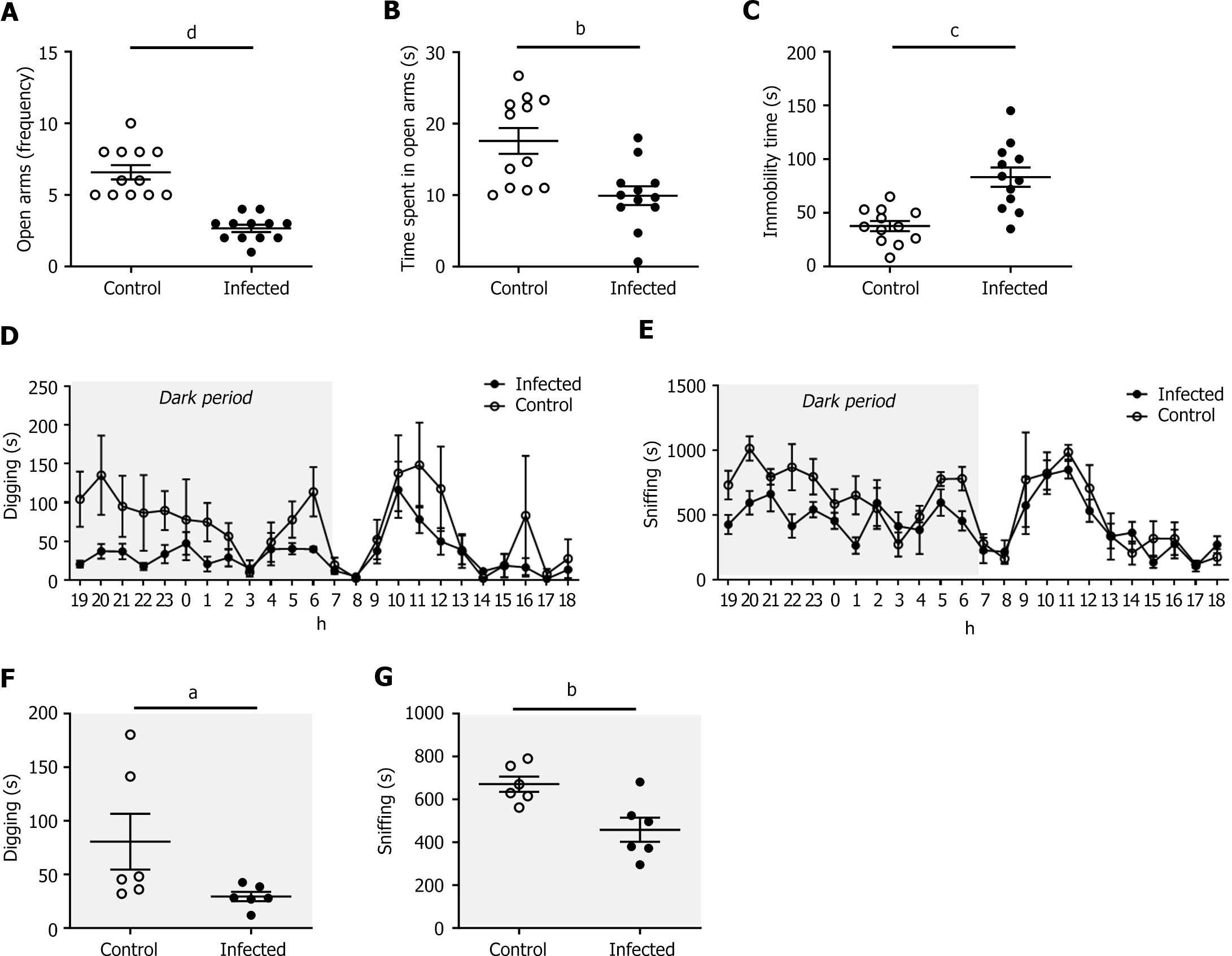
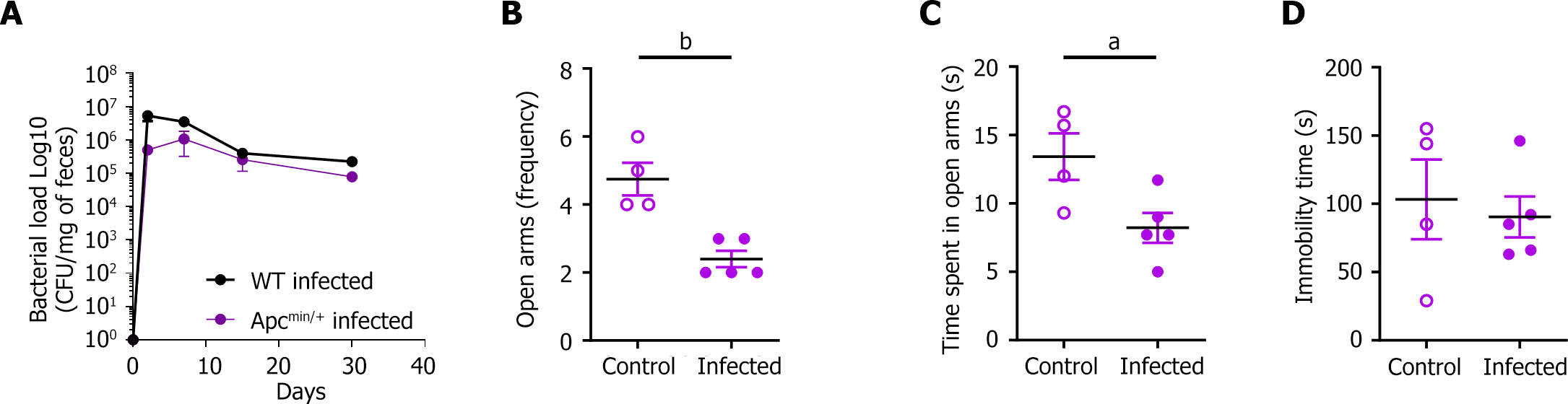
Infected mice made significantly fewer entries into and spent less time in the open arms of the EPM than did the control group at 15 DPI (2.7 ± 0.3 vs 6.6 ± 0.5 entries, P < 0.001; 9.9 ± 1.3 vs 17.6 ± 1.8 s, P < 0.01; Figure 4A and B).
These results were confirmed in the CRC susceptibility APCmin/+ mouse model (Figure 5). In addition, at 35 DPI, immobility time during the FST was longer in infected mice (83.3 ± 8.9 vs 37.7 ± 4.7 s, P < 0.001; Figure 4C). Taken together, these results indicate that the infected mice exhibited significant anxiety- and depressive-like behaviors.
We analyzed the impact of CoPEC infection on animal behavior at 20 DPI with a PhenoTyper® device. The infected mice did not differ in terms of the distance traveled or the time spent in the hidden zone (data not shown). However, the total duration of digging (P < 0.05) and sniffing (P < 0.01) was significantly shorter during the dark period (Figure 4D-G), suggesting anxiety- and depressive-like behaviors.
The involvement of the intestinal microbiota, particularly Enterobacteria, in CRC pathogenesis, anxiety and depression has become increasingly apparent in recent decades. In the present study, we showed, in a limited cohort, that anxiety and depressive disorders were significantly more prevalent in colon cancer patients colonized by CoPEC. Interestingly, we determined that the first psychiatric disorder occurred several years before the colon cancer diagnosis, as previously reported. Severe depressive symptoms were shown to be associated with an increased risk of CRC[30]. Preclinical models were used to confirm our clinical results. Since psychiatric disorders seemed to occur before carcinogenesis in our clinical study, we chronically infected WT mice with a CoPEC reference strain and observed a significant increase in anxiety-like behaviors in the EPM test after infection. In addition, sniffing and digging times measured with a PhenoTyper® device were significantly decreased, and immobility periods during the FST were increased after infection, suggesting anxiety- and depressive-like behaviors. The same effects on anxiety-like behaviors have also been observed in the context of colon carcinogenesis (Figure 5). These preclinical data indicated that chronic CoPEC infection could affect host behavior before colon cancer development. We hypothesized that CoPEC could colonize the gut of neonatal or young hosts as previously described[31]. This infection can induce perturbations in gut homeostasis, which may have some consequences for disturbances in the microbiota-gut-brain axis. Alterations in the gut epithelial barrier were already measured after CoPEC infection in a rat model[31]. We showed here that CoPEC infection induced a persistent increase in the level of fecal lipocalin-2, which is a marker of low-grade inflammation[32]. These disturbances (intestinal barrier alteration and inflammation) induced by CoPEC infection are known pathways associated with the anxiety- and depressive-like behaviors[33] suggesting that CoPEC infection could play a role in the physiopathology of these disorders. In addition, a recently published study showed that CoPEC could killed specifically enteric pathogens or commensals in the gut[34]. Effect of the CoPEC on intestinal homeostasis could be a direct effect on mucosa or by targeting microbiota leading to a pro-inflammatory dysbiosis. Further studies will be performed to evaluate dysbiosis and neuroinflammation in our chronical CoPEC-infected preclinical model. Our study has several limitations, particularly because of the low number of included patients and the high dropout rate. This study was proposed to colon cancer patients several years after surgery. Patients were elderly; sometimes lived far from the hospital; and were often refractory to additional surveys, notably those surveys concerning their psychiatric history. All these reasons may explain the low number of included patients. These first results require confirmation on a larger cohort. We could use the ongoing Metabiote clinical trial, which aims to study the prognostic value of CoPEC as markers for prediction of oncologic outcomes in CRC[35]. This prospective cohort could be used to verify these results on a larger number of patients. We then used preclinical models to support these clinical observations. A reverse translational research approach confirmed our clinical results. The number of animal behavioral studies, behavioral tests and even devices developed for targeted analysis of these behaviors are increasing, and these tests are being used and documented in the scientific community. Even if additional studies will be essential to investigate the involved mechanisms, such as the impact of colibactin and/or CoPEC-associated virulence factors, our reverse translational research approach confirmed our clinical results.
Our results support a significant role of Enterobacteriaceae in the development of psychiatric disorders. Although further mechanistic studies are planned, these initial results suggest that CoPEC infection could induce microbiota-gut-brain axis disturbances in addition to their procarcinogenic effects.
We thank Joana Boureau and Pierre Sauvanet for their technical assistance and Abdelkrim Alloui (Animal Facilities) for animal care.
| 1. | Carabotti M, Scirocco A, Maselli MA, Severi C. The gut-brain axis: interactions between enteric microbiota, central and enteric nervous systems. Ann Gastroenterol. 2015;28:203-209. [PubMed] |
| 2. | Thomas S, Izard J, Walsh E, Batich K, Chongsathidkiet P, Clarke G, Sela DA, Muller AJ, Mullin JM, Albert K, Gilligan JP, DiGuilio K, Dilbarova R, Alexander W, Prendergast GC. The Host Microbiome Regulates and Maintains Human Health: A Primer and Perspective for Non-Microbiologists. Cancer Res. 2017;77:1783-1812. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 241] [Article Influence: 30.1] [Reference Citation Analysis (0)] |
| 3. | Keshavarzian A, Green SJ, Engen PA, Voigt RM, Naqib A, Forsyth CB, Mutlu E, Shannon KM. Colonic bacterial composition in Parkinson's disease. Mov Disord. 2015;30:1351-1360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 680] [Cited by in RCA: 896] [Article Influence: 89.6] [Reference Citation Analysis (0)] |
| 4. | Scheperjans F, Aho V, Pereira PA, Koskinen K, Paulin L, Pekkonen E, Haapaniemi E, Kaakkola S, Eerola-Rautio J, Pohja M, Kinnunen E, Murros K, Auvinen P. Gut microbiota are related to Parkinson's disease and clinical phenotype. Mov Disord. 2015;30:350-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1044] [Cited by in RCA: 1068] [Article Influence: 97.1] [Reference Citation Analysis (0)] |
| 5. | Yu M, Jia H, Zhou C, Yang Y, Zhao Y, Yang M, Zou Z. Variations in gut microbiota and fecal metabolic phenotype associated with depression by 16S rRNA gene sequencing and LC/MS-based metabolomics. J Pharm Biomed Anal. 2017;138:231-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 264] [Article Influence: 33.0] [Reference Citation Analysis (0)] |
| 6. | Knuesel T, Mohajeri MH. The Role of the Gut Microbiota in the Development and Progression of Major Depressive and Bipolar Disorder. Nutrients. 2021;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 65] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 7. | Nikolova VL, Smith MRB, Hall LJ, Cleare AJ, Stone JM, Young AH. Perturbations in Gut Microbiota Composition in Psychiatric Disorders: A Review and Meta-analysis. JAMA Psychiatry. 2021;78:1343-1354. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 281] [Cited by in RCA: 427] [Article Influence: 106.8] [Reference Citation Analysis (0)] |
| 8. | Dinan TG, Cryan JF. The impact of gut microbiota on brain and behaviour: implications for psychiatry. Curr Opin Clin Nutr Metab Care. 2015;18:552-558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 199] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 9. | Yamawaki Y, Yoshioka N, Nozaki K, Ito H, Oda K, Harada K, Shirawachi S, Asano S, Aizawa H, Yamawaki S, Kanematsu T, Akagi H. Sodium butyrate abolishes lipopolysaccharide-induced depression-like behaviors and hippocampal microglial activation in mice. Brain Res. 2018;1680:13-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 136] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 10. | Defaye M, Nourrisson C, Baudu E, Lashermes A, Meynier M, Meleine M, Wawrzyniak I, Bonnin V, Barbier J, Chassaing B, Godfraind C, Gelot A, Barnich N, Ardid D, Bonnet M, Delbac F, Carvalho FA, Poirier P. Fecal dysbiosis associated with colonic hypersensitivity and behavioral alterations in chronically Blastocystis-infected rats. Sci Rep. 2020;10:9146. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 11. | Simpson CA, Diaz-Arteche C, Eliby D, Schwartz OS, Simmons JG, Cowan CSM. The gut microbiota in anxiety and depression - A systematic review. Clin Psychol Rev. 2021;83:101943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 599] [Cited by in RCA: 532] [Article Influence: 133.0] [Reference Citation Analysis (0)] |
| 12. | Alpert O, Begun L, Issac T, Solhkhah R. The brain-gut axis in gastrointestinal cancers. J Gastrointest Oncol. 2021;12:S301-S310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 13. | Matson V, Chervin CS, Gajewski TF. Cancer and the Microbiome-Influence of the Commensal Microbiota on Cancer, Immune Responses, and Immunotherapy. Gastroenterology. 2021;160:600-613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 240] [Article Influence: 60.0] [Reference Citation Analysis (0)] |
| 14. | Arthur JC, Perez-Chanona E, Mühlbauer M, Tomkovich S, Uronis JM, Fan TJ, Campbell BJ, Abujamel T, Dogan B, Rogers AB, Rhodes JM, Stintzi A, Simpson KW, Hansen JJ, Keku TO, Fodor AA, Jobin C. Intestinal inflammation targets cancer-inducing activity of the microbiota. Science. 2012;338:120-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1374] [Cited by in RCA: 1661] [Article Influence: 127.8] [Reference Citation Analysis (1)] |
| 15. | Bonnet M, Buc E, Sauvanet P, Darcha C, Dubois D, Pereira B, Déchelotte P, Bonnet R, Pezet D, Darfeuille-Michaud A. Colonization of the human gut by E. coli and colorectal cancer risk. Clin Cancer Res. 2014;20:859-867. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 364] [Cited by in RCA: 330] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 16. | Iyadorai T, Mariappan V, Vellasamy KM, Wanyiri JW, Roslani AC, Lee GK, Sears C, Vadivelu J. Prevalence and association of pks+ Escherichia coli with colorectal cancer in patients at the University Malaya Medical Centre, Malaysia. PLoS One. 2020;15:e0228217. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 78] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 17. | McCarthy AJ, Martin P, Cloup E, Stabler RA, Oswald E, Taylor PW. The Genotoxin Colibactin Is a Determinant of Virulence in Escherichia coli K1 Experimental Neonatal Systemic Infection. Infect Immun. 2015;83:3704-3711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 63] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 18. | Gagnière J, Bonnin V, Jarrousse AS, Cardamone E, Agus A, Uhrhammer N, Sauvanet P, Déchelotte P, Barnich N, Bonnet R, Pezet D, Bonnet M. Interactions between microsatellite instability and human gut colonization by Escherichia coli in colorectal cancer. Clin Sci (Lond). 2017;131:471-485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 39] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 19. | Bossuet-Greif N, Vignard J, Taieb F, Mirey G, Dubois D, Petit C, Oswald E, Nougayrède JP. The Colibactin Genotoxin Generates DNA Interstrand Cross-Links in Infected Cells. mBio. 2018;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 106] [Cited by in RCA: 162] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 20. | Lopès A, Billard E, Casse AH, Villéger R, Veziant J, Roche G, Carrier G, Sauvanet P, Briat A, Pagès F, Naimi S, Pezet D, Barnich N, Dumas B, Bonnet M. Colibactin-positive Escherichia coli induce a procarcinogenic immune environment leading to immunotherapy resistance in colorectal cancer. Int J Cancer. 2020;146:3147-3159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 75] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 21. | Pleguezuelos-Manzano C, Puschhof J, Rosendahl Huber A, van Hoeck A, Wood HM, Nomburg J, Gurjao C, Manders F, Dalmasso G, Stege PB, Paganelli FL, Geurts MH, Beumer J, Mizutani T, Miao Y, van der Linden R, van der Elst S; Genomics England Research Consortium, Garcia KC, Top J, Willems RJL, Giannakis M, Bonnet R, Quirke P, Meyerson M, Cuppen E, van Boxtel R, Clevers H. Mutational signature in colorectal cancer caused by genotoxic pks(+) E. coli. Nature. 2020;580:269-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 564] [Cited by in RCA: 715] [Article Influence: 143.0] [Reference Citation Analysis (0)] |
| 22. | Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59 Suppl 20:22-33;quiz 34. [PubMed] |
| 23. | Lecrubier Y, Sheehan D, Weiller E, Amorim P, Bonora I, Sheehan KH, Janavs J, Dunbar G. The Mini International Neuropsychiatric Interview (MINI). A short diagnostic structured interview: reliability and validity according to the CIDI. Eur psychiatr. 1997;12:224-231. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2316] [Cited by in RCA: 2416] [Article Influence: 483.2] [Reference Citation Analysis (0)] |
| 24. | Lempérière T, Lépine JP, Rouillon F, Hardy P, Ades J, Luaute JP, Ferrand I. [Comparison of various tools for evaluating depression apropos of a study on Athymil 30 mg]. Ann Med Psychol (Paris). 1984;142:1206-1214. [PubMed] |
| 25. | Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21041] [Cited by in RCA: 22831] [Article Influence: 351.2] [Reference Citation Analysis (0)] |
| 26. | Hamilton M. The assessment of anxiety states by rating. Br J Med Psychol. 1959;32:50-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6169] [Cited by in RCA: 6770] [Article Influence: 102.6] [Reference Citation Analysis (0)] |
| 27. | Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377-381. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38562] [Cited by in RCA: 36692] [Article Influence: 2293.3] [Reference Citation Analysis (0)] |
| 28. | Buc E, Dubois D, Sauvanet P, Raisch J, Delmas J, Darfeuille-Michaud A, Pezet D, Bonnet R. High prevalence of mucosa-associated E. coli producing cyclomodulin and genotoxin in colon cancer. PLoS One. 2013;8:e56964. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 401] [Cited by in RCA: 404] [Article Influence: 33.7] [Reference Citation Analysis (0)] |
| 29. | Meynier M, Baudu E, Rolhion N, Defaye M, Straube M, Daugey V, Modoux M, Wawrzyniak I, Delbac F, Villéger R, Méleine M, Borras Nogues E, Godfraind C, Barnich N, Ardid D, Poirier P, Sokol H, Chatel JM, Langella P, Livrelli V, Bonnet M, Carvalho FA. AhR/IL-22 pathway as new target for the treatment of post-infectious irritable bowel syndrome symptoms. Gut Microbes. 2022;14:2022997. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 39] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 30. | Kroenke CH, Bennett GG, Fuchs C, Giovannucci E, Kawachi I, Schernhammer E, Holmes MD, Kubzansky LD. Depressive symptoms and prospective incidence of colorectal cancer in women. Am J Epidemiol. 2005;162:839-848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 53] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 31. | Payros D, Secher T, Boury M, Brehin C, Ménard S, Salvador-Cartier C, Cuevas-Ramos G, Watrin C, Marcq I, Nougayrède JP, Dubois D, Bedu A, Garnier F, Clermont O, Denamur E, Plaisancié P, Theodorou V, Fioramonti J, Olier M, Oswald E. Maternally acquired genotoxic Escherichia coli alters offspring's intestinal homeostasis. Gut Microbes. 2014;5:313-325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 69] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 32. | Chassaing B, Srinivasan G, Delgado MA, Young AN, Gewirtz AT, Vijay-Kumar M. Fecal lipocalin 2, a sensitive and broadly dynamic non-invasive biomarker for intestinal inflammation. PLoS One. 2012;7:e44328. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 320] [Cited by in RCA: 430] [Article Influence: 33.1] [Reference Citation Analysis (0)] |
| 33. | Fond GB, Lagier JC, Honore S, Lancon C, Korchia T, Sunhary De Verville PL, Llorca PM, Auquier P, Guedj E, Boyer L. Microbiota-Orientated Treatments for Major Depression and Schizophrenia. Nutrients. 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 38] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 34. | Chen J, Byun H, Liu R, Jung IJ, Pu Q, Zhu CY, Tanchoco E, Alavi S, Degnan PH, Ma AT, Roggiani M, Beld J, Goulian M, Hsiao A, Zhu J. A commensal-encoded genotoxin drives restriction of Vibrio cholerae colonization and host gut microbiome remodeling. Proc Natl Acad Sci U S A. 2022;119:e2121180119. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 35. | Veziant J, Poirot K, Chevarin C, Cassagnes L, Sauvanet P, Chassaing B, Robin F, Godfraind C, Barnich N, Pezet D, Pereira B, Gagniere J, Bonnet M. Prognostic value of a combination of innovative factors (gut microbiota, sarcopenia, obesity, metabolic syndrome) to predict surgical/oncologic outcomes following surgery for sporadic colorectal cancer: a prospective cohort study protocol (METABIOTE). BMJ Open. 2020;10:e031472. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |