Published online May 28, 2024. doi: 10.3748/wjg.v30.i20.2709
Revised: April 18, 2024
Accepted: May 7, 2024
Published online: May 28, 2024
Processing time: 118 Days and 2.8 Hours
Constipation, a highly prevalent functional gastrointestinal disorder, induces a significant burden on the quality of patients' life and is associated with substantial healthcare expenditures. Therefore, identifying efficient therapeutic modalities for constipation is of paramount importance. Oxidative stress is a pivotal contributor to colonic dysmotility and is the underlying pathology responsible for constipation symptoms. Consequently, we postulate that hydrogen therapy, an emerging and promising intervention, can serve as a safe and efficacious treatment for constipation.
To determine whether hydrogen-rich water (HRW) alleviates constipation and its potential mechanism.
Constipation models were established by orally loperamide to Sprague-Dawley rats. Rats freely consumed HRW, and were recorded their 24 h total stool weight, fecal water content, and charcoal propulsion rate. Fecal samples were subjected to 16S rDNA gene sequencing. Serum non-targeted metabolomic analysis, malondialdehyde, and superoxide dismutase levels were determined. Colonic tissues were stained with hematoxylin and eosin, Alcian blue-periodic acid-Schiff, reactive oxygen species (ROS) immunofluorescence, and immunohistochemistry for cell growth factor receptor kit (c-kit), PGP 9.5, sirtuin1 (SIRT1), nuclear factor-erythroid-2-related factor 2 (Nrf2), and heme oxygenase-1 (HO-1). Quantitative real-time PCR and western blot analysis were conducted to determine the expression level of SIRT1, Nrf2 and HO-1. A rescue experiment was conducted by intraperitoneally injecting the SIRT1 inhibitor, EX527, into constipated rats. NCM460 cells were induced with H2O2 and treated with the metabolites to evaluate ROS and SIRT1 expression.
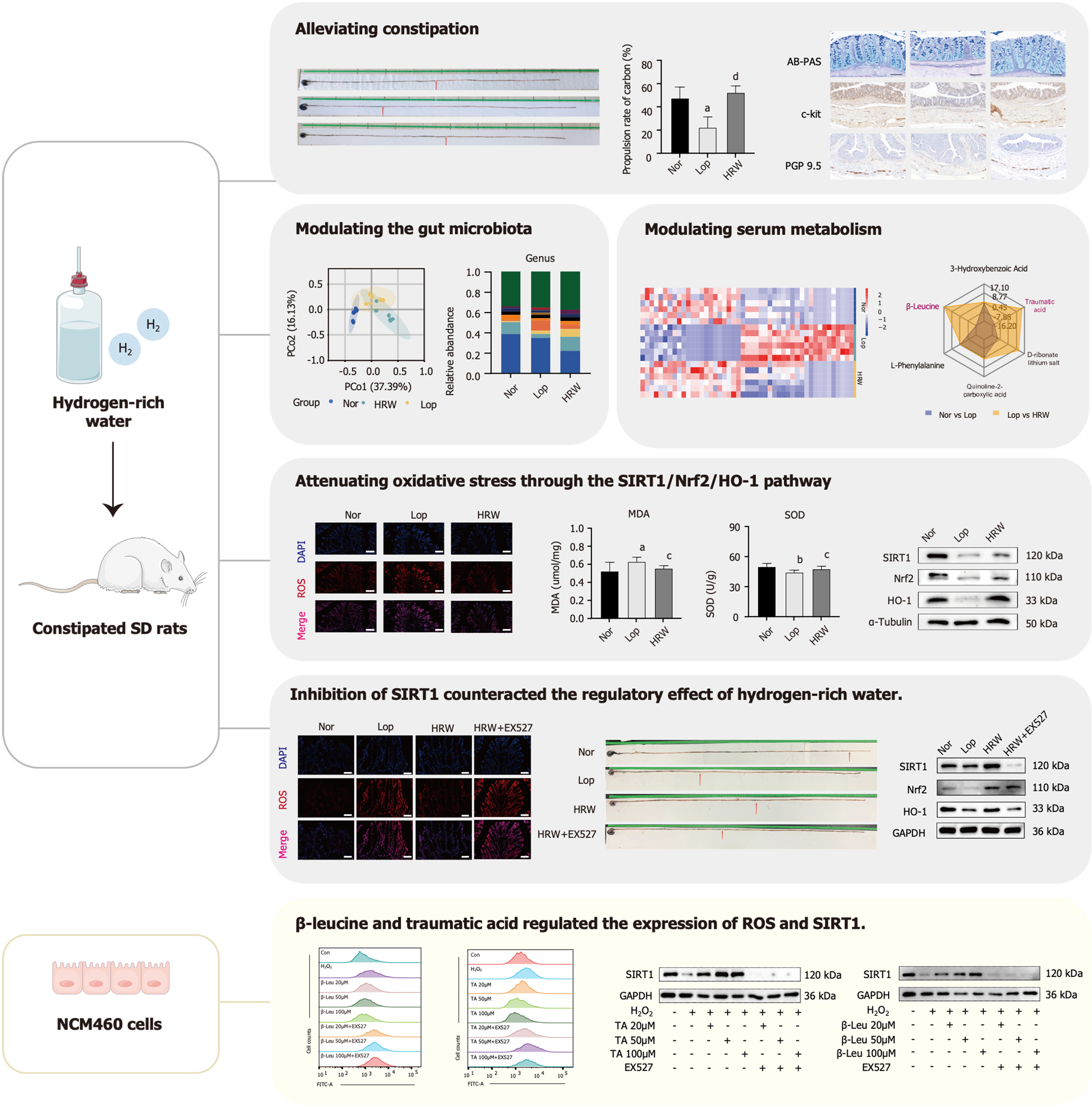
HRW alleviated constipation symptoms by improving the total amount of stool over 24 h, fecal water content, charcoal propulsion rate, thickness of the intestinal mucus layer, c-kit expression, and the number of intestinal neurons. HRW modulated intestinal microbiota imbalance and abnormalities in serum metabolism. HRW could also reduce intestinal oxidative stress through the SIRT1/Nrf2/HO-1 signaling pathway. This regulatory effect on oxidative stress was confirmed via an intraperitoneal injection of a SIRT1 inhibitor to constipated rats. The serum metabolites, β-leucine (β-Leu) and traumatic acid, were also found to attenuate H2O2-induced oxidative stress in NCM460 cells by up-regulating SIRT1.
HRW attenuates constipation-associated intestinal oxidative stress via SIRT1/Nrf2/HO-1 signaling pathway, modulating gut microbiota and serum metabolites. β-Leu and traumatic acid are potential metabolites that upregulate SIRT1 expression and reduce oxidative stress.
Core Tip: Constipation is a highly prevalent functional gastrointestinal disorder. Identifying cost-effective and efficient therapeutic modalities for constipation is of paramount importance. In the present study, hydrogen-rich water alleviated constipation by attenuating intestinal oxidative stress through the sirtuin1 (SIRT1)/nuclear factor-erythroid-2-related factor 2/heme oxygenase-1 signaling pathway and modulating gut microbiota and serum metabolites. β-leucine and traumatic acid were identified as potential metabolites for the treatment of constipation, inducing increased SIRT1 expression and decreased oxidative stress.
- Citation: Chen KD, Wang KL, Chen C, Zhu YJ, Tang WW, Wang YJ, Chen ZP, He LH, Chen YG, Zhang W. Hydrogen-rich water alleviates constipation by attenuating oxidative stress through the sirtuin1/nuclear factor-erythroid-2-related factor 2/heme oxygenase-1 signaling pathway. World J Gastroenterol 2024; 30(20): 2709-2725
- URL: https://www.wjgnet.com/1007-9327/full/v30/i20/2709.htm
- DOI: https://dx.doi.org/10.3748/wjg.v30.i20.2709
Constipation is a highly prevalent functional gastrointestinal disorder characterized by infrequent stools (fewer than 3 bowel movements per week), dry and hard stools, or difficulty in passing stools, which may significantly affect the quality of life and impose a remarkable economic burden[1]. Currently, constipation is initially managed through the administration of dietary fiber and suitable stimulants or osmotic laxatives, with subsequent consideration of gut secretagogues or prokinetic agents, if necessary[2]. However, these approaches have limitations, such as restricted long-term utility, high recurrence rates, challenges in achieving complete resolution, and potential impacts on the physical and mental well-being of patients[3]. Therefore, identifying suitable intervention measures is a top priority for the treatment of constipation.
Hydrogen therapy is an emerging and promising strategy that involves the use of molecular hydrogen as a new, safe, and effective therapeutic agent that exhibits remarkable therapeutic effects in many oxidative stress-related diseases owing to its bio-reductivity and homeostatic regulation abilities[4]. Hydrogen-rich water (HRW), a specialized type of water containing a precise concentration of dissolved hydrogen molecules (H2), has been reported to possess potent antioxidant, anti-inflammatory, and anti-apoptotic properties[5]. Oxidative stress is a key contributor to colonic dysmotility and leads to constipation[6]. Antioxidants, such as inulin, play a beneficial role in improving chronic constipation symptoms[7]. Thus, in this study, HRW was explored as a potential treatment with various advantages for constipation.
Mammalian Sirtuin1 (SIRT1) is an NAD+-dependent deacetylase that primarily regulates various physiological functions, including cellular stress response, energy metabolism and inflammatory response[8]. By promoting the nuclear translocation of nuclear factor-erythroid-2-related factor 2 (Nrf2), SIRT1 can enhance the cellular antioxidant capacity; however, Nrf2 can stimulate the transcriptional expression of heme oxygenase-1 (HO-1) to increase the intracellular antioxidant stress response. The combined effects of these three factors help maintain the oxidative balance in cells and protect them from oxidative stress damage via the inhibition of reactive oxygen species (ROS) production[9]. HRW was found to alleviate other diseases such as liver/renal injury and amyloid β-induced cytotoxicity, by reducing oxidative stress via SIRT1 regulation[10-12]. Thus, we hypothesized that HRW can relieve constipation via a mechanism involving the SIRT1/Nrf2/HO-1 signaling pathway to alleviate oxidative stress.
To test this hypothesis, we confirmed the activity of HRW in rats with loperamide-induced constipation (Lop). Thereafter, 16S rDNA gene sequencing and serum metabolomic analyses were conducted, and a SIRT1 inhibitor was used to determine whether HRW could relieve constipation symptoms and elevate oxidative stress levels in model rats. Finally, the serum metabolites, β-leucine (β-Leu) and traumatic acid, were screened to determine their ability to increase SIRT1 expression and reduce oxidative stress level in NCM460 cells. Overview of the workflow is shown in Figure 1.
HRW (H2 > 3.0 ppm) was provided by the Bauer Technology Company, Ltd. (Nanjing, China). Loperamide and β-Leu were purchased from Yuanye Biotechnology (Shanghai, China). Traumatic acid was purchased from Aladdin Biotechnology (Shanghai, China). EX527 (HY-15452) was purchased from MCE (NJ, United States). The MitoSOX staining (M36008, Invitrogen) was purchased from Thermo Fisher Scientific (Waltham, MA, United States). ROS assay kit (S0033S), lipid peroxidation malondialdehyde (MDA) assay kit (S0131S), and total superoxide dismutase (SOD) assay kit with WST-8 (S0101S) were purchased from Beyotime Biotechnology (Nanjing, China). Primary antibodies against cell growth factor receptor kit (c-kit; 18696-1-AP), PGP 9.5 (66230-1-Ig), Nrf2 (80593-1-RR), HO-1 (66743-1-Ig), and α-tubulin (66031-1-Ig) were purchased from Proteintech (Wuhan, China). The primary antibody against SIRT1 (ab110304) was purchased from Abcam (Cambridge, MA, United States). All primers for quantitative real-time PCR (qRT-PCR) were obtained from GenScript Biotechnology (Nanjing, China). Reverse transcription kits and SYBR Green qPCR Master Mix were provided by Vazyme (Nanjing, China).
Eighteen male Sprague-Dawley (SD) rats (8-wk old) were obtained from Nanjing Junke Biological Engineering Co. Ltd (Jiangsu, China) and housed under specific pathogen-free conditions. The rats were housed under a controlled temperature of 22 ± 1 °C, a humidity level of 55% ± 5%, and a 12 h light-dark cycle throughout the experiment.
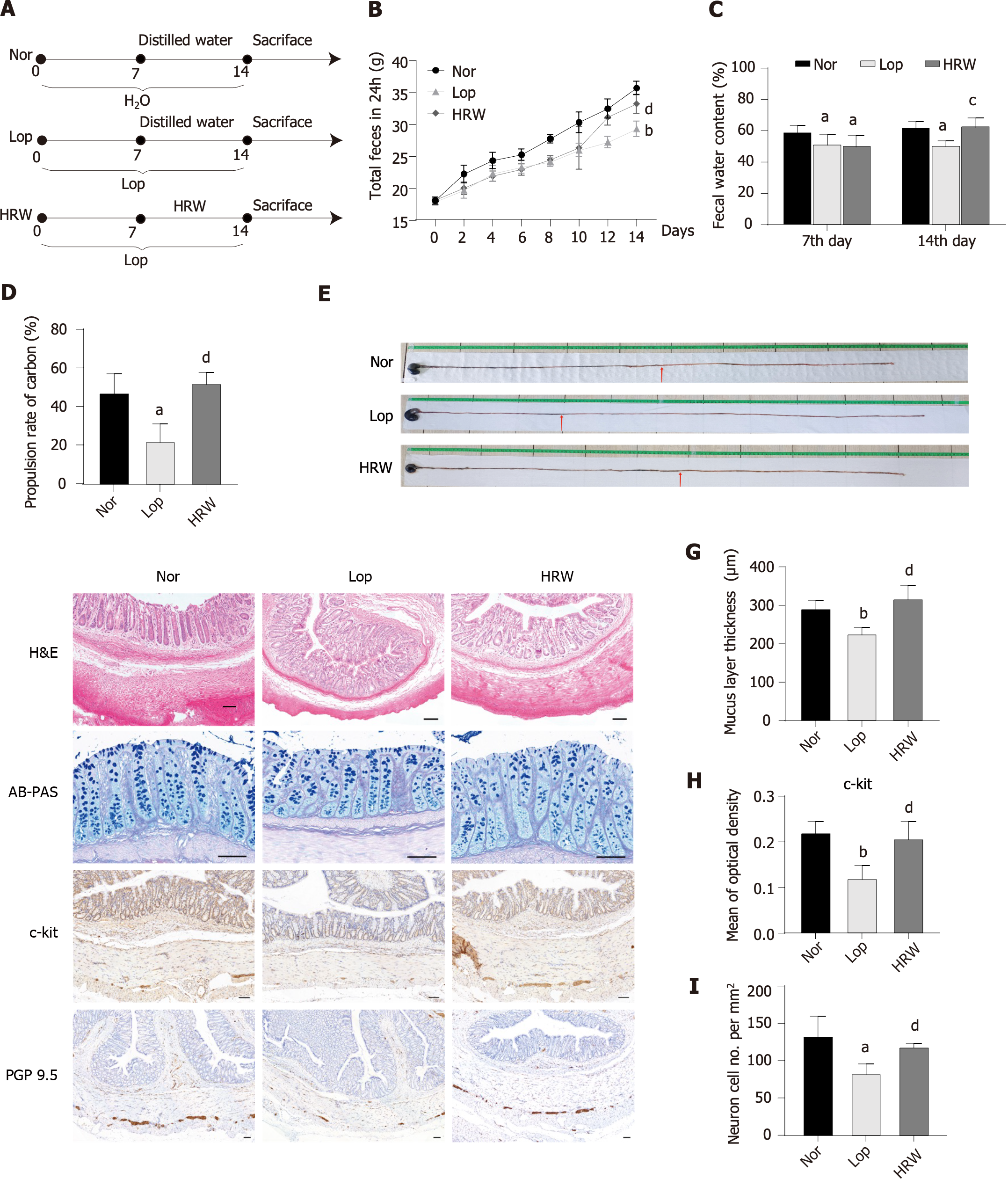
To establish a model of constipation, rats were administered loperamide, as described by Wang et al[13]. Following one week of acclimatization, rats were randomly divided into three experimental groups (n = 6): Normal (Nor), Lop, and HRW intervention. The process of animal experimentation is interpreted in Figure 2A. In the initial seven-day phase, the Nor group received clean water, while the Lop and HRW groups were orally administered 3 mg/kg/d loperamide. From day 8-14, the Nor and Lop groups were administered distilled water, while the HRW group received HRW. The total 24 h fecal output was recorded every other day, and the fecal water content was measured on day 7 and 14.
At the end of the 14-d animal study, a 12 h fasting period was carried out prior to the oral administration of 2 mL of activated carbon (5%). After 30 min, the rats were exsanguinated and separated to retrieve intact small intestine. The entire length of the small intestine, measured from the pylorus to the cecum, served as an indicator of the overall length, whereas the distance from the pylorus to the front edge of the carbon suspension indicated the transit extent of the carbon marker. The carbon propulsion rate was calculated as follows: (Transit length of carbon/full length of the small intestine) × 100%. Serum, small intestine, and colon samples from all rats were collected and stored at -80 °C for other analysis.
In a subsequent animal experiments, an additional group of rats was administered HRW and intraperitoneal injections of the SIRT1 inhibitor, EX527 (10 mg/kg qd)[14]. The remaining experimental procedures were performed as mentioned above.
A portion of the distal colon was fixed with 4% polyformaldehyde for histopathological examination. The fixed tissue was embedded in paraffin and sliced into 5 μm sections, and stained with hematoxylin and eosin (H&E) and Alcian blue-periodic acid-Schiff (AB-PAS). For immunohistochemical (IHC) staining, the slices were blocked with 5% goat serum, and stained with primary antibodies against c-kit (1:300), PGP 9.5 (1:5000), Nrf2 (1:500), HO-1 (1:1000), and SIRT1 (5 μg/mL). Polymeric secondary antibodies coupled to horseradish peroxidase and diaminobenzidine were used to visualize the stained sections. The tissue specimens were counterstained with hematoxylin and examined using a light microscopy. ImageJ software was used for the analysis, and the average optical density of the images was calculated.
Microbial DNA was extracted from colon samples using the PureLink™ genomic DNA kit (K182002; Thermo Fisher, Invitrogen, MA, United States). The DNA sample quality was assessed through agarose gel electrophoresis and quantification using a DNA detection kit (B17661; AAT Bioquest). The V3-V4 variable regions of the microbial DNA were expanded and sequenced on the Illumina NovaSeq platform using forward (5’-CCTAYGGGRBGCASCAG-3’) and reverse (5’-GGACTACNNGGGTATCTAAT-3’) primers. Statistical analyses and visualization of the sequencing data were refined to enhance the accuracy and clarity of the results. In particular, six samples from each experimental group were used to evaluate of gut microbiota.
Serum non-targeted metabolomics analysis was conducted using ultra-performance liquid chromatography (UPLC)-tandem mass spectrometry. An ExionLC™ AD system coupled with a QTRAP® system was employed for data acquisition. The samples were eluted on a Waters Acquity UPLC HSS T3 C18 column under the optimized conditions. The mobile phases consisted of 0.1% formic acid in water (A) and 0.1% formic acid in acetonitrile (B). The UPLC elution conditions were optimized as follows: Isocratic elution at 5%–90% B (0–11 min), 90% B (11–12 min), 90%–5% B (12–12.1 min), and 5% B (12.1–14 min). The flow rate was 0.4 mL/min, the temperatures of the column was maintained at 40 °C, and the sample injection volume was 2 µL. The pre-processed data underwent principal component analysis, and metabolites were identified using the Human Metabolome Database. Differential metabolites were selected based on the screening criteria, variable importance in the projection (VIP) ≥ 1 and P < 0.05. Pathway analyses were performed using the Kyoto Encyclopedia of Genes and Genomes (KEGG) database.
ROS levels in the colon were measured via MitoSOX staining (M36008; Thermo Fisher Scientific, Invitrogen, MA, United States). MitoSOX was excited by a laser at 510 nm and data were collected at the FSC, SSC, and 580 nm (FL2) channels. The levels of MDA and SOD in the serum or colon tissue of rats were determined using the lipid peroxidation MDA assay kit (S0131S) and total SOD assay kit with WST-8 (S0101S) procedure.
Total RNA was extracted from colon tissues using the fastpure cell/tissue total RNA isolation kit (RC112-01, Vazyme). The total RNA was reverse transcribed to cDNA utilizing reverse transcription kits (R302-01, Vazyme). qRT-PCR was performed using the SYBR Green qPCR master mix (Q121-02, Vazyme) and cDNA as the template. The Applied Biosystems™ 7500 Fast qPCR System (A30299; Thermo Fisher, Invitrogen, MA, United States) was used for qRT-PCR. The primer sequences for all target genes are listed in Table 1. Normalization of the fluorescence intensity was accomplished using GAPDH as an internal reference, and data analysis was performed using the 2−ΔΔCT method.
| Gene | Species | Forward primer (5′-3′) | Reverse primer (5′-3′) |
| SIRT1 | Human | CAAGAGGCCGCGGAGAGATG | CGGCCCATTGTCTCCTTCCC |
| Nrf2 | Human | TTCAGCCAGCCCAGCACATC | ACGGGAATGTCTGCGCCAAA |
| HO-1 | Human | CAAGCAGCTCTACCGCTCCC | GGGTGTTGAGTGGGGGCTTC |
| GAPDH | Human | CAATGCCTCCTGCACCACCA | GATGTTCTGGAGAGCCCCGC |
| SIRT1 | Rat | CCCGGACAGTTCCAGCCATC | GGCACCTAGGACACCGAGGA |
| Nrf2 | Rat | CCGATCTGCTCGGCGAAGTT | ACAAAGTGGTGCTGGCCTCC |
| HO-1 | Rat | AGAGGCCTGCTAGCCTGGTT | TGTGAGGACCCATCGCAGGA |
| GAPDH | Rat | TGTGGATGGCCCCTCTGGAA | TGACCTTGCCCACAGCCTTG |
The colon tissue was lysed using a lysis buffer including phosphatase inhibitor (1:100), protease inhibitor (1:1000), and phenylmethanesulfonylfluoride (PMSF, 1:100). The lysate was then centrifuged (4000 g, 4 °C, 10 min) and the supernatant was collected. The protein concentration in each sample was determined using the BCA approach. Following adjustment of the protein concentration and addition of the loading buffer, all samples were denatured by boiling in a metal bath at 100 °C for 10 min. Sample proteins were divided on 10% Vazyme premix gels and transferred onto polyvinylidene fluoride (PVDF) membranes. Following a 1 h blocking step, the PVDF membranes were incubated with primary antibodies overnight at 4 °C, washed four times, and incubated with the corresponding secondary antibodies for 2 h at room temperature. Western blot images were captured utilizing the Tanon 5200 imaging system and analyzed using ImageJ software.
The NCM460 cell line was obtained from iCell Biosciences Inc. (Shanghai, China). Cells were cultured in Roswell Park Memorial Institute 1640 medium. All media were supplemented with 10% (v/v) fetal bovine serum, 100 U penicillin, and 100 μg/mL streptomycin. All cell lines were cultured in a humidified 5% CO2 atmosphere at 37 °C.
For the cell counting kit-8 (CCK-8) cytotoxicity assays, cells were seeded in clear-bottom 96-well plates, treated with different concentrations of β-Leu and traumatic acid for 24 h, according to the manufacturer’s guidelines (K1018, APExBIO, Houston, TX, United States), and quantified using a Gemini XPS microplate reader (Molecular Devices, Silicon Valley, CA, United States).
The cells were seeded in clear-bottom 6-well plates at 105 cells per well and treated with 1 mmol/L H2O2 or 20 μM EX527 or different concentrations of β-Leu and traumatic acid for 6 h. To determine the intracellular levels of ROS, the cells were stained with antibodies against cell surface antigens and then incubated with DCFH-DA (S0033S; Beyotime Biotechnology, Shanghai, China) at 37 °C for 30 min[15]. Finally, the cells were analyzed using flow cytometry, and the mean fluorescence intensity of the ROS-reactive dichlorofluorescin was determined using the FlowJo software.
All data are expressed as mean ± SD. Statistical analyses were performed using the GraphPad Prism software (version 9). Differences between two groups were evaluated using a two-tailed t test, whereas differences among three groups were analyzed using a one-way ANOVA with Tukey's multiple-comparison test. A P value of less than 0.05 was considered to indicate statistically significance. The statistical methods of this study were reviewed by Ao Wang from the Department of medical statistics, the Affiliated Hospital of Nanjing University of Chinese Medicine.
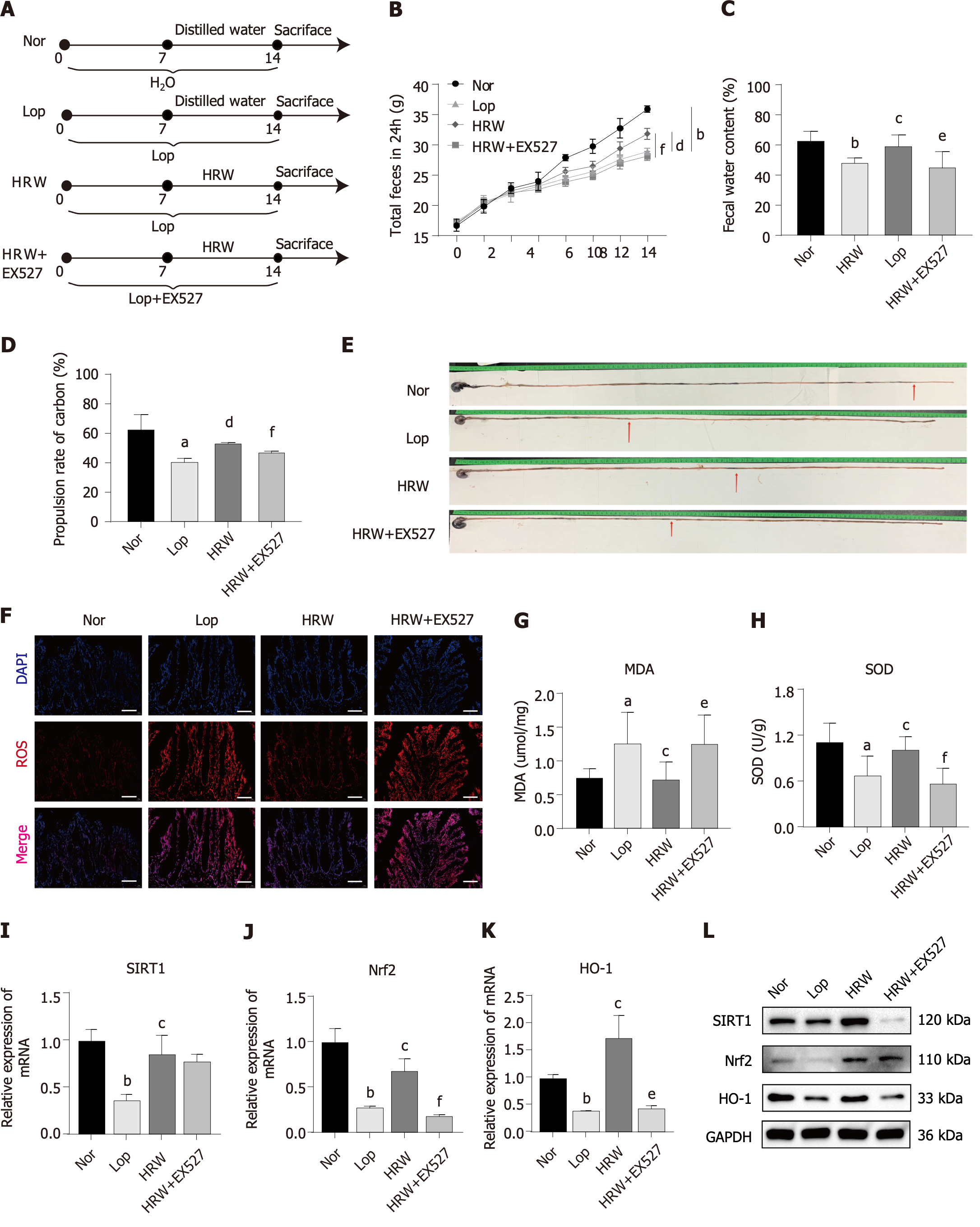
In this study, the effect of HRW was explored using a Lop model. A flow chart of the study is provided in Figure 2A. After seven days, the 24 h fecal total weight and fecal water content were found to decrease, indicating successful model construction of 18 SD rats (n = 6/group; Figure 2B and C). Following ad libitum consumption of HRW for seven days, the total amount, water content of the 24 h feces, and the charcoal propulsion rate increased compared to those in the untreated model animals (Figure 2B-E). Based on H&E staining, HRW improved the colonic villi and mucosal integrity by reducing looseness and breakage, restoring ileal epithelial integrity, and increasing muscle layer thickness in constipated rats. AB-PAS staining revealed a decrease in mucus layer thickness in constipated rats, which was reversed by HRW. Interstitial cells of Cajal, which are responsible for digestive tract motility and are closely associated with constipation, exhibited increased expression of the mast/stem c-kit following HRW consumption[16]. In addition, IHC staining of PGP9.5, a neurons marker[17], indicated that HRW increased the neuron count in constipated rats (Figure 2F-I). Overall, HRW can alleviate loperamide-induced constipation in rats.
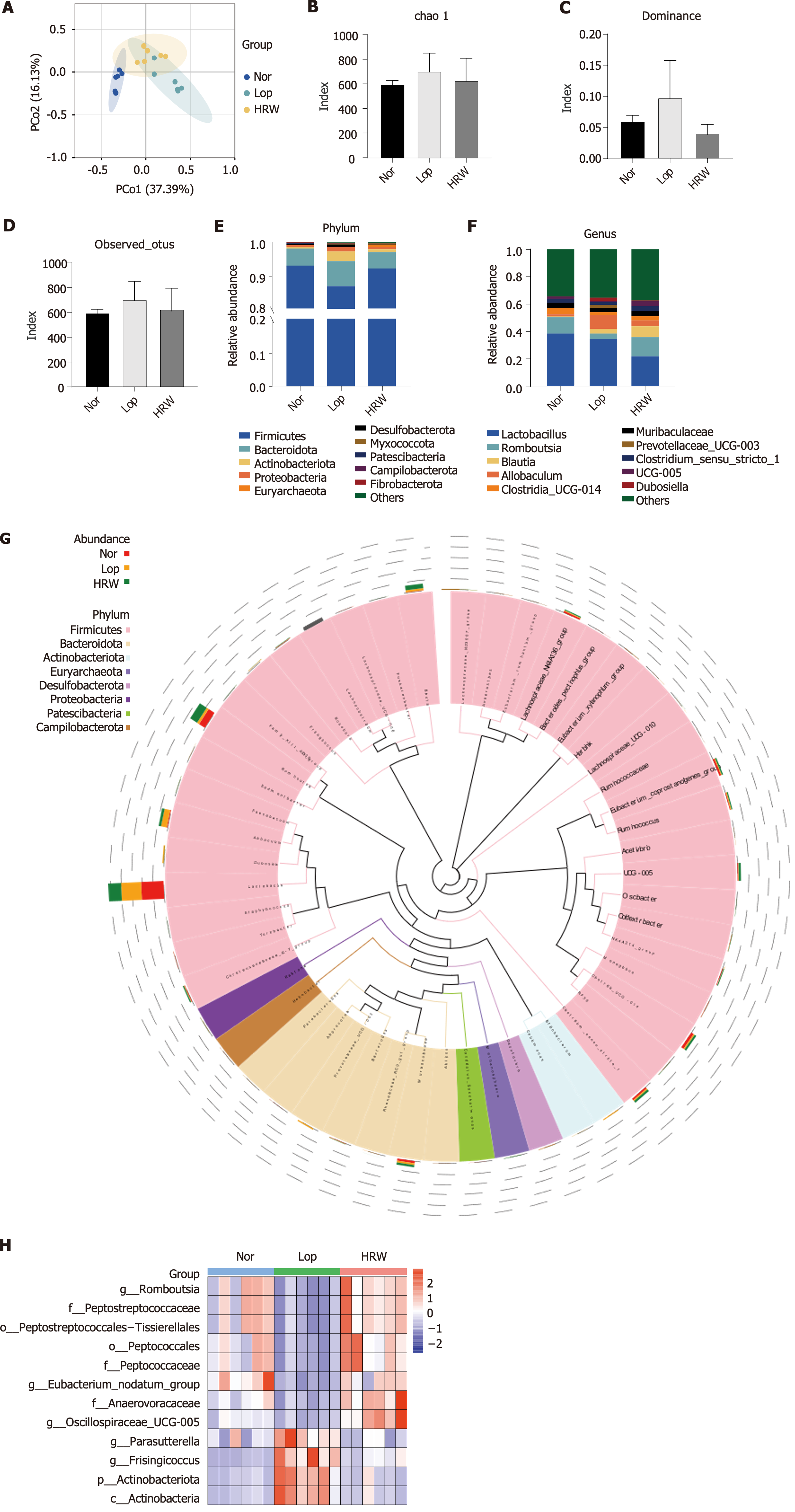
High-throughput sequencing of the 16S rDNA gene V3-V4 region of the gut microbiota revealed significant diversity and composition differences between the intestinal microbiota of rats treated with loperamide and that of Nor rats. PCoA indicated a clear separation between the Nor and the Lop groups. Notably, a partial correction trend was observed for constipated rats after intervention with HRW (Figure 3A). Based on the alpha diversity metrics, the Lop group had higher values on chao 1, dominance, and observed_otus indexes of the gut flora relative to the Nor group. Following HRW intervention, these indices decreased, resulting in a partial decrease in the richness and evenness of the gut flora (Figure 3B-D). The microbiota community structure at the phylum level is shown in Figure 3E. The identified microorganisms were mainly Firmicutes, Bacteroidetes, Proteobacteria, and Actinobacteria, with Firmicutes accounting for the largest proportion. Compared to the Nor rats, loperamide-induced constipated rats had a lower abundance of Firmicutes (92.58% vs 86.70%). Bacteroidetes (5.51% vs 7.50%) and Actinobacteria (0.57% vs 3.03%) were more abundant in model rats than Nor rats. Compared with the Lop group, the abundance of Firmicutes (86.70% vs 91.77%) increased whereas that of Bacteroidetes (7.50% vs 5.26%) and Actinobacteria (3.03% vs 0.81%) decreased in the HRW group. The dominant species in each group were selected and analyzed at the genus level to better understand the intestinal flora. As shown in Figure 3F and G, the relative abundances of Allobaculum (1.48% vs 9.95%), Prevotellaceae_UCG-003 (0.01% vs 2.11%), and Dubosiella (0.05% vs 2.42%) were significantly increased whereas the relative abundances of Romboutsia (11.88% vs 3.95%) and Clostridia_UCG-014 (4.73% vs 2.13%) were significantly decreased in the Lop group compared with the Nor group. Compared to the Lop group, the abundance of Romboutsia (3.95% vs 14.07%) and Clostridia_UCG-014 (2.13% vs 3.30%) increased whereas the abundance of Allobaculum (9.95% vs 4.24%), Prevotellaceae_UCG-003 (2.11% vs 0.05%), and Dubosiella (2.42% vs 0.30%) decreased in the HRW group. Finally, the differential flora between these groups were screened using a t test, with P < 0.05 as the threshold. Five bacteria, namely Romboutsia, Parasutterella, Frisingicoccusand, Eubac-terium_nodatum_group, and Oscillospiraceae_UCG-005, were identified (Figure 3H). Altogether, HRW can regulate gut flora disorder in constipated rats.
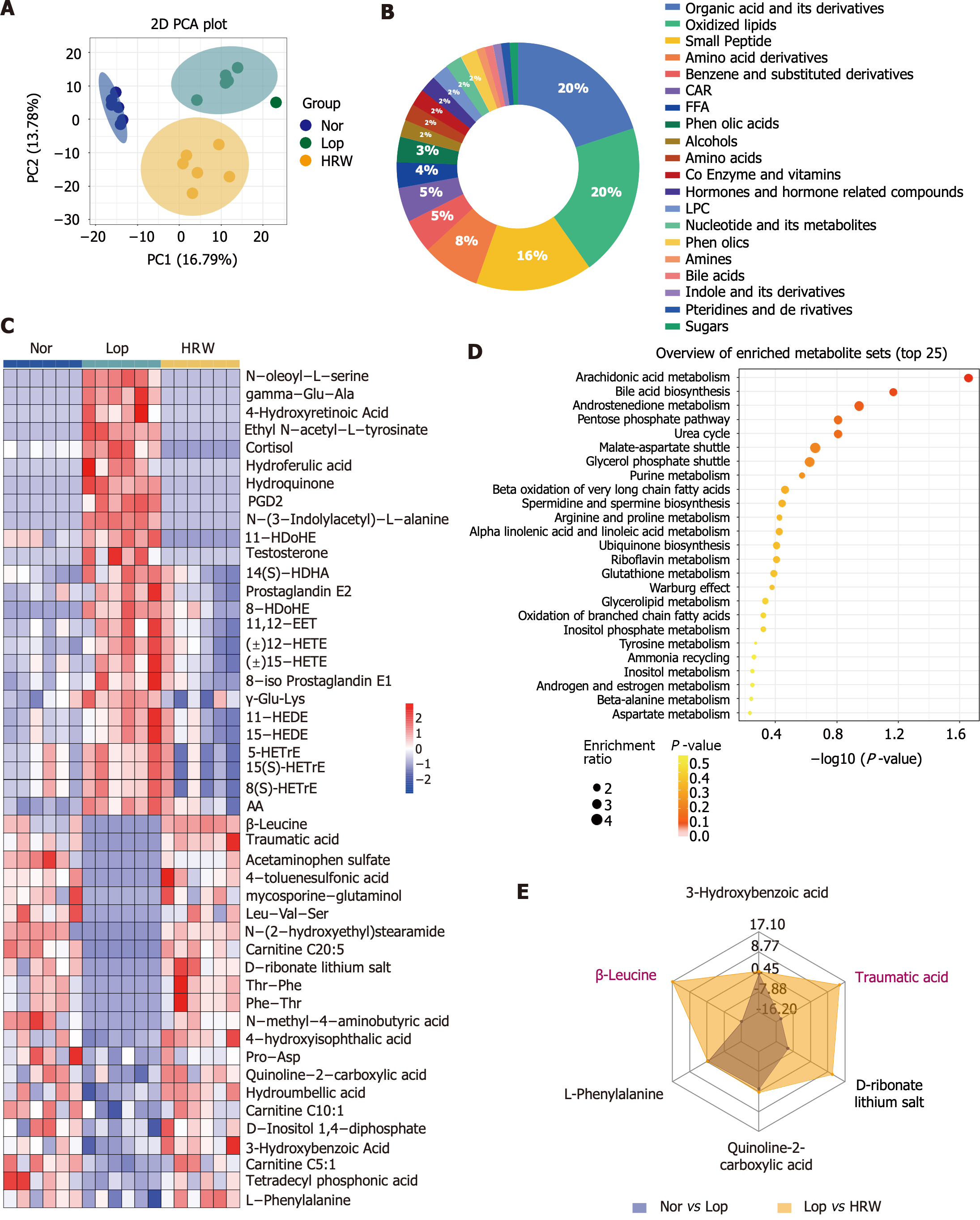
UPLC-QTRAP-tandem mass spectrometry was employed to detect metabolites in rat serum. PCA revealed a distinct separation and significant alterations in metabolomic profiles between the Nor and Lop groups. However, a remarkable trend of callback was observed in the HRW group compared to the Lop group (Figure 4A). Orthogonal partial least squares discriminant analysis indicated alterations in the metabolites profiles were altered. Based on the screening criteria, VIP ≥ 1 and P < 0.05, 244 differential metabolites were identified between the Nor and Lop groups, and 174 differential metabolites were found between the Lop and HRW groups. Of these, 90 differentiated metabolites were present in all three groups, including oxidized lipids (20%), organic acids and their derivatives (20%), and small peptides (15.6%; Figure 4B and C). KEGG enrichment analysis revealed that these differential metabolites were significantly enriched in the arachidonic acid metabolism, bile acid biosynthesis, and androstenedione metabolism pathways (Figure 4D). As shown in the radar map, the contents of β-Leu and traumatic acid were most significantly altered in the model and HRW groups (Figure 4E), suggesting that HRW may play a role in relieving constipation by altering metabolism in vivo. Therefore, the efficacies of β-Leu and traumatic acid need to be evaluated.
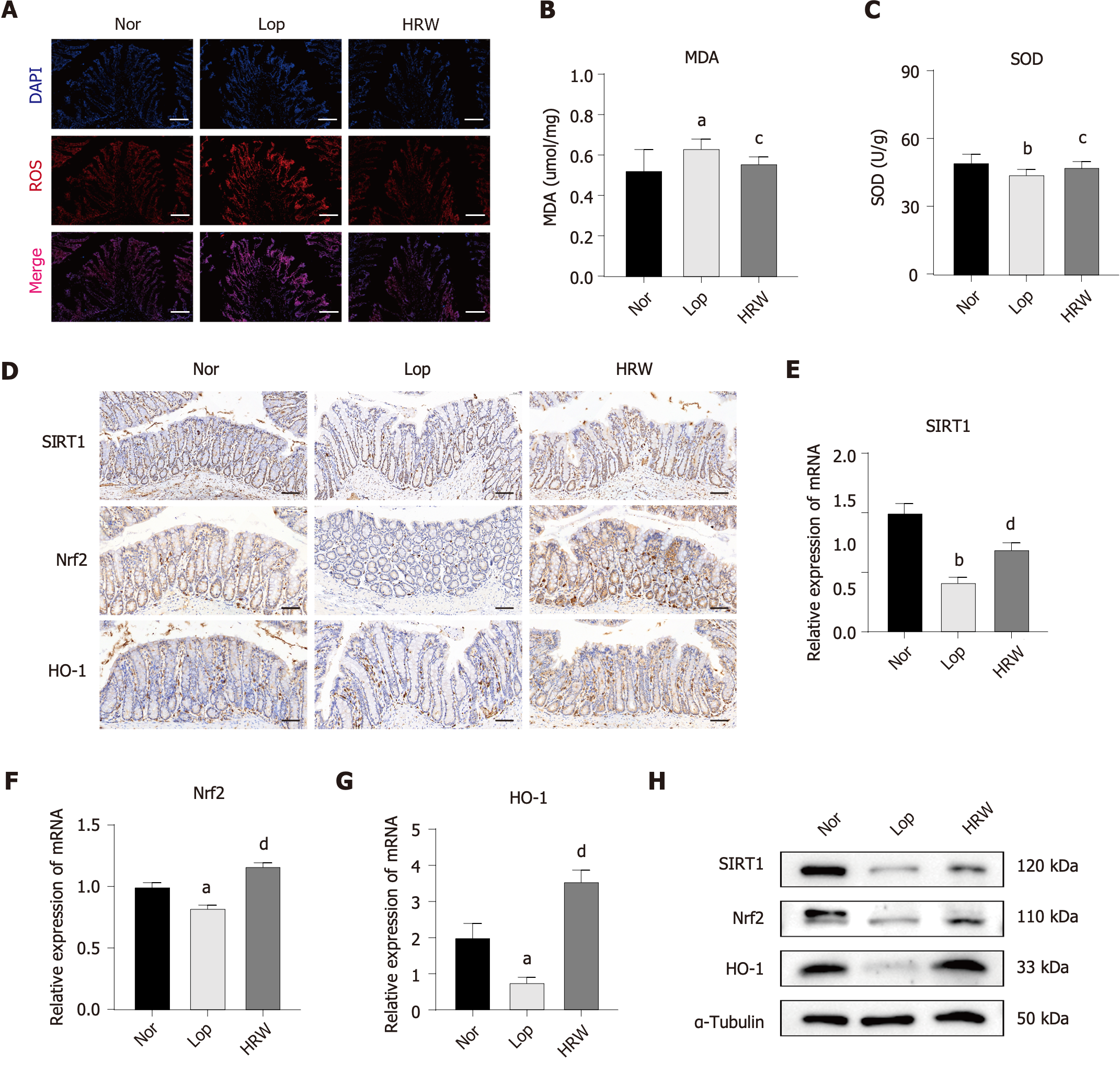
Increased oxidative stress levels are intimately connected with the occurrence and development of constipation[18]. Oxidative stress, defined as a disturbance in the pro-/antioxidant balance, is harmful to cells due to excessive ROS generation[14]. MDA, a product of lipid peroxidation, can indicate the severity of oxidative stress[19]. SOD is an important antioxidant enzyme that removes ROS[20]. Immunofluorescence (IF) staining revealed that ROS expression increased in constipated rats; however, this overexpression was markedly inhibited by HRW (Figure 5A). Compared to the control group, loperamide stimulation significantly increased the expression of MDA and decreased the expression of SOD in the model group. The expression levels of MDA and SOD were reversed by HRW (Figure 5B and C).
The decrease in SIRT1, Nrf2, and HO-1 causes an imbalance in intracellular oxidation, resulting in cellular oxidative stress damage[9]. The expression levels of these markers in the colon tissue of rats were further detected using an IHC assay. The levels of SIRT1, Nrf2, and HO-1 were markedly decreased in the constipated group; however, a remarkable increase was observed in the HRW group (Figure 5D). The expression levels of SIRT1, Nrf2, and HO-1 in the colon tissue of rats were validated using qRT-PCR and western blotting analysis. Notably, the results tended to be consistent with those of IHC staining (Figure 5E-H). The original western blots of Figure 5H are shown in the Supplementary Figure 1A.
To confirm that SIRT1 mediates the regulatory activity of HRW in constipation, loperamide-induced constipated rats were intraperitoneally injected with EX527, a SIRT1 inhibitor, to suppress SIRT1 expression. A flow chart illustrating the modeling and drug administration process is shown in Figure 6A. The regulatory effect of HRW in constipated rats was significantly counteracted in rats treated with the SIRT1 inhibitor according to the total amount of feces excreted within 24 h (Figure 6B), water content of feces (Figure 6C), carbon end propulsion distance (Figure 6D and E), and the expression of ROS, MDA, and SOD in the colons of rats (Figure 6F-H). Similarly, the suppressed expression of SIRT1 impeded the improvement of SIRT1 signaling (SIRT1, Nrf2, and HO-1) (Figure 6I-L) following HRW intervention. The original western blots of Figure 6L are shown in the Supplementary Figure 1B. Overall, the results indicate that SIRT1 is a crucial target by which HRW alleviates constipation in loperamide-induced rats.
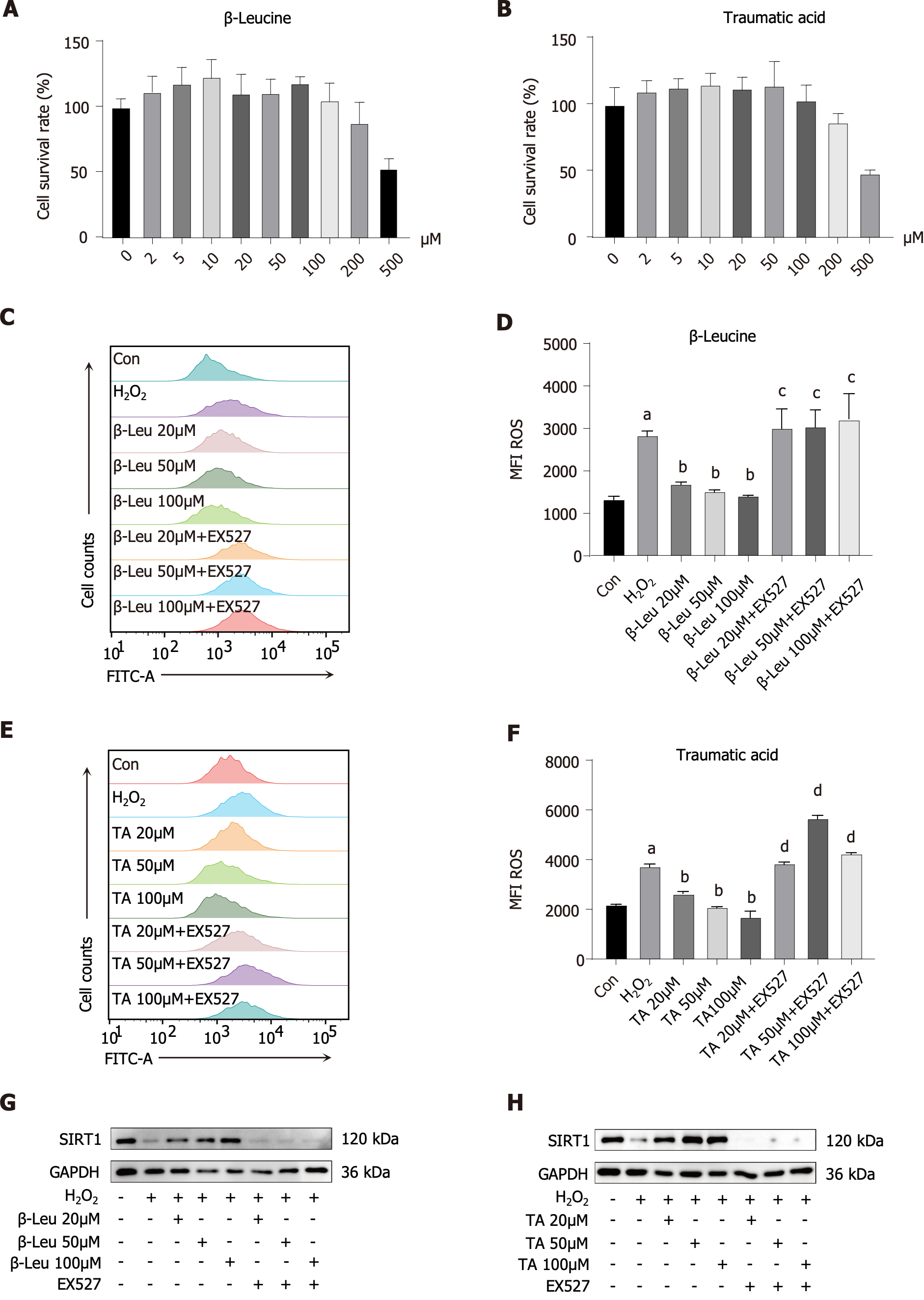
The serum metabolites, β-Leu and traumatic acid, were found to significantly increase after HRW intervention in loperamide-induced constipated rats. To validate whether these two metabolites were the basis for the effectiveness of HRW, NCM460 cells (a normal human colon mucosal epithelial cell line) was used. Based on the CCK-8 assay, administering appropriate concentrations of β-Leu and traumatic acid (20 μM, 50 μM, and 100 μM) did not affect normal cell growth (Figure 7A and B). Notably, H2O2 significantly induced excessive ROS in NCM460 cells; however, this induction was reversed by intervention with β-Leu and traumatic acid, suggesting that treatment with β-Leu and traumatic acid had stimulating rather than toxic effects on the viability of NCM460 cells. Following inhibition of SIRT1 expression, the effects of β-Leu and traumatic acid on the alleviation of oxidative stress were abolished (Figure 7C-F). Similarly, the variation in the expression levels of SIRT1 was confirmed via western blotting. Compared to the control group, H2O2 stimulation significantly decreased SIRT1 expression in the model group, which was increased in the β-Leu- and traumatic acid-treated groups, respectively. Of note, the expression of SIRT1 was reversed by the combination treatment of metabolites and EX527 (Figure 7G and H). The original western blots of Figure 7G and H are shown in the Supplementary Figure 1C.
Constipation is a prevalent gastrointestinal disorder characterized by difficulty in defecation and impaired intestinal motility. However, the current treatment options, such as oral laxatives, secretagogues, and prokinetic agents, have limitations, including short-term efficacy, high recurrence rates and inadequate curative effects[3]. The main approach for constipation management involves sufficient water intake. Magnesium and sulfate-rich water has been demonstrated to have beneficial laxative effects[21]. Hydrogen therapy has emerged as a promising therapeutic strategy owing to its biological reducibility and homeostatic regulatory abilities[4]. In fact, hydrogen therapy is a safe and effective therapeutic agent that has shown significant efficacy in various oxidative stress/inflammation-related diseases, including improving sepsis-related encephalopathy[22], promoting of diabetic wound healing[23], and preventing or treating of cardiovascular diseases[24]. Notably, HRW, which is a specialized type of water that can be consumed orally, and contains a precise concentration of dissolved H2, has been reported to possess potent antioxidant, anti-inflammatory, and anti-apoptotic properties[25]. In this study, HRW was found to relieve constipation symptoms by regulating intestinal flora disorders and serum metabolic abnormalities. Therefore, HRW may provide a new auxiliary method for the clinical treatment of constipation with advantages, such as convenient drinking, low price, and high acceptance.
Oxidative stress refers to the accumulation of free oxygen radicals to levels that exceed the ability of the antioxidant defense system, leading to oxidative damage to cells and tissues[26]. Oxidative stress can increase intestinal mucosal inflammation, destroy the intestinal barrier, reduce the mucus layer thickness, and affect intestinal peristalsis and water absorption[6]. Oxidative stress can also affect the function of the intestinal nervous system, inhibit the contraction of intestinal smooth muscles and the activity of ganglion cells, interfere with intestinal nerve conduction, slow intestinal peristalsis, and promote constipation[27]. H2 in HRW can rapidly diffuse through the transmembrane to reach the cell, react with toxic ROS, and prevent oxidative damage[5]. In the present study, HRW reduced the levels of ROS and MDA and increased the SOD content in constipated rats to reduce oxidative stress. HRW can also increase the thickness of the intestinal mucosal layer, increase the number of ICC and intestinal nerve cells, and alleviate the symptoms of constipation.
SIRT1, a member of the NAD+-dependent deacetylase family, has been extensively explored as a potential therapeutic target to attenuate oxidative stress and inflammation-induced disorders[8]. The deficiency of SIRT1 markedly increases ROS and inflammatory reactions[28], while modulation of the SIRT1/NF-kappaB pathway can suppress NLRP3 inflammasome and oxidative stress[29]. Increasing evidence has suggested that SIRT1 play a role in intestinal disease[30]. SIRT1 can regulate intestinal inflammation in aging mice by altering the gut microbiota[31], and achieve mitochondrial homeostasis through the NAD+-SIRT1 pathway to protect the intestinal barrier function in patients with severe malnourishment[32]. There are also reports emphasizing the dual role of SIRT1[33]; SIRT1 inhibitors can alleviate radiation-induced intestinal stem cell death, promote crypt recovery, and improve the survival rate of mice[34]. In this study, low SIRT1 expression was observed in the colons of constipated rats. Following intervention with HRW, the expression level of SIRT1 was upregulated. EX527, a SIRT1 inhibitor, also verified that HRW alleviates oxidative stress and improves constipation symptoms by regulating the SIRT1/Nrf2/HO-1 signaling pathway.
Two serum metabolites, β-Leu and traumatic acid, which can relieve constipation, were screened using non-target metabolomics. At present, studies revealing the correlation between β-Leu and oxidative stress, which deserves further exploration, are lacking. Traumatic acid reduces oxidative stress and enhances collagen biosynthesis in human skin fibroblasts[35]. In addition, traumatic acid can reduce the growth of breast cancer cells by influencing apoptosis through oxidative stress[36]. Overall, traumatic acid is an organic acid derivative that holds promise for the treatment of various chronic diseases. In this study, both of β-Leu and traumatic acid could attenuate H2O2-induced ROS in NCM460 cells. Further, the SIRT1 inhibitor validated their ability of these metabolites to decrease oxidative stress reaction, thereby regulating SIRT1 expression in vitro. In the future, the efficacy and safety of β-Leu and traumatic acid in animals will need to be further refined and validated. Hence, HRW alleviates constipation in rats by increasing the serum levels of β-Leu and traumatic acid and SIRT1 expression by reducing oxidative stress levels.
Five different bacteria, namely Oscillospiraceae_UCG-005, Romboutsia, Eubacterium_nodatum_group, Frisingicoccus and Parasutterella were identified using 16s rDNA sequencing. Romboutsia is associated with the production of short-chain fatty acids, which are important fuels for intestinal epithelial cells and play an important physiological function in intestinal motility and host metabolism[37]. Eubacterium sp. and Oscillospira sp. can produce butyrate, which plays a crucial role in enhancing colonic motility and suppressing intestinal inflammation[37,38]. In the present study, HRW was significantly enriched in potentially beneficial bacteria (Oscillospiraceae_UCG-005, Romboutsia and Eubact-erium_nodatum_group). In contrast, a positive correlation has been found between Parasutterella abundance and inflammation in constipated rats[39]. Constipation is a prevalent non-motor symptom observed in patients with Parkinson’s disease[40]. Frisingicoccus exhibits elevated abundance within the intestinal tract of patients diagnosed with Parkinson's disease and is closely associated with neuroinflammation and motor function[41]. Similarly, HRW was found to reduce certain pathogenic intestinal microbes (Frisingicoccus and Parasutterella).
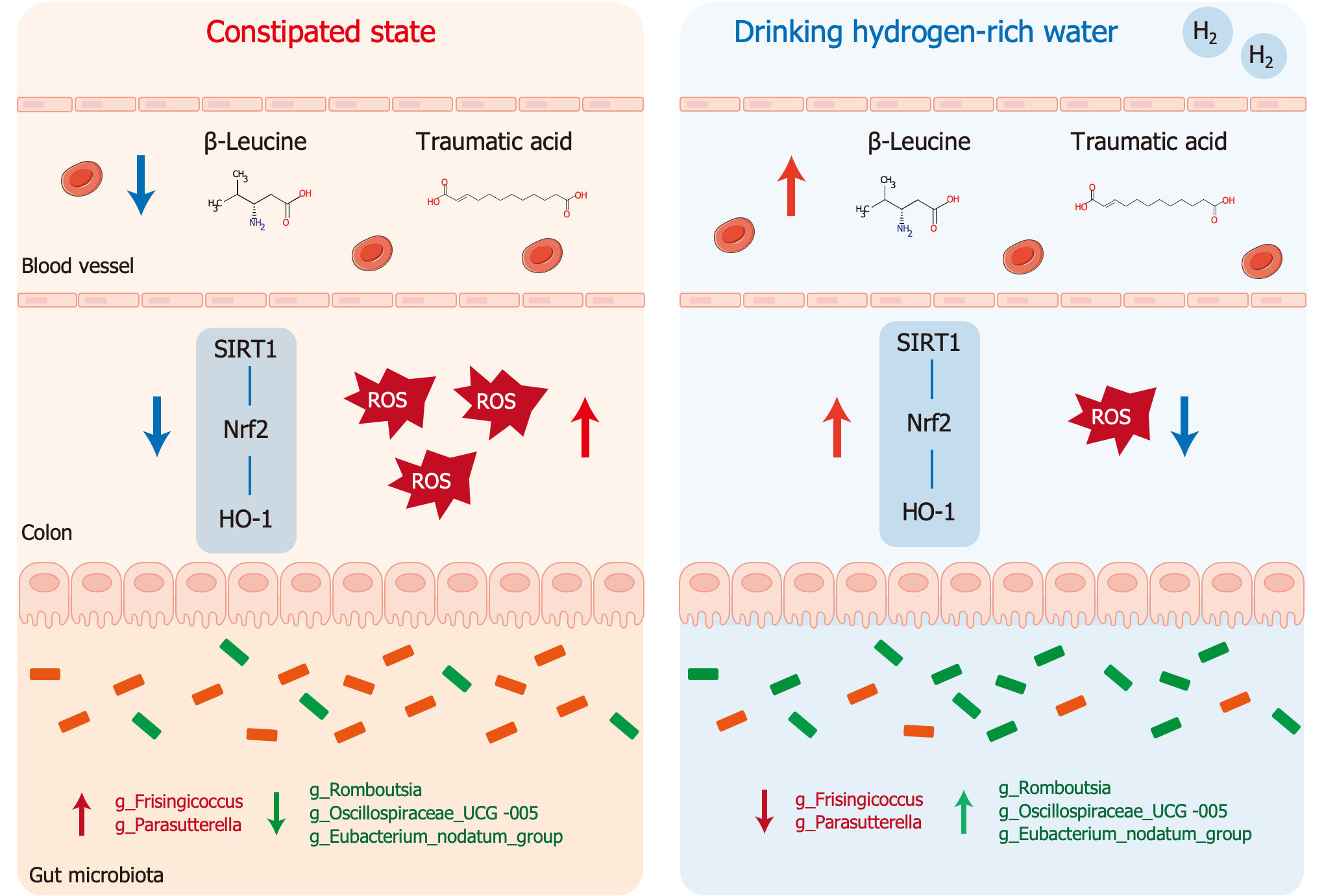
Altogether, HRW could reshape intestinal microbiota disorders, regulate serum metabolism abnormalities, and alleviate constipation by regulating the SIRT1/Nrf2/HO-1 signaling pathway to reduce oxidative stress levels in constipated rats (Figure 8). Notably, β-Leu and traumatic acid were identified as potential metabolites for the treatment of constipation as they increased SIRT1 expression and decrease the level of oxidative stress.
The authors would like to acknowledge the Jiangsu Province Key Laboratory of Tumor Systems Biology and Chinese Medicine for providing the laboratory space and instruments for experiments in vitro and in vivo.
| 1. | Black CJ, Drossman DA, Talley NJ, Ruddy J, Ford AC. Functional gastrointestinal disorders: advances in understanding and management. Lancet. 2020;396:1664-1674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 287] [Article Influence: 57.4] [Reference Citation Analysis (0)] |
| 2. | Bharucha AE, Lacy BE. Mechanisms, Evaluation, and Management of Chronic Constipation. Gastroenterology. 2020;158:1232-1249.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 342] [Article Influence: 68.4] [Reference Citation Analysis (1)] |
| 3. | Sayuk GS, Waldman SA, Brenner DM. Mechanisms of Action of Current Pharmacologic Options for the Treatment of Chronic Idiopathic Constipation and Irritable Bowel Syndrome With Constipation. Am J Gastroenterol. 2022;117:S6-S13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 16] [Article Influence: 5.3] [Reference Citation Analysis (1)] |
| 4. | Zhou G, Goshi E, He Q. Micro/Nanomaterials-Augmented Hydrogen Therapy. Adv Healthc Mater. 2019;8:e1900463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 76] [Article Influence: 12.7] [Reference Citation Analysis (1)] |
| 5. | Ohsawa I, Ishikawa M, Takahashi K, Watanabe M, Nishimaki K, Yamagata K, Katsura K, Katayama Y, Asoh S, Ohta S. Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals. Nat Med. 2007;13:688-694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1346] [Cited by in RCA: 1692] [Article Influence: 94.0] [Reference Citation Analysis (1)] |
| 6. | Song H, Guo R, Sun X, Kou Y, Ma X, Chen Y, Song L, Yuan C, Wu Y. Xylooligosaccharides from corn cobs alleviate loperamide-induced constipation in mice via modulation of gut microbiota and SCFA metabolism. Food Funct. 2023;14:8734-8746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (1)] |
| 7. | Lan J, Wang K, Chen G, Cao G, Yang C. Effects of inulin and isomalto-oligosaccharide on diphenoxylate-induced constipation, gastrointestinal motility-related hormones, short-chain fatty acids, and the intestinal flora in rats. Food Funct. 2020;11:9216-9225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 52] [Article Influence: 13.0] [Reference Citation Analysis (1)] |
| 8. | Wang F, Yao S, Xia H. SIRT1 is a key regulatory target for the treatment of the endoplasmic reticulum stress-related organ damage. Biomed Pharmacother. 2020;130:110601. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 34] [Article Influence: 6.8] [Reference Citation Analysis (1)] |
| 9. | Dang R, Wang M, Li X, Wang H, Liu L, Wu Q, Zhao J, Ji P, Zhong L, Licinio J, Xie P. Edaravone ameliorates depressive and anxiety-like behaviors via Sirt1/Nrf2/HO-1/Gpx4 pathway. J Neuroinflammation. 2022;19:41. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 299] [Article Influence: 99.7] [Reference Citation Analysis (1)] |
| 10. | Li SW, Takahara T, Que W, Fujino M, Guo WZ, Hirano SI, Ye LP, Li XK. Hydrogen-rich water protects against liver injury in nonalcoholic steatohepatitis through HO-1 enhancement via IL-10 and Sirt 1 signaling. Am J Physiol Gastrointest Liver Physiol. 2021;320:G450-G463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 27] [Article Influence: 6.8] [Reference Citation Analysis (1)] |
| 11. | Lin CL, Huang WN, Li HH, Huang CN, Hsieh S, Lai C, Lu FJ. Hydrogen-rich water attenuates amyloid β-induced cytotoxicity through upregulation of Sirt1-FoxO3a by stimulation of AMP-activated protein kinase in SK-N-MC cells. Chem Biol Interact. 2015;240:12-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 55] [Article Influence: 5.5] [Reference Citation Analysis (1)] |
| 12. | Xing Z, Pan W, Zhang J, Xu X, Zhang X, He X, Fan M. Hydrogen Rich Water Attenuates Renal Injury and Fibrosis by Regulation Transforming Growth Factor-β Induced Sirt1. Biol Pharm Bull. 2017;40:610-615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (1)] |
| 13. | Wang Z, Shi Y, Zeng S, Zheng Y, Wang H, Liao H, Song J, Zhang X, Cao J, Li C. Polysaccharides from Holothuria leucospilota Relieve Loperamide-Induced Constipation Symptoms in Mice. Int J Mol Sci. 2023;24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 14] [Reference Citation Analysis (1)] |
| 14. | Chen X, Bi M, Yang J, Cai J, Zhang H, Zhu Y, Zheng Y, Liu Q, Shi G, Zhang Z. Cadmium exposure triggers oxidative stress, necroptosis, Th1/Th2 imbalance and promotes inflammation through the TNF-α/NF-κB pathway in swine small intestine. J Hazard Mater. 2022;421:126704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 130] [Article Influence: 43.3] [Reference Citation Analysis (1)] |
| 15. | Wang S, Wang Z, Li Z, Zhang X, Zhang H, Zhang T, Meng X, Sheng F, Hou Y. Amelioration of systemic antitumor immune responses in cocktail therapy by immunomodulatory nanozymes. Sci Adv. 2022;8:eabn3883. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 30] [Article Influence: 10.0] [Reference Citation Analysis (1)] |
| 16. | Jin B, Ha SE, Wei L, Singh R, Zogg H, Clemmensen B, Heredia DJ, Gould TW, Sanders KM, Ro S. Colonic Motility Is Improved by the Activation of 5-HT(2B) Receptors on Interstitial Cells of Cajal in Diabetic Mice. Gastroenterology. 2021;161:608-622.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 35] [Article Influence: 8.8] [Reference Citation Analysis (1)] |
| 17. | Chen X, Qiu TT, Wang Y, Xu LY, Sun J, Jiang ZH, Zhao W, Tao T, Zhou YW, Wei LS, Li YQ, Zheng YY, Zhou GH, Chen HQ, Zhang J, Feng XB, Wang FY, Li N, Zhang XN, Jiang J, Zhu MS. A Shigella species variant is causally linked to intractable functional constipation. J Clin Invest. 2022;132. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (1)] |
| 18. | Kim JE, Choi YJ, Lee SJ, Gong JE, Lee YJ, Sung JE, Jung YS, Lee HS, Hong JT, Hwang DY. Antioxidant activity and laxative effects of tannin-enriched extract of Ecklonia cava in loperamide-induced constipation of SD rats. PLoS One. 2021;16:e0246363. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (1)] |
| 19. | de Mello Barros Pimentel MV, Bertolami A, Fernandes LP, Barroso LP, Castro IA. Could a lipid oxidative biomarker be applied to improve risk stratification in the prevention of cardiovascular disease? Biomed Pharmacother. 2023;160:114345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (1)] |
| 20. | Gui T, Luo L, Chhay B, Zhong L, Wei Y, Yao L, Yu W, Li J, Nelson CL, Tsourkas A, Qin L, Cheng Z. Superoxide dismutase-loaded porous polymersomes as highly efficient antioxidant nanoparticles targeting synovium for osteoarthritis therapy. Biomaterials. 2022;283:121437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 45] [Article Influence: 15.0] [Reference Citation Analysis (1)] |
| 21. | Bellini M, Tonarelli S, Barracca F, Rettura F, Pancetti A, Ceccarelli L, Ricchiuti A, Costa F, de Bortoli N, Marchi S, Rossi A. Chronic Constipation: Is a Nutritional Approach Reasonable? Nutrients. 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 41] [Article Influence: 10.3] [Reference Citation Analysis (1)] |
| 22. | Han Q, Bai Y, Zhou C, Dong B, Li Y, Luo N, Chen H, Yu Y. Effect of molecular hydrogen treatment on Sepsis-Associated encephalopathy in mice based on gut microbiota. CNS Neurosci Ther. 2023;29:633-645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 14] [Reference Citation Analysis (1)] |
| 23. | Chen S, Zhu Y, Xu Q, Jiang Q, Chen D, Chen T, Xu X, Jin Z, He Q. Photocatalytic glucose depletion and hydrogen generation for diabetic wound healing. Nat Commun. 2022;13:5684. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 70] [Reference Citation Analysis (1)] |
| 24. | LeBaron TW, Kura B, Kalocayova B, Tribulova N, Slezak J. A New Approach for the Prevention and Treatment of Cardiovascular Disorders. Molecular Hydrogen Significantly Reduces the Effects of Oxidative Stress. Molecules. 2019;24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 100] [Cited by in RCA: 83] [Article Influence: 13.8] [Reference Citation Analysis (1)] |
| 25. | Yamamoto H, Ichikawa Y, Hirano SI, Sato B, Takefuji Y, Satoh F. Molecular Hydrogen as a Novel Protective Agent against Pre-Symptomatic Diseases. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (1)] |
| 26. | Forman HJ, Zhang H. Targeting oxidative stress in disease: promise and limitations of antioxidant therapy. Nat Rev Drug Discov. 2021;20:689-709. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1299] [Cited by in RCA: 1471] [Article Influence: 367.8] [Reference Citation Analysis (0)] |
| 27. | Liu J, Wang S, Yi R, Long X, Luo G, Zhao X, He Y. LimosiLactobacillus pentosus Isolated from Mustard Relieves Drug-induced Constipation in Mice Fed a High-fat Diet by Modulating Enteric Neurotransmitter Function. Probiotics Antimicrob Proteins. 2023;15:1371-1381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 28. | Singh V, Ubaid S. Role of Silent Information Regulator 1 (SIRT1) in Regulating Oxidative Stress and Inflammation. Inflammation. 2020;43:1589-1598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 304] [Article Influence: 76.0] [Reference Citation Analysis (0)] |
| 29. | Ren B, Feng J, Yang N, Guo Y, Chen C, Qin Q. Ginsenoside Rg3 attenuates angiotensin II-induced myocardial hypertrophy through repressing NLRP3 inflammasome and oxidative stress via modulating SIRT1/NF-κB pathway. Int Immunopharmacol. 2021;98:107841. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 74] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 30. | Chen Y, Zhou D, Feng Y, Li B, Cui Y, Chen G, Li N. Association of sirtuins (SIRT1-7) with lung and intestinal diseases. Mol Cell Biochem. 2022;477:2539-2552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 31. | Wellman AS, Metukuri MR, Kazgan N, Xu X, Xu Q, Ren NSX, Czopik A, Shanahan MT, Kang A, Chen W, Azcarate-Peril MA, Gulati AS, Fargo DC, Guarente L, Li X. Intestinal Epithelial Sirtuin 1 Regulates Intestinal Inflammation During Aging in Mice by Altering the Intestinal Microbiota. Gastroenterology. 2017;153:772-786. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 142] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 32. | Ling C, Versloot CJ, Arvidsson Kvissberg ME, Hu G, Swain N, Horcas-Nieto JM, Miraglia E, Thind MK, Farooqui A, Gerding A, van Eunen K, Koster MH, Kloosterhuis NJ, Chi L, ChenMi Y, Langelaar-Makkinje M, Bourdon C, Swann J, Smit M, de Bruin A, Youssef SA, Feenstra M, van Dijk TH, Thedieck K, Jonker JW, Kim PK, Bakker BM, Bandsma RHJ. Rebalancing of mitochondrial homeostasis through an NAD(+)-SIRT1 pathway preserves intestinal barrier function in severe malnutrition. EBioMedicine. 2023;96:104809. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 18] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 33. | Devi K, Singh N, Jaggi AS. Dual role of sirtuin 1 in inflammatory bowel disease. Immunopharmacol Immunotoxicol. 2020;42:385-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 34. | Fu G, Chen S, Liang L, Li X, Tang P, Rao X, Pan M, Xu X, Li Y, Yao Y, Zhou Y, Gao J, Mo S, Cai S, Peng J, Zhang Z, Clevers H, Hua G. SIRT1 inhibitors mitigate radiation-induced GI syndrome by enhancing intestinal-stem-cell survival. Cancer Lett. 2021;501:20-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 35. | Jabłońska-Trypuć A, Pankiewicz W, Czerpak R. Traumatic Acid Reduces Oxidative Stress and Enhances Collagen Biosynthesis in Cultured Human Skin Fibroblasts. Lipids. 2016;51:1021-1035. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 42] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 36. | Jabłońska-Trypuć A, Krętowski R, Wołejko E, Wydro U, Butarewicz A. Traumatic acid toxicity mechanisms in human breast cancer MCF-7 cells. Regul Toxicol Pharmacol. 2019;106:137-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 37. | Mukherjee A, Lordan C, Ross RP, Cotter PD. Gut microbes from the phylogenetically diverse genus Eubacterium and their various contributions to gut health. Gut Microbes. 2020;12:1802866. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 240] [Cited by in RCA: 372] [Article Influence: 74.4] [Reference Citation Analysis (0)] |
| 38. | Gophna U, Konikoff T, Nielsen HB. Oscillospira and related bacteria - From metagenomic species to metabolic features. Environ Microbiol. 2017;19:835-841. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 297] [Article Influence: 37.1] [Reference Citation Analysis (0)] |
| 39. | Yang Z, Ye S, Xu Z, Su H, Tian X, Han B, Shen B, Liao Q, Xie Z, Hong Y. Dietary synbiotic ameliorates constipation through the modulation of gut microbiota and its metabolic function. Food Res Int. 2021;147:110569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 47] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 40. | Du Y, Li Y, Xu X, Li R, Zhang M, Cui Y, Zhang L, Wei Z, Wang S, Tuo H. Probiotics for constipation and gut microbiota in Parkinson's disease. Parkinsonism Relat Disord. 2022;103:92-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 54] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 41. | Jang JH, Yeom MJ, Ahn S, Oh JY, Ji S, Kim TH, Park HJ. Acupuncture inhibits neuroinflammation and gut microbial dysbiosis in a mouse model of Parkinson's disease. Brain Behav Immun. 2020;89:641-655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 145] [Article Influence: 29.0] [Reference Citation Analysis (0)] |