Published online Jan 14, 2024. doi: 10.3748/wjg.v30.i2.146
Peer-review started: August 22, 2023
First decision: November 20, 2023
Revised: November 28, 2023
Accepted: December 25, 2023
Article in press: December 25, 2023
Published online: January 14, 2024
Processing time: 143 Days and 7.9 Hours
Eosinophilic gastroenteritis (EGE) is a chronic recurrent disease with abnormal eosinophilic infiltration in the gastrointestinal tract. Glucocorticoids remain the most common treatment method. However, disease relapse and glucocorticoid dependence remain notable problems. To date, few studies have illuminated the prognosis of EGE and risk factors for disease relapse.
To describe the clinical characteristics of EGE and possible predictive factors for disease relapse based on long-term follow-up.
This was a retrospective cohort study of 55 patients diagnosed with EGE admitted to one medical center between 2013 and 2022. Clinical records were collected and analyzed. Kaplan-Meier curves and log-rank tests were conducted to reveal the risk factors for long-term relapse-free survival (RFS).
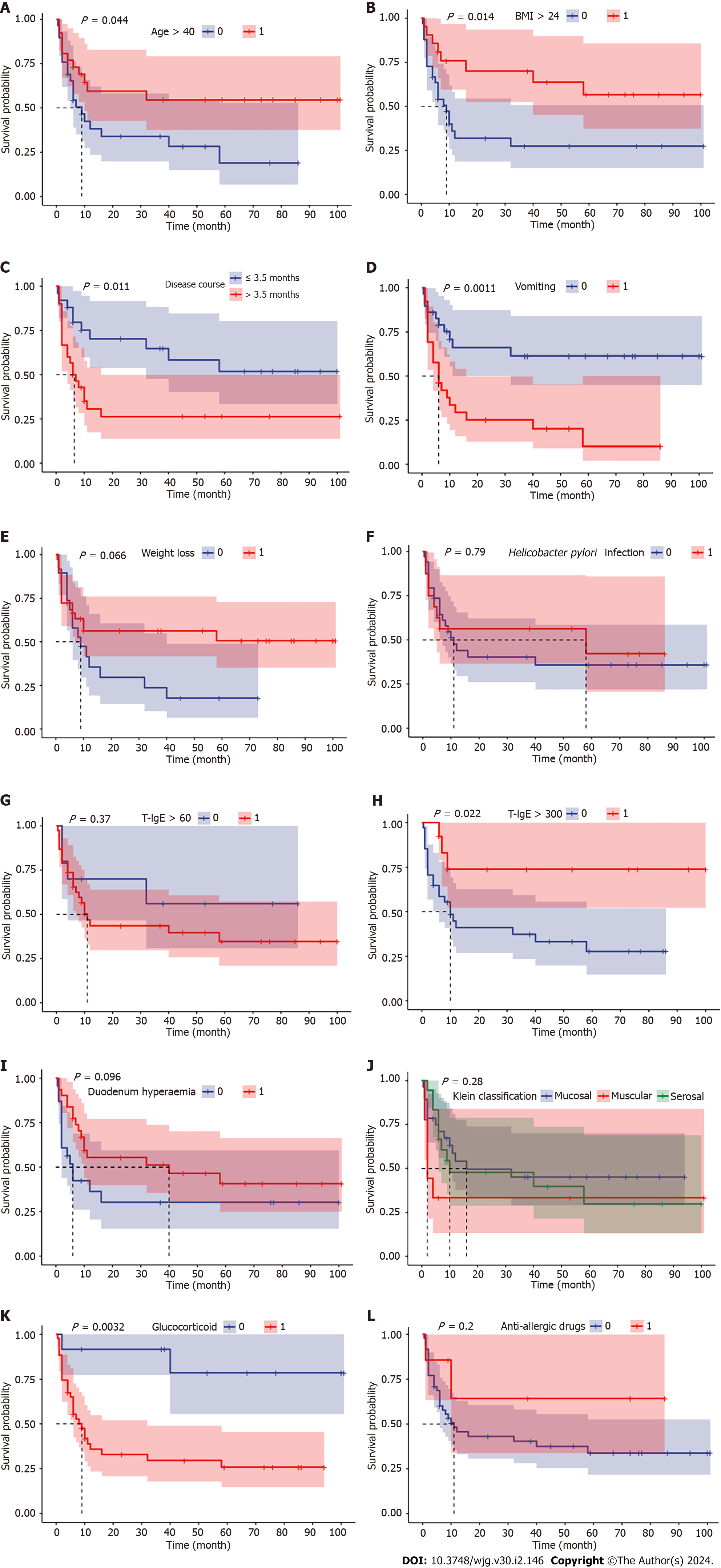
EGE showed a median onset age of 38 years and a slight female predominance (56.4%). The main clinical symptoms were abdominal pain (89.1%), diarrhea (61.8%), nausea (52.7%), distension (49.1%) and vomiting (47.3%). Forty-three (78.2%) patients received glucocorticoid treatment, and compared with patients without glucocorticoid treatments, they were more likely to have elevated serum immunoglobin E (IgE) (86.8% vs 50.0%, P = 0.022) and descending duodenal involvement (62.8% vs 27.3%, P = 0.046) at diagnosis. With a median follow-up of 67 mo, all patients survived, and 56.4% had at least one relapse. Six variables at baseline might have been associated with the overall RFS rate, including age at diagnosis < 40 years [hazard ratio (HR) 2.0408, 95% confidence interval (CI): 1.0082–4.1312, P = 0.044], body mass index (BMI) > 24 kg/m2 (HR 0.3922, 95%CI: 0.1916-0.8027, P = 0.014), disease duration from symptom onset to diagnosis > 3.5 mo (HR 2.4725, 95%CI: 1.220-5.0110, P = 0.011), vomiting (HR 3.1259, 95%CI: 1.5246-6.4093, P = 0.001), total serum IgE > 300 KU/L at diagnosis (HR 0.2773, 95%CI: 0.1204-0.6384, P = 0.022) and glucocorticoid treatment (HR 6.1434, 95%CI: 2.8446-13.2676, P = 0.003).
In patients with EGE, younger onset age, longer disease course, vomiting and glucocorticoid treatment were risk factors for disease relapse, whereas higher BMI and total IgE level at baseline were protective.
Core Tip: Disease relapse has been a long-standing concern for patients with eosinophilic gastroenteritis (EGE). Limited evidence has shown that predicting the course of EGE is complex and related to many factors. There is an urgent need to understand the long-term prognosis of EGE. Therefore, we aimed to describe the features of Chinese EGE patients and construct a model to predict disease relapse based on baseline clinical characteristics.
- Citation: Li KW, Ruan GC, Liu S, Xu TM, Ma Y, Zhou WX, Liu W, Zhao PY, Du ZR, Li J, Li JN. Long-term prognosis and its associated predictive factors in patients with eosinophilic gastroenteritis. World J Gastroenterol 2024; 30(2): 146-157
- URL: https://www.wjgnet.com/1007-9327/full/v30/i2/146.htm
- DOI: https://dx.doi.org/10.3748/wjg.v30.i2.146
Eosinophilic gastroenteritis (EGE) is a rare chronic disease characterized by eosinophilic infiltration in the gastrointestinal (GI) tract. EGE was first described by Kaijser in 1937 and, in 1970 by Klein et al[1], was classified as mucosal, muscular and serosal types based on the depth of eosinophilic infiltration in the GI tract. The prevalence of EGE reported in Western countries is 5.1-8.4/100000[2,3]. However, racial differences have been found, and EGE is more commonly seen in Asians than in Caucasians[4]. Although the overall pathogenesis of EGE is complicated and still not fully understood, it is widely considered to be related to a Th2-mediated allergic response, in which several molecules are involved, such as interleukin (IL)-4, IL-5, IL-13 and eotaxin[5-7].
Glucocorticoid and diet therapy remain the classical treatments for EGE. A considerable number of patients can achieve clinical remission after initial treatment, although more have recurrent disease or even develop glucocorticoid dependence. However, there are few studies about the long-term prognosis of EGE, most of which were case reports or case series. Some studies have suggested that EGE is related to the Klein classification[8,9]. However, there is more evidence that predicting the course of EGE is complicated and attributed to many factors. To meet this urgent need, this study aimed to clarify the long-term prognosis of EGE patients and to explore the predictive factors for disease relapse.
We retrospectively enrolled 55 patients with EGE who were diagnosed and hospitalized at Peking Union Medical College Hospital (PUMCH) between 2013 and 2022. EGE was defined by the criteria proposed by Talley et al[8], including GI symptoms, eosinophilic infiltration in one or more areas of GI biopsy specimens or ascites, and no evidence of other diseases that may lead to elevated eosinophils. Eosinophilic infiltration is defined as an eosinophil count > 20 per high-power field (HPF) in gastric and/or duodenal biopsies or an eosinophil count > 10% of the total white blood cells in ascites. The inclusion criteria were as follows: (1) Age ≥ 18 years; (2) comprehensive evaluation of clinical features, endoscopic findings and histological examinations confirming the diagnosis of EGE; (3) at least 6 mo of follow-up; and (4) signed informed consent. Exclusion criteria included: (1) Age < 18 years; and (2) clear evidence of parasite infections, inflammatory bowel diseases, high eosinophilia syndrome, connective tissue disease, malignant tumors, drug allergies and other diseases that could cause elevated eosinophil levels. Two experienced gastroenterologists (Li J and Li JN) reviewed the data and verified the EGE diagnosis.
The following baseline clinical data were collected from medical records: Age; sex; body mass index (BMI); duration from symptom onset to diagnosis; initial symptoms (e.g., abdominal pain, diarrhea, nausea, vomiting, distension, GI bleeding, weight loss, fever, and rash); complications (e.g., ascites and intestinal obstruction); comorbidities (e.g., Helicobacter pylori infection, hypertension, diabetes, and hepatitis B virus infection); allergic history (e.g., allergic rhinitis, asthma, and urticaria); laboratory test results (e.g. peripheral eosinophil count, hemoglobin, platelets, total serum immunoglobin E (IgE), high sensitivity C-reactive protein, erythrocyte sedimentation rate, antinuclear autoantibodies, antineutrophil cytoplasmic antibodies, and specific allergen tests); computed tomography features; and endoscopic findings.
All patients gave signed informed consent or oral consent, and the study was approved by the Institutional Review Board of Peking Union Medical College Hospital (Ethics approval number: I-23PJ1227).
The treatment and outcome of each patient were reviewed from medical records and updated via outpatient services or telephone calls. During follow-up, disease relapse was defined as recurrence of GI symptoms with elevated peripheral blood eosinophil levels. Adverse outcomes include GI surgery or death due to EGE or its complications. Loss of follow-up was defined as failing to contact patients by telephone > 3 times on different days in a week. According to follow-up, patients were divided into two groups based on the status of disease relapse. In addition, according to the efficacy of glucocorticoid treatment, the cohort was classified into glucocorticoid-dependent and non-glucocorticoid-dependent groups. Glucocorticoid dependence was defined as the failure to reduce glucocorticoids to the equivalent dose of prednisone of 10 mg/d within 3 mo after moderate or full doses of glucocorticoid use or a relapse within 3 mo of drug withdrawal.
Continuous variables were expressed as the mean and standard deviation for those fitting a normal distribution and were tested by Student’s t test for the comparison analysis between groups. Medians and quartiles [M (Q1, Q3)] were used for those not fitting a normal distribution, and the comparison analysis was conducted by the Mann-Whitney U test. Categorical variables were reported as numbers and percentages and compared by Fisher’s exact test between the groups. Relapse-free survival (RFS) was calculated from the date when the patient was first diagnosed with EGE at PUMCH until the date of disease relapse or the last follow-up. We plotted the survival curves by the Kaplan-Meier method and further explored possible risk factors by log-rank analysis. Receiver operating characteristic curves were used to find proper cutoff values and transform continuous variables to categorical variables. A Cox proportional hazards model was used to assess the association of clinical variables with RFS. Only variables with significance (P < 0.05) in the univariate analysis were included in the multivariate model. A forest plot was used to show the hazard ratios (HRs) of predictive variables in the Cox regression model. A two-tailed P < 0.05 was considered significant. All analyses were performed with R (version 4.1.2).
This study enrolled 55 patients with long-term follow-up. Clinical characteristics are shown in Table 1. Patients had a median age of 38 years at diagnosis, and females (56.4%) were slightly predominant. The median time interval from symptom onset to diagnosis was 5 mo. The most common GI symptom was abdominal pain (89.1%), followed by diarrhea (61.8%), nausea (52.7%), distension (49.1%) and vomiting (47.3%). Weight loss was commonly seen (65.5%). Eighteen patients (32.7%) had a history of allergic diseases, including allergic rhinitis (18.2%), asthma (20.0%) and urticaria (3.6%). Nine (16.4%) and 15 (27.3%) patients complained of a history of food and drug allergies, respectively. The most common complications were ascites (30.2%) and intestinal obstruction (14.5%). Sixteen patients (16/50, 32.0%) had Helicobacter pylori infection simultaneously. Elevated initial eosinophil levels were observed in 47 patients (85.5%) at diagnosis [1.16 (0.67–3.91) eosinophils/μL]. Additionally, 38 patients (79.2%) had increased total IgE.
| All patients (N = 55) | |||||
| Demographic characteristics | Complications | ESR (mm/h)2 | 4.00 [2.00, 8.00] | ||
| Sex, female | 31 (56.4) | Ascite | 17 (30.9) | Elevated ESR (> 15) | 7 (7/49, 14.3) |
| Age (yr)2 | 38.00 [25.50, 50.00] | Intestinal obstruction | 8 (14.5) | CRP (mg/L)2 | 1.62 [0.84, 4.38] |
| BMI (kg/m2)1 | 23.28 ± 3.96 | Comorbidities | Elevated hsCRP (> 3) | 11 (11/53, 20.8) | |
| BMI > 24 | 21 (38.9) | Hp infection | 16 (16/50, 32.0) | ANA (+) | 8 (8/47, 17.0) |
| Clinical manifestations | HTN | 8 (14.5) | ANCA (+) | 4 (4/46, 8.7) | |
| Disease duration (mo)2 | 5.00 [1.00, 30.00] | DM | 3 (5.5) | Allergen test (+) | 14 (14/28, 50.0) |
| Allergic history | 18 (32.7) | HBV infection | 4 (7.3) | Inhalant allergen | 8 (8/25, 32.0) |
| Allergic rhinitis | 10 (18.2) | Klein classification | Food allergen | 8 (8/27, 29.6) | |
| Asthma | 11 (20.0) | Mucosal type | 28 (50.9) | Endoscopy findings | |
| Urticaria | 2 (3.6) | Muscular type | 9 (16.4) | Duodenum involvement | 40 (40/54, 74.1) |
| Symptoms, n (%) | Serosal type | 18 (32.7) | Bulb | 27 (27/54, 50.0) | |
| Abdominal pain | 49 (89.1) | Laboratory tests | Post-bulb | 16 (16/54, 29.6) | |
| Diarrhea | 34 (61.8) | EOS (× 109/L)2 | 1.16 [0.67, 3.91] | Descending part | 29 (29/54, 55.6) |
| Nausea | 29 (52.7) | Hb (g/L)1 | 138.36 ± 14.81 | Hyperaemia | 31 (31/54, 57.4) |
| Vomiting | 26 (47.3) | PLT (× 109/L)1 | 279.29 ± 81.84 | Erosion | 13 (13/54, 24.1) |
| Distension | 27 (49.1) | Alb (g/L)2 | 40.00 [37.00, 42.00] | Ulcer | 5 (5/54, 9.3) |
| GI bleeding | 3 (5.5) | IgE (KU/L)2 | 152.00 [66.55, 335.50] | Therapy | |
| Weight loss | 36 (65.5) | > 60 | 38 (79.2) | Diet therapy | 7 (12.7) |
| Fever | 4 (7.3) | > 300 | 13 (27.1) | Anti-allergic agents | 7 (12.7) |
| Rash | 6 (10.9) | Immunosuppressors | 3 (5.5) | ||
The most commonly affected site of the GI tract under endoscopy was the duodenum (74.1%), which mainly presented as hyperemia (57.4%), erosion (24.1%) and ulceration (9.3%). The descending duodenum (55.6%) was more commonly involved than the duodenal bulb (50.0%) and retrobulbar duodenum (29.6%). In upper GI tissue biopsies, more than 20 eosinophils/HPF was considered to represent significant infiltration. In our cohort, 14 patients (25.5%) showed eosinophil infiltration in the stomach, and 30 (54.5%) showed eosinophil infiltration in the duodenum. Eight patients had a normal appearance on endoscopy examinations. Another important diagnostic procedure was the eosinophil count in ascites. Of the 17 patients complicated with ascites, 13 had accepted abdominocentesis and 10 (76.9%) of them had elevated eosinophils in ascites. However, three patients with elevated eosinophils in ascites had no elevated eosinophil infiltration in the gastric and duodenal biopsies, suggesting the need for increased ascites eosinophil counts to be included in the diagnostic criteria.
Twenty-eight patients (50.9%) were categorized into the mucosal type, nine (16.4%) into the muscular type and 18 (32.7%) into the serosal type based on the Klein classification.
Forty-three patients (78.2%) were treated with glucocorticoids, and all showed a clinical response. Glucocorticoids were used for the initial therapy in 35 patients (81.4%), while the remaining eight initiated glucocorticoids during follow-up. Other treatments included dietary therapy (12.7%), antiallergic drugs (13.2%) and immunosuppressants (5.5%). Patients receiving glucocorticoid treatment, compared with those who did not, had a significantly higher proportion with elevated IgE (86.8% vs 50.0%, P = 0.022) at diagnosis and were more likely to have descending duodenal involvement (62.8% vs 27.3%, P = 0.046) (Table 2, Supplementary Table 1). There were no significant differences observed between the two groups with regards to sex, disease duration, symptoms, Klein classification or the degree of eosinophil elevation and infiltration. Twenty-one patients (48.8%) were glucocorticoid dependent and had a significantly lower BMI (21.85 vs 24.51, P = 0.041). Additionally, duodenal ulcers were more frequently noted by endoscopy in glucocorticoid-dependent patients (19.0% vs 0.0%, P = 0.048).
| Non glucocorticoid treatment (n = 12) | Glucocorticoid treatment (n = 43) | P value | Non-glucocorticoid dependent (n = 22) | Glucocorticoid dependent (n = 21) | P value | |
| Sex (female) | 8 (66.7) | 23 (53.5) | 0.519 | 10 (45.5) | 13 (61.9) | 0.364 |
| Age (yr)2 | 44.00 [27.75, 48.75] | 36.00 [25.00, 50.00] | 0.838 | 36.00 [26.00, 53.25] | 36.00 [25.00, 48.00] | 0.780 |
| BMI (kg/m2)1 | 23.43 ± 2.84 | 23.24 (4.25) | 0.888 | 24.51 (4.55) | 21.85 (3.50) | 0.041a |
| Disease duration (mo) | 2.00 [1.00, 10.00] | 7.00 [1.00, 41.00] | 0.223 | 3.00 [1.00, 15.75] | 11.00 [4.00, 60.00] | 0.056b |
| Allergic history | 2 (16.7) | 16 (37.2) | 0.298 | 8 (36.4) | 8 (38.1) | 1.000 |
| Symptoms | ||||||
| Abdominal pain | 11 (91.7) | 38 (88.4) | 1.000 | 19 (86.4) | 19 (90.5) | 1.000 |
| Diarrhea | 8 (66.7) | 26 (60.5) | 0.750 | 12 (54.5) | 14 (66.7) | 0.537 |
| Vomiting | 3 (25.0) | 23 (53.5) | 0.108 | 12 (54.5) | 11 (52.4) | 1.000 |
| Weight loss | 10 (83.3) | 26 (60.5) | 0.183 | 14 (63.6) | 12 (57.1) | 0.760 |
| Hp infection | 5 (5/11, 45.5) | 11 (11/39, 28.2) | 0.297 | 6 (6/20, 30.0) | 5 (5/19, 26.3) | 1.000 |
| Klein classification | ||||||
| Mucosal type | 6 (50.0) | 22 (51.2) | 1.000 | 11 (50.0) | 11 (52.4) | 0.298 |
| Muscular type | 2 (16.7) | 7 (16.3) | 2 (9.1) | 5 (23.8) | ||
| Serosal type | 4 (33.3) | 14 (32.6) | 9 (40.9) | 5 (23.8) | ||
| EOS (× 109/L)2 | 0.76 [0.51, 2.32] | 1.48 [0.95, 4.00] | 0.124 | 2.46 [1.13, 3.96] | 1.01 [0.48, 3.99] | 0.065b |
| IgE (KU/L)2 | 54.50 [23.77, 629.75] | 166.00 [72.90, 298.00] | 0.406 | 234.00 [118.00, 343.25] | 118.00 [67.10,191.00] | 0.082b |
| Elevated IgE (> 60) | 5 (5/10, 50.0) | 33 (33/36, 86.8) | 0.022a | 17 (17/20, 85.0) | 16 (16/18, 88.9) | 1.000 |
| Duodenum involvement | 7 (7/11, 63.6) | 33 (33/43, 76.7) | 0.448 | 15 (68.2) | 18 (85.7) | 0.281 |
| Bulb | 5 (5/11, 45.5) | 21 (21/43, 48.8) | 1.000 | 9 (40.9) | 13 (61.9) | 0.227 |
| Post-bulb | 1 (1/11, 9.1) | 15 (15/43, 34.9) | 0.144 | 11 (50.0) | 4 (19.0) | 0.055b |
| Descending part | 3 (3/11, 27.3) | 27 (27/43, 62.8) | 0.046a | 12 (54.5) | 15 (71.4) | 0.347 |
| Duodenum endoscopic features | ||||||
| Hyperaemia | 6 (6/11, 54.5) | 25 (25/43, 58.1) | 1.000 | 14 (63.6) | 11 (52.4) | 0.543 |
| Erosion | 3 (3/11, 27.3) | 10 (10/43, 23.3) | 1.000 | 4 (18.2) | 6 (28.6) | 0.488 |
| Ulcer | 1 (1/11, 9.1) | 4 (4/43, 9.3) | 1.000 | 0 (0.0) | 4 (19.0) | 0.048a |
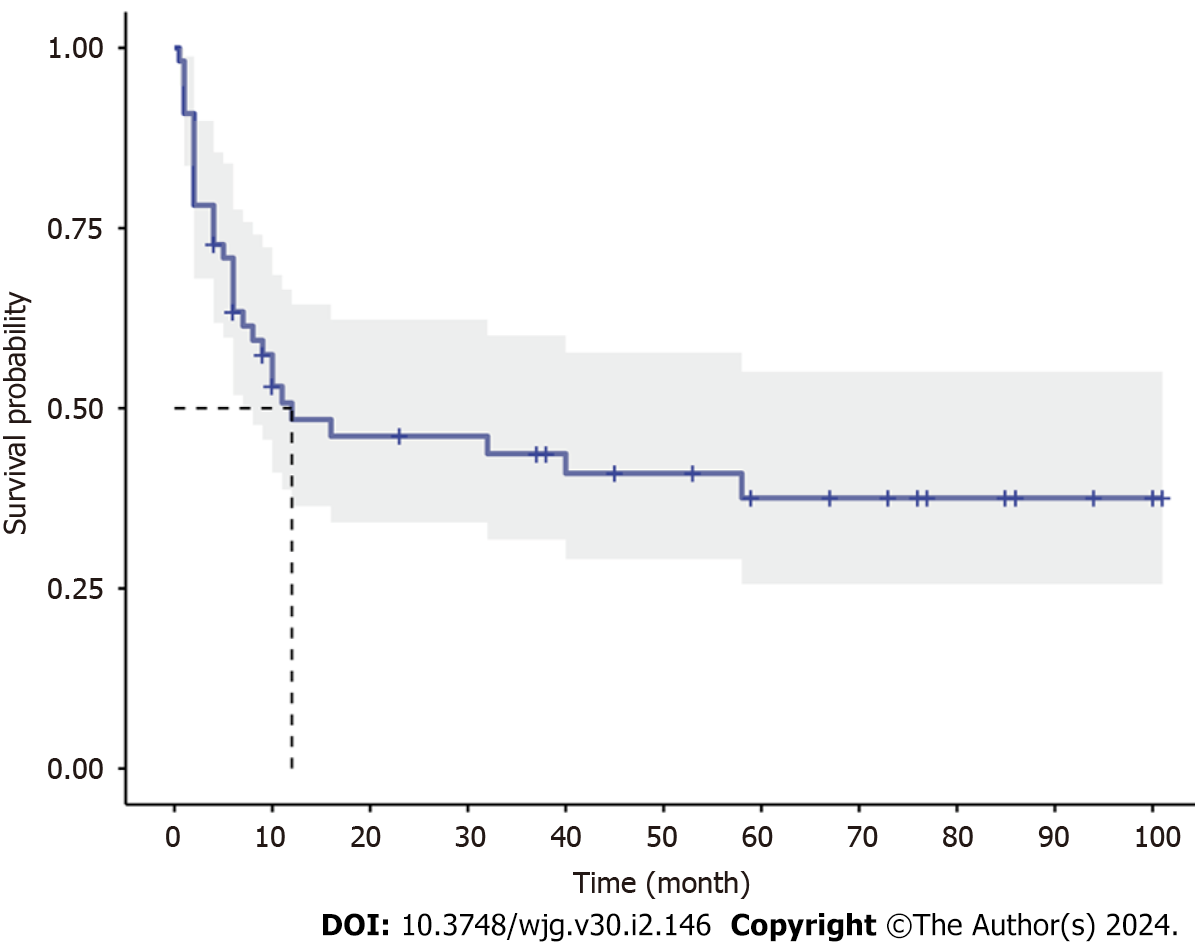
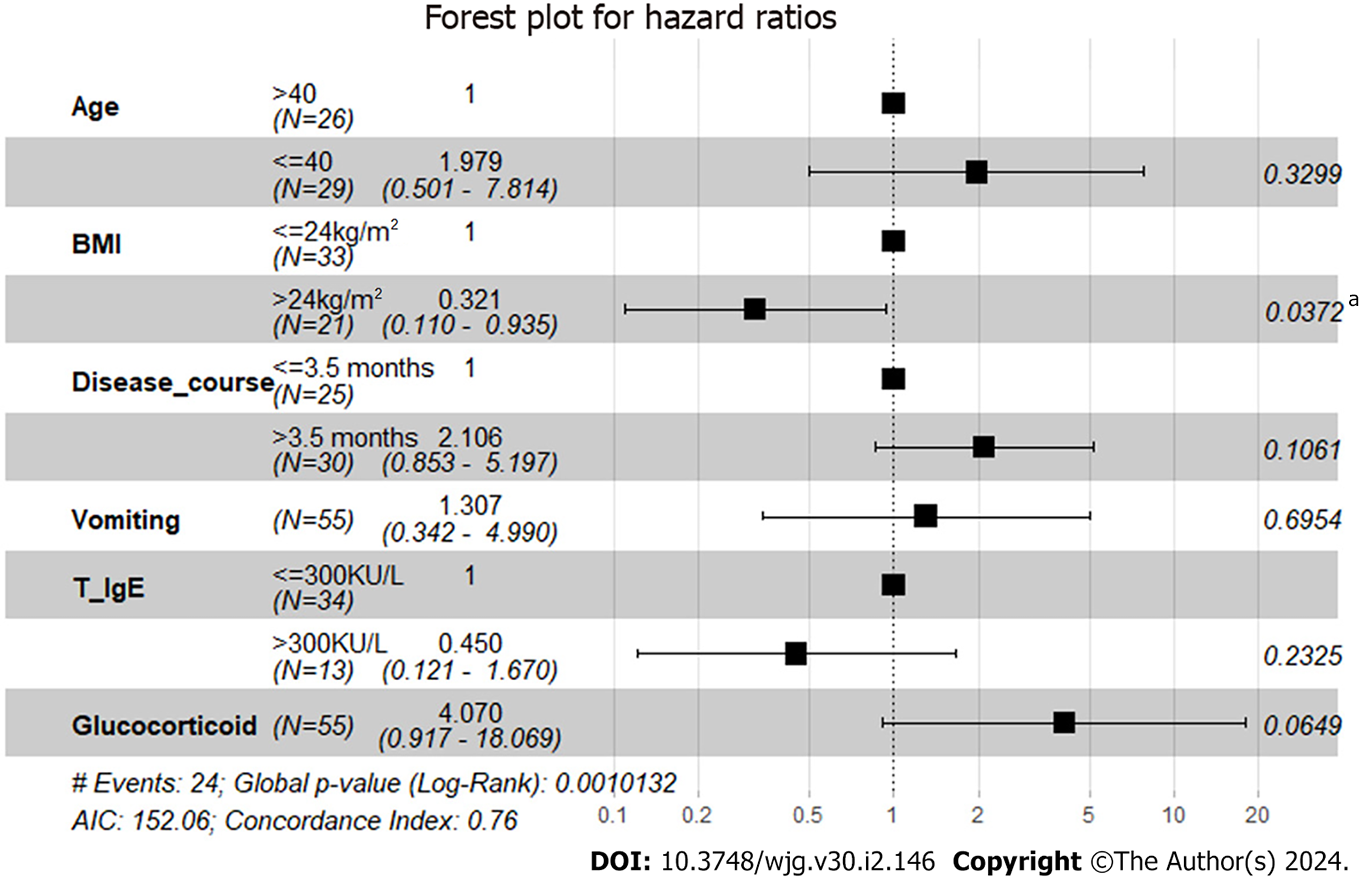
With a median follow-up of 67 (21.5-89.0) mo, all patients survived, and disease relapse after diagnosis was observed in 56.4% (31/55) of all patients and in 67.4% (29/43) of patients who were treated with glucocorticoids at diagnosis. The median RFS was 12 mo in EGE patients, and the incidences of RFS at 6, 12 and 24 mo were 63.4%, 48.4% and 46.1%, respectively (Figure 1). Patients with onset age < 40 years (HR 2.0408, 95%CI: 1.0082-4.1312, P = 0.044), a disease duration from symptom onset to diagnosis > 3.5 mo (HR 2.4725, 95%CI: 1.220-5.0110, P = 0.011), vomiting (HR 3.1259, 95%CI: 1.5246-6.4093, P = 0.001) and glucocorticoid treatment (HR 6.1434, 95%CI: 2.8446-13.2676, P = 0.003) were observed to be more likely to suffer from disease relapse during follow-up, while BMI > 24 kg/m2 (HR 0.3922, 95%CI: 0.1916-0.8027, P = 0.014) and baseline IgE level > 300 KU/L (HR 0.2773, 95%CI: 0.1204-0.6384, P = 0.022) tended to be protective factors for disease relapse (Figure 2). Multivariate analysis showed that higher BMI (HR 0.3206, 95%CI: 0.11-0.9347, P = 0.037) was independently significant for the prediction of less disease relapse (Table 3, Figure 3, Supplementary Table 2).
| Univariate analysis | Multivariate analysis | ||||
| HR (95%CI) | P value | Coef | HR (95%CI) | P value | |
| Age (yr) | |||||
| > 40 | 1 | 0.044a | 1 | 0.330 | |
| ≤ 40 | 2.0408 (1.0082-4.1312) | 0.6827 | 1.9791 (0.5013-7.8140) | ||
| BMI (kg/m2) | |||||
| ≤ 24 | 1 | 0.014a | 1 | 0.037a | |
| > 24 | 0.3922 (0.1916-0.8027) | -1.1375 | 0.3206 (0.11-0.9347) | ||
| Disease duration (mo) | |||||
| ≤ 3.5 | 1 | 0.011a | 1 | 0.106 | |
| > 3.5 | 2.4725 (1.2200-5.0110) | 0.7447 | 2.1058 (0.8533-5.1968) | ||
| Vomiting | |||||
| No | 1 | 0.001a | 1 | 0.695 | |
| Yes | 3.1259 (1.5246-6.4093) | 0.2677 | 1.3069 (0.3423-4.9904) | ||
| T-IgE (KU/L) | |||||
| ≤ 300 | 1 | 0.022a | 1 | 0.233 | |
| > 300 | 0.2773 (0.1204-0.6384) | -0.7995 | 0.4496 (0.121-1.6699) | ||
| Glucocorticoid treatment | |||||
| No | 1 | 0.003a | 1 | 0.065b | |
| Yes | 6.1434 (2.8446-13.2676) | 1.4038 | 4.0705 (0.917-18.0689) | ||
Establishing the long-term prognosis and predictive factors is valuable for clinical practice in EGE patients. This study conducted a median 67-mo follow-up and found that the median RFS was 12 mo, and the rates of RFS were quite high. Age at onset, BMI, disease duration from symptom onset to diagnosis, vomiting symptoms, serum level of IgE, and glucocorticoid treatment helped to predict disease prognosis. Higher BMI was independently significant for the prediction of less disease relapse based on multivariate analysis. These findings could facilitate the categorization of EGE patients into groups with different prognoses.
Consistent with previous studies[2,10], our patients showed a median onset in the third decade of life and a slight predominance in women. The diagnosis of EGE has long been considered difficult. In 2021, the United States Food and Drug Administration indicated a prolonged diagnosis delay of 4-9 years, which was probably due to delays in referral, endoscopic procedures, and the absence of biopsy[11,12]. However, the median duration from symptom onset to diagnosis in our study was only 5 mo. The physician’s knowledge of EGE might have contributed to the earlier diagnosis of EGE in our medical center, which is a tertiary medical center and the national medical center for refractory and rare diseases.
A significant percentage of patients had a history of allergic diseases or positive results in allergen tests, which is also comparable with previous studies from 23.8% to 63%[9,13-15], indicating the association between allergy and EGE pathogenesis. Nonspecific GI symptoms, such as abdominal pain, diarrhea and vomiting, are the main manifestations of EGE. However, in our cohort, weight loss was another dominant symptom, with a frequency of 65.5%. Extraintestinal manifestations such as pancreatitis, cholecystitis or splenic hypofunction[16-18] were not observed in our study. Peripheral eosinophil count elevation was observed in 85.6% of our patients but was neither necessary for diagnosis nor significant for disease severity. The proportion reported varied from 10.9% to 85.7%[9,10,19,20].
Endoscopy is one of the most important measures for EGE diagnosis. As research has described, EGE can affect any part of the GI tract, from the esophagus to the colon and rectum, but the small intestine and antrum are commonly affected[8,14,15]. This also agrees with our observations, and in our cohort, the duodenum was mostly involved. Previous studies reported that 62%-92.2% of patients showed a normal endoscopic appearance due to the patchy distribution of EGE[14,19,21]. In our study, only 14.5% of the patients had normal endoscopies. However, this does not deny the necessity for biopsies because eosinophilic infiltration was also frequently seen in endoscopically normal parts during routine biopsies from the antrum and descending duodenum in our cohort. Meanwhile, the endoscopic features of the stomach were not used for further analysis because > 30% of patients had Helicobacter pylori infection, which resulted in unavoidable bias for the interpretation of the endoscopic findings in the stomach.
In 1990, Talley et al[8] reported a 40-patient cohort in the Mayo Clinic with distributions of 44%, 12% and 39% for the mucosal, muscular and serosal types, respectively, by the Klein classification. In 2010, Chang et al[13] reported 59 new cases in the Mayo Clinic and suggested a shifting trend toward the mucosal type (52/59). However, muscular and serosal types made up almost half of the cohort (16.4% and 32.7%) in our study. Following an inward–outward pathway, EGE is considered to start in the mucosal layer; thus, the serosal type represents a more advanced stage. This may reflect the selection bias of our study in that patients with more severe symptoms were more commonly seen and treated in our center.
By suppressing the transcription of chemokines and eosinophilic growth factors, such as IL-3, IL-5, and granu
Only a few studies have reported the long-term outcomes of EGE, and small sample sizes and short follow-up times limit the conclusions that can be drawn from these studies. Between 42% and 50% of patients had spontaneous remission and remained asymptomatic without specific treatment in previous studies[9,14]. Chambrun et al identified three different courses of EGE after a 15-year follow-up: single flare (42%), recurring course (37%) and continuous course (21%). They found that more patients with the serosal type had a single flare, and predominant mucosal disease presented mostly a continuous course[9]. Sato et al[25] proposed that the chronicity of coexisting allergic disorders may be associated with the chronicity of EGE. However, in our study, the Klein classification and history of allergic diseases did not present a significant difference for disease relapse or glucocorticoid dependence. In 2021, Havlichek et al[10] divided patients into two groups by four clinical characteristics at diagnosis: weight loss, hypoalbuminemia, serosa involvement, and anemia. Patients in the severe group, who had one of the four characteristics, had a higher risk of chronic disease. However, we did not observe these characteristics to be significant prognostic factors for EGE relapse, probably because they grouped patients with preidentified risk factors and then validated them, while we directly filtered baseline variables by log-rank tests and had a longer follow-up time. Grandinetti et al[26] also reported that an increasing number of involved GI segments was associated with disease relapse, indicating the importance of multiple biopsies regardless of endoscopic manifestations in suspected EGE patients.
Notably, we identified six possible prognostic factors for disease relapse in EGE. As expected by clinical experience, patients with a younger onset age are more likely to have disease relapse, and a longer delay of diagnosis may contribute to the recurrent course, which emphasizes the importance of early recognition and diagnosis of EGE. In our previous work, we suggested a diagnostic flowchart for patients suspected of having EGE, including several key steps for a quicker and more accurate diagnosis[27]. Vomiting is a newly identified factor for EGE prognosis in our study. It may be a nonspecific GI symptom caused by muscular involvement. Our study indicates that if a patient has vomiting as one of the initial symptoms, he may be at higher risk for disease relapse. Consistent with observations in clinical practice, elevated total serum IgE is a protective prognostic factor for EGE relapse. The cutoff value (300 KU/L) is four times higher than the upper limit of normal (60 KU/L) in our center. IgE is produced by activated B cells and binds to the Fcε receptor I (FcεRI) on eosinophils and mast cells, inducing their degranulation. It can be hypothesized that in patients with a markedly elevated baseline IgE level, their FcεRIs on eosinophils and mast cells may be saturated and thus unable to function upon re-exposure to allergens. Glucocorticoid treatment is also a significant factor for disease relapse. Patients treated with glucocorticoids had a greater possibility of at least one relapse during our follow-up, which might be partially explained by their own greater disease burden with more involvement of the descending duodenum compared with patients without glucocorticoid treatment. The exact clinical value or pathogenesis needs to be validated and clarified in future studies.
For multivariate analysis, higher BMI was an independent protective factor for disease relapse in our study. However, from the survival analysis, we noticed that patients without weight loss were more likely to have a recurrent course, although the P value was not significant (0.066). This seems contradictory. Given the large proportion of patients with serosal involvement in our cohort, the measurement of BMI might have been affected by the large amount of ascites. While the effect of obesity on EGE has not been previously studied, several studies have pointed out that lower BMI in patients with eosinophilic esophagitis (EoE) might indicate more severe disease[28,29]. In addition to the possible association with EoE-induced dysphagia and thus malnutrition, studies found that this may be associated with tumor necrosis factor-α release from adipose tissue, which promotes a shifting trend from Th-2 to Th-1 responses[30]. The exact clinical value of obesity in EGE still needs further investigation.
Beyond the abovementioned limitations, there were some other limitations in our study. First, the findings were based on 55 patients from one medical center, which makes generalization difficult. Second, selection bias was also unavoidable because the patients were all hospitalized in one medical center. For example, there was a higher percentage of serosal type in this study. Third, the knowledge and clinical experience among different physicians during the long study period were variable, which directly affected the treatment decisions. Finally, the definition of disease relapse was mainly based on symptoms and did not include endoscopic and histological diagnostic criteria, which might have overestimated the relapse rate. Future prospective multicenter studies of EGE with confirmed and validated criteria of disease relapse should be conducted to fully address the clinical manifestations and long-term prognosis.
We have described the general clinical characteristics of Chinese EGE patients and their long-term outcomes and identified six baseline clinical variables that could predict prognosis.
Eosinophilic gastroenteritis (EGE) is a rare, chronic and recurrent disease with abnormal eosinophilic infiltration in gastrointestinal tract. Disease relapse and glucocorticoid dependence remain notable problems.
Few studies had illuminated the prognosis of EGE and risk factors for disease relapse. By exploring prognostic factors by baseline clinical data, we may identify patients who are more vulnerable for disease relapse at diagnosis and offer them individualized treatment.
To describe the clinical characteristics of EGE and possible predictive factors for disease relapse based on long-term follow-up.
This retrospective cohort study enrolled 55 EGE patients (2013-2022, Peking Union Medical College Hospital) and analyzed their clinical records. Kaplan-Meier analysis, Log-Rank test and Cox regression analysis were conducted to reveal the risk factors for long-term prognosis.
In our cohort, EGE showed a female predominance (56.4%) and its median onset age was 38 years old. Abdominal pain (89.1%) was most commonly seen. 78.2% of patients received glucocorticoid treatment in whom elevated serum immunoglobin E (IgE) at diagnosis (86.8% vs 50.0%, P = 0.022) and descending duodenum involvement (62.8% vs 27.3%, P = 0.046) were more frequently seen. The median follow-up time was 67 mo, during which 31 patients (56.4%) suffered from at least one flares of disease relapse. Six prognostic factors were figured out, including age at diagnosis < 40 years (hazard ratio (HR) 2.0408, 95% confidence interval (CI): 1.0082-4.1312, P = 0.044), body mass index > 24 kg/m2 (HR 0.3922, 95%CI: 0.1916-0.8027, P = 0.014), disease duration from symptom onset to diagnosis > 3.5 mo (HR 2.4725, 95%CI: 1.220-5.0110, P = 0.011), vomiting (HR 3.1259, 95%CI: 1.5246-6.4093, P = 0.001), total serum IgE > 300 KU/L at diagnosis (HR 0.2773, 95%CI: 0.1204-0.6384, P = 0.022) and glucocorticoid treatment (HR 6.1434, 95%CI: 2.8446-13.2676, P = 0.003).
We identified younger onset age, longer disease course, vomiting and glucocorticoid treatment to be risk factors for disease relapse, whereas higher body mass index and total-IgE level at baseline to be protective.
In future research, we tend to conduct multi-center prospective study to verify and improve our prognostic model and further explore more accurate biomarkers for disease relapse based on serum proteomics and intestinal microbiomes.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Caboclo JLF, Brazil S-Editor: Qu XL L-Editor: A P-Editor: Yu HG
| 1. | Klein NC, Hargrove RL, Sleisenger MH, Jeffries GH. Eosinophilic gastroenteritis. Medicine (Baltimore). 1970;49:299-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 434] [Cited by in RCA: 407] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 2. | Mansoor E, Saleh MA, Cooper GS. Prevalence of Eosinophilic Gastroenteritis and Colitis in a Population-Based Study, From 2012 to 2017. Clin Gastroenterol Hepatol. 2017;15:1733-1741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 146] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 3. | Jensen ET, Martin CF, Kappelman MD, Dellon ES. Prevalence of Eosinophilic Gastritis, Gastroenteritis, and Colitis: Estimates From a National Administrative Database. J Pediatr Gastroenterol Nutr. 2016;62:36-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 227] [Article Influence: 25.2] [Reference Citation Analysis (1)] |
| 4. | Ito J, Fujiwara T, Kojima R, Nomura I. Racial differences in eosinophilic gastrointestinal disorders among Caucasian and Asian. Allergol Int. 2015;64:253-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 5. | Hogan SP, Rosenberg HF, Moqbel R, Phipps S, Foster PS, Lacy P, Kay AB, Rothenberg ME. Eosinophils: biological properties and role in health and disease. Clin Exp Allergy. 2008;38:709-750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 551] [Cited by in RCA: 573] [Article Influence: 33.7] [Reference Citation Analysis (0)] |
| 6. | Stone KD, Prussin C. Immunomodulatory therapy of eosinophil-associated gastrointestinal diseases. Clin Exp Allergy. 2008;38:1858-1865. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 34] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 7. | Rothenberg ME. Eosinophilia. N Engl J Med. 1998;338:1592-1600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 721] [Cited by in RCA: 714] [Article Influence: 26.4] [Reference Citation Analysis (0)] |
| 8. | Talley NJ, Shorter RG, Phillips SF, Zinsmeister AR. Eosinophilic gastroenteritis: a clinicopathological study of patients with disease of the mucosa, muscle layer, and subserosal tissues. Gut. 1990;31:54-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 506] [Cited by in RCA: 514] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 9. | Pineton de Chambrun G, Gonzalez F, Canva JY, Gonzalez S, Houssin L, Desreumaux P, Cortot A, Colombel JF. Natural history of eosinophilic gastroenteritis. Clin Gastroenterol Hepatol. 2011;9:950-956.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 133] [Article Influence: 9.5] [Reference Citation Analysis (1)] |
| 10. | Havlichek D 3rd, Choung RS, Murray JA. Eosinophilic Gastroenteritis: Using Presenting Findings to Predict Disease Course. Clin Transl Gastroenterol. 2021;12:e00394. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 11. | Rothenberg ME, Hottinger SKB, Gonsalves N, Furuta GT, Collins MH, Talley NJ, Peterson K, Menard-Katcher C, Smith M, Hirano I, Genta RM, Chehade M, Gupta SK, Spergel JM, Aceves SS, Dellon ES. Impressions and aspirations from the FDA GREAT VI Workshop on Eosinophilic Gastrointestinal Disorders Beyond Eosinophilic Esophagitis and Perspectives for Progress in the Field. J Allergy Clin Immunol. 2022;149:844-853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 17] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 12. | Chehade M, Kamboj AP, Atkins D, Gehman LT. Diagnostic Delay in Patients with Eosinophilic Gastritis and/or Duodenitis: A Population-Based Study. J Allergy Clin Immunol Pract. 2021;9:2050-2059.e20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 47] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 13. | Chang JY, Choung RS, Lee RM, Locke GR 3rd, Schleck CD, Zinsmeister AR, Smyrk TC, Talley NJ. A shift in the clinical spectrum of eosinophilic gastroenteritis toward the mucosal disease type. Clin Gastroenterol Hepatol. 2010;8:669-75; quiz e88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 91] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 14. | Wong GW, Lim KH, Wan WK, Low SC, Kong SC. Eosinophilic gastroenteritis: Clinical profiles and treatment outcomes, a retrospective study of 18 adult patients in a Singapore Tertiary Hospital. Med J Malaysia. 2015;70:232-237. [PubMed] |
| 15. | Zhang L, Duan L, Ding S, Lu J, Jin Z, Cui R, McNutt M, Wang A. Eosinophilic gastroenteritis: clinical manifestations and morphological characteristics, a retrospective study of 42 patients. Scand J Gastroenterol. 2011;46:1074-1080. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 69] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 16. | Di Sabatino A, Aronico N, Giuffrida P, Cococcia S, Lenti MV, Vanoli A, Guerci M, Di Stefano M, Corazza GR. Association between defective spleen function and primary eosinophilic gastrointestinal disorders. J Allergy Clin Immunol Pract. 2018;6:1056-1058.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 17. | Hui CK. Eosinophilic gastroenteritis complicating acute calculus eosinophilic cholecystitis 6 months after cholecystectomy. J Dig Dis. 2018;19:693-695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 18. | Sheikh RA, Prindiville TP, Pecha RE, Ruebner BH. Unusual presentations of eosinophilic gastroenteritis: case series and review of literature. World J Gastroenterol. 2009;15:2156-2161. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 45] [Cited by in RCA: 54] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 19. | Hui CK, Hui NK. A Prospective Study on the Prevalence, Extent of Disease and Outcome of Eosinophilic Gastroenteritis in Patients Presenting with Lower Abdominal Symptoms. Gut Liver. 2018;12:288-296. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 20. | Yang XM, He SQ, Yang H, Zheng HH, Zhu LH, Zhou SK, Zhang Y. Clinical features and treatment outcomes of eosinophilic gastroenteritis : an analysis of 28 cases. Acta Gastroenterol Belg. 2019;82:5-10. [PubMed] |
| 21. | Pesek RD, Reed CC, Collins MH, Muir AB, Fulkerson PC, Menard-Katcher C, Falk GW, Kuhl J, Magier AZ, Ahmed FN, Demarshall M, Gupta A, Gross J, Ashorobi T, Carpenter CL, Krischer JP, Gonsalves N, Hirano I, Spergel JM, Gupta SK, Furuta GT, Rothenberg ME, Dellon ES; Consortium of Eosinophilic Gastrointestinal Disease Researchers (CEGIR). Association Between Endoscopic and Histologic Findings in a Multicenter Retrospective Cohort of Patients with Non-esophageal Eosinophilic Gastrointestinal Disorders. Dig Dis Sci. 2020;65:2024-2035. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 50] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 22. | Dellon ES, Peterson KA, Murray JA, Falk GW, Gonsalves N, Chehade M, Genta RM, Leung J, Khoury P, Klion AD, Hazan S, Vaezi M, Bledsoe AC, Durrani SR, Wang C, Shaw C, Chang AT, Singh B, Kamboj AP, Rasmussen HS, Rothenberg ME, Hirano I. Anti-Siglec-8 Antibody for Eosinophilic Gastritis and Duodenitis. N Engl J Med. 2020;383:1624-1634. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 222] [Cited by in RCA: 199] [Article Influence: 39.8] [Reference Citation Analysis (0)] |
| 23. | Assa'ad AH, Gupta SK, Collins MH, Thomson M, Heath AT, Smith DA, Perschy TL, Jurgensen CH, Ortega HG, Aceves SS. An antibody against IL-5 reduces numbers of esophageal intraepithelial eosinophils in children with eosinophilic esophagitis. Gastroenterology. 2011;141:1593-1604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 291] [Cited by in RCA: 323] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 24. | Foroughi S, Foster B, Kim N, Bernardino LB, Scott LM, Hamilton RG, Metcalfe DD, Mannon PJ, Prussin C. Anti-IgE treatment of eosinophil-associated gastrointestinal disorders. J Allergy Clin Immunol. 2007;120:594-601. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 176] [Cited by in RCA: 159] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 25. | Sato H, Honma T, Owaki T, Tominaga K, Yokoyama J, Terai S. Clinical and pathological profile of eosinophilic gastroenteritis. Eur J Gastroenterol Hepatol. 2019;31:157-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 26. | Grandinetti T, Biedermann L, Bussmann C, Straumann A, Hruz P. Eosinophilic Gastroenteritis: Clinical Manifestation, Natural Course, and Evaluation of Treatment with Corticosteroids and Vedolizumab. Dig Dis Sci. 2019;64:2231-2241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 59] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 27. | Li K, Ruan G, Liu S, Xu T, Guan K, Li J. Eosinophilic gastroenteritis: Pathogenesis, diagnosis, and treatment. Chin Med J (Engl). 2023;136:899-909. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 28. | Trovato A, Tsang T, Manem N, Donovan K, Gemoets DE, Ashley C, Dellon ES, Tadros M. The Impact of Obesity on the Fibrostenosis Progression of Eosinophilic Esophagitis in a U.S. Veterans Cohort. Dysphagia. 2023;38:866-873. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 29. | Wolf WA, Piazza NA, Gebhart JH, Rusin S, Covey S, Higgins LL, Beitia R, Speck O, Woodward K, Cotton CC, Runge TM, Eluri S, Woosley JT, Shaheen NJ, Dellon ES. Association Between Body Mass Index and Clinical and Endoscopic Features of Eosinophilic Esophagitis. Dig Dis Sci. 2017;62:143-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 30. | McLaughlin T, Ackerman SE, Shen L, Engleman E. Role of innate and adaptive immunity in obesity-associated metabolic disease. J Clin Invest. 2017;127:5-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 322] [Article Influence: 40.3] [Reference Citation Analysis (0)] |