Published online May 21, 2024. doi: 10.3748/wjg.v30.i19.2603
Revised: April 18, 2024
Accepted: April 22, 2024
Published online: May 21, 2024
Processing time: 117 Days and 20.1 Hours
The gut microbiota is strongly associated with radiation-induced gut damage. This study aimed to assess the effectiveness and safety of intestinal microecological transplantation for treating patients with chronic radiation enteritis.
A 64-year-old female with cervical cancer developed abdominal pain, diarrhea, and blood in the stool 1 year after radiotherapy. An electronic colonoscopy was performed to diagnose chronic radiation enteritis. Two courses of intestinal micro
Intestinal microecological transplantation is an effective treatment for relieving the clinical symptoms of chronic radiation enteritis by altering the composition of the intestinal flora. This study provides a new approach for treating patients with chronic radiation enteritis.
Core Tip: This study explores the efficacy of fecal microbiota transplantation (FMT) in a patient with chronic radiation enteritis. A 64-year-old patient experienced significant symptom relief and long-term remission after FMT. Microbial analysis revealed beneficial shifts in gut flora composition. This highlights FMT as a promising intervention for managing radiation-induced gastrointestinal complications, offering short-term relief and sustained benefits. Targeting intestinal microbiota presents a novel approach to improving patient outcomes and quality of life in chronic radiation enteritis, showing a potential paradigm shift in treatment strategies.
- Citation: Wang L, Li Y, Zhang YJ, Peng LH. Intestinal microecological transplantation for a patient with chronic radiation enteritis: A case report. World J Gastroenterol 2024; 30(19): 2603-2611
- URL: https://www.wjgnet.com/1007-9327/full/v30/i19/2603.htm
- DOI: https://dx.doi.org/10.3748/wjg.v30.i19.2603
Radiation enteritis is an intestinal complication caused by radiotherapy for pelvic, abdominal, and retroperitoneal malignancies affecting the small intestine, colon, and rectum. Radiation enteritis is divided into acute and chronic according to onset: acute enteritis may occur immediately or within 3 months after radiotherapy, and chronic enteritis may occur after 9–14 months, approximately 30 years, affecting the quality of life of patients[1]. The American Society of Colorectal Surgeons suggests treatments for chronic radiation enteritis, which mainly include formalin, aluminum thioglycollate, argon beam plasma coagulation, hyperbaric oxygen therapy, surgery, and other symptomatic treatments with limited efficacy[2]. Clinical studies have shown significant changes in the gut flora of patients after irradiation, such as an increase in Clostridium and other unclassified bacteria; a significant decrease in Firmicutes and Bacteroidetes; a sig
A 64-year-old female presented with intermittent abdominal pain, diarrhea, and blood in the stool that had been occurring for 1 year.
On April 9, 2020, the patient was diagnosed with cervical squamous cell carcinoma. Radiation therapy was commenced on April 27 with a 5000 cGy dose for external radiation therapy and four intermittent sessions of three-dimensional intracavitary radiotherapy between June 15 and June 29 with a 700 cGy dose. The patient experienced no discomfort during radiotherapy. In May 2021, the patient developed abdominal and colic pains. The patient had diluted watery bowel movements 7–8 times a day. The patient self-administered antidiarrheal drugs but did not experience significant relief. In June, the patient reported intermittent blood in her stool with a volume of 100 mL per occurrence. She visited a local hospital, where an electronic colonoscopy revealed rectal and sigmoid colonies. The medical history suggested chronic radiation enteritis, and mesalazine-soluble tablets and compound glutamine-soluble capsules were prescribed for symptomatic treatment. After receiving mesalazine enteric-coated tablets and compound glutamine enteric-coated capsules for symptomatic treatment, abdominal pain, diarrhea, and blood in the stool persisted, significantly affecting the patient's quality of life. The patient was admitted to our hospital for further treatment.
The patient has no previous medical history.
The patient and family histories were negative.
The abdomen exhibited tenderness in the mid and lower regions, with no other positive signs.
The patient's laboratory tests were within the normal range, including tests for blood routine, liver and kidney function, electrolytes, and tumor markers.
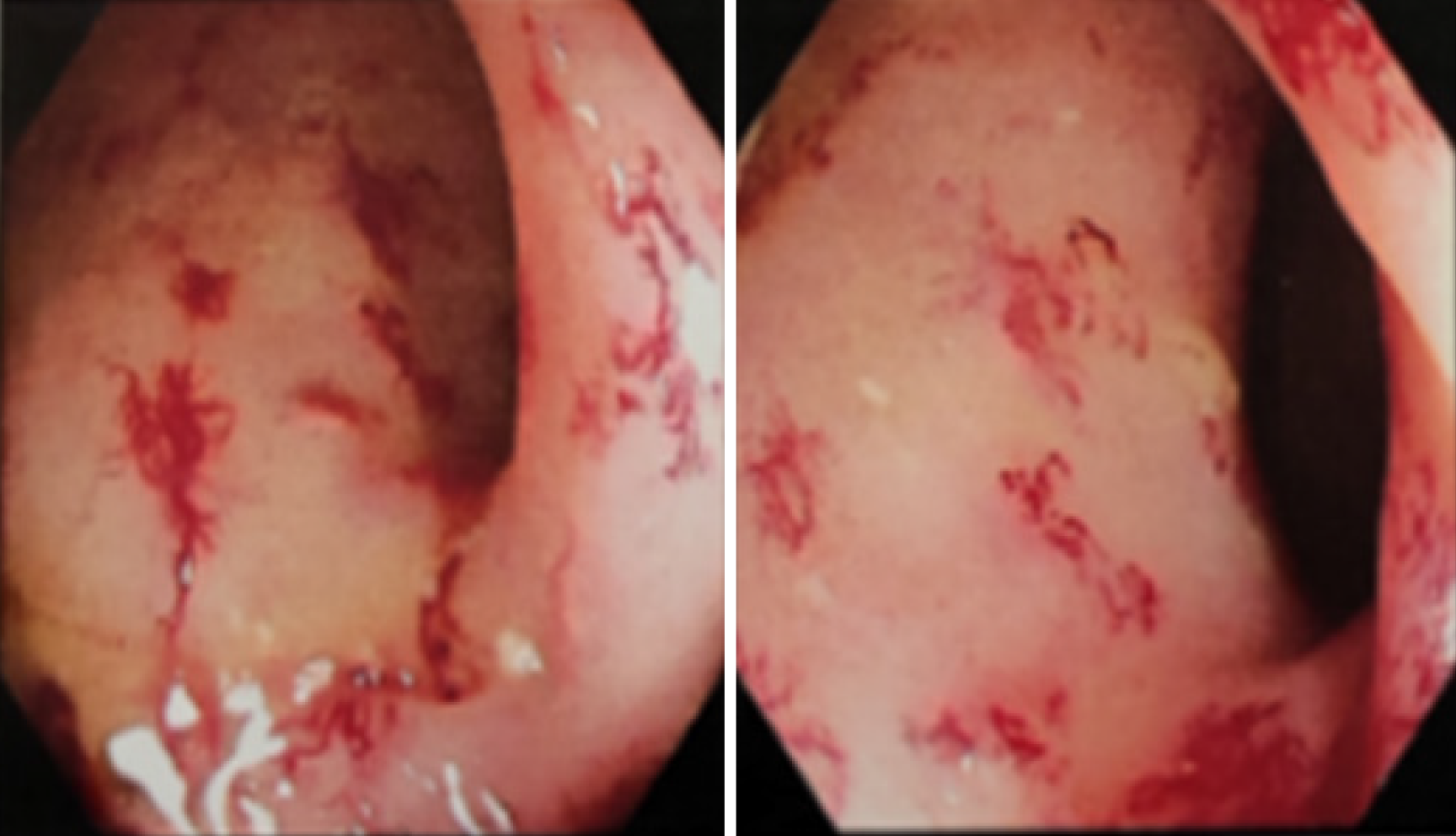
An electronic colonoscopy revealed diffuse congestion and edema of the mucosa 20 cm below the anus, multiple congestive spots, disappearance of the mucosal vascular texture, and multiple capillary dilatations. The patient was diagnosed with chronic radial enterocolitis (Figure 1).
The patient's medical history, clinical manifestations, and colonoscopy results were collectively utilized to facilitate the diagnosis of chronic radiation enteritis.
As the patient had received conventional symptomatic treatment with little success, she was informed about the indications and potential adverse effects of FMT in treating chronic radiation enteritis. Relevant studies and applications were also discussed. The patient agreed to undergo FMT and provided informed consent. The Ethics Committee of the First Medical Center of the General Hospital of the People's Liberation Army approved the use of FMT for the treatment of radiation enteritis (Ethics Approval Number: S2022-300-01).
Donor selection method: The research team conducted research, and a 27-year-old woman with no gastrointestinal symptoms, no history of infectious diseases, or family history was recruited[7,8]. The sample taken from the patient did not contain any of the following: Human immunodeficiency virus; Hepatitis A, B, C, and E; Pathogenic Escherichia coli; Shigella; Salmonella; Clostridium difficile toxin; Epstein-Barr virus; fungi; eggs; or encapsulation. The patient did not use antibiotics, probiotics, or other medications that affected the intestinal flora 4 wk before fecal donation.
The patient received FMT from a healthy participant with mesalazine enteric-coated tablets. The patient underwent two courses of FMT. The first course included a colonoscopic microbial product transplantation of 300 mL on August 16, 2022, an enema microbial product of 200 mL on August 18, and another enema microbial product of 200 mL on August 22. The second course included colonoscopic microbial product transplantation of 300 mL on October 24, 2022, and enema microbial product transplantation of 200 mL on October 26, 2022. A total of 200 mL of the microbiological enema product was administered.
Before and after each treatment and before and during the gut microbial transplantation, stool samples were collected from the patients for full-length 16S rRNA sequencing to examine the microbiota's diversity and composition at each time point. Donor stool samples were obtained.
Following the initial FMT, the patient experienced a significant improvement in diarrhea symptoms. The frequency of bowel movements decreased from 7–8 times per day to 2–3 times per day, blood in the stool was significantly reduced, and abdominal pain was significantly relieved. The patient’s diarrhea and abdominal pain were gradually alleviated after the first FMT course. However, 1 month later, blood in the stool reappeared, and a second course of transplantation was performed. At the end of the second course, the volume and frequency of blood in the stool decreased again, and remission was prolonged. The patient reported no diarrhea or abdominal pain one year after the second course of treatment. She occasionally noticed small amounts of blood in her stool; however, this did not significantly affect her daily life. The patient did not experience adverse reactions related to the FMT during the process.
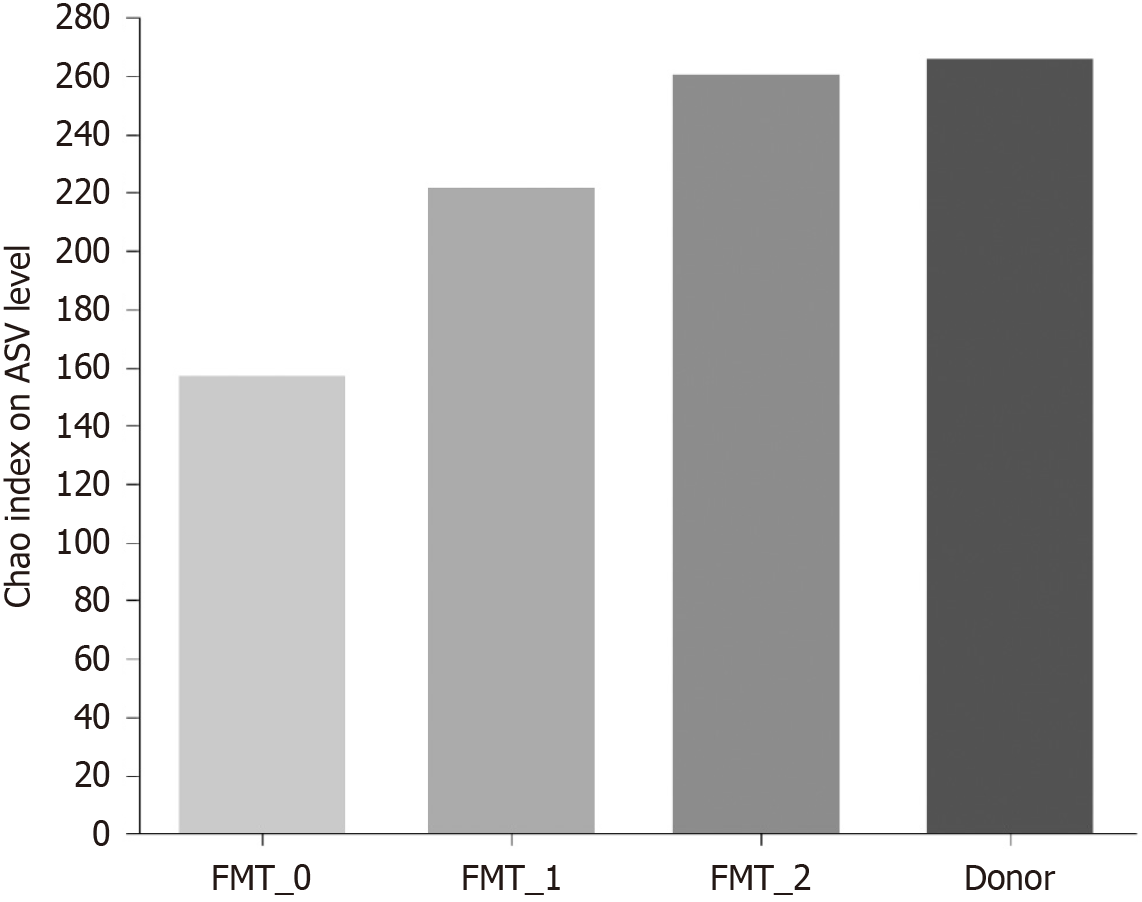
α-diversity and β-diversity of the bacterial flora: The Chao index was used to measure the alpha diversity of the bacterial community in the patients’ fecal specimens at the Amplicon Sequence Variant level (Figure 2). The alpha diversity was lowest before transplantation and increased significantly with the number of intestinal microecological transplants, similar to that of the donor.
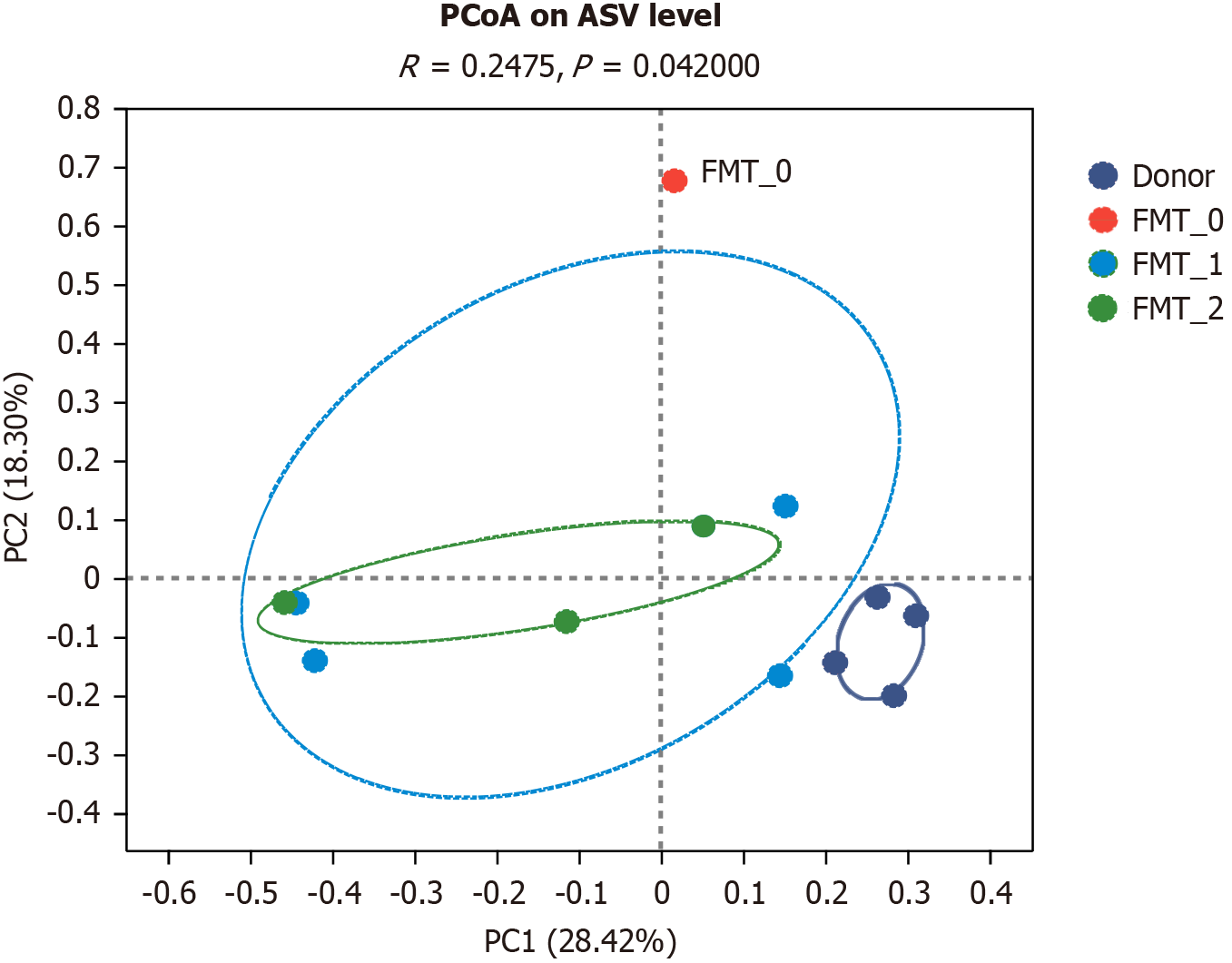
A study based on the Bray-Curtis principal coordinates analysis revealed that the donor, gut microbiota transplan
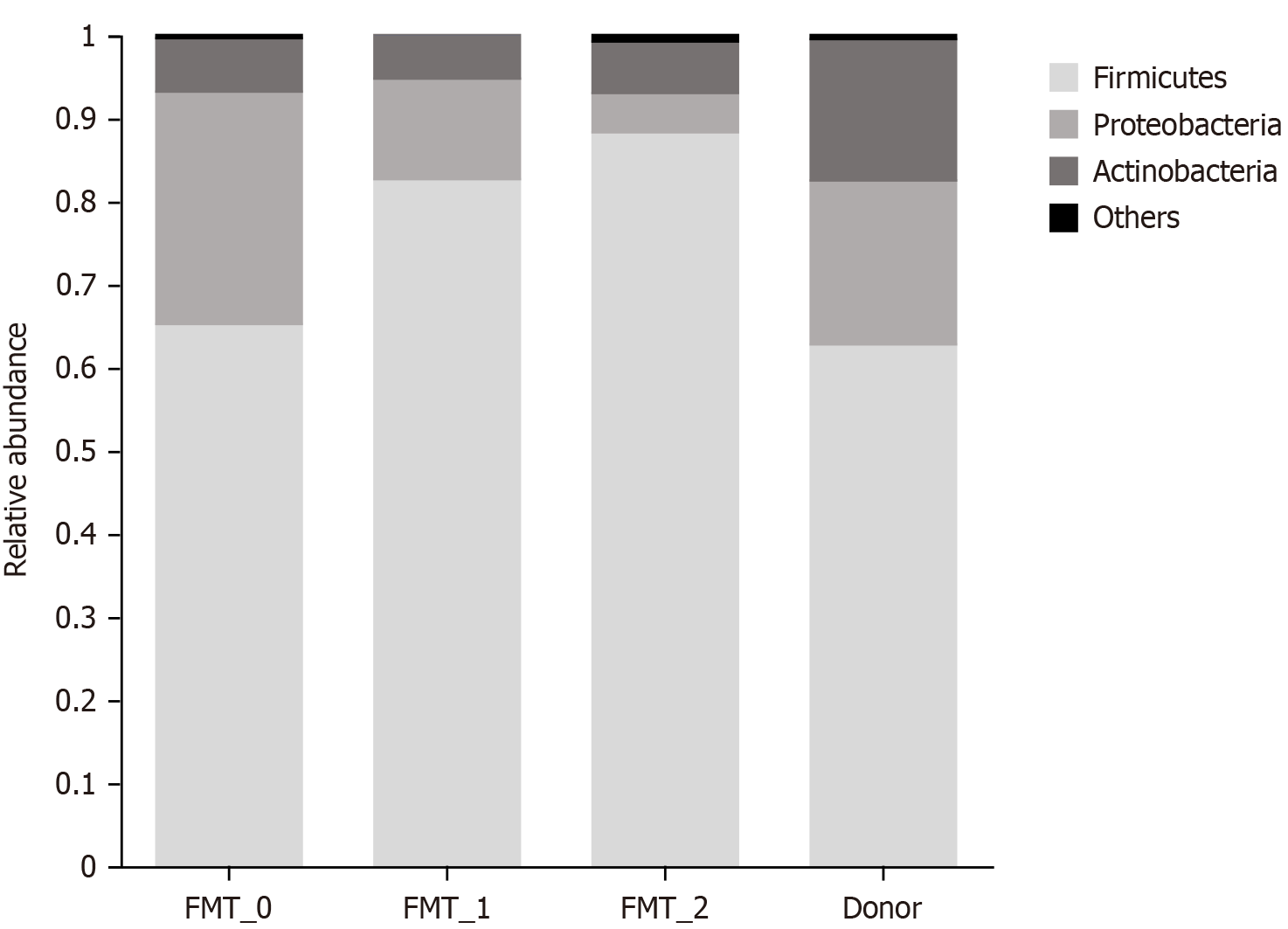
Comparison of dominant flora composition: Phylum levels: At the phylum level, the patient's gut microbiota contained fewer thick-walled phyla and more metaplastic phyla than the donor’s fecal composition before transplantation. How
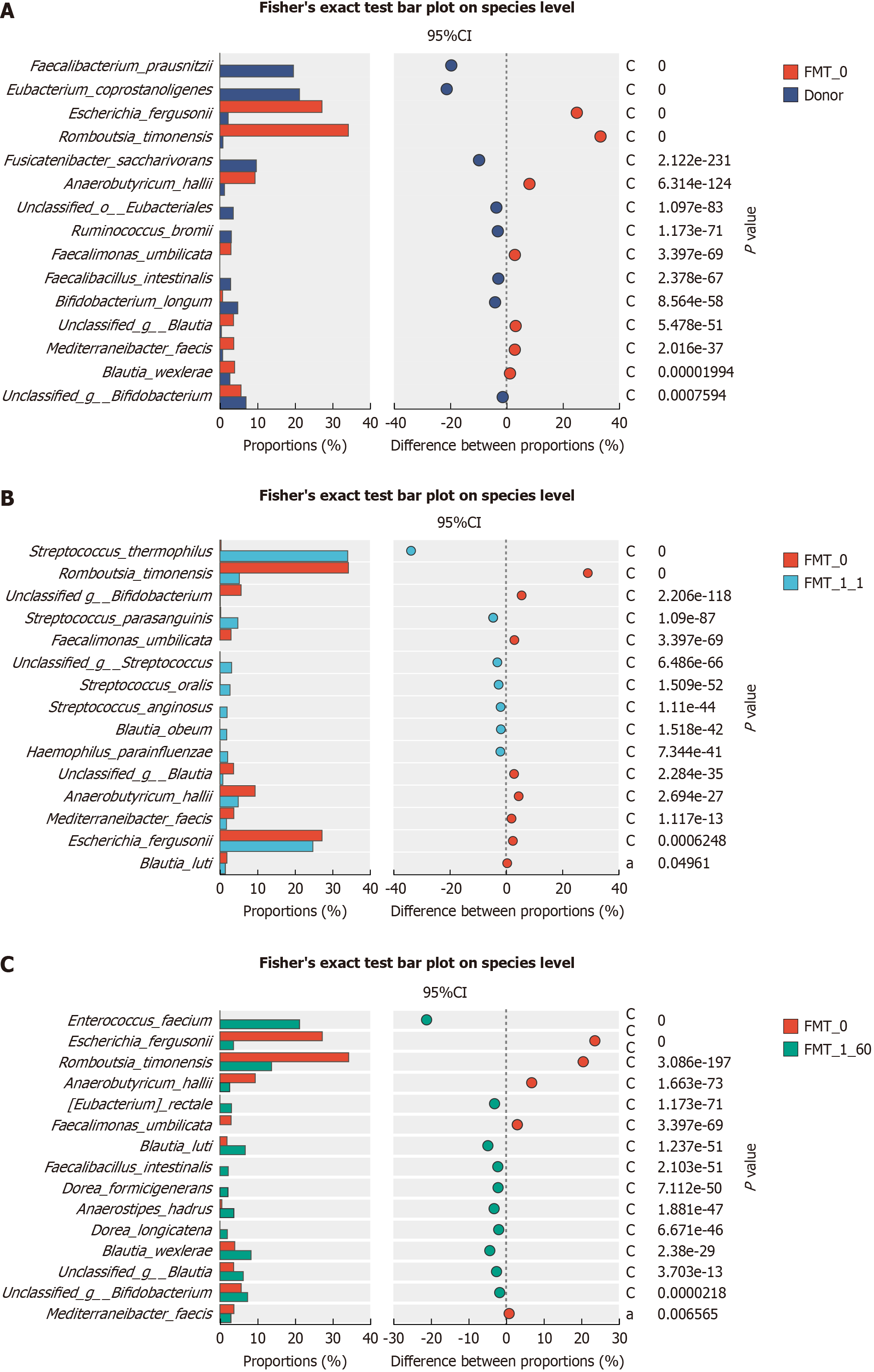
Species levels: Fisher’s exact test was used to assess the variability in the dominant flora. Some bacteria had a signi
Bacteria that increased 2 months after the first course of transplantation of the patient's gut microorganisms compared with the gut microorganisms before transplantation included Enterococcus faecium, Eubacterium rectale, Blautia luti, Fae
This case demonstrates that intestinal microbial transplantation provides significant relief in the short- and long-term remission of chronic radiculitis enterica. The patient experienced significant improvement in symptoms such as abdominal pain, diarrhea, and blood in the stool after the first course of intestinal microbial transplantation. Two months after the initial intestinal microbial transplantation, the patient's abdominal pain and diarrhea were alleviated. However, the patient had a small amount of blood in the stool, which disappeared shortly after the second course of intestinal microbial transplantation. One year after the second course, the patient's abdominal pain and diarrhea remained in remission, with occasional small amounts of blood in the stool. However, this did not affect the patient's quality of life.
Radiation enteritis development is linked to alterations in the composition of intestinal flora. A study of patients who underwent pelvic radiotherapy found that the number of Clostridium difficile and other unclassified bacteria increased, while the numbers of Firmicutes and Bacteroides bacteria significantly decreased[3]. Analysis of the fecal flora in this study showed similar results: before intestinal microbial transplantation, the diversity of the fecal flora was significantly lower than that of the healthy individual, with decreased Phylum Firmicutes and increased Proteobacteria. After intestinal micro
Following intestinal microcosm transplantation, the pathogenic bacteria Escherichia fergusonii and Romboutsia timonensis exhibited a significant decrease, which persisted at low levels even after 2 months. Conversely, the probiotic abundance significantly increased, with Streptococcus thermophilus and parasanguinis showing significant increases shortly after transplantation. Streptococcus thermophilus is a probiotic bacterium recognized for its antioxidant properties, which reduce the risk of some cancers and its anti-inflammatory, anti-mutagenic, and stimulatory effects on the intestinal immune system[19]. Streptococcus parasanguinis is a major colonizer of the intestinal tract of newborn infants and bacterial species in the adult small intestine. Streptococcus parasanguinis can moderately activate NFκB through TLR2/6 signaling, inducing the maturation, activation, and secretion of the cytokine IL-12 from human monocyte-derived dendritic cells[20]. This patient experienced significant symptoms shortly after gut flora transplantation, directly related to a decline in path
FMT can effectively treat patients with chronic radiation enteritis by providing short-term relief and long-term symptom remission. Furthermore, this case study elucidated the higher prevalence of pathogenic bacteria and lower prevalence of certain essential intestinal bacteria in patients with chronic radiation enteritis than in healthy individuals, as determined via sequencing three-generation full-length amplicons at the bacterial species level. The intestinal flora’s composition in patients changed significantly after FMT, corresponding to the alleviation of clinical symptoms. This provides further evidence for the effectiveness of FMT in treating patients with chronic radiation enteritis and improving quality of life. One limitation of this study was that the patient did not undergo further transplantation at a later stage for personal reasons. The patient was followed up via telephone for information on clinical symptoms. Based on the results of bacterial flora analysis, additional courses of intestinal microcosm transplantation could provide additional benefits.
In this study, transplanting intestinal microorganisms resulted in relief in the short- and long-term. Sequencing the entire amplicon at the strain level revealed an altered intestinal flora composition in patients with chronic radiation en
FMT is a valuable therapeutic option for chronic radiation enteritis. The significant symptom relief and long-term remission observed post-FMT underscore its efficacy in managing radiation-induced gastrointestinal complications. Microbial analysis further supports FMT's role in restoring a healthier gut flora composition. Based on these findings, we recommend considering FMT as part of the treatment algorithm for chronic radiation enteritis, particularly in cases refractory to conventional therapies. Because people with chronic radiation enteritis have very different symptoms, more research needs to be conducted to find the best way to treat them and how to perform intestinal microcosm trans
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report’s classification
Scientific Quality: Grade C, Grade C
Novelty: Grade A, Grade B
Creativity or Innovation: Grade A, Grade B
Scientific Significance: Grade B, Grade B
P-Reviewer: Rodrigo L, Spain; Snyder AM, United States S-Editor: Lin C L-Editor: A P-Editor: Zheng XM
| 1. | Dahiya DS, Kichloo A, Tuma F, Albosta M, Wani F. Radiation Proctitis and Management Strategies. Clin Endosc. 2022;55:22-32. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 27] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 2. | Paquette IM, Vogel JD, Abbas MA, Feingold DL, Steele SR; Clinical Practice Guidelines Committee of The American Society of Colon and Rectal Surgeons. The American Society of Colon and Rectal Surgeons Clinical Practice Guidelines for the Treatment of Chronic Radiation Proctitis. Dis Colon Rectum. 2018;61:1135-1140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 57] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 3. | Wang L, Wang X, Zhang G, Ma Y, Zhang Q, Li Z, Ran J, Hou X, Geng Y, Yang Z, Feng S, Li C, Zhao X. The impact of pelvic radiotherapy on the gut microbiome and its role in radiation-induced diarrhoea: a systematic review. Radiat Oncol. 2021;16:187. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 4. | Demers M, Dagnault A, Desjardins J. A randomized double-blind controlled trial: impact of probiotics on diarrhea in patients treated with pelvic radiation. Clin Nutr. 2014;33:761-767. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 149] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 5. | Garcia-Peris P, Velasco C, Hernandez M, Lozano MA, Paron L, de la Cuerda C, Breton I, Camblor M, Guarner F. Effect of inulin and fructo-oligosaccharide on the prevention of acute radiation enteritis in patients with gynecological cancer and impact on quality-of-life: a randomized, double-blind, placebo-controlled trial. Eur J Clin Nutr. 2016;70:170-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 59] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 6. | Nascimento M, Aguilar-Nascimento JE, Caporossi C, Castro-Barcellos HM, Motta RT. Efficacy of synbiotics to reduce acute radiation proctitis symptoms and improve quality of life: a randomized, double-blind, placebo-controlled pilot trial. Int J Radiat Oncol Biol Phys. 2014;90:289-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 7. | Zhao H, Shi Y, Luo X, Peng L, Yang Y, Zou L. The Effect of Fecal Microbiota Transplantation on a Child with Tourette Syndrome. Case Rep Med. 2017;2017:6165239. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 33] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 8. | Ren R, Sun G, Yang Y, Peng L, Zhang X, Wang S, Dou Y, Wang Z, Bo X, Liu Q, Li W, Fan N, Ma X. [A pilot study of treating ulcerative colitis with fecal microbiota transplantation]. Zhonghua Nei Ke Za Zhi. 2015;54:411-415. [PubMed] |
| 9. | Srinivas K, Ghatak S, Pyngrope DA, Angappan M, Milton AAP, Das S, Lyngdoh V, Lamare JP, Prasad MCB, Sen A. Avian strains of emerging pathogen Escherichia fergusonii are phylogenetically diverse and harbor the greatest AMR dissemination potential among different sources: Comparative genomic evidence. Front Microbiol. 2022;13:1080677. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 10. | Zang YM, Liu JF, Li G, Zhao M, Yin GM, Zhang ZP, Jia W. The first case of Escherichia fergusonii with biofilm in China and literature review. BMC Infect Dis. 2023;23:35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 11. | Dahal RH, Choi YJ, Kim S, Kim J. Differentiation of Escherichia fergusonii and Escherichia coli Isolated from Patients with Inflammatory Bowel Disease/Ischemic Colitis and Their Antimicrobial Susceptibility Patterns. Antibiotics (Basel). 2023;12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 12. | Park S, Wu X. Modulation of the Gut Microbiota in Memory Impairment and Alzheimer's Disease via the Inhibition of the Parasympathetic Nervous System. Int J Mol Sci. 2022;23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 19] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 13. | Ricaboni D, Mailhe M, Khelaifia S, Raoult D, Million M. Romboutsia timonensis, a new species isolated from human gut. New Microbes New Infect. 2016;12:6-7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 81] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 14. | Vallianou NG, Kounatidis D, Tsilingiris D, Panagopoulos F, Christodoulatos GS, Evangelopoulos A, Karampela I, Dalamaga M. The Role of Next-Generation Probiotics in Obesity and Obesity-Associated Disorders: Current Knowledge and Future Perspectives. Int J Mol Sci. 2023;24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 53] [Reference Citation Analysis (0)] |
| 15. | Shin SY, Park S, Moon JM, Kim K, Kim JW, Chun J, Lee TH, Choi CH; Microbiome Research Group of the Korean Society for Neurogastroenterology and Motility. Compositional Changes in the Gut Microbiota of Responders and Non-responders to Probiotic Treatment Among Patients With Diarrhea-predominant Irritable Bowel Syndrome: A Post Hoc Analysis of a Randomized Clinical Trial. J Neurogastroenterol Motil. 2022;28:642-654. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 16. | Lordan C, Thapa D, Ross RP, Cotter PD. Potential for enriching next-generation health-promoting gut bacteria through prebiotics and other dietary components. Gut Microbes. 2020;11:1-20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 111] [Cited by in RCA: 176] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 17. | Sasaki M, Schwab C, Ramirez Garcia A, Li Q, Ferstl R, Bersuch E, Akdis CA, Lauener R; CK-CARE study group, Frei R, Roduit C. The abundance of Ruminococcus bromii is associated with faecal butyrate levels and atopic dermatitis in infancy. Allergy. 2022;77:3629-3640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 30] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 18. | Yao S, Zhao Z, Wang W, Liu X. Bifidobacterium Longum: Protection against Inflammatory Bowel Disease. J Immunol Res. 2021;2021:8030297. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 113] [Article Influence: 28.3] [Reference Citation Analysis (0)] |
| 19. | Martinović A, Cocuzzi R, Arioli S, Mora D. Streptococcus thermophilus: To Survive, or Not to Survive the Gastrointestinal Tract, That Is the Question! Nutrients. 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 48] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 20. | Chen Q, Wu G, Chen H, Li H, Li S, Zhang C, Pang X, Wang L, Zhao L, Shen J. Quantification of Human Oral and Fecal Streptococcus parasanguinis by Use of Quantitative Real-Time PCR Targeting the groEL Gene. Front Microbiol. 2019;10:2910. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 21. | Benítez-Páez A, Gómez Del Pugar EM, López-Almela I, Moya-Pérez Á, Codoñer-Franch P, Sanz Y. Depletion of Blautia Species in the Microbiota of Obese Children Relates to Intestinal Inflammation and Metabolic Phenotype Worsening. mSystems. 2020;5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 235] [Cited by in RCA: 221] [Article Influence: 44.2] [Reference Citation Analysis (0)] |
| 22. | Islam SMS, Ryu HM, Sayeed HM, Byun HO, Jung JY, Kim HA, Suh CH, Sohn S. Eubacterium rectale Attenuates HSV-1 Induced Systemic Inflammation in Mice by Inhibiting CD83. Front Immunol. 2021;12:712312. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 30] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 23. | Liu N, Chen L, Yan M, Tao Q, Wu J, Chen J, Chen X, Zhang W, Peng C. Eubacterium rectale Improves the Efficacy of Anti-PD1 Immunotherapy in Melanoma via l-Serine-Mediated NK Cell Activation. Research (Wash D C). 2023;6:0127. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 21] [Reference Citation Analysis (0)] |
| 24. | Lu H, Xu X, Fu D, Gu Y, Fan R, Yi H, He X, Wang C, Ouyang B, Zhao P, Wang L, Xu P, Cheng S, Wang Z, Zou D, Han L, Zhao W. Butyrate-producing Eubacterium rectale suppresses lymphomagenesis by alleviating the TNF-induced TLR4/MyD88/NF-κB axis. Cell Host Microbe. 2022;30:1139-1150.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 99] [Article Influence: 33.0] [Reference Citation Analysis (0)] |
| 25. | Liu R, Zou Y, Wang WQ, Chen JH, Zhang L, Feng J, Yin JY, Mao XY, Li Q, Luo ZY, Zhang W, Wang DM. Gut microbial structural variation associates with immune checkpoint inhibitor response. Nat Commun. 2023;14:7421. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 9.5] [Reference Citation Analysis (0)] |