Published online May 21, 2024. doi: 10.3748/wjg.v30.i19.2512
Revised: April 8, 2024
Accepted: April 26, 2024
Published online: May 21, 2024
Processing time: 87 Days and 12.3 Hours
Hepatocellular carcinoma (HCC) is a high mortality neoplasm which usually appears on a cirrhotic liver. The therapeutic arsenal and subsequent prognostic outlook are intrinsically linked to the HCC stage at diagnosis. Notwithstanding the current deployment of treatments with curative intent (liver resection/local ablation and liver transplantation) in early and intermediate stages, a high rate of HCC recurrence persists, underscoring a pivotal clinical challenge. Emergent systemic therapies (ST), particularly immunotherapy, have demonstrate pro
Core Tip: This review provides an updated analysis (up to March 2024) of the current data about the new systemic therapies for the hepatocellular carcinoma (HCC) in the early and intermediate stages; specially focusing on the findings of neoadjuvant and adjuvant systemic therapies after a treatment with curative intent, for prevention of HCC recurrence. Finally, we discuss about the potential benefits of these new systemic therapies for early and intermediate stages of HCC and their future impact in the HCC treatment schedules.
- Citation: Urquijo-Ponce JJ, Alventosa-Mateu C, Latorre-Sánchez M, Castelló-Miralles I, Diago M. Present and future of new systemic therapies for early and intermediate stages of hepatocellular carcinoma. World J Gastroenterol 2024; 30(19): 2512-2522
- URL: https://www.wjgnet.com/1007-9327/full/v30/i19/2512.htm
- DOI: https://dx.doi.org/10.3748/wjg.v30.i19.2512
Cancer is the current leading cause of death, with 10 million deaths per year[1]. Among neoplasms, primary liver cancer ranks seventh in incidence (9.5 per 100000 inhabitants) and fourth in mortality (8.7 per 10000 inhabitants) worldwide. It accounts for 8.3% of all cancer deaths and is the most prevalent neoplasm in some places, such as Egypt or Southeast Asia[1-3].
The most common primary liver cancer, far exceeding other types (75%–85% of the total), is hepatocellular carcinoma (HCC)[2]. Its incidence and mortality rates are 2–3 times higher in men than in women and it usually appears in the sixth or seventh decades of life[4]. In addition, HCC usually occurs after liver cirrhosis, which increases its morbidity and mortality[5].
There are identified risk factors for the development of HCC such as the hepatitis B and C viruses, chronic alcohol consumption, and metabolic syndrome, all of which can be controlled as preventive measures[6-8]. Nevertheless, the incidence of HCC has increased or stabilized at its highest level in western countries (Europe, North America and Australia), due to the progressive increase in metabolic liver disease[8,9]. In the latter disease, HCC has a different molecular pathway of pathogenesis to the other aetiologies, thus hindering the early diagnosis and treatment of HCC[10-13], which can occur in patients without liver cirrhosis[14].
Use of the Barcelona Clinic Liver Cancer (BCLC) algorithm, which distinguishes between five stages (very early, early, intermediate, advanced and terminal) depending on the tumor burden, liver function and physical status of the patient, is deeply rooted in western countries. Its latest version was published in 2022[15]. The patient’s expected survival is conditioned by the treatment received and is significantly greater among patients with potentially resectable HCC who are candidates for treatment with curative intent (TCI)[16,17]. The latter consists of two options: Liver resection (LR) with local ablation as an alternative, and liver transplantation (LT). LT indications have traditionally been based on the Milan criteria, which have expanded over the years[18].
HCC patients can be classified into three groups under the BCLC system and according to their treatment (excluding the terminal stage). The first group involves TCI candidates. These are patients in early stages and select intermediate-stage cases, and have the highest expected survival (> 5 years)[15]. Despite this, their expected survival is limited by a high rate of post-surgical recurrence of up to 70% at the 5-year mark, due to microscopic vascular invasion and/or satellitosis[19-21].
The second group involves intermediate-stage patients who are not initially candidates for TCI but who may benefit from downstaging, i.e. reducing their stage by locoregional treatment to eventually receive an LT. There are several treatments used for downstaging[22-27] and the radiological response is usually assessed using mRECIST criteria[28].
The last group involves patients who are not candidates for these treatments, and has the aim of increasing their expected survival through systemic therapy (ST). These are patients in the advanced stage, as well as patients in the inter
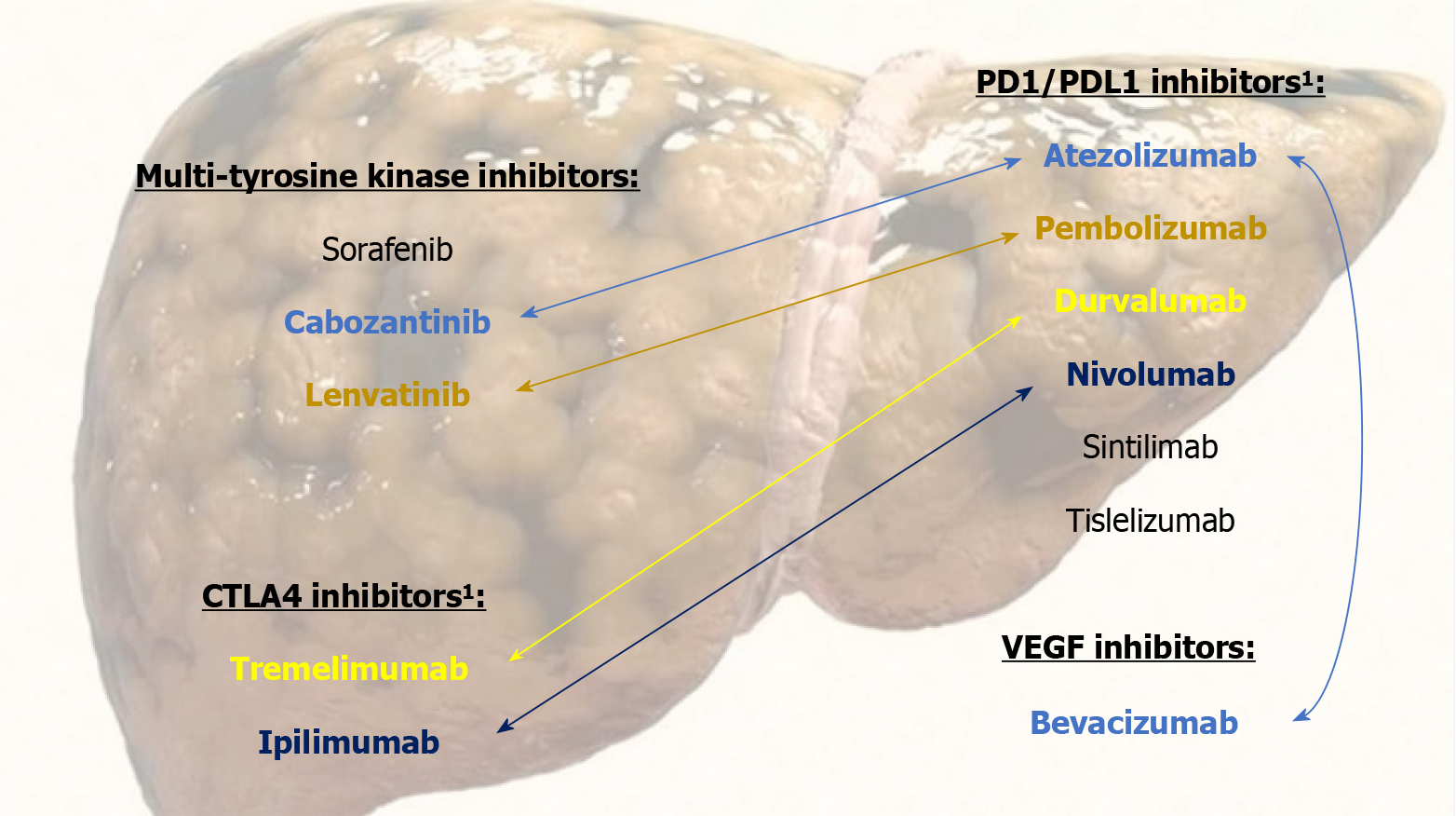
ST scenario has undergone a revolution in the last six years with the emergence of new drugs, as we briefly summarize in the following. In addition, the main studied combination of ST molecules and the approved first-line drugs median over
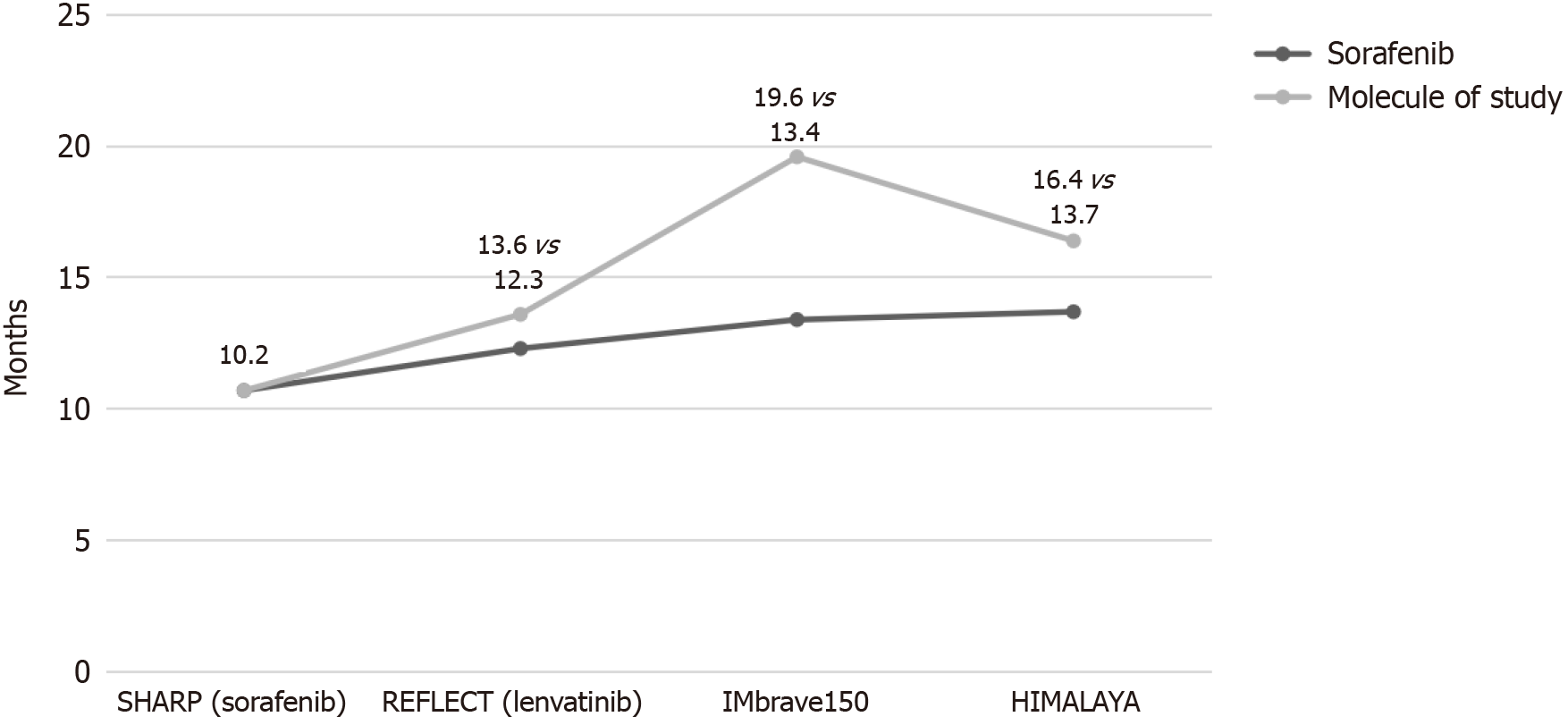
Sorafenib was the first one to achieve in a CT an OS greater than the placebo[30,31]. Subsequently, lenvatinib was the first to show non-inferiority to sorafenib in terms of OS as first-line treatment[32]; although other many drugs did not achieve this advantage[33-37]. The immunotherapy, drugs with immune checkpoint inhibition capabilities that are programmed for tumor cell removal by immune system stimulation[38-40], did not show a clear benefit of OS in monotherapy CTs[41-43]. However, the combination of the immunotherapy with the drugs of the previous groups presents a synergistic effect with clinical benefits[44].
In this respect, two combination therapies stand out that have shown a significant increase in OS. The phase III CT IMbrave150[45] compared atezolizumab + bevacizumab versus sorafenib in patients with advanced HCC, and showed a significantly higher OS in the first group. This combination was subsequently evaluated in real life in a cohort of Child-Pugh A-B cirrhosis[46], verifying the efficacy and tolerance profile shown in the CT. The phase III CT HIMALAYA[47] evaluated the double immunotherapy durvalumab + tremelimumab versus sorafenib showing a higher OS in the first group. In this same CT, durvalumab as monotherapy had OS similar to that of sorafenib.
In addition, ipilimumab + nivolumab achieved survival of 24 months among 40% of its subjects[48]. Lenvatinib + pem
The encouraging previous results of the new ST drugs have recently modified the treatment algorithm for advanced-stage HCC providing lengthy OS. Currently, atezolizumab + bevacizumab and durvalumab + tremelimumab are primarily indicated; as well as other first-, second- and third-line options available[15,17,51-54]. It should be noted that up to 50% of patients with HCC eventually receive ST[39].
Despite the potential benefits of these new drugs, ST is still bounded for advanced-stage HCC with the aim of increasing OS. Although the early and some intermediate stage HCC can be initially candidates for TCI, the OS in these group is clearly limited due to a high rate of post-surgical recurrence[19-21]. Furthermore, in the intermediate stage there is a subgroup of initially unresectable HCC that are not initially deemed eligible or failed to downstaging with locoregional treatment and cannot benefit from a TCI[15]. Thus, the therapeutic management of these stages is still a challenge, and new effective therapies are needed.
For all these reasons, the promising results of the new ST in HCC advanced-stage make it inevitable to consider its possible impact on potentially resectable HCC (early and intermediate stages as per BCLC), with the aims of increasing OS and recurrence-free survival (RFS) after TCI and the downstaging rate for LT or LR in initially unresectable tumors, both in neoadjuvant and adjuvant therapy[55]. For this purpose, it is highly relevant to analyze the recent studies, mainly CTs, that are being developed to evaluate the potential benefits of ST in this field.
Below, following a literature search updated to March 10th, 2024, focusing on our keywords, we show the main results from a selection of studies that we consider representative of these clinical scenarios.
CTs with the new ST drugs have used the term "major pathological response" (MPR) to define significant tumor necrosis following treatment, and although its definition varies among studies, it is usually > 70%.
Cabozantinib + nivolumab: Phase Ib CT for locally advanced/borderline HCC[56].
Fifteen patients with locally advanced HCC, who were initially ineligible for curative LR, were included and received cabozantinib + nivolumab neoadjuvant therapy. Eighty percent subsequently underwent LR, all with free resection margins. In addition, the surgical portion in five of these patients showed an MPR of > 90%. Grade 3 adverse events occurred in 13%.
Anti-programmed cell death protein 1 antibody (nivolumab, camrelizumab, pembrolizumab or sintilimab) + tyrosine kinase inhibitor (lenvatinib or apatinib): Series of cases in unresectable or advanced HCC[57].
A total of 63 with initially unresectable HCC received neoadjuvant combination therapy with these drugs. Of these, only 15.9% (10/63) were finally able to undergo LR, but the surgical portion showed partial MPR in 60% of these patients and complete MPR in the other 40%.
Anti-programmed cell death protein 1 antibody (pembrolizumab, toripalimab or sintilimab) + tyrosine kinase inhibitor (lenvatinib or apatinib): Pilot study in unresectable intermediate-stage HCC[58].
In this study with 10 Child-Pugh A patients with initially unresectable HCC, it was found that 80% were made eligible for LR following neoadjuvant therapy. Partial MPR was observed in 70% and complete MPR in 30% following treatment. Finally, the post-surgical RFS rate at the 12-month follow-up mark was 75%.
Nivolumab + ipilimumab: Phase Ib CT in resectable HCC[59].
After adjuvant therapy and subsequent LR, liver tissue was available for histological analysis in nine patients. Partial MPR (> 70%) was observed in 78% of patients and complete MPR in 22%. The six-month post-operative follow-up showed an RFS of 92%.
Dovitinib: Phase II CT in early- and intermediate-stage HCC[60].
A total of 24 patients received neoadjuvant therapy with dovitinib, followed by locoregional therapy. Following neoadjuvant therapy, the rate of partial or complete radiological response (as per mRECIST criteria) was 70% (7/10) in the early stage and 22% (2/9) in the intermediate stage; while the remaining patients showed radiological stability. In addition, seven patients were able to receive LT after neoadjuvant therapy.
Sorafenib: Phase II CT in resectable HCC[61].
Nineteen patients were included, with a 32% radiological response as per mRECIST and no cases of radiological progression being observed. After LR, disease-free surgical margins were observed in 88% of patients and an MPR ≥ 50% in 24%.
Atezolizumab + bevacizumab following TCI: Phase III CT in HCC with high risk of recurrence (IMbrave050)[62].
Multicenter study with 668 Child-Pugh A adult patients with HCC with a high risk of recurrence who underwent TCI via LR or local ablation. They were subsequently randomized to 12-month adjuvant therapy with atezolizumab + bevacizumab (n = 334) vs active follow-up (n = 334). At 12 months of follow-up, the RFS was higher in the adjuvant therapy group (78% vs 65%, hazard ratio 0.71, P value = 0.012). However, the mortality rate was also slightly higher in the adjuvant group (27 vs 20 patients). In addition, grade 3–4 adverse effects were more frequent in the adjuvant group (41% vs 13%), requiring the drug to be discontinued in 9% (29/334). This is the first phase III CT to show positive results for RFS with adjuvant therapy following TCI.
Sorafenib following TCI: Phase III CT[63].
More than 1000 patients were included, undergoing LR or ablation, followed by adjuvant therapy with sorafenib vs placebo. The RFS was similar in both groups (33.3 vs 33.7 months).
Nivolumab + ipilimumab: Phase II CT in resectable HCC[64].
Twenty-seven patients with resectable HCC were included, who were then randomized to two arms with neoadjuvant and adjuvant therapy with nivolumab (13 patients) vs nivolumab + ipilimumab (14 patients). Seventy-four percent finally received an LR (20/27) and grade 3–4 adverse effects were observed in both arms (43% vs 23%), not presenting delays to surgery.
An MPR > 70% was achieved in 6 patients (3 in each arm) with no tumor recurrence observed in these patients at 24 months of follow-up. Conversely, half of those with tumor necrosis < 70% did present a recurrence. The median RFS was 9.4 months in the nivolumab group versus 19.5 months in the nivolumab + ipilimumab group. It is noteworthy that four out of the seven patients who did not receive LR presented radiological progression; although we must take into account that this CT included 8 patients with HCC diameters > 10 cm.
Camrelizumab + apatinib: Phase II CT in resectable HCC[65].
Eighteen patients received neoadjuvant therapy, 17 of whom underwent LR and a final 13 completed adjuvant therapy. Six patients presented complete radiological response after neoadjuvant therapy, as per mRECIST criteria. After LR, an MPR > 90% was achieved in four patients. Grade ≥ 3 adverse events occurred in three patients. The recurrence rate was greater in the group that did not achieve these tumor necrosis rates.
Cemiplimab: Phase II CT in resectable HCC[66].
Twenty patients undergoing LR were included. An MPR > 70% was achieved in 20% and an MPR > 50% was achieved in 15%. Grade 3 adverse effects occurred in 33% of patients, with no grade 4–5 cases. This study found that immune infiltration in pre-treatment tumor specimens was greater in patients who achieved high rates of necrosis.
Dovitinib: Phase IIb/III CT in HCC outside of Milan criteria[67].
This trial included 74 patients with HCC exceeding Milan criteria, no macrovascular invasion or extrahepatic involvement, Child-Pugh ≤ B7, and < 50% of patients with an estimated post-transplant OS < 5 years. Patients underwent neoadjuvant therapy with dovitinib and subsequent downstaging with the treatment deemed appropriate by a multi
After dovitinib, the response rate as per mRECIST was 48%, including 13% with complete radiological remission. Despite reduction/discontinuation of the dovinitib dose in 83% of patients due to grade 3–4 adverse events, all patients were able to receive the planned locoregional treatment. OS was greater in the transplant group (34.8 vs 16.8 months).
Several studies have analyzed the combination of ST and locoregional treatments, with promising results. We highlight the phase III CT EMERALD-1[68] with unresectable HCC eligible for transarterial chemoembolization (TACE). Six hundred sixteen patients with early or intermediate HCC stages and no evidence of extrahepatic disease were randomized to durvalumab + bevacizumab + TACE, durvalumab + TACE or TACE alone. RFS was significantly improved for durvalumab + bevacizumab + TACE vs TACE alone (15.0 vs 8.2 months, P value = 0.032).
Worthy of mention is a CT comparing TACE vs TACE + sorafenib in unresectable HCC, which achieved a significantly higher RFS in the second group (13.5 vs 25.2 months)[69]. Another CT studied radioembolization with nivolumab adjuvant therapy in patients with unresectable HCC, presenting an encouraging response rate[70].
There is currently significant research activity in this field. Table 1 shows the main results of ongoing CTs, which can be found on clinicaltrials.gov and cilinicaltrialsregister.eu.
| Trial number (phase) | Treatment | Clinical condition | Patients included |
| NCT03510871 (II) | Nivolumab + ipilimumab | Surgical NeAd | Potentially resectable HCC with high-risk recurrence |
| NCT048 50040 (II) | Camrelizumab + apatinib + oxaliplatine | Surgical NeAd | Potentially resectable HCC with high-risk recurrence |
| NCT03578874 (II) | Sorafenib + capecitabine + oxaliplatine | Surgical NeAd | Unilobar no potentially curative HCC |
| NCT04174781 (II) | Sintilimab + TACE | Surgical NeAd | Unresectable HCC stage A/B BCLC |
| NCT01507064 (II) | Sorafenib + laser ablation vs laser ablation | Surgical NeAd | Unresectable HCC with single > 4 cm nodule |
| NCT04857684 (I) | Atezolizumab + bevacizumab + radiotherapy | Surgical NeAd | Resectable HCC |
| NCT04888546 (Ib) | Anlotinib + TQB2450 (PDL1 antibody) | Surgical NeAd | Potentially resectable HCC with high-risk recurrence |
| NCT04721132 (II) | Atezolizumab + bevacizumab | Surgical NeAd | Resectable HCC |
| NCT03847428 (III) | Durvalumab + bevacizumab | Surgical Ad | Potentially curative HCC (resection/ablation) |
| NCT03383458 (III) | Nivolumab | Surgical Ad | Potentially curative HCC (resection/ablation) |
| NCT03867084 (III) | Pembrolizumab | Surgical Ad | Potentially curative HCC (resection/ablation) |
| NCT04639180 (III) | Camrelizumab + apatinib | Surgical Ad | Potentially curative HCC (resection/ablation) |
| NCT05367687 (II) | Camrelizumab + apatinib vs camrelizumab | Surgical Ad | Potentially curative HCC (resection/ablation) |
| NCT05545124 (II) | Donafenib + tislelizumab | Surgical Ad | Potentially curative resection HCC |
| NCT05407519 (II) | Tislelizumab + sitravatinib | Surgical Ad | Potentially curative resection HCC |
| NCT04418401 (I) | Donafenib + anti-PD-1 antibody | Surgical Ad | Potentially curative resection HCC |
| NCT0544086 (II) | Tremelimumab NeAd + durvalumab Ad | Surgical NeAdAd | Resectable HCC |
| NCT03630640 (II) | Nivolumab NeAd + nivolumab Ad | Surgical NeAdAd | Resectable HCC |
| NCT04727307 (II) | Atezolizumab NeAdAd + bevacizumab Ad | Surgical NeAdAd | Ablation of HCC |
| NCT04930315 (II) | Camrelizumab NeAdAd + apatinib Ad | Surgical NeAdAd | Resectable HCC |
| NCT04834986 (II) | Tislelizumab NeAd + lenvatinib NeAdAd | Surgical NeAdAd | Resectable HCC |
| NCT04658147 (I) | Nivolumab NeAdAd vs nivolumab NeAdAd + relatlimab NeAdAd | Surgical NeAdAd | Resectable HCC |
| NCT05185739 (II) | Pembrolizumab NeAdAd + lenvatinib Ad | Surgical NeAdAd | Resectable HCC |
| NCT03867370 (Ib/II) | Toripalimab NeAdAd + lenvatinib NeAdAd | Surgical NeAdAd | Resectable HCC |
| NCT04615143 (II) | Tislelizumab NeAdAd + lenvatinib NeAdAd | Surgical NeAdAd | Resectable HCC |
| NCT04954339 (II) | Atezolizumab NeAdAd + bevacizumab NeAdAd | Surgical NeAdAd | Resectable HCC |
| NCT04521153 (II) | Camrelizumab NeAd + TACE + camrelizumab Ad + apatinib Ad | Surgical NeAdAd | Resectable HCC |
| NCT04425226 (UK) | Pembrolizumab + lenvatinib | NeAd LTD | Unresectable HCC |
| NCT04035876 (UK) | Camrelizumab + apatinib | NeAd LTD | Unresectable HCC |
The morbidity and mortality associated with HCC are significant in all BCLC stages, despite the wide range of treatments available. Therefore, the results we have observed with ST at early- and intermediate-stage CTs, with the intention of de
There is no doubt that the indications for ST are expanding, mainly due to its ability to induce tumor necrosis. In this respect, the American Association of the Study of the Liver guidelines[71], published following the results of IMbrave050, already recommends ST in patients at high risk of recurrence after LR or local ablation. In addition, the use of these drugs in neoadjuvant surgery allows for the possibility of identifying immunity tumor markers that are predictive of treatment response[72]. Some of these, such as immune infiltration, have already been suggested[66,73,74].
Even though the results available are promising, we believe they should be interpreted with caution. As we have seen, these mainly stem from phase I-II CTs with few patients, short follow-up periods and heterogeneous HCC samples. In addition, each CT analyzes a different drug or combination of drugs, and the methodology and objectives among them often vary. Finally, while the results are striking in terms of achieving MPR, their impact on OS and RFS in the long term has not been clearly defined.
The most representative study to date, the IMbrave050, can be used as example to illustrate these limitations. Despite showing superiority in RFS following TCI in the adjuvant group, the follow-up period was relatively short, so the duration of this beneficial effect is unknown. In addition, mortality during follow-up was low and similar in both groups, reason for which it will be a long time before we know their true impact on OS. Finally, a considerable percentage of grade ≥ 3 adverse effects was obtained in the adjuvant arm, which could be considered inadmissible depending on the OS and RFS that are ultimately reported.
Therefore, we need to know the OS and RFS results after long follow-up periods in current and future studies to be able to assess the actual potential of ST in the early and intermediate stages of HCC and, on this basis, be able to determine its indications and position among the treatment schedules. Important issues, such as the ideal moment for their use in patients with resectable tumors (before or after surgery), remain unresolved[55]. However, it is expected that in the next 5-years period several phase III CTs will be completed, and their results could shed a necessary light on those questions[75].
In our view, the future of HCC treatment will be based on personalized therapy as a function of patient and tumor characteristics. This therapy will allow a significant increase in OS and RFS, as well as a good treatment tolerance profile. However, to achieve personalized therapy, it will be necessary to define accurate and easy-to-use biomarkers, both of the host, as well as tumor-based or predictive of ST response. Research in this respect is currently flourishing[72,76-80].
Considering the findings described in this review, we posit that neoadjuvant and/or adjuvant ST with these novel drugs will play an important role in the personalized therapy for the early and intermediate stages of HCC.
Concerning the application of ST in the early and intermediate stages of HCC, the outcomes delineated in this review signal a promising enhancement in the prognosis for these patients in the near future. Nevertheless, deeper research in this field has to be done, before we are able to integrate this approach into clinical practice.
To María Beceiro, Ana Serrano-Prats and Salvadora Prats-Besó.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country of origin: Spain
Peer-review report’s classification
Scientific Quality: Grade B, Grade C
Novelty: Grade B, Grade C
Creativity or Innovation: Grade B, Grade B
Scientific Significance: Grade B, Grade B
P-Reviewer: Jiang L, China; Miao YD, China S-Editor: Li L L-Editor: A P-Editor: Zheng XM
| 1. | Ferlay J, Ervik M, Lam F, Colombet M, Mery L, Piñeros M, Znaor A, Soerjomataram I, Bray F. Global Cancer Observatory: Cancer Today. Lyon, France: International Agency for Research on Cancer (2020). [cited 10 November 2023]. Available from: https://gco.iarc.who.int/today/. |
| 2. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 64612] [Article Influence: 16153.0] [Reference Citation Analysis (176)] |
| 3. | Konyn P, Ahmed A, Kim D. Current epidemiology in hepatocellular carcinoma. Expert Rev Gastroenterol Hepatol. 2021;15:1295-1307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 166] [Article Influence: 41.5] [Reference Citation Analysis (0)] |
| 4. | McGlynn KA, Petrick JL, El-Serag HB. Epidemiology of Hepatocellular Carcinoma. Hepatology. 2021;73 Suppl 1:4-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 820] [Cited by in RCA: 1342] [Article Influence: 335.5] [Reference Citation Analysis (1)] |
| 5. | Devarbhavi H, Asrani SK, Arab JP, Nartey YA, Pose E, Kamath PS. Global burden of liver disease: 2023 update. J Hepatol. 2023;79:516-537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 840] [Reference Citation Analysis (4)] |
| 6. | Mak LY, Cruz-Ramón V, Chinchilla-López P, Torres HA, LoConte NK, Rice JP, Foxhall LE, Sturgis EM, Merrill JK, Bailey HH, Méndez-Sánchez N, Yuen MF, Hwang JP. Global Epidemiology, Prevention, and Management of Hepatocellular Carcinoma. Am Soc Clin Oncol Educ Book. 2018;38:262-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 157] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 7. | Sagnelli E, Macera M, Russo A, Coppola N, Sagnelli C. Epidemiological and etiological variations in hepatocellular carcinoma. Infection. 2020;48:7-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 152] [Article Influence: 30.4] [Reference Citation Analysis (0)] |
| 8. | Petrick JL, Florio AA, Znaor A, Ruggieri D, Laversanne M, Alvarez CS, Ferlay J, Valery PC, Bray F, McGlynn KA. International trends in hepatocellular carcinoma incidence, 1978-2012. Int J Cancer. 2020;147:317-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 315] [Cited by in RCA: 390] [Article Influence: 78.0] [Reference Citation Analysis (0)] |
| 9. | Valery PC, Laversanne M, Clark PJ, Petrick JL, McGlynn KA, Bray F. Projections of primary liver cancer to 2030 in 30 countries worldwide. Hepatology. 2018;67:600-611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 235] [Article Influence: 33.6] [Reference Citation Analysis (0)] |
| 10. | Llovet JM, Willoughby CE, Singal AG, Greten TF, Heikenwälder M, El-Serag HB, Finn RS, Friedman SL. Nonalcoholic steatohepatitis-related hepatocellular carcinoma: pathogenesis and treatment. Nat Rev Gastroenterol Hepatol. 2023;20:487-503. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 196] [Cited by in RCA: 176] [Article Influence: 88.0] [Reference Citation Analysis (0)] |
| 11. | Llovet JM, Kelley RK, Villanueva A, Singal AG, Pikarsky E, Roayaie S, Lencioni R, Koike K, Zucman-Rossi J, Finn RS. Hepatocellular carcinoma. Nat Rev Dis Primers. 2021;7:6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4432] [Cited by in RCA: 3878] [Article Influence: 969.5] [Reference Citation Analysis (3)] |
| 12. | Singal AG, Kanwal F, Llovet JM. Global trends in hepatocellular carcinoma epidemiology: implications for screening, prevention and therapy. Nat Rev Clin Oncol. 2023;20:864-884. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 344] [Article Influence: 172.0] [Reference Citation Analysis (1)] |
| 13. | Lodato F, Mazzella G, Festi D, Azzaroli F, Colecchia A, Roda E. Hepatocellular carcinoma prevention: a worldwide emergence between the opulence of developed countries and the economic constraints of developing nations. World J Gastroenterol. 2006;12:7239-7249. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 60] [Cited by in RCA: 61] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 14. | Bertot LC, Adams LA. Trends in hepatocellular carcinoma due to non-alcoholic fatty liver disease. Expert Rev Gastroenterol Hepatol. 2019;13:179-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 54] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 15. | Reig M, Forner A, Rimola J, Ferrer-Fàbrega J, Burrel M, Garcia-Criado Á, Kelley RK, Galle PR, Mazzaferro V, Salem R, Sangro B, Singal AG, Vogel A, Fuster J, Ayuso C, Bruix J. BCLC strategy for prognosis prediction and treatment recommendation: The 2022 update. J Hepatol. 2022;76:681-693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1904] [Cited by in RCA: 2604] [Article Influence: 868.0] [Reference Citation Analysis (59)] |
| 16. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol. 2018;69:182-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5593] [Cited by in RCA: 6056] [Article Influence: 865.1] [Reference Citation Analysis (3)] |
| 17. | Vogel A, Martinelli E; ESMO Guidelines Committee. Updated treatment recommendations for hepatocellular carcinoma (HCC) from the ESMO Clinical Practice Guidelines. Ann Oncol. 2021;32:801-805. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 297] [Article Influence: 74.3] [Reference Citation Analysis (1)] |
| 18. | Mehta N, Bhangui P, Yao FY, Mazzaferro V, Toso C, Akamatsu N, Durand F, Ijzermans J, Polak W, Zheng S, Roberts JP, Sapisochin G, Hibi T, Kwan NM, Ghobrial M, Soin A. Liver Transplantation for Hepatocellular Carcinoma. Working Group Report from the ILTS Transplant Oncology Consensus Conference. Transplantation. 2020;104:1136-1142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 158] [Article Influence: 31.6] [Reference Citation Analysis (0)] |
| 19. | Sala M, Fuster J, Llovet JM, Navasa M, Solé M, Varela M, Pons F, Rimola A, García-Valdecasas JC, Brú C, Bruix J; Barcelona Clinic Liver Cancer (BCLC) Group. High pathological risk of recurrence after surgical resection for hepatocellular carcinoma: an indication for salvage liver transplantation. Liver Transpl. 2004;10:1294-1300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 230] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 20. | Nevola R, Ruocco R, Criscuolo L, Villani A, Alfano M, Beccia D, Imbriani S, Claar E, Cozzolino D, Sasso FC, Marrone A, Adinolfi LE, Rinaldi L. Predictors of early and late hepatocellular carcinoma recurrence. World J Gastroenterol. 2023;29:1243-1260. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 103] [Cited by in RCA: 115] [Article Influence: 57.5] [Reference Citation Analysis (3)] |
| 21. | Tabrizian P, Jibara G, Shrager B, Schwartz M, Roayaie S. Recurrence of hepatocellular cancer after resection: patterns, treatments, and prognosis. Ann Surg. 2015;261:947-955. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 345] [Cited by in RCA: 658] [Article Influence: 65.8] [Reference Citation Analysis (0)] |
| 22. | De Luna W, Sze DY, Ahmed A, Ha BY, Ayoub W, Keeffe EB, Cooper A, Esquivel C, Nguyen MH. Transarterial chemoinfusion for hepatocellular carcinoma as downstaging therapy and a bridge toward liver transplantation. Am J Transplant. 2009;9:1158-1168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 106] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 23. | Chapman WC, Garcia-Aroz S, Vachharajani N, Fowler K, Saad N, Lin Y, Wellen J, Tan B, Khan AS, Doyle MB. Liver Transplantation for Advanced Hepatocellular Carcinoma after Downstaging Without Up-Front Stage Restrictions. J Am Coll Surg. 2017;224:610-621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 65] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 24. | Chapman WC, Majella Doyle MB, Stuart JE, Vachharajani N, Crippin JS, Anderson CD, Lowell JA, Shenoy S, Darcy MD, Brown DB. Outcomes of neoadjuvant transarterial chemoembolization to downstage hepatocellular carcinoma before liver transplantation. Ann Surg. 2008;248:617-625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 234] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 25. | Lewandowski RJ, Kulik LM, Riaz A, Senthilnathan S, Mulcahy MF, Ryu RK, Ibrahim SM, Sato KT, Baker T, Miller FH, Omary R, Abecassis M, Salem R. A comparative analysis of transarterial downstaging for hepatocellular carcinoma: chemoembolization versus radioembolization. Am J Transplant. 2009;9:1920-1928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 408] [Cited by in RCA: 437] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 26. | Hanje AJ, Yao FY. Current approach to down-staging of hepatocellular carcinoma prior to liver transplantation. Curr Opin Organ Transplant. 2008;13:234-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 24] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 27. | Ravaioli M, Grazi GL, Piscaglia F, Trevisani F, Cescon M, Ercolani G, Vivarelli M, Golfieri R, D'Errico Grigioni A, Panzini I, Morelli C, Bernardi M, Bolondi L, Pinna AD. Liver transplantation for hepatocellular carcinoma: results of down-staging in patients initially outside the Milan selection criteria. Am J Transplant. 2008;8:2547-2557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 291] [Cited by in RCA: 299] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 28. | Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30:52-60. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3353] [Cited by in RCA: 3302] [Article Influence: 220.1] [Reference Citation Analysis (36)] |
| 29. | Forner A, Reig ME, de Lope CR, Bruix J. Current strategy for staging and treatment: the BCLC update and future prospects. Semin Liver Dis. 2010;30:61-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 764] [Cited by in RCA: 866] [Article Influence: 57.7] [Reference Citation Analysis (0)] |
| 30. | Rimassa L, Santoro A. Sorafenib therapy in advanced hepatocellular carcinoma: the SHARP trial. Expert Rev Anticancer Ther. 2009;9:739-745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 103] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 31. | Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A, Schwartz M, Porta C, Zeuzem S, Bolondi L, Greten TF, Galle PR, Seitz JF, Borbath I, Häussinger D, Giannaris T, Shan M, Moscovici M, Voliotis D, Bruix J; SHARP Investigators Study Group. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9016] [Cited by in RCA: 10266] [Article Influence: 603.9] [Reference Citation Analysis (2)] |
| 32. | Kudo M, Finn RS, Qin S, Han KH, Ikeda K, Piscaglia F, Baron A, Park JW, Han G, Jassem J, Blanc JF, Vogel A, Komov D, Evans TRJ, Lopez C, Dutcus C, Guo M, Saito K, Kraljevic S, Tamai T, Ren M, Cheng AL. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018;391:1163-1173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3128] [Cited by in RCA: 3823] [Article Influence: 546.1] [Reference Citation Analysis (1)] |
| 33. | Bruix J, Qin S, Merle P, Granito A, Huang YH, Bodoky G, Pracht M, Yokosuka O, Rosmorduc O, Breder V, Gerolami R, Masi G, Ross PJ, Song T, Bronowicki JP, Ollivier-Hourmand I, Kudo M, Cheng AL, Llovet JM, Finn RS, LeBerre MA, Baumhauer A, Meinhardt G, Han G; RESORCE Investigators. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;389:56-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2160] [Cited by in RCA: 2710] [Article Influence: 338.8] [Reference Citation Analysis (0)] |
| 34. | Abou-Alfa GK, Meyer T, Cheng AL, El-Khoueiry AB, Rimassa L, Ryoo BY, Cicin I, Merle P, Chen Y, Park JW, Blanc JF, Bolondi L, Klümpen HJ, Chan SL, Zagonel V, Pressiani T, Ryu MH, Venook AP, Hessel C, Borgman-Hagey AE, Schwab G, Kelley RK. Cabozantinib in Patients with Advanced and Progressing Hepatocellular Carcinoma. N Engl J Med. 2018;379:54-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1630] [Cited by in RCA: 1767] [Article Influence: 252.4] [Reference Citation Analysis (0)] |
| 35. | Pazo Cid RA, Esquerdo G, Puertolas T, Calderero V, Gil I, Lao J, Millastre E, Alvarez-Alejandro M, Madani J, Anton A. Bevacizumab (BVZ) as second-line treatment after sorafenib (SFB) progression in patients (pts) with advanced hepatocellular carcinoma (HCC). J Clin Oncol. 2010;28:e14619. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 36. | Zhu AX, Kang YK, Yen CJ, Finn RS, Galle PR, Llovet JM, Assenat E, Brandi G, Pracht M, Lim HY, Rau KM, Motomura K, Ohno I, Merle P, Daniele B, Shin DB, Gerken G, Borg C, Hiriart JB, Okusaka T, Morimoto M, Hsu Y, Abada PB, Kudo M; REACH-2 study investigators. Ramucirumab after sorafenib in patients with advanced hepatocellular carcinoma and increased α-fetoprotein concentrations (REACH-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019;20:282-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1027] [Cited by in RCA: 1247] [Article Influence: 207.8] [Reference Citation Analysis (0)] |
| 37. | Zhu AX, Park JO, Ryoo BY, Yen CJ, Poon R, Pastorelli D, Blanc JF, Chung HC, Baron AD, Pfiffer TE, Okusaka T, Kubackova K, Trojan J, Sastre J, Chau I, Chang SC, Abada PB, Yang L, Schwartz JD, Kudo M; REACH Trial Investigators. Ramucirumab versus placebo as second-line treatment in patients with advanced hepatocellular carcinoma following first-line therapy with sorafenib (REACH): a randomised, double-blind, multicentre, phase 3 trial. Lancet Oncol. 2015;16:859-870. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 598] [Cited by in RCA: 652] [Article Influence: 65.2] [Reference Citation Analysis (0)] |
| 38. | Pinter M, Jain RK, Duda DG. The Current Landscape of Immune Checkpoint Blockade in Hepatocellular Carcinoma: A Review. JAMA Oncol. 2021;7:113-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 252] [Article Influence: 63.0] [Reference Citation Analysis (1)] |
| 39. | Llovet JM, Castet F, Heikenwalder M, Maini MK, Mazzaferro V, Pinato DJ, Pikarsky E, Zhu AX, Finn RS. Immunotherapies for hepatocellular carcinoma. Nat Rev Clin Oncol. 2022;19:151-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 1012] [Article Influence: 337.3] [Reference Citation Analysis (2)] |
| 40. | Ribas A, Wolchok JD. Cancer immunotherapy using checkpoint blockade. Science. 2018;359:1350-1355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2848] [Cited by in RCA: 4616] [Article Influence: 659.4] [Reference Citation Analysis (0)] |
| 41. | Yau T, Park JW, Finn RS, Cheng AL, Mathurin P, Edeline J, Kudo M, Harding JJ, Merle P, Rosmorduc O, Wyrwicz L, Schott E, Choo SP, Kelley RK, Sieghart W, Assenat E, Zaucha R, Furuse J, Abou-Alfa GK, El-Khoueiry AB, Melero I, Begic D, Chen G, Neely J, Wisniewski T, Tschaika M, Sangro B. Nivolumab versus sorafenib in advanced hepatocellular carcinoma (CheckMate 459): a randomised, multicentre, open-label, phase 3 trial. Lancet Oncol. 2022;23:77-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 741] [Article Influence: 185.3] [Reference Citation Analysis (0)] |
| 42. | Finn RS, Ryoo BY, Merle P, Kudo M, Bouattour M, Lim HY, Breder V, Edeline J, Chao Y, Ogasawara S, Yau T, Garrido M, Chan SL, Knox J, Daniele B, Ebbinghaus SW, Chen E, Siegel AB, Zhu AX, Cheng AL; KEYNOTE-240 investigators. Pembrolizumab As Second-Line Therapy in Patients With Advanced Hepatocellular Carcinoma in KEYNOTE-240: A Randomized, Double-Blind, Phase III Trial. J Clin Oncol. 2020;38:193-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1365] [Cited by in RCA: 1339] [Article Influence: 267.8] [Reference Citation Analysis (0)] |
| 43. | Zhu AX, Finn RS, Edeline J, Cattan S, Ogasawara S, Palmer D, Verslype C, Zagonel V, Fartoux L, Vogel A, Sarker D, Verset G, Chan SL, Knox J, Daniele B, Webber AL, Ebbinghaus SW, Ma J, Siegel AB, Cheng AL, Kudo M; KEYNOTE-224 investigators. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): a non-randomised, open-label phase 2 trial. Lancet Oncol. 2018;19:940-952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1184] [Cited by in RCA: 1895] [Article Influence: 270.7] [Reference Citation Analysis (0)] |
| 44. | Manegold C, Dingemans AC, Gray JE, Nakagawa K, Nicolson M, Peters S, Reck M, Wu YL, Brustugun OT, Crinò L, Felip E, Fennell D, Garrido P, Huber RM, Marabelle A, Moniuszko M, Mornex F, Novello S, Papotti M, Pérol M, Smit EF, Syrigos K, van Meerbeeck JP, van Zandwijk N, Yang JC, Zhou C, Vokes E. The Potential of Combined Immunotherapy and Antiangiogenesis for the Synergistic Treatment of Advanced NSCLC. J Thorac Oncol. 2017;12:194-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 188] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 45. | Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, Kudo M, Breder V, Merle P, Kaseb AO, Li D, Verret W, Xu DZ, Hernandez S, Liu J, Huang C, Mulla S, Wang Y, Lim HY, Zhu AX, Cheng AL; IMbrave150 Investigators. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N Engl J Med. 2020;382:1894-1905. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2542] [Cited by in RCA: 4689] [Article Influence: 937.8] [Reference Citation Analysis (2)] |
| 46. | D'Alessio A, Fulgenzi CAM, Nishida N, Schönlein M, von Felden J, Schulze K, Wege H, Gaillard VE, Saeed A, Wietharn B, Hildebrand H, Wu L, Ang C, Marron TU, Weinmann A, Galle PR, Bettinger D, Bengsch B, Vogel A, Balcar L, Scheiner B, Lee PC, Huang YH, Amara S, Muzaffar M, Naqash AR, Cammarota A, Personeni N, Pressiani T, Sharma R, Pinter M, Cortellini A, Kudo M, Rimassa L, Pinato DJ. Preliminary evidence of safety and tolerability of atezolizumab plus bevacizumab in patients with hepatocellular carcinoma and Child-Pugh A and B cirrhosis: A real-world study. Hepatology. 2022;76:1000-1012. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 138] [Cited by in RCA: 169] [Article Influence: 56.3] [Reference Citation Analysis (0)] |
| 47. | Abou-Alfa GK, Lau G, Kudo M, Chan SL, Kelley RK, Furuse J, Sukeepaisarnjaroen W, Kang YK, Dao TV, De Toni EN, Rimassa L, Breder V, Vasilyev A, Heurgué A, Tam VC, Mody K, Thungappa SC, Ostapenko Y, Yau T, Azevedo S, Varela M, Cheng AL, Qin S, Galle PR, Ali S, Gupta C, Makowsky M, Kurland JF, Negro A, Sangro B. Plain language summary of the HIMALAYA study: tremelimumab and durvalumab for unresectable hepatocellular carcinoma (liver cancer). Future Oncol. 2023;19:2505-2516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 48. | Yau T, Kang YK, Kim TY, El-Khoueiry AB, Santoro A, Sangro B, Melero I, Kudo M, Hou MM, Matilla A, Tovoli F, Knox JJ, Ruth He A, El-Rayes BF, Acosta-Rivera M, Lim HY, Neely J, Shen Y, Wisniewski T, Anderson J, Hsu C. Efficacy and Safety of Nivolumab Plus Ipilimumab in Patients With Advanced Hepatocellular Carcinoma Previously Treated With Sorafenib: The CheckMate 040 Randomized Clinical Trial. JAMA Oncol. 2020;6:e204564. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 908] [Cited by in RCA: 968] [Article Influence: 193.6] [Reference Citation Analysis (0)] |
| 49. | Llovet JM, Kudo M, Merle P, Meyer T, Qin S, Ikeda M, Xu R, Edeline J, Ryoo BY, Ren Z, Masi G, Kwiatkowski M, Lim HY, Kim JH, Breder V, Kumada H, Cheng AL, Galle PR, Kaneko S, Wang A, Mody K, Dutcus C, Dubrovsky L, Siegel AB, Finn RS; LEAP-002 Investigators. Lenvatinib plus pembrolizumab versus lenvatinib plus placebo for advanced hepatocellular carcinoma (LEAP-002): a randomised, double-blind, phase 3 trial. Lancet Oncol. 2023;24:1399-1410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 193] [Article Influence: 96.5] [Reference Citation Analysis (0)] |
| 50. | Yau T, Zagonel V, Santoro A, Acosta-Rivera M, Choo SP, Matilla A, He AR, Cubillo Gracian A, El-Khoueiry AB, Sangro B, Eldawy TE, Bruix J, Frassineti GL, Vaccaro GM, Tschaika M, Scheffold C, Koopmans P, Neely J, Piscaglia F. Nivolumab Plus Cabozantinib With or Without Ipilimumab for Advanced Hepatocellular Carcinoma: Results From Cohort 6 of the CheckMate 040 Trial. J Clin Oncol. 2023;41:1747-1757. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 39] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 51. | Gordan JD, Kennedy EB, Abou-Alfa GK, Beg MS, Brower ST, Gade TP, Goff L, Gupta S, Guy J, Harris WP, Iyer R, Jaiyesimi I, Jhawer M, Karippot A, Kaseb AO, Kelley RK, Knox JJ, Kortmansky J, Leaf A, Remak WM, Shroff RT, Sohal DPS, Taddei TH, Venepalli NK, Wilson A, Zhu AX, Rose MG. Systemic Therapy for Advanced Hepatocellular Carcinoma: ASCO Guideline. J Clin Oncol. 2020;38:4317-4345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 305] [Cited by in RCA: 417] [Article Influence: 83.4] [Reference Citation Analysis (1)] |
| 52. | Su GL, Altayar O, O'Shea R, Shah R, Estfan B, Wenzell C, Sultan S, Falck-Ytter Y. AGA Clinical Practice Guideline on Systemic Therapy for Hepatocellular Carcinoma. Gastroenterology. 2022;162:920-934. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 130] [Article Influence: 43.3] [Reference Citation Analysis (0)] |
| 53. | Vogel A, Saborowski A. Current strategies for the treatment of intermediate and advanced hepatocellular carcinoma. Cancer Treat Rev. 2020;82:101946. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 97] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 54. | Bruix J, Chan SL, Galle PR, Rimassa L, Sangro B. Systemic treatment of hepatocellular carcinoma: An EASL position paper. J Hepatol. 2021;75:960-974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 263] [Article Influence: 65.8] [Reference Citation Analysis (0)] |
| 55. | Vogel A, Grant RC, Meyer T, Sapisochin G, O'Kane GM, Saborowski A. Adjuvant and neoadjuvant therapies for hepatocellular carcinoma. Hepatology. 2023;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 14] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 56. | Ho WJ, Zhu Q, Durham J, Popovic A, Xavier S, Leatherman J, Mohan A, Mo G, Zhang S, Gross N, Charmsaz S, Lin D, Quong D, Wilt B, Kamel IR, Weiss M, Philosophe B, Burkhart R, Burns WR, Shubert C, Ejaz A, He J, Deshpande A, Danilova L, Stein-O'Brien G, Sugar EA, Laheru DA, Anders RA, Fertig EJ, Jaffee EM, Yarchoan M. Neoadjuvant Cabozantinib and Nivolumab Converts Locally Advanced HCC into Resectable Disease with Enhanced Antitumor Immunity. Nat Cancer. 2021;2:891-903. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 132] [Cited by in RCA: 232] [Article Influence: 58.0] [Reference Citation Analysis (0)] |
| 57. | Zhu XD, Huang C, Shen YH, Ji Y, Ge NL, Qu XD, Chen L, Shi WK, Li ML, Zhu JJ, Tan CJ, Tang ZY, Zhou J, Fan J, Sun HC. Downstaging and Resection of Initially Unresectable Hepatocellular Carcinoma with Tyrosine Kinase Inhibitor and Anti-PD-1 Antibody Combinations. Liver Cancer. 2021;10:320-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 162] [Article Influence: 40.5] [Reference Citation Analysis (0)] |
| 58. | Zhang W, Hu B, Han J, Wang Z, Ma G, Ye H, Yuan J, Cao J, Zhang Z, Shi J, Chen M, Wang X, Xu Y, Cheng Y, Tian L, Wang H, Lu S. Surgery After Conversion Therapy With PD-1 Inhibitors Plus Tyrosine Kinase Inhibitors Are Effective and Safe for Advanced Hepatocellular Carcinoma: A Pilot Study of Ten Patients. Front Oncol. 2021;11:747950. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 46] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 59. | Pinato DJ, Cortellini A, Sukumaran A, Cole T, Pai M, Habib N, Spalding D, Sodergren MH, Martinez M, Dhillon T, Tait P, Thomas R, Ward C, Kocher H, Yip V, Slater S, Sharma R. PRIME-HCC: phase Ib study of neoadjuvant ipilimumab and nivolumab prior to liver resection for hepatocellular carcinoma. BMC Cancer. 2021;21:301. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 58] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 60. | Woei-A-Jin FJSH, Weijl NI, Burgmans MC, Fariña Sarasqueta A, van Wezel JT, Wasser MNJM, Coenraad MJ, Burggraaf J, Osanto S. Neoadjuvant Treatment with Angiogenesis-Inhibitor Dovitinib Prior to Local Therapy in Hepatocellular Carcinoma: A Phase II Study. Oncologist. 2021;26:854-864. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 61. | Bouattour M, Fartoux L, Rosmorduc O, Scatton O, Vibert E, Costentin C, Soubrane O, Ronot M, Granier MM, De Gramont A, Belghiti J, Paradis V, Wendum D, Tijeras-Raballand A, Hadengue A, Brusquant D, Chibaudel B, Raymond E, Faivre SJ. BIOSHARE multicenter neoadjuvant phase 2 study: Results of pre-operative sorafenib in patients with resectable hepatocellular carcinoma (HCC)—From GERCOR IRC. J Clin Oncol. 2016;34:suppl.25. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 62. | Qin S, Chen M, Cheng AL, Kaseb AO, Kudo M, Lee HC, Yopp AC, Zhou J, Wang L, Wen X, Heo J, Tak WY, Nakamura S, Numata K, Uguen T, Hsiehchen D, Cha E, Hack SP, Lian Q, Ma N, Spahn JH, Wang Y, Wu C, Chow PKH; IMbrave050 investigators. Atezolizumab plus bevacizumab versus active surveillance in patients with resected or ablated high-risk hepatocellular carcinoma (IMbrave050): a randomised, open-label, multicentre, phase 3 trial. Lancet. 2023;402:1835-1847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 222] [Article Influence: 111.0] [Reference Citation Analysis (0)] |
| 63. | Bruix J, Takayama T, Mazzaferro V, Chau GY, Yang J, Kudo M, Cai J, Poon RT, Han KH, Tak WY, Lee HC, Song T, Roayaie S, Bolondi L, Lee KS, Makuuchi M, Souza F, Berre MA, Meinhardt G, Llovet JM; STORM investigators. Adjuvant sorafenib for hepatocellular carcinoma after resection or ablation (STORM): a phase 3, randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2015;16:1344-1354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 558] [Cited by in RCA: 788] [Article Influence: 78.8] [Reference Citation Analysis (0)] |
| 64. | Kaseb AO, Hasanov E, Cao HST, Xiao L, Vauthey JN, Lee SS, Yavuz BG, Mohamed YI, Qayyum A, Jindal S, Duan F, Basu S, Yadav SS, Nicholas C, Sun JJ, Singh Raghav KP, Rashid A, Carter K, Chun YS, Tzeng CD, Sakamuri D, Xu L, Sun R, Cristini V, Beretta L, Yao JC, Wolff RA, Allison JP, Sharma P. Perioperative nivolumab monotherapy versus nivolumab plus ipilimumab in resectable hepatocellular carcinoma: a randomised, open-label, phase 2 trial. Lancet Gastroenterol Hepatol. 2022;7:208-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 176] [Article Influence: 58.7] [Reference Citation Analysis (0)] |
| 65. | Xia Y, Tang W, Qian X, Li X, Cheng F, Wang K, Zhang F, Zhang C, Li D, Song J, Zhang H, Zhao J, Yao A, Wu X, Wu C, Ji G, Liu X, Zhu F, Qin L, Xiao X, Deng Z, Kong X, Li S, Yu Y, Xi W, Deng W, Qi C, Liu H, Pu L, Wang P, Wang X. Efficacy and safety of camrelizumab plus apatinib during the perioperative period in resectable hepatocellular carcinoma: a single-arm, open label, phase II clinical trial. J Immunother Cancer. 2022;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 118] [Cited by in RCA: 121] [Article Influence: 40.3] [Reference Citation Analysis (0)] |
| 66. | Marron TU, Fiel MI, Hamon P, Fiaschi N, Kim E, Ward SC, Zhao Z, Kim J, Kennedy P, Gunasekaran G, Tabrizian P, Doroshow D, Legg M, Hammad A, Magen A, Kamphorst AO, Shareef M, Gupta NT, Deering R, Wang W, Wang F, Thanigaimani P, Mani J, Troncoso L, Tabachnikova A, Chang C, Akturk G, Buckup M, Hamel S, Ioannou G, Hennequin C, Jamal H, Brown H, Bonaccorso A, Labow D, Sarpel U, Rosenbloom T, Sung MW, Kou B, Li S, Jankovic V, James N, Hamon SC, Cheung HK, Sims JS, Miller E, Bhardwaj N, Thurston G, Lowy I, Gnjatic S, Taouli B, Schwartz ME, Merad M. Neoadjuvant cemiplimab for resectable hepatocellular carcinoma: a single-arm, open-label, phase 2 trial. Lancet Gastroenterol Hepatol. 2022;7:219-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 139] [Article Influence: 46.3] [Reference Citation Analysis (0)] |
| 67. | Mazzaferro V, Citterio D, Bhoori S, Bongini M, Miceli R, De Carlis L, Colledan M, Salizzoni M, Romagnoli R, Antonelli B, Vivarelli M, Tisone G, Rossi M, Gruttadauria S, Di Sandro S, De Carlis R, Lucà MG, De Giorgio M, Mirabella S, Belli L, Fagiuoli S, Martini S, Iavarone M, Svegliati Baroni G, Angelico M, Ginanni Corradini S, Volpes R, Mariani L, Regalia E, Flores M, Droz Dit Busset M, Sposito C. Liver transplantation in hepatocellular carcinoma after tumour downstaging (XXL): a randomised, controlled, phase 2b/3 trial. Lancet Oncol. 2020;21:947-956. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 213] [Article Influence: 42.6] [Reference Citation Analysis (0)] |
| 68. | Lencioni R, Kudo M, Erinjeri J, Qin S, Ren Z, Chan S, Arai Y, Heo J, Mai A, Escobar J, Chuken YAL, Yoon J-H, Tak WY, Suttichaimongkol T, Bouattour M, Lin S-M, Zotkiewicz M, Udoye S, Cohen G, Sangro B. EMERALD-1: A phase 3, randomized, placebo-controlled study of transarterial chemoembolization combined with durvalumab with or without bevacizumab in participants with unresectable hepatocellular carcinoma eligible for embolization. J Clin Oncol. 2024;40:suppl. |
| 69. | Kudo M, Ueshima K, Ikeda M, Torimura T, Tanabe N, Aikata H, Izumi N, Yamasaki T, Nojiri S, Hino K, Tsumura H, Kuzuya T, Isoda N, Yasui K, Aino H, Ido A, Kawabe N, Nakao K, Wada Y, Yokosuka O, Yoshimura K, Okusaka T, Furuse J, Kokudo N, Okita K, Johnson PJ, Arai Y; TACTICS study group. Randomised, multicentre prospective trial of transarterial chemoembolisation (TACE) plus sorafenib as compared with TACE alone in patients with hepatocellular carcinoma: TACTICS trial. Gut. 2020;69:1492-1501. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 221] [Cited by in RCA: 502] [Article Influence: 100.4] [Reference Citation Analysis (0)] |
| 70. | Tai D, Loke K, Gogna A, Kaya NA, Tan SH, Hennedige T, Ng D, Irani F, Lee J, Lim JQ, Too CW, Ng MCH, Tham CK, Lam J, Koo SL, Chong HS, Goh GB, Huang HL, Venkatanarasimha N, Lo R, Chow PKH, Goh BKP, Chung A, Toh HC, Thng CH, Lim TKH, Yeong J, Zhai W, Chan CY, Choo SP. Radioembolisation with Y90-resin microspheres followed by nivolumab for advanced hepatocellular carcinoma (CA 209-678): a single arm, single centre, phase 2 trial. Lancet Gastroenterol Hepatol. 2021;6:1025-1035. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 84] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 71. | Singal AG, Llovet JM, Yarchoan M, Mehta N, Heimbach JK, Dawson LA, Jou JH, Kulik LM, Agopian VG, Marrero JA, Mendiratta-Lala M, Brown DB, Rilling WS, Goyal L, Wei AC, Taddei TH. AASLD Practice Guidance on prevention, diagnosis, and treatment of hepatocellular carcinoma. Hepatology. 2023;78:1922-1965. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 471] [Cited by in RCA: 743] [Article Influence: 371.5] [Reference Citation Analysis (23)] |
| 72. | Zhu AX, Abbas AR, de Galarreta MR, Guan Y, Lu S, Koeppen H, Zhang W, Hsu CH, He AR, Ryoo BY, Yau T, Kaseb AO, Burgoyne AM, Dayyani F, Spahn J, Verret W, Finn RS, Toh HC, Lujambio A, Wang Y. Molecular correlates of clinical response and resistance to atezolizumab in combination with bevacizumab in advanced hepatocellular carcinoma. Nat Med. 2022;28:1599-1611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 341] [Article Influence: 113.7] [Reference Citation Analysis (0)] |
| 73. | Losic B, Craig AJ, Villacorta-Martin C, Martins-Filho SN, Akers N, Chen X, Ahsen ME, von Felden J, Labgaa I, DʹAvola D, Allette K, Lira SA, Furtado GC, Garcia-Lezana T, Restrepo P, Stueck A, Ward SC, Fiel MI, Hiotis SP, Gunasekaran G, Sia D, Schadt EE, Sebra R, Schwartz M, Llovet JM, Thung S, Stolovitzky G, Villanueva A. Intratumoral heterogeneity and clonal evolution in liver cancer. Nat Commun. 2020;11:291. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 133] [Cited by in RCA: 257] [Article Influence: 51.4] [Reference Citation Analysis (0)] |
| 74. | Kim SI, Cassella CR, Byrne KT. Tumor Burden and Immunotherapy: Impact on Immune Infiltration and Therapeutic Outcomes. Front Immunol. 2020;11:629722. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 105] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 75. | Becht R, Kiełbowski K, Wasilewicz MP. New Opportunities in the Systemic Treatment of Hepatocellular Carcinoma-Today and Tomorrow. Int J Mol Sci. 2024;25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 76. | Greten TF, Villanueva A, Korangy F, Ruf B, Yarchoan M, Ma L, Ruppin E, Wang XW. Biomarkers for immunotherapy of hepatocellular carcinoma. Nat Rev Clin Oncol. 2023;20:780-798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 99] [Reference Citation Analysis (0)] |
| 77. | Wang Y, Deng B. Hepatocellular carcinoma: molecular mechanism, targeted therapy, and biomarkers. Cancer Metastasis Rev. 2023;42:629-652. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 161] [Article Influence: 80.5] [Reference Citation Analysis (0)] |
| 78. | Luo P, Yin P, Hua R, Tan Y, Li Z, Qiu G, Yin Z, Xie X, Wang X, Chen W, Zhou L, Li Y, Chen H, Gao L, Lu X, Wu T, Wang H, Niu J, Xu G. A Large-scale, multicenter serum metabolite biomarker identification study for the early detection of hepatocellular carcinoma. Hepatology. 2018;67:662-675. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 193] [Cited by in RCA: 284] [Article Influence: 40.6] [Reference Citation Analysis (0)] |
| 79. | Parikh ND, Tayob N, Singal AG. Blood-based biomarkers for hepatocellular carcinoma screening: Approaching the end of the ultrasound era? J Hepatol. 2023;78:207-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 92] [Article Influence: 46.0] [Reference Citation Analysis (0)] |
| 80. | Zhang N, Yang X, Piao M, Xun Z, Wang Y, Ning C, Zhang X, Zhang L, Wang S, Chao J, Lu Z, Wang H, Zhao H. Biomarkers and prognostic factors of PD-1/PD-L1 inhibitor-based therapy in patients with advanced hepatocellular carcinoma. Biomark Res. 2024;12:26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 35] [Reference Citation Analysis (0)] |