Published online May 21, 2024. doi: 10.3748/wjg.v30.i19.2505
Revised: March 18, 2024
Accepted: April 25, 2024
Published online: May 21, 2024
Processing time: 126 Days and 18.1 Hours
Chronic enteropathy associated with the SLCO2A1 gene (CEAS) is a complex gastroenterological condition characterized by multiple ulcers in the small intestine with chronic bleeding and protein loss. This review explores the potential mechanisms underlying the pathogenesis of CEAS, focusing on the role of SLCO2A1-encoded prostaglandin transporter OATP2A1 and its impact on prostaglandin E2 (PGE2) levels. Studies have suggested that elevated PGE2 levels contribute to mucosal damage, inflammation, and disruption of the intestinal barrier. The effects of PGE2 on macrophage activation and Maxi-Cl channel functionality, as well as its interaction with nonsteroidal anti-inflammatory drugs play crucial roles in the progression of CEAS. Understanding the balance between its protective and pro-inflammatory effects and the complex interactions within the gastrointestinal tract can shed light on potential therapeutic targets for CEAS and guide the development of novel, targeted therapies.
Core Tip: Chronic enteropathy associated with SLCO2A1 gene (CEAS) is a complex gastroenterological condition characterized by multiple ulcers in the small intestine with chronic bleeding and protein loss. This review explores potential mechanisms underlying the pathogenesis of CEAS, focusing on the role of SLCO2A1-encoded prostaglandin transporter OATP2A1 and its impact on prostaglandin E2 (PGE2) levels. Studies suggest that elevated PGE2 levels contribute to mucosal damage, inflammation, and disruption of the intestinal barrier. The effects of PGE2 on macrophage activation, Maxi-Cl channel functionality, and its interaction with nonsteroidal anti-inflammatory drugs also play crucial roles in the progression of CEAS. Understanding the delicate balance of its protective and pro-inflammatory effects as well as the complex interactions within the gastrointestinal tract can shed light on potential therapeutic targets for CEAS and guide the development of novel, targeted therapies.
- Citation: Xie ZX, Li Y, Yang AM, Wu D, Wang Q. Pathogenesis of chronic enteropathy associated with the SLCO2A1 gene: Hypotheses and conundrums. World J Gastroenterol 2024; 30(19): 2505-2511
- URL: https://www.wjgnet.com/1007-9327/full/v30/i19/2505.htm
- DOI: https://dx.doi.org/10.3748/wjg.v30.i19.2505
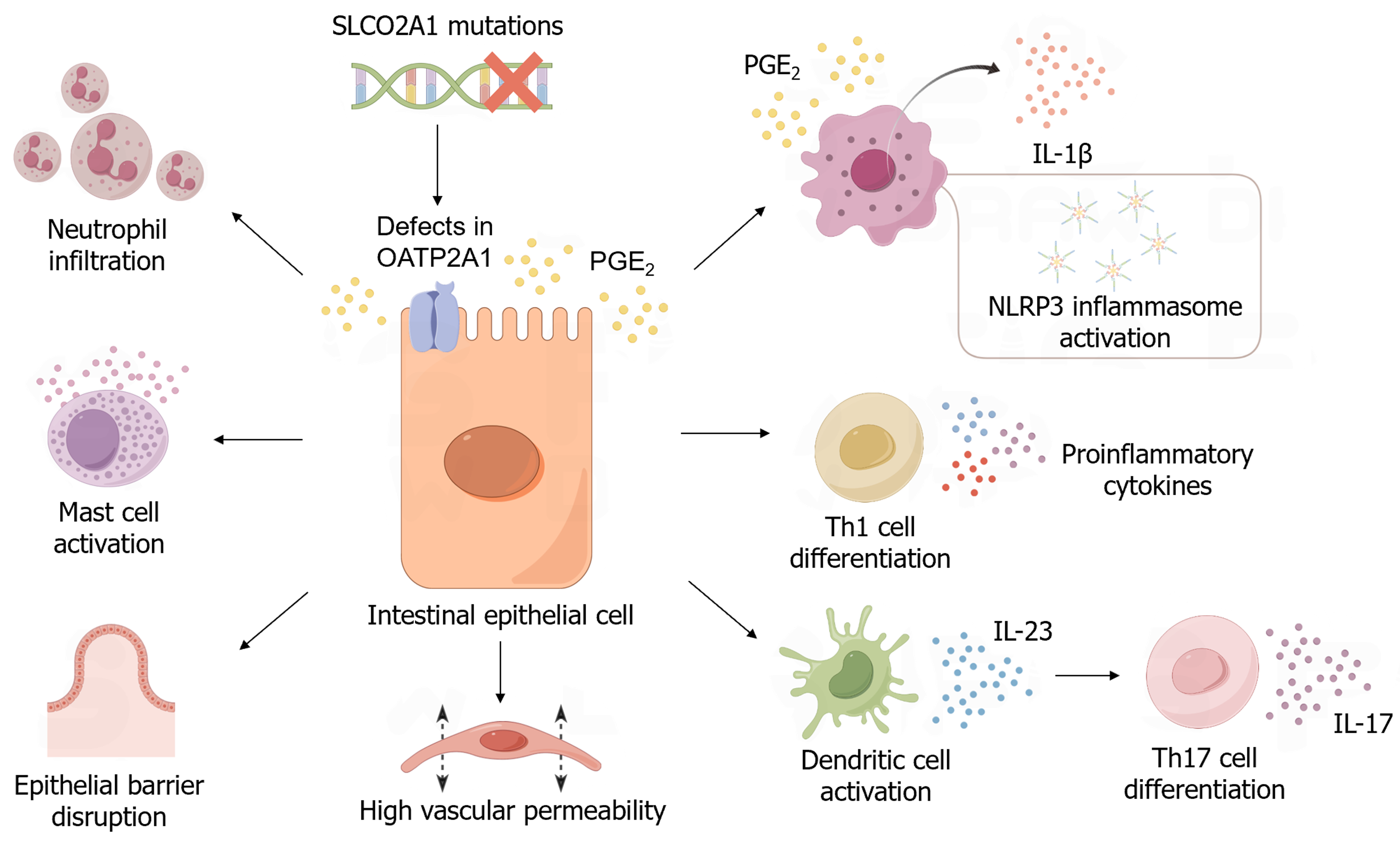
Chronic enteropathy associated with the SLCO2A1 gene (CEAS) is a gastroenterological disease characterized by multiple ulcers in the small intestine with chronic bleeding and protein loss. Before mutations in the SLCO2A1 gene were identified, CEAS was originally considered to be a chronic nonspecific multiple ulcer of the small intestine[1,2]. Although nearly all the segments of the stomach and small intestine can be affected in individuals with CEAS, involvement of the ileum (excluding the terminal segment) is most frequently observed[3]. Without genetic testing, the lack of histological specificity makes it difficult to distinguish CEAS from other gastrointestinal ulcerative diseases, such as Crohn’s disease and cryptogenic multifocal ulcerous stenosing enteritis. However, elevated serum levels of prostaglandin E2 (PGE2) and urinary levels of PGE-M can contribute to simplifying the differential diagnosis of CEAS from other ulcerative diseases[4]. Variants of the SLCO2A1 gene have also been reported to cause primary hypertrophic osteoarthropathy autosomal recessive 2 (PHOAR2), which is characterized by digit clubbing and osteoarthropathy[5-7]. Some male individuals with CEAS also share the symptoms of PHO, which partially assists with CEAS diagnosis. Although an increasingly profound understanding of CEAS is currently being gained, its pathogenesis is still unclear. In this review, we discuss recent findings on the possible mechanisms underlying the pathogenesis of CEAS (Figure 1).
Human SLCO2A1 is located on chromosome position 3q22.1-q22.2 and encodes OATP2A1 protein, a member of the solute carrier organic anion transporter family[8]. OATP2A1, which has 12 transmembrane domains, plays an essential role in the transport of prostanoids, especially PGE2[9]. Using lactate or other anions as a driving force, OATP2A1 promotes the uptake of prostaglandins into the cells, leading to degradation by 15-PGDH[10,11]. With an inwardly directed gradient of exchanged anions or inflammatory factors, OATP2A1 can act as an exporter of prostaglandins, thus exerting its bidirectional function[12]. OATP2A1 also mediates the trans-epithelial transport of prostaglandins by capturing them from the apical side and releasing them at the basal side, from where they can be released into the stroma[12-14]. OATP2A1 is also thought to remain in the cytoplasm of macrophages and load PGE2 into intracellular compartments to facilitate PGE2 secretion at a later stage[15]. Because of its role in PGE2 uptake and inactivation, defects in OATP2A1 caused by SLCO2A1 loss-of-function mutations lead to elevated serum levels of PGE2, which is considered a pathogenic hallmark of CEAS.
In addition to its known protective effect on the gastrointestinal mucosa, PGE2 has been shown to exhibit pro-inflammatory effects in several tissues, including the gastrointestinal tract (GIT)[16-18]. PGE2 induces vasodilation, activates mast cells, and induces histamine release through the EP receptor, causing acute inflammation with high vascular permeability and neutrophil infiltration. PGE2 also contributes to type 1 T helper (Th1) cell differentiation, which mediates autoimmune diseases[19]. Notably, a study based on an experimental inflammatory bowel disease (IBD) mouse model suggested that PGE2 promotes the production and release of interleukin (IL)-23 from activated dendritic cells, which leads to Th17 cell differentiation and IL-17 expression[20]. In addition to Th1 cells, Th17 cells have been reported as another important pathogenetic factor that causes tissue damage and inflammation in IBD[21]. High serum PGE2 levels in individuals with CEAS may disrupt the balance between pro- and anti-inflammatory signals in gastrointestinal tissues, leading to mucosal damage.
The gastrointestinal mucosal barrier is essential for protecting the GIT from damage. Intestinal epithelial cells and the tight junctions (TJs) between them constitute a mechanical barrier that prevents the invasion of the deeper layer of the bowel wall by pathogenic microorganisms and toxins. PGE2 has a dual effect on the gastrointestinal epithelial cell barrier. First, PGE2 protects the integrity of the intestinal mucosal barrier by promoting the differentiation of wound-associated epithelial cells from epithelial stem cells, which repair the wounded mucosa and induce regeneration[22,23]. Conversely, PGE2 has been reported to induce epithelial barrier disruption. A previous study showed that through EP1 and EP4 receptors, PGE2 induces an increase in paracellular permeability of Caco-2 cell monolayers by activating PLC-IP3-Ca2+ and the cAMP-PKA pathway, which may alter the distribution of occludin and zonula occludens-1[24]. PGE3 has been shown to exhibit a similar effect[25]. Another study suggested that PGE2 secreted by Entamoeba histolytica leads to a decrease in mucosal barrier integrity and elevated ion permeability through EP4 receptors, causing diarrhea[26]. Pathologically increased PGE2 levels may support its barrier-disrupting function and overwhelm its protective effect on the mucosal barrier, ultimately leading to the formation of ulcers.
Macrophages are regarded as key factors in maintaining gastrointestinal homeostasis by secreting a variety of cytokines and promoting the differentiation of certain immune cell subgroups[27,28]. An imbalance of intestinal macrophages leads to an excessive response to inflammatory stimuli, which is considered to underlie the pathogenesis of IBD[29,30]. As cells expressing EP2 and EP4 receptors, macrophages can potentially be regulated by PGE2. A positive correlation has been shown between PGE2 levels in rat colonic mucosa and the degree of macrophage infiltration[31]. A recent study showed that mice with Slco2a1-deficient macrophages are more sensitive to dextran sulfate sodium-induced colitis. Macrophages that fail to take up PGE2 for degradation are exposed to abnormally high levels of PGE2. Under the regulation of PGE2, macrophages secrete excessive levels of IL-1β, which is mediated by NOD-like receptor protein 3 (NLRP3) inflammasome activation[32]. As the involvement of the NLRP3 inflammasome and IL-1β in intestinal inflammation has been widely supported[33-35], the regulation of macrophages may also play a role in ulcer formation in CEAS. However, PGE2 has also been reported to act on ER4-expressing macrophages to promote the regeneration of intestinal epithelium[29,36]. In addition, PGE2 facilitates the polarization of M2 macrophages that exhibit anti-inflammatory effects and promote wound healing[37,38]. Therefore, further study is required to identify the specific effect of PGE2 on macrophages in CEAS as well as the underlying roles of certain receptors or cell subsets.
SLCO2A1 functions as a PG transporter, and is also an integral core component of the Maxi-Cl channel, which regulates the efflux of various important organic and inorganic molecules[39,40]. Maxi-Cl channels are permeable to glutathione and thus may contribute to the protection of cells from oxidative damage[41,42]. Maxi-Cl channels may also be able to mediate the transport of short-chain fatty acids in humans, as has been shown in ruminants[43,44]. Two mutations in individuals with PHO, G222R and P219L, were shown to completely abrogate the function of Maxi-Cl channels[39]. These findings indicate that loss of functional Maxi-Cl channels as a result of mutant SLCO2A1 may partially contribute to the intestinal lesions observed in CEAS.
Although nonsteroidal anti-inflammatory drugs (NSAIDs) can effectively alleviate the symptoms of PHO[45], they have been reported to exacerbate gastrointestinal lesions in individuals with CEAS[46]. This may be attributed to the inhibition of both COX-1 and COX-2, which maintain a constitutive level of PGE2 to protect the intestinal mucosa. Furthermore, NSAIDs induce the excessive generation of vasodilating molecules and mitochondrial oxidative stress, worsening the mucosal inflammation[47,48]. Interestingly, an indirect interaction between NSAIDs and OATP2A1 has also been reported. A study on a family affected by digit clubbing showed that heterozygous SLCO2A1 nonsense mutations are associated with resistance to NSAIDs[49]. Partial loss of OATP2A1 function causes relatively high extracellular PGE2 levels, which may cause a positive feedback loop that activates COX-2[50]. Collectively, NSAIDs have either stimulatory or inhibitory effects on OATP2A1, depending on their chemical structures[51,52]. Importantly, further analyses are required before the administration of NSAIDs to individuals carrying SLCO2A1 mutations.
In this review, we recapitulated the reported discoveries of CEAS pathogenesis, while also highlighting some inconsistencies within the findings and hypotheses. The protective function of PGE2 as well as its pro-inflammatory and barrier-disrupting effects are seemingly two extremes of a balanced mechanism, which maintains the normal state of the GIT at physiological concentrations. This equilibrium may explain why both low PGE2 levels caused by long-term NSAID usage and high PGE2 levels owing to transport deficiency can cause intestinal lesions. Excessive activation of macrophages is a potential mechanism underlying CEAS. However, mice with SLCO2A1-deficient macrophages failed to exhibit all the symptoms of systemic knockout mice[32], indicating that CEAS may not be explainable through a single hypothesis.
The failure of NSAIDs such as COX-2 inhibitors to alleviate gastrointestinal symptoms suggested that other factors in addition to PGE2 influence the disease status of CEAS. The integrity of the intestinal barrier is considered to play an important role in ulcerative diseases. However, further studies are required to assess the integrity of the intestinal barrier and the function of molecules that compose TJs in CEAS. In addition to PGE2, the intracellular and extracellular concentrations of other molecules that potentially depend on OATP2A1 transportation should be considered. These can be investigated using systemic and conditional SLCO2A1-knockout animal models. Additionally, considering that approximately 36% of PHOAR2 patients with homozygous and compound heterozygous SLCO2A1 loss-of-function mutations develop gastrointestinal symptoms[46], environmental factors and the basic physical condition of patients should be considered. This includes dietary habits, immune levels, and the composition of the intestinal flora.
A preponderance of female patients with CEAS have been reported compared to male patients, indicating that SLCO2A1 variations are more likely to lead to gastrointestinal symptoms in females[3]. Notably, elevated levels of PGE2 in follicles are essential for regulating ovulation. In response to changing PGE2 levels, OATP2A1 is periodically expressed in female reproductive tissues to transport PGs, participating in physiological processes such as ovulation, pregnancy, and delivery. Therefore, OATP2A1 dysfunction in the ovaries and uterus also contributes increased levels of circulating PGE2, which can exhibit a synergistic effect with PGE2 accumulation caused by OATP2A1 dysfunction in the GIT. Medical treatments that temporarily block the menstrual cycle may reduce gastrointestinal symptoms by partially easing the burden of PGE2 transport.
Because of the unclear pathogenesis of CEAS, conventional treatments for IBD, such as NSAIDs, glucocorticoids, immunosuppressants, and probiotics, are often ineffective, leading to patients considering surgery. Refractory diarrhea and hematochezia can lead to lowered hemoglobin and albumin levels, even with support through specific enteral or parenteral nutrition. Persistent abdominal pain, anemia, and malnutrition significantly compromise the quality of life of patients, amplifying the global disease burden of CEAS. Therefore, it is crucial to unravel the underlying pathogenesis of CEAS to enable the development of accessible, targeted, and efficacious therapeutics.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report’s classification
Scientific Quality: Grade A
Novelty: Grade A
Creativity or Innovation: Grade B
Scientific Significance: Grade A
P-Reviewer: Tanabe H, Japan S-Editor: Wang JJ L-Editor: A P-Editor: Cai YX
| 1. | Matsumoto T, Iida M, Matsui T, Yao T. Chronic nonspecific multiple ulcers of the small intestine: a proposal of the entity from Japanese gastroenterologists to Western enteroscopists. Gastrointest Endosc. 2007;66:S99-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 60] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 2. | Umeno J, Hisamatsu T, Esaki M, Hirano A, Kubokura N, Asano K, Kochi S, Yanai S, Fuyuno Y, Shimamura K, Hosoe N, Ogata H, Watanabe T, Aoyagi K, Ooi H, Watanabe K, Yasukawa S, Hirai F, Matsui T, Iida M, Yao T, Hibi T, Kosaki K, Kanai T, Kitazono T, Matsumoto T. A Hereditary Enteropathy Caused by Mutations in the SLCO2A1 Gene, Encoding a Prostaglandin Transporter. PLoS Genet. 2015;11:e1005581. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 112] [Cited by in RCA: 103] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 3. | Umeno J, Esaki M, Hirano A, Fuyuno Y, Ohmiya N, Yasukawa S, Hirai F, Kochi S, Kurahara K, Yanai S, Uchida K, Hosomi S, Watanabe K, Hosoe N, Ogata H, Hisamatsu T, Nagayama M, Yamamoto H, Abukawa D, Kakuta F, Onodera K, Matsui T, Hibi T, Yao T, Kitazono T, Matsumoto T; CEAS study group. Clinical features of chronic enteropathy associated with SLCO2A1 gene: a new entity clinically distinct from Crohn's disease. J Gastroenterol. 2018;53:907-915. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 47] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 4. | Matsuno Y, Umeno J, Esaki M, Hirakawa Y, Fuyuno Y, Okamoto Y, Hirano A, Yasukawa S, Hirai F, Matsui T, Hosomi S, Watanabe K, Hosoe N, Ogata H, Hisamatsu T, Yanai S, Kochi S, Kurahara K, Yao T, Torisu T, Kitazono T, Matsumoto T. Measurement of prostaglandin metabolites is useful in diagnosis of small bowel ulcerations. World J Gastroenterol. 2019;25:1753-1763. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 18] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 5. | Hou Y, Lin Y, Qi X, Yuan L, Liao R, Pang Q, Cui L, Jiang Y, Wang O, Li M, Dong J, Xia W. Identification of mutations in the prostaglandin transporter gene SLCO2A1 and phenotypic comparison between two subtypes of primary hypertrophic osteoarthropathy (PHO): A single-center study. Bone. 2018;106:96-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 6. | Seifert W, Kühnisch J, Tüysüz B, Specker C, Brouwers A, Horn D. Mutations in the prostaglandin transporter encoding gene SLCO2A1 cause primary hypertrophic osteoarthropathy and isolated digital clubbing. Hum Mutat. 2012;33:660-664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 60] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 7. | Zhang Z, Xia W, He J, Zhang Z, Ke Y, Yue H, Wang C, Zhang H, Gu J, Hu W, Fu W, Hu Y, Li M, Liu Y. Exome sequencing identifies SLCO2A1 mutations as a cause of primary hypertrophic osteoarthropathy. Am J Hum Genet. 2012;90:125-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 145] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 8. | Lu R, Kanai N, Bao Y, Schuster VL. Cloning, in vitro expression, and tissue distribution of a human prostaglandin transporter cDNA(hPGT). J Clin Invest. 1996;98:1142-1149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 181] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 9. | Pucci ML, Bao Y, Chan B, Itoh S, Lu R, Copeland NG, Gilbert DJ, Jenkins NA, Schuster VL. Cloning of mouse prostaglandin transporter PGT cDNA: species-specific substrate affinities. Am J Physiol. 1999;277:R734-R741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 17] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 10. | Chan BS, Satriano JA, Pucci M, Schuster VL. Mechanism of prostaglandin E2 transport across the plasma membrane of HeLa cells and Xenopus oocytes expressing the prostaglandin transporter "PGT". J Biol Chem. 1998;273:6689-6697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 106] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 11. | Chan BS, Endo S, Kanai N, Schuster VL. Identification of lactate as a driving force for prostanoid transport by prostaglandin transporter PGT. Am J Physiol Renal Physiol. 2002;282:F1097-F1102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 61] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 12. | Shirasaka Y, Shichiri M, Kasai T, Ohno Y, Nakanishi T, Hayashi K, Nishiura A, Tamai I. A role of prostaglandin transporter in regulating PGE₂ release from human bronchial epithelial BEAS-2B cells in response to LPS. J Endocrinol. 2013;217:265-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 13. | Nomura T, Lu R, Pucci ML, Schuster VL. The two-step model of prostaglandin signal termination: in vitro reconstitution with the prostaglandin transporter and prostaglandin 15 dehydrogenase. Mol Pharmacol. 2004;65:973-978. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 116] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 14. | Endo S, Nomura T, Chan BS, Lu R, Pucci ML, Bao Y, Schuster VL. Expression of PGT in MDCK cell monolayers: polarized apical localization and induction of active PG transport. Am J Physiol Renal Physiol. 2002;282:F618-F622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 15. | Shimada H, Nakamura Y, Nakanishi T, Tamai I. OATP2A1/SLCO2A1-mediated prostaglandin E2 loading into intracellular acidic compartments of macrophages contributes to exocytotic secretion. Biochem Pharmacol. 2015;98:629-638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 33] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 16. | Sheibanie AF, Khayrullina T, Safadi FF, Ganea D. Prostaglandin E2 exacerbates collagen-induced arthritis in mice through the inflammatory interleukin-23/interleukin-17 axis. Arthritis Rheum. 2007;56:2608-2619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 122] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 17. | Yao C, Hirata T, Soontrapa K, Ma X, Takemori H, Narumiya S. Prostaglandin E₂ promotes Th1 differentiation via synergistic amplification of IL-12 signalling by cAMP and PI3-kinase. Nat Commun. 2013;4:1685. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 90] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 18. | Morimoto K, Shirata N, Taketomi Y, Tsuchiya S, Segi-Nishida E, Inazumi T, Kabashima K, Tanaka S, Murakami M, Narumiya S, Sugimoto Y. Prostaglandin E2-EP3 signaling induces inflammatory swelling by mast cell activation. J Immunol. 2014;192:1130-1137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 100] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 19. | Tsuge K, Inazumi T, Shimamoto A, Sugimoto Y. Molecular mechanisms underlying prostaglandin E2-exacerbated inflammation and immune diseases. Int Immunol. 2019;31:597-606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 172] [Article Influence: 34.4] [Reference Citation Analysis (0)] |
| 20. | Sheibanie AF, Yen JH, Khayrullina T, Emig F, Zhang M, Tuma R, Ganea D. The proinflammatory effect of prostaglandin E2 in experimental inflammatory bowel disease is mediated through the IL-23-->IL-17 axis. J Immunol. 2007;178:8138-8147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 217] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 21. | Jiang P, Zheng C, Xiang Y, Malik S, Su D, Xu G, Zhang M. The involvement of TH17 cells in the pathogenesis of IBD. Cytokine Growth Factor Rev. 2023;69:28-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 76] [Article Influence: 38.0] [Reference Citation Analysis (0)] |
| 22. | Miyoshi H, VanDussen KL, Malvin NP, Ryu SH, Wang Y, Sonnek NM, Lai CW, Stappenbeck TS. Prostaglandin E2 promotes intestinal repair through an adaptive cellular response of the epithelium. EMBO J. 2017;36:5-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 180] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 23. | Kunikata T, Tanaka A, Miyazawa T, Kato S, Takeuchi K. 16,16-Dimethyl prostaglandin E2 inhibits indomethacin-induced small intestinal lesions through EP3 and EP4 receptors. Dig Dis Sci. 2002;47:894-904. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 75] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 24. | Rodríguez-Lagunas MJ, Martín-Venegas R, Moreno JJ, Ferrer R. PGE2 promotes Ca2+-mediated epithelial barrier disruption through EP1 and EP4 receptors in Caco-2 cell monolayers. Am J Physiol Cell Physiol. 2010;299:C324-C334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 58] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 25. | Rodríguez-Lagunas MJ, Ferrer R, Moreno JJ. Effect of eicosapentaenoic acid-derived prostaglandin E3 on intestinal epithelial barrier function. Prostaglandins Leukot Essent Fatty Acids. 2013;88:339-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 26. | Lejeune M, Moreau F, Chadee K. Prostaglandin E2 produced by Entamoeba histolytica signals via EP4 receptor and alters claudin-4 to increase ion permeability of tight junctions. Am J Pathol. 2011;179:807-818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 57] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 27. | Bain CC, Mowat AM. Macrophages in intestinal homeostasis and inflammation. Immunol Rev. 2014;260:102-117. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 357] [Cited by in RCA: 455] [Article Influence: 45.5] [Reference Citation Analysis (1)] |
| 28. | Mowat AM. To respond or not to respond - a personal perspective of intestinal tolerance. Nat Rev Immunol. 2018;18:405-415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 125] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 29. | Na YR, Stakenborg M, Seok SH, Matteoli G. Macrophages in intestinal inflammation and resolution: a potential therapeutic target in IBD. Nat Rev Gastroenterol Hepatol. 2019;16:531-543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 272] [Cited by in RCA: 593] [Article Influence: 98.8] [Reference Citation Analysis (0)] |
| 30. | Hegarty LM, Jones GR, Bain CC. Macrophages in intestinal homeostasis and inflammatory bowel disease. Nat Rev Gastroenterol Hepatol. 2023;20:538-553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 141] [Reference Citation Analysis (0)] |
| 31. | Yamashita S. Studies on changes of colonic mucosal PGE2 levels and tissue localization in experimental colitis. Gastroenterol Jpn. 1993;28:224-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 12] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 32. | Nakata R, Nakamura Y, Hosomi S, Okuda H, Nishida Y, Sugita N, Itani S, Nadatani Y, Otani K, Tanaka F, Kamata N, Taira K, Nagami Y, Tanigawa T, Watanabe T, Yamagami H, Nakanishi T, Fujiwara Y. Slco2a1 deficiency exacerbates experimental colitis via inflammasome activation in macrophages: a possible mechanism of chronic enteropathy associated with SLCO2A1 gene. Sci Rep. 2020;10:4883. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 33. | Zhen Y, Zhang H. NLRP3 Inflammasome and Inflammatory Bowel Disease. Front Immunol. 2019;10:276. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 487] [Cited by in RCA: 485] [Article Influence: 80.8] [Reference Citation Analysis (0)] |
| 34. | Ishiguro Y. Mucosal proinflammatory cytokine production correlates with endoscopic activity of ulcerative colitis. J Gastroenterol. 1999;34:66-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 143] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 35. | Kwon KH, Murakami A, Hayashi R, Ohigashi H. Interleukin-1beta targets interleukin-6 in progressing dextran sulfate sodium-induced experimental colitis. Biochem Biophys Res Commun. 2005;337:647-654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 94] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 36. | Na YR, Jung D, Stakenborg M, Jang H, Gu GJ, Jeong MR, Suh SY, Kim HJ, Kwon YH, Sung TS, Ryoo SB, Park KJ, Im JP, Park JY, Lee YS, Han H, Park B, Lee S, Kim D, Lee HS, Cleynen I, Matteoli G, Seok SH. Prostaglandin E(2) receptor PTGER4-expressing macrophages promote intestinal epithelial barrier regeneration upon inflammation. Gut. 2021;70:2249-2260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 55] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 37. | Xu M, Wang X, Li Y, Geng X, Jia X, Zhang L, Yang H. Arachidonic Acid Metabolism Controls Macrophage Alternative Activation Through Regulating Oxidative Phosphorylation in PPARγ Dependent Manner. Front Immunol. 2021;12:618501. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 63] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 38. | Kim YG, Udayanga KG, Totsuka N, Weinberg JB, Núñez G, Shibuya A. Gut dysbiosis promotes M2 macrophage polarization and allergic airway inflammation via fungi-induced PGE₂. Cell Host Microbe. 2014;15:95-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 225] [Cited by in RCA: 284] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 39. | Sabirov RZ, Merzlyak PG, Okada T, Islam MR, Uramoto H, Mori T, Makino Y, Matsuura H, Xie Y, Okada Y. The organic anion transporter SLCO2A1 constitutes the core component of the Maxi-Cl channel. EMBO J. 2017;36:3309-3324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 45] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 40. | Sabirov RZ, Islam MR, Okada T, Merzlyak PG, Kurbannazarova RS, Tsiferova NA, Okada Y. The ATP-Releasing Maxi-Cl Channel: Its Identity, Molecular Partners and Physiological/Pathophysiological Implications. Life (Basel). 2021;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 41. | Sabirov RZ, Kurbannazarova RS, Melanova NR, Okada Y. Volume-sensitive anion channels mediate osmosensitive glutathione release from rat thymocytes. PLoS One. 2013;8:e55646. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 42. | Forman HJ, Zhang H, Rinna A. Glutathione: overview of its protective roles, measurement, and biosynthesis. Mol Aspects Med. 2009;30:1-12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1947] [Cited by in RCA: 1665] [Article Influence: 104.1] [Reference Citation Analysis (0)] |
| 43. | Georgi MI, Rosendahl J, Ernst F, Günzel D, Aschenbach JR, Martens H, Stumpff F. Epithelia of the ovine and bovine forestomach express basolateral maxi-anion channels permeable to the anions of short-chain fatty acids. Pflugers Arch. 2014;466:1689-1712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 44. | Stumpff F. A look at the smelly side of physiology: transport of short chain fatty acids. Pflugers Arch. 2018;470:571-598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 104] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 45. | Giancane G, Diggle CP, Legger EG, Tekstra J, Prakken B, Brenkman AB, Carr IM, Markham AF, Bonthron DT, Wulffraat N. Primary Hypertrophic Osteoarthropathy: An Update on Patient Features and Treatment. J Rheumatol. 2015;42:2211-2214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 46. | Wang Q, Li YH, Lin GL, Li Y, Zhou WX, Qian JM, Xia WB, Wu D. Primary hypertrophic osteoarthropathy related gastrointestinal complication has distinctive clinical and pathological characteristics: two cases report and review of the literature. Orphanet J Rare Dis. 2019;14:297. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 47. | Bindu S, Mazumder S, Bandyopadhyay U. Non-steroidal anti-inflammatory drugs (NSAIDs) and organ damage: A current perspective. Biochem Pharmacol. 2020;180:114147. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 333] [Cited by in RCA: 891] [Article Influence: 178.2] [Reference Citation Analysis (0)] |
| 48. | Harirforoosh S, Asghar W, Jamali F. Adverse effects of nonsteroidal antiinflammatory drugs: an update of gastrointestinal, cardiovascular and renal complications. J Pharm Pharm Sci. 2013;16:821-847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 358] [Cited by in RCA: 486] [Article Influence: 44.2] [Reference Citation Analysis (0)] |
| 49. | Guda K, Fink SP, Milne GL, Molyneaux N, Ravi L, Lewis SM, Dannenberg AJ, Montgomery CG, Zhang S, Willis J, Wiesner GL, Markowitz SD. Inactivating mutation in the prostaglandin transporter gene, SLCO2A1, associated with familial digital clubbing, colon neoplasia, and NSAID resistance. Cancer Prev Res (Phila). 2014;7:805-812. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 50. | Díaz-Muñoz MD, Osma-García IC, Fresno M, Iñiguez MA. Involvement of PGE2 and the cAMP signalling pathway in the up-regulation of COX-2 and mPGES-1 expression in LPS-activated macrophages. Biochem J. 2012;443:451-461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 87] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 51. | Mandery K, Bujok K, Schmidt I, Wex T, Treiber G, Malfertheiner P, Rau TT, Amann KU, Brune K, Fromm MF, Glaeser H. Influence of cyclooxygenase inhibitors on the function of the prostaglandin transporter organic anion-transporting polypeptide 2A1 expressed in human gastroduodenal mucosa. J Pharmacol Exp Ther. 2010;332:345-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 52. | Kamo S, Nakanishi T, Aotani R, Nakamura Y, Gose T, Tamai I. Impact of FDA-Approved Drugs on the Prostaglandin Transporter OATP2A1/SLCO2A1. J Pharm Sci. 2017;106:2483-2490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |