Published online May 7, 2024. doi: 10.3748/wjg.v30.i17.2321
Revised: March 25, 2024
Accepted: April 9, 2024
Published online: May 7, 2024
Processing time: 76 Days and 2.2 Hours
The advent of cutting-edge systemic therapies has driven advances in the treatment of hepatocellular carcinoma (HCC), and therapeutic strategies with multiple modes of delivery have been shown to be more efficacious than mono
To evaluate the clinical efficacy of targeted therapy plus immunotherapy combined with hepatic arterial infusion chemotherapy (HAIC) of FOLFOX in patients with unresectable HCC.
We enrolled 53 patients with unresectable HCC who received a combination of targeted therapy, immunotherapy, and HAIC of FOLFOX between December 2020 and June 2021 and assessed the efficacy and safety of the treatment regimen.
The objective response rate was 60.4% (32/53), complete response was 24.5% (13/53), partial response was 35.9% (19/53), and stable disease was 39.6% (21/53). The median duration of response and median progression-free survival were 9.1 and 13.9 months, respectively. The surgical conversion rate was 34.0% (18/53), and 1-year overall survival was 83.0% without critical complicating diseases or adverse events (AEs).
The regimen of HAIC of FOLFOX, targeted therapy, and immunotherapy was curative for patients with unresectable HCC, with no serious AEs and a high rate of surgical conversion.
Core Tip: The therapeutic strategy of combining multiple modes of drug delivery for the treatment of hepatocellular carcinoma (HCC) has been shown to be more efficacious than single treatment modality, but the underlying mechanism of action has not been clarified. In this study, we observed the clinical efficacy of targeted therapy plus immunotherapy combined with FOLFOX hepatic artery infusion chemotherapy in the treatment of unresectable HCC, which provides a clinical basis for the clinical application of the combination of therapy in HCC.
- Citation: Lin ZP, Hu XL, Chen D, Huang DB, Zou XG, Zhong H, Xu SX, Chen Y, Li XQ, Zhang J. Efficacy and safety of targeted therapy plus immunotherapy combined with hepatic artery infusion chemotherapy (FOLFOX) for unresectable hepatocarcinoma. World J Gastroenterol 2024; 30(17): 2321-2331
- URL: https://www.wjgnet.com/1007-9327/full/v30/i17/2321.htm
- DOI: https://dx.doi.org/10.3748/wjg.v30.i17.2321
Globally, the third most prevalent cause of cancer-related mortality and the sixth most frequently occurring cancer type is primary hepatocellular carcinoma (HCC)[1]. The first-line treatment options for early-stage HCC are surgical resection and liver transplantation[2,3]. Most patients with early-stage HCC do not have obvious symptoms, and many of them have already developed middle- or late-stage disease at the time of diagnosis. Additionally, since malignant vascular invasion is considered a contraindication to surgical treatment, only 5%-10% of patients with HCC are suitable candidates[4]. There are a variety of treatment methods for unresectable HCC, including interventional therapy, ablation therapy, antitumor targeted therapy, antitumor immunotherapy, radiation therapy, and radioactive seed implantation therapy. The efficacy of hepatic arterial infusion chemotherapy (HAIC) for advanced HCC with vascular invasion or multiple intrahepatic lesions is highly regarded in clinical practice. The Japan Society of Hepatology recommends HAIC as the first-line option for HCC with portal vein tumor thrombus (PVTT)[5]. HCC tumors receive a dual blood supply from the portal vein and hepatic artery (primarily from the hepatic artery). HAIC involves injecting chemotherapeutic drugs directly into the HCC tumor through the hepatic artery. Although HAIC increases the concentration of anticancer drugs in tumor cells, the incidence of side effects is reduced due to the localized distribution. Therefore, HAIC may have a stronger antitumor effect and a lower incidence of adverse events (AEs) compared with other chemotherapeutic methods. Although sorafenib plus HAIC of FOLFOX was reported to improve the objective response rate (ORR) in patients with HCC rather than sorafenib alone, the survival duration remained inadequate[6].
The advent of cutting-edge systemic therapy has led to rapid advances in HCC treatment. Strategies involving medications with various modes of administration have proved much more effective than monotherapy, even though the causes of the success of such innovative HCC remedies remain poorly clarified. A two-drug approach, including concurrent or successive therapy with personalized treatment and immunotherapy, is among those protocols currently being explored[7-9]. The aim of this study was to retrospectively evaluate the effect of a regimen of targeted therapy, immunotherapy, and HAIC of FOLFOX.
We enrolled patients with HCC who underwent HAIC of FOLFOX combined with antitumor targeted therapy plus immunotherapy at Zhongshan People’s Hospital for HCC from December 2020 to June 2021. The inclusion criteria were as follows: (1) HCC diagnosed by ≥ 2 experienced physicians or pathologically diagnosed and no previous HCC treatment; (2) age 18-75 years old; (3) Child-Pugh class A or B; (4) Eastern Cooperative Oncology Group (ECOG) performance status grade 0 or 1; and (5) Barcelona Clinic Liver Cancer (BCLC) stage B or C. The exclusion criteria were: (1) White blood cell count < 2.0 × 109/L; (2) platelet count < 30 × 109/L; (3) serum total bilirubin > 51 μmol/L; (4) uncontrolled or systemic infection surrounding the lesion (excluding viral hepatitis); (5) severe liver, kidney, heart, or lung function insufficiency; (6) history of other malignant tumors; and (7) known history of human immunodeficiency virus infection.
This retrospective study was approved by the Ethics Committee of Zhongshan People’s Hospital (approval No. 2022-029). All study participants or their legal guardians provided informed written consent prior to study enrollment.
The patient was placed supine on the catheter bed, and the bilateral inguinal areas were disinfected. Then, the femoral artery (or radial artery or distal radial artery) was punctured under local anesthesia for the placement of an arterial sheath. The catheter was selectively inserted into the celiac artery and superior mesenteric artery, and angiography was used to observe the blood supply to the intrahepatic tumor. If the intrahepatic tumor was fed by a separate hepatic artery, the microcatheter was placed into the appropriate vascular target. If the intrahepatic tumor was fed by both hepatic arteries, the microcatheter was placed into the appropriate hepatic artery. In cases where the intrahepatic tumor was supplied by the left and right hepatic arteries and the gastroduodenal artery could not be avoided, the gastroduodenal artery underwent coil embolization before placing the microcatheter into the appropriate hepatic artery. Finally, the microcatheter was bandaged and fixed. FOLFOX chemotherapy was initiated upon return to the ward.
The regimen for HAIC of FOLFOX included oxaliplatin (85 mg/m2 intra-arterial infusion) for 2 h, followed by leucovorin (400 mg/m2 intravenous infusion) combined with 5-fluorouracil (400 mg/m2 intra-arterial infusion) for 1 h and then 2400 mg/m2 for 23 h, repeated every 3 wk. If the tumor shrunk significantly after HAIC treatment, the chemotherapy perfusion dose was appropriately reduced.
Lenvatinib (Eisai Co., Ltd.), sorafenib (Bayer), sintilimab (Innovent Biologics Suzhou Co., Ltd.), camrelizumab (Suzhou Suncadia Biopharmaceuticals Co., Ltd.), atezolizumab (Roche), and bevacizumab (Roche) were administered within 1 wk after the first HAIC treatment. The dosages were as follows: Lenvatinib, 8 mg [body weight (BW) < 60 kg] or 12 mg (BW ≥ 60 kg), orally, daily; sorafenib, 0.4 g, orally, daily; bevacizumab, 15 mg/kg, intravenous drip, once every 3 wk; sintilimab and camrelizumab, 200 mg, intravenous drip, once every 3 wk; and atezolizumab, 1200 mg, intravenous drip, once every 3 wk. Each drug was suspended, re-used (including dose reduction), or permanently discontinued according to the level of the corresponding drug-related AEs that occurred during treatment.
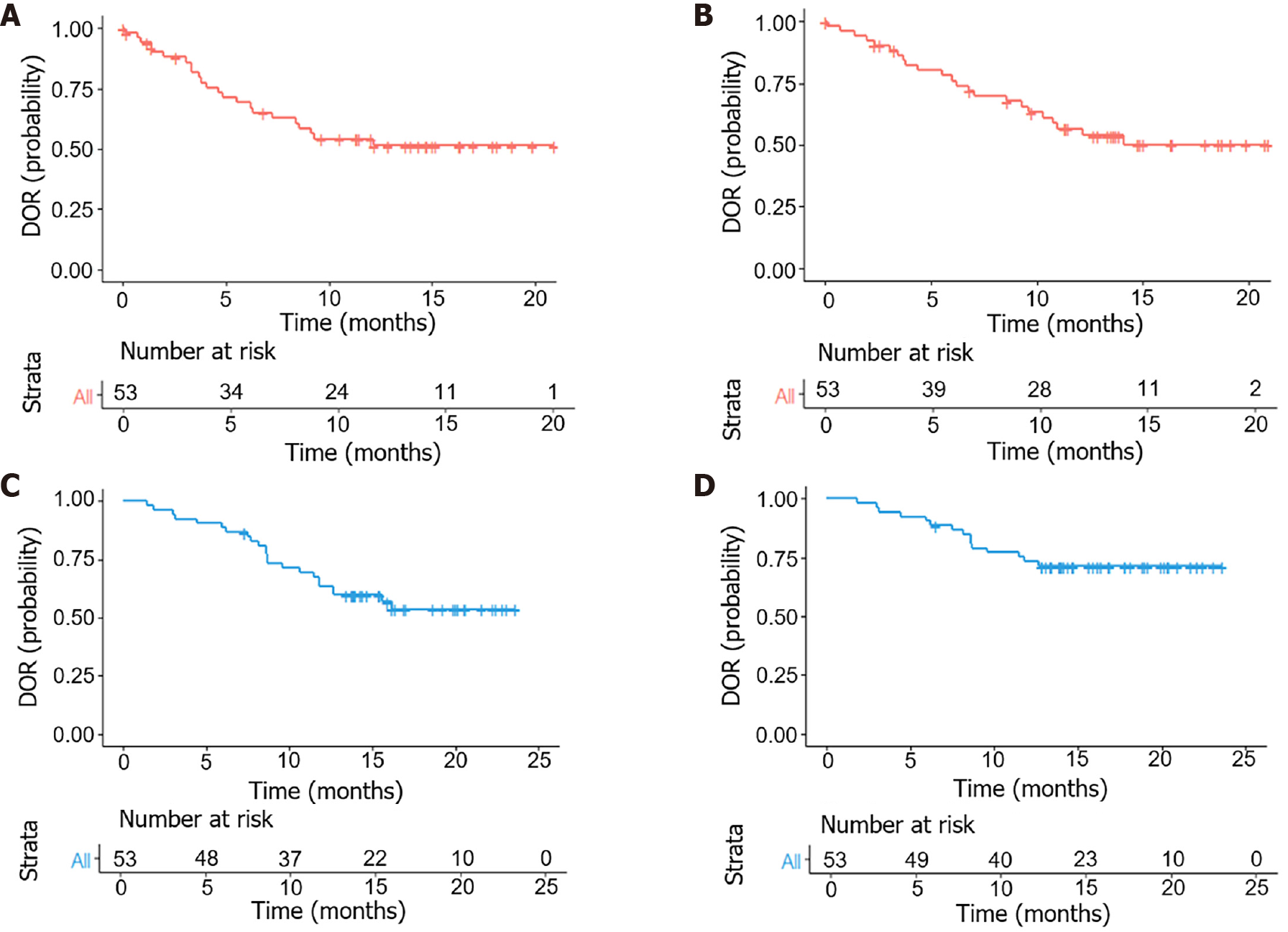
Each patient underwent enhanced magnetic resonance imaging or enhanced computed tomography of the upper abdomen; routine blood, liver function, and renal function tests; HCC index evaluations; and other examinations prior to HAIC. Curative effects included complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD) and were assessed using Response Evaluation Criteria in Solid Tumors (RECIST) 1.1 and modified RECIST (mRECIST)[10,11]. The ORR was calculated as the PR + CR, and the disease control rate (DCR) was calculated as the PR + CR + SD. The duration of response (DOR), surgical conversion rate, progression-free survival (PFS), 6-month and 12-month PFS, and safety were also determined.
Statistical analysis was performed using SPSS 20.0 software. Data were shown as means ± SD or range. Survival data were presented using Kaplan-Meier curves.
Of the 61 patients initially recruited, four withdrew after the first or second round of HAIC, and four showed poor compliance and did not come to our hospital for regular treatment on time (HAIC interval > 2 months). The final study cohort (n = 53) comprised 45 males and 8 females aged 51.6 (27.0-74.0) years. Of them, 45 (84.9%) had a history of hepatitis B, and 48 (90.1%) had a history of liver cirrhosis. The ECOG performance status was grade 0 in 26 (49.1%) and grade 1 in 27 (50.9%) cases, the BCLC stage was B in 27 (50.9%) and C in 26 (49.1%) cases, and there were 42 (79.2%) cases of Child-Pugh class A and 11 (20.8%) of Child-Pugh class B. Alpha-fetoprotein was elevated in 23 patients (43.4%) and was > 400 ng/mL in 30 (56.6%) patients. The tumor diameter was < 5 cm in 9 (16.9%), 5-10 cm in 18 (34.0%), and > 10 cm in 26 (46.7%) cases. The tumor capsule was complete in 28 (52.8%) and incomplete in 25 (47.2%) cases. Portal and/or hepatic vein tumor thrombus were present in 24 patients, including 5 cases each of PVTT in the second-order branches of the portal vein and the first-order branch of the portal vein, respectively, 11 cases of PVTT in the main trunk and/or a contralateral portal vein, and 6 cases of hepatic vein trunk tumor thrombus. Extrahepatic metastases were present in 12 patients (lung, 7; adrenal gland, 1; bone, 2; bone and lung, 2; and lymph nodes, 6; Table 1). The follow-up end date was June 30, 2022.
| Characteristics | Number | Proportion (%) |
| Gender | ||
| Male | 45 | 0.849 |
| Female | 8 | 0.151 |
| Age (yr) | ||
| < 50 | 22 | 0.415 |
| ≥ 50 | 31 | 0.585 |
| HBsAg | ||
| Positive | 45 | 0.849 |
| Negative | 8 | 0.151 |
| Liver cirrhosis | ||
| Yes | 48 | 0.901 |
| No | 5 | 0.099 |
| ECOG grade | ||
| 0 | 26 | 0.491 |
| 1 | 27 | 0.509 |
| Child-Pugh class | ||
| A | 42 | 0.792 |
| B | 11 | 0.208 |
| BCLC stage | ||
| B | 27 | 0.509 |
| C | 26 | 0.491 |
| Maximum tumor diameter (cm) | ||
| < 5 | 9 | 0.169 |
| 5-10 | 18 | 0.340 |
| > 10 | 26 | 0.491 |
| Tumor capsule | ||
| Complete | 28 | 0.528 |
| Incomplete | 25 | 0.472 |
| Alpha-fetoprotein (ng/mL) | ||
| < 400 ng/mL | 23 | 0.434 |
| ≥ 400 ng/mL | 30 | 0.566 |
| Number of tumors | ||
| ≤ 3 | 30 | 0.566 |
| > 3 | 23 | 0.434 |
| Vascular invasion | ||
| Vp2 | 5 | 0.099 |
| Vp3 | 5 | 0.099 |
| Vp4 | 11 | 0.208 |
| Vv2 | 6 | 0.113 |
| Vv3 | 0 | 0.000 |
| Extrahepatic metastases | ||
| Yes | 12 | 0.226 |
| No | 41 | 0.774 |
| Lymph node metastases | ||
| Yes | 6 | 0.113 |
| No | 47 | 0.887 |
Patients received 4-6 rounds of HAIC (average, 4.5 rounds), and the time interval between each round was 28.6 d (median time interval, 27.0 d). The average doses were as follows: Oxaliplatin, 79.9 mg/m2 (arterial infusion); leucovorin, 371.6 mg/m2 (intravenous infusion); 5-fluorouracil, 375.5 mg/m2 (intravenous infusion); and 5-fluorouracil, 2197.3 mg/m2 (arterial infusion).
The choice of targeted therapy drugs and immunotherapy drugs depended on the patient’s wishes and their economic status. There were 18 patients who received sorafenib combined with camrelizumab (sorafenib: 800.0 mg/d, average 666.7 mg/d; camrelizumab: 200 mg every 3 wk, intravenous drip); 10 chose sorafenib combined with sintilimab (sorafenib: 800.0 mg/d, average 640.0 mg/d; sintilimab: 200 mg every 3 wk, intravenous drip); 13 chose lenvatinib combined with camrelizumab (lenvatinib: average 10.8 mg/d; camrelizumab: 200 mg every 3 wk, intravenous drip); 11 chose lenvatinib combined with sintilimab (lenvatinib: < 60 kg BW, 8.0 mg/d and > 60 kg BW, 12.0 mg/d, average 10.5 mg/d; sintilimab: 200 mg every 3 wk, intravenous drip); and 1 chose atezolizumab combined with bevacizumab (atezolizumab: 1200 mg every 3 wk, intravenous drip; bevacizumab, 1100 mg every 3 wk, intravenous drip).
Assessment of the tumor treatment responses using mRECIST revealed that the number of cases of CR, PR, SD, and PD was 13 (24.5%), 19 (35.9%), 21 (39.6%), and 0 (0.0%), respectively, the ORR was 60.4%, and the DCR was 100.0%. Assessment according to RECIST v1.1 revealed that the number of cases of CR, PR, SD, and PD was 2 (3.8%), 23 (43.4%), 28 (52.8%), and 0 (0.0%), respectively, the ORR was 47.2%, and the DCR was 100.0% (Table 2).
| Variables | CR | PR | SD | PD | ORR | DCR |
| mRECIST | 13 (24.5) | 19 (35.9) | 21 (39.6) | 0 (0) | 32 (60.4) | 53 (100.0) |
| RECIST V1.1 | 2 (3.8) | 23 (43.4) | 28 (52.8) | 0 (0) | 25 (47.2) | 53 (100.0) |
AEs of different degrees were observed in all patients after receiving the combination therapy. Abdominal pain, nausea, vomiting, increased levels of transaminases, anorexia, fatigue, and diarrhea mainly occurred in the perioperative period of HAIC, and all patients recovered within a few days. No treatment-related grade 4 or 5 AEs were reported (Table 3).
| AEs | Any grade | Grade 1 | Grade 2 | Grade 3 |
| Leukopenia | 21 (39.62) | 11 (20.75) | 10 (18.87) | 0 |
| Thrombocytopenia | 12 (22.64) | 12 (22.64) | 0 (0.00) | 0 |
| Rash | 7 (13.21) | 3 (5.66) | 4 (7.55) | 0 |
| Itchy skin | 5 (9.43) | 5 (9.43) | 0 (0.00) | 0 |
| Hand-foot syndrome | 17 (32.08) | 10 (18.87) | 7 (13.21) | 0 |
| Increased serum ALT and AST levels | 25 (47.17) | 23 (43.40) | 2 (3.77) | 0 |
| Increased serum bilirubin | 8 (15.09) | 6 (11.32) | 2 (3.77) | 0 |
| Diarrhea | 5 (9.43) | 3 (5.66) | 2 (3.77) | 0 |
| Nausea, vomiting | 24 (45.28) | 13 (24.53) | 11 (20.75) | 0 |
| Proteinuria | 10 (18.87) | 4 (7.55) | 6 (11.32) | 0 |
| Hypothyroidism | 16 (30.19) | 0 (0.00) | 16 (30.19) | 0 |
| Gastrointestinal bleeding | 5 (9.43) | 0 (0.00) | 1 (1.88) | 4 (7.55) |
| Stomachache | 17 (32.08) | 10 (18.87) | 7 (13.21) | 0 |
| Hair loss | 4 (7.55) | 4 (7.55) | 0 (0.00) | 0 |
| Weight loss | 9 (16.98) | 9 (16.98) | 0 (0.00) | 0 |
| Decreased appetite | 25 (47.17) | 23 (43.40) | 2 (3.77) | 0 |
| Fatigue | 19 (35.85) | 19 (35.85) | 0 (0.00) | 0 |
| Hypertension | 24 (45.28) | 2 (3.77) | 22 (41.51) | 0 |
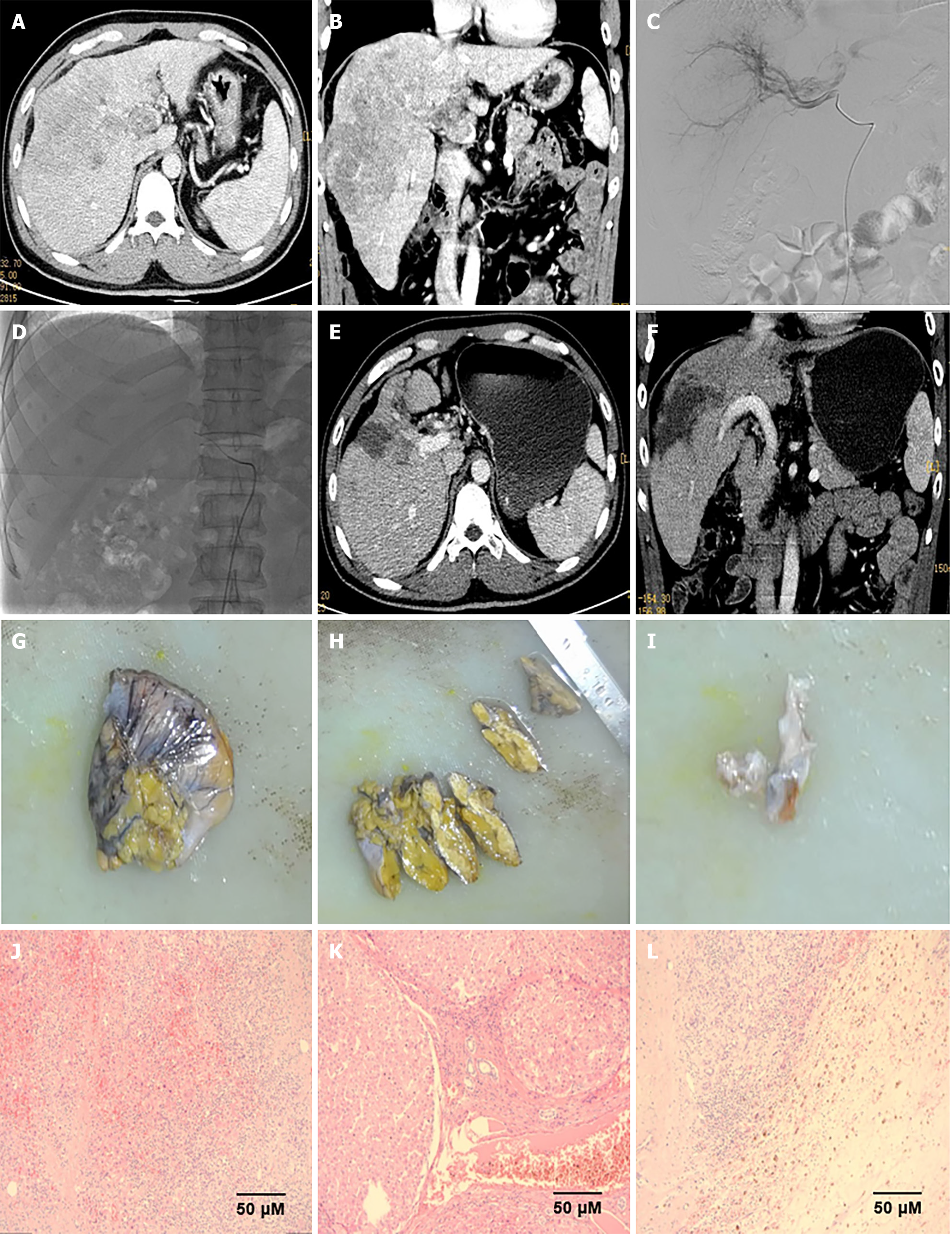
The follow-up period was 14.1 (3.8-23.6) months. PFS at 6- and 12 months was 88.7% and 62.3%, respectively. The median DOR was 9.1 months [95% confidence interval (95%CI): 8.40-not estimable (NE)] and 10.7 months (95%CI: 10.26-NE) according to mRECIST and RECIST v1.1, respectively. The median PFS (mPFS) was 13.9 months (95%CI: 12.63-NE), the 1-year overall survival (OS) rate was 83.0%, and a median OS (mOS) was not reached (95%CI: NE-NE) (Figure 1). Eighteen patients (34.0%) met the criteria for surgical resection, and all underwent surgery (Figure 2).
HCC is a serious threat to human life and has a high rate of morbidity and mortality. Despite the wide use of a combined therapeutic approach to HCC in recent years, there are no reports of > 2 therapies working together. In this study, we explored the clinical efficacy of targeted therapy plus immunotherapy combined with HAIC in patients with unresectable HCC. We found that HAIC of FOLFOX, targeted therapy, and immunotherapy were effective for patients with unresectable HCC.
In 2018, there were approximately 841000 new cases of HCC worldwide and 781000 associated deaths, of which around 50% of newly reported cases and associated deaths occurred in China[12,13]. The main treatments in early-stage HCC are surgical resection, liver transplantation, and ablation. Transcatheter arterial chemoembolization is effective in middle-stage unresectable HCC with multiple nodules. However, systemic treatments, such as targeted therapy and immunotherapy, are ineffective for some patients with late-stage HCC, and their prognosis is often poor[14]. One of the important means of interventional therapy for HCC is HAIC, and excellent results have been achieved using HAIC of an oxaliplatin-based sequential FOLFOX regimen to treat advanced HCC, making HAIC a more preferred method[15].
HAIC is an interventional treatment method. HCC tumors often have a rich blood supply, with > 95% originating from the hepatic artery, which justifies the treatment of HCC through the hepatic artery[16]. The direct infusion of drugs into the arterial blood supply of the tumor overcomes the physiological barriers through which some intravenous chemo
HAIC has been used for HCC treatment for over 60 years, and the earliest date can be traced back to 1961. Some scholars used femoral artery puncture and catheterization or right gastroepiploic artery incision and catheterization to infuse HCC therapeutics[18]. In 1986, there were reports of epirubicin-based HAIC for HCC treatment, but the effect was poor[19]. Around 2000, the cisplatin-based HAIC regimen was used abroad, resulting in the ORR reaching 27.6%-40.5%[20]. Because HAIC has long been used to treat HCC, the arterial perfusion schemes, doses, and number of cycles vary across countries and regions, and their therapeutic effects also differ. However, due to a lack of high-level evidence-based medicine, its clinical application is limited. In 2013, the EACH trial proved the curative effect of FOLFOX chemotherapy for HCC[21]. In 2018, Chinese scholars demonstrated an effective rate of 79.6% for HAIC of FOLFOX[15], and in 2022, an oxaliplatin-based FOLFOX4 regimen of systemic chemotherapy and HAIC therapy became the recommended treatment for advanced HCC[22].
Targeted therapy and immunotherapy have been widely used in clinical practice and are the main treatment methods for advanced tumors. However, their efficacy as a single application is low and does not meet the clinical needs. Thus, a combination of targeted therapy and immunotherapy has been proposed in an effort to develop a more effective treatment method. The current understanding of tumor pathogenesis involves not only the study of tumor cells but also the study of various cells and cytokines within cancer tissues, termed the tumor microenvironment (TME)[23]. For the indefinite proliferation of tumor cells, the TME must be hypoxic, with a low pH and low nutrient levels. This induces the formation of proangiogenic factors and an immunosuppressive microenvironment[24,25]. The TME both increases and decreases immunosuppression, while targeted therapy inhibits the migration and distant metastasis of immune cells. Immunotherapy stimulates the body’s immune system by affecting the target and enhancing the tumor suppression effect of immune cells.
Targeted therapy and immunotherapy have been widely used to treat unresectable HCC. Another option for patients with unresectable HCC is whole-body treatment. The phase III IMbrave150 clinical trial compared a regimen of atezolizumab and bevacizumab (n = 336) with that of sorafenib (n = 165) in 501 patients with advanced unresectable HCC[9]. The mOS in the combination regimen group was notably increased compared with the sorafenib regimen group (19.2 months vs 13.4 months, respectively), while in the 194 Chinese subgroups, the mOS and OS in the combination regimen group were 19.2 and 24 months, respectively, with the risk of death reduced by 47%. Most major guidelines prefer a regimen of atezolizumab and bevacizumab for late-stage HCC. The phase Ib KEYNOTE-524 clinical trial with a regimen of lenvatinib and pembrolizumab[26] reported the mOS and mPFS of 100 patients as 22 months and 9.3 months and the ORR and DCR as 46% and 88%, respectively. However, the OS and PFS of this regimen failed expectations. Study117, a phase Ib clinical study with a regimen of lenvatinib and nivolumab, demonstrated better results than a regimen of lenvatinib and pembrolizumab. For the 30 patients in the study, the CR was 10%, PR was 66.7%, ORR was 76.7%, and DCR was 96.7%[27]. A phase II/III clinical study compared a regimen of sintilimab and bevacizumab with that of sorafenib in 157 patients with unresectable or metastatic HCC[28]. Compared with the sorafenib regimen, the effect of the sintilimab and bevacizumab regimen was markedly better (mOS: NE vs 10.4 months), notably reduced the risk of mortality by 44%, and improved ORR by 20%. Targeted therapy and immunotherapy are also effective second-line treatments for unresectable HCC. A phase Ia/IIb clinical study with a regimen of apatinib and camrelizumab in advanced HCC[29] reported an mOS and mPFS of 3.4 and 9.3 months, respectively, an ORR of 46%, and a DCR of 88%.
The continuous exploration and progress of new treatment methods for advanced HCC has revealed that the effect brought about by a single treatment mode is inadequate. The upcoming therapies for progressed, incurable HCC are expected to increasingly integrate local and universal approaches. A prospective randomized controlled study suggested that a regimen of HAIC and sorafenib had better outcomes than that of sorafenib alone[6]. The OS of the combination regimen and sorafenib regimen was 13.37 months and 7.13 months, the PFS was 7.03 months and 2.60 months, and the ORR was 40.80% and 2.46%, respectively. Radical surgical resection was performed for 16 patients with the combined regimen and only 1 patient with the sorafenib regimen, and the success rate of surgical conversion was 12.8% and 0.8%, respectively. A single-center retrospective single-arm study reported the effect of HAIC of FOLFOX combined with targeted therapy and immunotherapy[30], in which the CR was 48.0%, PR was 48.0%, SD was 4.0%, and the ORR was as high as 96.0%. Furthermore, the median time to resolve was 50.5 d (95%CI: 31.02-64.00). Studies have shown that local therapy combined with systemic therapy has a better effect and a higher tumor remission rate.
Multiple treatment combinations can achieve surgical conversion in approximately 15%-20% of cases of unresectable HCC[31]. A potential multidisciplinary strategy for addressing severe HCC involves integrating HAIC-based locoregional treatment with selective treatment and immunotherapy[32]. Furthermore, a retrospective study with a regimen of HAIC and targeted therapy and immunotherapy showed that 15 of 25 (60.0%) patients underwent conversion to operable criteria; 1 refused surgery and the remaining 14 underwent surgical resection. Of them, 7 (28.0%) achieved pathological CR after resection, and the recurrence-free survival was 13.17 months[30]. A prospective, single-arm phase II clinical study on 30 patients with unresectable HCC treated with HAIC combined with sintilimab and bevacizumab biosimilar (IBI305) reported that R0 resections were achieved in all 14 (100.0%) patients who underwent surgical resection (2022 ASCO POSTER Abstract #4073). In this retrospective analysis, the surgical conversion rate did not achieve the above-mentioned results as expected. This might have been because a large proportion of the enrolled patients were BCLC grade C, the tumor burden was high, and there was a large number of patients with PVTT and extrahepatic metastases. Therefore, the surgical conversion rate was not significantly increased.
The common AEs of targeted therapy and immunotherapy combined with HAIC include decreased appetite, fatigue, leukopenia, thrombocytopenia, abdominal pain, nausea and vomiting, hypertension, diarrhea, liver function damage, and hand-foot syndrome, and effective relief occurs after medication. Patients undergoing curative-specific treatment most frequently experienced hypertension as an acute side effect[33,34], occurring in approximately 42% of patients receiving lenvatinib and 36% receiving combination therapy. Furthermore, the first side effect observed in patients with advanced HCC receiving combination therapy was hypertension[26]. Thus, it is recommended that blood pressure is monitored before combined treatment and antihypertensives be timely administered if blood pressure rises. If antihypertensive drugs are ineffective, the dosage of targeted drugs can be reduced or even terminated. In our study, the AEs in both groups were manageable, and no grade 4 or 5 AEs occurred.
The main limitation of our study is its single-center retrospective single-arm design, which leads to selection bias in treatment choice. Thus, higher-quality multicenter prospective studies are necessary to verify our findings. Second, the targeted therapy and immunotherapy drugs used by the patients in this study varied, and there were many different combinations. Third, patients had a large baseline range in terms of tumor stage and liver function, which may have affected clinical treatment effects and outcomes. Fourth, the follow-up period to assess OS was short (1 year), and a longer follow-up of OS is required. Fifth, the intervals between HAIC rounds were relatively long, which may have affected its efficacy.
Targeted therapy and immunotherapy combined with HAIC of FOLFOX is clinically effective for unresectable HCC, improves the surgical conversion rate, and has controllable AEs.
Conflict of interest statement: There is no conflict of interest to declare.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s classification
Scientific Quality: Grade B, Grade C
Novelty: Grade B, Grade B
Creativity or Innovation: Grade B, Grade B
Scientific Significance: Grade B, Grade B
P-Reviewer: Abbas HA, Egypt; Cillo U, Italy S-Editor: Chen YL L-Editor: A P-Editor: Yu HG
| 1. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 64583] [Article Influence: 16145.8] [Reference Citation Analysis (176)] |
| 2. | Ohama H, Hiraoka A, Tada F, Kato K, Fukunishi Y, Yanagihara E, Kato M, Saneto H, Izumoto H, Ueki H, Yoshino T, Kitahata S, Kawamura T, Kuroda T, Suga Y, Miyata H, Hirooka M, Abe M, Matsuura B, Ninomiya T, Hiasa Y. Comparison of Surgical Resection and Percutaneous Ultrasonographic Guided Radiofrequency Ablation for Initial Recurrence of Hepatocellular Carcinoma in Early Stage following Curative Treatment. Cancers (Basel). 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 3. | Endo Y, Sasaki K, Moazzam Z, Lima HA, Alaimo L, Munir MM, Shaikh CF, Schenk A, Kitago M, Pawlik TM. Liver transplantation for elderly patients with early-stage hepatocellular carcinoma. Br J Surg. 2023;110:1527-1534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 4. | Omata M, Cheng AL, Kokudo N, Kudo M, Lee JM, Jia J, Tateishi R, Han KH, Chawla YK, Shiina S, Jafri W, Payawal DA, Ohki T, Ogasawara S, Chen PJ, Lesmana CRA, Lesmana LA, Gani RA, Obi S, Dokmeci AK, Sarin SK. Asia-Pacific clinical practice guidelines on the management of hepatocellular carcinoma: a 2017 update. Hepatol Int. 2017;11:317-370. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1628] [Cited by in RCA: 1642] [Article Influence: 205.3] [Reference Citation Analysis (0)] |
| 5. | Ueshima K, Komemushi A, Aramaki T, Iwamoto H, Obi S, Sato Y, Tanaka T, Matsueda K, Moriguchi M, Saito H, Sone M, Yamagami T, Inaba Y, Kudo M, Arai Y. Clinical Practice Guidelines for Hepatic Arterial Infusion Chemotherapy with a Port System Proposed by the Japanese Society of Interventional Radiology and Japanese Society of Implantable Port Assisted Treatment. Liver Cancer. 2022;11:407-425. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 23] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 6. | He M, Li Q, Zou R, Shen J, Fang W, Tan G, Zhou Y, Wu X, Xu L, Wei W, Le Y, Zhou Z, Zhao M, Guo Y, Guo R, Chen M, Shi M. Sorafenib Plus Hepatic Arterial Infusion of Oxaliplatin, Fluorouracil, and Leucovorin vs Sorafenib Alone for Hepatocellular Carcinoma With Portal Vein Invasion: A Randomized Clinical Trial. JAMA Oncol. 2019;5:953-960. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 379] [Article Influence: 75.8] [Reference Citation Analysis (0)] |
| 7. | Finn RS, Merle P, Granito A, Huang YH, Bodoky G, Pracht M, Yokosuka O, Rosmorduc O, Gerolami R, Caparello C, Cabrera R, Chang C, Sun W, LeBerre MA, Baumhauer A, Meinhardt G, Bruix J. Outcomes of sequential treatment with sorafenib followed by regorafenib for HCC: Additional analyses from the phase III RESORCE trial. J Hepatol. 2018;69:353-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 226] [Cited by in RCA: 274] [Article Influence: 39.1] [Reference Citation Analysis (0)] |
| 8. | Kudo M. Combination Cancer Immunotherapy in Hepatocellular Carcinoma. Liver Cancer. 2018;7:20-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 63] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 9. | Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, Kudo M, Breder V, Merle P, Kaseb AO, Li D, Verret W, Xu DZ, Hernandez S, Liu J, Huang C, Mulla S, Wang Y, Lim HY, Zhu AX, Cheng AL; IMbrave150 Investigators. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N Engl J Med. 2020;382:1894-1905. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2542] [Cited by in RCA: 4687] [Article Influence: 937.4] [Reference Citation Analysis (2)] |
| 10. | He LN, Chen T, Fu S, Chen C, Jiang Y, Zhang X, Du W, Li H, Wang Y, Ali WAS, Zhou Y, Lin Z, Yang Y, Huang Y, Zhao H, Fang W, Zhang L, Hong S. Reducing number of target lesions for RECIST1.1 to predict survivals in patients with advanced non-small-cell lung cancer undergoing anti-PD1/PD-L1 monotherapy. Lung Cancer. 2022;165:10-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 11. | Llovet JM, Lencioni R. mRECIST for HCC: Performance and novel refinements. J Hepatol. 2020;72:288-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 474] [Cited by in RCA: 436] [Article Influence: 87.2] [Reference Citation Analysis (0)] |
| 12. | Li Y, Zou H, Zheng Z, Liu Z, Hu H, Wu W, Wang T. Advances in the Study of Bioactive Nanoparticles for the Treatment of HCC and Its Postoperative Residual Cancer. Int J Nanomedicine. 2023;18:2721-2735. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 13. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 55814] [Article Influence: 7973.4] [Reference Citation Analysis (132)] |
| 14. | Giannone F, Slovic N, Pessaux P, Schuster C, Baumert TF, Lupberger J. Inflammation-related prognostic markers in resected hepatocellular carcinoma. Front Oncol. 2023;13:1267870. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 15. | Lyu N, Lin Y, Kong Y, Zhang Z, Liu L, Zheng L, Mu L, Wang J, Li X, Pan T, Xie Q, Liu Y, Lin A, Wu P, Zhao M. FOXAI: a phase II trial evaluating the efficacy and safety of hepatic arterial infusion of oxaliplatin plus fluorouracil/leucovorin for advanced hepatocellular carcinoma. Gut. 2018;67:395-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 77] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 16. | Amoyav B, Bloom AI, Goldstein Y, Miller R, Sharam M, Fluksman A, Benny O. Drug-Eluting Porous Embolic Microspheres for Trans-Arterial Delivery of Dual Synergistic Anticancer Therapy for the Treatment of Liver Cancer. Adv Healthc Mater. 2023;12:e2301548. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 17. | Yuan Y, He W, Yang Z, Qiu J, Huang Z, Shi Y, Lin Z, Zheng Y, Chen M, Lau WY, Li B, Yuan Y. TACE-HAIC combined with targeted therapy and immunotherapy versus TACE alone for hepatocellular carcinoma with portal vein tumour thrombus: a propensity score matching study. Int J Surg. 2023;109:1222-1230. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 70] [Article Influence: 35.0] [Reference Citation Analysis (0)] |
| 18. | Provan JL, Stokes JF, Edwards D. Hepatic artery infusion chemotherapy in hepatoma. Br Med J. 1968;3:346-349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 19. | Nagasue N, Yukaya H, Okamura J, Kuroda C, Kubo Y, Hirai K, Tanikawa K, Okita K, Ando K, Tamura K. [Intra-arterial administration of epirubicin in the treatment of non-resectable hepatocellular carcinoma. Epirubicin Study Group for Hepatocellular Carcinoma]. Gan To Kagaku Ryoho. 1986;13:2786-2792. [PubMed] |
| 20. | Choi JH, Chung WJ, Bae SH, Song DS, Song MJ, Kim YS, Yim HJ, Jung YK, Suh SJ, Park JY, Kim DY, Kim SU, Cho SB. Randomized, prospective, comparative study on the effects and safety of sorafenib vs hepatic arterial infusion chemotherapy in patients with advanced hepatocellular carcinoma with portal vein tumor thrombosis. Cancer Chemother Pharmacol. 2018;82:469-478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 91] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 21. | Qin S, Bai Y, Lim HY, Thongprasert S, Chao Y, Fan J, Yang TS, Bhudhisawasdi V, Kang WK, Zhou Y, Lee JH, Sun Y. Randomized, multicenter, open-label study of oxaliplatin plus fluorouracil/leucovorin versus doxorubicin as palliative chemotherapy in patients with advanced hepatocellular carcinoma from Asia. J Clin Oncol. 2013;31:3501-3508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 375] [Article Influence: 31.3] [Reference Citation Analysis (0)] |
| 22. | Zhou J, Sun H, Wang Z, Cong W, Zeng M, Zhou W, Bie P, Liu L, Wen T, Kuang M, Han G, Yan Z, Wang M, Liu R, Lu L, Ren Z, Zeng Z, Liang P, Liang C, Chen M, Yan F, Wang W, Hou J, Ji Y, Yun J, Bai X, Cai D, Chen W, Chen Y, Cheng W, Cheng S, Dai C, Guo W, Guo Y, Hua B, Huang X, Jia W, Li Q, Li T, Li X, Li Y, Liang J, Ling C, Liu T, Liu X, Lu S, Lv G, Mao Y, Meng Z, Peng T, Ren W, Shi H, Shi G, Shi M, Song T, Tao K, Wang J, Wang K, Wang L, Wang X, Xiang B, Xing B, Xu J, Yang J, Yang Y, Ye S, Yin Z, Zeng Y, Zhang B, Zhang L, Zhang S, Zhang T, Zhang Y, Zhao M, Zhao Y, Zheng H, Zhou L, Zhu J, Zhu K, Shi Y, Xiao Y, Yang C, Wu Z, Dai Z, Cai J, Cai X, Shen F, Qin S, Teng G, Dong J, Fan J. Guidelines for the Diagnosis and Treatment of Primary Liver Cancer (2022 Edition). Liver Cancer. 2023;12:405-444. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 195] [Reference Citation Analysis (0)] |
| 23. | Hoshino A, Kim HS, Bojmar L, Gyan KE, Cioffi M, Hernandez J, Zambirinis CP, Rodrigues G, Molina H, Heissel S, Mark MT, Steiner L, Benito-Martin A, Lucotti S, Di Giannatale A, Offer K, Nakajima M, Williams C, Nogués L, Pelissier Vatter FA, Hashimoto A, Davies AE, Freitas D, Kenific CM, Ararso Y, Buehring W, Lauritzen P, Ogitani Y, Sugiura K, Takahashi N, Alečković M, Bailey KA, Jolissant JS, Wang H, Harris A, Schaeffer LM, García-Santos G, Posner Z, Balachandran VP, Khakoo Y, Raju GP, Scherz A, Sagi I, Scherz-Shouval R, Yarden Y, Oren M, Malladi M, Petriccione M, De Braganca KC, Donzelli M, Fischer C, Vitolano S, Wright GP, Ganshaw L, Marrano M, Ahmed A, DeStefano J, Danzer E, Roehrl MHA, Lacayo NJ, Vincent TC, Weiser MR, Brady MS, Meyers PA, Wexler LH, Ambati SR, Chou AJ, Slotkin EK, Modak S, Roberts SS, Basu EM, Diolaiti D, Krantz BA, Cardoso F, Simpson AL, Berger M, Rudin CM, Simeone DM, Jain M, Ghajar CM, Batra SK, Stanger BZ, Bui J, Brown KA, Rajasekhar VK, Healey JH, de Sousa M, Kramer K, Sheth S, Baisch J, Pascual V, Heaton TE, La Quaglia MP, Pisapia DJ, Schwartz R, Zhang H, Liu Y, Shukla A, Blavier L, DeClerck YA, LaBarge M, Bissell MJ, Caffrey TC, Grandgenett PM, Hollingsworth MA, Bromberg J, Costa-Silva B, Peinado H, Kang Y, Garcia BA, O'Reilly EM, Kelsen D, Trippett TM, Jones DR, Matei IR, Jarnagin WR, Lyden D. Extracellular Vesicle and Particle Biomarkers Define Multiple Human Cancers. Cell. 2020;182:1044-1061.e18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 356] [Cited by in RCA: 827] [Article Influence: 165.4] [Reference Citation Analysis (0)] |
| 24. | Martin JD, Cabral H, Stylianopoulos T, Jain RK. Improving cancer immunotherapy using nanomedicines: progress, opportunities and challenges. Nat Rev Clin Oncol. 2020;17:251-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 448] [Article Influence: 89.6] [Reference Citation Analysis (0)] |
| 25. | Sharonov GV, Serebrovskaya EO, Yuzhakova DV, Britanova OV, Chudakov DM. B cells, plasma cells and antibody repertoires in the tumour microenvironment. Nat Rev Immunol. 2020;20:294-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 422] [Article Influence: 84.4] [Reference Citation Analysis (0)] |
| 26. | Finn RS, Ikeda M, Zhu AX, Sung MW, Baron AD, Kudo M, Okusaka T, Kobayashi M, Kumada H, Kaneko S, Pracht M, Mamontov K, Meyer T, Kubota T, Dutcus CE, Saito K, Siegel AB, Dubrovsky L, Mody K, Llovet JM. Phase Ib Study of Lenvatinib Plus Pembrolizumab in Patients With Unresectable Hepatocellular Carcinoma. J Clin Oncol. 2020;38:2960-2970. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 661] [Cited by in RCA: 874] [Article Influence: 174.8] [Reference Citation Analysis (0)] |
| 27. | Kudo M, Ikeda M, Motomura K, Okusaka T, Kato N, Dutcus C, Hisai T, Suzuki M, Ikezawa H, Iwata T, Kumada H, Kobayashi M. A phase Ib study of lenvatinib (LEN) plus nivolumab (NIV) in patients (pts) with unresectable hepatocellular carcinoma (uHCC): Study 117. J Clin Oncol. 2020;38 Suppl 4:513. [RCA] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 45] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 28. | Ren Z, Xu J, Bai Y, Xu A, Cang S, Du C, Li Q, Lu Y, Chen Y, Guo Y, Chen Z, Liu B, Jia W, Wu J, Wang J, Shao G, Zhang B, Shan Y, Meng Z, Gu S, Yang W, Liu C, Shi X, Gao Z, Yin T, Cui J, Huang M, Xing B, Mao Y, Teng G, Qin Y, Xia F, Yin G, Yang Y, Chen M, Wang Y, Zhou H, Fan J; ORIENT-32 study group. Sintilimab plus a bevacizumab biosimilar (IBI305) versus sorafenib in unresectable hepatocellular carcinoma (ORIENT-32): a randomised, open-label, phase 2-3 study. Lancet Oncol. 2021;22:977-990. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 253] [Cited by in RCA: 669] [Article Influence: 167.3] [Reference Citation Analysis (1)] |
| 29. | Xu J, Zhang Y, Jia R, Yue C, Chang L, Liu R, Zhang G, Zhao C, Chen C, Wang Y, Yi X, Hu Z, Zou J, Wang Q. Anti-PD-1 Antibody SHR-1210 Combined with Apatinib for Advanced Hepatocellular Carcinoma, Gastric, or Esophagogastric Junction Cancer: An Open-label, Dose Escalation and Expansion Study. Clin Cancer Res. 2019;25:515-523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 359] [Article Influence: 51.3] [Reference Citation Analysis (0)] |
| 30. | Zhang J, Zhang X, Mu H, Yu G, Xing W, Wang L, Zhang T. Surgical Conversion for Initially Unresectable Locally Advanced Hepatocellular Carcinoma Using a Triple Combination of Angiogenesis Inhibitors, Anti-PD-1 Antibodies, and Hepatic Arterial Infusion Chemotherapy: A Retrospective Study. Front Oncol. 2021;11:729764. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 46] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 31. | Goto Y, Hisaka T, Sakai H, Takagi K, Fukutomi S, Akagi Y, Okuda K. Salvage Surgery for Initially Unresectable Locally Advanced Hepatocellular Carcinoma Downstaged by Hepatic Arterial Infusion Chemotherapy. Anticancer Res. 2020;40:4773-4777. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 32. | Zhang T, Merle P, Wang H, Zhao H, Kudo M. Combination therapy for advanced hepatocellular carcinoma: do we see the light at the end of the tunnel? Hepatobiliary Surg Nutr. 2021;10:180-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 43] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 33. | Bedrose S, Miller KC, Altameemi L, Ali MS, Nassar S, Garg N, Daher M, Eaton KD, Yorio JT, Daniel DB, Campbell M, Bible KC, Ryder M, Chintakuntlawar AV, Habra MA. Combined lenvatinib and pembrolizumab as salvage therapy in advanced adrenal cortical carcinoma. J Immunother Cancer. 2020;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 35] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 34. | Bruix J, Qin S, Merle P, Granito A, Huang YH, Bodoky G, Pracht M, Yokosuka O, Rosmorduc O, Breder V, Gerolami R, Masi G, Ross PJ, Song T, Bronowicki JP, Ollivier-Hourmand I, Kudo M, Cheng AL, Llovet JM, Finn RS, LeBerre MA, Baumhauer A, Meinhardt G, Han G; RESORCE Investigators. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;389:56-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2160] [Cited by in RCA: 2710] [Article Influence: 338.8] [Reference Citation Analysis (0)] |