Published online May 7, 2024. doi: 10.3748/wjg.v30.i17.2311
Revised: March 10, 2024
Accepted: April 15, 2024
Published online: May 7, 2024
Processing time: 98 Days and 9.5 Hours
Contrast-enhanced endoscopic ultrasound (CH-EUS) can overcome the limi
Core Tip: Contrast-enhanced endoscopic ultrasound may be better at identifying the targeted area in lesions by avoiding necrosis and vessels and improving the delineation of lesion margins. Guided fine-needle aspiration or fine-needle biopsy is simple and safe, but its benefit is limited to isoenhanced lesions or those with important surrounding fibrosis. These guided endoscopic ultrasound techniques can also be useful for guiding drainage in patients who have very echogenic content of pancreatic fluid collections or bile ducts.
- Citation: Gheorghiu MI, Seicean A, Pojoga C, Hagiu C, Seicean R, Sparchez Z. Contrast-enhanced guided endoscopic ultrasound procedures. World J Gastroenterol 2024; 30(17): 2311-2320
- URL: https://www.wjgnet.com/1007-9327/full/v30/i17/2311.htm
- DOI: https://dx.doi.org/10.3748/wjg.v30.i17.2311
Endoscopic ultrasound (EUS) has been developed as a valuable diagnostic technique for different intra-abdominal conditions, especially for tissue acquisition for the confirmation of oncologic diseases. A meta-analysis comprising 18 randomized controlled studies and 2718 patients with solid masses showed that the diagnostic accuracy of EUS-fine needle aspiration (EUS-FNA) was 67%-100%, the diagnostic accuracy of EUS-fine needle biopsy (EUS-FNB) was 69-100% and there was an advantage of using EUS-FNB needles [risk ratio: 0.94, 95% confidence interval (95%CI): 0.92-0.97; P = 0.0002][1]. Limited sample adequacy leads to false negative results, caused by technical difficulty due to the needle positioning (e.g., head/uncinate processus lesions) or intrinsic fibrosis of solid pancreatic masses[2]. Additionally, the presence of avascular areas inside pancreatic solid lesions was associated with a 72.9% sampling sensitivity, as compared to a 94.3% sampling sensitivity in lesions without avascular areas[3].
To improve and overcome the limitations of grayscale images, harmonic contrastenhanced EUS (CH-EUS) has become a promising imaging modality for visualizing microvessels inside targeted lesions for diagnostic or therapeutic purposes by using contrast microbubbles to enhance the low flow of capillary vascular network signals.
More than ten years ago, a study that combined EUS-FNA with CH-EUS guidance reported a sensitivity of 100% for diagnosing malignancies, and a hypoenhanced pattern was considered to indicate malignancy when there were false-negative FNA results[4]. In a subsequent study on 100 pancreatic solid lesions, CH-EUS helped to identify the target for EUS-FNA in the subgroup of mixed enhancement patterns (26% of adenocarcinoma cases) by targeting the hypoenhanced area, which led to a very good sensitivity of 95%[5].
Guiding CH-EUS-FNA was developed as an advanced medical procedure for improving the characterization of the margins and internal components of solid and cystic lesions and subsequently for improving the diagnostic accuracy.
Another rare application of CH-EUS is during the drainage of pancreatic fluid collections or bile ducts because it allows for better visualization of the structures that are to be crossed during puncture, but related data in the literature are scarce.
We performed a systematic search of the English literature published in the PubMed, Embase and Cochrane Library databases using the following keywords: “Contrastenhanced endoscopic ultrasound”, “contrast enhancement”, “contrastenhanced endosonography”, “biopsy”, “fine needle aspiration”, “fine needle biopsy”, “interventional EUS”, “radiofrequency ablation”, “pancreatic cancer”, “pancreatic fluid collection”, “drainage”, “walled-off necrosis”, “biliary drainage”, “gallbladder drainage”, “radiofrequency ablation”, and “tumour ablation”. All the authors participated in the search and selection of relevant studies.
According to recent guidelines, CH-EUS is recommended for the characterization of pancreatic solid lesions[6-10], especially inhomogeneous lesions[6]. The hypoenhanced pattern had a sensitivity of 93% and a specificity of 80% for the diagnosis of pancreatic adenocarcinoma[11]. In contrast, the hyperenhanced pattern was described in cases of benign lesions such as autoimmune pancreatitis, mass forming chronic pancreatitis or an intrapancreatic accessory spleen or in cases of neuroendocrine tumours or pancreatic metastases[12-16]. The presence of the hyperenhanced pattern had a sensitivity of 78.9% and a specificity of 98.0% for its ability to predict neuroendocrine tumours[4]. A hypoenhanced or heterogeneous aspect of neuroendocrine tumours is an indicator of a poor prognosis[15] and aggressiveness[17].
An improvement in the sensitivity and specificity of up to 94% was obtained using an artificial neural network model[18,19]. Moreover, the use of time-intensity curve analysis improved the conventional EUS diagnosis rate by using qualitative analysis (OR = 6) or quantitative analysis (OR = 10): Low peak enhancement and low in-slope in adenocarcinomas; high peak enhancement and normal in-slope in neuroendocrine tumours; and normal peak enhancement and low in-slope in cases of mass forming chronic pancreatitis[20]. Postprocessing analysis of the CHEUS timeintensity curve revealed that the contrast uptake ratio (representing the uptake of the mass vs the normal parenchyma) was significantly lower in adenocarcinoma than in mass-forming chronic pancreatitis, with a cut-off value of 0.17[21].
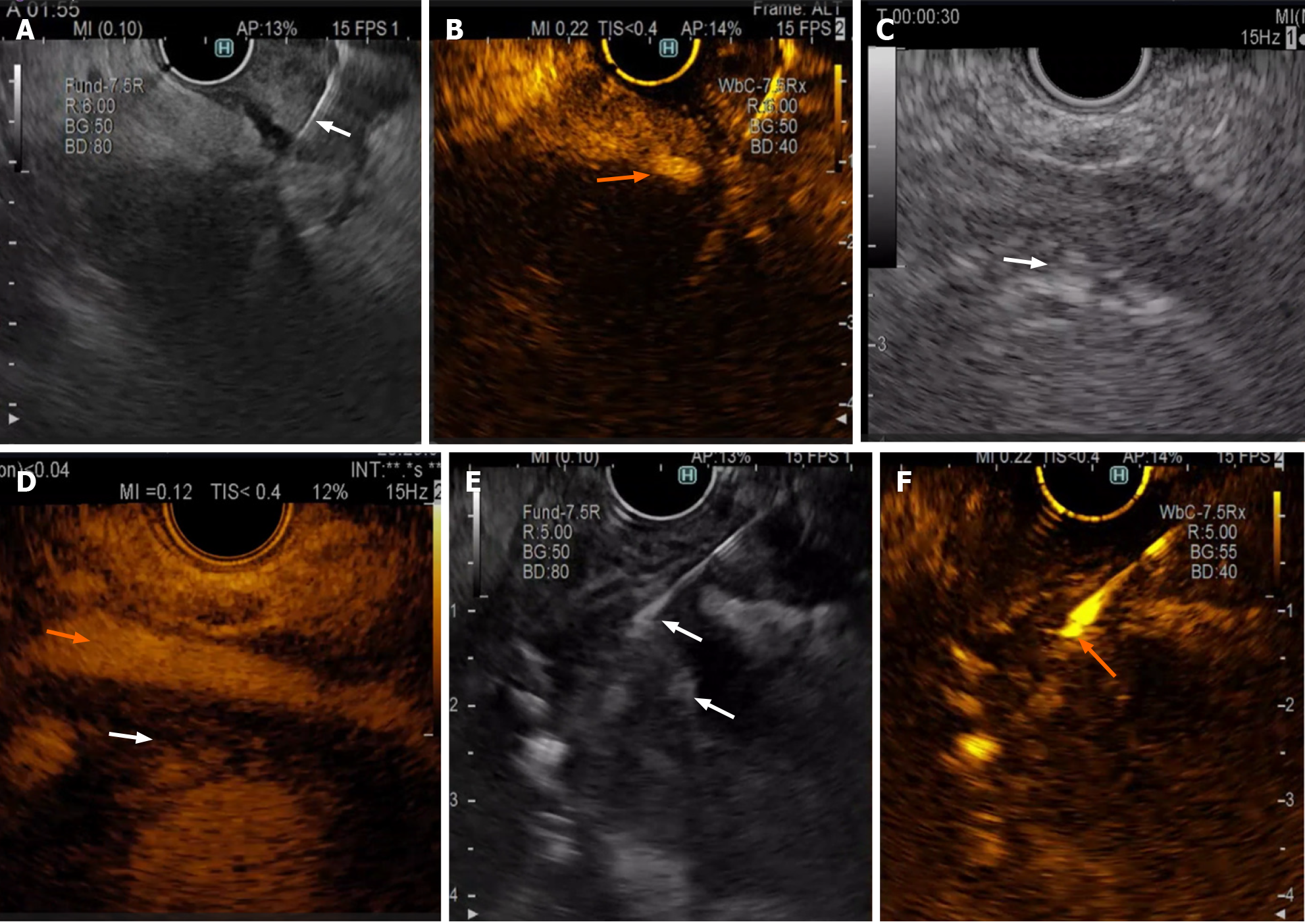
As the poorer contrast enhancement areas contained a greater number of necrotic and fibrotic cells[5,15,22], vessels should be avoided while using the contrast EUS for fine needle aspirate guidance in solid pancreatic lesions (Figure 1A and B) and nonenhanced regions, as they could represent an area of haemorrhage or necrosis.
While placing the needle, the areas with contrast uptake (the most hypoenhanced areas in hypoenhanced lesions or the most contrast-enhanced regions in hyper/isoenhanced lesions) should be targeted[12,23].
The large vessels are visible with contrast enhancement, so they are easily avoided, but the needle visibility could be impaired in cases where there is a deep lesion located away from the EUS probe.
There are nine studies on CH-EUS-FNA, seven of which have shown that compared with conventional EUS-FNA, CH-EUS-FNA has no statistical benefit (Table 1).
| Ref. | Study design | No. of pts CH-EUS/ EUS | Needle size (G), type | Contrast agent | Sensitivity (%), CH-EUS-FNA/B vs no CH-EUS-FNA/B | P value | Other findings |
| Hou et al[71], 2015 | R | 58/105 | 22, FNA | Sonovue | 81.6 vs 70.8 | NS | |
| Sugimoto et al[24], 2015 | RCT | 20/20 | 22, 25, FNA | Sonazoid | 90.0 vs 85.0 | NS | Adequate for the 1st pass only |
| Seicean et al[12], 2017 | P | 51 | 22, FNA | Sonovue | 82.9 vs 73.2 | NS | |
| Facciorusso et al[72], 2020 | R | 103/103 | 22, FNA | Sonovue | 87.6 vs 80.0 | NS | |
| Seicean et al[29], 2020 | RCT | 148 | 22, FBA | Sonovue | 87.6 vs 85.5 | NS | |
| Cho et al[26], 2021 | RCT | 120/120 | 19-25, FNA, FNB | Sonovue | 85.8 vs 88.3 | NS | Procore in 80% |
| Itonaga et al[30], 2020 | P | 93 | 22, FNA | Sonazoid | 84.9 vs 68.8 | 0.003 | Better adequacy in homogenous lesions and heterogenous lesions with non-enhancement areas |
| Lai et al[32], 2022 | R | 48/85 | 22, FNB | Sonazoid | 91.0 vs 90.0 | NS | 2.2 vs 3.6 passes |
| Kuo et al[23], 2023 | RCT | 59/59 | 22, FNB | Sonazoid | 100.0 vs 100.0 | NS | A nodule < 4 cm and a sample length > 1 cm improved the rate of diagnosis |
The first randomized controlled study used parallel small groups with twenty patients each and used up to five passes, and they compared Sonazoid contrastenhanced EUS to conventional EUS-guided FNA using 22 G and 25 G needles. The only significant difference was a higher diagnosis rate of the first pass with the use of contrast EUS (60% vs 25%), but the final diagnosis rate was similar in both groups[24]. Additionally, 25 G needles were used only in the conventional EUS-FNA group, but perhaps this did not influence the outcome, as the noninferiority of using 25 G needles as compared to 22 G needles for FNA has been demonstrated in a meta-analysis[25].
A second randomized controlled study involving parallel groups, which included 120 patients in the contrast EUS group (80% using first-generation EUS-FNB) and 120 patients in the conventional group, used needles of different sizes and up to five passes. In that study, no difference was revealed when contrast agent was used for guiding tissue acquisition (85.8% vs 88.3%)[26]. Neither the use of EUS-FNB needles nor the use of larger needles showed any difference in the multivariate analysis or in improving the diagnosis, although these needles are associated with a higher diagnostic yield[26]. The diagnostic accuracy of first-generation EUS-FNB was shown to be similar to that of EUSFNA[27,28], so the results of this study again reflect the same efficiency of EUS-guided tissue sampling, with and without contrast.
The third randomized controlled study included 148 patients who underwent crossover randomization; each patient had one EUS-FNA pass with contrast enhancement guidance and one EUS-FNA pass without contrast guidance. The diagnostic accuracy for each pass was 89.2% vs 88.5%, and the false-negative rate was similar for hypoenhanced or hyperenhanced lesions, for different mass locations and for masses of different sizes[29]. That study included 34 patients with chronic pancreatitis features and who had a pancreatic mass suspected to be malignant. The diagnostic rate of malignancy in this group was 85% for the CH-EUS-FNA passes compared to 79.4% for the EUS-FNA passes, which were lower rates than that for patients without chronic pancreatitis features (90.4% compared to 88.6%, respectively), but the difference was not significant[29].
Only one prospective study, including 93 patients, reported advantages in the use of CH-EUS-FNA using 22 G needles; however, that study reported a lower sensitivity than that reported in the literature (76.5% in the CH-EUS-FNA group vs 58.8% in the conventional EUS-FNA group), perhaps related to the limitations of only using two FNA passes only and the operator’s experience (300 EUS-FNA procedures)[30].
A preliminary meta-analysis that included only six studies (among which two were retrospective) and 701 patients reported superiority for CH-EUS-FNA[31], but subsequent studies[23,26,32] did not confirm this conclusion.
The results concerning the use of newer EUS-FNB needles were published recently. A retrospective study using Sonazoid contrast agent and second-generation EUSFNB needles achieved 91.7% accuracy in 48 patients with CH-EUS-FNB and 90.6% accuracy in 85 patients with conventional EUS-FNB; however, there was a lower number of passes in the CH-EUS-FNB group (2.21 ± 0.68 compared to 3.64 ± 1.20)[32]. There have been no prospective studies that have compared the time needed for each type of procedure (with and without contrast), but an additional 3 to 5 min are suggested for the preparation and observation of contrast agents[23,33], but the lower the number of passes might compensate for this needed time[32]. Another randomized controlled trial compared the fanning technique vs contrast-enhanced guidance for EUSFNB sampling and reported no differences in the sensitivity or accuracy (98% vs 100%). However, that study was monocentric, used up to five passes, and yielded false positive results; additionally, 24.6% of the samples were nondiagnostic on histology, which is unusual with the use of FNB needles[23].
Overall, based on the literature results, there is no demonstrable advantage with the use of routine CH-EUS tissue acquisition over conventional EUS tissue acquisition. Perhaps further research on chronic pancreatitis patients will show that the use of CH-EUS-FNB has advantages, especially when a differential diagnosis of a pancreatic mass is required or when it is used in patients with recent acute pancreatitis and suspicion of a pancreatic tumour.
Another potential field for CH-EUS tissue acquisition could be extravascular migratory metastasis (Figure 1C and D), which were identified by EUS-FNA as a perivascular soft-tissue cuff in 28% of 223 patients and changed the management of 29 patients[34].
Pancreatic cysts are mostly incidental findings, and there has been an increasing frequency of pancreatic cysts in recent years mainly because of improvements in the resolution of imaging methods. CH-EUS has limited yield for the characterization and differential diagnosis of pancreatic cystic lesions, but CH-EUS is recommended for the identification of mural nodules[6] because the contrast agent can highlight fine vascularisation while discharging nonenhanced mucin plugs[6,35,36].
A meta-analysis reported that the size of the mural nodule after contrast enhancement had a considerable effect on predicting malignancy, while another group reported a sensitivity of 97% and a specificity of 90% for the discrimination of malignant mural nodules[37,38]. Previous studies have shown that malignancy is present in 70%-100% of patients with mural nodules[35,39,40]. A size greater than 5 mm represents an absolute indication for surgery according to the European and Fukuoka guidelines[41,42], while the American guidelines recommend resection of cysts with mural nodules, regardless of their size[43].
Only one prospective study assessed the role of CH-EUS-FNA in cases of mural nodules within cysts. Among the 21 patients suspected of having mural nodules, only 13 had arterial enhancement, and only 10 (76.9%) had high dysplasia or carcinoma. The remaining patients had low or moderate dysplasia; the authors showed that not all the mural nodules were malignant; therefore, close follow-up is needed, regardless of the lesion size[44] (Figure 1E and F).
CH-EUS has been recommended for the guidance of tissue acquisition of liver masses in which a previous attempt had been negative[6]. CH-EUS allows the differentiation of millimetric lesions not visible via conventional imaging, and a longer follow-up into the late washout phase, over 240 s, is required for this purpose. Oh et al[45] published a retrospective series including 28 patients who underwent tissue acquisition after CH-EUS for confirmation. Metastases had typical non or hypo-enhancement behaviour; moreover, half of the neuroendocrine tumours presented early enhancement with early washout, while only 40% of the hepatocellular carcinomas presented hyperenhancement with delayed washout. The diagnostic value of CH-EUS-FNA was 86.7%[45], which is similar to other data from the literature that showed an 86.3% accuracy by using the same type of needle[46].
EUS tissue acquisition of suspected extrahepatic cholangiocarcinoma is indicated in selected cases of undetermined biliary strictures, unresectable tumours or metastatic masses forming cholangiocarcinomas[47-49]. A meta-analysis of 6 studies including 497 patients with extrahepatic biliary tumours and who underwent EUS-FNA revealed a diagnostic sensitivity of 76% and an accuracy of 94.5% for EUS-FNA[49]. However, the role that contrast-enhanced EUS-guided tissue acquisition played in improving the analysis of these lesions has not yet been identified.
The CH-EUS features suggestive of lymph node malignancy include heterogeneous hyperenhancement and fast washout[50], but the actual guidelines do not recommend routinely performing this technique in addition to EUS tissue acquisition for differentiating between malignant and benign lymph nodes[6]. In a cohort of 37 subjects with oesophageal cancer, the use of CH-EUS identified suspected lymph nodes that were not identified by conventional EUS and increased the rate of malignancy detection from 45% to 86%[51]. However, no studies on guiding CH-EUS tissue acquisition in lymph nodes or subepithelial lesions have been published.
The utility of intracavitary contrast transabdominal ultrasound for assessing drainage quality, such as in cases of stent dislodgements, the need for additional therapeutic procedures or the presence of communications with the surrounding structures, has been demonstrated by previous experience[52]. Additionally, complications such as fistulas associated with the biliary system, blood vessels, small or large intestine or to the peritoneal cavity were noted[53]. However, the daily usefulness of intracavity contrast in EUS-guided therapeutic procedures is less known.
The use of CH-EUS before endoscopic drainage was proposed for a better evaluation of the wall, which is less visible on standard EUS, especially when the content is thick (blood or pus)[54-56]. In such situations, CH-EUS shows the collection wall as an unenhanced structure and the vessels within the wall as hyperenhanced structures, and CH-EUS allows safe needle puncture as the first step of drainage[52]. The presence of vessels crossing the fluid collection area is also important because their visualization might change the drainage strategy (e.g., choosing plastic stents instead of lumen-apposing metal stents). Additionally, the interposition of the vascularized parenchyma between the gut wall and the fluid collection can be identified during CH-EUS. Moreover, CH-EUS can reveal necrosis as unenhanced areas and can differentiate the vascularized tissue within cystic pancreatic lesions to avoid a mis diagnosis and allow for the drainage of such lesions. However, large studies on its use during drainage are lacking.
The aim in the use of CEH-EUS to guide biliary drainage is to obtain better detection of a poorly visible intrahepatic bile duct due to echogenic, concentrated bile or intrabilliary neoplastic material[57]. The use of CH-EUS has been reported for only seven patients, and it allowed for clarifying the borders of the intrahepatic (3 patients) and extrahepatic (4 patients) bile ducts before planning EUS drainage[58-62].
EUS gallbladder drainage is contraindicated in cases of gangrenous cholecystitis with special transabdominal ultrasound features (decreased focal wall perfusion on colour Doppler, irregular mucosal outline, gallbladder wall thickening and delamination, gas within the gallbladder, absence of gallstones, and large pericholecystic collections) or computed tomography scan features (gas in the wall or lumen, intraluminal membranes, an irregular or absent wall)[63-66]. Its identification (disruptive layered structure, focal hypoenhanced gallbladder wall) could represent a potential application for CH-EUS prior to gallbladder drainage[60,62,63]. To our knowledge, no specific study in this area has been published.
The evaluation of residual stones in the common bile duct after endoscopic retrograde cholangio-pancreatography by the injection of a contrast agent through a nasobiliary tube was reported in 6 patients and confirmed by EUS cholangiography[67], but its usefulness remains anecdotical.
Contrast enhancement allows for real-time visualization of the perfusion of the target nodule in parallel with that of the normal parenchyma, similar to what occurs in hepatic conditions treated by ultrasoundguided procedures. Choi et al[68] evaluated the dynamics of perfusion of solid intraabdominal nodules 5-7 d after radiofrequency ablation and reported incomplete ablation in 12 of 19 patients. At the 1-year followup, a complete response was reported in 68.4% of patients, with a median of 2 sessions[68]. Similar to the use of CH-EUS after ablation of liver masses, its immediate use after ablative procedures might reveal incomplete ablations and prevent 3-6 months of waiting until a CT scan evaluation[69,70].
CH-EUS tissue acquisition is a promising advanced EUS technique that can improve the diagnosis in selected patients by differentiating tumoral vascularisation from the surrounding parenchyma, thus improving the histological results. Additionally, this technique reveals vessels that are not visible via colour Doppler EUS, and allows the identification of structures with thick content to be targeted for drainage while avoiding the puncture of these vessels. Additionally, CH-EUS assessment allows for timely evaluation of any tissue destruction after ablative procedures.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Romania
Peer-review report’s classification
Scientific Quality: Grade A
Novelty: Grade A
Creativity or Innovation: Grade B
Scientific Significance: Grade A
P-Reviewer: El-Karaksy H, Egypt S-Editor: Chen YL L-Editor: A P-Editor: Yu HG
| 1. | Li Z, Liu W, Xu X, Li P. A Meta-Analysis Comparing Endoscopic Ultrasound-guided Fine-needle Aspiration With Endoscopic Ultrasound-guided Fine-needle Biopsy. J Clin Gastroenterol. 2022;56:668-678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 2. | Togliani T, Lisotti A, Rinaldi R, Fornelli A, Pilati S, Passigato N, Fusaroli P. Tumor Location in the Head/Uncinate Process and Presence of Fibrosis Impair the Adequacy of Endoscopic Ultrasound-Guided Tissue Acquisition of Solid Pancreatic Tumors. Cancers (Basel). 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 3. | Kamata K, Takenaka M, Omoto S, Miyata T, Minaga K, Yamao K, Imai H, Sakurai T, Nishida N, Chikugo T, Chiba Y, Matsumoto I, Takeyama Y, Kudo M. Impact of avascular areas, as measured by contrast-enhanced harmonic EUS, on the accuracy of FNA for pancreatic adenocarcinoma. Gastrointest Endosc. 2018;87:158-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 4. | Kitano M, Kudo M, Yamao K, Takagi T, Sakamoto H, Komaki T, Kamata K, Imai H, Chiba Y, Okada M, Murakami T, Takeyama Y. Characterization of small solid tumors in the pancreas: the value of contrast-enhanced harmonic endoscopic ultrasonography. Am J Gastroenterol. 2012;107:303-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 241] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 5. | Gincul R, Palazzo M, Pujol B, Tubach F, Palazzo L, Lefort C, Fumex F, Lombard A, Ribeiro D, Fabre M, Hervieu V, Labadie M, Ponchon T, Napoléon B. Contrast-harmonic endoscopic ultrasound for the diagnosis of pancreatic adenocarcinoma: a prospective multicenter trial. Endoscopy. 2014;46:373-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 63] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 6. | Kitano M, Yamashita Y, Kamata K, Ang TL, Imazu H, Ohno E, Hirooka Y, Fusaroli P, Seo DW, Napoléon B, Teoh AYB, Kim TH, Dietrich CF, Wang HP, Kudo M; Working group for the International Consensus Guidelines for Contrast-Enhanced Harmonic Endoscopic Ultrasound. The Asian Federation of Societies for Ultrasound in Medicine and Biology (AFSUMB) Guidelines for Contrast-Enhanced Endoscopic Ultrasound. Ultrasound Med Biol. 2021;47:1433-1447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 7. | Saftoiu A, Napoleon B, Arcidiacono PG, Braden B, Burmeister S, Carrara S, Cui XW, Fusaroli P, Gottschalk U, Hocke M, Hollerbach S, Iglesias-Garcia J, Jenssen C, Kitano M, Larghi A, Oppong KW, Sahai AV, Sun S, Burmester E, Di Leo M, Petrone MC, Santos E, Teoh AYB, Hwang JH, Rimbas M, Sharma M, Puri R, Kahaleh M, Dietrich CF. Do we need contrast agents for EUS? Endosc Ultrasound. 2020;9:361-368. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 8. | Fusaroli P, Napoleon B, Gincul R, Lefort C, Palazzo L, Palazzo M, Kitano M, Minaga K, Caletti G, Lisotti A. The clinical impact of ultrasound contrast agents in EUS: a systematic review according to the levels of evidence. Gastrointest Endosc. 2016;84:587-596.e10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 66] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 9. | Iglesias-Garcia J, Lindkvist B, Lariño-Noia J, Abdulkader-Nallib I, Dominguez-Muñoz JE. Differential diagnosis of solid pancreatic masses: contrast-enhanced harmonic (CEH-EUS), quantitative-elastography (QE-EUS), or both? United European Gastroenterol J. 2017;5:236-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 63] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 10. | Iordache S, Costache MI, Popescu CF, Streba CT, Cazacu S, Săftoiu A. Clinical impact of EUS elastography followed by contrast-enhanced EUS in patients with focal pancreatic masses and negative EUS-guided FNA. Med Ultrason. 2016;18:18-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 31] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 11. | Yamashita Y, Shimokawa T, Napoléon B, Fusaroli P, Gincul R, Kudo M, Kitano M. Value of contrast-enhanced harmonic endoscopic ultrasonography with enhancement pattern for diagnosis of pancreatic cancer: A meta-analysis. Dig Endosc. 2019;31:125-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 69] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 12. | Seicean A, Badea R, Moldovan-Pop A, Vultur S, Botan EC, Zaharie T, Săftoiu A, Mocan T, Iancu C, Graur F, Sparchez Z, Seicean R. Harmonic Contrast-Enhanced Endoscopic Ultrasonography for the Guidance of Fine-Needle Aspiration in Solid Pancreatic Masses. Ultraschall Med. 2017;38:174-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 13. | Fusaroli P, Spada A, Mancino MG, Caletti G. Contrast harmonic echo-endoscopic ultrasound improves accuracy in diagnosis of solid pancreatic masses. Clin Gastroenterol Hepatol. 2010;8:629-34.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 152] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 14. | Braden B, Jenssen C, D'Onofrio M, Hocke M, Will U, Möller K, Ignee A, Dong Y, Cui XW, Sãftoiu A, Dietrich CF. B-mode and contrast-enhancement characteristics of small nonincidental neuroendocrine pancreatic tumors. Endosc Ultrasound. 2017;6:49-54. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 36] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 15. | Ishikawa R, Kamata K, Hara A, Tanaka H, Okamoto A, Yamazaki T, Nakai A, Omoto S, Minaga K, Yamao K, Takenaka M, Minami Y, Watanabe T, Chiba Y, Chikugo T, Matsumoto I, Takeyama Y, Matsukubo Y, Hyodo T, Kudo M. Utility of contrast-enhanced harmonic endoscopic ultrasonography for predicting the prognosis of pancreatic neuroendocrine neoplasms. Dig Endosc. 2021;33:829-839. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 16. | Kasono K, Hyodo T, Suminaga Y, Sugiura Y, Namai K, Ikoma A, Tamemoto H, Imawari M, Kawakami M, Ishikawa SE. Contrast-enhanced endoscopic ultrasonography improves the preoperative localization of insulinomas. Endocr J. 2002;49:517-522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 23] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 17. | Palazzo M, Napoléon B, Gincul R, Pioche M, Pujol B, Lefort C, Fumex F, Hautefeuille V, Fabre M, Cros J, Felce M, Couvelard A, Sauvanet A, Lévy P, Ruszniewski P, Palazzo L. Contrast harmonic EUS for the prediction of pancreatic neuroendocrine tumor aggressiveness (with videos). Gastrointest Endosc. 2018;87:1481-1488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 40] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 18. | Tang A, Tian L, Gao K, Liu R, Hu S, Liu J, Xu J, Fu T, Zhang Z, Wang W, Zeng L, Qu W, Dai Y, Hou R, Tang S, Wang X. Contrast-enhanced harmonic endoscopic ultrasound (CH-EUS) MASTER: A novel deep learning-based system in pancreatic mass diagnosis. Cancer Med. 2023;12:7962-7973. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 20] [Reference Citation Analysis (0)] |
| 19. | Săftoiu A, Vilmann P, Dietrich CF, Iglesias-Garcia J, Hocke M, Seicean A, Ignee A, Hassan H, Streba CT, Ioncică AM, Gheonea DI, Ciurea T. Quantitative contrast-enhanced harmonic EUS in differential diagnosis of focal pancreatic masses (with videos). Gastrointest Endosc. 2015;82:59-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 112] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 20. | Buxbaum J, Ko C, Varghese N, Lee A, Sahakian A, King K, Serna J, Lee H, Tchelepi H, Van Dam J, Duddalwar V. Qualitative and Quantitative Contrast-enhanced Endoscopic Ultrasound Improves Evaluation of Focal Pancreatic Lesions. Clin Gastroenterol Hepatol. 2020;18:917-925.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 21. | Seicean A, Badea R, Stan-Iuga R, Mocan T, Gulei I, Pascu O. Quantitative contrast-enhanced harmonic endoscopic ultrasonography for the discrimination of solid pancreatic masses. Ultraschall Med. 2010;31:571-576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 57] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 22. | Numata K, Ozawa Y, Kobayashi N, Kubota T, Shimada H, Nozawa A, Nakatani Y, Sugimori K, Matsuo K, Imada T, Tanaka K. Contrast-enhanced sonography of pancreatic carcinoma: correlations with pathological findings. J Gastroenterol. 2005;40:631-640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 58] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 23. | Kuo YT, Chu YL, Wong WF, Han ML, Chen CC, Jan IS, Cheng WC, Shun CT, Tsai MC, Cheng TY, Wang HP. Randomized trial of contrast-enhanced harmonic guidance versus fanning technique for EUS-guided fine-needle biopsy sampling of solid pancreatic lesions. Gastrointest Endosc. 2023;97:732-740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 24. | Sugimoto M, Takagi T, Hikichi T, Suzuki R, Watanabe K, Nakamura J, Kikuchi H, Konno N, Waragai Y, Watanabe H, Obara K, Ohira H. Conventional versus contrast-enhanced harmonic endoscopic ultrasonography-guided fine-needle aspiration for diagnosis of solid pancreatic lesions: A prospective randomized trial. Pancreatology. 2015;15:538-541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 60] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 25. | Guedes HG, Moura DTH, Duarte RB, Cordero MAC, Santos MELD, Cheng S, Matuguma SE, Chaves DM, Bernardo WM, Moura EGH. A comparison of the efficiency of 22G versus 25G needles in EUS-FNA for solid pancreatic mass assessment: A systematic review and meta-analysis. Clinics (Sao Paulo). 2018;73:e261. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 26. | Cho IR, Jeong SH, Kang H, Kim EJ, Kim YS, Cho JH. Comparison of contrast-enhanced versus conventional EUS-guided FNA/fine-needle biopsy in diagnosis of solid pancreatic lesions: a randomized controlled trial. Gastrointest Endosc. 2021;94:303-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 26] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 27. | Bang JY, Hawes R, Varadarajulu S. A meta-analysis comparing ProCore and standard fine-needle aspiration needles for endoscopic ultrasound-guided tissue acquisition. Endoscopy. 2016;48:339-349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 96] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 28. | Delconte G, Cavalcoli F, Magarotto A, Centonze G, Bezzio C, Cattaneo L, Rausa E, Kelly ME, Bonitta G, Milione M, Enzo M. Does ProCore Fine-Needle Biopsy Really Improve the Clinical Outcome of Endoscopic Ultrasound-Guided Sampling of Pancreatic Masses? Dig Dis. 2022;40:78-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 29. | Seicean A, Samarghitan A, Bolboacă SD, Pojoga C, Rusu I, Rusu D, Sparchez Z, Gheorghiu M, Al Hajjar N, Seicean R. Contrast-enhanced harmonic versus standard endoscopic ultrasound-guided fine-needle aspiration in solid pancreatic lesions: a single-center prospective randomized trial. Endoscopy. 2020;52:1084-1090. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 30. | Itonaga M, Kitano M, Kojima F, Hatamaru K, Yamashita Y, Tamura T, Nuta J, Kawaji Y, Shimokawa T, Tanioka K, Murata SI. The usefulness of EUS-FNA with contrast-enhanced harmonic imaging of solid pancreatic lesions: A prospective study. J Gastroenterol Hepatol. 2020;35:2273-2280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 31. | Facciorusso A, Mohan BP, Crinò SF, Ofosu A, Ramai D, Lisotti A, Chandan S, Fusaroli P. Contrast-enhanced harmonic endoscopic ultrasound-guided fine-needle aspiration versus standard fine-needle aspiration in pancreatic masses: a meta-analysis. Expert Rev Gastroenterol Hepatol. 2021;15:821-828. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 52] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 32. | Lai JH, Lin CC, Lin HH, Chen MJ. Is contrast-enhanced endoscopic ultrasound-guided fine needle biopsy better than conventional fine needle biopsy? A retrospective study in a medical center. Surg Endosc. 2022;36:6138-6143. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 33. | Cherciu Harbiyeli IF, Constantin A, Cazacu IM, Burtea DE, Gheorghe EC, Popescu CF, Bejinariu N, Georgescu CV, Pirici D, Ungureanu BS, Copăescu C, Săftoiu A. Technical Performance, Overall Accuracy and Complications of EUS-Guided Interventional Procedures: A Dynamic Landscape. Diagnostics (Basel). 2022;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 34. | Rustagi T, Gleeson FC, Chari ST, Lehrke HD, Takahashi N, Malikowski TM, Abu Dayyeh BK, Chandrasekhara V, Iyer PG, Kendrick ML, Pearson RK, Petersen BT, Rajan E, Smoot RL, Storm AC, Topazian MD, Truty MJ, Vege SS, Wang KK, Levy MJ. Safety, Diagnostic Accuracy, and Effects of Endoscopic Ultrasound Fine-Needle Aspiration on Detection of Extravascular Migratory Metastases. Clin Gastroenterol Hepatol. 2019;17:2533-2540.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 35. | Yamashita Y, Ueda K, Itonaga M, Yoshida T, Maeda H, Maekita T, Iguchi M, Tamai H, Ichinose M, Kato J. Usefulness of contrast-enhanced endoscopic sonography for discriminating mural nodules from mucous clots in intraductal papillary mucinous neoplasms: a single-center prospective study. J Ultrasound Med. 2013;32:61-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 71] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 36. | Ohno E, Hirooka Y, Kawashima H, Ishikawa T, Fujishiro M. Endoscopic ultrasonography for the evaluation of pancreatic cystic neoplasms. J Med Ultrason (2001). 2020;47:401-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 37. | Lisotti A, Napoleon B, Facciorusso A, Cominardi A, Crinò SF, Brighi N, Gincul R, Kitano M, Yamashita Y, Marchegiani G, Fusaroli P. Contrast-enhanced EUS for the characterization of mural nodules within pancreatic cystic neoplasms: systematic review and meta-analysis. Gastrointest Endosc. 2021;94:881-889.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 54] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 38. | Marchegiani G, Andrianello S, Borin A, Dal Borgo C, Perri G, Pollini T, Romanò G, D'Onofrio M, Gabbrielli A, Scarpa A, Malleo G, Bassi C, Salvia R. Systematic review, meta-analysis, and a high-volume center experience supporting the new role of mural nodules proposed by the updated 2017 international guidelines on IPMN of the pancreas. Surgery. 2018;163:1272-1279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 60] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 39. | Fusaroli P, Serrani M, De Giorgio R, D'Ercole MC, Ceroni L, Lisotti A, Caletti G. Contrast Harmonic-Endoscopic Ultrasound Is Useful to Identify Neoplastic Features of Pancreatic Cysts (With Videos). Pancreas. 2016;45:265-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 43] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 40. | Thornton GD, McPhail MJ, Nayagam S, Hewitt MJ, Vlavianos P, Monahan KJ. Endoscopic ultrasound guided fine needle aspiration for the diagnosis of pancreatic cystic neoplasms: a meta-analysis. Pancreatology. 2013;13:48-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 191] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 41. | European Study Group on Cystic Tumours of the Pancreas. European evidence-based guidelines on pancreatic cystic neoplasms. Gut. 2018;67:789-804. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1006] [Cited by in RCA: 893] [Article Influence: 127.6] [Reference Citation Analysis (1)] |
| 42. | Tanaka M, Fernández-Del Castillo C, Kamisawa T, Jang JY, Levy P, Ohtsuka T, Salvia R, Shimizu Y, Tada M, Wolfgang CL. Revisions of international consensus Fukuoka guidelines for the management of IPMN of the pancreas. Pancreatology. 2017;17:738-753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 868] [Cited by in RCA: 1154] [Article Influence: 144.3] [Reference Citation Analysis (1)] |
| 43. | Elta GH, Enestvedt BK, Sauer BG, Lennon AM. ACG Clinical Guideline: Diagnosis and Management of Pancreatic Cysts. Am J Gastroenterol. 2018;113:464-479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 282] [Cited by in RCA: 422] [Article Influence: 60.3] [Reference Citation Analysis (1)] |
| 44. | Olar MP, Bolboacă SD, Pojoga C, Moșteanu O, Gheorghiu M, Seicean R, Rusu I, Sparchez Z, Al Hajjar N, Seicean A. Clinical Utility of the Contrast-Enhanced Endoscopic Ultrasound Guided Fine Needle Aspiration in the Diagnosis of Pancreatic Cyst. Diagnostics (Basel). 2022;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 45. | Oh D, Seo DW, Hong SM, Jun JH, Song TJ, Park DH, Son BK, Lee SS, Lee SK, Kim MH. The usefulness of contrast-enhanced harmonic EUS-guided fine-needle aspiration for evaluation of hepatic lesions (with video). Gastrointest Endosc. 2018;88:495-501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 46. | Akay E, Atasoy D, Altınkaya E, Koç A, Ertan T, Karaman H, Caglar E. Endoscopic Ultrasound-Guided Fine Needle Aspiration Using a 22-G Needle for Hepatic Lesions: Single-Center Experience. Clin Endosc. 2021;54:404-412. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 47. | Khashab MA, Fockens P, Al-Haddad MA. Utility of EUS in patients with indeterminate biliary strictures and suspected extrahepatic cholangiocarcinoma (with videos). Gastrointest Endosc. 2012;76:1024-1033. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 43] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 48. | Pouw RE, Barret M, Biermann K, Bisschops R, Czakó L, Gecse KB, de Hertogh G, Hucl T, Iacucci M, Jansen M, Rutter M, Savarino E, Spaander MCW, Schmidt PT, Vieth M, Dinis-Ribeiro M, van Hooft JE. Endoscopic tissue sampling - Part 1: Upper gastrointestinal and hepatopancreatobiliary tracts. European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy. 2021;53:1174-1188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 93] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 49. | de Moura DTH, Ryou M, de Moura EGH, Ribeiro IB, Bernardo WM, Thompson CC. Endoscopic Ultrasound-Guided Fine Needle Aspiration and Endoscopic Retrograde Cholangiopancreatography-Based Tissue Sampling in Suspected Malignant Biliary Strictures: A Meta-Analysis of Same-Session Procedures. Clin Endosc. 2020;53:417-428. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 50. | Yoshida K, Iwashita T, Uemura S, Mita N, Iwata K, Mukai T, Yasuda I, Shimizu M. Efficacy of contrast-enhanced EUS for lymphadenopathy: a prospective multicenter pilot study (with videos). Gastrointest Endosc. 2019;90:242-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 51. | Liu JB, Machado P, Eisenbrey JR, Gummadi S, Forsberg F, Wessner CE, Kumar AR, Chiang A, Infantolino A, Schlachterman A, Kowalski T, Coben R, Loren D. Identification of sentinel lymph nodes in esophageal cancer patients using contrast-enhanced EUS with peritumoral injections. Endosc Ultrasound. 2023;12:362-368. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 52. | Ignee A, Jenssen C, Cui XW, Schuessler G, Dietrich CF. Intracavitary contrast-enhanced ultrasound in abscess drainage--feasibility and clinical value. Scand J Gastroenterol. 2016;51:41-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 53. | Heinzmann A, Müller T, Leitlein J, Braun B, Kubicka S, Blank W. Endocavitary contrast enhanced ultrasound (CEUS)--work in progress. Ultraschall Med. 2012;33:76-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 54. | Hori Y, Yoshida M, Hayashi K, Naitoh I, Kato A, Miyabe K, Kataoka H. Endoscopic drainage using a lumen-apposing metal stent under contrast-enhanced harmonic endoscopic ultrasonography guidance. Endoscopy. 2019;51:E187-E188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 55. | Minaga K, Takenaka M, Omoto S, Miyata T, Kamata K, Yamao K, Imai H, Watanabe T, Kitano M, Kudo M. A case of successful transluminal drainage of walled-off necrosis under contrast-enhanced harmonic endoscopic ultrasonography guidance. J Med Ultrason (2001). 2018;45:161-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 56. | Guo J, Saftoiu A, Vilmann P, Fusaroli P, Giovannini M, Mishra G, Rana SS, Ho S, Poley JW, Ang TL, Kalaitzakis E, Siddiqui AA, De La Mora-Levy JG, Lakhtakia S, Bhutani MS, Sharma M, Mukai S, Garg PK, Lee LS, Vila JJ, Artifon E, Adler DG, Sun S. A multi-institutional consensus on how to perform endoscopic ultrasound-guided peri-pancreatic fluid collection drainage and endoscopic necrosectomy. Endosc Ultrasound. 2017;6:285-291. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 79] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 57. | Iwashita T, Senju A, Tezuka R, Uemura S, Shimizu M. Contrast enhancement for undetectable intrahepatic bile duct to facilitate endoscopic ultrasound-guided hepaticogastrostomy. Endoscopy. 2023;55:E511-E512. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 58. | Tamura T, Kitano M. Role of CH-EUS as guidance for EUS-biliary drainage malignant obstruction. Minerva Gastroenterol (Torino). 2022;68:210-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 59. | Tamura T, Kitano M, Kawai M, Itonaga M, Okada KI, Yamaue H. Contrast-enhanced harmonic endoscopic ultrasound-guided drainage of a postoperative pancreatic fistula. Endoscopy. 2020;52:E174-E175. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 60. | Kamata K, Takenaka M, Kitano M, Omoto S, Miyata T, Minaga K, Yamao K, Imai H, Sakurai T, Nishida N, Kashida H, Chikugo T, Chiba Y, Nakai T, Takeyama Y, Lisotti A, Fusaroli P, Kudo M. Contrast-enhanced harmonic endoscopic ultrasonography for differential diagnosis of localized gallbladder lesions. Dig Endosc. 2018;30:98-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 43] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 61. | Choi JH, Seo DW, Choi JH, Park DH, Lee SS, Lee SK, Kim MH. Utility of contrast-enhanced harmonic EUS in the diagnosis of malignant gallbladder polyps (with videos). Gastrointest Endosc. 2013;78:484-493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 63] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 62. | Imazu H, Mori N, Kanazawa K, Chiba M, Toyoizumi H, Torisu Y, Koyama S, Hino S, Ang TL, Tajiri H. Contrast-enhanced harmonic endoscopic ultrasonography in the differential diagnosis of gallbladder wall thickening. Dig Dis Sci. 2014;59:1909-1916. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 48] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 63. | Okamoto K, Suzuki K, Takada T, Strasberg SM, Asbun HJ, Endo I, Iwashita Y, Hibi T, Pitt HA, Umezawa A, Asai K, Han HS, Hwang TL, Mori Y, Yoon YS, Huang WS, Belli G, Dervenis C, Yokoe M, Kiriyama S, Itoi T, Jagannath P, Garden OJ, Miura F, Nakamura M, Horiguchi A, Wakabayashi G, Cherqui D, de Santibañes E, Shikata S, Noguchi Y, Ukai T, Higuchi R, Wada K, Honda G, Supe AN, Yoshida M, Mayumi T, Gouma DJ, Deziel DJ, Liau KH, Chen MF, Shibao K, Liu KH, Su CH, Chan ACW, Yoon DS, Choi IS, Jonas E, Chen XP, Fan ST, Ker CG, Giménez ME, Kitano S, Inomata M, Hirata K, Inui K, Sumiyama Y, Yamamoto M. Tokyo Guidelines 2018: flowchart for the management of acute cholecystitis. J Hepatobiliary Pancreat Sci. 2018;25:55-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 556] [Cited by in RCA: 498] [Article Influence: 71.1] [Reference Citation Analysis (0)] |
| 64. | Corr P. Sonography of gangrenous cholecystitis. J Emerg Trauma Shock. 2012;5:82-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 65. | Bennett GL, Rusinek H, Lisi V, Israel GM, Krinsky GA, Slywotzky CM, Megibow A. CT findings in acute gangrenous cholecystitis. AJR Am J Roentgenol. 2002;178:275-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 120] [Article Influence: 5.2] [Reference Citation Analysis (1)] |
| 66. | Ripollés T, Martínez-Pérez MJ, Martin G, Vizuete J, Martínez-García R, Diez J, Martí E. Usefulness of contrast-enhanced US in the diagnosis of acute gangrenous cholecystitis: A comparative study with surgical and pathological findings. Eur J Radiol. 2016;85:31-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 67. | Wang Y, Yang Y, Wang K, Tang S. The Value of Contrast-Enhanced Ultrasound-Guided Contrast Injection via the Endoscopic Nasobiliary Drainage Duct in Diagnosing Residual Common Bile Duct Stones. Biomed Res Int. 2020;2020:3281241. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 68. | Choi JH, Seo DW, Song TJ, Park DH, Lee SS, Lee SK, Kim MH. Utility of Contrast-Enhanced Harmonic Endoscopic Ultrasound for the Guidance and Monitoring of Endoscopic Radiofrequency Ablation. Gut Liver. 2020;14:826-832. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 69. | Mangiavillano B, Auriemma F, Scaltrini F, Bianchetti M, Di Leo M, Carrara S, Repici A. Endoscopic Ultrasonography-Guided Radiofrequency Ablation for a Perianastomotic Neoplastic Colorectal Recurrence. Am J Gastroenterol. 2019;114:1709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 70. | Lisotti A, Piscaglia F, Fusaroli P. Contrast-enhanced harmonic endoscopic ultrasound-guided ethanol injection for a small hepatocellular carcinoma. Endoscopy. 2019;51:E317-E318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 71. | Hou X, Jin Z, Xu C, Zhang M, Zhu J, Jiang F, Li Z. Contrast-enhanced harmonic endoscopic ultrasound-guided fine-needle aspiration in the diagnosis of solid pancreatic lesions: a retrospective study. PLoS One. 2015;10:e0121236. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 33] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 72. | Facciorusso A, Cotsoglou C, Chierici A, Mare R, Crinò SF, Muscatiello N. Contrast-Enhanced Harmonic Endoscopic Ultrasound-Guided Fine-Needle Aspiration versus Standard Fine-Needle Aspiration in Pancreatic Masses: A Propensity Score Analysis. Diagnostics (Basel). 2020;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |