Published online May 7, 2024. doi: 10.3748/wjg.v30.i17.2302
Revised: March 9, 2024
Accepted: April 15, 2024
Published online: May 7, 2024
Processing time: 76 Days and 14.3 Hours
In this editorial, we discuss the article in the World Journal of Gastroenterology. The article conducts a meta-analysis of the diagnostic accuracy of the urea breath test (UBT), a non-invasive method for detecting Helicobacter pylori (H. pylori) infection in humans. It is based on radionuclide-labeled urea. Various methods, both invasive and non-invasive, are available for diagnosing H. pylori infection, inclu
Core Tip: This editorial comments on the article published in the World Journal of Gastroenterology, where it demonstrates that 13C-UBT is an accurate test procedure to detect Helicobacter pylori infection. It is a safe and simple test for the patient, providing clear positive or negative test results for the clinician in the majority of cases, making it the preferred non-invasive test in clinical settings. Furthermore, the provided article highlights the importance of accurate and careful choosing of urea dosage, timing of assessment, as well as techniques of measurement for 13C-UBT and 14C-UBT, thereby improving diagnostic accuracy.
- Citation: Said ZNA, El-Nasser AM. Evaluation of urea breath test as a diagnostic tool for Helicobacter pylori infection in adult dyspeptic patients. World J Gastroenterol 2024; 30(17): 2302-2307
- URL: https://www.wjgnet.com/1007-9327/full/v30/i17/2302.htm
- DOI: https://dx.doi.org/10.3748/wjg.v30.i17.2302
Helicobacter pylori (H. pylori) is a spiral-shaped Gram-negative, non-spore-forming bacterium that is microaerophilic and requires complex enriched growth media for its cultivation[1]. The bacterium typically colonizes the epithelium lining of the human stomach, particularly the gastric antrum. H. pylori are characterized by producing a powerful urease enzyme that hydrolyzes urea acquired in the diet to produce ammonia and carbon dioxide. This enzymatic process neutralizes gastric acidity and raises periplasmic pH to alkaline mediators, resulting in peptic ulcer (PU), gastritis, gastric adenocarcinoma, and low-grade B mucosa-associated lymphoma[1-4]. It is reported that about 50% of the world's population is infected with H. pylori. However, there are significant differences in the prevalence, incidence, age distribution, and outcomes of infection between developing and developed countries[2,5,6].
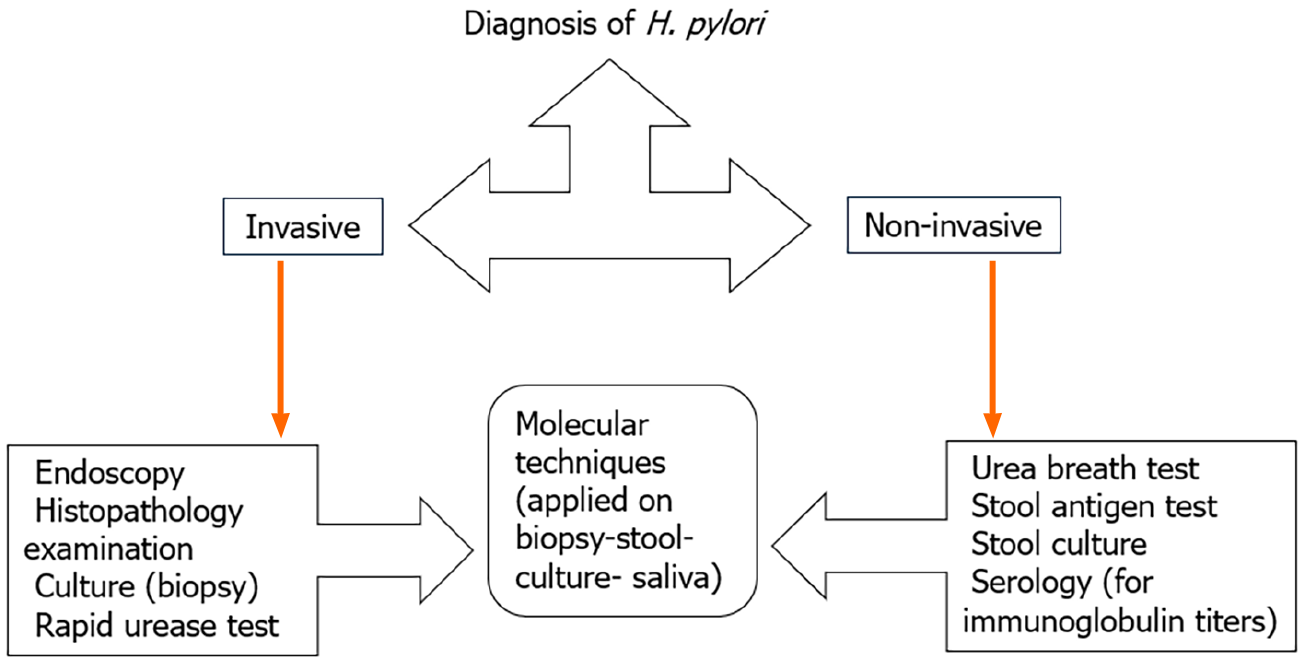
Diagnosis of patients infected with H. pylori can be achieved through non-invasive tests including serology, urea breath test (UBT) and stool antigen detection[7]. Alternatively, invasive techniques utilizing endoscopic gastric biopsy for histopathology and microbial identification tests including rapid urease test (RUT), bacterial culture and biochemical reactions can also be employed[3,8], as show in Figure 1 and Table 1[9-11]. The recommended protocol to identify H. pylori infection is a combination of RUT or microbiological culture with a histopathological examination of two different sites following having an endoscopic biopsy[12].
| Criteria | Invasive tests | Non-invasive tests |
| Technique | Endoscopy | UBT, stool antigen, serology |
| Sample | Biopsy | UB, stool, serum |
| Sampling error | ++ | Variable |
| Accuracy | The gold standard | Variable |
| Skill and experience | ++ | Easily applicable |
| Time of results | Short | Longer (variable) |
| Cost | ++ | + |
| Contraindications | May not be suitable for all patients | Suitable for most patients |
| Equipment and infrastructure | +++ | ++ |
| Availability | Available in specialized settings | Widely available in various healthcare settings |
| Patient discomfort | ++ | Minimum |
| Post-treatment monitoring | + | +++ |
UBT can play a useful role in diagnosing dyspeptic patients with specific criteria, such as those with any associated comorbidity that increase the risk of gastric endoscopy, intolerance to gastric endoscopy, diagnosed or doubtful cases of gastric atrophy[13]. Stool antigen tests are alternative non-invasive assays for detection of active of H. pylori infection. The proper selection of diagnostic method is based on several factors including cost, laboratory infrastructure, and the accompanied administration of medical treatment such as antibiotics or proton pump inhibitors, which may affect the test results. It was well known that serum antibody test results are variable geographically among population and may remain positive for an extended period after eradication of H. pylori, which limits their clinical value in confirming or ruling out current H. pylori infection[11].
Current guidelines recommend the use of UBT as a noninvasive test for diagnosing H. pylori infection and demonstrate eradication after treatment[14,15]. These recommendations are primarily documented by many researchers that demonstrate UBT sensitivity of 90%–96% and specificity of 88%–98%; in addition to its cost effectiveness and acceptance by patients compared to the invasive gold standard endoscopy[16,17]. Moreover, on comparison with other non-invasive tests, such as stool antigen test and serology, UBT exhibits the highest accuracy[17,18]. The UBT is particularly appropriate for all healthcare settings where endoscopy is not strictly indicated and for assessing the effectiveness of eradication therapy[19].
The 13C and 14C tests are two versions of the UBTs approved by the Food and Drug Administration. Both tests are cost-effective and provide real-time availability results[20]. Doctors like the use of 13C-UBT test since it is non-radioactive when compared to 14C-UBT. However, the latter uses a relatively low radiation dose (approximately 1 microCi) of a radioactive isotope. Despite this, 14C-UBT demonstrates a high degree of diagnostic accuracy[21].
Labeling urea with the 13C non-radioactive isotope is preferable as the test can be used frequently on the same patient and can be applied safely to children and pregnant females. Meanwhile, the UBT that uses 14C as a diagnostic test for H. pylori is still not approved by most health care authorities, as it is associated with a dose of radiation that might be hazardous. Thus, its use is contraindicated in some situations such as pregnancy and in young age groups[22,23]. The provided meta-analysis results also revealed high UBT performance, as well as high discrimination ability between patients and healthy individuals. However, the quality of this evidence is not strong enough due to heterogeneity that is explained by various factors such as using different reference standards, timing between capsule intake and test performance, or variation in the quality of the methodology of the included studies[24].
Beside the non-invasiveness of UBT, the test provides a comprehensive evaluation that is not dependent on the possible errors which may occur during sampling of gastric endoscopic biopsy because of the H. pylori patchy distribution[9]. Moreover, the biopsy-based tests largely depend on the skills and experience of the pathologist, with studies confirming internal observer variability[10,25]. On the other hand, results of UBT test can be impaired by the intake of H. pylori eradication medication, including antibiotics, proton pumps inhibitors, or bismuth. Additionally, it needs special CO2 tracing equipment, as well as infrastructure to deal with radioactive materials in the case of 14C-UBT. Consequently, it is considered an expensive test.
Although the UBT is well known for its high diagnostic accuracy in detection of H. pylori infection in dyspeptic patients, caution should always be taken in interpreting the test results due to significant limitations related to unexplained heterogeneity documented in several studies and assigned to various factors. For instance, the mouth normal flora urease enzyme activity may impair the UBT results; this can be avoided by advising each patient to wash his mouth before being tested[24]. The use of a nasogastric tube can be a solution. It is of note that both time of having the reading results following the meal intake and the cut-off value were not clarified in a lot of the recruited studies[11]. The nature of the radioactive isotopic meal and patients’ individual variations as age, gender, and anthropometric measures may also contribute to variability within and between different studies[26]. These factors may endorse the diversity persistence even after UBT type adjustment (13C vs14C) and method of measurement (infrared spectrometry vs radioisotope mass spectrometry). The recommendations for using UBT include precise considerations on adjusting dose of urea, timing of assessment, and measurement techniques for the 13C-UBT and 14C-UBT, so improving diagnostic accuracy.
Based on the availability of various diagnostic techniques, including invasive and non-invasive tests with different proportion of sensitivity and specificity for detection of H. pylori infection, the choice of one or more tests will depend on clinical conditions, clinical experience, cost-effectiveness, as well as sensitivity and specificity[27]. The diagnostic tests, as shown in Figure 1, are divided into two approaches: Invasive tests (upper endoscopy, histopathology, bacterial culture, and molecular techniques) and non-invasive tests (urea breath test, stool antigens, serological, and molecular tests) for diagnosing H. pylori infection[28]. UBT is considered as the gold standard non-invasive test for diagnosing H. pylori that shows high accuracy, sensitivity and specificity. It is of note that UBT has been used for nearly thirty years and remains the most accurate and most common non-invasive assay for diagnosing H. pylori infection[11]. The provided meta-analysis determines the UBT diagnostic accuracy for detecting H. pylori infection in adult dyspeptic patients and evaluates various variables related to the test accuracy, such as urea dose, assessment timing and selection of measurement technique for both 13C UBT & 14C UBT.
The provided results in the current meta-analysis highlighted the crucial importance of adjusting the appropriate dose of urea when performing the 13C-UBT for diagnosing H. pylori infection. The dose of twenty-five mg urea shows the highest sensitivity and specificity (98.85% & 99.13%) respectively[13]. As regards the timing of the assessment following urea administration, the results of the current meta-analysis revealed that the maximum sensitivity and specificity, both above 98%, are reached at 20-min following urea intake. The selection of assessment technique is also critical for accuracy of the test. Integrated Cavity Output Spectrometry (ICOS) is the most precise assessment test, with nearly equal sensitivity of and a specificity of (98.99% & 98.55%) respectively. However, it is of note that ICOS was assessed in one study only[29].
Results indicate that the dose of urea used in the 14C-UBT can also affect accuracy of the test. Particularly, five µCi dose of urea was evaluated in four studies and showed a sensitivity and a specificity of (99.21% & 93.43%) respectively. As regards the time for measurement after ingestion of the urea meal, conducting tests 15 min post urea intake constantly showed the highest sensitivity and specificity (98.39% & 98.71%) respectively. This shows that the 15-min time point is optimal for augmenting the test precision[30]. In terms of assessment techniques, the meta-analysis revealed differences in sensitivity and specificity. Liquid scintillation counting showed the highest sensitivity (98.79%) but at the expense of specificity (87.24%). On the other hand, Solid Scintillation UBT (scintillation counting) demonstrated higher specificity (97.46%) on expense of sensitivity (95.40%).
The trade-off between sensitivity and specificity must be put in mind in clinical setting, when selecting the assessment technique[31]. For instance, liquid scintillation counting is the recommended method when high sensitivity is essential to bypass missing true results, while solid scintillation counting could be a better selection, when high specificity is crucial to minimize false positives.
The meta-analysis showed that both 13C-UBT and 14C-UBT revealed high area under the curve values near 1.00, confirming their high precision and endorsing any of them as reliable diagnostic technique in clinical setting. However, the outperformance diagnostic accuracy of 13C-UBT over 14C-UBT is demonstrated by higher sensitivity, specificity, likelihood ratios, and area under the curve Table 2. 13C-UBT's Diagnostic odd ratio (DOR) significantly outperforms that of 14C-UBT (DOR), making it the preferred diagnostic tool for dyspeptic individuals with H. pylori infection[32].
| Test type | 14C-UBT | 13C-UBT | ||
| Optimal sensitivity | Optimal specificity | Optimal sensitivity | Optimal specificity | |
| Urea dose | 99.21% with 5 µCi | 93.43% with 5 µCi | 98.85% with 25 mg | 99.13% with 25 mg |
| Time of assessment after urea administration | 98.39% (15 min) | 98.71% (15 min) | 98.87% (20 min) | 98.14% (20 min) |
| Assessment technique | -98.79% (liquid scintillation counting); -95.40% (solid scintillation UBT) | -87.24% (liquid scintillation counting); -97.46% (solid scintillation UBT) | 98.99% (ICOS) | 98.55% (ICOS) |
| Overall accuracy, % | 96.15 | 89.84 | 96.60 | 96.93 |
| Safety | Not permitted | Used several times on the same patient and safe for children and pregnant females | ||
The authors conducted a thorough and comprehensive evaluation of the UBT, demonstrating a profound understanding of its diagnostic capabilities for H. pylori. The research reflects a rigorous methodology, highlighting the authors' commitment to scientific excellence in evaluating the accuracy and advantages of UBT in diagnosing H. pylori. The efforts of the authors are particularly appreciated for bridging the gap between research and clinical practice, providing valuable insights that can directly impact patient care and management. The study significantly contributes to the medical knowledge by advancing our understanding of UBT as a diagnostic tool, and potentially influencing future guidelines and protocols. The authors' acknowledgment of the limitations of their meta-analysis demonstrates intellectual honesty and contributes to the overall reliability of their research, guiding future investigations in this field. The collective efforts of the authors have a meaningful impact on advancing diagnostic approaches for H. pylori, highlighting their dedication to advancing medical science and improving healthcare outcomes.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country of origin: Egypt
Peer-review report’s classification
Scientific Quality: Grade C
Novelty: Grade B
Creativity or Innovation: Grade B
Scientific Significance: Grade B
P-Reviewer: Lv J, China S-Editor: Li L L-Editor: A P-Editor: Yu HG
| 1. | McColl KE. Clinical practice. Helicobacter pylori infection. N Engl J Med. 2010;362:1597-1604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 529] [Cited by in RCA: 550] [Article Influence: 36.7] [Reference Citation Analysis (0)] |
| 2. | Zamani M, Ebrahimtabar F, Zamani V, Miller WH, Alizadeh-Navaei R, Shokri-Shirvani J, Derakhshan MH. Systematic review with meta-analysis: the worldwide prevalence of Helicobacter pylori infection. Aliment Pharmacol Ther. 2018;47:868-876. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 565] [Cited by in RCA: 492] [Article Influence: 70.3] [Reference Citation Analysis (1)] |
| 3. | Chang WL, Yeh YC, Sheu BS. The impacts of H. pylori virulence factors on the development of gastroduodenal diseases. J Biomed Sci. 2018;25:68. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 127] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 4. | Asaka M, Dragosics BA. Helicobacter pylori and gastric malignancies. Helicobacter. 2004;9:35-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 5. | Torres J, Pérez-Pérez G, Goodman KJ, Atherton JC, Gold BD, Harris PR, la Garza AM, Guarner J, Muñoz O. A comprehensive review of the natural history of Helicobacter pylori infection in children. Arch Med Res. 2000;31:431-469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 166] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 6. | Lee JY, Kim N. Diagnosis of Helicobacter pylori by invasive test: histology. Ann Transl Med. 2015;3:10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 57] [Reference Citation Analysis (0)] |
| 7. | Stenström B, Mendis A, Marshall B. Helicobacter pylori--the latest in diagnosis and treatment. Aust Fam Physician. 2008;37:608-612. [PubMed] |
| 8. | Qureshi WA, Graham DY. Diagnosis and management of Helicobacter pylori infection. Clin Cornerstone. 1999;1:18-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 9. | Oztürk E, Yeşilova Z, Ilgan S, Arslan N, Erdil A, Celasun B, Ozgüven M, Dağalp K, Ovali O, Bayhan H. A new, practical, low-dose 14C-urea breath test for the diagnosis of Helicobacter pylori infection: clinical validation and comparison with the standard method. Eur J Nucl Med Mol Imaging. 2003;30:1457-1462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 10. | Morris A, Ali MR, Brown P, Lane M, Patton K. Campylobacter pylori infection in biopsy specimens of gastric antrum: laboratory diagnosis and estimation of sampling error. J Clin Pathol. 1989;42:727-732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 76] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 11. | Sabbagh P, Mohammadnia-Afrouzi M, Javanian M, Babazadeh A, Koppolu V, Vasigala VR, Nouri HR, Ebrahimpour S. Diagnostic methods for Helicobacter pylori infection: ideals, options, and limitations. Eur J Clin Microbiol Infect Dis. 2019;38:55-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 98] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 12. | Mentis A, Lehours P, Mégraud F. Epidemiology and Diagnosis of Helicobacter pylori infection. Helicobacter. 2015;20:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 120] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 13. | Gatta L, Ricci C, Tampieri A, Osborn J, Perna F, Bernabucci V, Vaira D. Accuracy of breath tests using low doses of 13C-urea to diagnose Helicobacter pylori infection: a randomised controlled trial. Gut. 2006;55:457-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 40] [Article Influence: 2.1] [Reference Citation Analysis (3)] |
| 14. | Suzuki T, Matsuo K, Sawaki A, Ito H, Hirose K, Wakai K, Sato S, Nakamura T, Yamao K, Ueda R, Tajima K. Systematic review and meta-analysis: importance of CagA status for successful eradication of Helicobacter pylori infection. Aliment Pharmacol Ther. 2006;24:273-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 45] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 15. | Sheu BS, Wu MS, Chiu CT, Lo JC, Wu DC, Liou JM, Wu CY, Cheng HC, Lee YC, Hsu PI, Chang CC, Chang WL, Lin JT. Consensus on the clinical management, screening-to-treat, and surveillance of Helicobacter pylori infection to improve gastric cancer control on a nationwide scale. Helicobacter. 2017;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 64] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 16. | Howden CW, Hunt RH. Guidelines for the management of Helicobacter pylori infection. Ad Hoc Committee on Practice Parameters of the American College of Gastroenterology. Am J Gastroenterol. 1998;93:2330-2338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 220] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 17. | Cutler AF, Havstad S, Ma CK, Blaser MJ, Perez-Perez GI, Schubert TT. Accuracy of invasive and noninvasive tests to diagnose Helicobacter pylori infection. Gastroenterology. 1995;109:136-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 330] [Cited by in RCA: 313] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 18. | Nocon M, Kuhlmann A, Leodolter A, Roll S, Vauth C, Willich SN, Greiner W. Efficacy and cost-effectiveness of the 13C-urea breath test as the primary diagnostic investigation for the detection of Helicobacter pylori infection compared to invasive and non-invasive diagnostic tests. GMS Health Technol Assess. 2009;5:Doc14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 19. | Gisbert JP, Pajares JM. Review article: 13C-urea breath test in the diagnosis of Helicobacter pylori infection -- a critical review. Aliment Pharmacol Ther. 2004;20:1001-1017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 257] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 20. | Leide-Svegborn S, Stenström K, Olofsson M, Mattsson S, Nilsson LE, Nosslin B, Pau K, Johansson L, Erlandsson B, Hellborg R, Skog G. Biokinetics and radiation doses for carbon-14 urea in adults and children undergoing the Helicobacter pylori breath test. Eur J Nucl Med. 1999;26:573-580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 28] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 21. | Raju GS, Smith MJ, Morton D, Bardhan KD. Mini-dose (1-microCi) 14C-urea breath test for the detection of Helicobacter pylori. Am J Gastroenterol. 1994;89:1027-1031. [PubMed] |
| 22. | Smith S, Boyle B, Brennan D, Buckley M, Crotty P, Doyle M, Farrell R, Hussey M, Kevans D, Malfertheiner P, Megraud F, Nugent S, O'Connor A, O'Morain C, Weston S, McNamara D. The Irish Helicobacter pylori Working Group consensus for the diagnosis and treatment of H. pylori infection in adult patients in Ireland. Eur J Gastroenterol Hepatol. 2017;29:552-559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 23. | Mahachai V, Vilaichone RK, Pittayanon R, Rojborwonwitaya J, Leelakusolvong S, Maneerattanaporn M, Chotivitayatarakorn P, Treeprasertsuk S, Kositchaiwat C, Pisespongsa P, Mairiang P, Rani A, Leow A, Mya SM, Lee YC, Vannarath S, Rasachak B, Chakravuth O, Aung MM, Ang TL, Sollano JD, Trong Quach D, Sansak I, Wiwattanachang O, Harnsomburana P, Syam AF, Yamaoka Y, Fock KM, Goh KL, Sugano K, Graham D. Helicobacter pylori management in ASEAN: The Bangkok consensus report. J Gastroenterol Hepatol. 2018;33:37-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 103] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 24. | Leal YA, Flores LL, Fuentes-Pananá EM, Cedillo-Rivera R, Torres J. 13C-urea breath test for the diagnosis of Helicobacter pylori infection in children: a systematic review and meta-analysis. Helicobacter. 2011;16:327-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 73] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 25. | Andersen LP, Kiilerick S, Pedersen G, Thoreson AC, Jørgensen F, Rath J, Larsen NE, Børup O, Krogfelt K, Scheibel J, Rune S. An analysis of seven different methods to diagnose Helicobacter pylori infections. Scand J Gastroenterol. 1998;33:24-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 42] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 26. | Ferwana M, Abdulmajeed I, Alhajiahmed A, Madani W, Firwana B, Hasan R, Altayar O, Limburg PJ, Murad MH, Knawy B. Accuracy of urea breath test in Helicobacter pylori infection: meta-analysis. World J Gastroenterol. 2015;21:1305-1314. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 124] [Cited by in RCA: 145] [Article Influence: 14.5] [Reference Citation Analysis (4)] |
| 27. | Kosunen TU, Seppälä K, Sarna S, Sipponen P. Diagnostic value of decreasing IgG, IgA, and IgM antibody titres after eradication of Helicobacter pylori. Lancet. 1992;339:893-895. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 261] [Cited by in RCA: 241] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 28. | Goossens H, Glupczynski Y, Burette A, Van den Borre C, DePrez C, Bodenmann J, Keller A, Butzler JP. Evaluation of a commercially available complement fixation test for diagnosis of Helicobacter pylori infection and for follow-up after antimicrobial therapy. J Clin Microbiol. 1992;30:3230-3233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 29. | Som S, Maity A, Banik GD, Ghosh C, Chaudhuri S, Daschakraborty SB, Ghosh S, Pradhan M. Excretion kinetics of 13C-urea breath test: influences of endogenous CO2 production and dose recovery on the diagnostic accuracy of Helicobacter pylori infection. Anal Bioanal Chem. 2014;406:5405-5412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 30. | Novis BH, Gabay G, Leichtmann G, Peri M, Bernheim J, Pomeranz IS. Two point analysis 15-minute 14C-urea breath test for diagnosing Helicobacter pylori infection. Digestion. 1991;50:16-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 31. | Wang X, Zhang S, Chua EG, He Y, Li X, Liu A, Chen H, Wise MJ, Marshall BJ, Sun D, Tay CY. A re-testing range is recommended for (13)C- and (14)C-urea breath tests for Helicobacter pylori infection in China. Gut Pathog. 2021;13:38. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 32. | Han YH, Zhang W, Wang YT, Xiong ZJ, Du Q, Xie Y, Lu H. Performance evaluation of a novel 14C-urea breath test (solid scintillation) for the diagnosis of helicobacter pylori infection. Medicine (Baltimore). 2023;102:e33107. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |