Published online Apr 28, 2024. doi: 10.3748/wjg.v30.i16.2258
Peer-review started: January 25, 2024
First decision: February 1, 2024
Revised: February 21, 2024
Accepted: March 28, 2024
Article in press: March 28, 2024
Published online: April 28, 2024
Processing time: 91 Days and 11.7 Hours
Irritable bowel syndrome (IBS) is one of the most frequent and debilitating conditions leading to gastroenterological referrals. However, recommended treatments remain limited, yielding only limited therapeutic gains. Chitin-glucan (CG) is a novel dietary prebiotic classically used in humans at a dosage of 1.5-3.0 g/d and is considered a safe food ingredient by the European Food Safety Authority. To provide an alternative approach to managing patients with IBS, we performed preclinical molecular, cellular, and animal studies to evaluate the role of chitin-glucan in the main pathophysiological mechanisms involved in IBS.
To evaluate the roles of CG in visceral analgesia, intestinal inflammation, barrier function, and to develop computational molecular models.
Visceral pain was recorded through colorectal distension (CRD) in a model of long-lasting colon hypersensitivity induced by an intra-rectal administration of TNBS [15 milligrams (mg)/kilogram (kg)] in 33 Sprague-Dawley rats. Intracolonic pressure was regularly assessed during the 9 wk-experiment (weeks 0, 3, 5, and 7) in animals receiving CG (n = 14) at a human equivalent dose (HED) of 1.5 g/d or 3.0 g/d and compared to negative control (tap water, n = 11) and positive control (phloroglucinol at 1.5 g/d HED, n = 8) groups. The anti-inflammatory effect of CG was evaluated using clinical and histological scores in 30 C57bl6 male mice with colitis induced by dextran sodium sulfate (DSS) administered in their drinking water during 14 d. HT-29 cells under basal conditions and after stimulation with lipopolysaccharide (LPS) were treated with CG to evaluate changes in pathways related to analgesia (µ-opioid receptor (MOR), cannabinoid receptor 2 (CB2), peroxisome proliferator-activated receptor alpha, inflammation [interleukin (IL)-10, IL-1b, and IL-8] and barrier function [mucin 2-5AC, claudin-2, zonula occludens (ZO)-1, ZO-2] using the real-time PCR method. Molecular modelling of CG, LPS, lipoteichoic acid (LTA), and phospholipomannan (PLM) was developed, and the ability of CG to chelate microbial pathogenic lipids was evaluated by docking and molecular dynamics simulations. Data were expressed as the mean ± SEM.
Daily CG orally-administered to rats or mice was well tolerated without including diarrhea, visceral hypersensitivity, or inflammation, as evaluated at histological and molecular levels. In a model of CRD, CG at a dosage of 3 g/d HED significantly decreased visceral pain perception by 14% after 2 wk of administration (P < 0.01) and reduced inflammation intensity by 50%, resulting in complete regeneration of the colonic mucosa in mice with DSS-induced colitis. To better reproduce the characteristics of visceral pain in patients with IBS, we then measured the therapeutic impact of CG in rats with TNBS-induced inflammation to long-lasting visceral hypersensitivity. CG at a dosage of 1.5 g/d HED decreased visceral pain perception by 20% five weeks after colitis induction (P < 0.01). When the CG dosage was increased to 3.0 g/d HED, this analgesic effect surpassed that of the spasmolytic agent phloroglucinol, manifesting more rapidly within 3 wk and leading to a 50% inhibition of pain perception (P < 0.0001). The underlying molecular mechanisms contributing to these analgesic and anti-inflammatory effects of CG involved, at least in part, a significant induction of MOR, CB2 receptor, and IL-10, as well as a significant decrease in pro-inflammatory cytokines IL-1b and IL-8. CG also significantly upregulated barrier-related genes including muc5AC, claudin-2, and ZO-2. Molecular modelling of CG revealed a new property of the molecule as a chelator of microbial pathogenic lipids, sequestering gram-negative LPS and gram-positive LTA bacterial toxins, as well as PLM in fungi at the lowesr energy conformations.
CG decreased visceral perception and intestinal inflammation through master gene regulation and direct binding of microbial products, suggesting that CG may constitute a new therapeutic strategy for patients with IBS or IBS-like symptoms.
Core Tip: Currently available irritable bowel syndrome (IBS) treatments are often inadequate. Chitin-glucan is a novel, well-tolerated, non-digestible prebiotic considered a safe food ingredient by the European Food Safety Authority. This study suggests new capacities of chitin-glucan to target most pathophysiological mechanisms of IBS and its therapeutic potential as a promising new generation of prebiotics for patients with IBS or IBS-like symptoms.
- Citation: Valibouze C, Dubuquoy C, Chavatte P, Genin M, Maquet V, Modica S, Desreumaux P, Rousseaux C. Chitin-glucan improves important pathophysiological features of irritable bowel syndrome. World J Gastroenterol 2024; 30(16): 2258-2271
- URL: https://www.wjgnet.com/1007-9327/full/v30/i16/2258.htm
- DOI: https://dx.doi.org/10.3748/wjg.v30.i16.2258
Irritable bowel syndrome (IBS) is a common functional gastrointestinal disorder with a worldwide prevalence of 5%-10%. It is associated with annual direct and indirect costs of more than 20 billion USD/year in the United States and is one of the leading causes of work absenteeism[1-3]. Although IBS represents a major burden for patients, the therapeutic strategies recommended by several gastroenterology societies (European, American, Canadian, Japanese, and British societies)[4-10] are often inadequate, leading to dissatisfaction among several patients with standard medical care[11,12].
Chitin-glucan is a novel, non-digestible dietary compound considered a safe food ingredient by the European Food Safety Authority (EFSA)[13]. It is the major component of the cell walls of the mycelium of Aspergillus niger (A. niger) fungi and is mainly composed of a branched β-1,3/1,6 glucan that is bound to chitin via a β-1, 4 linkage. Previous preclinical studies in rodent models[14,15], functional in vitro evaluation using the Simulator of the Human Intestinal Microbial Ecosystem model[16], and clinical exploration in healthy volunteers[17] have highlighted that oral administration of chitin-glucan at the EFSA-recommended dosage[13] induces a microbial signature of a prebiotic. Briefly, chitin-glucan is slowly fermented in all colon segments, leading to significant changes in gut microbiota composition, with a particular increase in the butyrate-producing genus Roseburia spp. and the Faecalibacterium genus, known as a fiber fermenter with strong intestinal anti-inflammatory properties[18].
To propose chitin-glucan treatment as an alternative approach for managing patients with IBS, we performed preclinical molecular, cellular, and animal studies to evaluate the role of chitin-glucan in the main physiopathological mechanisms responsible for IBS symptoms, including visceral analgesia, intestinal inflammation, and barrier function, together with the development of a computational molecular model.
CG from the cell wall of A. niger was obtained from Kitozyme SA (Herstal, Belgium). Rodents received a dose of 25 mg/kg body weight (BW)/d or 50 mg/kg BW/d of chitin-glucan [corresponding to a human equivalent dose (HED) of respectively 1.5 g/d and 3.0 g/d for a 70 kg man] by oral gavage once per day[14]. For in vitro studies, HT-29 cells were incubated with chitin-glucan at 500 micro (µ) g/mL or 1000 µg/mL corresponding to estimated luminal ileal concentrations of chitin-glucan calculated in healthy volunteers receiving the compound respectively at 1.5 g/d or 3.0 g/d.
TNBS-induced long-lasting visceral hypersensitivity is a reference model for screening novel treatments for visceral pain originating in the gastrointestinal tract[19].
Rats: Animal experiments were performed in accredited facilities at the Institut Pasteur, Lille, according to governmental guidelines. All studies were approved by the local investigational Ethics Review Board (Nord-Pas-de-Calais CEEA N°75, Lille, France; protocol reference numbers 352012 and 19-2009R), and the French government agreement n° APAFIS#16100-2018070309443695 v4 (colorectal distension, CRD) and APAFIS#9148-201901101416384 v1 (colitis). Three animals were housed per cage and had free access to standard rodent chow (Safe A04 P2,5) and tap water.
Male Sprague-Dawley rats, aged 5 wk and weighing 175 g to 200 g, were obtained from Janvier labs (France). Rats were randomized into different groups using a manual procedure and acclimated to the study conditions for at least 7 d before the beginning of the pre-treatment period. Upon completion of treatment, the animals were euthanized by cervical dislocation after gaseous anesthesia (isoflurane).
TNBS-induced visceral hypersensitivity: Rats were anesthetized for 2 h using a subcutaneous injection of xylazine at 12.5 mg/kg (Bayer, Rompun 2%) and ketamin at 25.0 mg/kg, (Virbac, Ketamin 1000; 100 mg/mL). Colitis was induced by intrarectal injection of TNBS (15 mg/kg) 8 cm from the anus. Using this dose of TNBS, rats develop transitory colitis followed by a wound healing period of 4-8 wk, during which macroscopic and histological inflammation disappears but hypersensitivity to CRD persists[20].
Evaluation of visceral pain by CRD: Nociception in the animals was assessed by measuring the intracolonic pressure required to induce a behavioral response during CRD caused by the inflation of the balloon introduced in the colon. This response is characterized by an elevation of the hind part of the animal’s body and clearly visible abdominal contractions corresponding to severe contractions[21-23]. Briefly, rats were anesthetized with volatile anesthesia (2% isoflurane), a balloon[21-23] was inserted intrarectally in a minimally invasive manner 7 cm from the anus, and the catheter was taped to the base of the tail. After 5 min, rats were placed in the middle of a 40 cm × 40 cm Plexiglas box, and the catheter was connected to an electronic barostat apparatus (Distender Series IIR™, G&J Electronics). Increasing pressure was continuously applied until pain behavior was displayed or a cut-off pressure of 80 mmHg was reached.
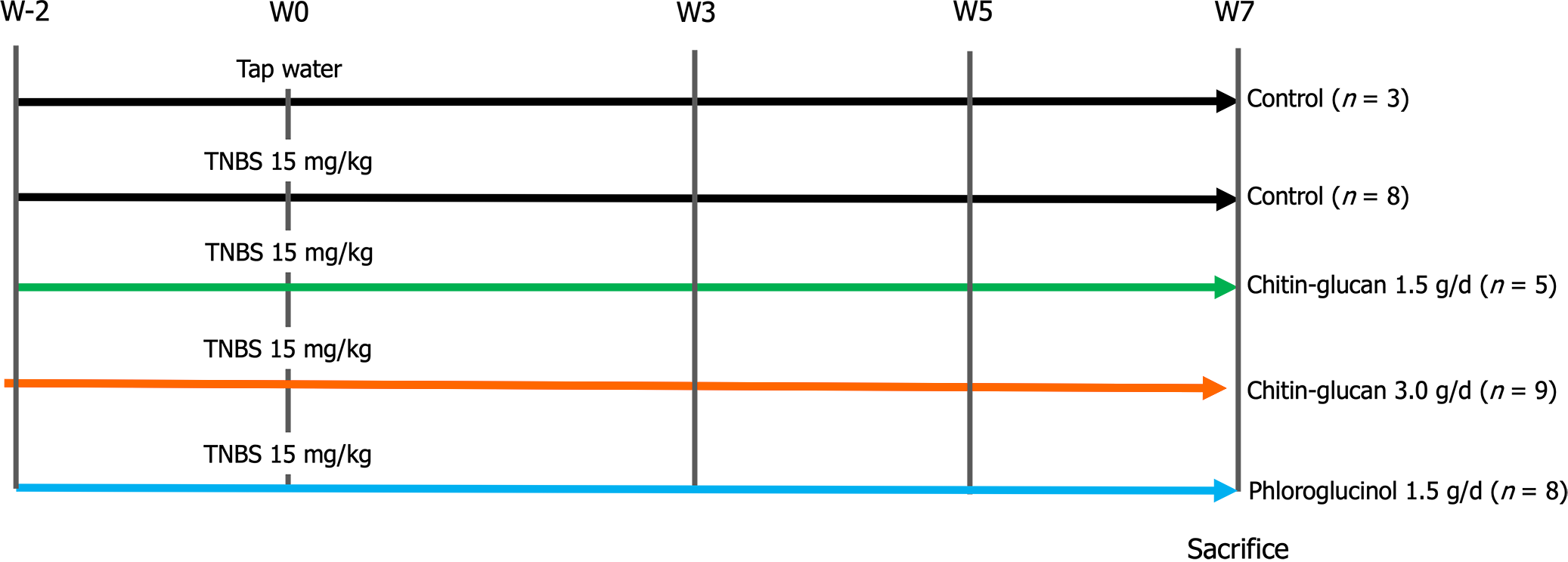
Experimental design: Animals were weighed and randomly distributed into five groups: One control group (n = 3); four groups with colitis, including a negative control group receiving tap water (n = 8); two groups of rats treated daily by oral gavage of chitin-glucan at 1.5 g/d (n = 5) or 3.0 g/d (n = 9); and a positive control group consisting of eight rats treated under the same conditions with phloroglucinol at 1.5 g/d (Figure 1). Visceral sensitivity was assessed at regular intervals throughout the 9-week experiment, with the first evaluation at week-2 corresponding to the basal condition, week 0 just before colitis induction, and weeks 3, 5, and 7 after colitis induction (Figure 1).
Mice: Nine-week-old C57BL/6 mice were obtained from Janvier labs (France). Animal experiments were conducted in accredited facilities at the Institut Pasteur, Lille, following governmental guidelines. All studies were approved by the local investigational Ethics Review Board (Nord-Pas-de-Calais CEEA N°75, Lille, France; protocol reference numbers 352012 and 19-2009R) and received French government agreement n° APAFIS#7542-20 17030609233680). Five animals were housed per cage with free access to standard rodent chow and tap water.
Induction of colitis by dextran sodium sulfate: C57BL/6 mice received 1.5% dextran sodium sulfate (DSS, 45kD; TDB Consultancy AB, Uppsala, Sweden, Ref DB001-42) in their drinking water for 14 d (from day 0 to day 14), followed by 3 d of regular water after the last administration of DSS.
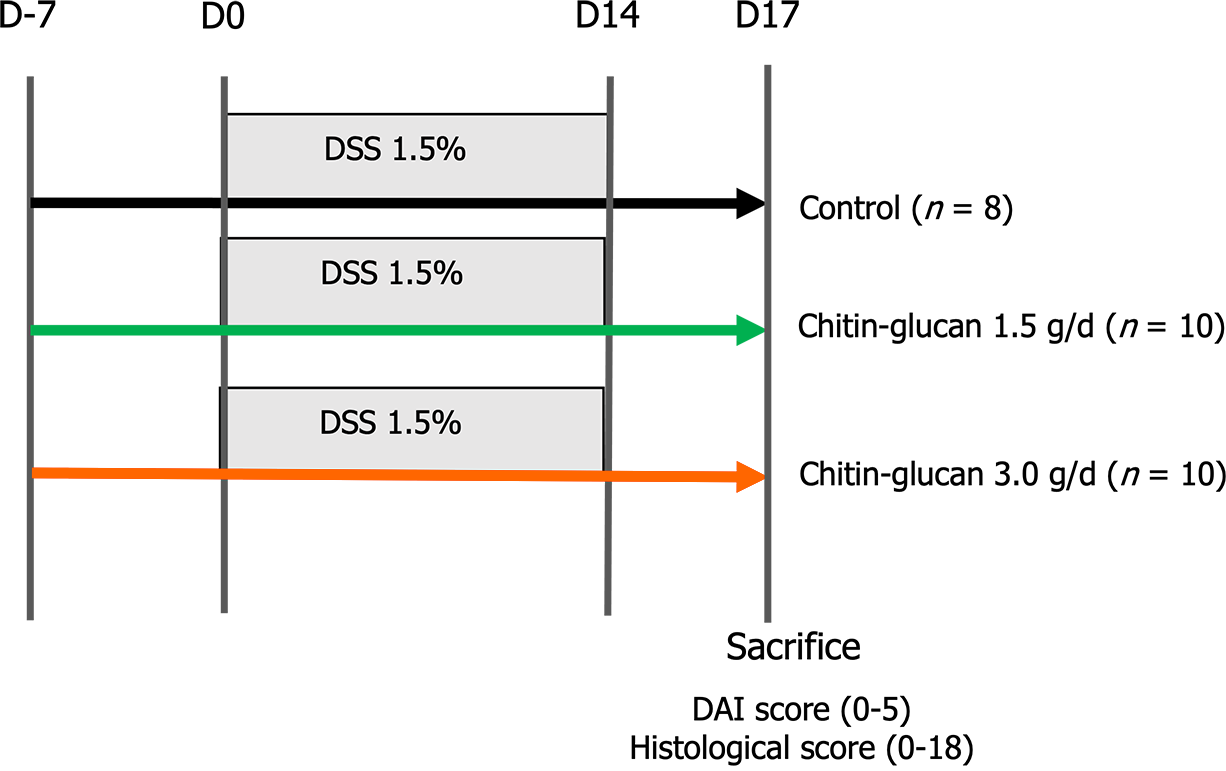
Experimental design: Mice were weighed and randomly distributed into 3 groups with colitis including a negative control receiving tap water (n = 8), and 2 groups of rats treated by daily oral gavage of chitin-glucan at 1.5 g/d (n = 10) or 3.0 g/d (n = 10). Preventive treatment with chitin-glucan was started 7 d before colitis induction on day 0 and continued until sacrifice at day 17[24] (Figure 2).
Clinical evaluation: The Disease Activity Index (DAI, 0-5) is a simple scoring system ranging from 0 to 5 used to determine the severity of colitis in mice[24]. It is calculated based on the evaluation of body weight loss compared to baseline at day 0 (0: No weight loss; 1: ≤ 10%; 2: > 10%), stool consistency (0: Well-formed pellets; 1: Soft pellets; 3: Liquid stools), and the presence of blood or occult blood in the feces (0-1) (Table 1). DAI was assessed by an investigator blinded to the protocol on day -7, day 0, and day 14. The presence of occult blood was recorded using the hemoccult method.
| Parameters | Scores |
| Weight loss | 0: No; 1: < 10%; 2: ≥ 10% |
| Stool consistency | 0: Regular; 1: Soft; 2: Diarrhea |
| Blood occurrence | 0: Absence; 1: Presence |
Histologic score (0-18): Transparietal colon samples were embedded in paraffin and stained with May-Grunwald-Giemsa stain. Multiparametric histologic scoring (0-10), as described by Dieleman et al[25], was performed blindly by two investigators. This score grades the severity and extent of inflammation, the intensity of cellular infiltrate in the mucosa, its extension in sub-mucosa layers, and the presence of epithelial lesions (Table 2).
| Parameters | Score | Description |
| Severity of inflammation | 0 | None |
| 1 | Slight | |
| 2 | Moderate | |
| 3 | Severe | |
| Extent | 0 | None |
| 1 | Mucosa | |
| 2 | Mucosa and submucosa | |
| 3 | Transmural | |
| Regeneration | 4 | No tissue repair |
| 3 | Surface epithelium not intact | |
| 2 | Regeneration with crypt depletion | |
| 1 | Almost complete regeneration | |
| 0 | Complete regeneration or normal tissue | |
| Crypt damage | 0 | None |
| 1 | Basal 1/3 damaged | |
| 2 | Basal 2/3 damaged | |
| 3 | Only surface epithelium intact | |
| 4 | Entire crypt and epithelium lost | |
| Involvement | 1 | 1%-25% |
| 2 | 26%-50% | |
| 3 | 51%-75% | |
| 4 | 76%-100% |
HT-29 cells (ATCC® HTB-38™, Molsheim, France), a human colon carcinoma-derived epithelial cell line, were cultured separately in Dulbecco’s Modified Eagle’s Medium (DMEM) with high glucose (4.5 g/L) containing 10% fetal calf serum (DMEM 10% FCS, PAA Laboratories, Les Mureaux, France), and antibiotics (streptomycin 100 μg/mL and penicillin 100 units/mL, Sigma Aldrich, St. Quentin Fallavier, France).
Experimental design: The biological effects of chitin-glucan were evaluated under basal conditions after incubating the cells with chitin-glucan for 3 h at 500 µg/mL or 1000 µg/mL. To mimic inflammatory conditions, HT-29 cells were incubated with lipopolysaccharide (LPS) at 10 ng/mL for 24 h, as previously described[26]. After washing, cells were incubated with culture medium containing chitin-glucan (500 µg/mL or 1000 µg/mL) and (LPS 10 nanog/mL) for an additional 3 h. All experiments were performed in triplicate and repeated thrice for reproducibility. Cell viability was determined using the trypan blue exclusion assay.
mRNA quantification in cells: Cells were homogenized, and the mRNA expression of the main analgesic-related genes [µ-opioid receptor (MOR), cannabinoid receptor 2 (CB2), peroxisome proliferator-activated receptor alpha (PPARa), inflammation-related genes interleukin (IL)-1b, IL-8, and IL-10], and intestinal barrier-related genes [MUC2, MUC5AC, zonula occludens (ZO)-1, ZO-2, and claudin-2] were assessed by quantitative RT-PCR. Briefly, total RNA was extracted using the Nucleospin RNA Kit (Macherey-Nagel, Hoerdt, France). After RNAse inactivation, total RNA was cleaned of traces of genomic DNA via DNase treatment and eluted in RNAse- and DEPC-free water. The RNA purity was evaluated by UV spectroscopy using a Nanodrop system at wavelengths ranging from 220 nm to 350 nm. One microgram of total RNA was used to perform quantitative RT-PCR using LightCycler FastStart DNA Master SYBR Green I (Roche diagnostics, Indianapolis, IN, United States), according to the manufacturer’s protocol. The sequences and relative NCBI reference sequences of the primer sets are listed in Table 3. For each reaction, a critical threshold cycle (Ct) value indicating the cycle number at which DNA amplification was determined. The relative gene expression value was calculated as E = 2-ΔCt, where ΔCt is the difference in crossing points between GAPDH and each gene.
| Human genes | Primer sequences (5’-3’) |
| GAPDH | F: 5’-GAC ACC CAC TCC TCC ACC TTT-3’ |
| R: 5’-TTG CTG TAG CCA AAT TCG TTG T- 3 | |
| IL-1b | F: 5’-GAT GCA CCT GTA CGA TCA CT-3’ |
| R: 5’-GAT GCA CCT GTA CGA TCA CT-3’ | |
| IL-8 | F: 5’-AAA TCA GGA AGG CTG CCA AGA-3’ |
| R: 5’-AAG GAA CCA TCT CAC TGT GTG TAA AC-3’ | |
| IL-10 | F: 5’-ACTTTAAGGGTTACCTGGGTTGC-3’ |
| R: 5’-TCACATGCGCCTTGATGTCTG-3’ | |
| PPARa | F: 5’-CCA GTA TTT AGG AAG CTG TCC-3’ |
| R: 5’-AAG TTC TTC AAG TAG CCC TCG-3’ | |
| MOR | F: 5’-GAT CAT GGC CCT CTA CTC CA-3’ |
| R: 5’-TGG TGG CAG TCT TCA TCT TG-3’ | |
| CB2 | F: 5’-GCT AAG TGC CCT GGA GAA CGT-3’ |
| R: 5’- TCA GCC CCA GCC AAG CT-3’ | |
| ZO-1 | F: 5’-AGA GCA ATG GAG GAA ACA GC-3’ |
| R: 5’-CCC CAC TCT GAA AAT GAG GA-3’ | |
| ZO-2 | F: 5’-GGA GCT GTC AGG TTG GCT C-3’ |
| R: 5’-GTC TCT GCC TCC GGA CAC T-3’ | |
| Claudin2 | F: 5’-AAG GCT CTG CAA AGA ACT GC-3’ |
| R: 5’-CTG CCA GGC TGA CTT CTC TC-3’ | |
| MUC2 | F: 5’-CTT CGA CGG ACT CTA CTA CAG C-3’ |
| R: 5’-CTT TGG TGT TGT TGC CAA AC-3’ | |
| MUC5AC | F: 5’-AGAGTGGGAGCTGGGAGAGAG-3’ |
| R: 5’-AGCTCA-GAGGACATATGGGAGGT-3’ |
Molecular modelling of chitin-glucan: Molecular modelling studies were performed using SYBYL software version 7.2 (SYBYL; 7.2 ed.; Tripos Associates Inc., 1699 South Hanley Road, St. Louis, MO 63144, United States). A three-dimensional model of the compounds was built from a standard fragment library, and their geometries were subsequently optimized using the Tripos force field[27], including the electrostatic term calculated from the Gasteiger and Hückel atomic charges. The Powell method available in the Maximin 2 procedure was used for energy minimization until the gradient value was < 0.001 kcalorie/mol.Å.
A chitin-glucan model composed of a chitin moiety containing six units of N-acetyl-D-glucosamine (33%) covalently linked to a ß-D-glucan moiety containing 14 units of D-glucose (67%) was built. Its ability to chelate LPS, lipoteichoic acid (LTA), and phospholipomannan (PLM) was evaluated using classical molecular dynamics (MD) simulations performed at a constant temperature (300 K) in vacuo for a total period of 2100 ps. The target temperature was achieved by slowly heating the system to 50 K for 4 ps. The atomic velocities were initialized to a Maxwell distribution consistent with the selected interval temperatures. The Verlet algorithm was used with time steps of 0.001 ps, and bond lengths were constrained to their equilibrium values using the SHAKE algorithm. The results (coordinates, energies, and velocities) were collected every 0.025 ps during the simulations. The last 2000 ps (corresponding to 80000 conformers) were analyzed. The most stable complexes were energy-minimized. Molecular surfaces were calculated using the MOLCAD module implemented in the SYBYL software.
All comparisons were performed using a Permutation Test for two independent samples. Statistical analyses were carried out using StatXact software (Cytel Inc., Cambridge, MA, United States). Differences were considered statistically significant if the P value was < 0.05.
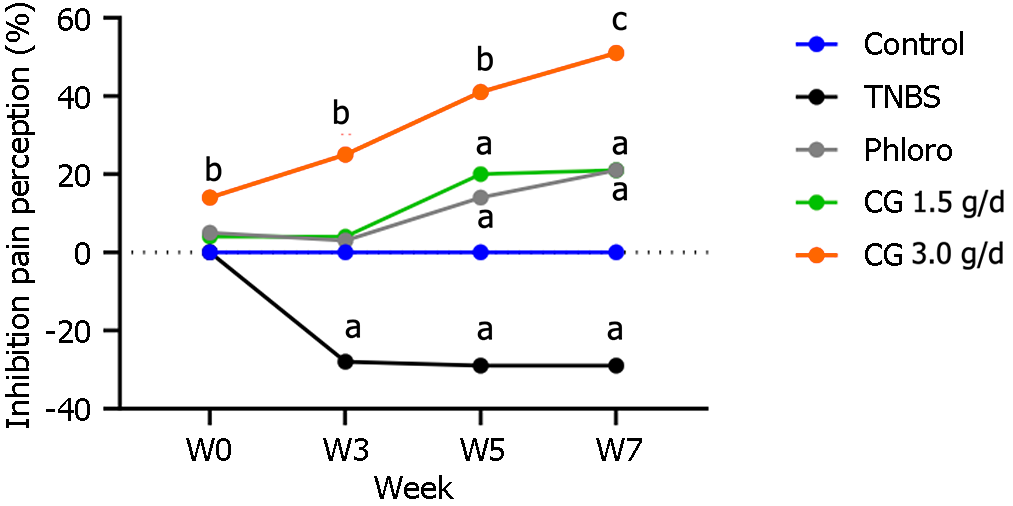
In control rats receiving tap water, a mean pressure of 46.0 mmHg ± 0.9 mmHg was required to induce pain. The two-week treatment with chitin-glucan at 3 g/d decreased normal visceral perception with a significant 14% increase in the pain threshold compared to untreated rats (52.5 mmHg ± 1.1 mmHg vs 46.0 mmHg ± 0.9 mmHg, P < 0.01). No significant modification of pain thresholds was observed in animals treated under the same conditions with chitin-glucan at 1.5 g/d (47.5 mmHg ± 0.8 mmHg vs 46.0 mmHg ± 0.9 mmHg, NS) or phloroglucinol (49.4 mmHg ± 1.2 mmHg vs 46.0 mmHg ± 0.9 mmHg, NS) compared to controls rats (Figure 3).
Compared to control rats, animals exposed to TNBS developed a significant and long-lasting hypersensitivity observed 3 wk after TNBS administration (36.2 mmHg ± 1.1 mmHg vs 50.0 mmHg ± 0.0 mmHg, -28%) (P < 0.01) and maintained at week 5 (34.4 mmHg ± 1.1 mmHg vs 48.3 mmHg ± 1.7 mmHg, -29%) (P < 0.01) and week 7 (33.1 mmHg ± 0.9 mmHg vs 46.7 mmHg ± 1.7 mmHg, -29%) (P < 0.01). A dose- and time-dependent analgesic effect of chitin-glucan was observed compared to control animals beginning from week 5 at 1.5 g/d (41.2 mmHg ± 1.2 mmHg vs 34.4 mmHg ± 1.1 mmHg, +20%) (P < 0.01) and from week 3 at 3.0 g/d (45.5 mmHg ± 1.4 mmHg vs 36.20 mmHg ± 1.12 mmHg, +25%) (P < 0.001). At the end of the experiment, the decrease in pain perception reached 51% in rats receiving chitin-glucan at 3.0 g/d (50.0 mmHg ± 1.6 mmHg vs 33.1 mmHg ± 0.9 mmHg, P < 0.0001). The kinetics and amplitude of the analgesic effects observed in rats receiving phloroglucinol were similar to those observed in animals treated with chitin-glucan at 1.5 g/d (Figure 3).
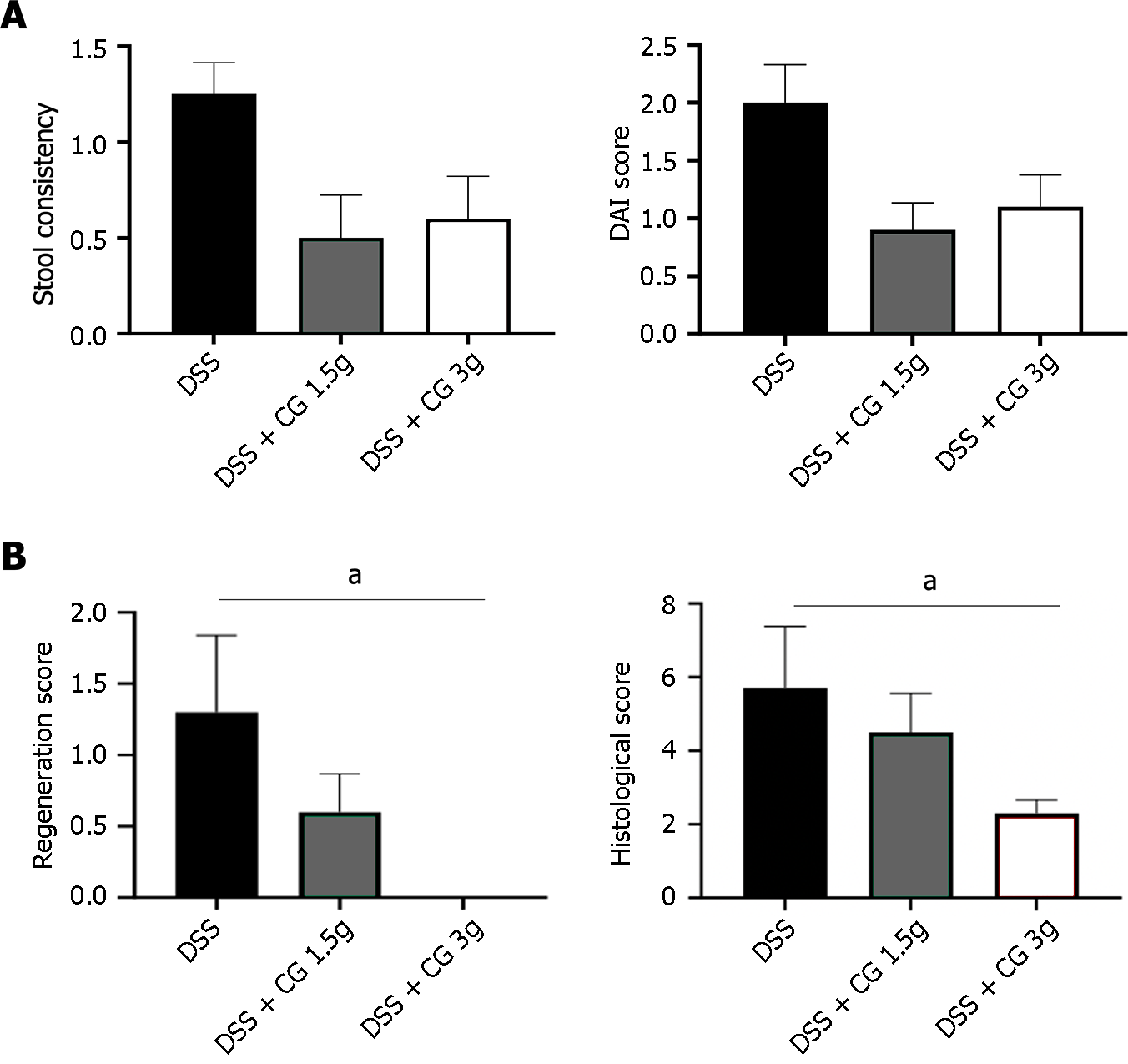
Disease activity index: Untreated mice with colitis had a DAI score of 2.0 ± 0.3, characterized by soft stools and episodic blood or occult blood in their stools. No significant weight loss was observed. Compared to untreated animals, chitin-glucan at 1.5 g/d or 3.0 g/d significantly improved the DAI score, leading to 50% decrease, with a restoration of stool consistency in most animals (Figures 4A).
Histological scores: Histologic lesions (5.7 ± 1.7) in untreated animals receiving DSS were characterized by slight to moderate inflammation, mainly located in the mucosa and submucosa, with damage to basal crypts and only partial tissue repair with crypt deletion. A dose-dependent decrease in histologic scores was observed in animals treated with chitin-glucan at 1.5 g/d (4.5 ± 1.0, NS) and 3.0 g/d (2.3 ± 0.4, P = 0.04) leading to a 50% decrease in inflammation severity scores (0.6 ± 0.1 vs 1.2 ± 0.4; P = 0.10), and limited inflammation to the epithelium (0.7 ± 0.2 vs 1.2 ± 0.3; P = 0.10) with complete regeneration of the mucosa without crypt damage (0.0 ± 0.0 vs 1.3 ± 0.5; P = 0.02) (Figure 4B).
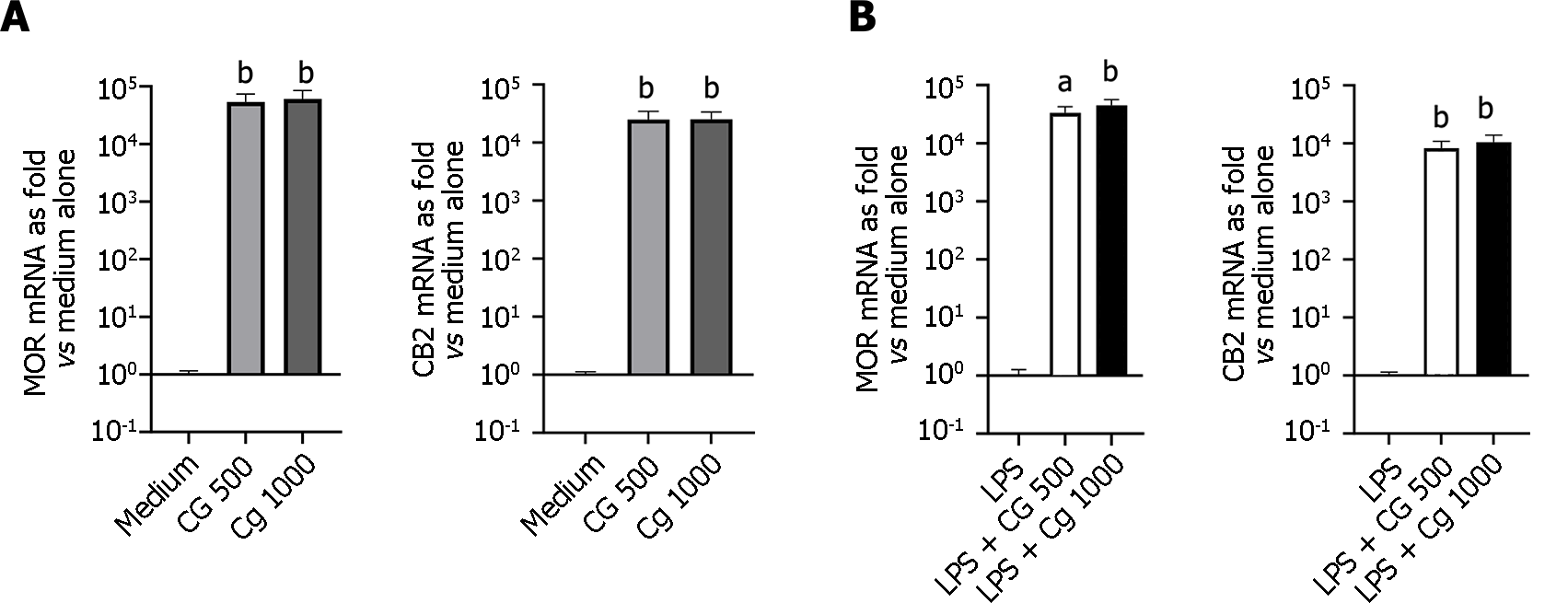
Modulation of analgesic-related receptors (MOR, CB2, and PPARa): Compared to untreated cells, a significant and similar increase of MOR mRNA levels was observed in HT-29 cells incubated with chitin-glucan at 500 µg/mL (5.39 × 104 ± 1.99 × 104vs 1.00 ± 0.16, P = 0.017) or 1000 µg/mL (6.07 × 104 ± 2.43 × 104vs 1.00 ± 0.16, P = 0.002) (Figure 5A). This increased expression of MOR mRNA was also observed at a similar level when LPS-stimulated HT-29 cells were incubated with chitin-glucan at 500 µg/mL (3.35 × 104 ± 8.95 × 103vs 1.00 ± 0.27, P = 0.017) or 1000 µg/mL (4.50 × 104 ± 1.13 × 104vs 1.00 ± 0.27, P = 0.004) (Figure 5B). Irrespective of the inflammatory status of HT-29 cells, a similar induction of CB2 mRNA expression was found in HT-29 cells after incubation with chitin-glucan at 500 µg/mL (2.51 × 104 ± 9.54 × 103vs 1.00 ± 0.12, P = 0.002) or 1000 µg/mL (2.52 × 104 ± 8.43 × 103vs 1.00 ± 0.12, P = 0.002) (Figures 5A and B). No relevant modification of PPARa mRNA levels was observed in quiescent or LPS-stimulated HT-29 cells after incubation with chitin-glucan, irrespective of the concentration used (500 µg/mL or 1000 µg/mL).
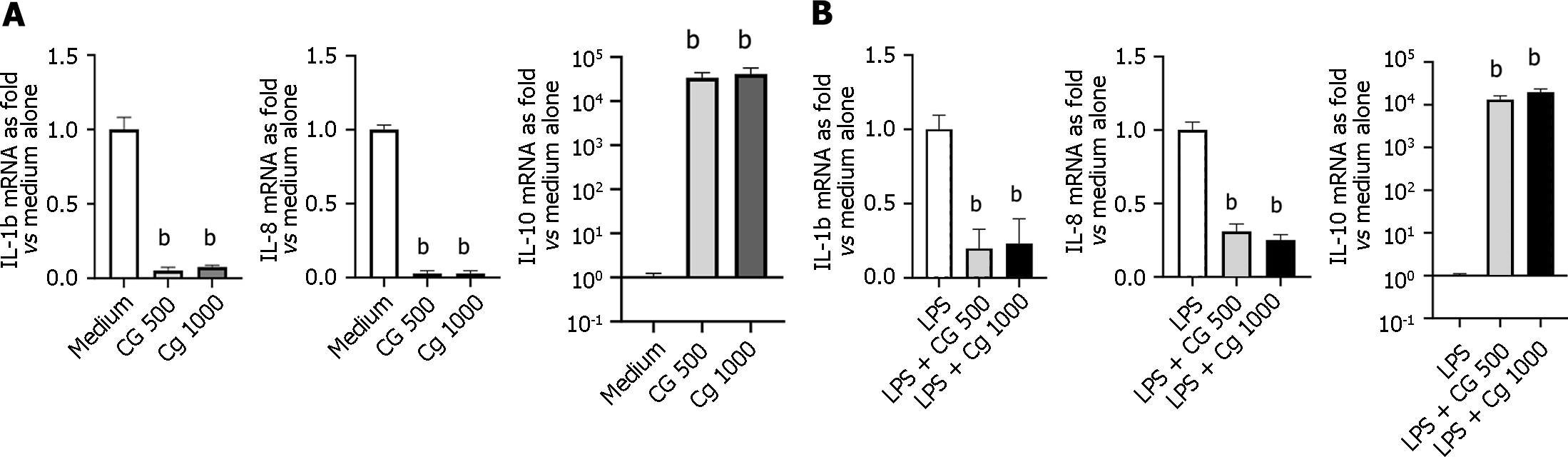
Modulation of inflammatory-related cytokines (IL-1b, IL-8, and IL-10): Treatment of HT-29 cells with CG at 500 µg/mL or 1000 µg/mL significantly and similarly decreased the basal production of inflammatory cytokines IL-1b (95% and 92%, respectively) and IL-8 mRNA (97% for both concentrations), along with a 4-log increase in the expression of IL-10 mRNA (3.4 × 104 ± 1.1 × 104; P = 0.002 and 4.1 × 104 ± 1.6 × 104; P = 0.002, respectively) (Figure 6A). Moreover, incubation of LPS-stimulated HT-29 cells with chitin-glucan at 500 µg/mL or 1000 µg/mL prevented the increased production of IL-1b and IL-8 mRNA while maintaining a strong induction of IL-10 mRNA expression (Figure 6B).
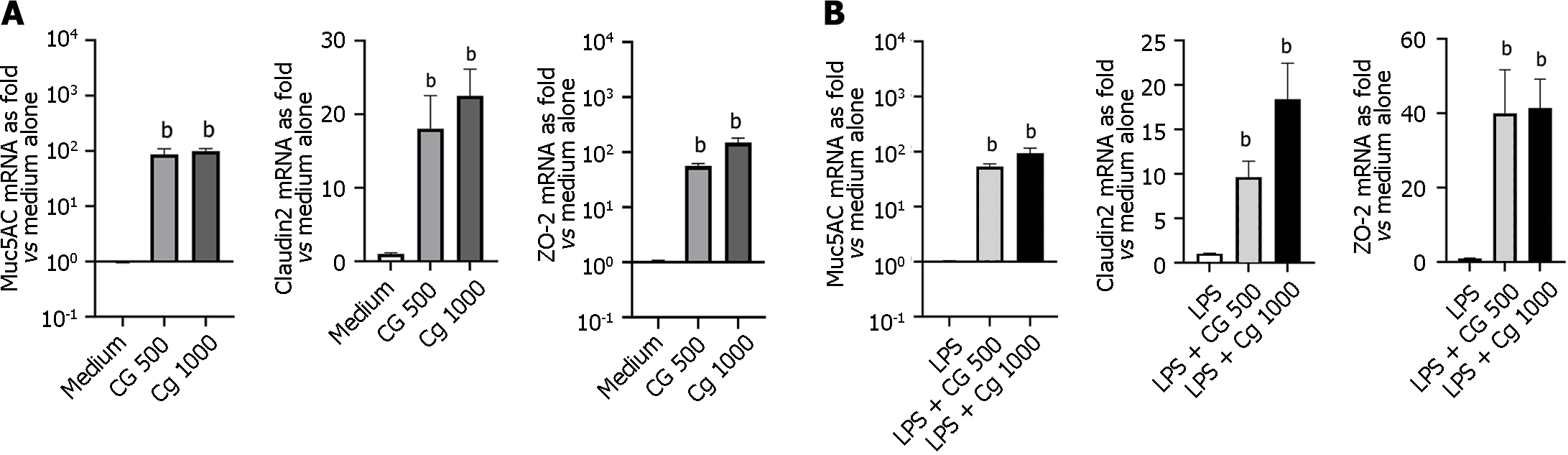
Modulation of intestinal barrier-related molecules (MUC2/5AC, ZO-1/2, and claudin-2): Compared to untreated HT-29 cells, those treated with chitin-glucan used at 500 or 1000 µg/mL induced the expression of MUC5AC (86.50 ± 22.80 vs 1.00 ± 0.10, P = 0.002 and 98.90 ± 10.70 vs 1.00 ± 0.05, P = 0.002, respectively), ZO-2 (56.60 ± 5.90 vs 1.00 ± 0.10, P = 0.002 and 150.60 ± 29.30 vs 1.00 ± 0.10, P = 0.005, respectively) and claudin-2 mRNA (18.00 ± 4.50 vs 1.00 ± 0.01, P = 0.008 and 22.50 ± 3.60 vs 1.00 ± 0.01, P = 0.008, respectively) (Figure 7A). Similar induction was observed with chitin-glucan in LPS-stimulated HT-29 cells (Figure 7B). No significant modification of MUC2 and ZO-1 mRNA levels was observed after treatment with CG (500 µg/mL or 1000 µg/mL), independently of the inflammatory status of HT-29 cells (Figure 7).
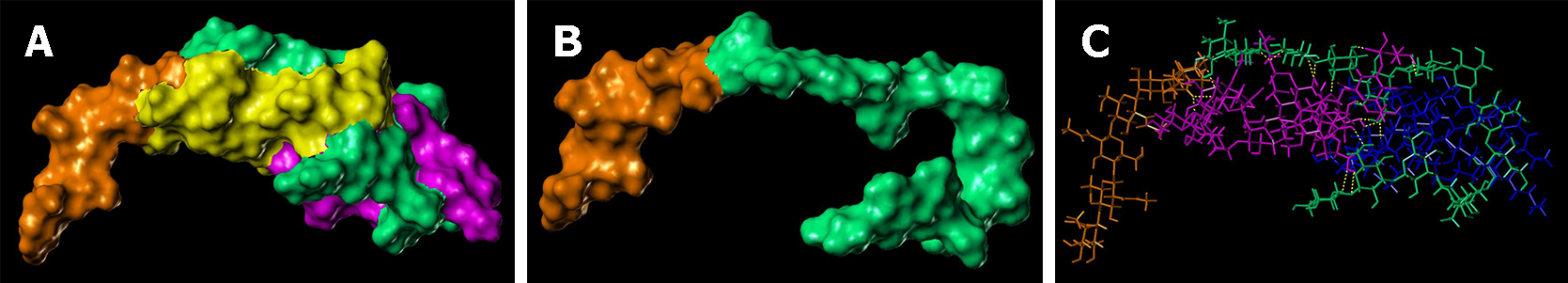
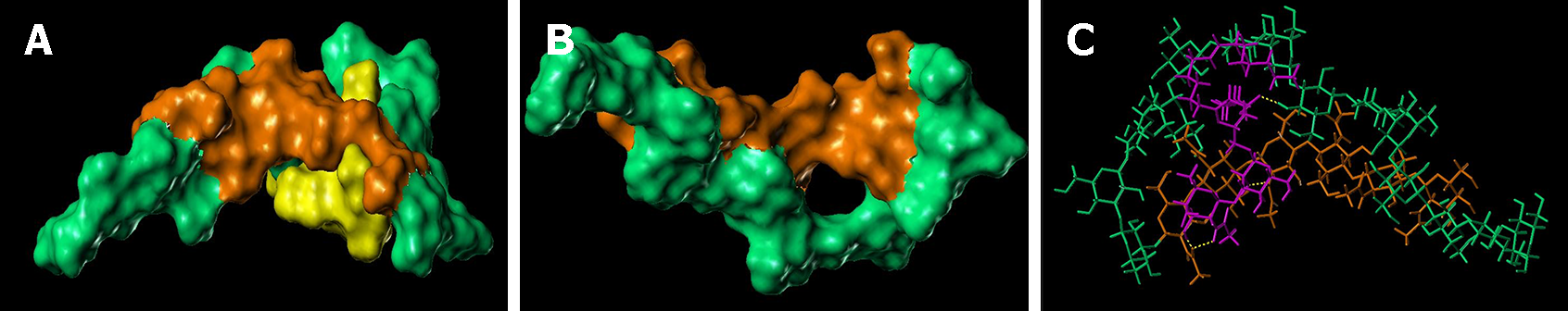
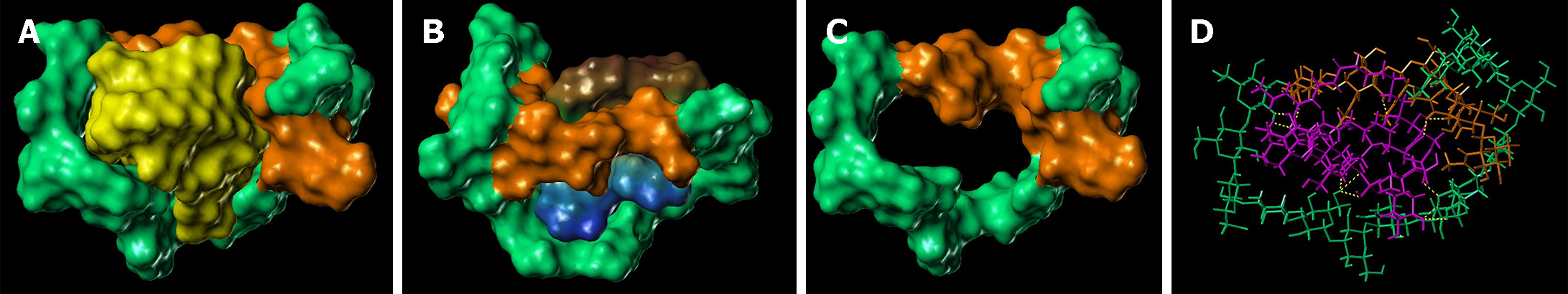
The lowest energy conformations from the MD trajectories showed the ability of chitin-glucan to chelate/complex both bacterial toxins (LPS and LTA) and fungal toxin (PLM). The high flexibility of the polysaccharide chain allowed it to wrap around LPS (Figure 8), LTA (Figure 9), and PLM (Figure 10). For LPS, the chitin-glucan folded into a hairpin to form a cavity hosting LPS in the middle (Figure 8B). LPS interacts with chitin-glucan via a network of 18 hydrogen bonds, mainly between the hydrophilic glucan moiety of chitin-glucan and the hydrophilic antigen O moiety of LPS (Figure 8C). The terminal part of the chitin-glucan moiety was wrapped around the lipophilic lipid A moiety of LPS (Figure 8C). Concerning LTA, chitin-glucan folded to form a small hole between the chitin and glucan moieties, occupied by the shortest lipid side chain of LTA (Figure 7B), and interacted with chitin-glucan via four hydrogen bonds (Figure 9C). For PLM, chitin-glucan formed a cavity hosting the PLM in its middle (Figure 10B). PLM was distributed on both sides of the cavity according to the degree of lipophilicity of its moieties (Figure 10C) and interacted with chitin-glucan via a network of 13 hydrogen bonds distributed around the cavity (Figure 10D).
IBS is a chronic functional gastrointestinal disorder diagnosed according to symptom-based criteria defined by the ROME IV classification[28]. IBS is a heterogeneous disorder with multiple physiopathological mechanisms[29]. Exposure to pathogenic organisms, changes in host-microbiota interactions, and disruption of the intestinal barrier can affect the gut-brain axis, triggering locally persistent low inflammation and altering visceral sensitivity[29]. Studies on the basic molecular mechanisms that enhance IBS management and facilitate the development of new, specific targeted treatments are important. In the present study, we investigated the functional and molecular gastrointestinal responses to chitin-glucan administration, particularly in relation to the main pathophysiological mechanisms of IBS. We showed that chitin-glucan decreased visceral pain perception and intestinal inflammation rapidly and significantly through master gene regulation in pain, inflammation, and intestinal barrier function, and may neutralize harmful substances in the intestinal lumen, such as microbial cell walls.
Visceral hypersensitivity is a key symptom of IBS. CRD in rats is the most widely used method for assessing visceral pain due to its ease of use and robust reproducibility[23,30]. In this model, we evaluated the functional characteristics of chitin-glucan, a novel dietary prebiotic used in humans at recommended dosages of 1.5 g/d and 3.0 g/d[13]. In untreated rats, a mean CRD of 46.0 mmHg ± 0.9 mmHg was required to induce pain, characterized by clearly visible abdominal contractions and elevation of the hind part of the animal’s body[21-23]. Oral administration of chitin-glucan at a HED of 3 g/d for 15 d decreased normal visceral perception, allowing for a significant 14% increase in the pain threshold under normal conditions. To more accurately evaluate the functional characteristics of chitin-glucan in the modulation of visceral pain, we induced intestinal hypersensitivity by local administration of TNBS. Compared to rats under normal conditions, intra-rectal administration of a low concentration of TNBS induces higher and long-lasting visceral hypersensitivity, making this model the reference for screening novel treatments for visceral pain originating within the gastrointestinal tract[19]. In rats with visceral hypersensitivity, oral administration of chitin-glucan (1.5 g/d decreased visceral perception by 20% five weeks after TNBS administration with a dose- and time-related analgesic effect leading, at 3.0 g/d, to a rapid 25% inhibition of pain perception three weeks after colon sensitization, reaching up to 50% at seven weeks. In the present study, we used phloroglucinol as a positive control under the same conditions as chitin-glucan. Phloroglucinol is a well-tolerated phenol derivative with antispasmodic properties that has long been used in clinical practice for painful gastrointestinal conditions, particularly in patients with IBS[31-33]. Using the most relevant, robust, and reliable animal model of chronic visceral pain to screen promising therapies to alleviate visceral pain[19], chitin-glucan at 3.0 g/d showed more rapid and two-fold superior analgesic effects compared to the positive control treatment phloroglucinol used at conventional dosages[31].
There are few effective analgesic options to manage chronic visceral pain, except for opioids, which require parenteral administration and have numerous adverse side effects. Compared to a previous study[23], the administration of chitin-glucan at 3.0 g/d resulted in an antinociceptive effect of the same magnitude as that induced by the subcutaneous administration of 3 mg morphine per kg body weight. Therefore, we hypothesized that Chitin-glucan induces the expression of receptors on epithelial cells, which locally control the transmission of nociceptive information to the intestinal nervous system. Opioid[34,35] and cannabinoid[36] systems are important pathways involved in visceral sensory signaling and intestinal motility. Most ligands activating the MOR and CB2R signal also target several other pathways involving PPAR-a to mediate synergistic antinociceptive activities[37,38]. Since these receptors are highly expressed in epithelial cells[23,39], we conducted a series of in vitro experiments using human intestinal HT-29 epithelial cells showing increased expression of MOR and CB2R mRNA, starting after cell incubation with different concentrations of chitin-glucan, without significant modification of PPARa mRNA levels.
Gut inflammation is a major risk factor for developing long-lasting visceral hypersensitivity[40]. Within the gastrointestinal tract, low-grade mucosal inflammation with variable-intensity immune activation is present in patients with IBS[41], particularly in those with post-infection IBS or IBS with diarrhea[5]. In rats with long-lasting visceral hypersensitivity induced by intra-rectal administration of TNBS, active inflammation of the colon is present a few days after TNBS exposure, followed by a recovery period of 4-8 wk, where overt signs of inflammation disappear, but hypersensitivity to CRD persists[19]. To gain insight into the mechanisms underlying the improvement of TNBS-induced visceral hypersensitivity in rats by chitin-glucan, we analyzed the effects of chitin-glucan specifically on colitis in one of the most commonly used models of intestinal inflammation induced by the oral administration of DSS[24,25]. We showed that oral administration of chitin-glucan provides a therapeutic benefit in established DSS-induced inflammatory lesions of the colon, decreasing the intensity of inflammation by 50%, leading to complete regeneration of the colonic mucosa without crypt damage, resolution of clinical manifestations, and restoration of stool consistency in most animals. In vitro, using human intestinal HT-29 epithelial cells, chitin-glucan used at clinically and biologically relevant concentrations was able to increase the expression of IL-10 mRNA, which is an important player in the regulation of intestinal inflammation[42]. Furthermore, the anti-inflammatory activity of chitin-glucan was investigated in LPS-stimulated HT-29 epithelial cells, showing potent inhibition of major inflammatory cytokine genes, such as IL-1b and IL-8. Regarding the importance of developing new treatments for IBS guided by physiopathology, the anti-inflammatory properties of chitin-glucan are potentially important, as they target a relevant physiopathological mechanism associated with IBS[29].
Increased intestinal permeability is a pathophysiological observation in IBS, observed mostly in the diarrhea-predominant patient subgroup[43,44]. The intestinal epithelium is the main protective barrier used to regulate the contact between luminal antigens, including microbe-derived molecules, and immune cells located below the lamina propria and submucosa. This epithelial barrier comprises occlusive intracellular tight junctions consisting of transmembrane proteins, such as claudins and zonula occludens molecules, as well as a dense film of mucus containing glycosylated glycoproteins called mucins, where MUC2, MUC3, MUC4, and MUC5AC are among the most expressed in the human colon or small bowel[45]. We previously demonstrated the protective effect of chitin-glucan fermentation products on inflammation-induced epithelial barrier disruption and the production of inflammatory cytokines[16]. In the present study, the incubation of HT-29 epithelial cells with chitin-glucan resulted in increased levels of MUC5AC, claudin-2, and ZO-2 mRNA. HT-29 cells share similarities with enterocytes of the human small intestine, and this cell line is a valuable in vitro model for studying molecules constituting tight junctions, mucin expression, and the intestinal epithelial response to bacterial infection[46]. Taken together, these findings suggest that in addition to chitin-glucan fermentation products, chitin-glucans may also have a direct effect on epithelial cells to preserve barrier integrity.
Microbial cell walls contain pathogenic lipids, including LPS and LTA, in gram-negative and gram-positive bacteria, and PLM in fungi[47]. These compounds are present in large quantities in the intestinal lumen and act as major ligands for Toll-like receptors (TLR-2-4-6) and other innate immune receptors that trigger inflammatory responses[48]. Therapeutic strategies involving intestinal sequestration and clearance of these pathogenic lipids have been successfully evaluated in patients with IBS using the mineral clay diosmectite[49]. Other pathogenic lipid-sequestering molecules have already been identified in marine chelicerates and crustaceans[50-53]. Chitin is found in the exoskeletons of marine invertebrates, insects, arachnids, and the cell walls of various fungi and algae[52]. Although chitin-glucan has antimicrobial activity against a large number of microorganisms[53], the mechanisms underlying these antibacterial and antifungal activities remain unknown. Therefore, we developed a molecular model of chitin-glucan and performed molecular docking and molecular dynamic simulations to evaluate the ability of chitin-glucan to chelate LPS, LTA, and PLM. In silico findings revealed that the high flexibility of chitin-glucan formed a cavity around LPS through a network of 18 hydrogen bonds interacting with the lipid A structure of LPS, which is the active and most conserved component of LPS, acting as a pathogen-associated molecular pattern[54]. Similar interactions were observed between chitin-glucan complexes and LTA or PLM. Collectively, these results suggest that the antimicrobial activity of chitin-glucan may be involved, at least in part, in a new property of the complex as a chelator of pathogenic lipids.
Current IBS treatments are often inadequate, and only a small percentage of patients are on prescription therapies, underscoring the potential market and need for additional therapeutic options. The multifactorial pathogenesis of IBS has led to the development of diverse treatment strategies. In the present study, the prebiotic chitin-glucan produced potent and non-tolerance-forming anti-nociception, together with the modulation of intestinal inflammation through intestinal intestinal master gene regulation and chelation of harmful microbial products in the gastrointestinal tract. These results advance our understanding of the mechanisms of action of chitin-glucans in the gut and augment the implementation of evidence-based chitin-glucan treatments in patients with IBS or IBS-like symptoms.
We thank the Charity European Foundation DigestScience for its help in the supervision of the study.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology & hepatology
Country/Territory of origin: France
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ong H, Malaysia S-Editor: Chen YL L-Editor: A P-Editor: Chen YX
| 1. | Camilleri M, Choi MG. Review article: irritable bowel syndrome. Aliment Pharmacol Ther. 1997;11:3-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 191] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 2. | Fortea J, Prior M. Irritable bowel syndrome with constipation: a European-focused systematic literature review of disease burden. J Med Econ. 2013;16:329-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 3. | Sethi S, Wadhwa V, LeClair J, Mikami S, Park R, Jones M, Sethi N, Brown A, Lembo A. In-patient discharge rates for the irritable bowel syndrome - an analysis of national trends in the United States from 1997 to 2010. Aliment Pharmacol Ther. 2013;38:1338-1346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 4. | Lacy BE, Pimentel M, Brenner DM, Chey WD, Keefer LA, Long MD, Moshiree B. ACG Clinical Guideline: Management of Irritable Bowel Syndrome. Am J Gastroenterol. 2021;116:17-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 462] [Article Influence: 115.5] [Reference Citation Analysis (0)] |
| 5. | Vasant DH, Paine PA, Black CJ, Houghton LA, Everitt HA, Corsetti M, Agrawal A, Aziz I, Farmer AD, Eugenicos MP, Moss-Morris R, Yiannakou Y, Ford AC. British Society of Gastroenterology guidelines on the management of irritable bowel syndrome. Gut. 2021;70:1214-1240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 276] [Article Influence: 69.0] [Reference Citation Analysis (0)] |
| 6. | Moayyedi P, Andrews CN, MacQueen G, Korownyk C, Marsiglio M, Graff L, Kvern B, Lazarescu A, Liu L, Paterson WG, Sidani S, Vanner S. Canadian Association of Gastroenterology Clinical Practice Guideline for the Management of Irritable Bowel Syndrome (IBS). J Can Assoc Gastroenterol. 2019;2:6-29. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 117] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 7. | Fukudo S, Okumura T, Inamori M, Okuyama Y, Kanazawa M, Kamiya T, Sato K, Shiotani A, Naito Y, Fujikawa Y, Hokari R, Masaoka T, Fujimoto K, Kaneko H, Torii A, Matsueda K, Miwa H, Enomoto N, Shimosegawa T, Koike K. Evidence-based clinical practice guidelines for irritable bowel syndrome 2020. J Gastroenterol. 2021;56:193-217. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 88] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 8. | Lembo A, Sultan S, Chang L, Heidelbaugh JJ, Smalley W, Verne GN. AGA Clinical Practice Guideline on the Pharmacological Management of Irritable Bowel Syndrome With Diarrhea. Gastroenterology. 2022;163:137-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 84] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 9. | Chang L, Sultan S, Lembo A, Verne GN, Smalley W, Heidelbaugh JJ. AGA Clinical Practice Guideline on the Pharmacological Management of Irritable Bowel Syndrome With Constipation. Gastroenterology. 2022;163:118-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 82] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 10. | Savarino E, Zingone F, Barberio B, Marasco G, Akyuz F, Akpinar H, Barboi O, Bodini G, Bor S, Chiarioni G, Cristian G, Corsetti M, Di Sabatino A, Dimitriu AM, Drug V, Dumitrascu DL, Ford AC, Hauser G, Nakov R, Patel N, Pohl D, Sfarti C, Serra J, Simrén M, Suciu A, Tack J, Toruner M, Walters J, Cremon C, Barbara G. Functional bowel disorders with diarrhoea: Clinical guidelines of the United European Gastroenterology and European Society for Neurogastroenterology and Motility. United European Gastroenterol J. 2022;10:556-584. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 83] [Article Influence: 27.7] [Reference Citation Analysis (0)] |
| 11. | Jakobsson Ung E, Ringstrom G, Sjövall H, Simrén M. How patients with long-term experience of living with irritable bowel syndrome manage illness in daily life: a qualitative study. Eur J Gastroenterol Hepatol. 2013;25:1478-1483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 12. | Bertram S, Kurland M, Lydick E, Locke GR 3rd, Yawn BP. The patient's perspective of irritable bowel syndrome. J Fam Pract. 2001;50:521-525. [PubMed] |
| 13. | EFSA Panel on Dietetic Products NaAN. Scientific opinion on the safety of ‘Chitin-glucan’ as a novel food ingredient. EFSA J. 2010;8:1687. [RCA] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 25] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 14. | Berecochea-Lopez A, Decordé K, Ventura E, Godard M, Bornet A, Teissèdre PL, Cristol JP, Rouanet JM. Fungal chitin-glucan from Aspergillus niger efficiently reduces aortic fatty streak accumulation in the high-fat fed hamster, an animal model of nutritionally induced atherosclerosis. J Agric Food Chem. 2009;57:1093-1098. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 15. | Neyrinck AM, Possemiers S, Verstraete W, De Backer F, Cani PD, Delzenne NM. Dietary modulation of clostridial cluster XIVa gut bacteria (Roseburia spp.) by chitin-glucan fiber improves host metabolic alterations induced by high-fat diet in mice. J Nutr Biochem. 2012;23:51-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 203] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 16. | Calatayud M, Verstrepen L, Ghyselinck J, Van den Abbeele P, Marzorati M, Modica S, Ranjanoro T, Maquet V. Chitin Glucan Shifts Luminal and Mucosal Microbial Communities, Improve Epithelial Barrier and Modulates Cytokine Production In Vitro. Nutrients. 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 17. | Rodriguez J, Neyrinck AM, Zhang Z, Seethaler B, Nazare JA, Robles Sánchez C, Roumain M, Muccioli GG, Bindels LB, Cani PD, Maquet V, Laville M, Bischoff SC, Walter J, Delzenne NM. Metabolite profiling reveals the interaction of chitin-glucan with the gut microbiota. Gut Microbes. 2020;12:1810530. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 40] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 18. | Martín R, Rios-Covian D, Huillet E, Auger S, Khazaal S, Bermúdez-Humarán LG, Sokol H, Chatel JM, Langella P. Faecalibacterium: a bacterial genus with promising human health applications. FEMS Microbiol Rev. 2023;47. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 182] [Reference Citation Analysis (0)] |
| 19. | Johnson AC, Farmer AD, Ness TJ, Greenwood-Van Meerveld B. Critical evaluation of animal models of visceral pain for therapeutics development: A focus on irritable bowel syndrome. Neurogastroenterol Motil. 2020;32:e13776. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 20. | Brenna Ø, Furnes MW, Drozdov I, van Beelen Granlund A, Flatberg A, Sandvik AK, Zwiggelaar RT, Mårvik R, Nordrum IS, Kidd M, Gustafsson BI. Relevance of TNBS-colitis in rats: a methodological study with endoscopic, histologic and Transcriptomic [corrected] characterization and correlation to IBD. PLoS One. 2013;8:e54543. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 60] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 21. | Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain. 1983;16:109-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5938] [Cited by in RCA: 6276] [Article Influence: 149.4] [Reference Citation Analysis (0)] |
| 22. | Bourdu S, Dapoigny M, Chapuy E, Artigue F, Vasson MP, Dechelotte P, Bommelaer G, Eschalier A, Ardid D. Rectal instillation of butyrate provides a novel clinically relevant model of noninflammatory colonic hypersensitivity in rats. Gastroenterology. 2005;128:1996-2008. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 130] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 23. | Rousseaux C, Thuru X, Gelot A, Barnich N, Neut C, Dubuquoy L, Dubuquoy C, Merour E, Geboes K, Chamaillard M, Ouwehand A, Leyer G, Carcano D, Colombel JF, Ardid D, Desreumaux P. Lactobacillus acidophilus modulates intestinal pain and induces opioid and cannabinoid receptors. Nat Med. 2007;13:35-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 525] [Cited by in RCA: 541] [Article Influence: 28.5] [Reference Citation Analysis (0)] |
| 24. | Cooper HS, Murthy SN, Shah RS, Sedergran DJ. Clinicopathologic study of dextran sulfate sodium experimental murine colitis. Lab Invest. 1993;69:238-249. [PubMed] |
| 25. | Dieleman LA, Palmen MJ, Akol H, Bloemena E, Peña AS, Meuwissen SG, Van Rees EP. Chronic experimental colitis induced by dextran sulphate sodium (DSS) is characterized by Th1 and Th2 cytokines. Clin Exp Immunol. 1998;114:385-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 747] [Cited by in RCA: 924] [Article Influence: 34.2] [Reference Citation Analysis (0)] |
| 26. | Funakoshi T, Yamashita K, Ichikawa N, Fukai M, Suzuki T, Goto R, Oura T, Kobayashi N, Katsurada T, Ichihara S, Ozaki M, Umezawa K, Todo S. A novel NF-κB inhibitor, dehydroxymethylepoxyquinomicin, ameliorates inflammatory colonic injury in mice. J Crohns Colitis. 2012;6:215-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 55] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 27. | Clark M, Cramer III RD, Van Opdenbosch N. Validation of the general purpose Tripos 5.2 force field. J Comput Chem. 1989;10:982-1012. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2344] [Cited by in RCA: 2351] [Article Influence: 65.3] [Reference Citation Analysis (0)] |
| 28. | Mearin F, Lacy BE, Chang L, Chey WD, Lembo AJ, Simren M, Spiller R. Bowel Disorders. Gastroenterology. 2016;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1781] [Cited by in RCA: 1893] [Article Influence: 210.3] [Reference Citation Analysis (3)] |
| 29. | Drossman DA. Functional Gastrointestinal Disorders: History, Pathophysiology, Clinical Features and Rome IV. Gastroenterology. 2016;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 50] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 30. | Esquerre N, Basso L, Dubuquoy C, Djouina M, Chappard D, Blanpied C, Desreumaux P, Vergnolle N, Vignal C, Body-Malapel M. Aluminum Ingestion Promotes Colorectal Hypersensitivity in Rodents. Cell Mol Gastroenterol Hepatol. 2019;7:185-196. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 31. | Jafri W, Yakoob J, Hussain S, Jafri N, Islam M. Phloroglucinol in irritable bowel syndrome. J Pak Med Assoc. 2006;56:5-8. [PubMed] |
| 32. | Annaházi A, Róka R, Rosztóczy A, Wittmann T. Role of antispasmodics in the treatment of irritable bowel syndrome. World J Gastroenterol. 2014;20:6031-6043. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 90] [Cited by in RCA: 80] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 33. | Shin SY, Cha BK, Kim WS, Park JY, Kim JW, Choi CH. The Effect of Phloroglucinol in Patients With Diarrhea-predominant Irritable Bowel Syndrome: A Randomized, Double-blind, Placebo-controlled Trial. J Neurogastroenterol Motil. 2020;26:117-127. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 34. | Philippe D, Chakass D, Thuru X, Zerbib P, Tsicopoulos A, Geboes K, Bulois P, Breisse M, Vorng H, Gay J, Colombel JF, Desreumaux P, Chamaillard M. Mu opioid receptor expression is increased in inflammatory bowel diseases: implications for homeostatic intestinal inflammation. Gut. 2006;55:815-823. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 73] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 35. | Philippe D, Dubuquoy L, Groux H, Brun V, Chuoï-Mariot MT, Gaveriaux-Ruff C, Colombel JF, Kieffer BL, Desreumaux P. Anti-inflammatory properties of the mu opioid receptor support its use in the treatment of colon inflammation. J Clin Invest. 2003;111:1329-1338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 69] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 36. | Wright K, Rooney N, Feeney M, Tate J, Robertson D, Welham M, Ward S. Differential expression of cannabinoid receptors in the human colon: cannabinoids promote epithelial wound healing. Gastroenterology. 2005;129:437-453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 238] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 37. | Kasatkina LA, Rittchen S, Sturm EM. Neuroprotective and Immunomodulatory Action of the Endocannabinoid System under Neuroinflammation. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 67] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 38. | Xu B, Zhang Q, Chen D, Zhang M, Zhang R, Zhao W, Qiu Y, Xu K, Xiao J, Niu J, Shi Y, Li N, Fang Q. OCP002, a Mixed Agonist of Opioid and Cannabinoid Receptors, Produces Potent Antinociception With Minimized Side Effects. Anesth Analg. 2023;136:373-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 39. | Luo Y, Xie C, Brocker CN, Fan J, Wu X, Feng L, Wang Q, Zhao J, Lu D, Tandon M, Cam M, Krausz KW, Liu W, Gonzalez FJ. Intestinal PPARα Protects Against Colon Carcinogenesis via Regulation of Methyltransferases DNMT1 and PRMT6. Gastroenterology. 2019;157:744-759.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 118] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 40. | Drewes AM, Olesen AE, Farmer AD, Szigethy E, Rebours V, Olesen SS. Gastrointestinal pain. Nat Rev Dis Primers. 2020;6:1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 178] [Article Influence: 35.6] [Reference Citation Analysis (0)] |
| 41. | Ford AC, Talley NJ. Mucosal inflammation as a potential etiological factor in irritable bowel syndrome: a systematic review. J Gastroenterol. 2011;46:421-431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 146] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 42. | Bugbee E, Wang AA, Gommerman JL. Under the influence: environmental factors as modulators of neuroinflammation through the IL-10/IL-10R axis. Front Immunol. 2023;14:1188750. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 43. | Dunlop SP, Hebden J, Campbell E, Naesdal J, Olbe L, Perkins AC, Spiller RC. Abnormal intestinal permeability in subgroups of diarrhea-predominant irritable bowel syndromes. Am J Gastroenterol. 2006;101:1288-1294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 336] [Cited by in RCA: 352] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 44. | Rao AS, Camilleri M, Eckert DJ, Busciglio I, Burton DD, Ryks M, Wong BS, Lamsam J, Singh R, Zinsmeister AR. Urine sugars for in vivo gut permeability: validation and comparisons in irritable bowel syndrome-diarrhea and controls. Am J Physiol Gastrointest Liver Physiol. 2011;301:G919-G928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 116] [Article Influence: 8.3] [Reference Citation Analysis (1)] |
| 45. | Inaba R, Vujakovic S, Bergstrom K. The gut mucus network: A dynamic liaison between microbes and the immune system. Semin Immunol. 2023;69:101807. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 46. | Martínez-Maqueda D, Miralles B, Recio I. HT29 Cell Line. In: The Impact of Food Bioactives on Health: in vitro and ex vivo models [Internet]. Cham (CH): Springer; 2015–. [PubMed] |
| 47. | Bentzer P, Russell JA, Walley KR. Advances in Sepsis Research. Clin Chest Med. 2015;36:521-530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 48. | Cinel I, Opal SM. Molecular biology of inflammation and sepsis: a primer. Crit Care Med. 2009;37:291-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 268] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 49. | Howell CA, Kemppinen A, Allgar V, Dodd M, Knowles CH, McLaughlin J, Pandya P, Whorwell P, Markaryan E, Yiannakou Y. Double-blinded randomised placebo controlled trial of enterosgel (polymethylsiloxane polyhydrate) for the treatment of IBS with diarrhoea (IBS-D). Gut. 2022;71:2430-2438. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 16] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 50. | Rosa RD, Vergnes A, de Lorgeril J, Goncalves P, Perazzolo LM, Sauné L, Romestand B, Fievet J, Gueguen Y, Bachère E, Destoumieux-Garzón D. Functional divergence in shrimp anti-lipopolysaccharide factors (ALFs): from recognition of cell wall components to antimicrobial activity. PLoS One. 2013;8:e67937. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 64] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 51. | Yang Y, Boze H, Chemardin P, Padilla A, Moulin G, Tassanakajon A, Pugnière M, Roquet F, Destoumieux-Garzón D, Gueguen Y, Bachère E, Aumelas A. NMR structure of rALF-Pm3, an anti-lipopolysaccharide factor from shrimp: model of the possible lipid A-binding site. Biopolymers. 2009;91:207-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 68] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 52. | Perez S, Wertz JL. Chitin and Chitosans in the bioeconomy. 1st ed. Boca Raton: CRC Press, 2022. [DOI] [Full Text] |
| 53. | Ngo DH, Kim SK. Antioxidant effects of chitin, chitosan, and their derivatives. Adv Food Nutr Res. 2014;73:15-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 171] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 54. | González-Fernández C, Basauri A, Fallanza M, Bringas E, Oostenbrink C, Ortiz I. Fighting Against Bacterial Lipopolysaccharide-Caused Infections through Molecular Dynamics Simulations: A Review. J Chem Inf Model. 2021;61:4839-4851. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |