Published online Apr 28, 2024. doi: 10.3748/wjg.v30.i16.2249
Peer-review started: January 25, 2024
First decision: February 8, 2024
Revised: February 18, 2024
Accepted: March 22, 2024
Article in press: March 22, 2024
Published online: April 28, 2024
Processing time: 92 Days and 3.9 Hours
This study aimed to identify characteristic gut genera in obese and normal-weight children (8-12 years old) using 16S rDNA sequencing. The research aimed to provide insights for mechanistic studies and prevention strategies for childhood obesity. Thirty normal-weight and thirty age- and sex-matched obese children were included. Questionnaires and body measurements were collected, and fecal samples underwent 16S rDNA sequencing. Significant differences in body mass index (BMI) and body-fat percentage were observed between the groups. Analysis of gut microbiota diversity revealed lower α-diversity in obese children. Di-fferences in gut microbiota composition were found between the two groups. Prevotella and Firmicutes were more abundant in the obese group, while Bacteroides and Sanguibacteroides were more prevalent in the control group.
To identify the characteristic gut genera in obese and normal-weight children (8-12-year-old) using 16S rDNA sequencing, and provide a basis for subsequent mechanistic studies and prevention strategies for childhood obesity.
Thirty each normal-weight, 1:1 matched for age and sex, and obese children, with an obese status from 2020 to 2022, were included in the control and obese groups, respectively. Basic information was collected through questionnaires and body measurements were obtained from both obese and normal-weight children. Fecal samples were collected from both groups and subjected to 16S rDNA sequencing using an Illumina MiSeq sequencing platform for gut microbiota diversity analysis.
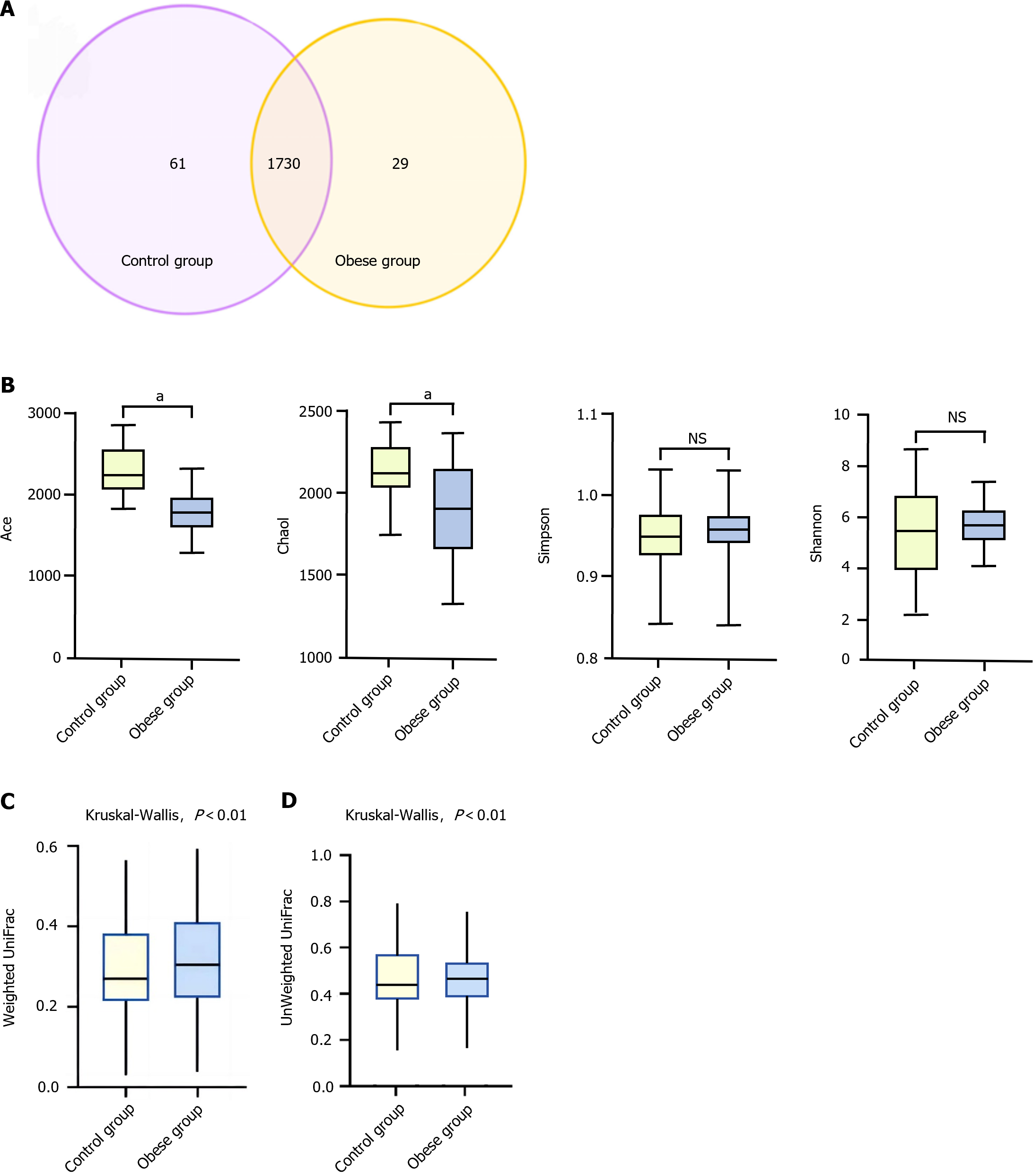
Significant differences in BMI and body-fat percentage were observed between the two groups. The Ace and Chao1 indices were significantly lower in the obese group than those in the control group, whereas differences were not significant in the Shannon and Simpson indices. Kruskal-Wallis tests indicated significant differences in unweighted and weighted UniFrac distances between the gut microbiota of normal-weight and obese children (P < 0.01), suggesting substantial disparities in both the species and quantity of gut microbiota between the two groups. Prevotella, Firmicutes, Bacteroides, and Sanguibacteroides were more abundant in the obese and control groups, respectively. Heatmap results demonstrated significant differences in the gut microbiota composition between obese and normal-weight children.
Obese children exhibited lower α-diversity in their gut microbiota than did the normal-weight children. Significant differences were observed in the composition of gut microbiota between obese and normal-weight children.
Core Tip: This study used 16S rDNA sequencing to identify characteristic gut genera in obese and normal-weight children. The findings revealed lower α-diversity in the gut microbiota of obese children compared to normal-weight children. Significant differences were observed in the composition of gut microbiota between the two groups, with Prevotella and Firmicutes being more abundant in the obese group, and Bacteroides and Sanguibacteroides more prevalent in the control group. These results provide insights into the potential role of gut microbiota in childhood obesity and may contribute to future mechanistic studies and prevention strategies.
- Citation: Li XM, Lv Q, Chen YJ, Yan LB, Xiong X. Association between childhood obesity and gut microbiota: 16S rRNA gene sequencing-based cohort study. World J Gastroenterol 2024; 30(16): 2249-2257
- URL: https://www.wjgnet.com/1007-9327/full/v30/i16/2249.htm
- DOI: https://dx.doi.org/10.3748/wjg.v30.i16.2249
Childhood obesity is a growing global health concern with an increasing prevalence worldwide. The World Health Organization defines childhood obesity as “a condition in which excess body fat negatively affects children’s health and well-being”. It is associated with various adverse health outcomes, including type 2 diabetes, cardiovascular diseases, and psychological disorders. The complex etiology of childhood obesity involves genetic, environmental, and behavioral factors. However, emerging evidence suggests that the gut microbiota, a community of microorganisms residing in the gastrointestinal tract, plays a significant role in the development of obesity. The human gut microbiota consists of trillions of microorganisms, including bacteria, viruses, fungi, and archaea, which interact with the host physiology, metabolism, and immune system. Recent advances in sequencing technologies, particularly 16S rRNA gene sequencing, have enabled researchers to comprehensively explore the composition and function of gut microbiota. Studies in adults have high-lighted the association between alterations in the gut microbiota and obesity; however, research in pediatric populations is essential to understand the early-life factors contributing to obesity.
Approximately 3.9 × 1013 bacteria in the human body, with the majority residing in the intestines, where approximately 1011 bacteria are found per gram of wet feces[1]. Studies have identified nearly 10 million non-redundant genes in the human intestinal tract, which is 150 times the size of the human genome, leading to the use of the term “human second genome” to describe the human intestinal microbiota. Therefore, human intestinal microbiota constitutes a unique ecological system with distinct microenvironments. The metabolic capacity of the gut microbiota far exceeds that of human cells and plays crucial roles in human health, including digestion, nutrition, metabolism, and immunity. Imbalances in the intestinal microbiota are associated with various diseases, including obesity and underweight status. A causal relationship exists between disruptions in the gut microbiota and obesity or underweight conditions. Multiple animal experiments have demonstrated the role of the host gut microbiota in energy acquisition and storage, potentially leading to obesity or underweight conditions. For instance, germ-free mice fed a high-fat diet exhibited lower weight gain than conventionally fed mice fed the same diet[2]. Germ-free mice transplanted with fecal microbiota from obese individuals exhibit obesity symptoms[3], whereas mice colonized with microbiota from malnourished children show symptoms of poor development and are underweight[4]. Numerous studies have indicated alterations in the composition and diversity of the gut microbiota in obese adults compared to those with normal weight[5,6]. However, inconsistencies exist regarding changes in the Firmicutes/Bacteroidetes ratio in the gut microbiota of obese individuals compared to those with normal weight[7].
Multiple studies have found that the composition, quantity, and proportion of gut microbiota in obese children undergo changes. Abdallah et al[8] reported that when comparing the gut microbiota of obese children to that of a control group, there were more bacteria from the phylum Firmicutes and fewer from the phylum Bacteroidetes. However, after controlling for dietary factors, when body weight decreased, the abundance of these two bacterial phyla was reversed. This suggests that the changes in the ratio of Bacteroidetes to Firmicutes in the gut are associated with childhood obesity. Kalliomäki et al[9] conducted a prospective study of 7-year-old children with an increased body mass index (BMI) and found that, compared to age-matched children with a normal BMI, children with a higher BMI had a reduced abundance of Bifidobacteria and an increased abundance of Enterobacteriaceae in their gut microbiota. Gao et al[10] discovered that, compared to school-aged children with normal weight, school-aged obese children had a decreased abundance of Bifidobacteria and an increased abundance of Escherichia coli in their feces. This resulted in a lower ratio between the two bacterial groups. These findings suggest a correlation between childhood obesity and an imbalance in the gut microbiota. Bifidobacteria are typical representatives of beneficial probiotics in the gut, whereas Escherichia coli can serve as a representative of pathogenic bacteria. An increased abundance of Escherichia coli is considered an important warning sign of the gut microbiota structure shifting towards a less favorable state for overall health. Both Bifidobacteria and Escherichia coli are commonly found in childhood gut microbiota, and their ratio can be used to assess the condition of the gut microbiota structure.
In obesity, there is an increase in taxa within the Bacteroidales order, such as Lactobacillus spp., Bifidobacterium spp., Bacteroides spp., and Enterococcus spp., as well as an elevated ratio of Firmicutes to Bacteroidetes and Enterobacteriaceae, while taxa within the Clostridia class, including Clostridium leptum and Enterobacter spp., are decreased[11-13]. Numerous studies suggest that an increased Firmicutes to Bacteroidetes ratio at the phylum level is a notable feature of the gut microbiota in individuals with obesity. Families such as Christensenellaceae and orders like Methanobacteriales, as well as genera including Lactobacillus, Bifidobacteria, and Akkermansia, are commonly regarded as probiotics, and their relative abundance typically correlates negatively with obesity. The gut microbiota regulates obesity by modulating energy absorption, central appetite, fat storage, chronic inflammation, and circadian rhythms[14]. The composition of the gut microbiota profoundly influences nutrient acquisition and energy regulation in the body, thus playing a pivotal role in the onset and progression of obesity and associated conditions[15,16]. Notably, the microbiota composition varies between infants and adults, as well as between obese and lean individuals. For instance, calorie-restricted diets can reduce the Firmicutes to Bacteroidetes ratio in the gut, while vegetarian diets have been found to increase Bacteroidetes and decrease Firmicutes, Bifidobacterium spp., Escherichia coli, Enterobacteriaceae, and Clostridia[17]. Consequently, targeting the gut microbiota presents a promising therapeutic avenue for addressing obesity[18].
This study aimed to investigate changes in gut microbiota composition in children with obesity using 16S rRNA gene sequencing technology. Understanding the relationship between childhood obesity and alterations in gut microbiota can shed light on potential therapeutic interventions and preventive strategies. Moreover, it may offer insights into the role of gut microbiota in childhood obesity-related metabolic disturbances.
This study employed a prospective cohort design to investigate the relationship between childhood obesity and the composition of the gut microbiota. Data collection will span a two-year period, allowing for the observation of long-term microbiota dynamics. A total of 60 children, aged 8 to 12 years, were recruited, and stratified into two age- and sex-matched groups: The obese group (defined as having a BMI percentile greater than or equal to the 95th percentile) and the normal-weight group.
The inclusion criteria for the obese group were based on BMI percentile, while the normal-weight group was selected to match for age and sex. Comprehensive baseline data, including age, sex, and lifestyle information, were collected from all participants. Clinical parameters, such as height, weight, waist circumference, and other relevant measurements were recorded. Stool samples will be collected using standardized procedures and immediately stored at -80 °C to preserve microbial DNA integrity.
Total microbial DNA was extracted from the fecal samples using a high-efficiency soil DNA extraction kit. The extraction procedure strictly followed the instructions provided in the manual. The extracted DNA was stored at -80 °C for future use.
Forty microliters of DNA from each sample were used for high-throughput sequencing on an Illumina MiSeq platform. Paired-end sequencing of the V3-V4 region of bacterial 16S rRNA was performed. The concentration and purity of DNA samples were evaluated using a UV spectrophotometry and agarose gel electrophoresis, respectively. Samples with DNA quantities greater than 500 ng were considered as qualified. The qualified samples were subjected to PCR amplification of the 16S rRNA gene V4 region using forward primer 347F (5’-CCT ACG GRR BGC ASC AGK VRV GAA T-3’) and reverse primer 806R (5’-GGA CTA CNV GGG TWT CTA ATC C-3’). PCR products were verified for specificity through agarose gel electrophoresis, and the purified PCR products were sequenced using an Illumina MiSeq M300 sequencer (Illumina Inc., United States) for paired-end 250 bp sequencing.
Raw sequencing data were filtered using VSEARCH software to remove low-quality fragments. The PCR products were assembled, and duplicate, tag, and primer sequences were removed to obtain the optimized sequences. Operational taxonomic units (OTUs) were clustered with 97% sequence similarity. Representative sequences and corresponding taxonomic information for each OTU were extracted using the QIIME software. Alpha diversity indices, namely Ace, Chao1, Shannon, and Simpson indices, were calculated using R software to assess the diversity and richness of the gut microbiota within each sample. Beta diversity, which describes the diversity between samples, was analyzed using various tools, including R, QIIME, and Mothur software, considering OTU abundance, bacterial alpha diversity, beta diversity, and taxonomic composition at different taxonomic levels.
Diversity analyses, including alpha diversity (e.g., Shannon diversity index) and beta diversity (e.g., Bray-Curtis distance matrix), were performed using QIIME 2 and R packages. The resulting data were used for species annotation and classification to generate the OTU or ASV tables. Differential abundance analysis was conducted using statistical tools such as DESeq2 or LEfSe to identify significant differences in microbial communities between the obese and normal-weight groups. Functional prediction of the gut microbiota can be achieved using PICRUSt or other relevant tools. Correlation analysis was performed using Pearson or Spearman correlation methods to explore the associations between microbial composition and clinical parameters.
To investigate the differences in gastrointestinal microbiota between obese and normal-weight children, 30 samples each from healthy children and obese children were collected from the hospital. BMI, which is widely used to measure obesity, continues to be a practical tool for large-scale population studies and clinical screening. Specific information regarding the children is presented in Table 1, which shows the differences in BMI values and body fat content between the two groups of children.
| Indicator | Control (n = 30) | Obese group (n = 30) | P value |
| Age (yr) | 10.67 ± 1.36 | 10.29 ± 1.84 | > 0.05 |
| Height (cm) | 116.82 ± 1.97 | 118.27 ± 1.25 | > 0.05 |
| Weight (kg) | 23.90 ± 0.82 | 33.89 ± 0.17 | < 0.05 |
| Body mass index | 18.96 ± 0.19 | 24.35 ± 0.79 | < 0.05 |
| Percentage of body fat (%) | 16.49 ± 0.81 | 23.74 ± 0.63 | < 0.05 |
In the control group of healthy children, there were 1791 OTUs, with 61 unique OTUs. In contrast, the obese group contained 1759 OTUs, with 29 unique OTUs (Figure 1A). Comparatively, the richness of OTUs in the obese children group decreased at various taxonomic levels compared to that in the control group. Alpha diversity analysis of the gut microbiota in the study subjects showed that, in comparison to the control group, obese children had lower Ace and Chao1 indices, while the differences in the Shannon and Simpson indices were not statistically significant (Figure 1B).
The relative abundances of the top five genera in the gut microbiota of the control group (normal-weight children) and obese group were analyzed. These data highlight the significant differences in the gut microbiota composition between the control and obese groups. In the control group, Bacteroides and Faecalibacterium had higher relative abundances, whereas Prevotella and Firmicutes were more abundant in the obese group (Table 2). These differences indicate distinct compositional variances in the gut microbiota of obese children compared with normal-weight children, possibly related to the development of obesity and its associated metabolic disorders.
| Control group | Obese group |
| Bacteroides | Prevotella |
| Sanguibacteroides | Firmicutes |
| Faecalibacterium | Bacteroides |
| Pseudoramibacter | Peptoclostridium |
| Plesiomonas | Faecalibacterium |
The overall structure of the gut microbiota in normal-weight and obese children was analyzed based on two different beta diversity metrics: Unweighted UniFrac and Weighted UniFrac (Figure 1C and D). UniFrac distances were calculated based on the evolutionary tree of each OTU, reflecting differences in the gut microbiota between samples based on their evolutionary relationships. Unweighted UniFrac distances consider only the presence or absence of OTUs in the microbial community, without considering their abundance. In contrast, weighted UniFrac distances incorporate OTU abundance into the calculations. Kruskal-Wallis tests revealed significant differences (P < 0.01) in Unweighted UniFrac and Weighted UniFrac distances between the gut microbiota of normal-weight and obese children, indicating significant variations in both the species and quantity of gut microbiota between these two groups.
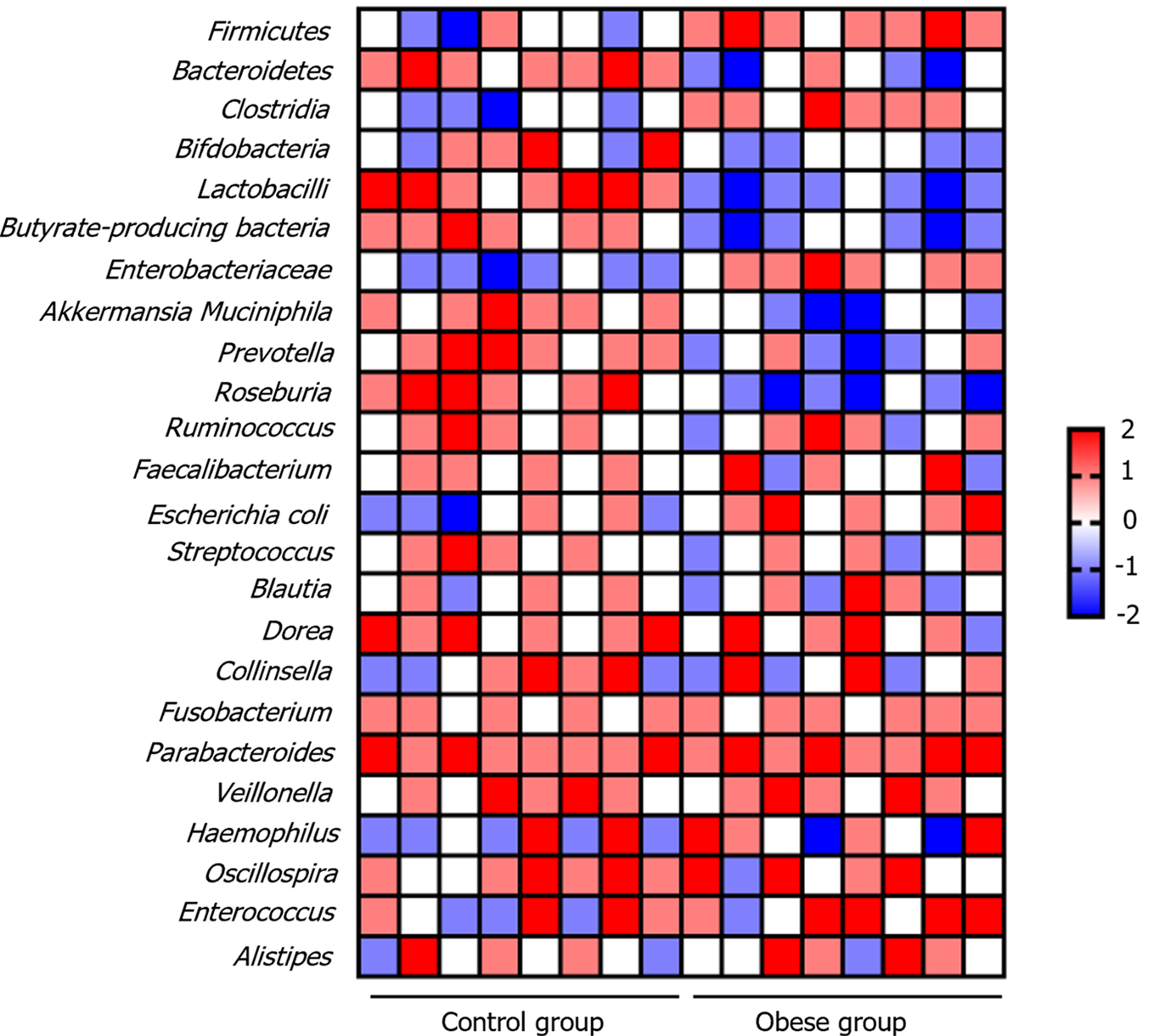
There were significant differences in the gut microbiota between the obese and normal-weight children. The results indicate that in obese children, the relative abundance of Firmicutes bacteria increases, while Bacteroidetes bacteria decrease, leading to an elevated Firmicutes/Bacteroidetes ratio. Additionally, there was an increase in Clostridia (involved in cellulose breakdown), a decrease in beneficial probiotics such as Bifidobacteria and Lactobacilli, and a reduction in butyrate-producing bacteria, which are responsible for producing beneficial short-chain fatty acids (SCFAs), in obese children. In cases of metabolic syndrome, there is an increase in the Enterobacteriaceae family. Conversely, Akkermansia muciniphila, a bacterium usually beneficial for gut health, decreased in obese children (Figure 2). These observations highlight the potential role of the gut microbiota in the development of obesity. However, further research is required to explore the specific impact of these changes on health.
Apart from these trends, some gut microbiota showed no significant differences between the obese and normal-weight children. These relatively stable microbiota included Ruminococcus, Faecalibacterium, Blautia, Dorea, Collinsella, Fusobacterium, Parabacteroides, Veillonella, Haemophilus, Oscillospira, Enterococcus, and Alistipes. Although some of these may exhibit minor changes in different studies, the magnitude of these changes is typically small, making them difficult to confirm. This stability emphasizes the complexity of gut microbiota, with different bacterial groups showing individual variations that are likely influenced by factors such as lifestyle and dietary habits. Therefore, when studying the microbiota of obese children, it is crucial to comprehensively consider this diversity to gain a more holistic understanding of the relationship between the gut microbiota and obesity development.
The results of this study revealed significant differences in the gut microbiota of obese and normal-weight children. The gut microbiota of obese children exhibits multifaceted changes that may play a crucial role in the development of obesity and related metabolic disorders. First, we observed an increase in the relative abundance of Firmicutes and a decrease in Bacteroidetes in obese children, leading to an elevated Firmicutes/Bacteroidetes ratio. This alteration is commonly associated with obesity, suggesting that energy metabolism in obese children may be influenced by gut microbiota[19]. Increased abundance of Firmicutes is typically linked to more efficient energy absorption from food, potentially contributing to energy intake and storage in obese children[20]. Second, the proliferation of Clostridia bacteria may accelerate cellulose breakdown, further enhancing energy absorption in obese children. Conversely, the reduction in beneficial bacteria, such as Bifidobacteria and Lactobacilli, as well as a decrease in butyrate-producing bacteria, could weaken the intestinal barrier function, disrupt the immune system, and increase chronic inflammation. These factors may create favorable conditions for the development of obesity and its related metabolic disorders[21-23]. Additionally, with the progression of metabolic syndrome, there was a significant increase in Enterobacteriaceae family, further elevating the risk of chronic inflammation[24-26]. Simultaneously, Akkermansia muciniphila, a bacterium that is usually beneficial for intestinal health, decreases in obese children. This reduction may lead to mucosal layer damage and exacerbation of intestinal inflammation. However, it is worth noting that some gut microbiota showed no significant differences between the obese and normal-weight children. This stability emphasizes the complexity of gut microbiota, suggesting that individuals may react differently to obesity. Such variations may be influenced by various factors, including individual lifestyle and dietary habits.
The prevalence of overweightness and obesity in children and adolescents is becoming an increasingly serious public health concern. Childhood overweight and obesity are caused by multiple factors including genetic background, diet, and lifestyle. Furthermore, the gut microbiota and its metabolites play crucial roles in the progression of childhood overweight and obesity. Alpha diversity plays a significant role in the study of gut microbiota in obese children. Alpha diversity primarily reflects the abundance and diversity of the microbial species within an individual. According to studies conducted both domestically and abroad, the alpha diversity of the gut microbiota in obese adults is typically low, which has been widely confirmed[27]. Similarly, in the present study, we observed that the ACE and Chao1 indices (used to estimate the total number of species in a community) were significantly lower in obese children than those in normal-weight children, particularly in obese boys, where the Chao1 index exhibited a more significant decrease. However, consistent with some research findings[28], differences in the Shannon and Simpson indices between the obese and control groups were not significant.
Alterations in the gut microbiota reveal an imbalance in the ecosystem in obese children. Our results demonstrated significant differences in the gut microbiota composition between obese and normal-weight children. In obese children, there was an increase in the relative abundances of Firmicutes and Clostridia, whereas those of Bacteroidetes, Bifidobacteria, and Lactobacilli decreased. These changes could stem from various factors; for instance, obese children often undergo more antibiotic treatments in early life, which may have a lasting impact on the composition of their gut microbiota[29]. These alterations may lead to mucosal barrier dysfunction in the intestines of obese children, including the S100 calcium-binding proteins S100A8 and S100A9[30]. Simultaneously, the abnormal production and absorption of specific metabolites such as SCFAs and bile acids can affect energy metabolism and weight control[19]. The gut microbiota and its metabolites differ significantly between obese and normal-weight individuals. The reduced abundance of various Akkermansia species that metabolize glutamate is associated with a higher risk of obesity. Additionally, the gut microbiota of obese adolescents exhibits enhanced carbohydrate oxidation capabilities[31]. Changes in the gut microbiota have been linked to childhood obesity and non-alcoholic fatty liver disease. The biosynthesis of SCFAs, amino acids, and lipopolysaccharides is negatively correlated with insulin resistance (IR), whereas peptidoglycan biosynthesis pathways are positively correlated with IR[32]. Therefore, studying alterations in the gut microbiota of obese children is of great significance.
The study revealed significant differences between obese and normal-weight children, including higher BMI and body-fat percentage in obese children. While the Ace and Chao1 indices indicated lower species richness in the obese group, the Shannon and Simpson indices showed no significant diversity differences. Moreover, Kruskal-Wallis tests highlighted significant dissimilarities in both unweighted and weighted UniFrac distances between the gut microbiota of normal-weight and obese children (P < 0.01). Prevotella and Firmicutes were more abundant in obese children, while Bacteroides and Sanguibacteroides were prevalent in normal-weight children, as evidenced by heatmap results. These findings suggest distinct microbial profiles associated with obesity in children, implicating the potential for targeted interventions to modulate gut microbiota composition and inform individualized treatment strategies for childhood obesity. Longitudinal monitoring of gut microbiota alongside BMI changes may offer insights into intervention effectiveness and guide adjustments to treatment plans over time. Although the study made some important findings in comparing the gut microbiota of obese and normal-weight children, there are several limitations. Firstly, the sample size of the study was relatively small, including only 30 obese children and 30 normal-weight children, which may limit the generalizability and statistical significance of the results. Secondly, the study only utilized 16S rDNA sequencing technology for microbial composition analysis, which may restrict the understanding of microbial functions and metabolic activities. Additionally, the study did not comprehensively control for children’s dietary habits, lifestyles, and environmental factors, which could influence the composition and abundance of the gut microbiota. Furthermore, the study did not explore the causal relationship between gut microbiota and childhood obesity, making it unclear whether changes in gut microbiota are the cause or the result of obesity. Therefore, future research needs larger sample sizes, more in-depth methods, and comprehensive controls to validate and expand these findings, thus enhancing our understanding of the relationship between childhood obesity and gut microbiota.
In summary, our study revealed the diversity and complexity of the gut microbiota in obese children. These microbial changes may affect energy metabolism, the immune system, and intestinal barrier function in obese children, providing new insights into the development of obesity and related metabolic diseases. However, further research is needed to elucidate the specific relationship between these changes and the pathological processes related to obesity, and whether they can serve as targets for intervention strategies.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology & hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Mazzola M, Italy; Saze Z, Japan S-Editor: Chen YL L-Editor: A P-Editor: Chen YX
| 1. | Sender R, Fuchs S, Milo R. Are We Really Vastly Outnumbered? Revisiting the Ratio of Bacterial to Host Cells in Humans. Cell. 2016;164:337-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1022] [Cited by in RCA: 1290] [Article Influence: 143.3] [Reference Citation Analysis (0)] |
| 2. | Bäckhed F, Manchester JK, Semenkovich CF, Gordon JI. Mechanisms underlying the resistance to diet-induced obesity in germ-free mice. Proc Natl Acad Sci U S A. 2007;104:979-984. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2086] [Cited by in RCA: 1876] [Article Influence: 104.2] [Reference Citation Analysis (0)] |
| 3. | Ridaura VK, Faith JJ, Rey FE, Cheng J, Duncan AE, Kau AL, Griffin NW, Lombard V, Henrissat B, Bain JR, Muehlbauer MJ, Ilkayeva O, Semenkovich CF, Funai K, Hayashi DK, Lyle BJ, Martini MC, Ursell LK, Clemente JC, Van Treuren W, Walters WA, Knight R, Newgard CB, Heath AC, Gordon JI. Gut microbiota from twins discordant for obesity modulate metabolism in mice. Science. 2013;341:1241214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2415] [Cited by in RCA: 2720] [Article Influence: 226.7] [Reference Citation Analysis (0)] |
| 4. | Blanton LV, Charbonneau MR, Salih T, Barratt MJ, Venkatesh S, Ilkaveya O, Subramanian S, Manary MJ, Trehan I, Jorgensen JM, Fan YM, Henrissat B, Leyn SA, Rodionov DA, Osterman AL, Maleta KM, Newgard CB, Ashorn P, Dewey KG, Gordon JI. Gut bacteria that prevent growth impairments transmitted by microbiota from malnourished children. Science. 2016;351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 420] [Cited by in RCA: 547] [Article Influence: 60.8] [Reference Citation Analysis (0)] |
| 5. | Yun Y, Kim HN, Kim SE, Heo SG, Chang Y, Ryu S, Shin H, Kim HL. Comparative analysis of gut microbiota associated with body mass index in a large Korean cohort. BMC Microbiol. 2017;17:151. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 132] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 6. | Sun L, Ma L, Ma Y, Zhang F, Zhao C, Nie Y. Insights into the role of gut microbiota in obesity: pathogenesis, mechanisms, and therapeutic perspectives. Protein Cell. 2018;9:397-403. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 134] [Cited by in RCA: 185] [Article Influence: 30.8] [Reference Citation Analysis (0)] |
| 7. | Marchesi JR, Adams DH, Fava F, Hermes GD, Hirschfield GM, Hold G, Quraishi MN, Kinross J, Smidt H, Tuohy KM, Thomas LV, Zoetendal EG, Hart A. The gut microbiota and host health: a new clinical frontier. Gut. 2016;65:330-339. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1341] [Cited by in RCA: 1556] [Article Influence: 172.9] [Reference Citation Analysis (0)] |
| 8. | Abdallah Ismail N, Ragab SH, Abd Elbaky A, Shoeib AR, Alhosary Y, Fekry D. Frequency of Firmicutes and Bacteroidetes in gut microbiota in obese and normal weight Egyptian children and adults. Arch Med Sci. 2011;7:501-507. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 167] [Cited by in RCA: 180] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 9. | Kalliomäki M, Collado MC, Salminen S, Isolauri E. Early differences in fecal microbiota composition in children may predict overweight. Am J Clin Nutr. 2008;87:534-538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 721] [Cited by in RCA: 716] [Article Influence: 42.1] [Reference Citation Analysis (0)] |
| 10. | Gao X, Jia R, Xie L, Kuang L, Feng L, Wan C. Obesity in school-aged children and its correlation with gut E.coli and Bifidobacteria: a case-control study. BMC Pediatr. 2015;15:64. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 54] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 11. | Song W, Wen R, Liu T, Zhou L, Wang G, Dai X, Shi L. Oat-based postbiotics ameliorate high-sucrose induced liver injury and colitis susceptibility by modulating fatty acids metabolism and gut microbiota. J Nutr Biochem. 2024;125:109553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 12. | Chen D, Yang Z, Chen X, Huang Y, Yin B, Guo F, Zhao H, Huang J, Wu Y, Gu R. Effect of Lactobacillus rhamnosus hsryfm 1301 on the Gut Microbiota and Lipid Metabolism in Rats Fed a High-Fat Diet. J Microbiol Biotechnol. 2015;25:687-695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 34] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 13. | de La Serre CB, Ellis CL, Lee J, Hartman AL, Rutledge JC, Raybould HE. Propensity to high-fat diet-induced obesity in rats is associated with changes in the gut microbiota and gut inflammation. Am J Physiol Gastrointest Liver Physiol. 2010;299:G440-G448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 628] [Cited by in RCA: 680] [Article Influence: 45.3] [Reference Citation Analysis (0)] |
| 14. | Gérard P. Gut microbiota and obesity. Cell Mol Life Sci. 2016;73:147-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 362] [Cited by in RCA: 343] [Article Influence: 38.1] [Reference Citation Analysis (0)] |
| 15. | Régnier M, Van Hul M, Knauf C, Cani PD. Gut microbiome, endocrine control of gut barrier function and metabolic diseases. J Endocrinol. 2021;248:R67-R82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 102] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 16. | Ding RX, Goh WR, Wu RN, Yue XQ, Luo X, Khine WWT, Wu JR, Lee YK. Revisit gut microbiota and its impact on human health and disease. J Food Drug Anal. 2019;27:623-631. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 111] [Cited by in RCA: 174] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 17. | Tomova A, Bukovsky I, Rembert E, Yonas W, Alwarith J, Barnard ND, Kahleova H. The Effects of Vegetarian and Vegan Diets on Gut Microbiota. Front Nutr. 2019;6:47. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 334] [Cited by in RCA: 399] [Article Influence: 66.5] [Reference Citation Analysis (0)] |
| 18. | Lee P, Yacyshyn BR, Yacyshyn MB. Gut microbiota and obesity: An opportunity to alter obesity through faecal microbiota transplant (FMT). Diabetes Obes Metab. 2019;21:479-490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 102] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 19. | Magne F, Gotteland M, Gauthier L, Zazueta A, Pesoa S, Navarrete P, Balamurugan R. The Firmicutes/Bacteroidetes Ratio: A Relevant Marker of Gut Dysbiosis in Obese Patients? Nutrients. 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 418] [Cited by in RCA: 1301] [Article Influence: 260.2] [Reference Citation Analysis (0)] |
| 20. | Porras D, Nistal E, Martínez-Flórez S, Pisonero-Vaquero S, Olcoz JL, Jover R, González-Gallego J, García-Mediavilla MV, Sánchez-Campos S. Protective effect of quercetin on high-fat diet-induced non-alcoholic fatty liver disease in mice is mediated by modulating intestinal microbiota imbalance and related gut-liver axis activation. Free Radic Biol Med. 2017;102:188-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 287] [Cited by in RCA: 374] [Article Influence: 46.8] [Reference Citation Analysis (0)] |
| 21. | Fujisaka S, Watanabe Y, Tobe K. The gut microbiome: a core regulator of metabolism. J Endocrinol. 2023;256. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 65] [Article Influence: 32.5] [Reference Citation Analysis (0)] |
| 22. | Zhao Q, Hou D, Fu Y, Xue Y, Guan X, Shen Q. Adzuki Bean Alleviates Obesity and Insulin Resistance Induced by a High-Fat Diet and Modulates Gut Microbiota in Mice. Nutrients. 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 67] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 23. | Lim S, Sohn M, Florez JC, Nauck MA, Ahn J. Effects of Initial Combinations of Gemigliptin Plus Metformin Compared with Glimepiride Plus Metformin on Gut Microbiota and Glucose Regulation in Obese Patients with Type 2 Diabetes: The INTESTINE Study. Nutrients. 2023;15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 24. | Feng L, Zhang W, Shen Q, Miao C, Chen L, Li Y, Gu X, Fan M, Ma Y, Wang H, Liu X, Zhang X. Bile acid metabolism dysregulation associates with cancer cachexia: roles of liver and gut microbiome. J Cachexia Sarcopenia Muscle. 2021;12:1553-1569. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 49] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 25. | Villanueva-Millan MJ, Leite G, Wang J, Morales W, Parodi G, Pimentel ML, Barlow GM, Mathur R, Rezaie A, Sanchez M, Ayyad S, Cohrs D, Chang C, Rashid M, Hosseini A, Fiorentino A, Weitsman S, Chuang B, Chang B, Pichetshote N, Pimentel M. Methanogens and Hydrogen Sulfide Producing Bacteria Guide Distinct Gut Microbe Profiles and Irritable Bowel Syndrome Subtypes. Am J Gastroenterol. 2022;117:2055-2066. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 67] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 26. | Amador-Lara F, Andrade-Villanueva JF, Vega-Magaña N, Peña-Rodríguez M, Alvarez-Zavala M, Sanchez-Reyes K, Toscano-Piña M, Peregrina-Lucano AA, Del Toro-Arreola S, González-Hernández LA, Bueno-Topete MR. Gut microbiota from Mexican patients with metabolic syndrome and HIV infection: An inflammatory profile. J Appl Microbiol. 2022;132:3839-3852. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 27. | Koutoukidis DA, Jebb SA, Zimmerman M, Otunla A, Henry JA, Ferrey A, Schofield E, Kinton J, Aveyard P, Marchesi JR. The association of weight loss with changes in the gut microbiota diversity, composition, and intestinal permeability: a systematic review and meta-analysis. Gut Microbes. 2022;14:2020068. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 69] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 28. | Grech A, Collins CE, Holmes A, Lal R, Duncanson K, Taylor R, Gordon A. Maternal exposures and the infant gut microbiome: a systematic review with meta-analysis. Gut Microbes. 2021;13:1-30. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 102] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 29. | Cho I, Yamanishi S, Cox L, Methé BA, Zavadil J, Li K, Gao Z, Mahana D, Raju K, Teitler I, Li H, Alekseyenko AV, Blaser MJ. Antibiotics in early life alter the murine colonic microbiome and adiposity. Nature. 2012;488:621-626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1092] [Cited by in RCA: 1209] [Article Influence: 93.0] [Reference Citation Analysis (0)] |
| 30. | Willers M, Ulas T, Völlger L, Vogl T, Heinemann AS, Pirr S, Pagel J, Fehlhaber B, Halle O, Schöning J, Schreek S, Löber U, Essex M, Hombach P, Graspeuntner S, Basic M, Bleich A, Cloppenborg-Schmidt K, Künzel S, Jonigk D, Rupp J, Hansen G, Förster R, Baines JF, Härtel C, Schultze JL, Forslund SK, Roth J, Viemann D. S100A8 and S100A9 Are Important for Postnatal Development of Gut Microbiota and Immune System in Mice and Infants. Gastroenterology. 2020;159:2130-2145.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 84] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 31. | Goffredo M, Mass K, Parks EJ, Wagner DA, McClure EA, Graf J, Savoye M, Pierpont B, Cline G, Santoro N. Role of Gut Microbiota and Short Chain Fatty Acids in Modulating Energy Harvest and Fat Partitioning in Youth. J Clin Endocrinol Metab. 2016;101:4367-4376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 118] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 32. | Orsso CE, Peng Y, Deehan EC, Tan Q, Field CJ, Madsen KL, Walter J, Prado CM, Tun HM, Haqq AM. Composition and Functions of the Gut Microbiome in Pediatric Obesity: Relationships with Markers of Insulin Resistance. Microorganisms. 2021;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |