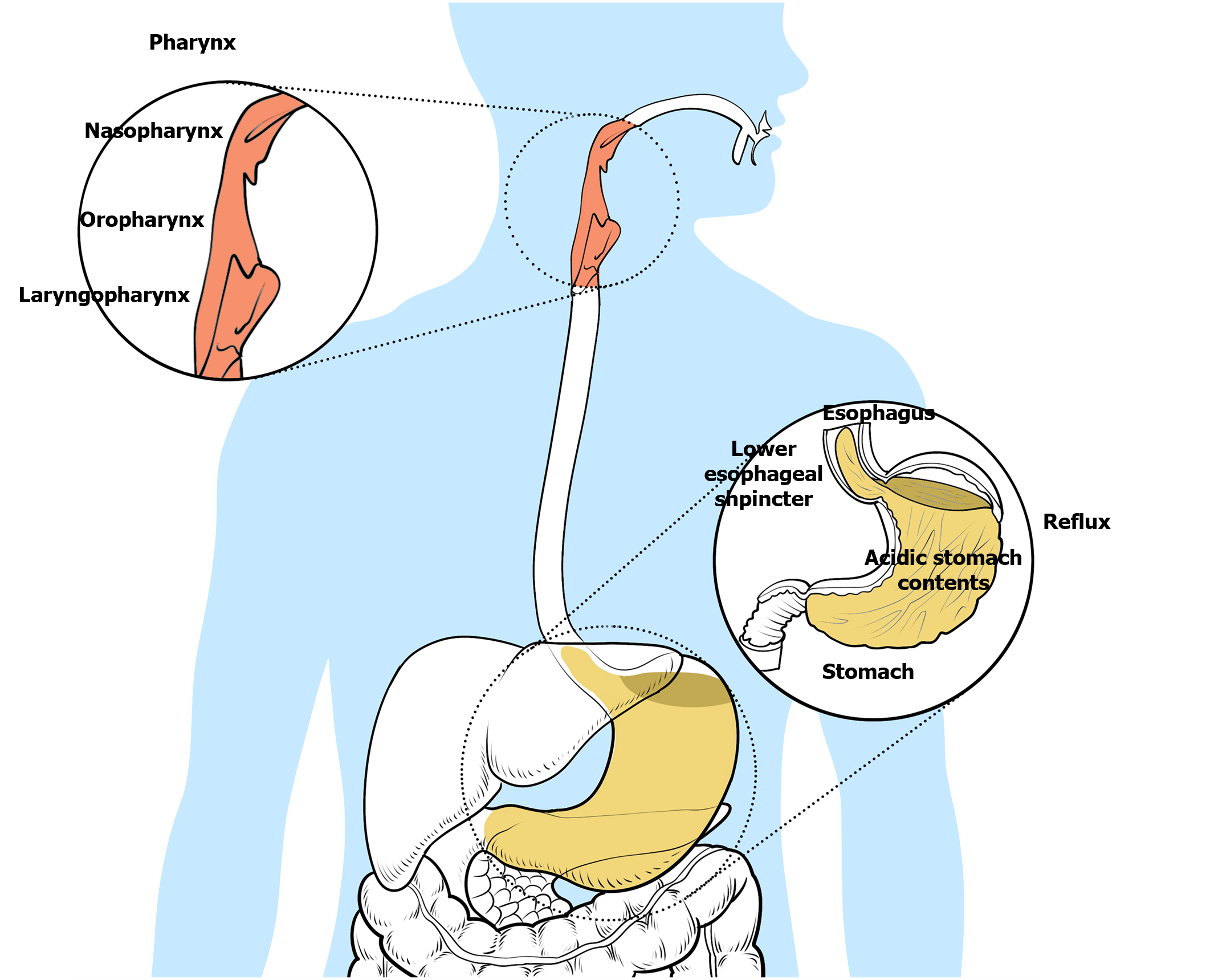
INTRODUCTION
Laryngopharyngeal reflux disease (LPRD) is an inflammatory disease caused by gastroduodenal contents regurgitating into the pharynx, stimulating and damaging the pharyngeal mucosa, and remains one of the most complex and socially relevant problems in modern medicine[1]. Reflux of stomach contents into the upper aerodigestive tract causes many clinical symptoms, including hoarseness, cough, sore throat, a feeling of throat obstruction, and excessive throat mucus. The stomach contents usually include gastric acid, nonacid substances, bile and pepsin. Reflux of the upper respiratory tract mainly involves the pharynx, larynx and nasal cavity. The mucosal changes of the pharynx and larynx mainly include interarytenoid mucosal hyperemia and edema, posterior commissure hyperplasia and vocal cord edema. More serious conditions include ulcers, granulomas and laryngeal compartment disappearance, although these are rare. LPRD has become a societal burden in recent years[2]. It has been found that 15% of otorhinolaryngology outpatient patients have laryngeal reflux. In one study, 50% of patients with laryngeal and voice disorders were diagnosed with LPRD through 24-h dual-probe pH monitoring.
However, because of the variety of symptoms and signs, the current limited diagnostic methods and the lack sensitivity or specificity, LPRD is sometimes treated empirically and without a correct diagnosis. At the same time, its pathophysiological mechanism has not been clarified. In the reflux theory, factors that cause LPRD include inhaling pepsin, trace amounts of stomach and bile acids, which can damage the throat and cause inflammation. The resistance of the laryngeal forces to these substances is weak, so some patients may not respond well to proton pump inhibitors (PPIs) alone. In addition, in reflex theory, acidic stomach contents are postulated to stimulate the vagus nerve at the distal end of the esophagus to induce laryngeal. Therefore, different pathological mechanisms lead to differences in the clinical manifestations of LPRD, and there are also differences in treatment strategies. Recent studies have found that the disease is closely related to other common diseases, such as sinusitis, otitis media, asthma and laryngeal cancer[3]. Although LPRD has gradually developed in recent years, its prevalence has become more evident in this decade. Patients with LPRD who experience severe clinical manifestations can have increased physiological and psychological burden, and impaired quality of life and emotional state. LPRD is considered an extraesophageal manifestation of gastroesophageal reflux disease (GERD). The present article reviews the characteristics, pathophysiology, diagnostic work-up, and new therapeutic strategies for LPRD, and investigates the association between LPRD and GERD.
PATHOPHYSIOLOGY OF LPRD
In 2002, the American Academy of Otolaryngology Head and Neck Surgery first proposed the concept of LPRD[4]. Currently, the definition of LPRD is still controversial and there is disagreement between specialists about its pathogenesis. Many researchers believe that LPRD is a form of extraesophageal symptoms of GERD, and its pathogenesis is roughly similar to GERD[5]. But, otolaryngologists consider LPRD to be an independent disorder. It is widely believed that the pathogenesis of LPRD includes reflux theory, reflex theory, behavioral changes and psychological factors.
Reflux theory
Reflux refers to the backflow of stomach contents to the pharynx and larynx, causing direct damage to mucosal tissue. Under physiological conditions, the body has an antireflux mechanism, including the upper esophageal sphincter (UES), lower esophageal sphincter, diaphragmic foot, esophageal peristalsis associated with swallowing, and acid resistance and clearance ability of the esophageal mucosa[6]. The antireflux mechanism prevents gastric contents from regurgitating into the pharynx and directly damaging the pharyngeal mucosal tissue. However, under pathogenic conditions, the antireflux mechanisms can be damaged individually or simultaneously, causing the gastric contents to regurgitate to the laryngopharyngeal mucosa. Studies have shown that the esophageal mucosa can resist 50 potential episodes of reflux per day without causing tissue damage[7]. However, the laryngopharyngeal mucosa can be damaged by four reflux episodes per day. This indicates that the laryngopharyngeal mucosa is more fragile and more sensitive to stimulation. At the same time, the gastric contents of reflux are complex, including hydrochloric acid, pepsin, bile and trypsin. These different substances cause the symptoms and pathogenesis of laryngopharyngeal injury. This is why, although PPIs are the leading choice for drug treatment of LPRD, up to 40% of patients with LPRD do not obtain relief[8]. The effect of PPIs on non-acidic LPRD is not satisfactory. Therefore, the development of drugs targeting non-acidic LPRD is very urgent. A survey showed that the prevalence of non-acidic and mixed LPRD reached 25.4% and 35.5%, respectively[9]. Considering the complexity of LPRD, it is necessary to study the action mechanism of different reflux substances on LPRD. The main factors affecting the severity of LPRD include the composition, duration and frequency of regurgitation.
Reflex theory
The pharynx is an essential organ in humans that connects the mouth with the respiratory and digestive systems. It is important for both breathing and swallowing. Because the esophageal and bronchial trees have the same embryonic origin, there is a common vagal reflex pathway between them. In the physiological state, if the nerve receptors in the pharynx are stimulated by acidic substances, it can cause bronchospasm, accumulation of sticky mucus, involuntary swallowing, glottal closure reflex and cough reflex, through nerve reflexes[10]. Once LPRD occurs, patients often cough and clear their throat in order to relieve throat discomfort. The above actions further aggravate throat mucosal edema and damage, resulting in throat sensory disorders, and then enter a vicious cycle of chronic persistent cough and throat clearing.
Connection between LPRD and related respiratory diseases
LPRD is an inflammatory reaction that occurs in the mucosa of respiratory organs such as larynx and pharynx. Reflux of stomach contents outside the esophagus may cause diseases of the upper and lower airways. Current studies[11-13] have shown that LPRD is associated with a variety of upper and lower airway diseases, such as chronic pharyngitis, chronic laryngitis, laryngeal contact granuloma, paroxysmal laryngeal spasm, space edema, vocal cord leukoplasia, glottic laryngeal cancer, chronic cough, asthma, pediatric subglottic stenosis, secreted otitis media, sinusitis and sleep apnea hypopnea syndrome.
LPRD and sinusitis
There is a relationship between LPRD and chronic recurrent sinusitis (CRS). Although CRS and LPRD have different courses, they can coexist in the same patient. In case-control studies, patients with LPRD were found to be at risk of developing sinusitis one year after diagnosis. DelGaudio et al[14] found that patients with refractory CRS had a higher incidence of LPRD than control patients had. The pathogenesis of CRS is still unclear, but most scholars believe that it is related to mucosal injury caused by direct stimulation, pepsin action and autonomic hyper-responsiveness caused by reflux. The above factors all cause edema and retention of secretions in the mucosa of the nasal cavity and sinuses, and then secondary infection[15]. LPRD can cause sneezing, runny nose, nasal congestion and a series of similar allergic rhinitis symptoms of throat reflux rhinitis.
LPRD and vocal fold polyps
Recent studies have shown that laryngeal reflux is associated with vocal cord polyps, but whether it is an independent risk factor is unclear. Kantas et al[16] found that antipharyngeal reflux played an important role in preventing the incidence of vocal cord polyps and reducing the recurrence rate of vocal cord polyps, and proposed that pharyngeal reflux was related to the damage of vocal cord regeneration. At present, research on laryngeal reflux and vocal cord polyps is limited, and the specific mechanism of laryngeal reflux causing vocal cord polyps is still unclear. The epithelium is the first barrier of the vocal cords for resistance to foreign irritants, and studies have shown that laryngeal epithelial injury caused by laryngeal reflux is the main pathogenesis[17]. First, gastric acid, pepsin, trypsin, bile salt and gas-troduodenal protein directly stimulate the throat mucosa, but the larynx lacks the defense mechanism against gastric and duodenal reflux. Second, the reflux stimulates the distal mucosal chemoreceptors of the esophagus to cause vagal nerve reflex, which leads to indirect laryngeal injury such as coughing and throat clearing, but the mechanism is still controversial. Some studies have located pepsin in vocal cord polyps, and speculated that the mechanism of laryngeal reflux participating in the pathogenesis of vocal cord polyps may be that pepsin promotes the aggregation of immune cells and leads to the increase of local cytokines. Bile acids disrupt the epithelial barrier function of the esophagus by regulating the expression of claudin (CLDN)1 and CLDN4 and increasing the gap between epithelial cells[18]. However, the expression of CLDN1 and CLDN4 in vocal cord polyp tissue is increased, and the reflux bile acids may destroy the epithelial barrier of the vocal cords and induce the occurrence of vocal cord polyps.
LPRD and asthma
In 40%-80% of patients with asthma, laryngoscopy also found LPRD[19]. There is a growing belief that sinusitis and asthma can co-occur, but often asthma symptoms caused by LPRD are overlooked. Therefore, with the exception of asthma and/or allergic rhinitis, pH probe detection is recommended for the diagnosis of LPRD patients. In addition, the use of beta agonists has become possible. Laryngeal reflux may be induced by reducing the contractile force of the lower esophageal sphincter (LES)[20]. Studies on this have been inconsistent. Some studies suggest that severe asthma may aggravate LPRD, but the incidence of LPRD is similar in children with asthma. Based on the current level of research, the feasibility of simultaneous treatment needs to be considered in the future for the reality that LPRD and asthma often occur together.
Relationship between LPRD and stomach contents
Different substances of reflux have different pathological mechanisms. Compared nonacidic and mixed regurgitation of LPRD, the patients with mixed regurgitation of LPRD were more severe cough then nonacidic regurgitation[21].
Hydrochloric acid
Hydrochloric acid is the main determinant of reflux symptoms. Acid in gastric juices regurgitate and contact the laryngeal tissues, causing damage and inflammation to the epithelium of the laryngeal mucosa. In general, the throat mucosa is more sensitive to acid stimulation than the esophagus. Small amounts of acid may also cause damage to the throat mucosa. Carbononic anhydrase III (CA III) secretes bicarbonate, regulates pH, and neutralizes stomach acid. In one study, it was found that some LPRD patients lacked CA III in the throat tissue, stomach acid could not be neutralized, and pH value was unbalanced[22]. E-cadherin is a transmembrane glycoprotein, which affects the intercellular adhesion of epithelial tissue and forms an anti-permeability barrier to prevent the penetration of solutes[23]. Hydrochloric acid can down-regulate the expression of E-cadherin, improve the intercellular permeability and weaken the barrier function of throat mucosa.
Pepsin
The abnormal secretion and activation of pepsin may play an important role in the pathogenesis of LPRD. Pepsin, which is obtained by the conversion of pepsinogen, is the main factor that causes cell damage and protein hydrolysis. Normally, pepsin is not detected in the mucosa of the larynx in normal people. When gastric contents regurgitate into the throat, pepsin enters the throat, and at different pH environments, the signaling pathways that disrupt the integrity of the epithelial barrier include E-cadherin, CA III, nuclear factor (NF)-κB and interleukin (IL)-8[24]. It causes mucosal damage in the throat and induces inflammation. Roh et al[25] found that under acidic conditions (pH 1-2), pepsin and bile acids had more serious damage to subglottic tissue. In addition to damaging the laryngeal mucosa, pepsin may also cause chronic inflammation of surrounding tissues such as vocal cord polyps, tonsil hypertrophy, otitis media, and laryngeal tumors.
Bile acid
Bile acid reflux is the main cause of laryngeal injury. Bile acids are normally secreted by the liver to maintain fat digestion and absorption, regulate inflammation, and affect intestinal flora. The bile acid will be protonated under the action of hydrochloric acid, enhancing their cytotoxic effects and can penetrate and dissolve cell membranes. The main mechanism of bile acid induced throat mucosal injury is as follows[26]. Bile acid induces cell epithelial–mesenchymal transformation (EMT) and induces transforming growth factor-β1 (TGF-β1), matric metalloproteinase (MMP)-9 and fibronectin to increase. It reduces expression of E-cadherin, leading to laryngotracheal scar formation and tracheal stenosis. Bile acids can cause NF-κB activation, DNA/RNA damage, and induce abnormal expression of tumor factors[27].
Trypsin
The laryngeal mucosa is damaged by trypsin reflux[28]. Trypsin is secreted by pancreatic cells mainly in the form of proenzyme. And zymogen acts as the main activator of protease activating receptor-2 (PAR-2). PAR-2 affects the functionality of LES. LES dysfunction is considered to be the main factor inducing LPRD. Trypsin activates PAR-2, induces IL-8 and transient receptor potential vanilloid (TRPV) secretion, and causes epithelial barrier dysfunction in the throat[29].
Taken together, the pathogenesis of LPRD is caused by a variety of factors, including bile acids, pepsin, acids and trypsin, as shown in Figure 1. At present, the mechanism of the interaction of various reflux substances is not clear.
Figure 1 Pathogenesis of laryngopharyngeal reflux.
Laryngopharyngeal reflux disease is an inflammatory disease of the upper aerodigestive tract caused by reflux of gastroduodenal content. The stomach contents usually include gastric acid, nonacid substances, bile and pepsin. Reflux of the upper respiratory tract mainly involves the pharynx, larynx and nasal cavity.
CLINICAL FEATURES AND DIAGNOSIS OF LPRD
Clinical features of LPRD
LPR lacks specific symptoms and signs, which can be manifested as sore throat globus pharyngeus, chronic throat clearing, and dysphonia[30]. The initial stages of LPR are characterized by hoarseness, globus pharyngeus, excessive mucus in the throat, chronic cough and persistent throat clearing. If LPRD is not treated in time, it will be complicated with laryngeal granuloma and vocal cord polyps. It can develop from GERD, along with the typical symptoms of GERD. GER may also be absent and present only with throat discomfort. Different from the characteristics of patients with esophageal reflux, LPRD mostly occurs when patients remain upright or during the day, while esophageal peristalsis and gastric acid clearance was within the normal range. However, regurgitation in GERD patients is usually in the supine position. At night, it is often accompanied by esophageal peristalsis disorder and prolonged exposure to gastric acid. The two have different clinical characteristics.
Diagnosis of LPRD
LPRD is a common disease with a vast number of clinical symptoms that are sometimes treated empirically and without a correct diagnosis. There is disagreement in the diagnosis of LPRD between specialists in different area about its definition. The specificity of the diagnostic methods reported in the literature is not ideal, which faces great challenges[31]. Currently, diagnostic methods commonly used include medical history, physical examination, fibrolaryngoscopy, 24-h pH monitoring, 24-h multichannel intraluminal impedance (MII), esophageal manometry, biomolecular marker detection, pepsin detection nuclide scanning, esophagography, reflux scale scoring and experimental treatment. The 24-h MII can identify gas, liquid, or a mixture of both, and can detect acid reflux and non-acid reflux, so it is recommended as the preferred method for the diagnosis of LPRD. However, 24-h MII is expensive, limiting its popularity in the clinic. Pepsin, bile acid, and MMP are easy to detect. However in different research centers, their detection standards, sensitivity and specificity are different. Therefore, they are still not suitable for clinical diagnosis of LPRD. The pepsin-positive threshold and the timing of saliva specimen collection are inconclusive. Na et al[32] suggested that the best time to collect saliva is when LPRD patients have just woken. De Corso et al[33] believed that bile acid was the most suitable for the diagnosis of LPRD, and its sensitivity and positive predictive value both exceeded 80%. Salivary bile acid is one of the indexes to evaluate LPRD grade[34]. Hoppo et al[35] believed that Sep70 was a predictive indicator, and the absence of Sep70 meant that hypopharyngeal cells were damaged. In addition, the Sep70/pepsin ratio can be used to predict LPRD damage with a sensitivity of over 90%. The disadvantage is that the specificity is low, and further clinical research is needed. Moreover, the sensitivity and specificity of MMP-7 as a marker were found to be 71.43% and 79.75%[36], respectively. When MMP-7 is combined with pepsin, its sensitivity and specificity exceed 80%.
24-h pH monitoring
Twenty-four-hour pH monitoring of the hypopharynx and esophagus is the gold standard for the diagnosis of LPRD[37]. A catheter with a pH monitoring probe is placed at the lower end of the esophagus through the patient’s nose and oropharynx, and the catheter is fixed to monitor reflux and removed on the next day. It is a currently accepted form of diagnosis and has become an acceptable method for most researchers to significantly improve patient compliance. The greatest advantage of this method is that intermittent reflux can be recorded by daily measurement of patients, and the pH change in the esophagus can be objectively recorded in the physiological state, so as to determine whether there is reflux, and to distinguish physiological and pathological reflux. The hypopharyngeal–esophageal multichannel intraluminal impedance catheter with dual pH (HEMII-pH) has been used to monitor hypopharyngeal reflux in patients with LPR. HEMII-pH monitoring can differentiate LPR and GERD. There is no universal definition of pH for pharyngeal acid reflux. Some analysts believe that pH 5 can be judged as the defining diagnostic value. pH 5 indicates damaged laryngeal epithelium, and pH < 4 damaged cells in the esophageal epithelium. pH < 4 is considered to be highly sensitive and specific.
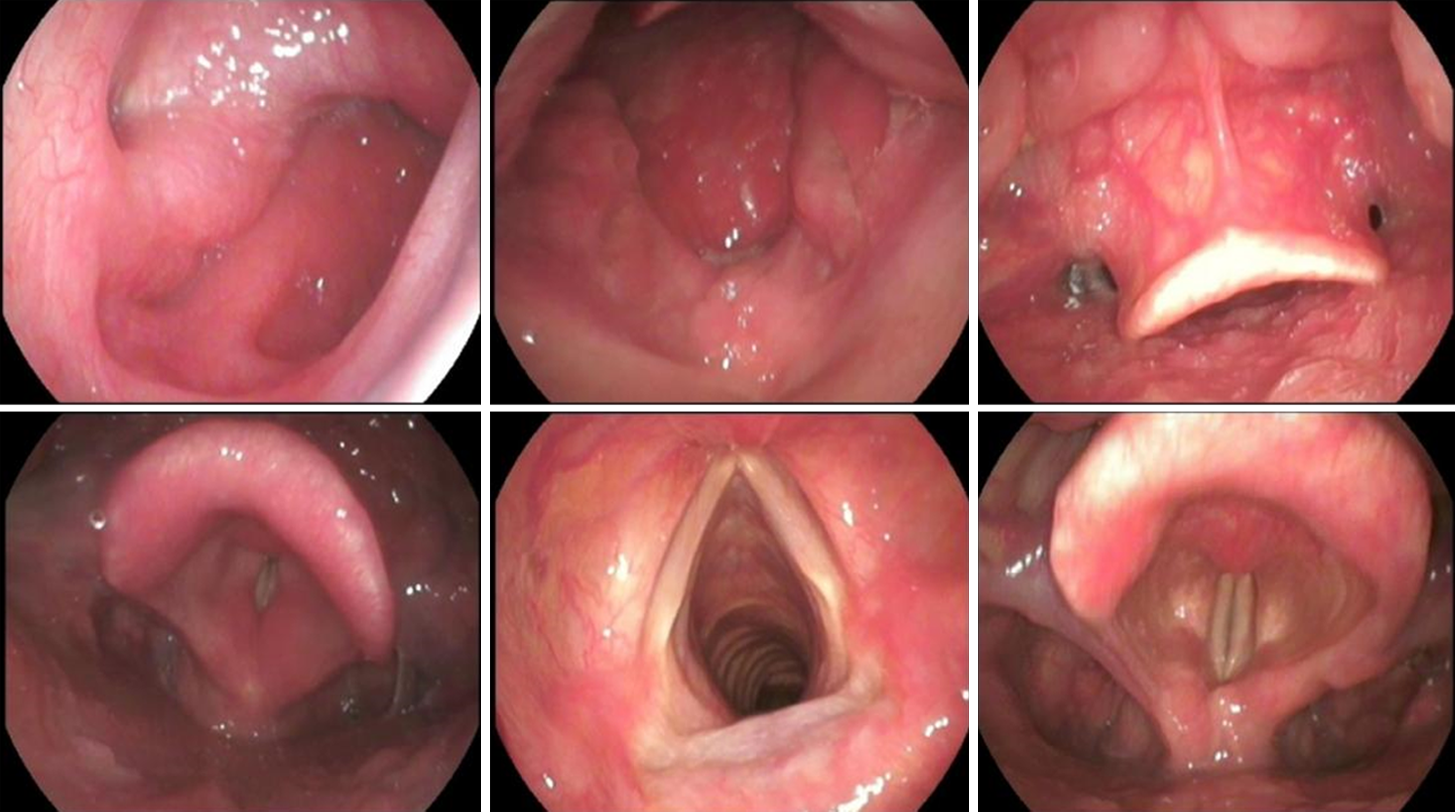
Fibrolaryngoscopy
Currently, fibrolaryngoscopy is the most commonly used method of examination (Figure 2). Fibrolaryngoscopy can also rule out other nasal and throat lesions such as laryngeal cancer, allergic rhinitis or sinusitis. Fibrolaryngoscopy has diagnostic value for LPRD. Endoscopic findings include subglottic edema, diffuse laryngeal edema, laryngeal ventricle disappearance, granuloma, contact ulcer and vocal cord lesion. Belafsky et al[38] proposed reflux discovery score (RFS) and reflux Symptom Index (RSI) for screening LPRD. The RSI scale includes nine symptoms. It is rated on a scale of 0–5 depending on the severity. The RFS scale is a score given by clinicians according to the characteristics of electronic laryngoscopy images. In LPRD patients, due to long-term repeated stimulation of reflux, the throat mucosal tissue is damaged, and various characteristics such as mucosal erythema, edema and posterior connective hyperplasia are shown by laryngoscopy. It is proved that RSI/RFS scale has high reliability and good clinical practical value. LPRD can be diagnosed by RSI > 13 and/or RFS > 7 points[39].
Figure 2 Fibrolaryngoscopy diagnostic value for laryngopharyngeal reflux disease.
Fibrolaryngoscopy showed granulations of the posterior wall of the nasopharynx and edema of the retrocricoid region, epiglottis erythema, and posterior commissure hypertrophy, and laryngeal erythema.
TREATMENT OF LPR
Routine intervention – behavioral adjustment of life and diet
Lifestyle behavior and dietary habits of patients can be adjusted to maximize improvement of LPR symptoms[40]. Patients who followed diet and lifestyle recommendations had significantly greater improvements in RSI compared with patients who did not. In a retrospective study, researchers found that patients with LPRD who took anti-reflux drugs and took behavioral change measures improved their RSI scores significantly (mean 32 d of first follow-up), while the RSI scores of patients in the control group taking only antireflux drugs (mean 62 d of first follow-up) did not improve significantly. Recommended lifestyle habits include: wearing loose clothing on a daily basis; standing as upright as possible for 30 min after eating; not eating or drinking 2–3 h before bedtime; chewing gum to increase saliva secretion; controlling blood pressure; quitting smoking; eating slowly; avoiding talking while eating; avoiding drugs such as aspirin, progesterone, corticosteroids, and nonsteroidal anti-inflammatory drugs and fried foods and fatty animal products such as chicken/fish and meat.
Drug treatment
There is no reliable treatment for LPRD because its pathogenesis is not clear. To study the etiology of LPRD, it is necessary to consider the influence of self and environmental factors[3]. The acid-suppressing PPIs are currently the main drug used for the treatment of LPRD and are suitable for LPRD patients with GERD symptoms. PPIs combined with gastroenterokinetic drugs is the most common clinical treatment for LPRD, and they are recommended for at least 8 wk.
PPIs can inhibit the secretion of gastric acid, down-regulate the activity of pepsin, damage the throat mucosa, and finally achieve the treatment of lesions and alleviate symptoms. Some analysts believe that PPIs twice daily are the best choice to treat this disease[41]. Therefore, the study of pathogenesis of LPRD has become the research focus at home and abroad.
A recent multicenter study found that patients with different phenotypes, such as no LPRD or GERD, LPRD/GERD with hiatic hernia, reflex cough, LPRD with mild GERD, etc., were most responsive to PPIs[42]. This was followed by LPRD and LPRD with mild reflux and reflux cough. Therefore, it is speculated that classification based on phenotype may be more conducive to matching patients and corresponding treatment methods. Esophagopharyngeal reflux may not be entirely acidic. Previous research has suggested that acid reducers may not be effective for patients with nonacid reflux events. A mixed response to PPIs depends on their underlying complicating disease pathology. Despite the failure of empiric PPI treatment, 24-h MI-pH testing is still considered necessary. If significant acid reflux occurs, the PPI regimen needs to be optimized, for example, by increasing the dose, extending the duration of treatment and adjusting the time of administration. Patients with acid reflux who do not respond to PPIs may try switching to potassium-competitive acid blockers[43].
H2 receptor antagonists are commonly used in the treatment of LPRD. Due to the short duration of action of the drug, the acid inhibition efficacy is lower, and its status is slightly lower than that of PPIs[44]. The study found that 51 percent and 54 percent of anti-reflux prod patients who took 20 mg of omeprazole and 20mg of famotidine at night experienced relief from symptoms, respectively. Eighty-three percent of patients improved after taking 20mg of omeprazole at night.
Alginate is an oral drug. It prevents reflux of gastric acid or non-acid substances by forming a viscous mechanical barrier on the surface of the stomach contents, ultimately reducing the contact of the stomach contents with the eso-phagus or pharynx[45]. It works regardless of whether the reflux is acidic or nonacidic. In addition, alginate inhibits pepsin and bile salts. Alginate also improves symptoms in patients with LPRD. In a randomized controlled trial, patients treated with alginate showed significant improvement in symptoms at 2, 4 and 6 months after treatment.
Baclofen can inhibit LES relaxation and prevent acidic and non-acidic reflux[46]. In one study, Baclofen was found to significantly reduce the duration of reflux, the incidence of GER, and the incidence of LES relaxation compared to placebo.
Considering the different mechanisms of action of reflux substances in LPRD, a variety of receptor antagonists and enzyme inhibitors have emerged as new inhibitors, such as trypsin inhibitors, protease activating receptor 2 (PAR-2) antagonists, pepsin inhibitors or receptor antagonists, NF-κB antagonists, TRPV1 antagonists and MMP inhibitors. Hossain thinks PAR-2 may play an important role in unresolved heartburn symptoms after PPI treatment. TRPV1 and PAR-2 antagonists have the potential to be targeted agents for ameliorating LPRD-induced heartburn and pain[47]. Yoshida et al[48] found that MEK inhibitors and p38 inhibitors reduce IL-6 or IL-8 secretion through MAPK signaling pathway, thereby reducing esophageal inflammation and achieving the treatment of LPRD.
Surgical intervention
Experts suggest that surgery may be considered for patients with refractory extraesophageal symptoms treated with medication in the latest American Gastroenterological Association guidelines[49].
In a retrospective controlled trial, Swoger et al[50] evaluated the difference between surgical treatment and PPI. They found significant differences in fundus dilation. Fundus dilation is associated with improved RSI scores and is expected to be an effective treatment for patients with LPR. However, how to judge the effect of surgery and accurately select the right patients for surgical treatment is a difficult problem, worthy of further study. It would be irresponsible to recommend that every LPR patient undergo major abdominal surgery. Previous studies often assessed the effect of treatment according to the subjective judgment of patients, which has great subjectivity and individual differences. Therefore, when selecting and evaluating the effect of treatment, patient-reported results must be given priority in a patient-centered approach, and reflux and symptoms must be evaluated in detail in combination with pH monitoring and RSI score. Although fundoplication is more effective than PPI, it is more risky. The most common complaint is dysphagia. In one study[51], all patients experienced dysphagia after surgery. These patients may have dysphagia in the initial postoperative period, but it resolves spontaneously after 2 wk, and 13 patients (4.53%) reported prolonged dysphagia after surgery. And the second most common complaint was postoperative gas/abdominal distension. One study[52] reported that abdominal distension occurred in all 12 patients during the first 2 wk. Sahin et al[53] found that postoperative complications mainly included emphysema (10.8%), intraoperative hemorrhage (4.4%), pleural displacement (2.9%), etc. 2.4% patients needed a second operation due to postoperative complications, and 0.4% patients needed a second operation due to surgical failure.
For granulomas in the laryngeal cavity that are large and may affect normal breathing, surgical treatment is required and antacid therapy can be performed after surgery. A number of recent data have confirmed that esophageal diseases such as hiatal hernia can lead to an increase in the incidence of LRPD and refractory extraesophageal symptoms. The main symptoms of primary esophageal diseases are significantly improved after receiving standard laparoscopic surgery. The fundoplication is achieved by reconstructing the gastroesophageal junction area and re-establishing the barrier function[54]. Currently, fundus folding has relatively obvious value. Overall, endoscopic and surgical interventions are considered as the last line of care for patients with LPRD, and only some patients should be considered for surgical or endoscopic interventions.
Nonsurgical treatment
The UES external pressure device is a new treatment for LPRD[55]. Reflux Band (Somna Therapeutics, Germantown, WI, United States) has received United States Food and Drug Administration approval. It has been reported that patients with typical reflux symptoms and supresophageal symptoms have impaired esophageal and UES responses that mimic reflux and are therefore at greater risk for esophagopharyngeal reflux. After wearing an external UES compression device at night for 2–4 wk, RSI scores improved significantly.
Speech therapy
In addition to medication, speech therapy is proven to be effective and is recommended by CHEST guidelines[56]. The treatment is achieved through speech and breathing training. In one study[57], it was found that compared with a control group, patients who received 6 months of PPI therapy and 3-5 sessions of breathing therapy and after treatment with speech therapy and guidance on a healthy lifestyle such as relaxation, exercise, diet, and stress management, patients showed significant improvement in upper respiratory tract breathing and cough. The results suggest that speech therapy may potentially improve laryngeal allergy symptoms in patients with chronic cough. It was found that 100% of patients experienced an improvement in their cough symptoms. Further research in this field is still needed in the future.
Behavioral therapy
In cognitive behavioral therapy[58], psychiatrists use a range of behavioral therapies, including stress management, cognitive reconstruction, coping strategies, problem solving, and anxiety management, to improve patients' throat symptoms, which have been shown to be a safe and effective treatment option. It was reported that hypnotherapy was used to treat] patients with allergic laryngeal symptoms and foreign body sensation in the pharynx[59,60]. The patients experienced a significant reduction in the severity and symptoms of throat discomfort after relaxation breathing therapy, which involves adjusting breathing and relaxing muscles, and esophageal-oriented hypnotic-assisted relaxation therapy. In another study, nine patients with functional heartburn who received esophageal directed hypnotherapy seven times a week experienced significant improvements in heartburn symptoms, visceral anxiety, and quality of life. In a recent study, it was found that, based on the available evidence, hypnotherapy for patients presenting with dysphagia, foreign body sensation, indigestion, and functional heartburn has been comprehensively studied in patients with bowel disease as a form of cognitive behavioral therapy (CBT). Future research is needed in behavioral therapy for laryngeal hypersensitivity and laryngeal dysfunction.
ASSOCIATION BETWEEN LPRD AND GERD
LPRD is considered to be a substantially part of the extraesophageal manifestations of GERD. Koufman[61] first reported an important epidemiological study on LPRD in 1991. In that study, LPR was initially differentiated from GERD based on dual-probe pH monitoring in the esophagus and pharynx. Many existing studies have shown that the prevalence of LPRD is generally higher in GERD patients[62]. Clinical symptoms of GERD include regurgitation and heartburn, resulting from esophageal mucosal disruption caused by gastric content reflux. However, some patients with GERD have no symptoms of LPRD. According to the results of pH monitoring, the incidence of GERD in LPRD was 52.7%, while the incidence of LPRD in GERD was 46.3%[42]. Otolaryngologists believe that LPRD is an independent, but common in their practice, upper airway disorder. GER and LPR have similar pathological mechanisms, but their clinical manifestations and 24-h double pH-probe monitoring are different. Patients with GERD have a lower proportion of nonacid and mixed LPRD compared with LPRD/GERD patients. The recent use of HEMII-pH monitoring gave evidence that physiology may differ between LPRD and GERD. LPRD is induced by daytime and upright gaseous weakly or nonacid hypopharyngeal reflux. A series of symptoms beyond the esophagus may be caused by nonacid content such as bile, pepsin and trypsin l[6]. However, the presence of GERD is strongly associated with acid reflux in the esophagus. However, due to the reflux of gastric contents, the treatment plan with acid suppression with PPIs is similar. Helicobacter pylori (H. pylori) is found in many sites, including laryngeal mucosa and interarytenoid region[63]. For cases complicated with H. pylori infection, this should be considered as a cause of LPRD.
PROSPECT
For LPRD caused by different regurgitation substances, 24-h MII-pH test, salivary pepsin and bile acid test are usually performed clinically. On the one hand, it can determine the cause of the disease, on the other hand, it can determine the cause of the disease, record the therapeutic effect of the drug, and then provide the best treatment plan for the patient. For patients with refractory LPRD, multidisciplinary evaluation is required in conjunction with otolaryngologists and gastroenterologists. The efficacy of PPIs in some patients is not satisfactory, indicating that the pathogenesis of non-acidic components in throat mucosal injury needs further study. Furthermore, the reliability of biomarkers such as pepsin and bile acids for the diagnosis and prognosis of LPRD needs to be further evaluated. At the same time, more clinical prospective studies are needed to evaluate the selection of laparoscopic surgical treatment methods and indications in order to provide more effective treatment strategies for the patients.