Published online Apr 21, 2024. doi: 10.3748/wjg.v30.i15.2118
Peer-review started: January 18, 2024
First decision: February 9, 2024
Revised: February 19, 2024
Accepted: March 27, 2024
Article in press: March 27, 2024
Published online: April 21, 2024
Processing time: 91 Days and 23.8 Hours
During emergency endoscopic retrograde cholangiopancreatography (ERCP), the safety and feasibility of performing one-stage endoscopic treatment for patients with acute cholangitis (AC) due to choledocholithiasis are unclear.
To investigate the safety and feasibility of one-stage endoscopic treatment for moderate to severe AC.
We enrolled all patients diagnosed with moderate to severe cholangitis due to common bile duct stones from January 2019 to July 2023. The outcomes were compared in this study between patients who underwent ERCP within 24 h and those who underwent ERCP 24 h later, employing a propensity score (PS) frame
In total, we included 254 patients and categorized them into two groups based on the time elapsed between admission and intervention: The urgent group (≤ 24 h, n = 102) and the elective group (> 24 h, n = 152). Ninety-three pairs of patients with similar characteristics were selected by PS matching. The urgent ERCP group had more ICU admissions (34.4% vs 21.5%, P = 0.05), shorter ICU stays (3 d vs 9 d, P < 0.001), fewer antibiotic use (6 d vs 9 d, P < 0.001), and shorter hospital stays (9 d vs 18.5 d, P < 0.001). There were no significant differences observed in adverse events, in-hospital mortality, recurrent cholangitis occurrence, 30-d readmission rate or 30-d mortality.
Urgent one-stage ERCP provides the advantages of a shorter ICU stay, a shorter duration of antibiotic use, and a shorter hospital stay.
Core Tip: We investigated the safety and feasibility of one-stage endoscopic treatment for moderate to severe acute cholangitis. Our study found that patients who underwent endoscopic retrograde cholangiopancreatography within 24 h had a shorter intensive care unit stay, a shorter duration of antibiotic use, and a shorter hospital stay.
- Citation: Zhou Y, Zhang YQ, Huang SJ, Liang Y, Liang X, Wali M, Feng YD. Urgent one-stage endoscopic treatment for choledocholithiasis related moderate to severe acute cholangitis: A propensity score-matched analysis. World J Gastroenterol 2024; 30(15): 2118-2127
- URL: https://www.wjgnet.com/1007-9327/full/v30/i15/2118.htm
- DOI: https://dx.doi.org/10.3748/wjg.v30.i15.2118
Acute cholangitis (AC) is a severe and life-threatening infection that affects the biliary tract. It is a significant digestive disorder characterized by rapid onset and is common. Approximately 10%-29% of people with AC develop sepsis[1,2], and approximately 5% of patients progress to septic shock[3]. In severe cases, AC can be fatal. Currently, the Tokyo Guideline 2018 (TG18) criteria are used to diagnose and categorize ACs as mild, moderate, or severe cholangitis[4]. The primary cause of AC is biliary obstruction, which is often caused by cholelithiasis. Approximately 53% of patients with severe AC (SAC) require admission to the intensive care unit (ICU)[5].
While treating SAC, fluid resuscitation and antibiotics need to be administered as initial therapy. In addition, emergent biliary decompression is necessary to improve clinical outcomes[4]. The primary treatment choice for AC is endoscopic retrograde cholangiopancreatography (ERCP), which benefits approximately 90% of patients[6-10]. It is essential to adhere to the principle of “the sooner, the better” when performing ERCP treatment for AC. However, emergency ERCP biliary drainage in patients with severe cholangitis is associated with a significantly high risk of morbidity and mortality. In cases of early ERCP for AC associated with choledocholithiasis, patients with severe cholangitis are frequently subjected to brief procedures such as endoscopic nasobiliary drainage (ENBD) or stenting[9,11,12]. Nevertheless, this additional ERCP procedure not only prolongs the duration of hospital stay but also increases associated risks[12-16]. It is unclear whether single-stage stone removal is feasible for individuals with AC. The optimal timing for ERCP is yet a matter of debate. Therefore, we retrospectively examined and evaluated patients who underwent ERCP for moderate to SAC with choledocholithiasis. The aim was to assess the feasibility and safety of urgent single-stage stone removal for moderate to SAC.
The study was conducted at Zhongda Hospital Affiliated with Southeast University. The study was approved by the Ethics Committee (2019ZDSYLL094-P01). All methodologies employed in this study strictly adhered to the pertinent guidelines and regulations.
We collected data from the endoscopic reporting system for all patients who underwent ERCP procedures following admission to the emergency department between January 2019 and July 2023. The inclusion criteria were as follows: (1) Diagnosed with AC in accordance with the TG13 or TG18[17,18]; (2) Aged > 18 years; and (3) Willing to undergo ERCP.
The exclusion criteria were as follows: (1) Had mild AC; (2) Did not undergo endoscopic retrograde lithotomy; and (3) Had non-common bile duct (CBD) stones detected via cholangiopancreatography.
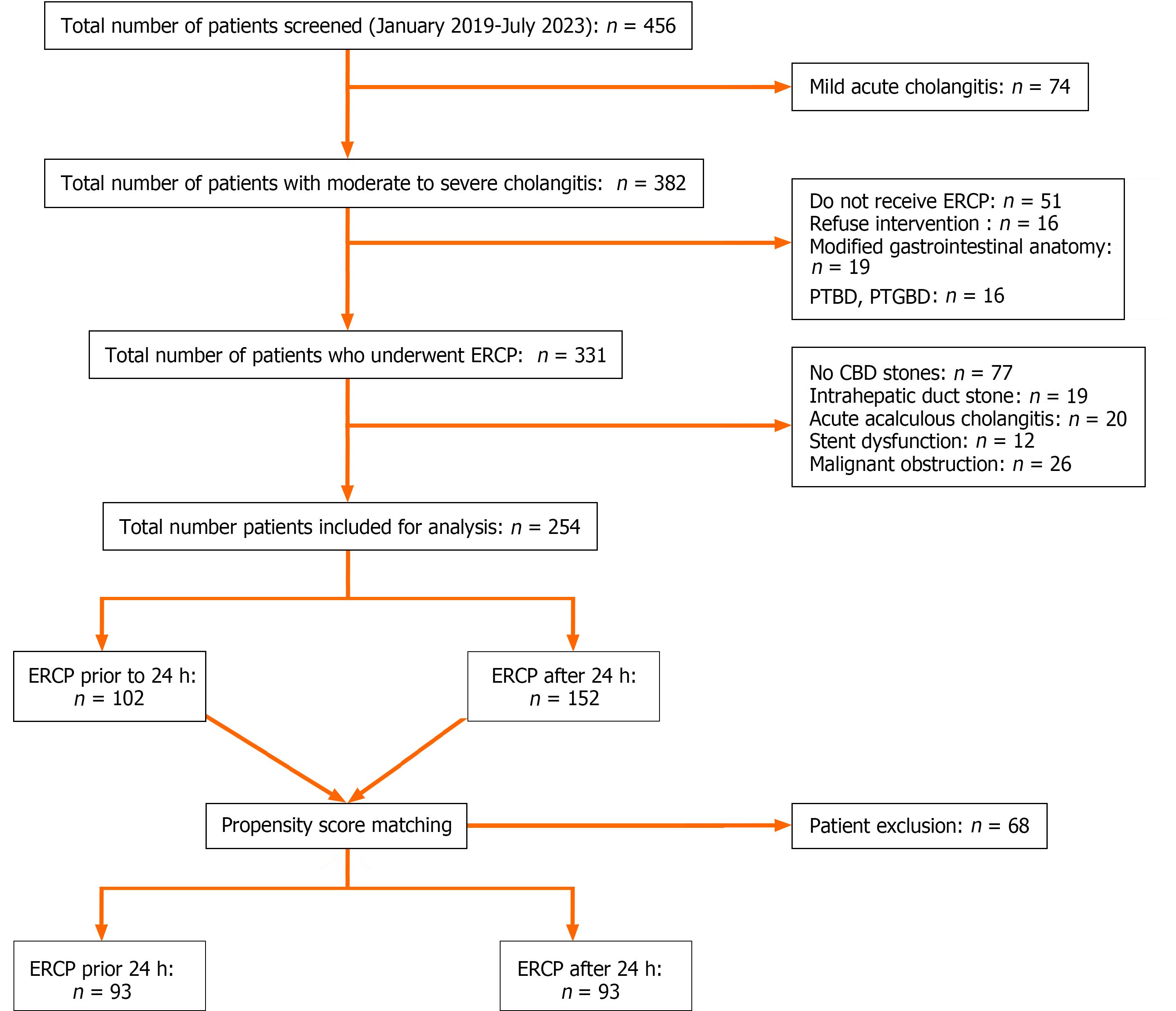
We retrieved data for all emergency ERCP procedures performed from January 2019 to July 2023 from the endoscopy reporting system, all patients were diagnosed with moderate to severe cholangitis due to CBD stones. The flowchart of this study is listed as Figure 1. The patients in the study were categorized into two groups based on the time between admission and intervention. These patients were classified into urgent (≤ 24 h, n = 102) and elective (> 24 h, n = 152) ERCP groups. The time span from admission to intervention was considered the time between registration in the emergency room and ERCP. We then sorted and reviewed patient demographic data, presenting symptoms, and ERCP outcomes. These data included the date, time between symptom onset and ERCP, admission and ERCP procedures, as well as laboratory data upon admission, such as the white blood cell (WBC) count, platelets (PLT), total bilirubin (TB), international normalized ratio (INR), creatinine (Cr), serum albumin, C-reactive protein (CRP), and neutrophil/lymphocyte ratio. The time span for biliary drainage was calculated as the duration between admission and the ERCP procedure. Furthermore, postoperative follow-up data were acquired through outpatient examinations or postoperative telephone follow-ups conducted after discharge. Disease severity was graded using the TG18 severity scale[18].
Prior to ERCP, we monitored the patients’ vital signs, established intravenous access, and administered empiric antibiotic therapy with third-generation cephalosporins. In instances where patients exhibited postshock symptoms, the execution of ERCP was postponed until their condition improved. Urgent ERCP was considered the primary treatment for patients who did not respond to drug therapy or who had moderate to severe disease. Prior to commencing the procedure, all participants provided informed consent. ERCP procedures were performed under general anesthesia and supervised by an anesthesiologist. During ERCP, the maternal endoscope used in this procedure was a therapeutic duodenoscope (Olympus TJF-260, Tokyo, Japan).
In the initial step, we established biliary access. Conventional biliary cannulation was attempted by using a sphincterotome (Microtech, Nanjing, China) and a 0.035-inch guidewire (Microtech, Nanjing, China). Successful biliary access was confirmed by observing visible bile aspiration, and bile samples were extracted for bacterial cultivation upon the manifestation of turbid bile flow. Subsequently, a 3-mm endoscopic sphincterotomy (EST) combined with endoscopic papillary balloon dilation was performed to establish a proper biliary orifice. The balloon was gradually inflated with 0.9% saline solution to the proposed pressure or until the biliary wall could be seen. For the extraction of stones, we employed either a basket or a balloon; for larger stones, mechanical lithotripsy was employed at the discretion of the endoscopist. In cases of cannulation failure, percutaneous transhepatic biliary drainage was explored as an alternative therapeutic option. Subsequently, a nasal biliary drainage tube was placed, and bile acid samples were collected for bacterial culture on postoperative days 1 and 2. The decision to drain the nasal biliary tube was contingent upon the patient’s clinical condition. Adverse reactions after drainage were classified according to the ASGE dictionary[19] and included post-ERCP pancreatitis (PEP), bleeding, and infection, among others.
The quantitative parameters are reported as either the mean (with range) or median (with interquartile range), depending on the distribution. Categorical variables are presented as the frequency and percentage. The propensity score (PS) framework was used to compare the clinical endpoints of ERCP within 24 h of onset and 24 h after onset. The PS method was used to create a new dataset in which the probability of ERCP occurring within 24 h of or after its occurrence was equal (as in a purely randomized trial) to balance the baseline characteristics of patients. First, multivariate logistic regression was used to predict the probability of ERCP within 24 h (i.e., estimated PS), controlling for the following prespecified covariates: Sex, age, Charlson Comorbidity Index (CCI) score, previous discharge ERCP, history of gallbladder surgery, TB, albumin, Cr, the INR, the PLT, the WBC, and the Tokyo score. The 1:1 nearest neighbor matching algorithm was used to match the two groups (urgent group and elective group) without substitution, and the caliper was 0.2[20] of the PS standard deviation of the logit score. The clinical endpoints were subsequently compared between the two groups in the matched datasets. Statistical analysis, including the χ2 test, one-way analysis of variance (ANOVA) and multivariate linear regression, was performed using the Statistical Package for Social Sciences (SPSS, Inc., version 27.0 for Windows, Chicago, IL, United States). A P value of < 0.05 was considered to indicate statistical significance.
From January 2019 to July 2023, a total of 456 patients with acute cholangitis were screened. Among these, 74, 16, 19 and 16 patients were excluded due to mild acute cholangitis, refusal of endoscopic treatment, upper gastrointestinal anatomy changes, and preference of PTBD or PTGBD, respectively. Additionally, 19, 20, 12 and 26 patients were excluded due to intrahepatic stone, acute acalculous cholecystitis, dysfunction of previous biliary stents and malignant obstructions, respectively. Consequently, 254 patients were included, 102 (40.2%) of whom underwent ERCP within 24 h of presentation and 152 (59.8%) after 24 h. The mean age was 69.47 (± 15.81) years, 47.6% were male, and 100% had choledocholithiasis-related cholangitis. The mean CCI score was 1 (0-7), and ERCP was performed for a mean time span of 48 (1-312) h. Cholangitis severity was categorized per Tokyo guidelines: Score 1 = 0%, score 2 = 72%, and score 3 = 28%. Table 1 shows the baseline patient characteristics before and after PS matching. After PS matching, 93 pairs of patients with similar traits were selected (Algorithm 1, Table 1). The proportion of patients who underwent one-step stone extraction after matching was consistent (100% vs 100%, P = 1) (Table 1).
| Before matching | After matching | |||||||
| Total, n = 254 | ERCP ≤ 24 h, n = 102 | ERCP > 24 h, n = 152 | P value | Total, n = 186 | ERCP ≤ 24 h, n = 93 | ERCP > 24 h, n = 93 | P value | |
| Age, yr | 69.47 ± 15.81 | 70.73 ± 15.24 | 68.63 ± 16.18 | 0.362 | 70.32 ± 15.39 | 71.05 ± 15.26 | 69.58 ± 15.56 | 0.515 |
| Male sex, n (%) | 121 (47.6) | 58 (56.9) | 63 (41.4) | 0.016 | 94 (50.5) | 54 (58.1) | 40 (43) | 0.04 |
| CCI | 1 (0-7) | 1 (0-5) | 1 (0-7) | 0.108 | 1 (0-7) | 1 (0-3) | 1 (0-7) | 0.187 |
| Past medical history | ||||||||
| ERCP, n (%) | 30 (11.8) | 13 (12.7) | 17 (11.2) | 0.706 | 22 (11.8) | 13 (14) | 9 (9.7) | 0.364 |
| Cholecystectomy, n (%) | 67 (26.4) | 27 (26.5) | 40 (26.3) | 0.978 | 44 (23.7) | 22 (23.7) | 22 (23.7) | 1 |
| Lab values | ||||||||
| WBC count as/μL | 10.32 ± 6.71 | 12.57 ± 6.61 | 8.81 ± 6.37 | < 0.001 | 10.58 ± 7.03 | 12.11 ± 6.44 | 9.06 ± 7.3 | 0.003 |
| Platelet count as/μL | 173.96 ± 71.08 | 164.21 ± 73.35 | 180.5 ± 68.99 | 0.594 | 168.11 ± 71.33 | 164.85 ± 74.65 | 171.38 ± 68.09 | 0.534 |
| CRP in mg/L | 75.17 ± 76.03 | 94.9 ± 79.32 | 61.75 ± 70.95 | < 0.001 | 78.53 ± 76.6 | 89.66 ± 76.37 | 76.37 ± 75.75 | 0.079 |
| NLR (%) | 7.94 (0.81-106.31) | 15.67 (1.36-106.31) | 6.9 (0.805-64.13) | < 0.001 | 18.545 (2.55-64.13) | 19.87 (11.2) | 16.42 (2.55-64.13) | < 0.001 |
| INR | 1.2 ± 0.22 | 1.23 ± 0.97 | 1.14 ± 0.92 | < 0.001 | 1.21 ± 0.23 | 1.25 ± 0.27 | 1.17 ± 0.17 | 0.012 |
| D2 polymers | 657 (0.21-26652) | 1567 (76-26652) | 504 (0.38-15502) | < 0.001 | 1193.5 (479-15502) | 1455 (479-4811) | 832 (504-15502) | 0.005 |
| Creatinine in mg/dL | 0.826 (0.34-8.32) | 1.01 (0.34-5.86) | 0.76 (0.44-8.32) | < 0.001 | 1.10 (0.77-5.86) | 1.15 (0.77-5.86) | 1.02 (0.79-3.1) | 0.013 |
| TB in mg/dL | 2.61 (0.28-22.52) | 3.7 (0.29-15.02) | 2.14 (0.28-22.52) | 0.021 | 3.58 ± 2.98 | 3.78 ± 2.8 | 3.38 ± 3.14 | 0.363 |
| AST in U/L | 108.5 (13-4051) | 128.5 (13-744) | 104 (15-4051) | 0.168 | 118 (40-539) | 131 (40-497) | 116 (46-539) | 0.53 |
| ALT in U/L | 201.68 ± 206.61 | 196.67 ± 173.10 | 205.03 ± 226.84 | 0.652 | 222 (68-512) | 259 (68-512) | 209 (88-479) | 0.55 |
| γ-GT in U/L | 406.66 ± 354.98 | 425.8 ± 376.89 | 393.82 ± 340.16 | 0.463 | 383.61 ± 332.61 | 405.97 ± 347.74 | 361.25 ± 361.25 | 0.361 |
| Albumin in g/dL | 35.8 (15.9-46.6) | 35.9 (15.9-48.7) | 37.8 (25.1-49.1) | 0.033 | 33.7 (24.3-36.1) | 32.6 (24.3-35.7) | 33.8 (25.4-36.1) | 0.163 |
| Tokyo Score | ||||||||
| 3 | 48 (28) | 39 (38.2) | 32 (21.1) | 0.003 | 58 (31.2) | 35 (37.6) | 23 (24.7) | 0.058 |
| 2 | 206 (72) | 63 (61.8) | 120 (78.9) | 128 (68.8) | 58 (62.4) | 70 (75.3) | ||
| ERCP procedure | ||||||||
| Door to ERCP time in h | 48 (1-312) | 8.5 (1-24) | 120 (27-312) | < 0.001 | 25.5 (1-312) | 9 (1-24) | 120 (27-312) | < 0.001 |
| ERCP procedure time (min) | 60 (25-780) | 60 (30-200) | 60 (30-335) | 0.714 | 60 (26-780) | 60 (30-200) | 60 (30-335) | 0.52 |
| One-stage ERCP, n (%) | 254 (100) | 102 (100) | 152 (100) | 1 | 186 (100) | 93 (100) | 93 (100) | 1 |
| CBD, n (%) | 254 (100) | 102 (100) | 152 (100) | 1 | 186 (100) | 93 (100) | 93 (100) | 1 |
| Stones size (mm) | 8 (2-25) | 9 (2-25) | 8 (2-25) | 0.222 | 8 (2-25) | 9 (2-25) | 8 (2-25) | 0.368 |
| Multiple stones, n (%) | 109 (42.9) | 35 (34.3) | 74 (48.7) | 0.023 | 79 (42.5) | 32 (34.4) | 47 (50.5) | 0.026 |
| Common bile duct width (mm) | 13 (4-33) | 14 (4-33) | 14 (5-33) | 0.016 | 13 (4-33) | 13 (4-33) | 12.1 (5-25) | 0.038 |
| EST, n (%) | 177 (69.7) | 75 (73.5) | 102 (67.1) | 0.275 | 128 (68.8) | 69 (74.2) | 59 (63.4) | 0.113 |
| EPBD, n (%) | 204 (80.3) | 90 (88.2) | 114 (75) | 0.009 | 149 (80.1) | 83 (89.2) | 66 (71) | 0.002 |
| Pancreatic stent placement, n (%) | 21 (8.3) | 5 (4.9) | 16 (10.5) | 0.111 | 15 (8.1) | 5 (5.4) | 10 (10.8) | 0.178 |
| Nasal Biliary Drainage Catheter placement, n (%) | 251 (98.8) | 100 (98) | 151 (99.3) | 0.346 | 183 (98.4) | 91 (97.8) | 92 (98.9) | 0.561 |
| HLL, n (%) | 21 (8.3) | 11 (10.8) | 10 (6.6) | 0.233 | 16 (8.6) | 11 (11.8) | 5 (5.4) | 0.117 |
Our primary outcome was ICU admission rate, ICU length of stay, and duration of antibiotic use (Table 2). The results derived from our analysis of a PS-matched population indicated a significant difference in ICU admission rates between the urgent ERCP group and the elective ERCP group (34.4% vs 21.5%, P = 0.05). Importantly, there was a significant difference in ICU stay length between the urgent ERCP and elective ERCP groups, with the urgent group having a shorter stay (3 d vs 9 d, P < 0.001). Additionally, compared with those in the elective group, the patients in the urgent ERCP group had a shorter duration of antibiotic use (6 d vs 9 d, P < 0.001). Univariate linear regression analysis of ICU stay length revealed independent correlations with variables, including WBC [95% confidence interval (CI): 0.18-0.82, P = 0.003], CRP (95%CI: 0.01-0.08, P = 0.015), Cr (95%CI: 4.22-8.28, P < 0.001), age (95%CI: -0.66 to -0.07, P = 0.016), and the time span of ERCP (hours) (95%CI: 0.04-0.06, P < 0.001). Additionally, ICU stay length was not significantly correlated with one-stage endoscopic treatment, EST, ENBD, adverse events, 30-d readmission, or recurrent cholangitis (Table 3). Multivariate linear regression analysis of the matched data revealed significant correlations between ERCP delay time (95%CI: 0.03-0.06, P < 0.001), Cr level (95%CI: 0.07-3.56, P = 0.041), and ICU stay length.
| Before matching | After matching | |||||||
| Total, n = 254 | ERCP ≤ 24 h, | ERCP > 24 h, | P value | Total, n = 186 | ERCP ≤ 24 h, | ERCP > 24 h, | P value | |
| ERCP intervention type, n (%) | ||||||||
| Complete stone removal | 250 (98.4) | 101 (99) | 149 (98) | 0.533 | 184 (98.9) | 92 (98.9) | 92 (98.9) | 1 |
| Biliary stent insertion | 4 (1.6) | 1 (1) | 3 (2) | 0.533 | 2 (1.1) | 1 (1.1) | 1 (1.1) | 1 |
| Technical success rate, n (%) | 250 (98.4) | 101(99) | 149 (98) | 0.533 | 183 (98.4) | 92 (98.9) | 91 (98) | 0.561 |
| ERCP failure, n (%) | 4 (1.6) | 1 (1) | 3 (2) | 0.533 | 3 (1.6) | 1 (1.1) | 2 (2.2) | 1 |
| Duration of antibiotic use (d) | 7 (1-28) | 6 (2-15) | 8 (2-26) | < 0.001 | 7 (2-28) | 6 (2-18) | 9 (2-28) | < 0.001 |
| In-hospital mortality, n (%) | 3 (1.2) | 0 | 3 (2) | 0.153 | 2 (1.1) | 0 | 2 (2.2) | 0.155 |
| 30-d mortality, n (%) | 7 (2.8) | 2 (2) | 5 (3.3) | 0.526 | 5 (2.7) | 2 (2.2) | 3 (3.2) | 0.65 |
| Recurrent cholangitis, n (%) | 7 (2.8) | 3 (2.9) | 4 (2.6) | 0.883 | 6 (3.2) | 3 (3.2) | 3 (3.2) | 1 |
| LOHS, (d) | 10 (3-71) | 9 (3-39) | 18 (5-71) | < 0.001 | 9 (3-71) | 9 (3-39) | 18.5 (7-71) | < 0.001 |
| Required ICU stay, n (%) | 61 (24) | 33 (32.4) | 28 (18.4) | 0.011 | 52 (28) | 32 (34.4) | 20 (21.5) | 0.05 |
| ICU stay length, (d) | 9 (1-71) | 3 (1-15) | 8 (1-71) | 0.003 | 4.5 (1-71) | 3 (1-15) | 9 (1-71) | < 0.001 |
| 30 d readmission, n (%) | 33 (13) | 15 (14.7) | 18 (11.8) | 0.506 | 29 (15.6) | 14 (15.1) | 15 (16.1) | 0.84 |
| ERCP-related complications, n (%) | 42 (16.5) | 18 (17.7) | 24 (15.8) | 0.696 | 29 (15.6) | 16 (17.2) | 13 (14) | 0.544 |
| PEP | 23 (9.1) | 9 (8.8) | 14 (9.2) | 0.916 | 17 (9.1) | 9 (9.7) | 8 (8.6) | 0.799 |
| Cholangitis | 9 (3.5) | 6 (5.9) | 3 (2) | 0.099 | 7 (3.8) | 5 (5.4) | 2 (2.2) | 0.248 |
| Bleeding | 6 (2.4) | 4 (3.9) | 2 (1.3) | 0.18 | 4 (2.2) | 3 (3.2) | 1 (1.1) | 0.312 |
| Others | 2 (0.8) | 2 (2) | 0 | 0.083 | 2 (1.1) | 2 (2.2) | 0 | 0.155 |
| Univariate analysis | Multivariate analysis | |||
| OR (95%CI) | P value | OR (95%CI) | P value | |
| WBC count | 0.503 (0.185 to 0.821) | 0.003 | -0.092 (-0.305 to 0.12) | 0.387 |
| Platelet count | -0.031 (-0.069 to 0.007) | 0.105 | ||
| CRP | 0.046 (0.009 to 0.082) | 0.015 | 0.002 (0.021 to 0.024) | 0.877 |
| NLR | -0.006 (-0.181 to 0.169) | 0.945 | ||
| INR | -4.986 (-15.932 to 5.961) | 0.365 | ||
| TB | -0.101 (-1.26 to 1.058) | 0.862 | ||
| Cr | 6.248 (4.216 to 8.281) | < 0.001 | 1.818 (0.073 to 3.564) | 0.042 |
| Albumin | 0.569 (0.043 to 1.095) | 0.035 | 0.02 (-0.308 to 0.347) | 0.905 |
| ALT | -0.005 (-0.017 to 0.007) | 0.375 | ||
| AST | -0.001 (-0.007 to 0.005) | 0.789 | ||
| Multiple stones | -3.31 (-9.87 to 3.249) | 0.316 | ||
| CCI | 1.466 (-0.713 to 3.644) | 0.183 | ||
| Age | -0.367 (-0.663 to -0.072) | 0.016 | -0.086 (-0.256 to 0.083) | 0.312 |
| Severity of AC | 3.188 (-3.434 to 9.809) | 0.338 | ||
| Time to ERCP | 0.051 (0.340 to 0.059) | < 0.001 | 0.044 (0.033 to 0.056) | < 0.001 |
| Common bile duct width | -0.002 (-0.34 to 0.335) | 0.988 | ||
According to our analysis of the PS-matched population (Table 2), the length of hospital stay (LOHS) in the urgent group was significantly shorter than that in the elective group (9 d vs 18.5 d, P < 0.001). The two groups exhibited no significant differences in 30-d readmission (15.1% vs 16.1%, P = 0.84), recurrent cholangitis (2.9% vs 2.6%, P = 0.883), in-hospital mortality (0% vs 2.2%, P = 0.155), 30-d mortality (2.2% vs 3.2%, P = 0.65), adverse events after ERCP (17.65% vs 15.79%, P = 0.696), PEP (8.82% vs 9.21%, P = 0.916), bleeding (3.9% vs 1.3%, P = 0.180), biliary tract infection (5.9% vs 1.97%, P = 0.099), or other ERCP-related adverse events (1.96% vs 0, P = 0.083).
After PS matching (Table 4), 58 patients in the cohort presented with severe biliary tract infection according to a Tokyo score of 3. Among these patients, 60.3% underwent ERCP within 24 h of onset, while 39.7% underwent ERCP after 24 h. Subsequently, we compared outcomes between the urgent ERCP group and the elective ERCP group within the subset of patients who experienced severe cholangitis. No significant difference in ICU admission rates was observed between the two groups (60% vs 47.8%, P = 0.362). The urgent group had a significantly shorter ICU stay than did the elective group (4 d vs 11 d, P = 0.014), a significantly shorter duration of antibiotic use (17.1% vs 17.4%, P = 0.98), and a markedly shorter LOHS (9 d vs 20 d, P < 0.001). Additionally, within 30 d, there were no significant differences between the two subgroups in terms of readmission (17.1% vs 17.4%, P = 0.98), in-hospital mortality (0% vs 4.3%, P = 0.213), 30-d mortality (5.7% vs 8.7%, P = 0.661), occurrence of adverse events after ERCP (22.86% vs 13.04%, P = 0.351), PEP (8.57% vs 4.35%, P = 0.535), bleeding (2.86% vs 4.35%, P = 0.761), biliary tract infection (8.57% vs 0%, P = 0.149), or occurrence of other ERCP-related adverse events (2.86% vs 0, P = 0.414).
| Patients with Grade III AC | Total, n = 58 | ERCP ≤ 24 h, n = 35 | ERCP > 24 h, n = 23 | P value |
| Duration of antibiotic use (d) | 8 (3-28) | 7 (3-15) | 11 (3-28) | 0.004 |
| In-hospital mortality, n (%) | 1 (1.7) | 0 | 1 (4.3) | 0.213 |
| 30-d mortality, n (%) | 4 (6.9) | 2 (5.7) | 2 (8.7) | 0.661 |
| Recurrent cholangitis, n (%) | 4 (6.9) | 2 (5.7) | 2 (8.7) | 0.661 |
| LOHS, (d) | 13 (6-71) | 9 (6-17) | 20 (14-71) | < 0.001 |
| Required ICU stay, n (%) | 32 (55.2) | 21 (60) | 11 (47.8) | 0.362 |
| ICU stay length, (d) | 6 (1-71) | 4 (1-15) | 11 (1-71) | 0.014 |
| 30 d readmission, n (%) | 10 (17.2) | 6 (17.1) | 4 (17.4) | 0.98 |
| ERCP-related complications, n (%) | 11 (19) | 8 (22.9) | 3 (13) | 0.351 |
| PEP | 4 (6.9) | 3 (8.6) | 1 (4.3) | 0.535 |
| Cholangitis | 3 (5.2) | 3 (8.6) | 0 | 0.149 |
| Bleeding | 2 (3.4) | 1 (2.9) | 1 (4.3) | 0.761 |
| Others | 1 (1.7) | 1 (2.9) | 0 | 0.414 |
Numerous studies have been conducted to determine the best timing for biliary decompression in patients with AC. However, the advantages of urgent one-stage endoscopic procedures via ERCP for treating moderate to severe cholangitis associated with CBD stones still need further clarification[20-23]. We comprehensively analyzed the characteristics and diagnostic findings of 254 patients diagnosed with AC who were admitted to Zhongda Hospital of Southeast University over the past four years. Within our PS-matched population, multivariate regression analysis was used to identify independent predictors of ICU stay length, including preoperative Cr levels and delay in performing ERCP. Notably, elective ERCP was associated with a longer duration of ICU stay (3 d vs 8 d, P < 0.001) and a prolonged course of antibiotic treatment (6 d vs 9 d, P < 0.001). Additionally, elective ERCP resulted in an increased LOHS (9 d vs 18.5 d, P < 0.001). Similar findings were observed in the unadjusted cohort analysis: ICU stay length (3 d vs 8 d, P = 0.003), antibiotic duration (6 d vs 8 d, P < 0.001), and LOHS (9 d vs 18 d, P < 0.001).
Our investigation concentrated on patients who underwent single-stage endoscopic procedures for AC. In our PS-matched population, the mortality rate was 2.7%. This figure aligns with the findings reported by Park et al[12] and Zhang et al[14]. Notably, our observation rate was lower than the 5%-11% range documented in other studies[11,21]. One plausible rationale for this variance may stem from the fact that all subjects in our study exclusively underwent single-stage endoscopic procedures, potentially contributing to the observed lower mortality rate. Notably, single-stage endoscopic procedures exhibit both safety and efficacy in addressing biliary drainage and CBD stone clearance in individuals with AC. Previous studies have revealed that one-stage endoscopic treatment has a high cure rate and low complication rate in patients with mild to moderate cholangitis. In a multicenter retrospective study conducted by our team in 2019, the safety and efficacy of this approach were reaffirmed, particularly in patients with severe complications[14]. Eto et al[24] also reported a cure rate of 90% within 4 d of single-stage treatment for AC (45 out of 50 patients), as well as complete stone clearance achieved in all patients and a complication rate of only 10% (5 out of 50 individuals). This approach effectively reduces the risks associated with two-stage ERCP procedures. Our study included 254 patients who underwent urgent single-stage endoscopic procedures, all of which resulted in complete stone clearance and a low complication rate of 16.5%. These results suggested that single-stage treatment can be an effective and safe method for treating moderate to SAC associated with stone removal.
In 2023, Hedjoudje et al[22] conducted an analytical study based on a substantial database that included 85 patients with severe cholangitis. These patients underwent drainage within 24 h, while the remaining 51 patients underwent drainage 24 h later. The study revealed that the elective ERCP procedure was linked to higher mortality rates (13.0% vs 45.5%, P < 0.001), prolonged length of ICU stays (4.61 d vs 7.41 d, P = 0.004), and increased LOHS. In a retrospective study conducted by Muangkaew et al[25], a cohort of patients diagnosed with acute biliary pancreatitis associated with cholangitis was analyzed. Of these, 67 out of 95 patients underwent drainage within 72 h. The study revealed no statistically significant differences in mortality, ERCP-related complications, or disease-related complications between the early and elective ERCP groups. However, the early ERCP (< 72 h) group had a shorter LOHS (6.3 ± 4.4 d) than did the elective ERCP group (9.8 ± 6.1 d; P = 0.002). The difference in mortality outcomes between the two studies may be attributed to the study of Hedjoudje et al[22], patients specifically with severe cholangitis were enrolled, which potentially resulted in significantly greater mortality rates than those in the study of Muangkaew et al[25]. This discrepancy may partially explain the differences in mortality outcomes between the two studies.
Given the relatively low mortality rate observed among cholangitis patients in our study, our primary outcome measures included the ICU admission rate, ICU length of stay, and duration of antibiotic use. After analyzing multiple factors within our matched cohort, we found that for every hour of delay in ERCP, patients’ ICU stay increased by 0.033 d. Such a prolonged ICU stay not only contributes to increased hospital expenses but also amplifies the risks of hospital-acquired infections and associated adverse events. Our research underscores the imperative for urgent ERCP in patients experiencing moderate to SAC. The delay in receiving ERCP correlates with extended hospital and ICU stays, aligning with findings from prior investigations[11,21,22,25,26]. Nevertheless, we observed a heightened ICU admission rate in the urgent ERCP group, potentially attributed to the greater prevalence of severe cases in that cohort (34.4% vs 21.5%, P = 0.05). After surgery, medical practitioners typically move patients with severe biliary tract inflammation to the ICU for stabilization. Contrary to this norm, our study demonstrated that patients receiving urgent ERCP exhibited a shorter ICU stay (3 d vs 8 d, P < 0.001), with no discernible differences in post-ERCP prognostic indicators between the two groups. Despite a higher percentage of severe patients in the urgent group, patients in this subset recovered faster post surgery. Additionally, we assessed the duration of antibiotic usage among patients who underwent ERCP. Patients in the urgent group had a significantly shorter duration of antibiotic usage than did those in the nonurgent group (7 d vs 16 d, P < 0.001). Simultaneously, our results indicate a reduction in overall hospitalization within the urgent group. These findings collectively affirm the quicker postoperative recovery observed in the urgent group. Furthermore, our multifactorial linear analysis of ICU stay length revealed that Cr levels had a significant impact on ICU stay.
There are several limitations to our research. First, there may be inherent selection bias present, and the results of our research may only reflect the clinical situation within our facility because this was a retrospective single-center study. Second, we implemented strict inclusion and exclusion criteria, which led to a relatively small sample size. To address these issues, further large-scale clinical studies are necessary to confirm our findings.
To summarize, urgent one-stage endoscopic treatment is feasible and safe for patients with moderate to SAC. Our research also showed that if ERCP is performed more than 24 h after admission for moderate to SAC, it may lead to longer stays in the ICU and hospital.
Thanks to Ya-Dong Feng for the case design; Ying-Qiu Zhang and Shuai-Jing Huang on the collection and analysis of data; revisions to article by Yan Liang and Masoom Wali. Thanks for all participants for their contributions.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Covantsev S, Russia S-Editor: Wang JJ L-Editor: A P-Editor: Cai YX
| 1. | Baykara N, Akalın H, Arslantaş MK, Hancı V, Çağlayan Ç, Kahveci F, Demirağ K, Baydemir C, Ünal N; Sepsis Study Group. Epidemiology of sepsis in intensive care units in Turkey: a multicenter, point-prevalence study. Crit Care. 2018;22:93. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 86] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 2. | Annane D, Aegerter P, Jars-Guincestre MC, Guidet B; CUB-Réa Network. Current epidemiology of septic shock: the CUB-Réa Network. Am J Respir Crit Care Med. 2003;168:165-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 421] [Cited by in RCA: 424] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 3. | Leligdowicz A, Dodek PM, Norena M, Wong H, Kumar A; Co-operative Antimicrobial Therapy of Septic Shock Database Research Group. Association between source of infection and hospital mortality in patients who have septic shock. Am J Respir Crit Care Med. 2014;189:1204-1213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 189] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 4. | Kiriyama S, Kozaka K, Takada T, Strasberg SM, Pitt HA, Gabata T, Hata J, Liau KH, Miura F, Horiguchi A, Liu KH, Su CH, Wada K, Jagannath P, Itoi T, Gouma DJ, Mori Y, Mukai S, Giménez ME, Huang WS, Kim MH, Okamoto K, Belli G, Dervenis C, Chan ACW, Lau WY, Endo I, Gomi H, Yoshida M, Mayumi T, Baron TH, de Santibañes E, Teoh AYB, Hwang TL, Ker CG, Chen MF, Han HS, Yoon YS, Choi IS, Yoon DS, Higuchi R, Kitano S, Inomata M, Deziel DJ, Jonas E, Hirata K, Sumiyama Y, Inui K, Yamamoto M. Tokyo Guidelines 2018: diagnostic criteria and severity grading of acute cholangitis (with videos). J Hepatobiliary Pancreat Sci. 2018;25:17-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 468] [Cited by in RCA: 424] [Article Influence: 60.6] [Reference Citation Analysis (0)] |
| 5. | Lavillegrand JR, Mercier-Des-Rochettes E, Baron E, Pène F, Contou D, Favory R, Préau S, Galbois A, Molliere C, Miailhe AF, Reignier J, Monchi M, Pichereau C, Thietart S, Vieille T, Piton G, Preda G, Abdallah I, Camus M, Maury E, Guidet B, Dumas G, Ait-Oufella H. Acute cholangitis in intensive care units: clinical, biological, microbiological spectrum and risk factors for mortality: a multicenter study. Crit Care. 2021;25:49. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 6. | Nishino T, Hamano T, Mitsunaga Y, Shirato I, Shirato M, Tagata T, Shimada M, Yoshida S, Mitsunaga A. Clinical evaluation of the Tokyo Guidelines 2013 for severity assessment of acute cholangitis. J Hepatobiliary Pancreat Sci. 2014;21:841-849. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 7. | Buxbaum JL, Buitrago C, Lee A, Elmunzer BJ, Riaz A, Ceppa EP, Al-Haddad M, Amateau SK, Calderwood AH, Fishman DS, Fujii-Lau LL, Jamil LH, Jue TL, Kwon RS, Law JK, Lee JK, Naveed M, Pawa S, Sawhney MS, Schilperoort H, Storm AC, Thosani NC, Qumseya BJ, Wani S. ASGE guideline on the management of cholangitis. Gastrointest Endosc. 2021;94:207-221.e14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 35] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 8. | An Z, Braseth AL, Sahar N. Acute Cholangitis: Causes, Diagnosis, and Management. Gastroenterol Clin North Am. 2021;50:403-414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 32] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 9. | Yokoe M, Takada T, Strasberg SM, Solomkin JS, Mayumi T, Gomi H, Pitt HA, Garden OJ, Kiriyama S, Hata J, Gabata T, Yoshida M, Miura F, Okamoto K, Tsuyuguchi T, Itoi T, Yamashita Y, Dervenis C, Chan AC, Lau WY, Supe AN, Belli G, Hilvano SC, Liau KH, Kim MH, Kim SW, Ker CG; Tokyo Guidelines Revision Committee. TG13 diagnostic criteria and severity grading of acute cholecystitis (with videos). J Hepatobiliary Pancreat Sci. 2013;20:35-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 302] [Cited by in RCA: 271] [Article Influence: 22.6] [Reference Citation Analysis (1)] |
| 10. | Navuluri R, Hoyer M, Osman M, Fergus J. Emergent Treatment of Acute Cholangitis and Acute Cholecystitis. Semin Intervent Radiol. 2020;37:14-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 11. | Lee F, Ohanian E, Rheem J, Laine L, Che K, Kim JJ. Delayed endoscopic retrograde cholangiopancreatography is associated with persistent organ failure in hospitalised patients with acute cholangitis. Aliment Pharmacol Ther. 2015;42:212-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 60] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 12. | Park CS, Jeong HS, Kim KB, Han JH, Chae HB, Youn SJ, Park SM. Urgent ERCP for acute cholangitis reduces mortality and hospital stay in elderly and very elderly patients. Hepatobiliary Pancreat Dis Int. 2016;15:619-625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 13. | Sato J, Nakahara K, Morita R, Morita N, Suetani K, Michikawa Y, Kobayashi S, Itoh F. Efficacy and Safety of Single-Session Endoscopic Stone Removal for Acute Cholangitis Associated with Choledocholithiasis. Can J Gastroenterol Hepatol. 2018;2018:3145107. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 14. | Zhang X, Li G, Pan L, Chen Y, Shi R, Xu W, Zhou K, Cheng Y, Feng Y, Zhou A, Zhao K. The efficacy and safety of one-stage endoscopic treatment for ascending acute cholangitis caused by choledocholithiasis with severe comorbidities. Surg Endosc. 2020;34:3963-3970. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 15. | Liang CM, Chiu YC, Lu LS, Wu CK, Sou FM, Chiu SM, Lee YC, Huang PY, Chuah SK, Kuo CM. Early and Direct Endoscopic Stone Removal in the Moderate Grade of Acute Cholangitis with Choledocholithiasis Was Safe and Effective: A Prospective Study. Life (Basel). 2022;12. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 16. | Ito T, Sai JK, Okubo H, Saito H, Ishii S, Kanazawa R, Tomishima K, Watanabe S, Shiina S. Safety of immediate endoscopic sphincterotomy in acute suppurative cholangitis caused by choledocholithiasis. World J Gastrointest Endosc. 2016;8:180-185. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 11] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 17. | Yokoe M, Hata J, Takada T, Strasberg SM, Asbun HJ, Wakabayashi G, Kozaka K, Endo I, Deziel DJ, Miura F, Okamoto K, Hwang TL, Huang WS, Ker CG, Chen MF, Han HS, Yoon YS, Choi IS, Yoon DS, Noguchi Y, Shikata S, Ukai T, Higuchi R, Gabata T, Mori Y, Iwashita Y, Hibi T, Jagannath P, Jonas E, Liau KH, Dervenis C, Gouma DJ, Cherqui D, Belli G, Garden OJ, Giménez ME, de Santibañes E, Suzuki K, Umezawa A, Supe AN, Pitt HA, Singh H, Chan ACW, Lau WY, Teoh AYB, Honda G, Sugioka A, Asai K, Gomi H, Itoi T, Kiriyama S, Yoshida M, Mayumi T, Matsumura N, Tokumura H, Kitano S, Hirata K, Inui K, Sumiyama Y, Yamamoto M. Tokyo Guidelines 2018: diagnostic criteria and severity grading of acute cholecystitis (with videos). J Hepatobiliary Pancreat Sci. 2018;25:41-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 769] [Cited by in RCA: 685] [Article Influence: 97.9] [Reference Citation Analysis (0)] |
| 18. | Miura F, Okamoto K, Takada T, Strasberg SM, Asbun HJ, Pitt HA, Gomi H, Solomkin JS, Schlossberg D, Han HS, Kim MH, Hwang TL, Chen MF, Huang WS, Kiriyama S, Itoi T, Garden OJ, Liau KH, Horiguchi A, Liu KH, Su CH, Gouma DJ, Belli G, Dervenis C, Jagannath P, Chan ACW, Lau WY, Endo I, Suzuki K, Yoon YS, de Santibañes E, Giménez ME, Jonas E, Singh H, Honda G, Asai K, Mori Y, Wada K, Higuchi R, Watanabe M, Rikiyama T, Sata N, Kano N, Umezawa A, Mukai S, Tokumura H, Hata J, Kozaka K, Iwashita Y, Hibi T, Yokoe M, Kimura T, Kitano S, Inomata M, Hirata K, Sumiyama Y, Inui K, Yamamoto M. Tokyo Guidelines 2018: initial management of acute biliary infection and flowchart for acute cholangitis. J Hepatobiliary Pancreat Sci. 2018;25:31-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 174] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 19. | Cotton PB, Eisen GM, Aabakken L, Baron TH, Hutter MM, Jacobson BC, Mergener K, Nemcek A Jr, Petersen BT, Petrini JL, Pike IM, Rabeneck L, Romagnuolo J, Vargo JJ. A lexicon for endoscopic adverse events: report of an ASGE workshop. Gastrointest Endosc. 2010;71:446-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1238] [Cited by in RCA: 1841] [Article Influence: 122.7] [Reference Citation Analysis (1)] |
| 20. | Parikh MP, Wadhwa V, Thota PN, Lopez R, Sanaka MR. Outcomes Associated With Timing of ERCP in Acute Cholangitis Secondary to Choledocholithiasis. J Clin Gastroenterol. 2018;52:e97-e102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 33] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 21. | Khashab MA, Tariq A, Tariq U, Kim K, Ponor L, Lennon AM, Canto MI, Gurakar A, Yu Q, Dunbar K, Hutfless S, Kalloo AN, Singh VK. Delayed and unsuccessful endoscopic retrograde cholangiopancreatography are associated with worse outcomes in patients with acute cholangitis. Clin Gastroenterol Hepatol. 2012;10:1157-1161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 92] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 22. | Hedjoudje A, Cheurfa C, Et Talby M, Levy P, Prat F, Piton G. Outcomes and predictors of delayed endoscopic biliary drainage for severe acute cholangitis due to choledocholithiasis in an intensive care unit. Dig Liver Dis. 2023;55:763-770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 23. | Tan M, Schaffalitzky de Muckadell OB, Laursen SB. Association between early ERCP and mortality in patients with acute cholangitis. Gastrointest Endosc. 2018;87:185-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 54] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 24. | Eto K, Kawakami H, Haba S, Yamato H, Okuda T, Yane K, Hayashi T, Ehira N, Onodera M, Matsumoto R, Matsubara Y, Takagi T, Sakamoto N; Hokkaido Interventional EUS/ERCP study (HONEST) group. Single-stage endoscopic treatment for mild to moderate acute cholangitis associated with choledocholithiasis: a multicenter, non-randomized, open-label and exploratory clinical trial. J Hepatobiliary Pancreat Sci. 2015;22:825-830. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 25. | Muangkaew P, Kamalaporn P, Mingphruedhi S, Rungsakulkij N, Suragul W, Vassanasiri W, Tangtawee P. Outcomes of delayed endoscopic retrograde cholangiopancreatography in patients with acute biliary pancreatitis with cholangitis. Asian J Surg. 2020;43:913-918. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 26. | Iqbal U, Khara HS, Hu Y, Khan MA, Ovalle A, Siddique O, Sun H, Shellenberger MJ. Emergent versus urgent ERCP in acute cholangitis: a systematic review and meta-analysis. Gastrointest Endosc. 2020;91:753-760.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 38] [Article Influence: 7.6] [Reference Citation Analysis (1)] |