Published online Apr 14, 2024. doi: 10.3748/wjg.v30.i14.2006
Peer-review started: December 7, 2023
First decision: January 24, 2024
Revised: February 5, 2024
Accepted: March 25, 2024
Article in press: March 25, 2024
Published online: April 14, 2024
Processing time: 127 Days and 8 Hours
The success of liver resection relies on the ability of the remnant liver to regenerate. Most of the knowledge regarding the pathophysiological basis of liver regeneration comes from rodent studies, and data on humans are scarce. Additionally, there is limited knowledge about the preoperative factors that influence postoperative regeneration.
To quantify postoperative remnant liver volume by the latest volumetric software and investigate perioperative factors that affect posthepatectomy liver regenera
A total of 268 patients who received partial hepatectomy were enrolled. Patients were grouped into right hepatectomy/trisegmentectomy (RH/Tri), left hepa
The numbers of patients in the RH/Tri, LH, Seg, and Sub/Non groups were 41, 53, 99 and 75, respectively. The RI plateaued at 3 months in the LH, Seg, and Sub/Non groups, whereas the RI increased until 12 months in the RH/Tri group. According to our multivariate analysis, the preoperative albumin-bilirubin (ALBI) score was an independent factor for low regeneration at 3 months [odds ratio (OR) 95%CI = 2.80 (1.17-6.69), P = 0.02; per 1.0 up] and 12 months [OR = 2.27 (1.01-5.09), P = 0.04; per 1.0 up]. Multivariate analysis revealed that only liver resection percentage [OR = 1.03 (1.00-1.05), P = 0.04] was associated with delayed regeneration. Furthermore, multivariate analysis demonstrated that the preoperative ALBI score [OR = 2.63 (1.00-1.05), P = 0.02; per 1.0 up] and liver resection percentage [OR = 1.02 (1.00-1.05), P = 0.04; per 1.0 up] were found to be independent risk factors associated with volume restoration failure.
Liver regeneration posthepatectomy was determined by the resection percentage and preoperative ALBI score. This knowledge helps surgeons decide the timing and type of rehepatectomy for recurrent cases.
Core Tip: Insights into posthepatectomy liver regeneration in humans are limited. We quantified liver volumes using the latest volumetric software and investigated perioperative factors that affect posthepatectomy liver regeneration. It was revealed that liver regeneration continues after 3 months of hepatectomy with more than one-fourth of the liver resection and decline in preoperative liver function, reflected by the albumin-bilirubin (ALBI) score, is associated with decreased regeneration. Furthermore, restoring to the original volume depended on the combination of the preoperative ALBI score and liver resection percentage. With this knowledge, surgeons can select an appropriate hepatectomy type with rehepatectomy in mind after intrahepatic recurrence.
- Citation: Takahashi K, Gosho M, Miyazaki Y, Nakahashi H, Shimomura O, Furuya K, Doi M, Owada Y, Ogawa K, Ohara Y, Akashi Y, Enomoto T, Hashimoto S, Oda T. Preoperative albumin-bilirubin score and liver resection percentage determine postoperative liver regeneration after partial hepatectomy. World J Gastroenterol 2024; 30(14): 2006-2017
- URL: https://www.wjgnet.com/1007-9327/full/v30/i14/2006.htm
- DOI: https://dx.doi.org/10.3748/wjg.v30.i14.2006
Liver regeneration after partial hepatectomy proceeds through the action of several cells that compose the liver, including hepatocytes, bile duct epithelial cells, hepatic sinusoidal endothelial cells, Kupffer cells, and hepatic stellate cells[1]. Hepatocytes are normally dormant but undergo one or two rounds of cell division after hepatectomy. The onset of hepatocyte cell division varies among animal species, with that of rats beginning 24 h after hepatectomy and that of humans and mice beginning 48 h after hepatectomy. Growth factors and cytokines such as hepatocyte growth factor (HGF), tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), transforming growth factor-α, and epidermal growth factor are involved in this process[1]. Both of these growth factors and cytokines activate downstream growth signaling pathways, ultimately leading to the transition from quiescent cells in G0 phase to G1/S phase and initiation of the cell cycle. In rodents, the remnant liver after 70% partial hepatectomy returns to a size of 100% in 7 to 10 d, and in humans, the liver mass is believed to be completely restored in 3 to 6 months[1-3]. Even small resections with a resection percentage of 10% or less are followed by eventual restoration of the liver to its full size[3].
Liver regeneration is an important topic not only for basic science but also for clinicians because liver regeneration failure could cause serious complications that could lead to patient mortality. Although this topic has been well described, most related studies have been conducted in animal models, and there are limited data on this topic in humans[1]. Furthermore, most of those previous reports focused on liver regeneration only after major hepatectomy[4,5]; few reports provided insights into liver regeneration after minor hepatectomy, and few studies have compared regeneration between hepatectomy types. Moreover, the volumetric assessments in most of the previous studies were performed before the development of computer-based volumetry or used prototype volumetry before the early 2000s. In this study, we quantified postoperative remnant liver volumes using the latest volumetric software in patients who underwent partial hepatectomy and had various backgrounds. We observed postoperative parenchymal regeneration according to hepatectomy type and identified perioperative factors that affect postoperative liver regeneration.
Between January 2006 and October 2022, 762 patients with hepatocellular carcinoma (HCC), cholangiocellular carcinoma (CCC), combined HCC and CCC (CHC), colorectal metastasis, or other diseases underwent partial hepatectomy at the University of Tsukuba Hospital (Tsukuba, Japan). Patient records were identified by an administrative database. Patients who (1) were less than 14 years old; (2) underwent partial hepatectomy with concomitant gastrointestinal surgical procedures, biliary reconstruction, or splenectomy; (3) underwent partial hepatectomy with a liver resection percentage of less than 10%; (4) had macroscopic evidence of noncurative resection; (5) had incomplete records, including postoperative computed tomography (CT) data; and (6) had recurrence were excluded. All the data for the current study were collected in accordance with the University of Tsukuba Hospital Institutional Review Board.
Major hepatectomy was defined as right trisegmentectomy, left trisegmentectomy, right hepatectomy or left hepatectomy (LH), while minor hepatectomy was defined as segmentectomy (Seg), subsegmentectomy, or nonanatomical hepa
In 2006, an IDT-16 multidetector row CT scanner (Philips, Netherlands) was used to obtain images with a slice thickness of 5 mm. From 2007 to 2013, a Brilliance 64 multidetector row CT scanner (Philips, Netherlands) was used to obtain images with slice thicknesses of 2-5 mm. From 2014 to the present, an iCT 128 multidetector row CT scanner (Philips, Netherlands) was used to obtain 1-2 mm image slices. Medical image analysis software was used for volumetry (Synapse Vincent® version 6.7; Fujifilm Global, Tokyo, Japan). Volumetric values were obtained by the inherent software volume rendering algorithm. Accurate simulation of liver resection was performed postoperatively by manually tracing the resected border on CT images based on intraoperative images of the resected liver plane, resected specimen, and medical records. The total liver volume (TLV), resected liver volume (RLV), tumor volume (Tuv), and postoperative liver volume were automatically measured. Postoperative liver volume was measured at 3 months (Supplementary Figure 1), 6 months, and 12 months after surgery. The total functional liver volume (TFLV) was calculated by subtracting Tuv from the TLV, and the RLV was calculated by subtracting Tuv from the postoperative reconstructed resected specimen volume. The liver resection percentage (%), regeneration index (RI) (%), remnant liver growth rate (fold) and late regeneration rate (fold) were calculated according to the following formulas.
Liver resection percentage (%) = [postoperative reconstructed resected specimen volume (mL)-Tuv (mL)]/[TFLV (mL)] × 100.
RI (%) = [postoperative liver volume (mL)]/[TFLV (mL)] × 100.
Remnant liver growth rate (fold) = [postoperative remnant liver volume (mL)]/[TFLV (mL) - RLV (mL)].
Late regeneration rate (fold) = [RI at 6 months (%) - RI at 3 months (%)]/[RI at 6 months (%)].
The patients were classified into the “low regeneration” group based on the RI at 3 months and 12 months, which was < the 25th percentile. “Delayed liver regeneration” was defined as a late regeneration rate ≥ the 25th percentile. “Restoration to the original volume” was defined as regeneration of the remnant liver to more than 90% of the TFLV.
Categorical variables were compared using the chi-square test or Fisher’s exact test. Continuous variables are expressed as the median, minimum, or maximum. Pearson’s correlation coefficients were calculated, and unpaired t tests were used to compare two groups. Comparisons among more than three groups were performed using one-way analysis of variance. Significant differences were examined using the Bonferroni-Dunn multiple comparison post hoc test. Low regeneration, delayed regeneration and volume restoration failure were analyzed using univariate and multivariate logistic regression models. Receiver operating characteristic (ROC) analysis was also conducted using the constructed multivariate logistic model. Independent predictors in the multivariate model were selected based on the results of the univariate analysis (P < 0.10). P < 0.05 was considered to indicate statistical significance. All the statistical analyses were performed using SPSS 29.0 (SPSS, Inc., Chicago, IL, United States).
A total of 268 patients who underwent partial hepatectomy were included. Patients were classified according to the hepatectomy type: Right hepatectomy and trisegmentectomy (RH/Tri) group, LH group, Seg group, and subsegmentectomy and nonanatomical hepatectomy (Sub/Non) group. The numbers of patients in the RH/Tri, LH, Seg, and Sub/Non groups were 41, 53, 99 and 75, respectively (Table 1). All patients demonstrated similar baseline characteristics except for body mass index (BMI) (kg/m2), background disease, hepatitis C virus positivity, liver resection percentage (%), heavy alcohol history and preoperative platelet count (µL) (Table 1).
| RH/Tri group (n = 41) | LH group (n = 53) | Seg group (n = 99) | Sub/Non group (n = 75) | P value | |
| Baseline characteristics | |||||
| Age | 68 (16-80) | 69 (14-86) | 68 (46-83) | 70 (52-90) | 0.06 |
| Sex, male, yes | 29 (71) | 33 (62) | 73 (74) | 58 (77) | 0.29 |
| BMI (kg/m2) | 21.8 (15.4-31.3) | 22.3 (15.2-29.4) | 230 (15.5-32.8) | 23.4 (17.8-35.5) | 0.009 |
| Background disease | |||||
| HCC | 21 (51) | 14 (26) | 63 (64) | 56 (75) | < 0.001 |
| CCC & CHC | 4 (10) | 19 (36) | 9 (9) | 8 (11) | - |
| Colorectal liver metastasis | 10 (24) | 6 (11) | 16 (16) | 11 (15) | - |
| Others | 6 (15) | 14 (26) | 11 (11) | 0 (0) | - |
| HCV, yes | 6 (15) | 9 (17) | 26 (26) | 29 (39) | 0.01 |
| Liver resection percentage | 52 (21-71) | 32 (13-49) | 27 (10-56) | 15 (10-32) | < 0.001 |
| Diabetes mellitus, yes | 13 (32) | 16 (30) | 34 (34) | 33 (44) | 0.36 |
| Renal complication | 3 (7) | 3 (6) | 6 (6) | 6 (8) | 0.94 |
| Heavy alcoholic1 | 15 (37) | 9 (17) | 39 (39) | 32 () | 0.03 |
| ICG 15 min | 10.7 (0.1-81.1) | 9.0 (1.4-66.4) | 10.1 (1.3-80.0) | 11.5 (2.8-33.4) | 0.65 |
| Preoperative ALBI score | -2.56 (-3.46 to -1.40) | -2.73 (-3.50 to -0.69) | -2.85 (-3.75 to -1.51) | -2.78 (-3.38 to -1.82) | 0.07 |
| Preoperative platelet count (/uL) | 205 (95-562) | 198 (78-504) | 186 (61-542) | 182 (91-396) | 0.02 |
| Liver cirrhosis (F4), yes | 4 (10) | 2 (4) | 18 (18) | 9 (12) | 0.08 |
| Laparoscopic hepatectomy | 3 (7) | 2 (38) | 13 (13) | 7 (9) | 0.28 |
| Re-hepatectomy | 3 (7) | 4 (8) | 5 (5) | 7 (9) | 0.74 |
| Preoperative Chemotherapy | 10 (24) | 9 (17) | 15 (15) | 5 (7) | 0.06 |
| Postoperative outcomes | |||||
| Operation time (min) | 383 (218-644) | 354 (183-629) | 388 (151-742) | 341 (153-584) | 0.008 |
| Intraoperative blood loss (mL) | 680 (110-13360) | 378 (68-3660) | 525 (0-32150) | 470 (0-1894) | 0.25 |
| RBC transfusion | 6 (15) | 3 (6) | 8 (8) | 3 (4) | 0.19 |
| Total Pringle time (min) | 56 (0-179) | 45 (0-131) | 75 (0-202) | 78 (0-167) | < 0.001 |
| Posthepatectomy liver failure grade B/C | 10 (24) | 3 (6) | 7 (7) | 3 (4) | 0.001 |
| Complication CD grade ≥ 3 | 9 (22) | 10 (19) | 22 (22) | 12 (16) | 0.75 |
| Length of Hospital stay (d) | 16 (7-41) | 11 (5-54) | 11 (6-40) | 11 (6-167) | 0.46 |
Regarding postoperative outcomes, there were significant differences in operation time (min), total Pringle maneuver time (min), and PHLF grade B/C occurrence rate (%) among the groups. In particular, the incidence rate of PHLF was significantly greater in the RH/Tri group (Table 1).
The RI plateaued at 3 months in the LH, Seg and Sub/Non groups, whereas the RI continued to increase until 12 months in the RH/Tri group (Figure 1A). A comparison of the RIs among these four groups at 3 months showed that the RIs were significantly lower in the RH/Tri group than in the other groups (77% vs 85% vs 90% vs 92%, Figure 1B). However, there was no difference in the RI between the RH/Tri group and the LH group (85% and 87%, P = 0.12) at 12 months; both of these values are below the criterion for restoring the original volume. On the other hand, the RIs in the RH/Tri group were significantly lower than those in the Seg group and the Sub/Non group (92% and 93%, Figure 1B), both of which met the criterion of restoring the original volume.
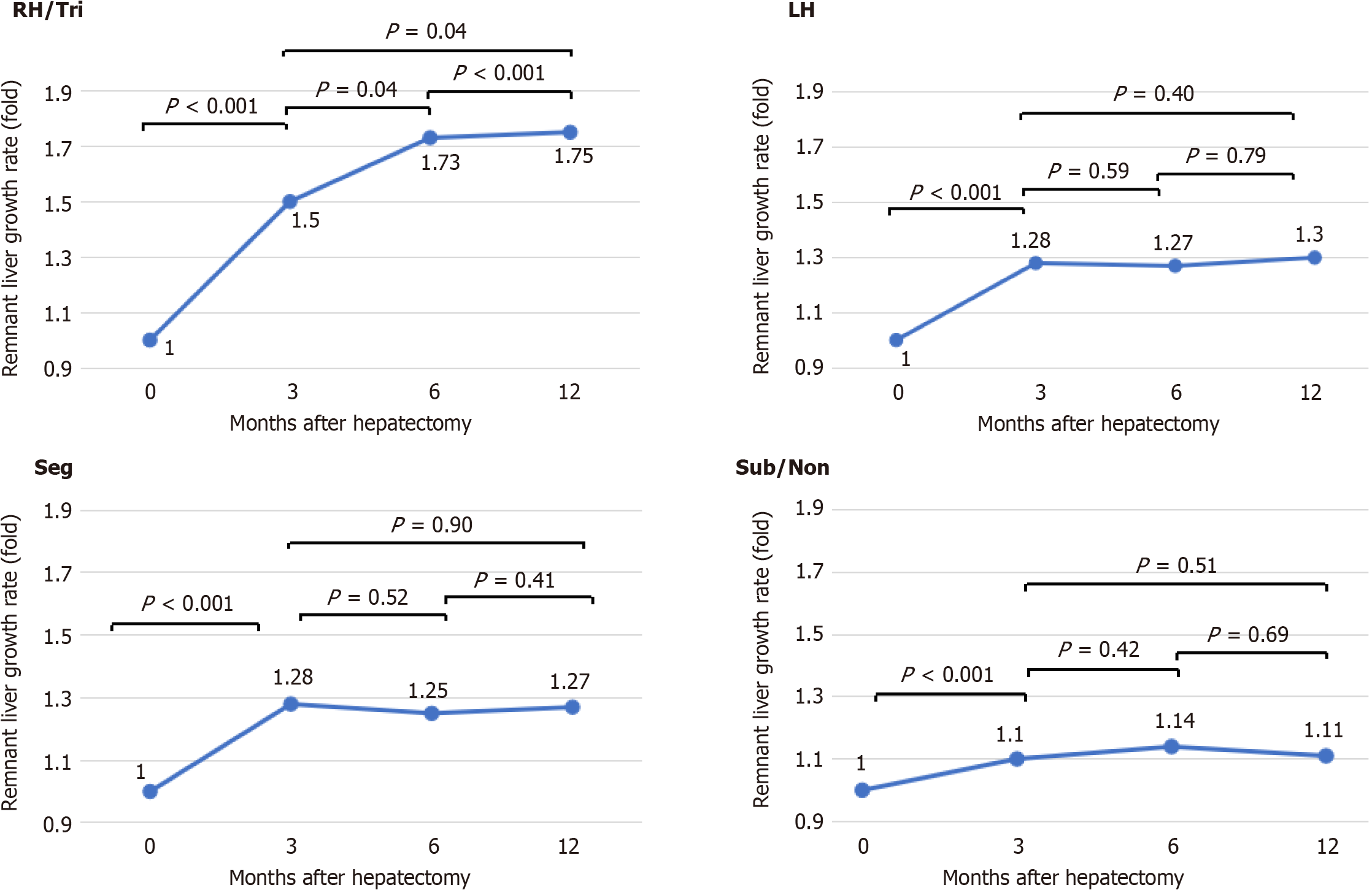
The remnant liver growth rate plateaued at 3 months in the LH, Seg and Sub/Non groups, whereas the remnant liver growth rate continued to increase until 12 months in the RH/Tri group (Figure 2).
Although the postoperative ALBI scores in the Seg group tended to increase during the early period postsurgery, the postoperative ALBI scores in general were not significantly different at any time point in either group at 3, 6 or 12 months posthepatectomy (Supplementary Figure 2A). There was no difference in the postoperative ALBI score among the four groups at 3 or 12 months (Supplementary Figure 2B).
Univariate analysis revealed that a background disease of CCC or CHC (compared with that of HCC), a preoperative ALBI score (per 1.0 up), rehepatectomy and a postoperative complication according to the Clavien-Dindo (CD) classification of grade 3 or higher were associated with low regeneration at 3 months (Table 2). Multivariate analysis demonstrated that the preoperative ALBI score [per 1.0 up, odds ratio (OR) 95%CI = 2.80 (1.17-6.69), P = 0.02] and a postoperative complication CD classification of grade 3 or higher [OR = 0.29 (0.10-0.82), P = 0.02] were found to be independent risk factors for low regeneration at 3 months. ROC analysis demonstrated an area under the ROC curve (AUROC) of 0.60 for the ALBI score, which was the highest among the representative liver function parameters, Child-Pugh score, model for end-stage liver disease (MELD) score, MELD-Na score, and indocyanine green 15-min rate (0.53, 0.56, 0.58, 0.55, respectively), and its cutoff value was -2.69.
| 3-months (n = 187) | 12-months (n = 192) | |||||||
| Univariate OR (95%CI) | P value | Multivariate OR (95%CI) | P value | Univariate OR (95%CI) | P value | Multivariate OR (95%CI) | P value | |
| Age > 75, yes | 0.69 (0.30-1.56) | 0.37 | - | - | 0.48 (0.19-1.24) | 0.13 | - | - |
| Sex, male, yes | 1.40 (0.66-32.94) | 0.38 | - | - | 0.79 (0.39-1.62) | 0.79 | - | - |
| BMI >28 kg/m2, yes | 0.19 (0.02-1.44) | 0.11 | - | - | 0.42 (0.09-1.94) | 0.42 | - | - |
| Background, HCC | - | Ref. | - | Ref. | ||||
| CCC & CHC | 0.30 (0.09-1.08) | 0.07 | 0.31 (0.08-1.17) | 0.08 | 0.81 (0.31-2.07) | 0.65 | - | - |
| Colorectal liver metastasis | 0.98 (0.41-2.38) | 0.96 | 0.88 (0.35-2.21) | 0.79 | 0.88 (0.34-2.27) | 0.79 | - | - |
| Others | 0.76 (0.31-2.37) | 0.76 | 0.93 (0.30-2.88) | 0.90 | 0.97 (0.35-2.71) | 0.96 | - | - |
| HCV positive, yes | 0.63 (0.29-1.38) | 0.25 | - | - | 1.18 (0.58-2.39) | 0.65 | ||
| Diabetes mellitus, yes | 0.93 (0.47-1.83) | 0.84 | - | - | 0.77 (0.39-1.52) | 0.45 | - | - |
| Renal complication, yes | 2.50 (0.73-8.60) | 0.15 | - | - | 0.51 (0.11-2.37) | 0.39 | - | - |
| Heavy alcoholic, yes | 1.10 (0.56-2.18) | 0.77 | - | - | 0.86 (0.44-1.69) | 0.66 | - | - |
| Preoperative platelet count < 100 × 103/L, yes | 2.19 (0.47-10.13) | 0.32 | 5.30 (1.22-23.09) | 0.03 | 5.01 (1.14-21.95) | 0.03 | ||
| Preoperative ALBI score (per 1.0 up) | 2.85 (1.21-6.71) | 0.02 | 2.80 (1.17-6.69) | 0.02 | 2.33 (1.05-5.18) | 0.04 | 2.27 (1.01-5.09) | 0.04 |
| ICG 15 minutes rate (per 1.0 up) | 0.97 (0.93-1.01) | 0.15 | - | - | 1.00 (0.97-1.04) | 0.92 | - | - |
| Liver cirrhosis (F4), yes | 1.22 (0.47-23.14) | 0.69 | - | - | 1.42 (0.51-3.97) | 0.51 | - | - |
| Preoperative chemotherapy, yes | 0.66 (0.25-1.73) | 0.40 | - | - | 0.84 (0.32-2.24) | 0.73 | - | - |
| Re-hepatectomy, yes | 2.65 (0.84-8.33) | 0.09 | 2.81 (0.84-9.47) | 0.09 | 0.28 (0.04-2.25) | 0.23 | - | - |
| Liver resection percentage (per 1.0% up) | 1.01 (0.99-1.04) | 0.22 | - | - | 1.01 (0.99-1.04) | 0.24 | - | - |
| Intraoperative blood loss > 1000mL, yes | 1.73 (0.83-3.62) | 0.14 | - | - | 1.04 (0.47-2.34) | 0.92 | - | - |
| RBC transfusion, yes | 0.75 (0.20-2.82) | 0.67 | - | - | 0.81 (0.22-3.02) | 0.75 | - | - |
| Pringle time > 90 min, yes | 1.07 (0.49-2.34) | 0.86 | - | - | 1.09 (0.51-2.31) | 0.83 | - | - |
| Posthepatectomy liver failure grade B/C, yes | 0.58 (0.16-2.10) | 0.41 | - | - | 0.72 (0.21-2.93) | 0.72 | - | - |
| Postoperative complication CD grade ≥ 3, yes | 0.31 (0.11-0.84) | 0.02 | 0.29 (0.10-0.82) | 0.02 | 1.79 (0.83-3.87) | 0.14 | - | - |
Univariate analysis revealed that a preoperative platelet count < 100 × 103/µL and a preoperative ALBI score (per 1.0 up) were associated with low regeneration at 12 months (Table 2). Multivariate analysis demonstrated that both a preoperative platelet count < 100 × 103/µL [OR = 5.01 (1.14-21.95), P = 0.03} and a preoperative ALBI score [per 1.0 up, OR = 2.27 (1.01-5.09), P = 0.04] were found to be independent risk factors for low regeneration at 12 months. ROC analysis demonstrated an AUROC of 0.57 for the ALBI score, and its cutoff value was -2.73.
Pathologically, F3 and F4 were classified into the severe fibrosis group. In the RH/Tri and LH groups, patients in the severe fibrosis group tended to have delayed liver regeneration, but the final liver volume at 12 months was not different. On the other hand, in the Seg and Sub/Non groups, the RI did not significantly differ between the severe fibrosis group and the nonsevere liver fibrosis group at any time point (Supplementary Figure 3). The preoperative ALBI score and F stage were not correlated (r = 0.08, P = 0.22).
Univariate analysis revealed that only the liver resection percentage [OR = 1.03 (1.00-1.05), P = 0.04] was associated with delayed regeneration (Table 3). ROC analysis demonstrated an AUROC of 0.61 for the liver resection percentage, and its cutoff value was 26%.
| Delayed regeneration (total n = 153) | Volume restoration failure (total n = 191) | |||||||
| Univariate OR (95%CI) | P value | Multivariate OR (95%CI) | P value | Univariate OR (95%CI) | P value | Multivariate OR (95%CI) | P value | |
| Age > 75, yes | 0.67 (0.27-1.68) | 0.45 | - | - | 0.94 (0.46-1.94) | 0.88 | - | - |
| Gender, male, yes | 1.14 (0.48-2.67) | 0.77 | - | - | 0.76 (0.40-1.45) | 0.41 | - | - |
| BMI > 28 kg/m2, yes | 0.22 (0.03-1.74) | 0.15 | - | - | 0.44 (0.14-1.46) | 0.18 | - | - |
| Background, HCC | - | Ref. | - | Ref. | ||||
| CCC & CHC | 0.49 (0.13-1.82) | 0.29 | - | - | 1.64 (0.73-3.65) | 0.23 | 0.40 (0.15-1.11) | 0.08 |
| Colorectal liver metastasis | 1.83 (0.73-4.56) | 0.19 | - | - | 0.88 (0.34-1.90) | 0.81 | 0.71 (0.22-2.26) | 0.56 |
| Others | 1.10 (0.27-4.42) | 0.90 | - | - | 2.39 (0.95-6.00) | 0.07 | 0.28 (0.08-0.97) | 0.44 |
| HCV positive, yes | 0.61 (0.26-1.47) | 0.27 | - | - | 0.70 (0.37-1.34) | 0.28 | - | - |
| Diabetes mellitus, yes | 0.64 (0.30-1.39) | 0.26 | - | - | 0.55 (0.30-1.00) | 0.05 | 0.69 (0.36-1.35) | 0.28 |
| Renal complication, yes | 0.89 (0.18-4.48) | 0.88 | - | - | 1.15 (0.37-3.56) | 0.81 | - | - |
| Heavy alcoholic, yes | 0.86 (0.39-1.88) | 0.71 | - | - | 0.84 (0.46-1.53) | 0.56 | - | - |
| Preoperative platelet count < 100 × 103/uL, yes | 1.60 (0.28-9.11) | 0.60 | - | - | 4.17 (0.82-21.21) | 0.09 | 4.34 (0.81-23.40) | 0.09 |
| Preoperative ALBI score (per 1.0 up) | 1.13 (0.46-2.81) | 0.79 | - | - | 2.76 (1.27-5.97) | 0.01 | 2.63 (1.15-6.04) | 0.02 |
| ICG 15 min rate (per 1.0 up) | 1.00 (0.97-1.04) | 0.99 | - | - | 1.00 (0.98-1.04) | 0.58 | - | - |
| Liver cirrhosis (F4), yes | 1.94 (0.70-5.38) | 0.20 | - | - | 1.22 (0.47-3.15) | 0.69 | - | - |
| Preoperative chemotherapy, yes | 2.01 (0.80-5.03) | 0.14 | - | - | 0.80 (0.34-1.87) | 0.60 | - | - |
| Re-hepatectomy, yes | 1.67 (0.40-7.03) | 0.49 | - | - | 0.74 (0.21-2.62) | 0.64 | - | - |
| Liver resection percentage (per 1.0% up) | 1.03 (1.00-1.05) | 0.04 | 1.03 (1.00-1.05) | 0.04 | 1.03 (1.01-1.05) | 0.008 | 1.02 (1.00-1.05) | 0.04 |
| Intraoperative blood loss > 1000 mL, yes | 1.35 (0.58-3.15) | 0.49 | - | - | 1.41 (0.69-2.86) | 0.34 | - | - |
| RBC transfusion, yes | 0.55 (0.12-2.58) | 0.58 | - | - | 1.04 (0.35-3.12) | 0.95 | - | - |
| Pringle time > 90 min, yes | 1.82 (0.80-4.14) | 0.16 | - | - | 0.91 (0.46-1.81) | 0.78 | - | - |
| Posthepatectomy liver failure grade B/C, yes | 0.95 (0.24-3.60) | 0.92 | - | - | 1.83 (0.61-5.50) | 0.28 | - | - |
| Postoperative complication CD grade ≥ 3, yes | 0.54 (0.19-1.53) | 0.24 | - | - | 1.97 (0.95-4.07) | 0.07 | 1.80 (0.83-3.91) | 0.14 |
Univariate analysis revealed that other background diseases (compared with HCC), the presence of diabetes mellitus, a preoperative platelet count < 100 × 103/µL, a preoperative ALBI score (per 1.0 up), liver resection percentage, and postoperative complication CD classification of grade 3 or higher were associated with volume restoration failure. The multivariate analysis demonstrated that the preoperative ALBI score [per 1.0 up, OR = 2.63 (1.15-6.04), P = 0.02] and the liver resection percentage [OR = 1.02 (1.00-1.05), P = 0.04] were found to be independent risk factors associated with volume restoration failure.
A heatmap for predicting the probability of volume restoration failure was constructed by combining the preoperative ALBI score and liver resection percentage (Figure 3). The gradient shows the risk level: Blue indicates a low risk of volume restoration failure (less than 10%), whereas red indicates a high risk of volume restoration failure (higher than 90%). As the ALBI score and liver resection percentage increased, the risk of volume restoration failure increased.
The novelty of this study is that liver regeneration after partial hepatectomy was determined by the preoperative liver function reserve reflected by the ALBI score, and prolonged regenerative activity was dependent on the liver resection percentage. It was also acknowledged that although the remnant liver volume after minor hepatectomy could return to its initial size, the residual liver volume after major resection does not necessarily return to its original volume. Furthermore, the degree to which the original volume was restored after hepatectomy was determined by the preoperative ALBI score and the liver resection percentage. This knowledge, along with our heatmap, can aid surgeons in deciding the timing and type of hepatectomy after intrahepatic recurrence.
Liver resection success relies on the ability of the remnant liver to regenerate. Most of the knowledge regarding the pathophysiological basis of liver regeneration comes from rodent studies, and data on humans are scarce. Among the limited reports on humans, early studies in the 1970s and 1980s revealed that mitotic activity occurred 3 d after partial hepatectomy, and cellular proliferation continued for several weeks[8]. The liver remnant returned to its original size in approximately 3 to 6 months. At that time, the liver volume was calculated by multiplying the liver area measured by hand using tracing paper and a CT width greater than 1 cm[8]. This could cause significant mismatches in the extraction results, depending on the examiners. On the other hand, studies from the 1990s to the early 2000s reported different results based on the experience in donor hepatectomy. Using prototype CT-volumetric software, they reported that the volume of the normal liver after major hepatectomy increased up to 70%-80% in 6 months and up to 85%-90% in 12 months; however, the liver did not reach its initial volume[9-11]. In our study, we used the latest CT-volumetric software, Synapse Vincent version 6.7, to measure postoperative remnant liver volume. This software calculates liver volume based on a high-speed and precise image-processing algorithm that uses an image processing method[12]. Using two different regeneration parameters (the RI and remnant liver growth rate), the remnant liver continued to regenerate until 12 months after right hepatectomy/trisegmentectomy. On the other hand, regeneration stopped less than 3 months after LH and minor hepatectomy. The remnant liver volume 12 months after RH/Tri increased but was not significantly different from that after LH and did not return to the original volume, while the liver volume after minor hepatectomy returned to almost the initial size. Regardless of the type of hepatectomy, postoperative liver function at 3, 6, and 12 months did not differ from preoperative liver function. In addition, a prolonged regenerative response was related to the amount of liver removed, especially for patients who underwent more than one-fourth resection. The difference in these regenerative reactions depends on the hepatectomy type, and it has been reported that the regenerative response of hepatocytes is altered by different resection rates[13,14]. In animal studies, hepatocytes removed via 1/3 hepatectomy were less proliferatively active and quieter and had a slower response than hepatocytes removed via 2/3 hepatectomy, and the rege
Liver regeneration after hepatectomy is modified by various factors, such as age, sex, BMI, degree of liver fibrosis, native liver disease, chemotherapy, preoperative portal pressure, and steatosis[4,19-21]. In animal models, platelets promoted liver regeneration by enhancing the production of TNF-α and IL-6 and accelerating hepatocyte mitosis in the acute phase after hepatectomy[22]. It has previously been shown that hepatic fibrosis has a negative impact on liver regeneration; i.e., the liver regeneration in cirrhotic livers is significantly slower and less complete than that in noncirrhotic livers[20,23]. This was explained by the hypothesis that the regeneration-promoting factors TNF-α, IL-6 and HGF were significantly lower in cirrhotic livers than in normal livers[24]. However, some cirrhotic livers display a fair degree of regenerative capacity[19,25]. In fact, in our study, the severely fibrotic liver eventually regenerated to a point that was comparable to that of a normal liver. Interestingly, the progression of liver fibrosis did not directly affect postoperative low regeneration or delayed regeneration; rather, the preoperative ALBI score was more influential. Considering cutoff values of -2.69 and -2.73 at 3 months and 12 months, posthepatectomy liver regeneration could be attenuated in patients with preoperative liver function and an ALBI grade 2a or higher. This was because the progression of liver fibrosis does not always correlate with liver function, and liver function usually decreases after cirrhosis has progressed to advanced stages. Moreover, the attenuation of liver regeneration in patients with a low preoperative liver function reserve could be attributed to the disturbance of the intrahepatic microcirculation of materials such as oxygen, proteins, and growth factors in hepatocytes[26]. This condition is called “sinusoidal capillarization”, which results in the defenestration of hepatic sinusoidal endothelial cells and the formation of a continuous basement membrane, compromising the bidirectional exchange of materials between sinusoids and hepatocytes and resulting in hepatocellular dysfunction. Sinusoidal capillarization is a reversible condition that is usually recognized in chronic liver diseases, such as alcoholic liver disease, nonalcoholic fatty liver disease, and chronic hepatitis viral disease. However, sinusoidal capillarization does not necessarily correlate with the fibrotic stage[25], which might explain why the preoperative ALBI score correlated more with low regeneration than with severe fibrosis. Another interesting finding of this study was that postoperative complication CD classifications of grade 3 or higher were related to greater regeneration at 3 months postsurgery. This might be because proinflammatory cytokines such as IL-6 and TNF-α released from Kupffer cells or liver sinusoidal endothelial cells also promote cytokines conducive to liver regeneration[1].
Parenchymal sparing hepatectomy has been proposed for its ability to prevent PHLF and its oncological efficacy against postoperative recurrence[27]. Owing to the improved accuracy of preoperative and intraoperative imaging modalities that are not limited to open hepatectomies, “parenchymal sparing hepatectomy” is widely applied in minimally invasive surgeries such as laparoscopic and robot-assisted hepatectomies. However, its effectiveness in performing hepatectomies adjacent to major large vessels and for accessing posterior superior segments (S7 and S8) has still not been established[28]. In accordance with “parenchymal sparing hepatectomy”, surgeons will try to resect livers with the smallest volume possible. However, when the tumor is located close to a major blood vessel or deep inside the liver, it is often difficult to decide whether to choose major hepatectomy or Seg, which are technically simpler, but the resection volumes are larger, or subsegmentectomy or nonanatomical hepatectomy, which are sometimes technically more complicated, but the resection volumes are smaller. In our study, although the remnant liver volume after minor hepatectomy returned to the initial size, the residual liver volume after major hepatectomy did not return to its original volume. Based on these results, from the perspective of liver regeneration, it may be preferable to avoid major hepatectomies and instead choose minor hepatectomies when the minor hepatectomy can completely remove the tumor. However, since the liver regenerates to the original volume after minor hepatectomy, there is no need to forcibly choose more complicated minor hepatectomy procedures, such as subsegmentectomy or nonanatomical resection, as long as the preoperative liver function is sufficient to avoid severe PHLF, which can be assessed by preoperative surgical planning[29]. Instead, Seg, which is usually performed with a simpler resection procedure, might be preferable. This might be especially true in cases of minimally invasive surgeries that require the creation of complicated resection planes in a limited surgical view. This approach not only prolongs the operation time but can also cause intraoperative and postoperative complications such as bleeding and bile leakage. Specifically, robot-assisted hepatectomy requires the use of an inflexible scope, which obscures the surgical view in hepatectomies in the subphrenic area, such as in S7, S8, and S4a. Therefore, the usefulness of “parenchymal sparing hepatectomy” can vary depending on the patient and modality.
This study has several limitations. First, the data were retrospective in nature and were collected from a single center with a small sample size. Second, although we have evaluated different percentages of partial hepatectomy patients overall, the question remains as to whether this potentially different mode of regeneration can be assessed in the same way. In addition to the difference in the number of hepatocytes involved in regeneration depending on the resection rate, it was also reported that the remnant liver following 30% partial hepatectomy regenerated solely by hypertrophy without cell division; hypertrophy and subsequent proliferation equally contributed to regeneration after 70% partial hepa
Liver regeneration after partial hepatectomy was determined by the liver resection percentage and the preoperative liver function reserve reflected by the ALBI score. With this knowledge and our heatmap, surgeons can choose the appropriate hepatectomy type on a case- and modality-specific basis, with rehepatectomy in mind after intrahepatic recurrence.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: The Japanese Society of Gastroenterology, G0323676.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Elchaninov AV, Russia; Massimi M, Italy S-Editor: Li L L-Editor: A P-Editor: Zhao YQ
| 1. | Michalopoulos GK, DeFrances MC. Liver regeneration. Science. 1997;276:60-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2649] [Cited by in RCA: 2467] [Article Influence: 88.1] [Reference Citation Analysis (0)] |
| 2. | Yagi S, Hirata M, Miyachi Y, Uemoto S. Liver Regeneration after Hepatectomy and Partial Liver Transplantation. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 121] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 3. | Michalopoulos GK, Bhushan B. Liver regeneration: biological and pathological mechanisms and implications. Nat Rev Gastroenterol Hepatol. 2021;18:40-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 647] [Cited by in RCA: 573] [Article Influence: 143.3] [Reference Citation Analysis (1)] |
| 4. | Shimada M, Matsumata T, Maeda T, Itasaka H, Suehiro T, Sugimachi K. Hepatic regeneration following right lobectomy: estimation of regenerative capacity. Surg Today. 1994;24:44-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 5. | Akamatsu N, Sugawara Y, Kaneko J, Sano K, Imamura H, Kokudo N, Makuuchi M. Effects of middle hepatic vein reconstruction on right liver graft regeneration. Transplantation. 2003;76:832-837. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 72] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 6. | Wakabayashi G, Cherqui D, Geller DA, Abu Hilal M, Berardi G, Ciria R, Abe Y, Aoki T, Asbun HJ, Chan ACY, Chanwat R, Chen KH, Chen Y, Cheung TT, Fuks D, Gotohda N, Han HS, Hasegawa K, Hatano E, Honda G, Itano O, Iwashita Y, Kaneko H, Kato Y, Kim JH, Liu R, López-Ben S, Morimoto M, Monden K, Rotellar F, Sakamoto Y, Sugioka A, Yoshiizumi T, Akahoshi K, Alconchel F, Ariizumi S, Benedetti Cacciaguerra A, Durán M, Garcia Vazquez A, Golse N, Miyasaka Y, Mori Y, Ogiso S, Shirata C, Tomassini F, Urade T, Wakabayashi T, Nishino H, Hibi T, Kokudo N, Ohtsuka M, Ban D, Nagakawa Y, Ohtsuka T, Tanabe M, Nakamura M, Tsuchida A, Yamamoto M. The Tokyo 2020 terminology of liver anatomy and resections: Updates of the Brisbane 2000 system. J Hepatobiliary Pancreat Sci. 2022;29:6-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 102] [Article Influence: 34.0] [Reference Citation Analysis (0)] |
| 7. | Rahbari NN, Garden OJ, Padbury R, Brooke-Smith M, Crawford M, Adam R, Koch M, Makuuchi M, Dematteo RP, Christophi C, Banting S, Usatoff V, Nagino M, Maddern G, Hugh TJ, Vauthey JN, Greig P, Rees M, Yokoyama Y, Fan ST, Nimura Y, Figueras J, Capussotti L, Büchler MW, Weitz J. Posthepatectomy liver failure: a definition and grading by the International Study Group of Liver Surgery (ISGLS). Surgery. 2011;149:713-724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1224] [Cited by in RCA: 1719] [Article Influence: 122.8] [Reference Citation Analysis (0)] |
| 8. | Nagasue N, Yukaya H, Ogawa Y, Kohno H, Nakamura T. Human liver regeneration after major hepatic resection. A study of normal liver and livers with chronic hepatitis and cirrhosis. Ann Surg. 1987;206:30-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 209] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 9. | Haga J, Shimazu M, Wakabayashi G, Tanabe M, Kawachi S, Fuchimoto Y, Hoshino K, Morikawa Y, Kitajima M, Kitagawa Y. Liver regeneration in donors and adult recipients after living donor liver transplantation. Liver Transpl. 2008;14:1718-1724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 97] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 10. | Pomfret EA, Pomposelli JJ, Gordon FD, Erbay N, Lyn Price L, Lewis WD, Jenkins RL. Liver regeneration and surgical outcome in donors of right-lobe liver grafts. Transplantation. 2003;76:5-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 140] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 11. | Pascher A, Sauer IM, Walter M, Lopez-Haeninnen E, Theruvath T, Spinelli A, Neuhaus R, Settmacher U, Mueller AR, Steinmueller T, Neuhaus P. Donor evaluation, donor risks, donor outcome, and donor quality of life in adult-to-adult living donor liver transplantation. Liver Transpl. 2002;8:829-837. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 111] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 12. | Ohshima S. Volume analyzer synapse Vincent for liver analysis. J Hepatobiliary Pancreat Sci. 2014;21:235-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 97] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 13. | Meier M, Andersen KJ, Knudsen AR, Nyengaard JR, Hamilton-Dutoit S, Mortensen FV. Liver regeneration is dependent on the extent of hepatectomy. J Surg Res. 2016;205:76-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 38] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 14. | Mitchell C, Nivison M, Jackson LF, Fox R, Lee DC, Campbell JS, Fausto N. Heparin-binding epidermal growth factor-like growth factor links hepatocyte priming with cell cycle progression during liver regeneration. J Biol Chem. 2005;280:2562-2568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 128] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 15. | Al-Ghamdi TH, Atta IS, El-Refaei M. Role of interleukin 6 in liver cell regeneration after hemi-hepatectomy, correlation with liver enzymes and flow cytometric study. Clin Exp Hepatol. 2020;6:42-48. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 16. | Bucher NL, Swaffield MN. The rate of incorporation of labeled thymidine into the deoxyribonucleic acid of regenerating rat liver in relation to the amount of liver excised. Cancer Res. 1964;24:1611-1625. [PubMed] |
| 17. | Yamada A, Kawata S, Tamura S, Kiso S, Higashiyama S, Umeshita K, Sakon M, Taniguchi N, Monden M, Matsuzawa Y. Plasma heparin-binding EGF-like growth factor levels in patients after partial hepatectomy as determined with an enzyme-linked immunosorbent assay. Biochem Biophys Res Commun. 1998;246:783-787. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 18. | Inoue Y, Fujii K, Ishii M, Kagota S, Tomioka A, Hamamoto H, Osumi W, Tsuchimoto Y, Masubuchi S, Yamamoto M, Asai A, Komeda K, Shimizu T, Asakuma M, Fukunishi S, Hirokawa F, Narumi Y, Higuchi K, Uchiyama K. Volumetric and Functional Regeneration of Remnant Liver after Hepatectomy. J Gastrointest Surg. 2019;23:914-921. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 19. | Shirabe K, Motomura T, Takeishi K, Morita K, Kayashima H, Taketomi A, Ikegami T, Soejima Y, Yoshizumi T, Maehara Y. Human early liver regeneration after hepatectomy in patients with hepatocellular carcinoma: special reference to age. Scand J Surg. 2013;102:101-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 20. | Aierken Y, Kong LX, Li B, Liu XJ, Lu S, Yang JY. Liver fibrosis is a major risk factor for liver regeneration: A comparison between healthy and fibrotic liver. Medicine (Baltimore). 2020;99:e20003. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 21. | Gupta A, Patil NS, Mohapatra N, Benjamin J, Thapar S, Kumar A, Rastogi A, Pamecha V. Lifestyle Optimization Leads to Superior Liver Regeneration in Live Liver Donors and Decreases Early Allograft Dysfunction in Recipients: A Randomized Control Trial. Ann Surg. 2023;278:e430-e439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 22. | Matsuo R, Nakano Y, Ohkohchi N. Platelet administration via the portal vein promotes liver regeneration in rats after 70% hepatectomy. Ann Surg. 2011;253:759-763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 96] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 23. | Chen MF, Hwang TL, Hung CF. Human liver regeneration after major hepatectomy. A study of liver volume by computed tomography. Ann Surg. 1991;213:227-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 117] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 24. | Shan YS, Hsieh YH, Sy ED, Chiu NT, Lin PW. The influence of spleen size on liver regeneration after major hepatectomy in normal and early cirrhotic liver. Liver Int. 2005;25:96-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 25. | Ogata T, Okuda K, Ueno T, Saito N, Aoyagi S. Serum hyaluronan as a predictor of hepatic regeneration after hepatectomy in humans. Eur J Clin Invest. 1999;29:780-785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 26. | Mak KM, Kee D, Shin DW. Alcohol-associated capillarization of sinusoids: A critique since the discovery by Schaffner and Popper in 1963. Anat Rec (Hoboken). 2022;305:1592-1610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 27. | Torzilli G, McCormack L, Pawlik T. Parenchyma-sparing liver resections. Int J Surg. 2020;82S:192-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 41] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 28. | Katagiri H, Nitta H, Kanno S, Umemura A, Takeda D, Ando T, Amano S, Sasaki A. Safety and Feasibility of Laparoscopic Parenchymal-Sparing Hepatectomy for Lesions with Proximity to Major Vessels in Posterosuperior Liver Segments 7 and 8. Cancers (Basel). 2023;15. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 29. | Takahashi K, Gosho M, Kim J, Shimomura O, Miyazaki Y, Furuya K, Akashi Y, Enomoto T, Hashimoto S, Oda T. Prediction of Posthepatectomy Liver Failure with a Combination of Albumin-Bilirubin Score and Liver Resection Percentage. J Am Coll Surg. 2022;234:155-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 30. | Miyaoka Y, Ebato K, Kato H, Arakawa S, Shimizu S, Miyajima A. Hypertrophy and unconventional cell division of hepatocytes underlie liver regeneration. Curr Biol. 2012;22:1166-1175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 286] [Cited by in RCA: 344] [Article Influence: 26.5] [Reference Citation Analysis (0)] |