Published online Apr 14, 2024. doi: 10.3748/wjg.v30.i14.1990
Peer-review started: December 21, 2023
First decision: January 30, 2024
Revised: February 12, 2024
Accepted: March 19, 2024
Article in press: March 19, 2024
Published online: April 14, 2024
Processing time: 113 Days and 3.6 Hours
Gastric cancer is a common malignant tumor of the digestive tract, and endosco
To analyze the features of gastric mucosal tumors at different differentiation levels, and to explore the prognostic factors of ESD.
We retrospectively studied 301 lesions in 285 patients at The Second Affiliated Hospital of Xi'an Jiaotong University from 2014 to 2021, according to the latest Japanese guidelines (sixth edition), and divided them into low-grade intraepithelial neoplasia (LGIN), high-grade intraepithelial neoplasia (HGIN), and differentiated and undifferentiated early carcinoma. They are followed up by endoscopy, chest and abdominal computed tomography at 3, 6 and 12 months after ESD. We compared clinicopathologic characteristics, ESD efficacy, and complications with different degrees of differentiation, and analyzed the related factors associated with ESD.
HGIN and differentiated carcinoma patients were significantly older compared with LGIN patients (P < 0.001) and accounted for more 0-IIc (P < 0.001), atrophic gastritis was common (P < 0.001), and irregular microvascular patterns (IMVPs) and demarcation lines (DLs) were more obvious (P < 0.001). There was more infiltration in the undifferentiated carcinoma tissue (P < 0.001), more abnormal folds and poorer mucosal peristalsis (P < 0.001), and more obvious IMVPs, irregular microsurface patterns and DLs (P < 0.05) than in the LGIN and HGIN tissues. The disease-free survival rates at 2, 5, and 8 years after ESD were 95.0%, 90.1%, and 86.9%, respectively. Undifferentiated lesions (HR 5.066), white moss (HR 7.187), incomplete resection (HR 3.658), and multiple primary cancers (HR 2.462) were significantly associated with poor prognosis.
Differentiations of gastric mucosal tumors have different epidemiological and endoscopic characteristics, which are closely related to the safety and efficacy of ESD.
Core Tip: Endoscopic diagnosis and treatment of early gastric cancer is a hot topic in gastroenterology. By analyzing gastric mucosal tumors treated with endoscopic submucosal dissection (ESD) at a high-volume center in Northwest China, this study investigated the differences in characteristics of gastric mucosal tumors with different degrees of differentiation and the predictors of ESD efficacy and safety, providing data support for the further development of endoscopic technology.
- Citation: Zhu HY, Wu J, Zhang YM, Li FL, Yang J, Qin B, Jiang J, Zhu N, Chen MY, Zou BC. Characteristics of early gastric tumors with different differentiation and predictors of long-term outcomes after endoscopic submucosal dissection. World J Gastroenterol 2024; 30(14): 1990-2005
- URL: https://www.wjgnet.com/1007-9327/full/v30/i14/1990.htm
- DOI: https://dx.doi.org/10.3748/wjg.v30.i14.1990
Gastric cancer is a malignant tumor of the gastrointestinal tract with high morbidity and mortality. The annual incidence and death rate of gastric cancer in China account for nearly half of the global incidence. The early symptoms of gastric cancer are relatively difficult to identify, and they are usually found in the advanced stage. Surgery and drug treatment are less effective than other methods, so early diagnosis and treatment are crucial measures for improving the survival rate of gastric cancer patients. Early intervention can lead to a 5-year survival rate exceeding 90%[1-3].
Gastric epithelial neoplasia can be divided into nonneoplastic lesions, uncertain neoplastic lesions, low-grade intraepithelial neoplasia (LGIN), high-grade intraepithelial neoplasia (HGIN), early gastric cancer (EGC), and advanced cancer based on the Vienna classification. EGC is a superficial cancer confined to the mucosa and submucosa, with or without lymph node metastasis[4]. Japanese scholars have classified gastric cancer into differentiated and undifferentiated types based on the tissue source. The differentiated types included tubular and papillary adenocarcinomas, while the undifferentiated types included poorly differentiated adenocarcinomas, mucinous adenocarcinomas, and signet ring cell carcinomas[5]. It is widely accepted that intraepithelial neoplasia is a precancerous lesion of gastric cancer that progresses through a process known as the Correa cascade reaction. However, this concept requires further research, and the occurrence and development of gastric cancer and its influencing factors are still unclear[6].
Endoscopic submucosal dissection (ESD) is a minimally invasive technique that originated in Japan for the treatment of EGC. The high safety and short-term efficacy of ESD have led to its use worldwide. Despite the great success of ESD, the endoscopic treatment of gastric cancer is still progressing, and the indications are constantly being updated. According to the latest guidelines in Japan, previous expanded indications are included in absolute indications: Differentiated intramucosal cancer, without ulcers, of any size; differentiated intramucosal cancer, with ulcers, ≤ 3 cm; and undifferentiated intramucosal cancer, without ulcers, ≤ 2 cm. Reports of curative resection rates for ESD vary widely. Lee et al[7] reported an uncurative resection rate of 6.6%-28.4% after ESD. The long-term efficacy of ESD is still under observation and summarized, with discrepancies in different reports[8,9], and the risk factors affecting the efficacy and prognosis of ESD still need to be clarified.
Analysis of the epidemiological characteristics of gastric mucosal tumors with different degrees of differentiation may be important for revealing the developmental pattern and influencing factors of gastric cancer. The outcomes of endoscopic resection are closely related to the degree of differentiation, so accurate preoperative judgment of differentiation type is highly important for successful treatment[10]. This study incorporates epidemiological, pathological, and endoscopic features to compare the differences among intraepithelial neoplasia and differentiated and undifferentiated early cancers, and can help to explore the features and risk factors for gastric mucosal tumors of different differentiation types, which is beneficial for early and accurate diagnosis and prognosis.
This study included EGC patients who underwent ESD at our center from March 2014 to December 2021. We collected patient information through the hospital HIS system. The inclusion criteria for patients were as follows: (1) Patients diagnosed with early gastric tumors based on gastroscopy and biopsy; (2) the relevant imaging examination had no contraindications, such as lymph node or distant metastasis, the patient met the indications for ESD, and informed consent was signed before surgery; and (3) the study protocol was approved by the Medical Ethics Committee (approval number: 2023530). The exclusion criteria for patients were as follows: (1) Patients had gastric mucosal tumors and did not undergo ESD; (2) patients had incomplete follow-up data; and (3) patients had serious cardiovascular, hematologic, neuropsychiatric disease and severe liver and kidney dysfunction.
Preoperative preparation: Endoscopic examinations, such as white light, magnified endoscopy with narrow-band imaging (ME + NBI), and chemical staining (indigo carmine and acetic acid staining), were performed to determine the extent and depth of the lesion, and endoscopic ultrasonography was used to clarify the depth of tumor infiltration. The diet was restricted before surgery, antiplatelet drugs were stopped for 1 wk, and intravenous general anesthesia was initiated.
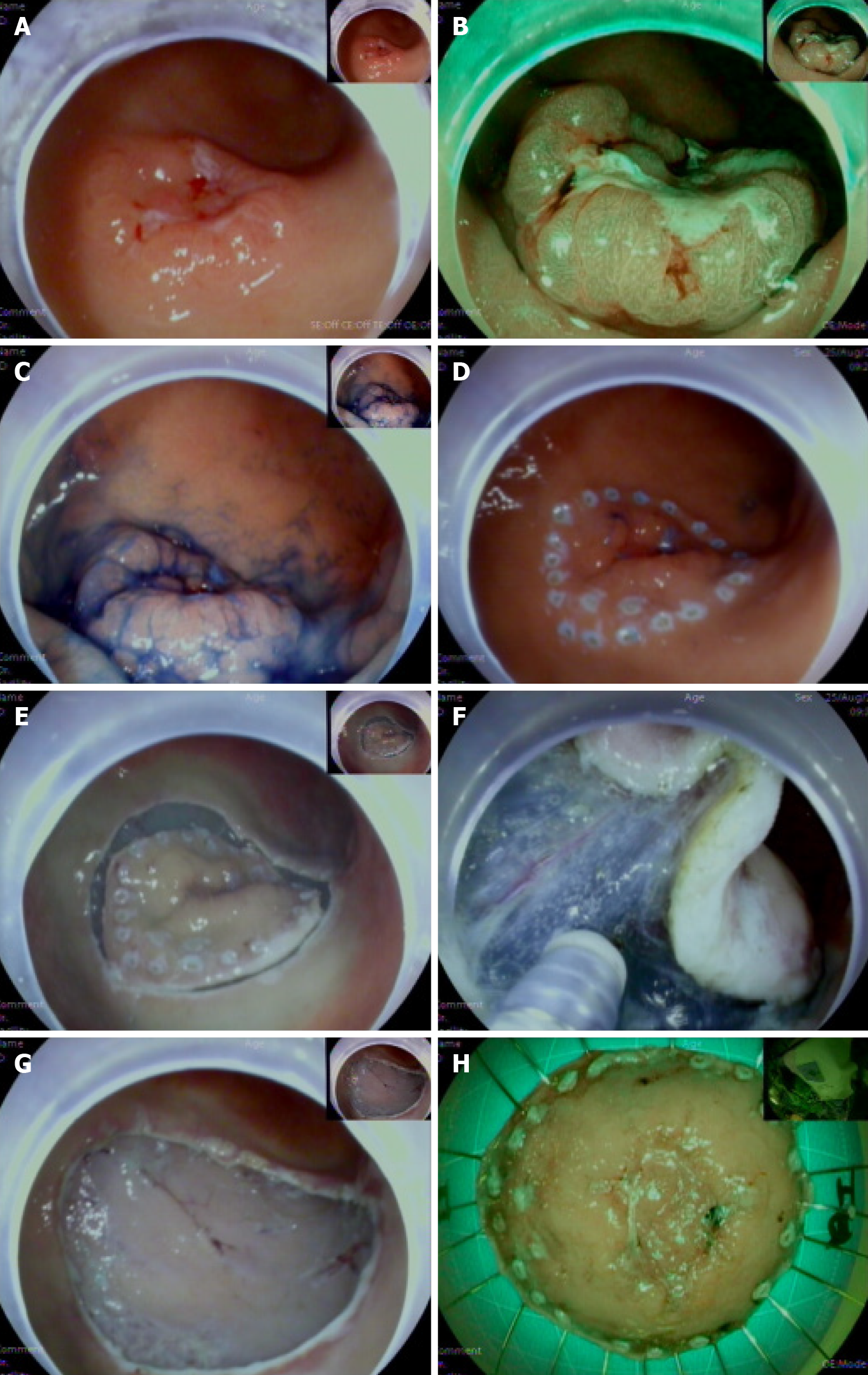
Procedure: The lesion boundary was determined 0.3-0.5 cm from the edge of the lesion, and multiple submucosal injections of 1 mL of 0.3% indigo carmine, 1 mL of epinephrine, and 100 mL of saline mixture were administered until the lesion was raised. The mucosa was cut along the marked point with a needle knife, the submucosal layer was peeled, and the bleeding was properly stopped. The specimen was sent to the pathology department to clarify the pathological nature and whether the margin was involved (Figure 1).
Postoperative management: Included fasting for 1-2 d after the operation; the use of antibiotics, gastric mucosal protectors, and acid suppressants; and the observation of wound healing and hospitalization for 2-3 d after the operation. Endoscopy, computed tomography (CT) or magnetic resonance imaging, and serum tumor marker data were regularly reviewed at 3, 6, and 12 months and annually after discharge to monitor for recurrence or metastasis.
According to the Paris classification, gastric mucosal tumors can be subgrouped into the following six types: Polypoid/protruded (type 0-I), superficial elevated (type 0-IIa), flat (type 0-IIb), superficial depressed (type 0-IIc), superficial depressed area in an elevated lesion (type 0-IIa+IIc) and excavated (type 0-III). The depth of tumor invasion was measured under a microscope: (1) M1, tumor limited to the mucosal epithelium; (2) M2, tumor limited to the lamina propria without involvement of the muscularis mucosa; (3) M3, tumor involving the muscularis mucosa; (4) SM1, tumor infiltrated into the superficial submucosa (from the muscularis mucosa to the submucosa < 500 μm); and (5) SM2, tumor involved the deep submucosa (≥ 500 μm in submucosa). In this study, tumors were rarely located in the lamina propria of the mucosa, so M1 and M2 were divided into one group.
The main complications after ESD were bleeding, perforation, and stenosis. Early delayed bleeding was defined as bleeding that was endoscopically visible within 48 h after ESD. Patients with clinical manifestations of melena, hematemesis, or a significant decrease in hemoglobin levels need emergency endoscopy or surgical hemostasis. Bleeding events treated with hemostatic forceps during ESD were excluded. Delayed bleeding was defined as bleeding detected more than 48 h after ESD. Perforation was defined as the absence of the gastric wall under endoscopy, yellow adipose tissue could be observed directly, or free gas could be found on the abdominal X-ray. Stenosis was diagnosed when the endoscope could not easily reach the cardia through the lower esophagus.
En bloc resection was defined as complete endoscopic removal of the lesion. Complete resection meant that the lesion was removed in its entirety and there was no histopathological evidence of tumor involvement in the lateral or vertical margins. Curative resection was defined as meeting eCuraA and eCuraB, and the evaluation system includes: eCuraA: The tumor was En bloc resection, negative horizontal and vertical margins, without lymphatic and vascular invasion and meets one of the following criteria: (1) Postoperative pathology showed that the tumor was pT1a with predominantly differentiated carcinoma without ulceration; (2) postoperative pathology showed that the tumor was pT1a with predominantly undifferentiated carcinoma without ulceration and a long diameter ≤ 2 cm; and (3) postoperative pathology showed that the tumor was pT1a with predominantly differentiated carcinoma with ulceration and a long diameter ≤ 3 cm. eCuraB: The tumor was resected en bloc. Postoperative pathology revealed that the tumor was pT1b but infiltrated < 500 μm, with predominantly differentiated carcinoma, a long diameter ≤ 3 cm, negative horizontal and vertical margins, and no lymphatic or vascular invasion. eCuraC: Includes eCuraC1 and eCuraC2. eCuraC1 refers to differentiated cancers that satisfied some of the conditions of eCuraA or eCuraB but were not treated with en bloc resection or had positive margins; eCuraC2 refers to those that did not satisfy the conditions of eCuraA, eCuraB, or eCuraC1.
For eCuraA and eCuraB, Japanese guidelines recommend endoscopy once or twice a year (eCuraB resection patients should also undergo abdominal ultrasound or CT to determine metastasis); in principle, eCuraC1 resection patients could undergo ESD or gastrectomy, while eCuraC2 resection patients were required to undergo additional gastrectomy with lymphadenectomy. If patients refused additional resection, they were advised to follow up with endoscopy and CT after 3-6 months. Tumors found at the same site within 1 year after ESD were local recurrence, tumors found at other sites were simultaneous tumors, and more than one year later, the tumors found at other sites were metachronous tumors. Disease-free survival (DFS) was defined as the survival rate without recurrence, metastasis, or death from any cause.
SPSS 26.0 was used for statistical analysis, and GraphPad Prism 9.0 was used for plotting the data. Measurement data were compared by ANOVA if they were normally distributed and had a chi-square test. Comparisons between two groups were performed using the LSD test, and the Kruskal-Wallis rank sum test was used if the data were not normally distributed. Outcome unordered categorical data were compared using the chi-square test or Fisher exact test, and outcome ordered categorical data were compared using the Kruskal-Wallis rank sum test. The missing data among the measurement data were analyzed via the SPSS multiple interpolation method, and the missing data among the categorical data were analyzed via the virtual variable method, where the missing data were reassigned to a new classification. Logistic regression was used in multifactor analysis, the forward or backward method was selected for single-factor analysis with more variables, and the input method was selected for fewer variables. The overall survival rate and DFS rate were estimated by the Kaplan-Meier method and log-rank test, and survival-related multifactorial analysis was performed by Cox regression. A P value of < 0.05 was considered to indicate statistical significance.
This study included the clinical data of 285 patients with 301 Lesions treated with ESD at our hospital from March 2014 to December 2021.
Compared with LGIN patients, HGIN patients were significantly older (average age: 57 years vs average age: 63 years, P < 0.001), had more smoking habits (27.1% vs 49.0%, P = 0.004), and had more atrophic gastritis (75.0% vs 93.0%, P < 0.001). A higher proportion of patients with differentiated early cancer were male (63.5% vs 82.0%, P = 0.013), were older (average age: 57 years vs average age: 63 years, P < 0.001), had more smoking habits (27.1% vs 50.6%, P = 0.004), and had more atrophic gastritis (75.0% vs 94.4%, P < 0.001) than LGIN patients (Table 1).
| LGIN (n = 96) | HGIN (n = 100) | Differentiated-type (n = 89) | Undifferentiated-type (n = 16) | P value | |
| Gender | 0.013 | ||||
| Male | 61 (63.5)a | 76 (76.0)a,b | 73 (82.0)b | 9 (56.3)a,b | |
| Female | 35 (36.5)a | 24 (24.0)a,b | 16 (18.0)b | 7 (43.8)a,b | |
| Age (yr) (range) | 57 (55-59)a | 63 (61-65)b | 63 (61-65)b | 64 (59-68)a,b | < 0.001 |
| Comorbidity | |||||
| Diabetes | 6 (6.3) | 15 (15.0) | 9 (10.1) | 0 (0.0) | 0.108 |
| Hypertension | 17 (17.7) | 31 (31.0) | 19 (21.3) | 4 (25.0) | 0.161 |
| Cardiovascular disease | 5 (5.2) | 8 (8.0) | 9 (10.1) | 2 (12.5) | 0.572 |
| Liver cirrhosis | 5 (5.2) | 1 (1.0) | 2 (2.2) | 0 (0.0) | 0.265 |
| Chronic pulmonary disease | 2 (2.1) | 6 (6.0) | 1 (1.1) | 0 (0.0) | 0.175 |
| Reflux esophagitis | 15 (15.6) | 12 (12.0) | 11 (12.4) | 3 (18.8) | 0.794 |
| Barrett’s esophagus | 4 (4.2) | 2 (2.0) | 1 (1.1) | 0 (0.0) | 0.489 |
| Atrophic gastritis | 72 (75.0)a | 93 (93.0)b | 84 (94.4)b | 13 (81.3)a,b | < 0.001 |
| Previous gastric cancer | 1 (1.0) | 0 (0.0) | 2 (2.2) | 0 (0.0) | 0.461 |
| Smoking history | 26 (27.1)a | 49 (49.0)b | 45 (50.6)b | 6 (37.5)a,b | 0.004 |
| Drinking history | 24 (25.0) | 31 (31.0) | 24 (27.0) | 3 (18.8) | 0.673 |
| Family history | 15 (15.6) | 11 (11.0) | 19 (21.3) | 0 (0.0) | 0.074 |
Compared with LGIN patients, HGIN patients had fewer lesions located in the antrum area (69.8% vs 48.0%, P = 0.006), fewer type 0-I lesions (25.0% vs 3.0%, P < 0.001), and more type 0-IIc lesions (20.8% vs 39.0%, P < 0.001). Differentiated early cancer patients had fewer lesions located in the antrum area (69.8% vs 48.3%, P = 0.006), a larger volume (Z =
| LGIN (n = 96) | HGIN (n = 100) | Differentiated-type (n = 89) | Undifferentiated-type (n = 16) | P value | |
| Size (cm) (range) | 2.7 (2.2-3.7)a | 3.2 (2.4-3.7)a,b | 3.7 (2.7-4.2)b | 3.5 (2.8-4.6)b | 0.001 |
| Location | 0.006 | ||||
| Top 1/3 | 19 (19.8) | 30 (30.0) | 33 (37.1) | 2 (12.5) | |
| Medium 1/3 | 10 (10.4) | 22 (22.0) | 13 (14.6) | 5 (31.3) | |
| Lower 1/3 | 67 (69.8)a | 48 (48.0)b | 43 (48.3)b | 9 (56.3)a,b | |
| Paris classification | < 0.001 | ||||
| 0-I | 24 (25.0)a | 3 (3.0)b | 3 (3.4)b | 2 (12.5)a,b | |
| 0-IIa | 19 (19.8) | 14 (14.0) | 11 (12.4) | 0 (0.0) | |
| 0-IIb | 9 (9.4) | 11 (11.0) | 9 (10.1) | 0 (0.0) | |
| 0-IIc | 20 (20.8)a | 39 (39.0)b | 39 (43.8)b | 8 (50.0)a,b | |
| 0-III | 1 (1.0) | 1 (1.0) | 1 (1.1) | 0 (0.0) | |
| 0-IIa+ IIc | 23 (24.0) | 32 (32.0) | 26 (29.2) | 6 (37.5) | |
| Invasion depth | < 0.001 | ||||
| M1M2 | 96 (100.0)a | 100 (100.0)a | 43 (48.3)b | 5 (31.3)b | |
| M3 | 0 (0.0)a | 0 (0.0)a | 37 (41.6)b | 6 (37.5)b | |
| SM1 | 0 (0.0)a,b | 0 (0.0)b | 6 (6.7)a,c | 2 (12.5)c | |
| SM2 | 0 (0.0)a | 0 (0.0)a | 3 (3.4)a,b | 3 (18.8)b | |
| Presence of H. pylori | 37 (48.7) | 38 (52.8) | 32 (56.1) | 4 (40.0) | 0.727 |
| Presence of ulceration | 6 (6.3) | 6 (6.0) | 7 (7.9) | 4 (25.0) | 0.058 |
Spontaneous bleeding was more frequent in differentiated early cancers than in LGIN lesions (3.1% vs 20.2%, P < 0.001). Patients with undifferentiated early cancer had significantly more abnormal folds and mucosal peristalsis (elevation and extension of the lesion mucosa during suction and insufflation in the gastric cavity) than patients with LGIN or HGIN (3.1% vs 25.0%, 2.1% vs 18.8%, P < 0.001; 1.0% vs 25.0%, 1.0% vs 18.8%, P < 0.001). Compared with that of HGIN patients, the mucosal peristalsis of differentiated early cancer patients was more abnormal according to white light endoscopy (1.0% vs 10.1%, P < 0.001) (Table 3).
| LGIN (n = 96) | HGIN (n = 100) | Differentiated-type (n = 89) | Undifferentiated-type (n = 16) | P value | |
| Nodularity | 65 (67.7) | 65 (65.0) | 60 (67.4) | 11 (68.8) | 0.974 |
| Redness | 51 (53.1) | 50 (50.0) | 60 (67.4) | 7 (43.8) | 0.059 |
| Whiteness | 7 (7.3) | 5 (5.0) | 2 (2.2) | 0 (0.0) | 0.324 |
| White moss | 10 (10.4) | 14 (14.0) | 15 (16.9) | 2 (12.5) | 0.646 |
| Spontaneous bleeding | 3 (3.1)a | 11 (11.0)a,b | 18 (20.2)b | 0 (0.0)a,b | 0.001 |
| Abnormal fold | 3 (3.1)a | 1 (1.0)a | 6 (6.7)a,b | 4 (25.0)b | < 0.001 |
| Poor peristalsis | 2 (2.1)a,b | 1 (1.0)b | 9 (10.1)a,c | 3 (18.8)c | < 0.001 |
Compared with those in LGIN patients, the irregular microvascular patterns, and demarcation lines (DLs) in patients with HGIN and differentiated early cancer were more obvious (38.5% vs 66.2%, 63.0% vs 86.3%, P < 0.001; 38.5% vs 77.8%, 63.0% vs 90.6%, P < 0.001). Patients with undifferentiated early cancer had more irregular microvascular and microsurface patterns and DLs than patients with LGIN (38.5% vs 100.0%, 49.2% vs 90.9%, and 63.0% vs 91.7%, P < 0.05) (Table 4).
Undifferentiated early cancers exceeded ESD indications more often than LGIN (5.2% vs 87.5%, P < 0.001), with a higher percentage of noncurative resections (13.5% vs 87.5%, P < 0.001), and a higher incidence of postoperative complications (stenosis) (5.2% vs 25.0%, P = 0.007). More differentiated, undifferentiated early cancer exceeded ESD indications compared to HGIN (3.0% vs 13.5%, P < 0.001; 3.0% vs 87.5%, P < 0.001). Undifferentiated early cancer patients, compared with HGIN patients, had a higher percentage of noncurative resections (13.0% vs 87.5%, P < 0.001) and a greater risk of postoperative hemorrhage (1.0% vs 18.8%, P = 0.002) and stenosis (0.0% vs 6.3%, P < 0.001). Compared with differentiated early cancers, undifferentiated early cancers were more likely to beyond the ESD indications (13.5% vs 87.5%, P < 0.001), with a greater risk of postoperative hemorrhage (2.2% vs 18.8%, P = 0.002) and stenosis (0.0% vs 6.3%, P < 0.001), and a higher percentage of ESD noncurative resections (21.3% vs 87.5%, P < 0.001) (Table 5).
| LGIN (n = 96) | HGIN (n = 100) | Differentiated-type (n = 89) | Undifferentiated-type (n = 16) | P value | |
| Absolute indication | 91 (94.8)a,b | 97 (97.0)b | 77 (86.5)a | 2 (12.5)c | < 0.001 |
| En bloc resection | 93 (96.9) | 98 (98.0) | 88 (98.9) | 16 (100.0) | 0.729 |
| Complete resection | 88 (91.7) | 90 (90.0) | 79 (88.8) | 13 (81.3) | 0.630 |
| Curative resection | 83 (86.5)a | 87 (87.0)a | 70 (78.7)a | 2 (12.5)b | < 0.001 |
| Complication | 5 (5.2)a | 5 (5.0)a | 3 (3.4)a | 4 (25.0)b | 0.007 |
| Bleeding | 3 (3.1)a,b | 1 (1.0)b | 2 (2.2)b | 3 (18.8)a | 0.002 |
| Perforation | 2 (2.1) | 4 (4.0) | 2 (2.2) | 1 (6.3) | 0.710 |
| Stenosis | 0 (0.0)a | 0 (0.0)a | 0 (0.0)a | 1 (6.3)b | < 0.001 |
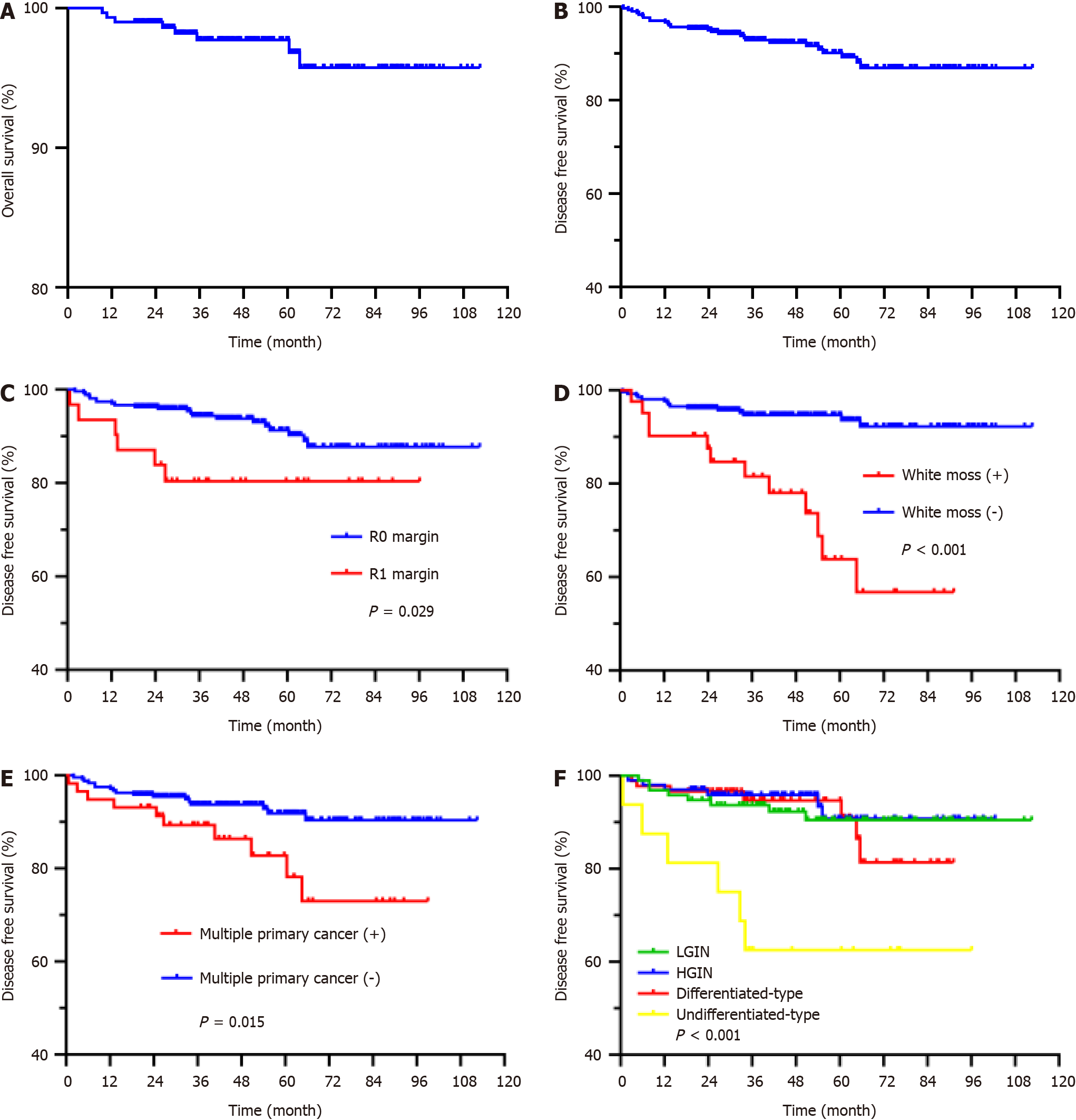
The median follow-up period was 49 months (range 0.6-112.5 months), with overall survival rates of 99.0%, 97.7%, and 95.7% at 2, 5, and 8 years, respectively, and DFS rates of 95.0%, 90.1% and 86.9%, respectively (Figure 1). Patients with differentiated and undifferentiated early cancers had a lower body mass index (BMI) than those with LGIN (average: 23.87 vs 22.37, P = 0.003; average: 23.87 vs 21.44, P = 0.003). Compared with patients with LGIN, HGIN and differentiated early cancer, the percentage of undifferentiated early cancer with poor long-term outcome (recurrence, metastasis or death) was higher (8.3% vs 37.5%, P < 0.001; 6.0% vs 37.5%, P < 0.001; 7.9% vs 37.5%, P < 0.001), and undifferentiated early cancer was more likely to metastasize than HGIN and differentiated early cancer (0.0% vs 18.8%, P < 0.001; 2.2% vs 18.8%, P < 0.001) (Table 6).
| LGIN (n = 96) | HGIN (n = 100) | Differentiated-type (n = 89) | Undifferentiated-type (n = 16) | P value | |
| BMI (range) | 23.87 (23.19-24.54)a | 23.09 (22.41-23.77)a,b | 22.37 (21.71-23.02)b | 21.44 (20.08-22.79)b | 0.003 |
| Adverse outcome | 8 (8.3)a | 6 (6.0)a | 7 (7.9)a | 6 (37.5)b | < 0.001 |
| Recurrence | 5 (5.2) | 6 (6.0) | 6 (6.7) | 2 (12.5) | 0.734 |
| Metastasis | 3 (3.1)a,b | 0 (0.0)b | 2 (2.2)b | 3 (18.8)a | < 0.001 |
| Multiple primary cancer | 24 (25.0) | 15 (15.0) | 15 (16.9) | 4 (25.0) | 0.276 |
Barrett esophagus (OR 7.805) and lesion location (lower third of the stomach) (OR 2.399) were independent risk factors for high preoperative pathological diagnosis, and BMI (OR 0.906) was an independent risk factor for postoperative pathological upgrading. The depth of lesion invasion (M1M2 OR 11.200, M3 OR 7.600) was significantly associated with complete resection. Based on the eCura system, lesion size, degree of differentiation, depth of invasion, ulceration, and complete resection were considered influencing factors for curative resection. Our study revealed that poor mucosal peristalsis (OR 0.185), ulcers (OR 0.073), and irregular microsurface patterns (IMSPs) (OR 0.410) were significantly associated with curative resection. Pathology (signet ring cell carcinoma OR 32.627, mucinous adenocarcinoma OR 49.855) and the Paris classification (III OR 30.406) were significantly associated with complications. Undifferentiated lesions (HR 5.066), white moss (HR 7.187), incomplete resection (HR 3.658), and multiple primary cancers (HR 2.462) were significantly associated with poor prognosis (Figure 2, Tables 7-12).
| Variable | Category | P value | Hazard ratio | 95%CI |
| Differentiation degree | Undifferentiated-type | 0.004 | 5.066 | 1.688-15.200 |
| LGIN | 1 | |||
| White moss | Yes | < 0.001 | 7.187 | 3.122-16.546 |
| No | 1 | |||
| Complete resection | No | 0.012 | 3.658 | 1.337-10.010 |
| Yes | 1 | |||
| Multiple primary cancer | Yes | 0.030 | 2.462 | 1.090-5.562 |
| No | 1 |
| Variable | Category | P value | Odds ratio | 95%CI |
| Invasion depth | M1M2 | 0.004 | 11.200 | 2.120-59.163 |
| M3 | 0.032 | 7.600 | 1.192-48.437 | |
| SM2 | - | 1 | - |
| Variable | Category | P value | Odds ratio | 95%CI |
| Peristaltic condition | Poor | 0.009 | 0.185 | 0.052-0.653 |
| Normal | 1 | |||
| Microsurface pattern | Irregular | 0.043 | 0.410 | 0.173-0.972 |
| Regular | 1 | |||
| Ulceration | Yes | < 0.001 | 0.073 | 0.026-0.207 |
| No | 1 |
| Variable | Categor | P value | Odds ratio | 95%CI |
| Histologic type | Signet ring cell (sig) | 0.032 | 32.627 | 1.357-784.415 |
| Mucinous (muc) | 0.006 | 49.855 | 3.051-814.724 | |
| LGIN | - | 1 | - | |
| Paris classification | 0-III | 0.020 | 30.406 | 1.695-545.565 |
| 0-I | 1 |
| Variable | Category | P value | Odds ratio | 95%CI |
| Location | Lower 1/3 | 0.037 | 2.399 | 1.055-5.455 |
| Top 1/3 | 1 | |||
| Barrett’s esophagus | Yes | 0.026 | 7.805 | 1.277-47.689 |
| No | 1 |
| Variable | P value | Odds ratio | 95%CI |
| BMI | 0.022 | 0.906 | 0.833-0.986 |
Currently, Japanese scholars believe that the development of differentiated gastric cancer involves multiple stages and gradual progression (Correa cascade), usually due to long-term chronic inflammation stimulating the differentiation of gastric mucosal glandular neck stem cells to the intestinal epithelium, intestinal metaplasia and dysplasia, which ultimately induces gastric cancer occurrence. However, undifferentiated gastric cancer cells do not undergo this process and directly form diffuse cancer cells. Scientists have shown that this change is related to the destruction of intercellular connections caused by a lack of E-cadherin expression[11]. Since some people will not continue to progress even if they develop intestinal metaplasia or intraepithelial neoplasia, some scholars believe that different differentiation types of gastric mucosal tumors might have primary genetic causes, interact with the environment, and ultimately determine the development direction of gastric cancer[6,12]. In this study, we retrospectively analyzed the cases of patients who underwent ESD at a high-volume center in Northwest China within 8 years and compared the differences in the characteristics of LGIN, HGIN, and differentiated and undifferentiated cancers, to provide ideas for further exploration of the mechanism of gastric cancer and analysis of the efficacy and related influencing factors of ESD treatment in Northwest China.
From this study, it was clear that the greater the differentiation degree of the lesion was, the more likely it was to develop into depression, the greater the depth of invasion, and the more obvious the NBI + ME abnormality. Among them, patients with differentiated tumors were older than those with intraepithelial neoplasia; most were male patients and had a longer history of smoking. Most of these patients had a history of atrophic gastritis, which is in line with the classical evolution of gastric cancer. However, there was no significant difference in age or smoking history between patients with undifferentiated tumors and those with intraepithelial neoplasia, and the number of lesions that developed from nonatrophic gastritis increased, suggesting that age, smoking history, and atrophic gastritis may not be necessary factors. Patients with undifferentiated tumors were more likely to have poor mucosal peristalsis and abnormal folds under white light endoscopy, which might be related to their deep invasion and large extent. The efficacy, safety, and long-term follow-up outcomes of ESD were worse than those of other types of tumors.
Our results suggested that ESD was a safe and effective treatment. The en bloc resection rate, complete resection rate, and curative resection rate were 98%, 90%, and 80.4%, respectively. There were 59 cases of noncurative resection, among which 31 patients underwent noncomplete resection, 11 patients underwent immediate additional gastrectomy, 4 patients had no obvious residual cancer tissue after surgery, and 2 patients died of liver metastasis; the remaining 48 patients did not receive additional treatments, partly because they did not satisfy the conditions of curative resection of the eCura but pathology suggested complete resection, and partly because of poor quality of life after surgery, advanced age, comorbidities and other reasons. During the follow-up of patients without additional treatment, 5 patients experienced recurrence, 4 patients died, and 3 patients experienced tumor metastasis. Combined with the findings of previous studies, the survival outcome of patients who underwent noncurative resection or additional surgery was better than that of patients who did not undergo additional surgery, but there was no difference between the two in this study because there were fewer patients who underwent noncurative resection or additional surgery, including 1 patient with recurrence and 2 patients with metastasis that resulted in death. Choi et al[13] reported no significant difference in overall survival or DFS between patients treated with additional surgery and those followed up after ESD alone, which is consistent with the findings of our study. However, additional studies have suggested that noncurative resection via ESD, especially in patients with lymphovascular invasion or positive vertical margins with submucosal invasion, should include further surgical treatment, which is safe and effective, and that results in better survival outcomes[14-17].
The incidences of bleeding, perforation, and stenosis after ESD treatment were 3%, 3%, and 0.3%, respectively, and the only patient with postoperative stenosis had a lesion located at the cardia, which was considered to be related to postoperative scar contracture. There were 19 patients (6.3%) with recurrence, including 3 patients with simultaneous tumors who underwent complete resection by ESD or endoscopic mucosal resection; 6 patients with metachronous tumors; 2 patients who underwent major gastrectomy; 2 patients who underwent ESD treatment again; and 2 patients who underwent regular gastroscopy to assess the lesions. Another 10 patients had local recurrence; 3 of these patients underwent ESD surgery again; 3 patients were transferred to the surgery department for gastrectomy; and another 4 patients underwent regular gastroscopy. The pathologic result for all untreated patients after recurrence was LGIN, which was temporarily treated with oral medication according to the patient’s wishes and condition, with regular follow-up gastroscopy and additional surgery if necessary. There were 8 metastases (2.7%), including 5 liver metastases, 1 retroperitoneal metastasis, 1 lung metastasis, and 1 lymph node metastasis. The overall survival rates at 2, 5, and 8 years were 99.0%, 97.7%, and 95.7%, respectively, and the DFS rates were 95.0%, 90.1%, and 86.9%, respectively.
Choi et al[18] assessed 522 EGC lesions treated with ESD, the en bloc resection rate was 97.1%, and the local recurrence rate was 1.8% (median follow-up was 24 months) for lesions with absolute indications; the en bloc resection rate was 96.1%, and the local recurrence rate was 7.0% for lesions with expanded indications. No metastasis was observed at any of the follow-ups. Kosaka et al[19] followed up EGC patients treated with ESD for 5-9 years and reported that the en bloc resection rate, curative resection rate, and local recurrence rate of lesions meeting the absolute indications were 98.0%, 96.0%, and 0.3%, respectively; and the en bloc resection rate, curative resection rate, and local recurrence rate of expanded indication lesions were 89.7%, 72.0%, and 3.7%, respectively; and there were no deaths. Nakamura et al[20] studied 1332 lesions treated with ESD; the en bloc resection rate was 99.0%, the curative resection rate was 96.4%, and the local recurrence rate was 0.2% (median follow-up was 29.5 months) for lesions of absolute indication; the en bloc resection rate, curative resection rate, and local recurrence rate of expanded indication lesions were 97.4%, 93.4%, and 0.9%, respectively; and there was 1 case of liver metastasis (0.2%). This study used the latest Japanese guidelines, which categorized previous expanded indications as absolute indications; thus, the results were consistent, indicating that the ESD level in our hospital had reached the standard, which provided a strong guarantee for the treatment of gastric cancer in Northwest China. Bhandari et al[21], in Western countries, reported that the 2-year recurrence-free survival rate after ESD for EGCs was 94%, but this rate decreased to 83% at 5 years. A total of 722 EGCs in 697 patients treated with ESD in Kim’s study showed a 5-year follow-up overall survival rate of 96.6%, a DFS rate of 90.6%, a local recurrence rate of 0.9%, a metachronous tumor incidence of 7.8%, and a distant metastasis incidence of 0.5%[22]. The long-term follow-up results of the above study were consistent with our study, but the recurrence and metastasis rates were low. These findings were related to the 34 patients in this study who did not meet the absolute indications for ESD, possibly because of the patients’ wishes. These findings also indicated that ESD technology was gradually improving and that doctors were gradually challenging and exploring the best indications. We should disseminate knowledge of the high recurrence risk after ESD for EGC patients so that patients have a positive attitude toward completing postoperative monitoring on time, which will further improve the diagnosis and treatment of gastric cancer in China[23]. Na et al[24] showed that postoperative bleeding occurred in 325 (5.8%) of 5629 ESD-treated EGC patients. Oda et al[25] reported that there were 11 cases (3.6%) of perforation in 303 EGC patients treated with ESD, which was roughly equal to the probability of occurrence in this study. Most cases of stenosis that occur during ESD are located in the cardia. According to the study by Cao et al[26], stenosis occurred in 22 patients (1.9%) of 1133 Lesions, 18 of whom had cardia cancer; these findings suggest that ESD of cardia tumors, similar to esophageal tumors, should be used to prevent stenosis and dilation if necessary.
Our data revealed a significant discrepancy between the preoperative biopsy pathology and postoperative specimen pathology results, with a concordance rate of only 46.8%; 16.6% of the patients were diagnosed with high pathological grade (postoperative pathological downgrade) and 36.5% were diagnosed with low diagnostic grade (postoperative pathological escalation). Multivariate analysis revealed that, compared with the consistent pathology of patients before and after ESD, Barrett’s esophagus and lesion location were found to be independent risk factors for preoperative pathological hypertension, and BMI was found to be an independent risk factor for postoperative pathological upgrading. The high judgment may be due to the biopsy revealing the most obvious structural abnormality with the naked eye, that is, the traditional definition of “one point cancer”, and the biopsy completes the treatment process. Barrett’s esophagus is a gastroesophageal reflux-induced lesion that manifests as the degeneration of esophageal squamous epithelium into columnar epithelium and is considered to be a risk factor for the development of the adenocarcinoma of the esophagogastric conjunction. Therefore, when patients have Barrett’s esophagus, the mucosa located in the cardia should be observed carefully to prevent missed diagnoses, but the diagnosis and treatment should not be overly rigorous. Lesions located in the antral and pyloric regions of the stomach are susceptible to high pathological grade, probably because this area is a high incidence area for gastric cancer, and inflammation in the stomach caused by Helicobacter pylori bacteria most often occurs here, with varied manifestations, which can easily arouse physician’s suspicion. A lower BMI was associated with postoperative pathological escalation, and relevant studies have shown that a low BMI was associated with more aggressive gastric cancer. Our study showed that, compared with those of LGIN patients, the malignancy of undifferentiated early cancer was greater, differentiated early cancer patients were older and had a longer medical history; additionally, most of these patients had atrophic gastritis, which affected the patients’ eating and nutrient absorption, resulting in a relatively low BMI. Therefore, when there is more weight loss, we should reasonably suspect that the malignancy of the lesions is greater.
Among the 1541 patients studied by Ryu et al[27], the concordance rate of pathological diagnosis before and after ESD was 31.1%, the postoperative pathological upgrading rate was 23.8%, and the degradation rate was 7.3%. The factors related to postoperative pathological upgrading of LGIN lesions were central depression (OR 2.959), surface nodule (OR 6.581), and surface redness (OR 6.399). The factors related to postoperative pathological escalation of HGIN lesions were central depression (OR 1.999), surface nodules (OR 1.733), surface redness (OR 2.283), lesions located in the upper 1/3 of the stomach (OR 3.989), and lesion sizes ≥ 10 mm (OR 2.200). Ryu et al[28] reported a pathological concordance rate of 76.3%, a diagnostic upgrade rate of 66.5%, and a downgrade rate of 9.8% in 427 patients with HGIN who underwent ESD. Central depression (OR 4.151), surface nodules (OR 5.582), surface redness (OR 2.926), lesion sites in the upper 1/3 of the stomach (OR 3.894), and tumor sizes ≥ 10 mm (OR 2.287) were associated with postoperative pathology upgrading to EGC. Surface nodules (OR 2.746), submucosal fibrosis (OR 3.958), lesion sites in the upper 1/3 of the stomach (OR 6.652), and tumor sizes ≥ 10 mm (OR 4.935) were significantly associated with invasive submucosal cancers. There were some differences between the results of this study and those of the above studies, which might be related to the small sample size and the lack of stratified analysis; therefore, the sample size needs to be expanded for further research. Most doctors rely on preoperative biopsy pathology when making treatment decisions, but current research has suggested that additional inconsistencies occur; therefore, experts need to determine the optimal treatment plan by combining white light, NBI + ME, ultrasound endoscopy, and patient conditions.
Cox regression showed that the factors affecting DFS (no outcomes such as tumor recurrence, metastasis, or death) were the degree of differentiation, white moss, multiple primary cancers, and complete resection. Undifferentiated cancers grow diffusely and are prone to have undetectable margin remnants. White moss more often combined with ulcers, suggesting deeper infiltration, and according to the eCura system, ESD treatment requires the lesion to be differentiated into intramucosal carcinoma and ≤ 3 cm in length. This study introduced the concept of multiple primary cancers, also known as duplicated cancers, which refer to the simultaneous or successive occurrence of two or more primary malignant tumors in a single or multiple organs of the same host. When intraepithelial neoplasia or carcinoma also existed in other parts of the body, postoperative recurrence, metastasis, or death were more likely to occur, which deserved our attention, and indicated that if patients had a history of tumors in other parts of the body, close monitoring was more necessary, and we should not ignore the chest and abdominal CT and tumor marker screening, in addition to gastroscopy. Patients who underwent incomplete resection via ESD were more likely to have adverse outcomes, which was consistent with the findings of other studies, and required good preoperative evaluation and intraoperative rigor.
This study suggested that the depth of lesion invasion was an independent risk factor for complete resection of early EGCs. Doctors should accurately determine the edge of the lesion before ESD surgery, with the help of NBI + ME endoscopy, ultrasound endoscopy, staining, and other technologies, and not be too close to avoid residual margins.
In this study, poor gastric mucosal peristalsis under white light endoscopy, ulcers, and IMSP were found to be independent risk factors for curative resection. Poor gastric mucosal peristalsis under white light indicates a poor degree of lesion differentiation and invasive growth of cancer cells, such as “leather stomach”, which is a typical manifestation of signet ring cell carcinoma. The presence of ulcers indicated deep infiltration. The Japanese scholar Kishino et al[29] proposed that microvasculature and microsurfaces should be taken into account but focused more on whether the structures were regular or had disappeared during the endoscopic identification of EGC. The microsurface was divided into the pit structure (pit) of the gastric fundus and the villus structure (villi) of the gastric antrum, and it was believed that the glandular duct was the culprit and that the blood vessel was the accomplice in the development of gastric cancer. The results of this study suggested that lesions with IMSP were more likely to undergo noncurative resection, which confirmed Kishino’s theory that IMSP might indicate a longer development time and a greater degree of malignancy in gastric cancer patients. Therefore, when poor gastric mucosal peristalsis, ulcers, or IMSP occur, the biological behavior of the tumor should be vigilant, and whether the patient meets the indications for curative resection via ESD should be strictly assessed.
The pathological classification and Paris classification of lesions were found to be independent risk factors for complications after ESD. Patients with signet ring cell carcinoma and mucinous adenocarcinoma were more prone to complications such as bleeding, perforation, and stenosis. In this study, 1 out of 2 patients with signet ring cell carcinoma had hemorrhages, 1 out of 3 patients with mucinous adenocarcinoma had perforations, and 1 out of 3 patients with mucinous adenocarcinoma had hemorrhages and stenoses. These findings may be related to the small number of lesions, and it is necessary to expand the sample size for further exploration. Perforation occurred in 2 of the 3 Paris type 0-III, because these lesions had deeper infiltration and were often associated with ulceration, which increased the difficulty of submucosal dissection.
This study used the latest Japanese guidelines (sixth edition). According to the old guidelines, some of our lesions were extended or beyond the scope of indications. After the guidelines were updated, 34 lesions that still extended or exceeded the indications were not curatively resected, but some lesions were stable during follow-up, such as differentiated carcinoma with ulcers > 3 cm, differentiated carcinoma with invasion depth of SM1 > 3 cm, and undifferentiated intramucosal carcinoma > 2 cm. These types of lesions suggested a higher probability of lymph node metastasis in the available studies; however, a larger number of patients were still doing well during subsequent follow-up, and with the improvement of the skills of endoscopists, the indications for ESD might further expand, requiring further studies.
This study has several limitations: (1) In this study, bleeding, perforation, and stenosis occurred in 9, 9, and 1 patients, respectively. The number of patients was small when analyzing the risk factors for each complication individually, so a combined analysis was performed, which might lack the precision of analyzing the factors affecting the occurrence of each complication; (2) the lack of complete endoscopic follow-up data for all study subjects was a major limitation of most published studies on gastric ESD. Our study was no exception, and to overcome this limitation, we conducted a rigorous DFS analysis, including the time factor of the occurrence event, which could effectively reduce the impact of lost follow-up data; and (3) this was a single-center study, which might lack representativeness of ESD efficacy across Northwest China; therefore, a joint multicenter study might be more useful.
Despite these limitations, we could draw the following conclusions after carefully analyzing the relevant data. Gastric mucosal tumors with different degrees of differentiation had different characteristics that were closely related to ESD indications and need to be further explored to provide a clinical basis for the pathogenesis, diagnosis and treatment of gastric mucosal tumors. The findings of this study suggested a high rate of curative resection and few adverse events associated with early gastric tumors after ESD. After noncurative resection, additional surgical resection and lymph node dissection should be considered according to the patient’s condition to potentially improve long-term survival. All patients should closely monitored after ESD to detect recurrence and metastasis promptly.
Timely intervention in early gastric cancer can improve the 5-year survival rate to more than 90%. Endoscopic submucosal dissection (ESD) is a safe and mature endoscopic treatment method, but its indications, postoperative management strategies and related influencing factors are still under exploration.
The aim of this study was to explore the underlying factors affecting the development of gastric mucosal tumors, the efficacy of ESD and the underlying scientific prevention and treatment strategies after surgery.
The epidemiological, clinical, and endoscopic features and ESD efficacy of gastric mucosal tumors with different degrees of differentiation were analyzed by stratification, and the related risk factors affecting preoperative diagnosis, ESD efficacy and long-term disease-free survival (DFS) were explored.
According to the latest Japanese guidelines (sixth edition), 301 patients with gastric mucosal tumors treated with ESD at our center from 2014 to 2021 were enrolled, and followed up by endoscopy and chest and abdominal computed tomography at 3, 6 and 12 months after surgery for monitoring, and the data were retrospectively analyzed.
The greater the degree of differentiation of the lesion is, the more likely the lesion is to develop into depression, the deeper the infiltration depth, the more obvious the magnified endoscopy with narrow-band imaging (ME + NBI) abnormality, and the more postoperative complications and adverse outcomes there are. The overall survival rates at 2, 5 and 8 years were 99.0%, 97.7% and 95.7%, respectively, and the DFS rates were 95.0%, 90.1% and 86.9%, respectively. Undifferentiated lesions (HR 5.066), coating with white moss (HR 7.187), incomplete resection (HR 3.658), and multiple primary cancers (HR 2.462) were risk factors for poor prognosis.
Before ESD, it is necessary to strictly screen lesions that meet the indications and be aware of the risk factors that affect the efficacy of ESD. Patients with high-risk factors should be followed up more closely after surgery to identify any recurrence and metastasis in a timely manner. After noncurative resection, additional surgical resection and lymph node dissection should be performed according to the patient’s condition.
A large-scale, multicenter retrospective study in Northwest China is needed to increase the sample size and the number of positive outcomes, and further exploration of treatment options for patients with noncurative resections is necessary.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Cesaretti M, Italy S-Editor: Qu XL L-Editor: A P-Editor: Yuan YY
| 1. | Isobe Y, Nashimoto A, Akazawa K, Oda I, Hayashi K, Miyashiro I, Katai H, Tsujitani S, Kodera Y, Seto Y, Kaminishi M. Gastric cancer treatment in Japan: 2008 annual report of the JGCA nationwide registry. Gastric Cancer. 2011;14:301-316. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 194] [Cited by in RCA: 245] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 2. | Ajani JA, Bentrem DJ, Besh S, D'Amico TA, Das P, Denlinger C, Fakih MG, Fuchs CS, Gerdes H, Glasgow RE, Hayman JA, Hofstetter WL, Ilson DH, Keswani RN, Kleinberg LR, Korn WM, Lockhart AC, Meredith K, Mulcahy MF, Orringer MB, Posey JA, Sasson AR, Scott WJ, Strong VE, Varghese TK Jr, Warren G, Washington MK, Willett C, Wright CD, McMillian NR, Sundar H; National Comprehensive Cancer Network. Gastric cancer, version 2.2013: featured updates to the NCCN Guidelines. J Natl Compr Canc Netw. 2013;11:531-546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 303] [Cited by in RCA: 372] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 3. | Ishii N, Shiratori Y, Ishikane M, Omata F. Population effectiveness of endoscopy screening for mortality reduction in gastric cancer. DEN Open. 2024;4:e296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 4. | Japanese Gastric Cancer Association. Japanese Gastric Cancer Treatment Guidelines 2021 (6th edition). Gastric Cancer. 2023;26:1-25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 199] [Cited by in RCA: 602] [Article Influence: 301.0] [Reference Citation Analysis (2)] |
| 5. | Nagtegaal ID, Odze RD, Klimstra D, Paradis V, Rugge M, Schirmacher P, Washington KM, Carneiro F, Cree IA; WHO Classification of Tumours Editorial Board. The 2019 WHO classification of tumours of the digestive system. Histopathology. 2020;76:182-188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2554] [Cited by in RCA: 2428] [Article Influence: 485.6] [Reference Citation Analysis (3)] |
| 6. | Yeoh KG, Tan P. Mapping the genomic diaspora of gastric cancer. Nat Rev Cancer. 2022;22:71-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 108] [Article Influence: 36.0] [Reference Citation Analysis (0)] |
| 7. | Lee H, Lee HH, Song KY, Park CH, Lee J. Negative Impact of Endoscopic Submucosal Dissection on Short-Term Surgical Outcomes of Subsequent Laparoscopic Distal Gastrectomy for Gastric Cancer. Ann Surg Oncol. 2020;27:313-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 8. | Tanabe S, Ishido K, Matsumoto T, Kosaka T, Oda I, Suzuki H, Fujisaki J, Ono H, Kawata N, Oyama T, Takahashi A, Doyama H, Kobayashi M, Uedo N, Hamada K, Toyonaga T, Kawara F, Tanaka S, Yoshifuku Y. Long-term outcomes of endoscopic submucosal dissection for early gastric cancer: a multicenter collaborative study. Gastric Cancer. 2017;20:45-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 86] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 9. | Nishizawa T, Yahagi N. Long-Term Outcomes of Using Endoscopic Submucosal Dissection to Treat Early Gastric Cancer. Gut Liver. 2018;12:119-124. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 78] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 10. | Okada K, Fujisaki J, Yoshida T, Ishikawa H, Suganuma T, Kasuga A, Omae M, Kubota M, Ishiyama A, Hirasawa T, Chino A, Inamori M, Yamamoto Y, Yamamoto N, Tsuchida T, Tamegai Y, Nakajima A, Hoshino E, Igarashi M. Long-term outcomes of endoscopic submucosal dissection for undifferentiated-type early gastric cancer. Endoscopy. 2012;44:122-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 107] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 11. | Wang Q, Qi C, Min P, Wang Y, Ye F, Xia T, Zhang Y, Du J. MICAL2 contributes to gastric cancer cell migration via Cdc42-dependent activation of E-cadherin/β-catenin signaling pathway. Cell Commun Signal. 2022;20:136. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 12. | Sugano K, Moss SF, Kuipers EJ. Gastric Intestinal Metaplasia: Real Culprit or Innocent Bystander as a Precancerous Condition for Gastric Cancer? Gastroenterology. 2023;165:1352-1366.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 36] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 13. | Choi JY, Jeon SW, Cho KB, Park KS, Kim ES, Park CK, Chung YJ, Kwon JG, Jung JT, Kim EY, Kim KO, Jang BI, Lee SH, Park JB, Yang CH. Non-curative endoscopic resection does not always lead to grave outcomes in submucosal invasive early gastric cancer. Surg Endosc. 2015;29:1842-1849. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 37] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 14. | Hu ZH, Wang JT, Li RX, Wang GJ, Gao BL. Short-term efficacy of additional laparoscopic-assisted radical gastrectomy after non-curative endoscopic submucosal dissection for early gastric cancer. Langenbecks Arch Surg. 2023;408:354. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 15. | Kim ER, Lee H, Min BH, Lee JH, Rhee PL, Kim JJ, Kim KM, Kim S. Effect of rescue surgery after non-curative endoscopic resection of early gastric cancer. Br J Surg. 2015;102:1394-1401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 71] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 16. | Suzuki S, Gotoda T, Hatta W, Oyama T, Kawata N, Takahashi A, Yoshifuku Y, Hoteya S, Nakagawa M, Hirano M, Esaki M, Matsuda M, Ohnita K, Yamanouchi K, Yoshida M, Dohi O, Takada J, Tanaka K, Yamada S, Tsuji T, Ito H, Hayashi Y, Shimosegawa T. Survival Benefit of Additional Surgery After Non-curative Endoscopic Submucosal Dissection for Early Gastric Cancer: A Propensity Score Matching Analysis. Ann Surg Oncol. 2017;24:3353-3360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 48] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 17. | Suzuki H, Oda I, Abe S, Sekiguchi M, Nonaka S, Yoshinaga S, Saito Y, Fukagawa T, Katai H. Clinical outcomes of early gastric cancer patients after noncurative endoscopic submucosal dissection in a large consecutive patient series. Gastric Cancer. 2017;20:679-689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 81] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 18. | Choi MK, Kim GH, Park DY, Song GA, Kim DU, Ryu DY, Lee BE, Cheong JH, Cho M. Long-term outcomes of endoscopic submucosal dissection for early gastric cancer: a single-center experience. Surg Endosc. 2013;27:4250-4258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 86] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 19. | Kosaka T, Endo M, Toya Y, Abiko Y, Kudara N, Inomata M, Chiba T, Takikawa Y, Suzuki K, Sugai T. Long-term outcomes of endoscopic submucosal dissection for early gastric cancer: a single-center retrospective study. Dig Endosc. 2014;26:183-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 101] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 20. | Nakamura K, Honda K, Akahoshi K, Ihara E, Matsuzaka H, Sumida Y, Yoshimura D, Akiho H, Motomura Y, Iwasa T, Komori K, Chijiiwa Y, Harada N, Ochiai T, Oya M, Oda Y, Takayanagi R. Suitability of the expanded indication criteria for the treatment of early gastric cancer by endoscopic submucosal dissection: Japanese multicenter large-scale retrospective analysis of short- and long-term outcomes. Scand J Gastroenterol. 2015;50:413-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 21. | Bhandari P, Abdelrahim M, Alkandari AA, Galtieri PA, Spadaccini M, Groth S, Pilonis ND, Subhramaniam S, Kandiah K, Hossain E, Arndtz S, Bassett P, Siggens K, Htet H, Maselli R, Kaminski MF, Seewald S, Repici A. Predictors of long-term outcomes of endoscopic submucosal dissection of early gastric neoplasia in the West: a multicenter study. Endoscopy. 2023;55:898-906. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 14] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 22. | Kim SG, Park CM, Lee NR, Kim J, Lyu DH, Park SH, Choi IJ, Lee WS, Park SJ, Kim JJ, Kim JH, Lim CH, Cho JY, Kim GH, Lee YC, Jung HY, Lee JH, Chun HJ, Seol SY. Long-Term Clinical Outcomes of Endoscopic Submucosal Dissection in Patients with Early Gastric Cancer: A Prospective Multicenter Cohort Study. Gut Liver. 2018;12:402-410. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 55] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 23. | Yang XY, Wang C, Hong YP, Zhu TT, Qian LJ, Hu YB, Teng LH, Ding J. Knowledge, attitude, and practice of monitoring early gastric cancer after endoscopic submucosal dissection. World J Gastrointest Surg. 2023;15:1751-1760. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 24. | Na JE, Lee YC, Kim TJ, Lee H, Won HH, Min YW, Min BH, Lee JH, Rhee PL, Kim JJ. Utility of a deep learning model and a clinical model for predicting bleeding after endoscopic submucosal dissection in patients with early gastric cancer. World J Gastroenterol. 2022;28:2721-2732. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 1] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 25. | Oda I, Saito D, Tada M, Iishi H, Tanabe S, Oyama T, Doi T, Otani Y, Fujisaki J, Ajioka Y, Hamada T, Inoue H, Gotoda T, Yoshida S. A multicenter retrospective study of endoscopic resection for early gastric cancer. Gastric Cancer. 2006;9:262-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 291] [Cited by in RCA: 314] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 26. | Cao S, Zou T, Sun Q, Liu T, Fan T, Yin Q, Fan X, Jiang J, Raymond D, Wang Y, Zhang B, Lv Y, Zhang X, Ling T, Zhuge Y, Wang L, Zou X, Xu G, Huang Q. Safety and long-term outcomes of early gastric cardiac cancer treated with endoscopic submucosal dissection in 499 Chinese patients. Therap Adv Gastroenterol. 2020;13:1756284820966929. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 27. | Ryu DG, Choi CW, Kang DH, Kim HW, Park SB, Kim SJ, Nam HS. Pathologic outcomes of endoscopic submucosal dissection for gastric epithelial neoplasia. Medicine (Baltimore). 2018;97:e11802. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 28. | Ryu DG, Choi CW, Kang DH, Kim HW, Park SB, Kim SJ, Nam HS. Clinical outcomes of endoscopic submucosa dissection for high-grade dysplasia from endoscopic forceps biopsy. Gastric Cancer. 2017;20:671-678. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 29. | Kishino T, Oyama T, Funakawa K, Ishii E, Yamazato T, Shibagaki K, Miike T, Tanuma T, Kuwayama Y, Takeuchi M, Kitamura Y. Multicenter prospective study on the histological diagnosis of gastric cancer by narrow band imaging-magnified endoscopy with and without acetic acid. Endosc Int Open. 2019;7:E155-E163. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |