Published online Apr 14, 2024. doi: 10.3748/wjg.v30.i14.1963
Peer-review started: December 31, 2023
First decision: January 10, 2024
Revised: January 23, 2024
Accepted: March 21, 2024
Article in press: March 21, 2024
Published online: April 14, 2024
Processing time: 102 Days and 20.4 Hours
Digestion and intestinal absorption allow the body to sustain itself and are the emblematic functions of the bowel. On the flip side, functions also arise from its role as an interface with the environment. Indeed, the gut houses microorganisms, collectively known as the gut microbiota, which interact with the host, and is the site of complex immune activities. Its role in human pathology is complex and scientific evidence is progressively elucidating the functions of the gut, especially regarding the pathogenesis of chronic intestinal diseases and inflammatory conditions affecting various organs and systems. This editorial aims to highlight and relate the factors involved in the pathogenesis of intestinal and systemic inflammation.
Core Tip: The pathophysiology and pathogenesis of inflammatory bowel diseases, functional bowel diseases and inflammatory diseases affecting other organs and systems is being defined. The gut is intended to be the site where inflammatory processes with systemic implications are triggered. A wide-ranging view is required to clarify these pathways with the aim of increasing differential diagnosis, early diagnosis, and treat
- Citation: Panarese A. Bowel function and inflammation: Is motility the other side of the coin? World J Gastroenterol 2024; 30(14): 1963-1967
- URL: https://www.wjgnet.com/1007-9327/full/v30/i14/1963.htm
- DOI: https://dx.doi.org/10.3748/wjg.v30.i14.1963
Bowel disease is a substantial and growing factor driving access to medical care, with notable economic and social impacts, especially in the context of chronic inflammatory diseases[1]. These diseases encompass not only inflammatory bowel disease (IBD), but also other intestinal inflammations, such as diverticulosis and functional disorders[2]. Additionally, chronic systemic and organ inflammation is also increasing, carrying both epidemiological and clinical implications[3]. Currently, the pathogenetic role of the gut in chronic systemic inflammation depends on the function of the gut barrier. This functionality is related to the composition of the gut microbiota and the activity of tight junctions, influenced by inflammatory factors, diet, hormones, and the enteric system[4]. The gut barrier disruption, the “leaky gut”, contributes to the development and progression of metabolic, ischemic, neoplastic, neurodegenerative and autoimmune systemic diseases with a substantial epidemiological impact[5-8].
The established role of gut microbiota/microbiome and intercellular junctions prompts a comprehensive exploration of all the factors influencing them. The rapidly accumulating volume of publications serves to enrich our understanding of biological processes and review data[9]. If environmental factors act unconditionally on all individuals, with variations across geographical area[2,5,9-11], among host factors, the genome is the most important. It determines intestinal nutrient absorption and availability, intrinsic intestinal motility, expression of structural proteins (including those of intercellular junctions), interaction with the gut microbiota, and immune response[3,7,8,12-14]. Gut and systemic inflammation, resulting from impaired gut immune activity, are primarily determined by genome[10,12-15]. The potential of intestinal inflammation to induce systemic inflammation may be attributed to the number of exogenous factors. These factors act over a very large surface, involving multiple types of cells and tissues[8-11,13,16]. The well-being of the human body depends on the homeostasis of the intestinal balance, which is unique in many respects. In the presence of favorable genetic characteristics exogenous factors have the potential to shift the immune response towards an inflammatory/autoimmune direction, carrying systemic implications[2-5,9,14,15,17].
Genome is the main determinant of gut biological processes for several reasons. First, the host genomics has an impact on the gut microbiota/microbiome[15-17]. Second, the genome determines the gut barrier, a dynamic structure that serves as a defense mechanism by shielding the intestinal structures and processes from external aggression[12,13,18]. The microbiota, located in the intestinal lumen, at the interface with the intestinal epithelium, interacts with the gut barrier[16,19]. Third, intestinal immune activity, protected by the gut barrier, also has characteristics conferred by the genome[3,13,14,16,20]. Therefore, exogenous factors, as well as the microbiota, interact with a genetically set immune response[21,22]. The expression of structural, enzymatic and functional proteins, including those involved in the gut barrier, immune response, digestive function and absorption, and neuromuscular components, depends on genetic characteristics[23,24].
The fact that family history is relevant in the onset of intestinal and systemic/organ diseases (inflammation, malabsorption, allergies and neuromuscular diseases) confirms the relevance of the genome, for genetic syndromes such as idiopathic chronic intestinal pseudo-obstruction (mutation in ACTG2, ERBB2-3, etc.) and for diseases in which immune and oxidative stress is a determining factor[25]. Obviously, phenotypic expression is influenced by exogenous factors that interfere with the immune activities of the lamina propria by crossing the gut barrier, as well as the genome[2,5,9,26]. In the presence of these factors, including the microbiota and the intestinal barrier, stromal cells, fibroblasts, endothelial cells, and inflammatory and immune cells, alter their interactions[2,4,5,11,13,16,27].
Among the factors that act on intestinal biological processes, a phenotypic manifestation of the genome, the microbiota play a crucial role in both physiological and pathological conditions[2,4,5,7]. Various factors modify the microbiota and intestinal activities (the intestinal barrier, immune response, neuromuscular activity). Ultimately, these modifications can amplify or inhibit the inflammatory cascade[2,5,9,28-30]. Among the factors, vitamin D plays an important protective role because vitamin D signaling strengthens the gut barrier by upregulating the tight junctions of intestinal epithelial cells, and increasing the production of mucin and antibacterial peptides; downregulates dendritic cells activity; induces the differentiation and function of tolerogenic rather than pro-inflammatory T cells [increases the production of anti-inflammatory cytokines interleukin (IL)-4/IL-10 and decreases IL-17/IL-6/IL-2/interferon γ/tumor necrosis factor α][30]. Vitamin D/vitamin D receptor (VDR), by influencing both innate and adaptive immunity, plays a role in regulating the intestinal inflammation switch[30,31].
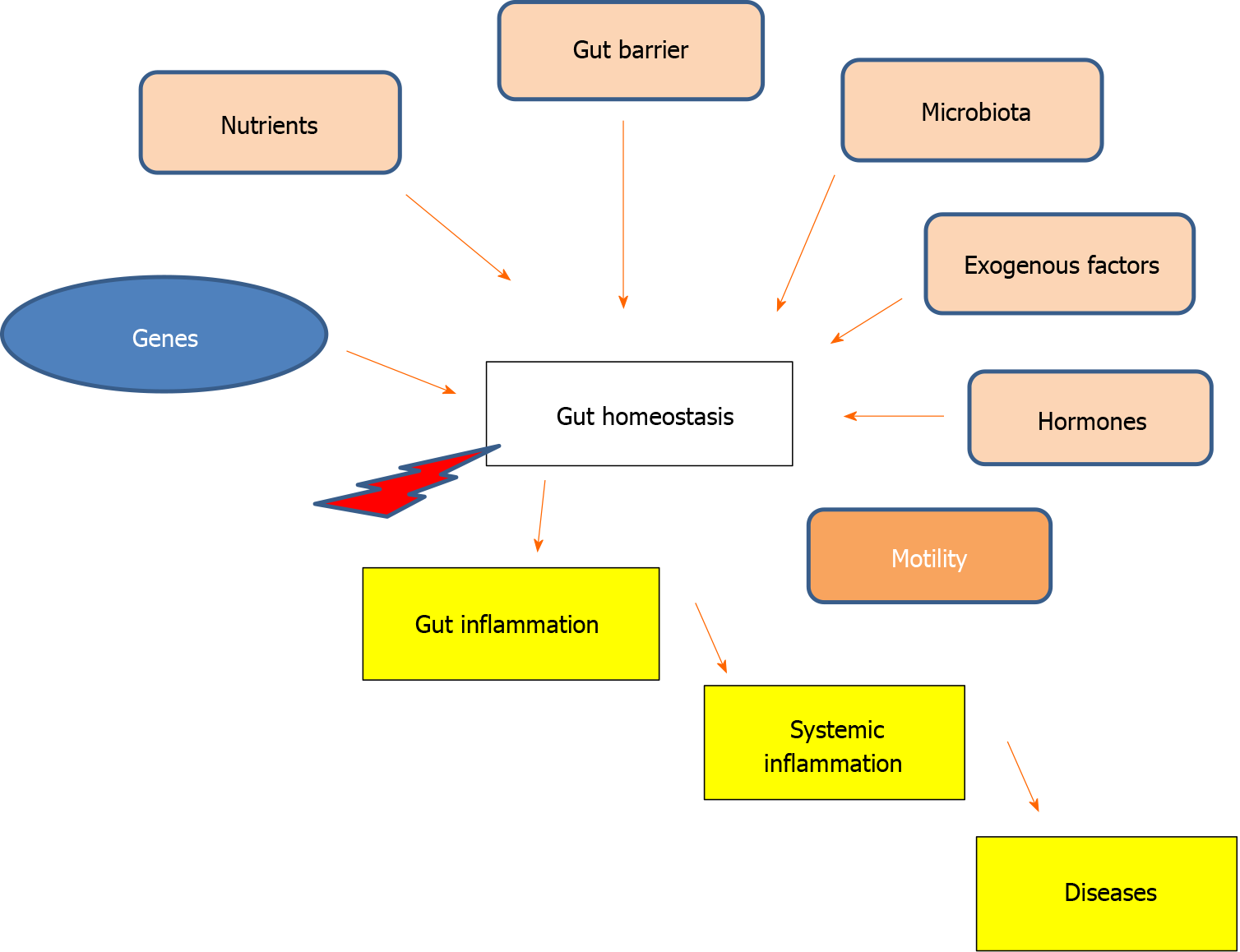
Scientific literature affirms the association between leaky gut and T cell dysfunction with the onset of conditions such as diabetes, cancers, depression and cardiovascular disease. Additionally, factors such obesity, diet, psychosocial stress, and early life stress are implicated in these associations[6,19,20]. Moreover, vitamin D signaling is related to type 2 diabetes, nonalcoholic fatty liver disease, multiple sclerosis and others autoimmune diseases, neurodegenerative diseases, allergies, cancers, IBDs, and chronic intestinal constipation[29,31-33]. Vitamin D deficiency may lead to gut dysbiosis and endotoxemia, potentially leading to systemic inflammation[22,30,31]. Essentially, vitamin D/VDR is involved in the pathogenesis of intestinal inflammation, with repercussions on systemic inflammation[30,31]. However, at the root of the pathogenetic sequence leading to diseases “related” to vitamin D deficiency there may be a defect in intestinal motility. Chronically reduced, whether idiopathic or secondary, intestinal motility can result in decreased absorption of vitamin D and other nutrients due to dysbiosis. Under favorable conditions, this scenario may lead to chronic intestinal and systemic inflammation[32]. By considering these premises, we can aim to prevent or treat diseases by modifying factors that reduce intestinal motility[25,34-41] (Figure 1).
Gut homeostasis depends on the balance between phenotype characteristics and exogenous factors, which collectively foster the stability of the microbiota. Clinical trials are required to validate the pathogenetic role of intestinal motility in impairing gut homeostasis consequently leading to inflammation with systemic involvement.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author’s Membership in Professional Societies: European Society of Gastrointestinal Endoscopy; Italian Society of Digestive Endoscopy; IG-IBD; Italian Society of Gastroenterology.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Banerjee A, United States; Poddighe D, Kazakhstan S-Editor: Wang JJ L-Editor: A P-Editor: Yuan YY
| 1. | Agrawal M, Jess T. Implications of the changing epidemiology of inflammatory bowel disease in a changing world. United European Gastroenterol J. 2022;10:1113-1120. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 100] [Article Influence: 33.3] [Reference Citation Analysis (0)] |
| 2. | Sanmarco LM, Chao CC, Wang YC, Kenison JE, Li Z, Rone JM, Rejano-Gordillo CM, Polonio CM, Gutierrez-Vazquez C, Piester G, Plasencia A, Li L, Giovannoni F, Lee HG, Faust Akl C, Wheeler MA, Mascanfroni I, Jaronen M, Alsuwailm M, Hewson P, Yeste A, Andersen BM, Franks DG, Huang CJ, Ekwudo M, Tjon EC, Rothhammer V, Takenaka M, de Lima KA, Linnerbauer M, Guo L, Covacu R, Queva H, Fonseca-Castro PH, Bladi MA, Cox LM, Hodgetts KJ, Hahn ME, Mildner A, Korzenik J, Hauser R, Snapper SB, Quintana FJ. Identification of environmental factors that promote intestinal inflammation. Nature. 2022;611:801-809. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 92] [Article Influence: 30.7] [Reference Citation Analysis (0)] |
| 3. | Furman D, Campisi J, Verdin E, Carrera-Bastos P, Targ S, Franceschi C, Ferrucci L, Gilroy DW, Fasano A, Miller GW, Miller AH, Mantovani A, Weyand CM, Barzilai N, Goronzy JJ, Rando TA, Effros RB, Lucia A, Kleinstreuer N, Slavich GM. Chronic inflammation in the etiology of disease across the life span. Nat Med. 2019;25:1822-1832. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1084] [Cited by in RCA: 2762] [Article Influence: 460.3] [Reference Citation Analysis (0)] |
| 4. | Di Vincenzo F, Del Gaudio A, Petito V, Lopetuso LR, Scaldaferri F. Gut microbiota, intestinal permeability, and systemic inflammation: a narrative review. Intern Emerg Med. 2024;19:275-293. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 349] [Article Influence: 349.0] [Reference Citation Analysis (0)] |
| 5. | Malesza IJ, Malesza M, Walkowiak J, Mussin N, Walkowiak D, Aringazina R, Bartkowiak-Wieczorek J, Mądry E. High-Fat, Western-Style Diet, Systemic Inflammation, and Gut Microbiota: A Narrative Review. Cells. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 408] [Article Influence: 102.0] [Reference Citation Analysis (0)] |
| 6. | Martel J, Chang SH, Ko YF, Hwang TL, Young JD, Ojcius DM. Gut barrier disruption and chronic disease. Trends Endocrinol Metab. 2022;33:247-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 262] [Article Influence: 87.3] [Reference Citation Analysis (0)] |
| 7. | Mou Y, Du Y, Zhou L, Yue J, Hu X, Liu Y, Chen S, Lin X, Zhang G, Xiao H, Dong B. Gut Microbiota Interact With the Brain Through Systemic Chronic Inflammation: Implications on Neuroinflammation, Neurodegeneration, and Aging. Front Immunol. 2022;13:796288. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 208] [Article Influence: 69.3] [Reference Citation Analysis (0)] |
| 8. | Liu X, Liu Y, Liu J, Zhang H, Shan C, Guo Y, Gong X, Cui M, Li X, Tang M. Correlation between the gut microbiome and neurodegenerative diseases: a review of metagenomics evidence. Neural Regen Res. 2024;19:833-845. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 28] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 9. | Usuda H, Okamoto T, Wada K. Leaky Gut: Effect of Dietary Fiber and Fats on Microbiome and Intestinal Barrier. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 155] [Article Influence: 38.8] [Reference Citation Analysis (0)] |
| 10. | Chang CS, Kao CY. Current understanding of the gut microbiota shaping mechanisms. J Biomed Sci. 2019;26:59. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 100] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 11. | Andersen-Civil AIS, Arora P, Williams AR. Regulation of Enteric Infection and Immunity by Dietary Proanthocyanidins. Front Immunol. 2021;12:637603. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 12. | Wibbe N, Ebnet K. Cell Adhesion at the Tight Junctions: New Aspects and New Functions. Cells. 2023;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 23] [Reference Citation Analysis (0)] |
| 13. | Spalinger MR, Sayoc-Becerra A, Santos AN, Shawki A, Canale V, Krishnan M, Niechcial A, Obialo N, Scharl M, Li J, Nair MG, McCole DF. PTPN2 Regulates Interactions Between Macrophages and Intestinal Epithelial Cells to Promote Intestinal Barrier Function. Gastroenterology. 2020;159:1763-1777.e14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 101] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 14. | Kline EM, Houser MC, Herrick MK, Seibler P, Klein C, West A, Tansey MG. Genetic and Environmental Factors in Parkinson's Disease Converge on Immune Function and Inflammation. Mov Disord. 2021;36:25-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 87] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 15. | Qin Y, Havulinna AS, Liu Y, Jousilahti P, Ritchie SC, Tokolyi A, Sanders JG, Valsta L, Brożyńska M, Zhu Q, Tripathi A, Vázquez-Baeza Y, Loomba R, Cheng S, Jain M, Niiranen T, Lahti L, Knight R, Salomaa V, Inouye M, Méric G. Combined effects of host genetics and diet on human gut microbiota and incident disease in a single population cohort. Nat Genet. 2022;54:134-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 288] [Article Influence: 96.0] [Reference Citation Analysis (1)] |
| 16. | Kayama H, Okumura R, Takeda K. Interaction Between the Microbiota, Epithelia, and Immune Cells in the Intestine. Annu Rev Immunol. 2020;38:23-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 447] [Article Influence: 111.8] [Reference Citation Analysis (0)] |
| 17. | Kolde R, Franzosa EA, Rahnavard G, Hall AB, Vlamakis H, Stevens C, Daly MJ, Xavier RJ, Huttenhower C. Host genetic variation and its microbiome interactions within the Human Microbiome Project. Genome Med. 2018;10:6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 92] [Cited by in RCA: 119] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 18. | Yao Y, Kim G, Shafer S, Chen Z, Kubo S, Ji Y, Luo J, Yang W, Perner SP, Kanellopoulou C, Park AY, Jiang P, Li J, Baris S, Aydiner EK, Ertem D, Mulder DJ, Warner N, Griffiths AM, Topf-Olivestone C, Kori M, Werner L, Ouahed J, Field M, Liu C, Schwarz B, Bosio CM, Ganesan S, Song J, Urlaub H, Oellerich T, Malaker SA, Zheng L, Bertozzi CR, Zhang Y, Matthews H, Montgomery W, Shih HY, Jiang J, Jones M, Baras A, Shuldiner A, Gonzaga-Jauregui C, Snapper SB, Muise AM, Shouval DS, Ozen A, Pan KT, Wu C, Lenardo MJ. Mucus sialylation determines intestinal host-commensal homeostasis. Cell. 2022;185:1172-1188.e28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 138] [Article Influence: 46.0] [Reference Citation Analysis (0)] |
| 19. | Di Tommaso N, Gasbarrini A, Ponziani FR. Intestinal Barrier in Human Health and Disease. Int J Environ Res Public Health. 2021;18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 286] [Article Influence: 71.5] [Reference Citation Analysis (1)] |
| 20. | Montgomery TL, Künstner A, Kennedy JJ, Fang Q, Asarian L, Culp-Hill R, D'Alessandro A, Teuscher C, Busch H, Krementsov DN. Interactions between host genetics and gut microbiota determine susceptibility to CNS autoimmunity. Proc Natl Acad Sci U S A. 2020;117:27516-27527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 77] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 21. | Kopp EB, Agaronyan K, Licona-Limón I, Nish SA, Medzhitov R. Modes of type 2 immune response initiation. Immunity. 2023;56:687-694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 37] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 22. | Geuking MB, Burkhard R. Microbial modulation of intestinal T helper cell responses and implications for disease and therapy. Mucosal Immunol. 2020;13:855-866. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 23. | Li X, Li W, Zeng M, Zheng R, Li M. Network-based methods for predicting essential genes or proteins: a survey. Brief Bioinform. 2020;21:566-583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 67] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 24. | Poddighe D, Capittini C. The Role of HLA in the Association between IgA Deficiency and Celiac Disease. Dis Markers. 2021;2021:8632861. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 25. | Stavely R, Ott LC, Rashidi N, Sakkal S, Nurgali K. The Oxidative Stress and Nervous Distress Connection in Gastrointestinal Disorders. Biomolecules. 2023;13. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 26. | Guo T, Li X. Machine learning for predicting phenotype from genotype and environment. Curr Opin Biotechnol. 2023;79:102853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 26] [Reference Citation Analysis (0)] |
| 27. | Nolan LS, Rimer JM, Good M. The Role of Human Milk Oligosaccharides and Probiotics on the Neonatal Microbiome and Risk of Necrotizing Enterocolitis: A Narrative Review. Nutrients. 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 69] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 28. | Wang J, Thingholm LB, Skiecevičienė J, Rausch P, Kummen M, Hov JR, Degenhardt F, Heinsen FA, Rühlemann MC, Szymczak S, Holm K, Esko T, Sun J, Pricop-Jeckstadt M, Al-Dury S, Bohov P, Bethune J, Sommer F, Ellinghaus D, Berge RK, Hübenthal M, Koch M, Schwarz K, Rimbach G, Hübbe P, Pan WH, Sheibani-Tezerji R, Häsler R, Rosenstiel P, D'Amato M, Cloppenborg-Schmidt K, Künzel S, Laudes M, Marschall HU, Lieb W, Nöthlings U, Karlsen TH, Baines JF, Franke A. Genome-wide association analysis identifies variation in vitamin D receptor and other host factors influencing the gut microbiota. Nat Genet. 2016;48:1396-1406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 413] [Cited by in RCA: 466] [Article Influence: 51.8] [Reference Citation Analysis (0)] |
| 29. | Vernia F, Valvano M, Longo S, Cesaro N, Viscido A, Latella G. Vitamin D in Inflammatory Bowel Diseases. Mechanisms of Action and Therapeutic Implications. Nutrients. 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 72] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 30. | Sassi F, Tamone C, D'Amelio P. Vitamin D: Nutrient, Hormone, and Immunomodulator. Nutrients. 2018;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 312] [Cited by in RCA: 484] [Article Influence: 69.1] [Reference Citation Analysis (0)] |
| 31. | L Bishop E, Ismailova A, Dimeloe S, Hewison M, White JH. Vitamin D and Immune Regulation: Antibacterial, Antiviral, Anti-Inflammatory. JBMR Plus. 2021;5:e10405. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 169] [Cited by in RCA: 179] [Article Influence: 44.8] [Reference Citation Analysis (0)] |
| 32. | Panarese A, Pesce F, Porcelli P, Riezzo G, Iacovazzi PA, Leone CM, De Carne M, Rinaldi CM, Shahini E. Chronic functional constipation is strongly linked to vitamin D deficiency. World J Gastroenterol. 2019;25:1729-1740. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 37] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 33. | Khatoon S, Kalam N, Rashid S, Bano G. Effects of gut microbiota on neurodegenerative diseases. Front Aging Neurosci. 2023;15:1145241. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 23] [Reference Citation Analysis (0)] |
| 34. | Giambra V, Pagliari D, Rio P, Totti B, Di Nunzio C, Bosi A, Giaroni C, Gasbarrini A, Gambassi G, Cianci R. Gut Microbiota, Inflammatory Bowel Disease, and Cancer: The Role of Guardians of Innate Immunity. Cells. 2023;12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 35. | Katsirma Z, Dimidi E, Rodriguez-Mateos A, Whelan K. Fruits and their impact on the gut microbiota, gut motility and constipation. Food Funct. 2021;12:8850-8866. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 55] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 36. | Ding N, Zhang X, Zhang XD, Jing J, Liu SS, Mu YP, Peng LL, Yan YJ, Xiao GM, Bi XY, Chen H, Li FH, Yao B, Zhao AZ. Impairment of spermatogenesis and sperm motility by the high-fat diet-induced dysbiosis of gut microbes. Gut. 2020;69:1608-1619. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 206] [Article Influence: 41.2] [Reference Citation Analysis (0)] |
| 37. | Fournier N, Fabre A. Smooth muscle motility disorder phenotypes: A systematic review of cases associated with seven pathogenic genes (ACTG2, MYH11, FLNA, MYLK, RAD21, MYL9 and LMOD1). Intractable Rare Dis Res. 2022;11:113-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 15] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 38. | Zhernakova DV, Wang D, Liu L, Andreu-Sánchez S, Zhang Y, Ruiz-Moreno AJ, Peng H, Plomp N, Del Castillo-Izquierdo Á, Gacesa R, Lopera-Maya EA, Temba GS, Kullaya VI, van Leeuwen SS; Lifelines Cohort Study, Xavier RJ, de Mast Q, Joosten LAB, Riksen NP, Rutten JHW, Netea MG, Sanna S, Wijmenga C, Weersma RK, Zhernakova A, Harmsen HJM, Fu J. Host genetic regulation of human gut microbial structural variation. Nature. 2024;625:813-821. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 67] [Article Influence: 67.0] [Reference Citation Analysis (0)] |
| 39. | Wang S, Hou H, Tang Y, Zhang S, Wang G, Guo Z, Zhu L, Wu J. An overview on CV2/CRMP5 antibody-associated paraneoplastic neurological syndromes. Neural Regen Res. 2023;18:2357-2364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 40. | Sarfo BO, Kopdag H, Pott MC, Stiedenroth L, Nahrstedt U, Schäfer H, von Wichert G. Postinfectious T-lymphocytic enteral leiomyositis as a rare cause of chronic intestinal pseudoobstruction. Z Gastroenterol. 2021;59:326-330. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (1)] |
| 41. | Radocchia G, Neroni B, Marazzato M, Capuzzo E, Zuccari S, Pantanella F, Zenzeri L, Evangelisti M, Vassallo F, Parisi P, Di Nardo G, Schippa S. Chronic Intestinal Pseudo-Obstruction: Is There a Connection with Gut Microbiota? Microorganisms. 2021;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |