Published online Apr 14, 2024. doi: 10.3748/wjg.v30.i14.1934
Peer-review started: November 30, 2023
First decision: February 2, 2024
Revised: February 3, 2024
Accepted: March 25, 2024
Article in press: March 25, 2024
Published online: April 14, 2024
Processing time: 134 Days and 4.5 Hours
Olympus Corporation developed texture and color enhancement imaging (TXI) as a novel image-enhancing endoscopic technique. This topic highlights a series of hot-topic articles that investigated the efficacy of TXI for gastrointestinal disease identification in the clinical setting. A randomized controlled trial demonstrated improvements in the colorectal adenoma detection rate (ADR) and the mean number of adenomas per procedure (MAP) of TXI compared with those of white-light imaging (WLI) observation (58.7% vs 42.7%, adjusted relative risk 1.35, 95%CI: 1.17-1.56; 1.36 vs 0.89, adjusted incident risk ratio 1.48, 95%CI: 1.22-1.80, respectively). A cross-over study also showed that the colorectal MAP and ADR in TXI were higher than those in WLI (1.5 vs 1.0, adjusted odds ratio 1.4, 95%CI: 1.2-1.6; 58.2% vs 46.8%, 1.5, 1.0-2.3, respectively). A randomized controlled trial demonstrated non-inferiority of TXI to narrow-band imaging in the colorectal mean number of adenomas and sessile serrated lesions per procedure (0.29 vs 0.30, difference for non-inferiority -0.01, 95%CI: -0.10 to 0.08). A cohort study found that scoring for ulcerative colitis severity using TXI could predict relapse of ulcerative colitis. A cross-sectional study found that TXI improved the gastric cancer detection rate compared to WLI (0.71% vs 0.29%). A cross-sectional study revealed that the sensitivity and accuracy for active Helicobacter pylori gastritis in TXI were higher than those of WLI (69.2% vs 52.5% and 85.3% vs 78.7%, res
Core Tip: Olympus Corporation has developed texture and color enhancement imaging (TXI) as a novel image-enhancing endoscopy technique. This highlights a series of hot-topic articles investigating the usefulness of TXI for gastrointestinal disease diagnosis in clinical practice. TXI showed improvement compared with white-light imaging (WLI) and non-inferiority compared with narrow-band imaging in detecting colorectal neoplasia. TXI observation can predict the relapse of ulcerative colitis. TXI improved gastric cancer detection and diagnostic accuracy for active Helicobacter pylori gastritis compared to WLI. In conclusion, TXI can improve detection and qualitative diagnosis.
- Citation: Toyoshima O, Nishizawa T, Hata K. Topic highlight on texture and color enhancement imaging in gastrointestinal diseases. World J Gastroenterol 2024; 30(14): 1934-1940
- URL: https://www.wjgnet.com/1007-9327/full/v30/i14/1934.htm
- DOI: https://dx.doi.org/10.3748/wjg.v30.i14.1934
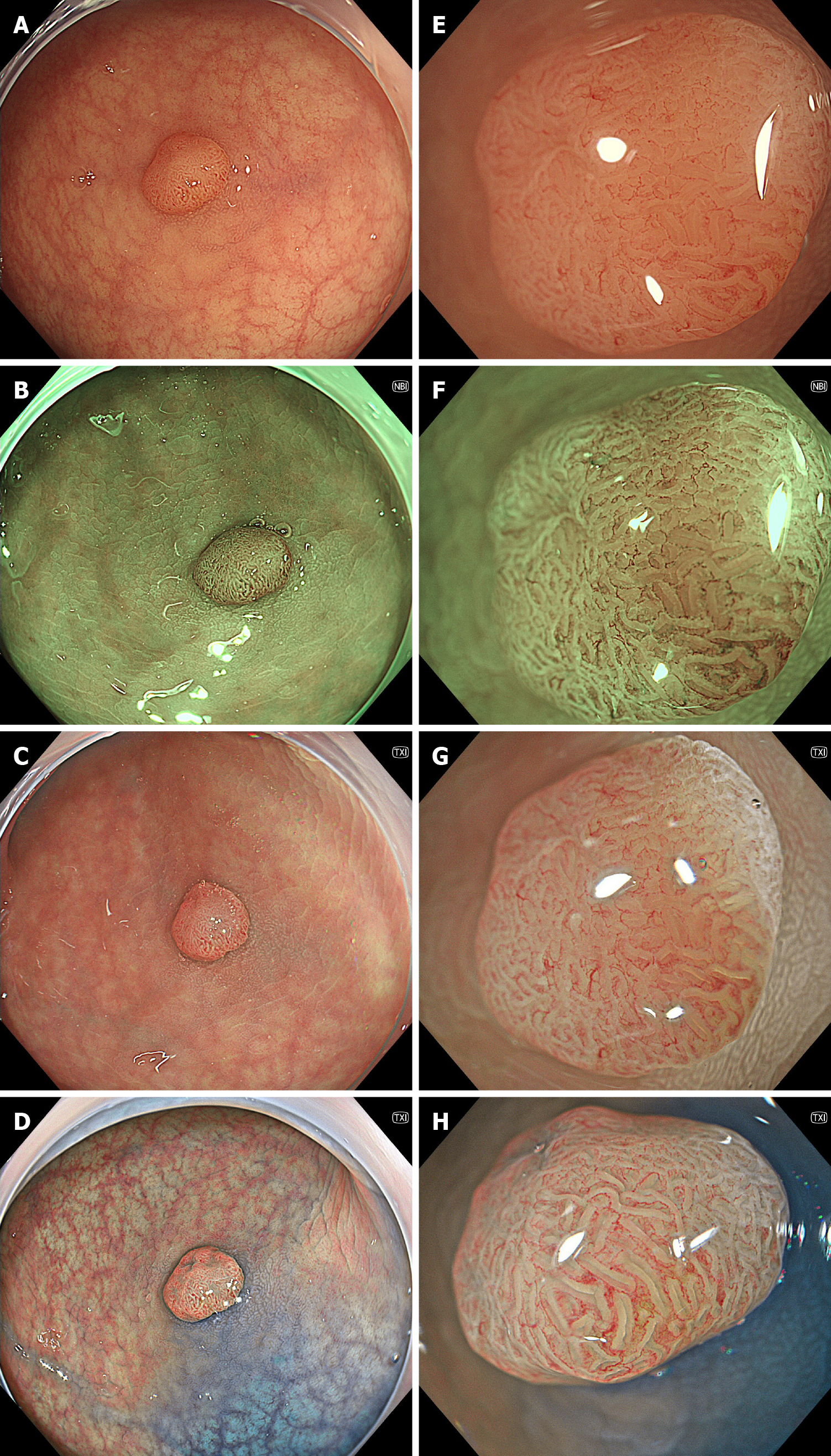
Image-enhanced endoscopy (IEE) improves the diagnosis of gastrointestinal lesions that are challenging in conventional white-light imaging (WLI). Narrow-band imaging (NBI) developing as an IEE modality is effective in diagnosing gastrointestinal disease[1-3]. Following NBI, blue-light imaging and linked color imaging (LCI) have been developed as our new IEE modalities. The utility of BLI and LCI has also been reported extensively[4-6]. Texture and color enhancement imaging (TXI), which is a novel technique to enhance images, was developed in the new endoscopy system EVIS X1 (Olympus Corporation, Tokyo, Japan) in 2020. TXI enhances three image factors, namely texture, brightness, and color, in WLI. This enhancement helps to define subtle tissue differences by applying the retinex-based method. TXI has two modes (i.e., modes 1 and 2). TXI mode 2 is composited with brightness adjustment in dark regions and texture enhancement for subtle contrast. Additionally, TXI mode 1 applies color enhancement to define slight color contrasts more clearly[7,8]. Representative endoscopic images of colonic neoplasm are shown in Figure 1.
Many studies investigating the visibility and color differences of TXI have been published. For example, visibility scores and color differences of TXI for colorectal neoplasia[9-12], gastric neoplasia[13-18], gastritis[13,19], Barrett’s esophagus[20,21], pharyngeal and esophageal cancer[22], duodenal neoplasia[23], and the papilla of Vater[24] have been assessed. However, few studies have evaluated the detection and diagnosis of lesions using TXI. Therefore, this study highlights recent reports. We selected six comparative studies that examined the effects of TXI on neoplasia detection, disease prognosis, and diagnostic accuracy (Table 1).
| Ref. | Publication year | Study design | Country | No. of patients | Endpoint | Comparison | Main result |
| Antonelli et al[25] | 2023 | Randomized control study | Italy, Germany, and Japan | 747 | Detection of colorectal neoplasia | TXI vs WLI | Improvement |
| Yoshida et al[27] | 2023 | Randomized control study | Japan | 381 | Detection of colorectal neoplasia | TXI vs NBI | Non-inferiority |
| Sakamoto et al[28] | 2023 | Cross-over study | Japan | 470 | Detection of colorectal neoplasia | TXI vs WLI | Improvement |
| Hayashi et al[29] | 2023 | Cohort study | Japan | 146 | Relapse of ulcerative colitis | TXI score 2 vs TXI scores 0-1 | Predictable |
| Kemmoto et al[30] | 2024 | Cross-sectional study | Japan | 13440 | Detection of gastric cancer | TXI (mode 2) vs WLI | Improvement |
| Kitagawa et al[31] | 2023 | Cross-sectional study | Japan | 60 | Diagnostic accuracy for active Helicobacter pylori gastritis | TXI (mode 1) vs WLI | Improvement |
Two randomized controlled trials (RCTs) examined the utility of TXI in colorectal neoplasia detection. Antonelli et al[25] conducted an international multicenter RCT (Italy, Germany, and Japan) assessing colorectal adenoma detection using TXI compared to WLI. Patients were randomly assigned to two arms, those who underwent colonoscopy using TXI or WLI. A total of 747 patients were enrolled (mean age 62.3 years; 50.2% male). Adenoma detection rate (ADR) was higher in the TXI group compared with the WLI group (58.7% vs 42.7%; adjusted relative risk 1.35, 95%CI: 1.17-1.56, P = 0.001). The detection rate of adenomas < 10 mm was higher with TXI than with WLI (37.1% vs 24.5%). The TXI group had a higher proportion of patients with high-risk polyps compared to the WLI group (26.7% vs 19.9%, 1.31, 1.01-1.71, P = 0.046). High-risk polyps were defined as at least one adenoma ≥ 10 mm or with high-grade dysplasia, or at least five adenomas, or any serrated polyp ≥ 10 mm or with dysplasia, according to the most recent European Society of Gastrointestinal Endoscopy guideline[26]. The mean number of adenomas per procedure (MAP) with TXI was larger than that with WLI (1.36 vs 0.89; adjusted incident risk ratio 1.48, 95%CI: 1.22-1.80, P < 0.001). This is the first RCT to compare colorectal neoplasia detection using TXI and WLI. Researchers demonstrated that TXI had a higher ADR than WLI and was useful for colorectal adenoma detection. They also reported that TXI increased the detection rate of small adenomas. Furthermore, TXI provided a larger MAP and a higher high-risk polyp detection rate, indicating that a different surveillance interval after colonoscopy is recommended compared to WLI.
Yoshida et al[27] performed a multicenter RCT evaluating the efficacy of an additional 30-s (Add-30-s) observation of the right-sided colon (i.e., cecum to ascending colon) using TXI compared with NBI observation. Patients were assigned to either the TXI or NBI group. The right colon was first observed with WLI in both groups; then, the right colon was examined with Add-30-s observations using either TXI or NBI. Three-hundred fifty-eight patients were enrolled (mean age 68.3 years, 63.4% male). This study showed the non-inferiorities of TXI to NBI (0.29 vs 0.30, difference for non-inferiority -0.01, 95%CI: -0.10 to 0.08, P = 0.02) in the mean number of adenomas and sessile serrated lesions per procedure (MASP). The difference in MAP between TXI and NBI groups was also significant for non-inferiority (0.23 vs 0.24, -0.01, -0.09 to 0.007, P = 0.01). Multivariable analyses showed no significant differences in MASPs and MAPs between the TXI and NBI groups, regardless of bowel preparation, endoscopes, and endoscopist level. Increases in the ADR, adenoma and sessile serrated lesion detection rate, and polyp detection rate for the right colon from WLI to TXI were 10.2%, 13.0%, and 15.3%, respectively, and from WLI to NBI were 10.5%, 12.7%, and 13.8%, respectively. The increases in the TXI and NBI groups were not significantly different. This is the first non-inferiority RCT comparing TXI and NBI for colorectal neoplasia detection. The authors recommend that either TXI or NBI be used for Ad-30-s observation.
Sakamoto et al[28] conducted a retrospective cross-over study that compared colorectal neoplasia detection using TXI and WLI. They repeated the right colon observation twice with WLI or TXI as the first observation in the WLI and TXI groups, respectively. Then, the right-sided colon was re-examined as a second look using TXI or WLI, whichever was not used for the first observation. The remaining colorectal mucosa was examined using the first observation method. This study included 470 patients (mean age 64.0 years, 64.0% male). Multivariable analyses showed that the MAP and ADR in the TXI group were higher than those in the WLI group (1.5 vs 1.0, adjusted odds ratio 1.4, 95%CI: 1.2-1.6, P < 0.001; 58.2% vs 46.8%, 1.5, 1.0-2.3, P = 0.044), regardless of patient demographic characteristics, withdrawal time, bowel preparation, and endoscopes. TXI detected more adenomas in the ascending colon with a non-polypoid morphology and a size of 6-9 mm. Fewer non-polypoid lesions were missed in the TXI group than in the WLI group (16.6% vs 30.6%). This cross-over study indicated that TXI was more suitable than WLI for the detection of adenomas, especially small non-polypoid adenomas, in the ascending colon.
A cohort study established a score using the TXI as a predictor of ulcerative colitis (UC) relapse and investigated its usefulness. Hayashi et al[29] did a prospective single-center, single-arm cohort study. They developed the TXI scores as follows: Score 0 = no accentuated redness; score 1 = accentuated redness; and score 2 = accentuated redness and poor visibility of deep vessels. The endoscopic images of 146 patients with UC in remission were reviewed. Patients with a TXI score of 2 had lower UC relapse-free rates than those with TXI scores of 0 and 1 (log-rank test, P < 0.01). When pathologic remission was defined as Matts grade ≤ 2, the rate of pathologic remission decreased with higher TXI scores (TXI score 0, 95.2%; TXI score 1, 67.8%; TXI score 2, 40.0%, respectively, P = 0.01). In multivariate analysis, TXI score 2 was associated with UC relapse (hazard ratio 4.16, 95%CI: 1.72-10.04, P < 0.01), whereas the Mayo endoscopic subscore (MES) was not. Furthermore, the relapse-free rate was lower in MES 1 with a TXI score of 2 than in MES 0 or MES 1 with a TXI score of 0 or 1. The authors concluded that TXI could identify populations with poor prognosis in MES 1, for whom treatment intensification has been controversial.
Two studies have investigated the efficacy of TXI in treating gastric lesions. Kemmoto et al[30] performed a cross-sectional study analyzing gastric cancer (GC) detection during gastroscopic screening. They compared the GC detection rate in TXI mode 2 with that of the WLI observations. A total of 13440 patients (median age 57 years; 60.6% male) were included in this study. The GC detection rate was higher in the TXI group than in the WLI group (0.71% vs 0.29%), especially among patients who had undergone Helicobacter pylori (H. pylori) eradication (1.36% vs 0.43%). The positive predictive value of GC on biopsy was higher in the TXI group than in the WLI group (11.0% vs 4.9%). Furthermore, the Expert-WLI group, which was limited to patients who underwent WLI by three endoscopists with the highest GC detection rates, was compared with the TXI group. The detection rates of GC after H. pylori eradication in the lower stomach, with 0-IIc endoscopic morphology according to the Paris classification, and with the histologically differentiated type were higher in the TXI group than in the Expert-WLI group (0.87% vs 0.17%, 1.36% vs 0.43%, and 1.36% vs 0.52%, respectively). The authors concluded that TXI mode 2 improved the detection of GC after H. pylori eradication in the L-region with superficially depressed and differentiated types. This study demonstrated the usefulness of TXI observation in GC screening.
Kitagawa et al[31] examined the diagnostic accuracy of H. pylori infections in the stomach. The patients were divided into three groups based on H. pylori infection status: Current, past, and non-infection. The diagnostic accuracy for H. pylori infection status was compared in TXI with WLI observation. Endoscopic images of 60 patients (median age 73 years; 68.3% male) were reviewed. The sensitivity and accuracy for current H. pylori infection in TXI were higher than those of WLI (69.2% vs 52.5%, P = 0.012; 85.3% vs 78.7%, P = 0.034). The diagnostic odds ratio of diffuse redness for current infection, map-like redness for past infection, and regular arrangement of collecting venules for non-infection in TXI observation were higher than those in WLI observation (56.21 vs 22.00, 10.97 vs 6.29, and 42.25 vs 25.24, respectively). TXI may be useful for diagnosing H. pylori infection during gastroscopy.
We have highlighted recent reports examining the usefulness of TXI for gastrointestinal diseases in clinical practice (Table 1). For colorectal neoplasia, an RCT and a cross-over study demonstrated that TXI had a higher ADR and MAP than WLI, and an RCT showed that TXI had non-inferiority to NBI in terms of MASP and MAP. A scoring system using the TXI was shown to be useful in predicting UC relapse in a cohort study. For the stomach, TXI was reported to improve GC detection and diagnostic accuracy of active H. pylori gastritis compared to WLI. TXI can selectively enhance brightness in dark areas of an endoscopic image and highlight subtle tissue differences, such as slight morphological or color changes, without over-enhancement[7]. These characteristics of TXI may aid in making more accurate diagnoses.
Further validation of the detection of neoplastic and inflammatory lesions using TXI is required. Furthermore, investigations on whether the use of TXI endoscopy is associated with better patient prognosis are desirable. Studies have shown that endoscopy with magnification, dye spraying[9,10,32,33], and artificial intelligence[34-36] is useful for diagnosing neoplasia and inflammation, and validation of these modalities in combination with TXI is expected. Therapeutic applications of TXI are expected in the future[37]. The two modes of TXI have different features, so they should be separately described in future studies. There have been many reports from Japan, and further international research is required. In conclusion, TXI can improve the detection and qualitative diagnosis of gastrointestinal lesions.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Altonbary AY, Egypt S-Editor: Li L L-Editor: A P-Editor: Yuan YY
| 1. | Muto M, Minashi K, Yano T, Saito Y, Oda I, Nonaka S, Omori T, Sugiura H, Goda K, Kaise M, Inoue H, Ishikawa H, Ochiai A, Shimoda T, Watanabe H, Tajiri H, Saito D. Early detection of superficial squamous cell carcinoma in the head and neck region and esophagus by narrow band imaging: a multicenter randomized controlled trial. J Clin Oncol. 2010;28:1566-1572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 427] [Cited by in RCA: 524] [Article Influence: 34.9] [Reference Citation Analysis (0)] |
| 2. | Kobayashi S, Yamada M, Takamaru H, Sakamoto T, Matsuda T, Sekine S, Igarashi Y, Saito Y. Diagnostic yield of the Japan NBI Expert Team (JNET) classification for endoscopic diagnosis of superficial colorectal neoplasms in a large-scale clinical practice database. United European Gastroenterol J. 2019;7:914-923. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 75] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 3. | Cho JH, Jeon SR, Jin SY, Park S. Standard vs magnifying narrow-band imaging endoscopy for diagnosis of Helicobacter pylori infection and gastric precancerous conditions. World J Gastroenterol. 2021;27:2238-2250. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 5] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (1)] |
| 4. | Suzuki T, Hara T, Kitagawa Y, Takashiro H, Nankinzan R, Sugita O, Yamaguchi T. Linked-color imaging improves endoscopic visibility of colorectal nongranular flat lesions. Gastrointest Endosc. 2017;86:692-697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 82] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 5. | Fujiyoshi T, Miyahara R, Funasaka K, Furukawa K, Sawada T, Maeda K, Yamamura T, Ishikawa T, Ohno E, Nakamura M, Kawashima H, Nakaguro M, Nakatochi M, Hirooka Y. Utility of linked color imaging for endoscopic diagnosis of early gastric cancer. World J Gastroenterol. 2019;25:1248-1258. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 24] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (1)] |
| 6. | Cheema HI, Tharian B, Inamdar S, Garcia-Saenz-de-Sicilia M, Cengiz C. Recent advances in endoscopic management of gastric neoplasms. World J Gastrointest Endosc. 2023;15:319-337. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 4] [Cited by in RCA: 7] [Article Influence: 3.5] [Reference Citation Analysis (1)] |
| 7. | Sato T. TXI: Texture and Color Enhancement Imaging for Endoscopic Image Enhancement. J Healthc Eng. 2021;2021:5518948. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 72] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 8. | Wang Y, Sun CY, Scott L, Wu DD, Chen X. Texture and color enhancement imaging for detecting colorectal adenomas: Good, but not good enough. World J Gastrointest Endosc. 2022;14:471-473. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (1)] |
| 9. | Nishizawa T, Toyoshima O, Yoshida S, Uekura C, Kurokawa K, Munkhjargal M, Obata M, Yamada T, Fujishiro M, Ebinuma H, Suzuki H. TXI (Texture and Color Enhancement Imaging) for Serrated Colorectal Lesions. J Clin Med. 2021;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 10. | Toyoshima O, Nishizawa T, Yoshida S, Yamada T, Odawara N, Matsuno T, Obata M, Kurokawa K, Uekura C, Fujishiro M. Texture and color enhancement imaging in magnifying endoscopic evaluation of colorectal adenomas. World J Gastrointest Endosc. 2022;14:96-105. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 15] [Cited by in RCA: 14] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 11. | Tamai N, Horiuchi H, Matsui H, Furuhashi H, Kamba S, Dobashi A, Sumiyama K. Visibility evaluation of colorectal lesion using texture and color enhancement imaging with video. DEN Open. 2022;2:e90. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 12. | Hiramatsu T, Nishizawa T, Kataoka Y, Yoshida S, Matsuno T, Mizutani H, Nakagawa H, Ebinuma H, Fujishiro M, Toyoshima O. Improved visibility of colorectal tumor by texture and color enhancement imaging with indigo carmine. World J Gastrointest Endosc. 2023;15:690-698. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 13. | Ishikawa T, Matsumura T, Okimoto K, Nagashima A, Shiratori W, Kaneko T, Oura H, Tokunaga M, Akizue N, Ohta Y, Saito K, Arai M, Kato J, Kato N. Efficacy of Texture and Color Enhancement Imaging in visualizing gastric mucosal atrophy and gastric neoplasms. Sci Rep. 2021;11:6910. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 37] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 14. | Abe S, Yamazaki T, Hisada IT, Makiguchi ME, Yoshinaga S, Sato T, Nonaka S, Suzuki H, Oda I, Saito Y. Visibility of early gastric cancer in texture and color enhancement imaging. DEN Open. 2022;2:e46. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 26] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 15. | Kawasaki A, Yoshida N, Nakanishi H, Tsuji S, Takemura K, Doyama H. Usefulness of third-generation narrow band imaging and texture and color enhancement imaging in improving visibility of superficial early gastric cancer: A study using color difference. DEN Open. 2023;3:e186. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 10] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 16. | Koyama Y, Sugimoto M, Kawai T, Mizumachi M, Yamanishi F, Matsumoto S, Suzuki Y, Nemoto D, Shinohara H, Ichimiya T, Muramatsu T, Kagawa Y, Matsumoto T, Madarame A, Morise T, Uchida K, Yamaguchi H, Kono S, Naito S, Fukuzawa M, Itoi T. Visibility of early gastric cancers by texture and color enhancement imaging using a high-definition ultrathin transnasal endoscope. Sci Rep. 2023;13:1994. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 17. | Shijimaya T, Tahara T, Uragami T, Yano N, Tokutomi Y, Uwamori A, Nishimon S, Kobayashi S, Matsumoto Y, Nakamura N, Okazaki T, Takahashi Y, Tomiyama T, Honzawa Y, Fukata N, Fukui T, Naganuma M. Usefulness of texture and color enhancement imaging (TXI) in early gastric cancer found after Helicobacter pylori eradication. Sci Rep. 2023;13:6899. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 18. | Futakuchi T, Dobashi A, Horiuchi H, Furuhashi H, Matsui H, Hara Y, Kobayashi M, Ono S, Tamai N, Gomisawa K, Yamauchi T, Suka M, Sumiyama K. Texture and color enhancement imaging improves the visibility of gastric neoplasms: clinical trial with image catalogue assessment using conventional and newly developed endoscopes. BMC Gastroenterol. 2023;23:389. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 19. | Sugimoto M, Kawai Y, Morino Y, Hamada M, Iwata E, Niikura R, Nagata N, Koyama Y, Fukuzawa M, Itoi T, Kawai T. Efficacy of high-vision transnasal endoscopy using texture and colour enhancement imaging and narrow-band imaging to evaluate gastritis: a randomized controlled trial. Ann Med. 2022;54:1004-1013. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 19] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 20. | Sugimoto M, Kawai Y, Akimoto Y, Hamada M, Iwata E, Murata M, Mizuno H, Niikura R, Nagata N, Fukuzawa M, Itoi T, Kawai T. Third-Generation High-Vision Ultrathin Endoscopy Using Texture and Color Enhancement Imaging and Narrow-Band Imaging to Evaluate Barrett's Esophagus. Diagnostics (Basel). 2022;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 21. | Ikeda A, Takeda T, Ueyama H, Uemura Y, Iwano T, Yamamoto M, Uchida R, Utsunomiya H, Oki S, Suzuki N, Abe D, Yatagai N, Akazawa Y, Ueda K, Asaoka D, Shibuya T, Hojo M, Nojiri S, Nagahara A. Comparison of Texture and Color Enhancement Imaging with White Light Imaging in 52 Patients with Short-Segment Barrett's Esophagus. Med Sci Monit. 2023;29:e940249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 22. | Dobashi A, Ono S, Furuhashi H, Futakuchi T, Tamai N, Yamauchi T, Suka M, Sumiyama K. Texture and Color Enhancement Imaging Increases Color Changes and Improves Visibility for Squamous Cell Carcinoma Suspicious Lesions in the Pharynx and Esophagus. Diagnostics (Basel). 2021;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 26] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 23. | Okimoto K, Matsumura T, Maruoka D, Kurosugi A, Shiratori W, Nagashima A, Ishikawa T, Kaneko T, Kanayama K, Akizue N, Ohta Y, Taida T, Saito K, Kato J, Kato N. Magnified endoscopy with texture and color enhanced imaging with indigo carmine for superficial nonampullary duodenal tumor: a pilot study. Sci Rep. 2022;12:10381. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 24. | Miyaguchi K, Mizuide M, Tanisaka Y, Fujita A, Jinushi R, Hiromune K, Ogawa T, Saito Y, Tashima T, Mashimo Y, Imaeda H, Ryozawa S. Distinguishing the papilla of Vater during biliary cannulation using texture and color enhancement imaging: A pilot study. DEN Open. 2023;3:e125. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 25. | Antonelli G, Bevivino G, Pecere S, Ebigbo A, Cereatti F, Akizue N, Di Fonzo M, Coppola M, Barbaro F, Walter BM, Sharma P, Caruso A, Okimoto K, Antenucci C, Matsumura T, Zerboni G, Grossi C, Meinikheim M, Papparella LG, Correale L, Costamagna G, Repici A, Spada C, Messmann H, Hassan C, Iacopini F. Texture and color enhancement imaging versus high definition white-light endoscopy for detection of colorectal neoplasia: a randomized trial. Endoscopy. 2023;55:1072-1080. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 10.0] [Reference Citation Analysis (1)] |
| 26. | Hassan C, Antonelli G, Dumonceau JM, Regula J, Bretthauer M, Chaussade S, Dekker E, Ferlitsch M, Gimeno-Garcia A, Jover R, Kalager M, Pellisé M, Pox C, Ricciardiello L, Rutter M, Helsingen LM, Bleijenberg A, Senore C, van Hooft JE, Dinis-Ribeiro M, Quintero E. Post-polypectomy colonoscopy surveillance: European Society of Gastrointestinal Endoscopy (ESGE) Guideline - Update 2020. Endoscopy. 2020;52:687-700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 312] [Article Influence: 62.4] [Reference Citation Analysis (0)] |
| 27. | Yoshida N, Inagaki Y, Inada Y, Kobayashi R, Tomita Y, Hashimoto H, Dohi O, Hirose R, Inoue K, Murakami T, Morimoto Y, Okuyama Y, Morinaga Y, Itoh Y. Additional 30-Second Observation of the Right-Sided Colon for Missed Polyp Detection With Texture and Color Enhancement Imaging Compared with Narrow Band Imaging: A Randomized Trial. Am J Gastroenterol. 2023;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Reference Citation Analysis (1)] |
| 28. | Sakamoto T, Ikematsu H, Tamai N, Mizuguchi Y, Takamaru H, Murano T, Shinmura K, Sasabe M, Furuhashi H, Sumiyama K, Saito Y. Detection of colorectal adenomas with texture and color enhancement imaging: Multicenter observational study. Dig Endosc. 2023;35:529-537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 20] [Reference Citation Analysis (0)] |
| 29. | Hayashi Y, Takabayashi K, Kato M, Tojo A, Aoki Y, Hagihara Y, Yoshida K, Yoshimatsu Y, Kiyohara H, Sugimoto S, Nanki K, Mikami Y, Sujino T, Mutaguchi M, Kawaguchi T, Hosoe N, Yahagi N, Ogata H, Kanai T. Usefulness of texture and color enhancement imaging in assessing mucosal healing in patients with ulcerative colitis. Gastrointest Endosc. 2023;97:759-766.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Reference Citation Analysis (0)] |
| 30. | Kemmoto Y, Ozawa SI, Sueki R, Furuya K, Shirose D, Wakao S, Shindo K, Nagata A, Sato T. Higher detectability of gastric cancer after Helicobacter pylori eradication in texture and color enhancement imaging mode 2 in screening endoscopy. DEN Open. 2024;4:e279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 31. | Kitagawa Y, Koga K, Ishigaki A, Nishii R, Sugita O, Suzuki T. Endoscopic diagnosis of Helicobacter pylori gastritis using white light imaging and texture and color enhancement imaging. Endosc Int Open. 2023;11:E136-E141. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 32. | Waki K, Kanesaka T, Michida T, Ishihara R, Tanaka Y. Improved visibility of early gastric cancer by using a combination of chromoendoscopy and texture and color enhancement imaging. Gastrointest Endosc. 2022;95:800-801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 33. | Takabayashi K, Kato M, Sugimoto S, Yahagi N, Kanai T. Texture and color enhancement imaging in combination with indigo carmine dye spraying to highlight the border of flat ulcerative colitis-associated neoplasia. Gastrointest Endosc. 2022;95:1273-1275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 34. | Joseph J, LePage EM, Cheney CP, Pawa R. Artificial intelligence in colonoscopy. World J Gastroenterol. 2021;27:4802-4817. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 5] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 35. | Taghiakbari M, Mori Y, von Renteln D. Artificial intelligence-assisted colonoscopy: A review of current state of practice and research. World J Gastroenterol. 2021;27:8103-8122. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 47] [Cited by in RCA: 32] [Article Influence: 8.0] [Reference Citation Analysis (5)] |
| 36. | Du RC, Ouyang YB, Hu Y. Research trends on artificial intelligence and endoscopy in digestive diseases: A bibliometric analysis from 1990 to 2022. World J Gastroenterol. 2023;29:3561-3573. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (2)] |
| 37. | Nabi Z, Chavan R, Ramchandani M, Darisetty S, Reddy DN. Endoscopic submucosal dissection and tunneling procedures using novel image-enhanced technique. VideoGIE. 2022;7:158-163. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |