Published online Apr 7, 2024. doi: 10.3748/wjg.v30.i13.1871
Peer-review started: January 9, 2024
First decision: January 17, 2024
Revised: January 30, 2024
Accepted: March 13, 2024
Article in press: March 13, 2024
Published online: April 7, 2024
Processing time: 84 Days and 16.6 Hours
Real-world data on tofacitinib (TOF) covering a period of more than 1 year for a sufficient number of Asian patients with ulcerative colitis (UC) are scarce.
To investigate the long-term efficacy and safety of TOF treatment for UC, including clinical issues.
We performed a retrospective single-center observational analysis of 111 UC patients administered TOF at Hyogo Medical University as a tertiary inflammatory bowel disease center. All consecutive UC patients who received TOF between May 2018 and February 2020 were enrolled. Patients were followed up until August 2020. The primary outcome was the clinical response rate at week 8. Secondary outcomes included clinical remission at week 8, cumulative persistence rate of TOF administration, colectomy-free survival, relapse after tapering of TOF and predictors of clinical response at week 8 and week 48.
The clinical response and remission rates were 66.3% and 50.5% at week 8, and 47.1% and 43.5% at week 48, respectively. The overall cumulative clinical remission rate was 61.7% at week 48 and history of anti-tumor necrosis factor-alpha (TNF-α) agents use had no influence (P = 0.25). The cumulative TOF persistence rate at week 48 was significantly lower in patients without clinical remission than in those with remission at week 8 (30.9% vs 88.1%; P < 0.001). Baseline partial Mayo Score was significantly lower in responders vs non-responders at week 8 (odds ratio: 0.61, 95% confidence interval: 0.45-0.82, P = 0.001). Relapse occurred in 45.7% of patients after TOF tapering, and 85.7% of patients responded within 4 wk after re-increase. All 6 patients with herpes zoster (HZ) developed the infection after achieving remission by TOF.
TOF was more effective in UC patients with mild activity at baseline and its efficacy was not affected by previous treatment with anti-TNF-α agents. Most relapsed patients responded again after re-increase of TOF and nearly half relapsed after tapering off TOF. Special attention is needed for tapering and HZ.
Core Tip: This is a retrospective single-center observational study to investigate the long-term efficacy and safety of tofacitinib (TOF) treatment for ulcerative colitis (UC), including clinical issues. TOF is more effective in low-activity UC patients and its efficacy is not affected by previous treatment with anti-tumor necrosis factor-alpha agents. Most patients in the clinical remission group at week 8 could continue TOF over a long follow-up period. Most relapsed patients responded again after re-increase of TOF and nearly half relapsed after tapering off TOF. Although most patients continue TOF without severe adverse events, careful monitoring for herpes zoster is necessary.
- Citation: Kojima K, Watanabe K, Kawai M, Yagi S, Kaku K, Ikenouchi M, Sato T, Kamikozuru K, Yokoyama Y, Takagawa T, Shimizu M, Shinzaki S. Real-world efficacy and safety of tofacitinib treatment in Asian patients with ulcerative colitis. World J Gastroenterol 2024; 30(13): 1871-1886
- URL: https://www.wjgnet.com/1007-9327/full/v30/i13/1871.htm
- DOI: https://dx.doi.org/10.3748/wjg.v30.i13.1871
Ulcerative colitis (UC) is an idiopathic disease causing chronic mucosal inflammation in the colorectum. The pathogenesis of UC is not fully established, but genetic factors and environmental factors are thought to be intricately intertwined with each other[1]. In addition, some factors may cause a dysregulated immune response in the intestinal tract that is involved in the onset and persistence of inflammation as a multifactorial disease[1]. Although various therapeutic agents have been developed, insufficient efficacy for remission induction and remission maintenance is observed. Several treatment options are currently available as advanced therapy, including calcineurin inhibitors, anti-tumor necrosis factor-alpha (TNF-α) agents (infliximab, adalimumab, golimumab), anti-α4β7 integrin antibodies (vedolizumab), and anti-interleukin-12/23 submit p40 antibodies (ustekinumab) for patients with intractable UC. In 2018, the small molecule Janus kinase (JAK) inhibitor tofacitinib (TOF) was approved for use in Japan. The selective JAK1 inhibitors filgotinib and upadacitinib were also recently approved in Japan.
Cytokines are involved in both intestinal homeostasis and pathologic processes associated with inflammatory bowel disease (IBD)[2]. TOF prevents the activation of JAK, phosphorylation of signal transducer and activator of transcription (STAT), and translocation to the nucleus to activate gene transcription, and thus downregulates cytokine production[3]. The JAK/STAT pathway plays an important role in cell growth and survival, differentiation, and proliferation. JAK consists of JAK1, JAK2, JAK3, and tyrosine kinase 2, and TOF is a non-selective pan-JAK inhibitor, targeting mainly JAK1/JAK3[4,5]. TOF is orally bioavailable and has predictable pharmacokinetics and no immunogenicity in contrast to monoclonal antibody therapies such as the anti-TNF-α agents used for the treatment of IBD[2].
The efficacy and safety of TOF for moderately to severely active UC were globally shown in a phase II trial; and 2 phase III induction studies (OCTAVE Induction 1 and 2); a phase III maintenance study (OCTAVE Sustain); and an open-label, long-term extension study (OCTAVE Open)[6-8]. In Japanese patients, post hoc analyses of TOF treatment in 2 studies (OCTAVE Induction 1 and OCTAVE Sustain) are reported[9]. The importance of real-world data, however, has become increasingly recognized in recent years because the setting of a clinical (drug) trial is a special situation[10]. Additionally, genetic backgrounds and phenotypes of IBD differ considerably between Asian and Western patients[11,12]; thus, it is critical to determine the appropriate treatment targets, outcomes, and responses in populations with different genetic backgrounds[13]. It is well-known that the risk of herpes zoster (HZ) as an adverse event associated with TOF is greater in Asian UC patients than in Western UC patients[14]. The United States Food and Drug Administration noted the risk of major adverse cardiovascular events, malignancy, and thrombosis for JAK inhibitors in patients with other immune-mediated diseases, e.g., rheumatoid arthritis[15]. It is possible, however, that the difference in the risks depends on the disease or geographical region.
Only 1 published study of more than 100 Asian UC cases with a 1-year observation period focusing on the efficacy and safety of TOF is available from Korea[16]. Real-world data, however, including clinical issues of TOF, such as relapse rate after tapering TOF or efficacy of re-increasing the dosage of TOF, with a sufficient number of subjects are essential for optimizing treatment with TOF and comparing with the other data of JAK1 inhibitors to optimize the JAK inhibitor treatment strategies in Asian UC patients. The aim of this investigation was to evaluate the clinical efficacy, safety, and clinical issues related to TOF in more than 100 Japanese UC patients with a median observation period of more than 1 year in our specialized IBD center.
We performed a retrospective single-center observational analysis to investigate the efficacy and safety of TOF for intractable UC patients at the Hyogo Medical University as a tertiary IBD center. This investigation was approved by the Institutional Ethics Committee at our institute (number 3030).
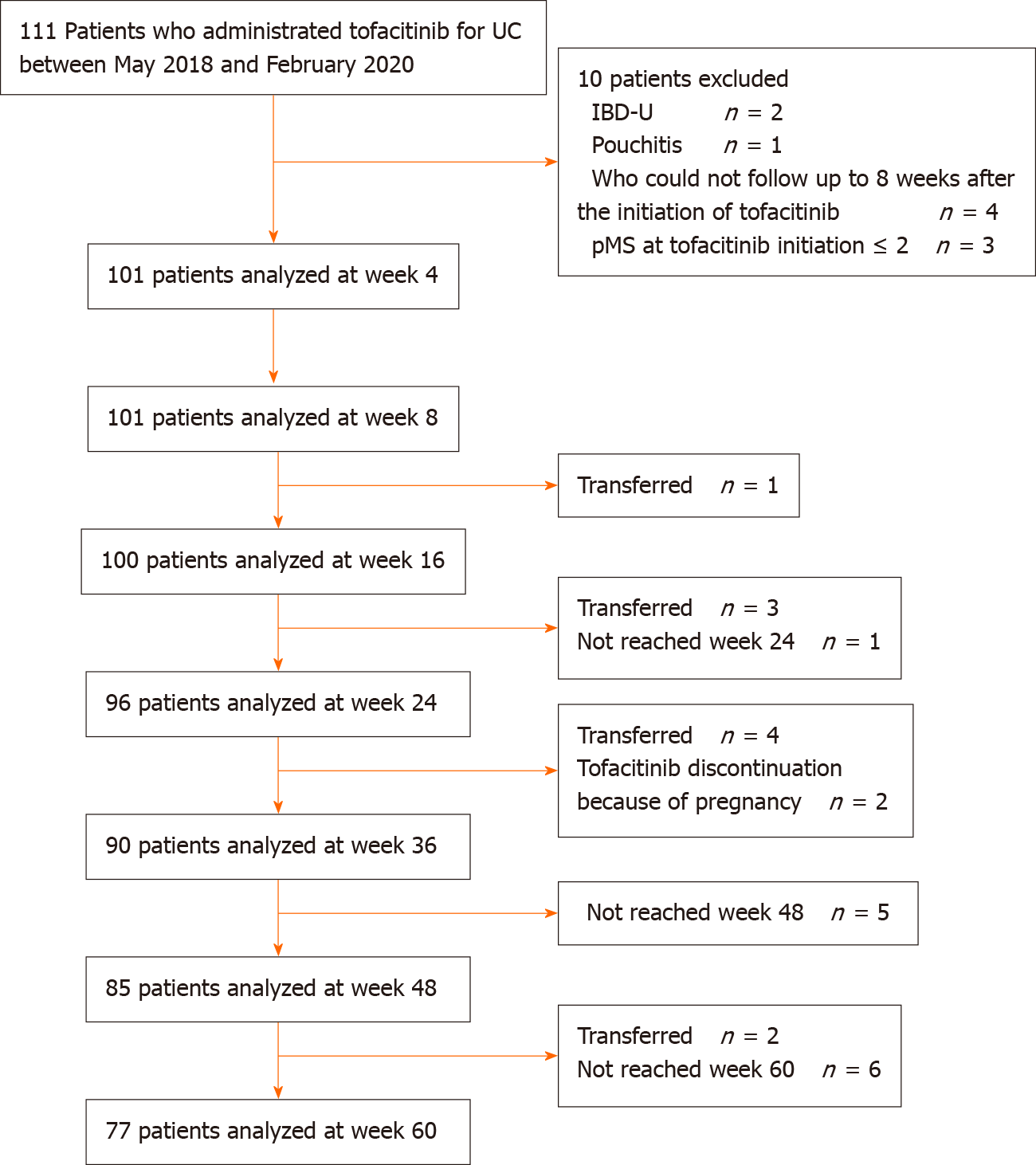
All consecutive UC patients who received TOF between May 2018 and February 2020 were enrolled. Patients were followed up until August 2020. The diagnosis of UC was based on the diagnostic criteria of the Research Committee on Inflammatory Bowel Disease in Japan[17]. For evaluation of the efficacy, we excluded patients with IBD-unclassified, patients who underwent total colectomy, and patients who could not be followed up ≥ 8 wk after the initiation of TOF. Patients with a partial Mayo Score (pMS) ≤ 2 at TOF initiation were also excluded[18]. For the safety evaluation, all patients who were administered TOF were included. Patients aged ≤ 16 years were excluded from assessment of the efficacy and safety of TOF.
All data on patient demographics were retrieved from electronic medical records. Data were collected on age, sex, duration of UC, disease extent according to the Montreal classification[19], family history of IBD, smoking status, comorbidities, past history of HZ, disease activity according to the total or pMS[18], endoscopic activity according to the Mayo endoscopic subscore, intractability to steroids, details of previous and concomitant UC therapies, and details of TOF treatment (dosage and duration). Laboratory data included white blood cell count, lymphocyte count, hemoglobin, serum albumin, C-reactive protein (CRP), and total cholesterol levels. Potential adverse events related to TOF were recorded in all patients who received at least 1 dose of TOF.
All patients were administered TOF (10 mg p.o.) twice daily as induction therapy. After achieving clinical remission, the dosage of TOF was tapered and then withdrawn according to the patient’s condition. If relapse occurred after tapering or withdrawing, dose escalation or re-administration were accepted.
The primary outcome was the proportion of patients with a clinical response (defined as a decrease in pMS ≥ 2 points from baseline with an accompanying decrease in the rectal bleeding subscore ≥ 1 point or an absolute rectal bleeding subscore ≤ 1 point). Secondary outcomes included clinical remission (defined as pMS ≤ 2, with no subscore > 1 and a rectal bleeding subscore of 0) at week 8[20], steroid-free remission (defined as clinical remission without corticosteroid use at that time point), cumulative remission rate among patients who achieved clinical remission at week 8, cumulative persistence rate of TOF administration, colectomy-free survival, relapse after tapering of TOF (defined as increasing dosage of TOF or concomitant treatment drug, addition of treatment drug, or an increase in a pMS of at least 2 points or an increase with absolute rectal bleeding subscore ≥ 1 point from the time of TOF reduction), and predictors of clinical response at week 8 and week 48. A delayed responder was defined as a patient who did not achieve clinical remission at week 8 but had achieved clinical remission by week 24. Complete endoscopic remission was defined as a Mayo endoscopic score (MES) of 0. Endoscopic remission was defined as a MES ≤ 1 and endoscopic response was defined as a decrease from baseline in the MES of at least 1 point. Endoscopic efficacy at week 16 was assessed in 25 patients who underwent endoscopy by week 16, and the other 14 clinical non-responders who did not undergo endoscopy until week 16 were regarded as endoscopic non-responders with MES ≥ 2 at week 16. Patients who did not undergo colonoscopy were excluded from the evaluation of endoscopic efficacy despite being in clinical remission. Treatment failure was defined as TOF discontinuation due to symptomatic recurrence or adverse events, or additional therapy. Patients considered to have failed treatment were counted as non-responders. For example, a patient who discontinued TOF at week 8 was classified as a non-responder until week 60. If TOF was discontinued due to the desire to have children or the patient was transferred, it was censored at that time. The follow-up period was defined as the time between the initiation of TOF and the last visit or discontinuation of TOF until August 2020.
In the descriptive statistics of quantitative variables, the median and interquartile range (IQR) were calculated depending on whether the data were normally distributed. Categorical variables are presented as percentage with 95% confidence intervals (CI). We used the Mann–Whitney U test to compare the quantitative variables and Fisher’s exact test to compare categorical variables for patients in the responder and non-responder groups at week 8 and week 48. Paired t test was used to determine the level of significance of change in total cholesterol levels over time during the treatment of TOF. Survival was analyzed using the Kaplan-Meier method and log-rank test. P values for log-rank trend tests were examined whether increased score of pMS at baseline stratified by number of previous anti-TNF-α agent failures associate with the cumulative remission rate or persistence rate of TOF administration. Variables associated with predictors of the baseline characteristics of the efficacy at week 8 were explored using a logistic regression model. P values < 0.05 were considered as statistically significant. Statistical analyses were performed with EZR ver. 1.38 (Saitama Medical Center, Jichi Medical University, Saitama, Japan), which is a graphical user interface for R (The R Foundation for Statistical Computing, Vienna, Austria)[21].
The study included 111 patients treated with TOF between May 2018 and February 2020. Among them, 101 patients were included in the assessment of the efficacy of TOF after excluding 10 patients (Figure 1). The median follow-up time was 365 (IQR: 231–500) d. Patients' baseline characteristics are shown in Table 1. The median age was 35.0 (IQR: 28.0–47.0) years, 60 (59.4%) patients were male, and the median disease duration was 4.8 (IQR: 1.5–10.0) years. Most patients had pancolitis-type UC (82.2%). Seven (6.9%) patients had a family history of IBD and 14 (13.9%) were current smokers. Seven (6.9%) patients had hypertension, 6 (5.9%) had dyslipidemia, 3 (3.0%) had diabetes, and 2 (2.0%) had thrombosis. Only 1 (1.0%) patient had a past history of HZ. Forty-eight (47.5%) patients were steroid-dependent and 51 (50.5%) were steroid-refractory, and 23 (22.8%) received systemic corticosteroid therapy at TOF administration. Seventy (69.3%) patients had previously received an immunomodulator, 27 (26.7%) received cytapheresis, 31 (30.7%) received tacrolimus, and 67 (66.3%) received biologics. Sixty-three (62.4%) patients received anti-TNF-α agents, 11 (10.9%) received vedolizumab, and 7 (6.9%) received both. The number of anti-TNF-α agent failures was 44 (43.6%) patients with 1 failure, 17 (16.8%) patients with 2 failures, and 2 (2.0%) patients with 3 failures. The median pMS was 6.0 (IQR: 4.0-7.0) and median CRP was 0.3 (IQR: 0.1-1.0) mg/dL. At baseline, 54 (53.5%) patients underwent endoscopy, with a median MES of 2.0 (IQR: 2.0-3.0) and a median total Mayo Score of 9.0 (IQR: 7.0-10.0).
| Characteristic | n = 101 |
| Age, years, median (IQR) | 35 (28.0, 47.0) |
| Sex | |
| Male, n (%) | 60 (59.4) |
| Female, n (%) | 41 (40.6) |
| Duration of UC, years, median (IQR) | 4.8 (1.5, 10.0) |
| Disease extent, n (%) | |
| Left-sided colitis | 18 (17.8) |
| Pancolitis | 83 (82.2) |
| Family history of IBD, n (%) | 7 (6.9) |
| Smoking classification, n (%) | |
| Never smoked | 66 (65.3) |
| Current smoker | 14 (13.9) |
| Ex-smoker | 21 (20.8) |
| Comorbidities, n (%) | |
| Hypertension | 7 (6.9) |
| Dyslipidemia | 6 (5.9) |
| Diabetes mellitus | 3 (3.0) |
| Thrombosis | 2 (2.0) |
| Past history of herpes zoster, n (%) | 1 (1.0) |
| Total Mayo score (n = 54), median (IQR) | 9.0 (7.0,10.0) |
| Partial Mayo score, median (IQR) | 6.0 (4.0, 7.0) |
| Mayo endoscopic subscore (n = 54), median (IQR) | 2.0 (2.0, 3.0) |
| 1, n (%) | 3.0 (5.0, 6.0) |
| 2, n (%) | 26 (48.1) |
| 3, n (%) | 25 (46.3) |
| Intractability, n (%) | |
| Steroid dependent | 48 (47.5) |
| Steroid refractory | 51 (50.5) |
| C-reactive protein (mg/dL), median (IQR) | 0.3 (0.1, 1.0) |
| Albumin (g/dL), median (IQR) | 3.9 (3.5, 4.2) |
| White blood cells (/μL), median (IQR) | 7180.0 (5490.0, 9160.0) |
| Lymphocytes (/μL), median (IQR) | 1506.0 (1083.0, 1874.0) |
| Hemoglobin (g/dL), median (IQR) | 12.3 (11.1, 13.8) |
| Total Cholesterol (mg/dL) (n = 71), median (IQR) | 179.0 (163.0, 199.0) |
| Concomitant drugs at baseline, n (%) | |
| 5-aminosalicylic acid | 68 (67.3) |
| Systemic corticosteroid | 23 (22.8) |
| Topical corticosteroid | 22 (21.8) |
| History of treatment at baseline | |
| Previous corticosteroid, n (%) | 99 (98.0) |
| Previous immunomodulator, n (%) | 70 (69.3) |
| Previous cytapheresis, n (%) | 27 (26.7) |
| Previous tacrolimus, n (%) | 31 (30.7) |
| Previous biologics, n (%) | 67 (66.3) |
| Anti-TNF-α agent, n (%) | 63 (62.4) |
| Infliximab | 38 (37.6) |
| Adalimumab | 27 (26.7) |
| Golimumab | 19 (18.8) |
| Number of anti-TNF-α agent failure, n (%) | |
| 1 agent | 44 (43.6) |
| 2 agents | 17 (16.8) |
| 3 agents | 2 (2.0) |
| Vedolizumab, n (%) | 11 (10.9) |
| Anti-TNF-α agent and vedolizumab, n (%) | 7 (6.9) |
The proportion of patients with a clinical response, clinical remission, and steroid-free remission was 61.4% (62/101), 36.6% (37/101), 29.7% (30/101), respectively, at week 4, and 66.3% (67/101), 50.5% (51/101), 46.5% (47/101), respectively, at week 8 (Figure 2). After week 16, all patients with clinical remission achieved steroid-free remission. Seven (6.9%) patients were delayed responders. The proportion of patients with clinical response and (steroid-free) clinical remission was 47.1% (40/85) and 43.5% (37/85), respectively, at week 48, and 39.0% (30/77) and 37.7% (29/77), respectively, at week 60.
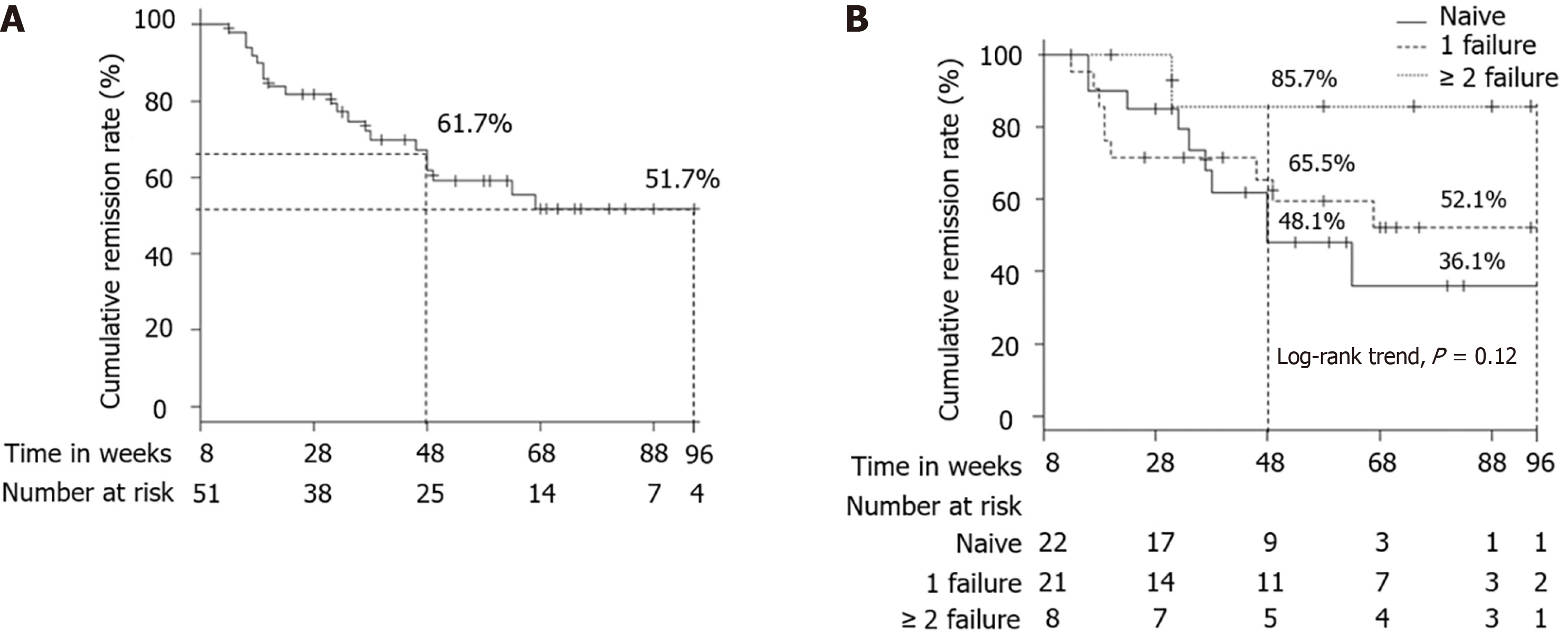
The cumulative remission rate in patients who achieved clinical remission at week 8 was 61.7% at week 48 and 51.7% at week 96 (Figure 3A). According to a previous history of anti-TNF-α agent failure (naive vs 1 failure vs ≥ 2 failure), the cumulative remission rate was 48.1% vs 65.5% vs 85.7% at week 48, and 36.1% vs 52.1% vs 85.7% at week 96 (Figure 3B). The cumulative remission rate did not differ significantly among the 3 groups (log-rank trend test, P = 0.12).
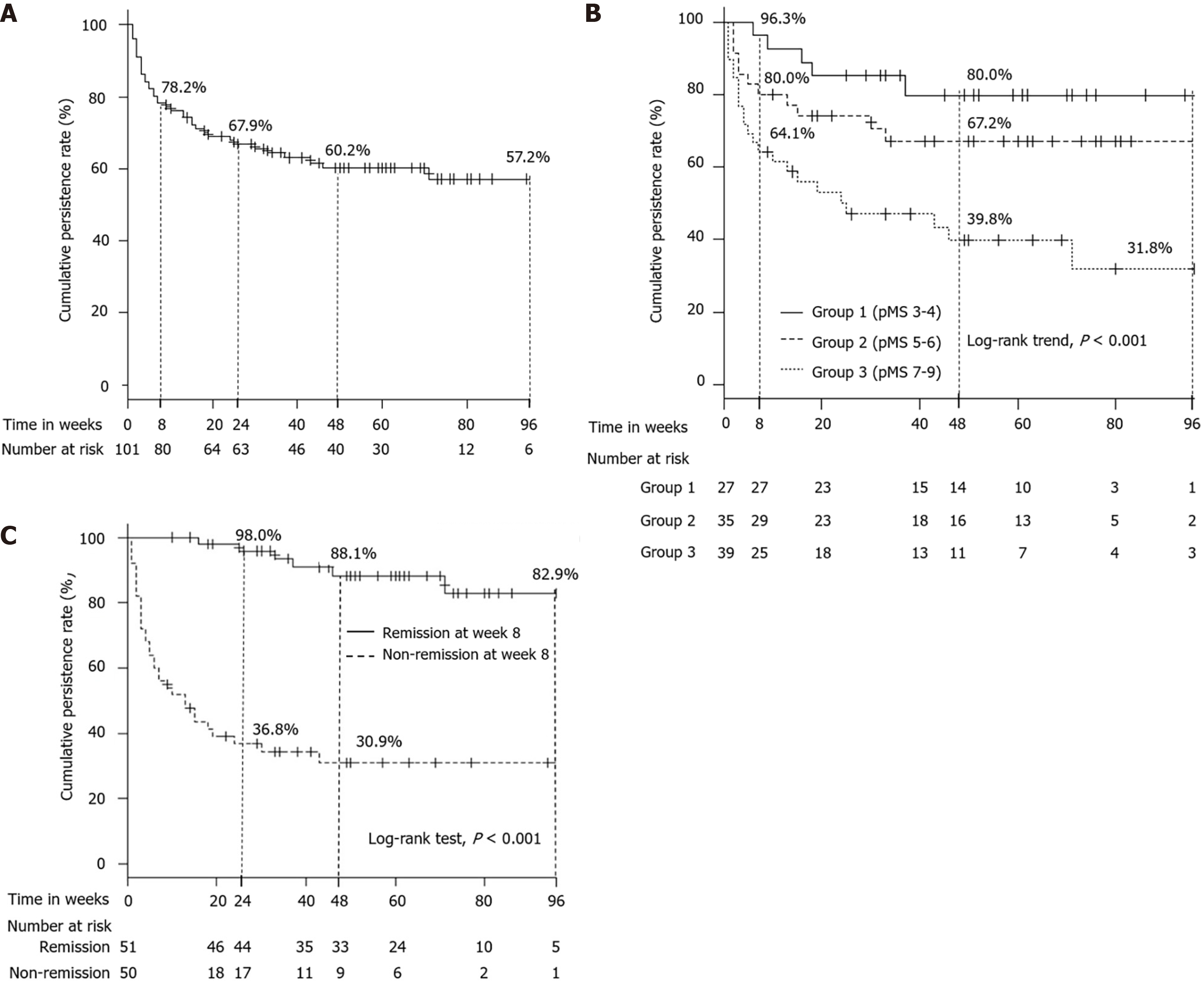
On the other hand, the cumulative persistence rate of TOF administration was 78.2% at week 8, 67.9% at week 24, 60.2% at week 48, and 57.2% at week 96 (Figure 4A). According to the raw data of pMS at baseline, we divided participants into 3 groups (Group 1: pMS 3-4, Group 2: pMS 5-6, Group 3: pMS 7-9), and the cumulative persistence rate of TOF administration among 3 groups, respectively, was 96.3%, 80.0%, and 64.1% at week 8; 80.0%, 67.2%, and 39.8% at week 48; and 80.0%, 67.2%, and 31.8% at week 96 (log-rank trend test, P < 0.001) (Figure 4B). Additionally, the cumulative persistence rate of TOF administration between patients who were in clinical remission and non-clinical remission at week 8 was, respectively, 98.0% and 36.8% at week 24, 88.1% and 30.9% at week 48, 82.9% and 30.9% at week 96 (P < 0.001; Figure 4C).
Nine (8.9%) patients required a colectomy during the follow-up period; 2 due to primary failure of TOF and 7 due to failure of remission maintenance therapy after withdrawing the TOF. After excluding cases that transferred, could not be followed up, or whose treatment was discontinued due to pregnancy (Figure 1), the colectomy-free survival rate was 91.9% at week 48 and 89.1% at week 96 (Figure 5).
For endoscopic efficacy at week 16, the complete endoscopic remission rate was 7.7% (3/39), the endoscopic remission rate was 23.1% (9/39), and the endoscopic response rate was 38.5% (15/39).
Clinical and demographic baseline characteristics between responders and non-responders at week 8 are shown in Table 2. Baseline pMS of responders at week 8 was significantly lower than that of non-responders [5.0 (IQR: 4.0-7.0) vs 7.0 (IQR: 5.3-8.0), P < 0.001]. In multivariate analysis, lower baseline pMS was independently associated with responders at week 8 [Odds ratio (OR): 0.61, 95%CI: 0.45-0.82, P = 0.001].
| Variable | Univariate analyses | Multivariate analyses | ||||
| Non-responder (n = 34) | Responder (n = 67) | P value | Odds ratio | 95%CI | P value | |
| Age, years, median (IQR) | 32.5 (27.5, 46.0) | 37.0 (28.0, 47.5) | 0.27 | 1.02 | 0.99-1.05 | 0.28 |
| Sex (M/F), n | 20/14 | 40/27 | 1.00 | |||
| Baseline partial Mayo score, median (IQR) | 7.0 (5.3, 8.0) | 5.0 (4.0, 7.0) | < 0.001 | 0.61 | 0.45-0.82 | 0.001 |
| Disease duration, years, median (IQR) | 6.2 (1.7, 9.9) | 4.5 (1.6, 10.0) | 0.84 | |||
| Disease extent, n (%) | ||||||
| Left-sided colitis | 8 (23.5) | 10 (14.9) | ||||
| Extensive colitis | 26 (76.5) | 57 (85.1) | 0.29 | |||
| Family history of IBD, n (%) | 3 (8.8) | 4 (6.0) | 0.69 | |||
| Current smoker, n (%) | 5 (14.7) | 9 (13.4) | 1.00 | |||
| Intractability (dependent/refractory), n | 16/18 | 32/33 | 1.00 | |||
| Albumin, g/dL, median (IQR) | 3.9 (3.5, 4.3) | 3.9 (3.6, 4.2) | 0.88 | |||
| C-reactive protein, mg/dL, median (IQR) | 0.2 (0.1, 0.7) | 0.3 (0.1, 1.1) | 0.49 | |||
| White blood cells, /μL, median (IQR) | 6660.0 (5107.5, 8415.0) | 7260.0 (5760.0, 9460.0) | 0.15 | 1.00 | 1.00-1.00 | 0.17 |
| Hemoglobin, g/dL, median (IQR) | 12.4 (11.1, 13.7) | 12.3 (11.2, 14.0) | 1.00 | |||
| Baseline systemic corticosteroid use, n (%) | 8 (23.5) | 15 (22.4) | 1.00 | |||
| Previous immunomodulator, n (%) | 24 (70.6) | 46 (68.7) | 1.00 | |||
| Previous tacrolimus, n (%) | 11 (32.4) | 20 (29.9) | 0.82 | |||
| Previous biologics, n (%) | ||||||
| Previous anti TNF-α agent | 22 (64.7) | 41 (61.2) | 0.83 | |||
| Previous vedolizumab | 4 (11.8) | 7 (10.4) | 1.00 | |||
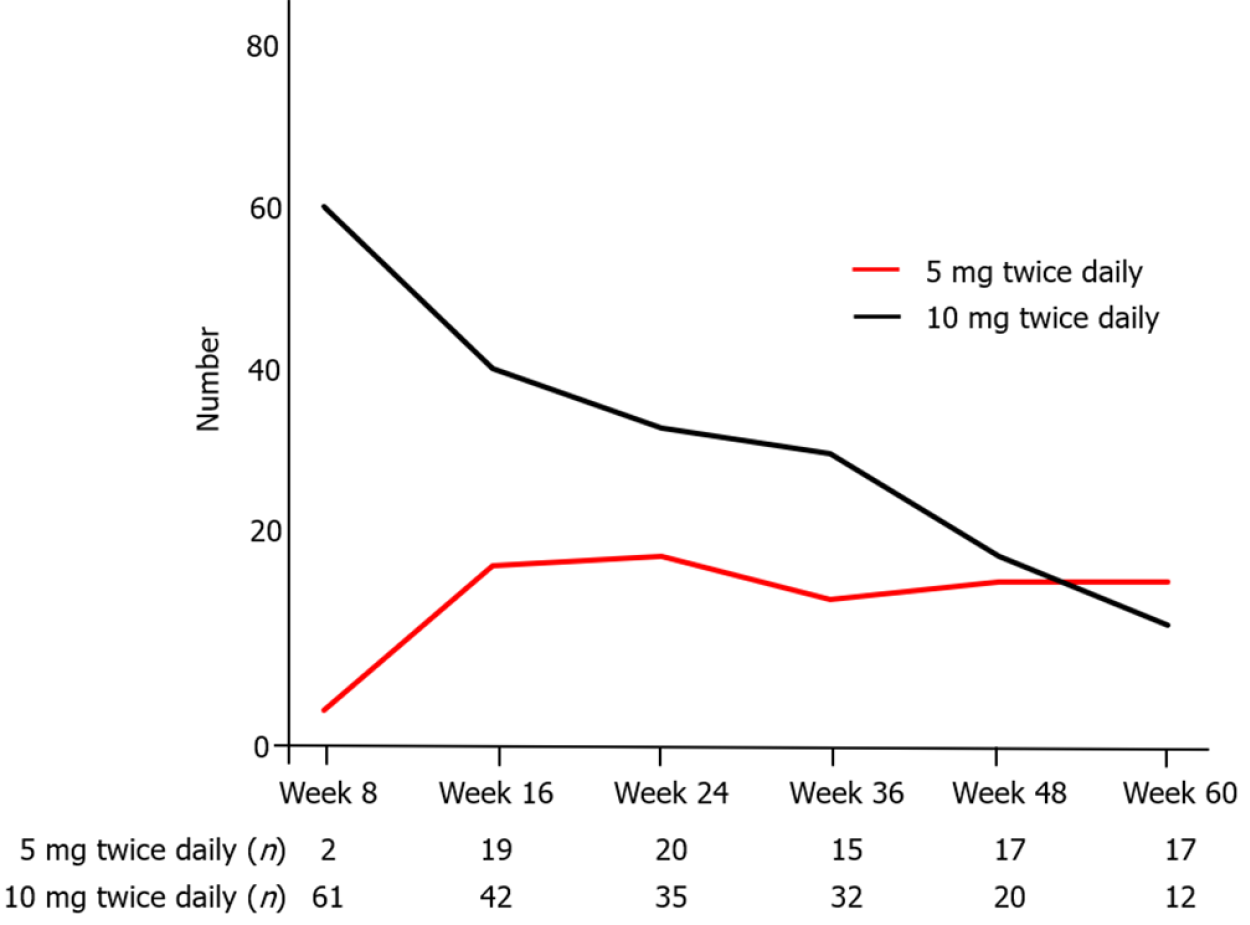
Figure 6 shows the number of patients treated with 5 mg or 10 mg twice daily at each time point in whole patients. Seventy-one (70.3%) patients were administered TOF at week 8. Among of them, 36 (35.6%) underwent tapering of TOF (5 mg twice daily) after achieving clinical remission, and the median period from baseline to the beginning of tapering was 92 (IQR: 63–120) d. The relapse rate after tapering (excluding 1 patient that withdrew from TOF due to an adverse event) was 45.7% (16/35) (Figure 7A). In these 16 patients with relapse, 14 re-increased the dosage of TOF. The clinical response and remission rate of re-increasing the dosage was, respectively, 85.7% (12/14) and 57.1% (8/14) at week 4, 84.6 % (11/13) and 69.2 % (9/13) at week 12 (Figure 7B). The median period from beginning of tapering to re-increasing the dosage was 95 (IQR: 38–216) d.
All 111 patients who received TOF were included in the safety evaluation and observed for 80.5 patient-years. A total of 58 adverse events occurred during the follow-up period, 38 (34.2%) patients experienced at least 1 adverse event. All adverse events are shown in Table 3. Seven (6.3%) patients discontinued TOF due to adverse events; 3 (2.7%) with fever, and 1 each (0.9%) with bronchitis, elevation of creatine kinase, lower leg edema, general fatigue, or sore throat.
| Safety profile | Incidence rates per 100 patient-years | |
| Hypercholesterolemia | 17 (15.3) | 21.1 |
| Herpes zoster | 6 (5.4) | 7.5 |
| Upper respiratory tract infection | 5 (4.5) | 6.2 |
| Fever | 4 (3.6) | 5.0 |
| Headache | 3 (2.7) | 3.7 |
| Influenza | 3 (2.7) | 3.7 |
| Lower leg edema | 2 (1.8) | 2.5 |
| General fatigue | 2 (1.8) | 2.5 |
| Sore throat | 2 (1.8) | 2.5 |
| Bronchitis | 1 (0.9) | 1.2 |
| Herpes labialis | 1 (0.9) | 1.2 |
| Norovirus enteritis | 1 (0.9) | 1.2 |
| Clostridioides difficile colitis | 1 (0.9) | 1.2 |
| Folliculitis | 1 (0.9) | 1.2 |
| Palpitations | 1 (0.9) | 1.2 |
| Joint pain | 1 (0.9) | 1.2 |
| General pain | 1 (0.9) | 1.2 |
| Dizziness | 1 (0.9) | 1.2 |
| Sleepiness | 1 (0.9) | 1.2 |
| Deep vein thrombosis | 1 (0.9) | 1.2 |
| Dysplasia | 1 (0.9) | 1.2 |
| Elevation of creatine kinase | 1 (0.9) | 1.2 |
| Abnormal liver function tests | 1 (0.9) | 1.2 |
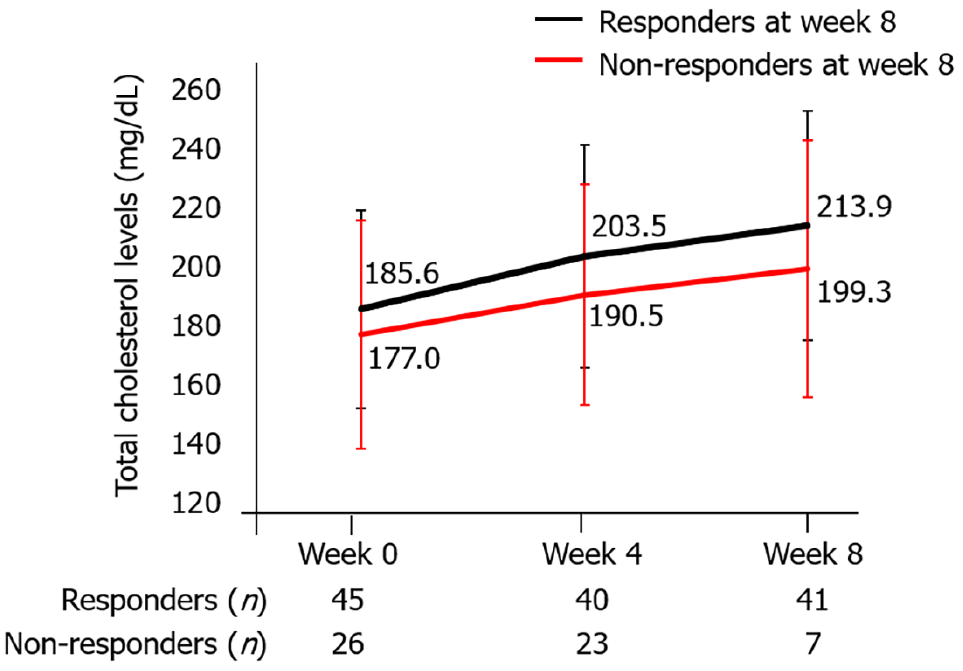
The most common adverse event was hypercholesterolemia in 17 (15.3%) patients, and all 17 patients were administered an oral antihyperlipidemic drug. Total cholesterol levels were not significantly different at weeks 0, 4, and 8 between responders and non-responders at week 8 (Figure 8).
In terms of infectious disease, HZ developed in 6 patients, upper respiratory tract infection in 5, influenza in 3, herpes labialis in 1, norovirus enteritis in 1, and Clostridioides difficile colitis in 1. Among patients with HZ, the median age was 51 (IQR: 35–54) years, and the median lymphocyte count was 1466 (IQR: 1167–1750) (/μL) (Table 4). There were no specific features for TOF dosage, duration of TOF administration, or concomitant drug in these patients. All patients with HZ developed it while in the clinical remission stage. The median age [50 (IQR: 34–54) vs 35 (IQR: 28–47) years, P = 0.20] and lymphocyte count [1369 (IQR: 1131–1504) vs 1485 (IQR: 1064–1892) (/μL), P = 0.45] were also not different in patients with HZ and without HZ (Figure 9). All patients that had HZ were treated with oral medications. Three patients continued TOF administration, 2 patients were withdrawn temporarily, and 1 was discontinued permanently. HZ occurred in multiple dermatomes in only 1 patient, and postherpetic neuralgia remained in 1 patient.
| Case | Age | Sex | Prior TNF-α failure | TOF dose (mg/day) | Week of onset | pMS at onset | Lymphocyte count (/μL) | Concomitant drug | Therapy for HZ | TOF therapy | Area of HZ | Postherpetic neuralgia |
| 1 | 68 | F | (+) | 10 | 30 | 1 | 1362 | 5ASA; PPI; ARB | OAD | Continuation | Lower leg | (+) |
| 2 | 30 | F | (+) | 20 | 5 | 0 | 1570 | Prednisolone 10 mg | OAD | Resumption after drug withdrawal | Head; Neck; Back | (-) |
| 3 | 25 | F | (+) | 10 | 23 | 1 | 1102 | 5ASA | OAD | Continuation | Back | (-) |
| 4 | 48 | M | (+) | 10 | 31 | 1 | 1810 | 5ASA | OAD | Resumption after drug withdrawal | Abdomen | (-) |
| 5 | 54 | F | (-) | 20 | 5 | 1 | 2127 | Iron preparations | OAD | Discontinuation | Buttocks | (-) |
| 6 | 53 | M | (+) | 10 | 26 | 0 | 922 | (-) | OAD | Continuation | Back | (-) |
Deep vein thrombosis was observed in 1 patient; a 53-year-old man with emphysema developed deep vein thrombosis in the left soleus vein with an unknown trigger. He was examined with ultrasonography because his D-dimer was slightly elevated (from 0.6 μg/mL to 2.4 μg/mL). The size of the thrombus was unchanged throughout the observation period even while continuing TOF without administering an antithrombotic agent.
The main purpose of our study was to evaluate the efficacy and safety of TOF in the real-world. Our study provides support for the efficacy of both short-term and long-term TOF treatment in active UC patients. Two-thirds of the patients had a clinical response and half achieved clinical remission at week 8. In addition, 44% of patients achieved corticosteroid-free remission at week 48 and the cumulative remission rate at week 48 among those patients in clinical remission at week 8 was 62%.
These results are fairly consistent with previously reported real-world studies. With regard to short-term efficacy, Honap et al[22] reported that a clinical response [defined as the reduction of The Simple Clinical Colitis Activity Index (SCCAI) or pMS ≥ 3] was achieved in 74% at week 8 and clinical remission (SCCAI ≤ 2 or pMS ≤ 1) was achieved in 57% of patients in a multicenter retrospective study involving 134 UC patients (83% previously treated with biologics). Similarly, Chaparro et al[23] reported that a clinical response (reduction in pMS ≥ 3 points and at least 30% from baseline, with a decrease ≥ 1 point in the rectal bleeding subscale) was achieved in 60% at week 8 and clinical remission (pMS < 2) was achieved in 31% of patients in a multicenter prospective study involving 113 UC patients (100% previously treated with biologics). With regard to long-term efficacy, Lair-Mehiri et al[24] reported steroid-free clinical remission (pMS < 3 with a combined stool frequency and rectal bleeding subscore ≤ 1) in 34% of patients at week 48 in a multicenter retrospective study involving 38 UC patients (100% previously treated with biologics). Chaparro et al[23] reported that 38% of patients who achieved remission at week 8 relapsed over time (median of exposure to TOF, 44 wk).
In the OCTAVE Induction 1 and 2 trials (almost 50% previously treated with anti-TNF-α agent), 19% and 17% of UC patients treated with TOF achieved remission at week 8, and 60% and 55% achieved a clinical response at week 8, respectively. In the OCTAVE Sustain trial, which included patients who had a clinical response to induction therapy, the sustained remission rate at week 52 was 37% in the 5 mg TOF twice-daily group and 47% in the 10 mg TOF twice-daily group[7]. Although our results appear to be better than these results, the OCTAVE trials included endoscopic results and had a more stringent definition of efficacy, unlike real-world studies, which may explain this discrepancy.
Predictors of clinical response were investigated in other studies. Honap et al[22] reported that a younger age (OR: 1.04, 95%CI: 1.01-1.07) and higher CRP (OR: 0.292, 95%CI: 0.121-0.655) at baseline were associated with non-response at week 8. Biemans et al[25] reported that prior exposure to vedolizumab (OR: 0.327, 95%CI: 0.100-0.907) and SCCAI per point (OR: 0.825, 95%CI: 0.686-0.992) were associated with a reduced corticosteroid-free clinical remission rate at week 24. In the present study, we showed that a higher pMS at baseline was associated with non-responders at week 8 in multivariate analysis (OR: 0.61, 95%CI: 0.45-0.82, P = 0.001). Patients with higher activity tend to have a lower drug persistence rate, so it is more beneficial for patients with lower activity. Also, we revealed that patients who achieved clinical remission at week 8 continued TOF for a longer time than those who did not. The proportion of patients who had previously failed an anti-TNF-α agent in our study was lower than in the other real-world studies. Drug persistence was not significantly different between anti-TNF-α agent-naive and -failure patients, but the cumulative remission rate tended to be higher in patients who had previously failed anti-TNF-α agents.
In our study, although the relapse rate after tapering TOF was 46%, the proportion of patients who achieved a clinical remission after dose re-escalation was 57% at week 4 and 69% at week 12. Sands et al[26] reported that 35.1% and 49.1% of patients after dose escalation recaptured clinical remission at months 2 and 12, strongly suggesting that efficacy may be recaptured by dose re-escalation and making it a possible option for patients undergoing relapse.
With regard to safety, the most common adverse events in our study were hypercholesterolemia and infectious diseases such as HZ and upper respiratory tract inflammation. It was previously reported that the risk of HZ is increased in UC patients receiving TOF, especially in the elderly, Asians, and patients who previously failed anti-TNF-α agents[14]. The overall cohort (phase II and OCTAVE Induction 1 and 2, OCTAVE Sustain, OCTAVE Open) reported that 5.6% of patients developed HZ during TOF use[14]. In the present study, 6 (5.4%) patients developed HZ, a higher percentage than previously reported in Europe[23,25]. All patients were treated with oral antiviral therapy, but the oldest patient (68 years) developed postherpetic neuralgia. One patient switched to alternative therapy because of an insufficient clinical response, and others continued TOF after recovering from HZ.
Lipid levels reversibly increase during TOF administration in UC patients as well as in patients with rheumatoid arthritis and psoriatic arthritis[27-29]. Consistent with this finding, in our study, total cholesterol levels were significantly higher at week 4 and week 8 compared with baseline, but no severe cardiovascular events were observed. IBD patients have a higher risk of venous thromboembolism events compared with non-IBD patients, with a relative risk of 2.27[30]. In a United States Food and Drug Administration post-marketing requirement safety study including patients with rheumatoid arthritis aged at least 50 years old with at least 1 cardiovascular risk factor, patients treated with 10 mg TOF twice daily had a higher frequency of pulmonary embolism and death than patients treated with 5 mg TOF twice daily or with anti-TNF-α agent[31].
The present study has several limitations. First, the study was conducted retrospectively at a single center, with the inherent risk of bias and data missing for some patients. There is a possibility of selection bias to administrate TOF by each physician. Second, as endoscopy was not mandatory at baseline and follow-up, endoscopic data were only available in a small number of patients, and efficacy was judged only on the basis of clinical symptoms, so the evaluation may be insufficient. A strength of our study is that it is a large real-world study in an Asian population that demonstrated the efficacy and safety including related clinical needs of TOF. Shin et al[16] reported also analyzed more than 100 patients of Asian UC treated with TOF, but they did not analyze the efficacy of TOF according to the history of anti-TNF-α agent failure, relapse rate at dose reduction and the efficacy at dose re-escalation. In addition, while most of the included patients in the other studies had previously failed anti-TNF-α agents, only 62% of anti-TNF-α failure patients were included in our study. Therefore, we were able to determine whether previous treatment with anti-TNF-α agents affected the TOF efficacy. We analyzed for the first time in more than 100 Asian UC patients that the efficacy of TOF was effective irrespective to prior history of anti-TNF-α agents, relapse rate after tapering of TOF and effective rate for reinduction of TOF. These data is essential for clinical strategy of TOF treatment. The observation period of present study was one year, but we are planning to report further data for 3-year follow-up in the near future.
In conclusion, TOF is more effective in low-activity UC patients in real practice and its efficacy is not affected by previous treatment with anti-TNF-α agents. Most patients in the clinical remission group at week 8 could continue TOF over a long follow-up period. Relapse occurred in 45.7% of patients after tapering TOF, but 85.7% of those patients recaptured a response to TOF by week 4. Although most patients continue TOF without severe adverse events, careful monitoring for HZ is necessary. Further studies are needed to investigate predictive factors for a response to TOF treatment and the positioning of TOF in many treatment options for active UC.
Various therapeutic agents are currently available as advanced therapies. Tofacitinib (TOF) was approved to treat the patients with intractable ulcerative colitis (UC) as first Janus kinase inhibitor in Japan.
Real-world data for the efficacy and safety of TOF treatment covering a period of more than 1 year with a sufficient number of Asian patients with UC are scarce. We investigated to optimize TOF treatment by using data of our specialized IBD center.
The aim of this study is to investigate the efficacy and safety of TOF treatment covering a period of more than 1 year in patients with intractable UC.
We performed a retrospective single-center observational analysis of 111 UC patients administered TOF between May 2018 and February 2020. The primary outcome was the clinical response rate at week 8.
The overall cumulative clinical remission rate was 61.7% at week 48 and history of anti- tumor necrosis factor-alpha (TNF-α) agents use had no influence (P = 0.25). Baseline partial Mayo Score was significantly lower in responders compared with non-responders at week 8 (odds ratio: 0.61, 95% confidence interval: 0.45-0.82, P = 0.001). Relapse occurred in 45.7% of patients after TOF tapering, and 85.7% of patients responded within 4 wk after re-increase.
TOF was more effective in UC patients with mild activity at baseline and its efficacy was not affected by previous treatment with anti-TNF-α agents. Although careful monitoring for herpes zoster is necessary, most patients continue TOF without severe adverse events.
Future prospective studies with a large number of UC patients and long follow-up periods are needed in clinical practice and we should consider the positioning of TOF in many treatment options for active UC.
Nagase K (Hyogo Medical University) kindly supported this work.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: American College of Gastroenterology; American Gastroenterological Association; American Society for Gastrointestinal Endoscopy; The Japanese Society of Gastroenterology; European Crohn's and Colitis Organization; Asian Organization for Crohn’s and Colitis; Japanese Society of Internal Medicine.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Han J, China S-Editor: Fan JR L-Editor: A P-Editor: Yu HG
| 1. | Ordás I, Eckmann L, Talamini M, Baumgart DC, Sandborn WJ. Ulcerative colitis. Lancet. 2012;380:1606-1619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1151] [Cited by in RCA: 1542] [Article Influence: 118.6] [Reference Citation Analysis (5)] |
| 2. | Salas A, Hernandez-Rocha C, Duijvestein M, Faubion W, McGovern D, Vermeire S, Vetrano S, Vande Casteele N. JAK-STAT pathway targeting for the treatment of inflammatory bowel disease. Nat Rev Gastroenterol Hepatol. 2020;17:323-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 425] [Article Influence: 85.0] [Reference Citation Analysis (0)] |
| 3. | Danese S, Grisham M, Hodge J, Telliez JB. JAK inhibition using tofacitinib for inflammatory bowel disease treatment: a hub for multiple inflammatory cytokines. Am J Physiol Gastrointest Liver Physiol. 2016;310:G155-G162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 130] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 4. | Thoma G, Nuninger F, Falchetto R, Hermes E, Tavares GA, Vangrevelinghe E, Zerwes HG. Identification of a potent Janus kinase 3 inhibitor with high selectivity within the Janus kinase family. J Med Chem. 2011;54:284-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 80] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 5. | Cordes F, Foell D, Ding JN, Varga G, Bettenworth D. Differential regulation of JAK/STAT-signaling in patients with ulcerative colitis and Crohn's disease. World J Gastroenterol. 2020;26:4055-4075. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 68] [Cited by in RCA: 68] [Article Influence: 13.6] [Reference Citation Analysis (3)] |
| 6. | Sandborn WJ, Ghosh S, Panes J, Vranic I, Su C, Rousell S, Niezychowski W; Study A3921063 Investigators. Tofacitinib, an oral Janus kinase inhibitor, in active ulcerative colitis. N Engl J Med. 2012;367:616-624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 585] [Cited by in RCA: 631] [Article Influence: 48.5] [Reference Citation Analysis (0)] |
| 7. | Sandborn WJ, Su C, Sands BE, D'Haens GR, Vermeire S, Schreiber S, Danese S, Feagan BG, Reinisch W, Niezychowski W, Friedman G, Lawendy N, Yu D, Woodworth D, Mukherjee A, Zhang H, Healey P, Panés J; OCTAVE Induction 1, OCTAVE Induction 2, and OCTAVE Sustain Investigators. Tofacitinib as Induction and Maintenance Therapy for Ulcerative Colitis. N Engl J Med. 2017;376:1723-1736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 896] [Cited by in RCA: 1186] [Article Influence: 148.3] [Reference Citation Analysis (0)] |
| 8. | Sandborn WJ, Lawendy N, Danese S, Su C, Loftus EV Jr, Hart A, Dotan I, Damião AOMC, Judd DT, Guo X, Modesto I, Wang W, Panés J. Safety and efficacy of tofacitinib for treatment of ulcerative colitis: final analysis of OCTAVE Open, an open-label, long-term extension study with up to 7.0 years of treatment. Aliment Pharmacol Ther. 2022;55:464-478. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 111] [Article Influence: 37.0] [Reference Citation Analysis (0)] |
| 9. | Suzuki Y, Watanabe M, Matsui T, Motoya S, Hisamatsu T, Yuasa H, Tabira J, Isogawa N, Tsuchiwata S, Arai S, Hibi T. Tofacitinib as Induction and Maintenance Therapy in Japanese Patients with Active Ulcerative Colitis. Inflamm Intest Dis. 2019;4:131-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 10. | D'Amico F, Parigi TL, Fiorino G, Peyrin-Biroulet L, Danese S. Tofacitinib in the treatment of ulcerative colitis: efficacy and safety from clinical trials to real-world experience. Therap Adv Gastroenterol. 2019;12:1756284819848631. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 50] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 11. | Liu JZ, van Sommeren S, Huang H, Ng SC, Alberts R, Takahashi A, Ripke S, Lee JC, Jostins L, Shah T, Abedian S, Cheon JH, Cho J, Dayani NE, Franke L, Fuyuno Y, Hart A, Juyal RC, Juyal G, Kim WH, Morris AP, Poustchi H, Newman WG, Midha V, Orchard TR, Vahedi H, Sood A, Sung JY, Malekzadeh R, Westra HJ, Yamazaki K, Yang SK; International Multiple Sclerosis Genetics Consortium; International IBD Genetics Consortium, Barrett JC, Alizadeh BZ, Parkes M, Bk T, Daly MJ, Kubo M, Anderson CA, Weersma RK. Association analyses identify 38 susceptibility loci for inflammatory bowel disease and highlight shared genetic risk across populations. Nat Genet. 2015;47:979-986. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1898] [Cited by in RCA: 1861] [Article Influence: 186.1] [Reference Citation Analysis (0)] |
| 12. | Park SJ, Kim WH, Cheon JH. Clinical characteristics and treatment of inflammatory bowel disease: a comparison of Eastern and Western perspectives. World J Gastroenterol. 2014;20:11525-11537. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 95] [Cited by in RCA: 103] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 13. | Ng SC. Emerging Trends of Inflammatory Bowel Disease in Asia. Gastroenterol Hepatol (N Y). 2016;12:193-196. [PubMed] |
| 14. | Winthrop KL, Melmed GY, Vermeire S, Long MD, Chan G, Pedersen RD, Lawendy N, Thorpe AJ, Nduaka CI, Su C. Herpes Zoster Infection in Patients With Ulcerative Colitis Receiving Tofacitinib. Inflamm Bowel Dis. 2018;24:2258-2265. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 132] [Cited by in RCA: 171] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 15. | Núñez P, Quera R, Yarur AJ. Safety of Janus Kinase Inhibitors in Inflammatory Bowel Diseases. Drugs. 2023;83:299-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 39] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 16. | Shin SH, Oh K, Hong SN, Lee J, Oh SJ, Kim ES, Na SY, Kang SB, Koh SJ, Bang KB, Jung SA, Jung SH, Kim KO, Park SH, Yang SK, Choi CH, Ye BD. Real-life effectiveness and safety of tofacitinib treatment in patients with ulcerative colitis: a KASID multicenter cohort study. Therap Adv Gastroenterol. 2023;16:17562848231154103. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 17. | Nakase H, Uchino M, Shinzaki S, Matsuura M, Matsuoka K, Kobayashi T, Saruta M, Hirai F, Hata K, Hiraoka S, Esaki M, Sugimoto K, Fuji T, Watanabe K, Nakamura S, Inoue N, Itoh T, Naganuma M, Hisamatsu T, Watanabe M, Miwa H, Enomoto N, Shimosegawa T, Koike K. Evidence-based clinical practice guidelines for inflammatory bowel disease 2020. J Gastroenterol. 2021;56:489-526. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 89] [Cited by in RCA: 306] [Article Influence: 76.5] [Reference Citation Analysis (0)] |
| 18. | Schroeder KW, Tremaine WJ, Ilstrup DM. Coated oral 5-aminosalicylic acid therapy for mildly to moderately active ulcerative colitis. A randomized study. N Engl J Med. 1987;317:1625-1629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1958] [Cited by in RCA: 2250] [Article Influence: 59.2] [Reference Citation Analysis (0)] |
| 19. | Satsangi J, Silverberg MS, Vermeire S, Colombel JF. The Montreal classification of inflammatory bowel disease: controversies, consensus, and implications. Gut. 2006;55:749-753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1970] [Cited by in RCA: 2349] [Article Influence: 123.6] [Reference Citation Analysis (2)] |
| 20. | Lewis JD, Chuai S, Nessel L, Lichtenstein GR, Aberra FN, Ellenberg JH. Use of the noninvasive components of the Mayo score to assess clinical response in ulcerative colitis. Inflamm Bowel Dis. 2008;14:1660-1666. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 733] [Cited by in RCA: 711] [Article Influence: 41.8] [Reference Citation Analysis (0)] |
| 21. | Kanda Y. Investigation of the freely available easy-to-use software 'EZR' for medical statistics. Bone Marrow Transplant. 2013;48:452-458. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9275] [Cited by in RCA: 13270] [Article Influence: 1105.8] [Reference Citation Analysis (0)] |
| 22. | Honap S, Chee D, Chapman TP, Patel M, Kent AJ, Ray S, Sharma E, Kennedy J, Cripps S, Walsh A, Goodhand JR, Ahmad T, Satsangi J, Irving PM, Kennedy NA; LEO [London, Exeter, Oxford] IBD Research Consortium. Real-world Effectiveness of Tofacitinib for Moderate to Severe Ulcerative Colitis: A Multicentre UK Experience. J Crohns Colitis. 2020;14:1385-1393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 94] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 23. | Chaparro M, Garre A, Mesonero F, Rodríguez C, Barreiro-de Acosta M, Martínez-Cadilla J, Arroyo MT, Manceñido N, Sierra-Ausín M, Vera-Mendoza I, Casanova MJ, Nos P, González-Muñoza C, Martínez T, Boscá-Watts M, Calafat M, Busquets D, Girona E, Llaó J, Martín-Arranz MD, Piqueras M, Ramos L, Surís G, Bermejo F, Carbajo AY, Casas-Deza D, Fernández-Clotet A, García MJ, Ginard D, Gutiérrez-Casbas A, Hernández L, Lucendo AJ, Márquez L, Merino-Ochoa O, Rancel FJ, Taxonera C, López Sanromán A, Rubio S, Domènech E, Gisbert JP. Tofacitinib in Ulcerative Colitis: Real-world Evidence From the ENEIDA Registry. J Crohns Colitis. 2021;15:35-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 74] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 24. | Lair-Mehiri L, Stefanescu C, Vaysse T, Laharie D, Roblin X, Rosa I, Treton X, Abitbol V, Amiot A, Bouguen G, Dib N, Fumery M, Pariente B, Carbonnel F, Peyrin-Biroulet L, Simon M, Viennot S, Bouhnik Y. Real-world evidence of tofacitinib effectiveness and safety in patients with refractory ulcerative colitis. Dig Liver Dis. 2020;52:268-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 60] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 25. | Biemans VBC, Sleutjes JAM, de Vries AC, Bodelier AGL, Dijkstra G, Oldenburg B, Löwenberg M, van Bodegraven AA, van der Meulen-de Jong AE, de Boer NKH, Srivastava N, West RL, Römkens TEH, Horjus Talabur Horje CS, Jansen JM, van der Woude CJ, Hoekstra J, Weersma RK, van Schaik FDM, Hoentjen F, Pierik MJ; Dutch Initiative on Crohn and Colitis (ICC). Tofacitinib for ulcerative colitis: results of the prospective Dutch Initiative on Crohn and Colitis (ICC) registry. Aliment Pharmacol Ther. 2020;51:880-888. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 73] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 26. | Sands BE, Armuzzi A, Marshall JK, Lindsay JO, Sandborn WJ, Danese S, Panés J, Bressler B, Colombel JF, Lawendy N, Maller E, Zhang H, Chan G, Salese L, Tsilkos K, Marren A, Su C. Efficacy and safety of tofacitinib dose de-escalation and dose escalation for patients with ulcerative colitis: results from OCTAVE Open. Aliment Pharmacol Ther. 2020;51:271-280. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 73] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 27. | Sands BE, Taub PR, Armuzzi A, Friedman GS, Moscariello M, Lawendy N, Pedersen RD, Chan G, Nduaka CI, Quirk D, Salese L, Su C, Feagan BG. Tofacitinib Treatment Is Associated With Modest and Reversible Increases in Serum Lipids in Patients With Ulcerative Colitis. Clin Gastroenterol Hepatol. 2020;18:123-132.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 78] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 28. | Kremer JM, Bloom BJ, Breedveld FC, Coombs JH, Fletcher MP, Gruben D, Krishnaswami S, Burgos-Vargas R, Wilkinson B, Zerbini CA, Zwillich SH. The safety and efficacy of a JAK inhibitor in patients with active rheumatoid arthritis: Results of a double-blind, placebo-controlled phase IIa trial of three dosage levels of CP-690,550 versus placebo. Arthritis Rheum. 2009;60:1895-1905. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 413] [Cited by in RCA: 440] [Article Influence: 27.5] [Reference Citation Analysis (0)] |
| 29. | Gladman DD, Charles-Schoeman C, McInnes IB, Veale DJ, Thiers B, Nurmohamed M, Graham D, Wang C, Jones T, Wolk R, DeMasi R. Changes in Lipid Levels and Incidence of Cardiovascular Events Following Tofacitinib Treatment in Patients With Psoriatic Arthritis: A Pooled Analysis Across Phase III and Long-Term Extension Studies. Arthritis Care Res (Hoboken). 2019;71:1387-1395. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 42] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 30. | Weng MT, Park SH, Matsuoka K, Tung CC, Lee JY, Chang CH, Yang SK, Watanabe M, Wong JM, Wei SC. Incidence and Risk Factor Analysis of Thromboembolic Events in East Asian Patients With Inflammatory Bowel Disease, a Multinational Collaborative Study. Inflamm Bowel Dis. 2018;24:1791-1800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 48] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 31. | US Food and Drug Administration. Safety trial finds risk of blood clots in the lungs and death with higher dose of tofacitinib (Xeljanz, Xeljanz XR) in rheumatoid arthritis patients; FDA to investigate. 2019. [cited May 29, 2019]. Available from: https://www.fda.gov/media/120485/download. |