Published online Mar 28, 2024. doi: 10.3748/wjg.v30.i12.1727
- This article has been corrected.
- See: World J Gastroenterol. Feb 14, 2025; 31(6): 102800
Peer-review started: November 27, 2023
First decision: January 17, 2024
Revised: January 30, 2024
Accepted: March 13, 2024
Article in press: March 13, 2024
Published online: March 28, 2024
Processing time: 121 Days and 22.1 Hours
Sarcopenia may be associated with hepatocellular carcinoma (HCC) following hepatectomy. But traditional single clinical variables are still insufficient to predict recurrence. We still lack effective prediction models for recent recurrence (time to recurrence < 2 years) after hepatectomy for HCC.
To establish an interventable prediction model to estimate recurrence-free survival (RFS) after hepatectomy for HCC based on sarcopenia.
We retrospectively analyzed 283 hepatitis B-related HCC patients who underwent curative hepatectomy for the first time, and the skeletal muscle index at the third lumbar spine was measured by preoperative computed tomography. 94 of these patients were enrolled for external validation. Cox multivariate analysis was per-formed to identify the risk factors of postoperative recurrence in training cohort. A nomogram model was developed to predict the RFS of HCC patients, and its predictive performance was validated. The predictive efficacy of this model was evaluated using the receiver operating characteristic curve.
Multivariate analysis showed that sarcopenia [Hazard ratio(HR) = 1.767, 95%CI: 1.166-2.678, P < 0.05], alpha-fetoprotein ≥ 40 ng/mL (HR = 1.984, 95%CI: 1.307-3.011, P < 0.05), the maximum diameter of tumor > 5 cm (HR = 2.222, 95%CI: 1.285-3.842, P < 0.05), and hepatitis B virus DNA level ≥ 2000 IU/mL (HR = 2.1, 95%CI: 1.407-3.135, P < 0.05) were independent risk factors associated with postoperative recurrence of HCC. Based on the sarcopenia to assess the RFS model of hepatectomy with hepatitis B-related liver cancer disease (SAMD) was established combined with other the above risk factors. The area under the curve of the SAMD model was 0.782 (95%CI: 0.705-0.858) in the training cohort (sensitivity 81%, specificity 63%) and 0.773 (95%CI: 0.707-0.838) in the validation cohort. Besides, a SAMD score ≥ 110 was better to distinguish the high-risk group of postoperative recurrence of HCC.
Sarcopenia is associated with recent recurrence after hepatectomy for hepatitis B-related HCC. A nutritional status-based prediction model is first established for postoperative recurrence of hepatitis B-related HCC, which is superior to other models and contributes to prognosis prediction.
Core Tip: Our focus on the factors that can intervene or improve the adverse outcomes of postoperative recurrence in patients with hepatitis B-related hepatocellular carcinoma (HCC) and establish a more effective model for predicting recurrence. Our study found Sarcopenia is remarkably associated with recent recurrence after hepatectomy for hepatitis B-related HCC. The SAMD model based on sarcopenia established in this study emphasizes the assessment and monitor of sarcopenia in hepatitis B-related HCC patients and effectively assists clinicians in closely to identify and monitor high-risk populations to improve the recurrence outcome of hepatitis B-related HCC patients after surgery through multi angle intervention measures.
- Citation: Peng H, Lei SY, Fan W, Dai Y, Zhang Y, Chen G, Xiong TT, Liu TZ, Huang Y, Wang XF, Xu JH, Luo XH. Assessing recent recurrence after hepatectomy for hepatitis B-related hepatocellular carcinoma by a predictive model based on sarcopenia. World J Gastroenterol 2024; 30(12): 1727-1738
- URL: https://www.wjgnet.com/1007-9327/full/v30/i12/1727.htm
- DOI: https://dx.doi.org/10.3748/wjg.v30.i12.1727
Hepatocellular carcinoma (HCC) is one of the most common malignancies worldwide. For patients with early-stage HCC, hepatic resection, and liver transplantation offer the most reasonable expectation for curative treatment[1]. Unfortunately, the prognosis of HCC patients following curative resection remains dismal due to the high postoperative recurrence rate. It is reported that 40%-70% of HCC cases present with disease recurrence within 5 years[2,3]. Currently, there is no effective therapy to prevent the recurrence of HCC, which makes early identification and timely treatment of the high-risk populations for HCC recurrence crucial to improving the prognosis of HCC.
Recurrence after hepatectomy for HCC is associated with many factors. Several prognostic scoring systems and models, such as assessment for surveillance interval score (AS score)[4], early recurrence after surgery for liver tumor (ERASL-pre), and model of recurrence after liver transplant (pre-MORAL)[5,6], have been developed to predict the risk of HCC recurrence preoperatively. However, it is difficult to guide clinically targeted interventions to factors related to the tumor. Nutritional status is crucial in determining the prognosis of HCC patients. Objective nutritional assessment and timely nutritional intervention may improve patient prognosis. Sarcopenia is a representative indicator of nutrition, associated with morbidity and mortality in various pathology, including colorectal, gastric, and liver[7,8]. Studies have also found that sarcopenia is a risk factor for the recurrence of HCC after curative treatment[9,10]. It's worth noting that measuring the skeletal muscle index (SMI) of the third lumbar spine (L3) by abdominal computed tomography (CT) is the most objective method for assessing sarcopenia in liver disease[11]. This study aims to establish a comprehensive prediction model for HCC recurrence based on sarcopenia to improve the prediction of early recurrence risk in HCC patients undergoing hepatectomy, which can provide a reference for the formulation of comprehensive treatment plans for patients.
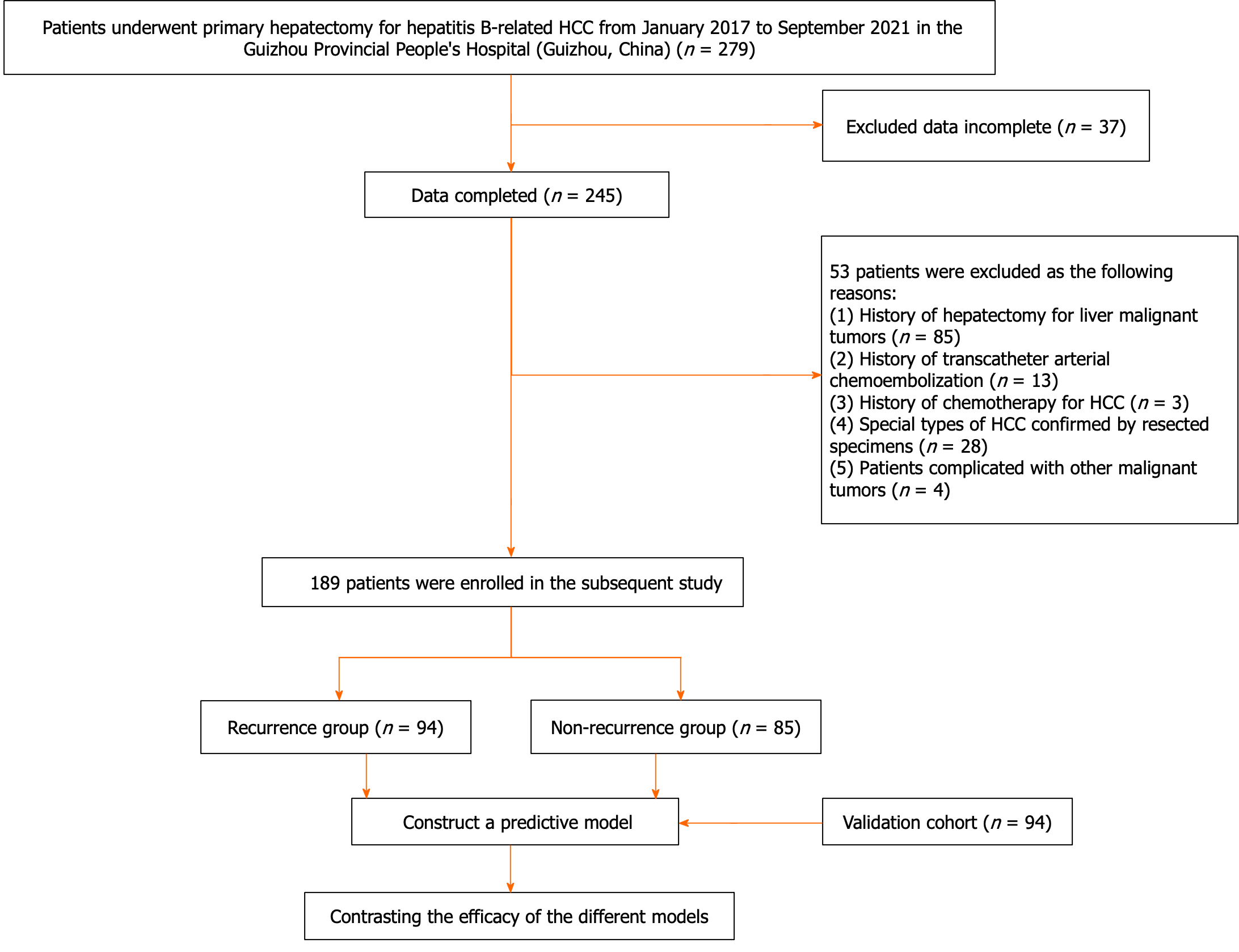
All patients who underwent primary hepatectomy for HCC at the Guizhou Provincial People's Hospital between January 2017 and September 2021 were retrospectively collected. The inclusion criteria included: (1) First hepatectomy for HCC; (2) no extrahepatic metastasis; (3) above 18 years old; and (4) history of chronic hepatitis B with positive hepatitis B surface antigen. The exclusion criteria included: (1) Data incomplete; (2) history of hepatectomy for liver malignancies; (3) history of transcatheter arterial chemoembolization for HCC; (4) history of chemotherapy for HCC; (5) particular subtypes of HCC confirmed by resected specimens (such as intrahepatic cholangiocarcinoma and combined hepatocellular-cholangiocarcinoma); and (6) patients complicated with other malignant tumors. Detailed patient information is shown in Figure 1. Specifically, 189 patients in the training cohort were hospitalized at the second ward of hepatobiliary surgery; Moreover, 94 patients hospitalized in the third ward of hepatobiliary surgery at the Guizhou Provincial People's Hospital were enrolled for external validation.
The demographic data and laboratory parameters of all patients were extracted by reviewing the medical records. All biochemical and pathological indicators were determined in our laboratory. The laboratory parameters included alpha-fetoprotein (AFP), alanine-aminotransferase (ALT), total bilirubin, albumin (ALB), international normalized ratio (INR), leukocyte, platelet count (PLT), serum Hepatitis B virus deoxyribonucleic acid (HBV DNA). The pathological characteristics of HCC including the degree of differentiation, liver capsular invasion, and microvascular invasion (MVI) were recorded. The maximum tumor diameter and the third lumbar-skeletal muscle area (SMA) were measured by CT before hepatectomy. Sarcopenia was assessed by the L3-SMI. Existing prediction models: AS score = 0.176 × age (year) + 17.279 × INR–21.887[4]; ERASL-pre score = 0.818 × Gender (0: Female, 1: Male) + 0.447 × Albumin-Bilirubin (ALBI) grade (0: Grade 1; 1: Grade 2 or 3) + 0.100 × ln (Serum AFP in µg/L) + 0.580 × ln (Tumor size in cm) + 0.492 × Tumor number (0: Single; 1: Two or three; 2: Four or more)[5]; pre-MORAL score = tumor size > 3 cm (Score: 3) + AFP ≥ 200 ng/mL (Score: 4) + and the neutrophil-lymphocyte ratio > 5 (Score: 6)[6]; Cut-offs to generate the risk groups: AS score ≥ 9.26, ERASL-pre score > 3.521 (high), ERASL-pre score > 3.521 (high), and pre-MORAL score > 7 (high). Use these models to rate in our dataset.
In our institute, the selection of patients with HCC for hepatectomy was based on considering tumor factor, liver functional status, and patient factor. A curative resection was defined as a complete resection of all macroscopically evident tumors. The absence of tumor cells along the parenchymal transection line was confirmed histologically. No tumors remained on CT or serological features in the remnant liver at 2 months after the operation. HCC recurrence was defined as the recurrence of HCC after radical treatment of HCC. Recent recurrence [time to recurrence (TTR) < 2 years] and forward recurrence (TTR ≥ 2 years) by TTR[12]. All recurrent tumors were new lesions diagnosed by radiological features typical of HCC in the CT scans[13].
Using CT images, SMA and SMI at the L3 vertebra could be calculated and analyzed to evaluate skeletal muscle mass. Data at CT were obtained at baseline. L3-SMI was calculated using the following formula: L3-SMI = L3 SMA (cm2)/the square of the patient's height (m2). The diagnostic criteria of sarcopenia were determined based on the Japan Society of Hepatology Guidelines for Sarcopenia in Liver Disease[14]. L3-SMI of female and male patients were < 38 cm2/m2 and < 42 cm2/m2, respectively.
The follow-up imaging results of patients after HCC resection were reviewed every 3-6 months. Tumor recurrence was defined as new lesions in the liver detected by liver ultrasound, CT, or magnetic resonance imaging, with elevated serum AFP.
The present study was approved by the Ethics Committee of Guizhou Provincial People’s Hospital and performed according to the ethical guidelines of the 1975 Declaration of Helsinki. The requirement for informed consent was waived due to the retrospective nature of the study and the anonymity of the data.
Categorical variables were presented in number and percentage (%), and variables were compared using the chi-square test. Continuous variables are presented as mean ± standard deviation or median (interquartile range). Quantitative variables in normal distribution were compared by student's t-test or Mann-Whitney nonparametric U test. recurrence-free survival (RFS) was calculated by the Kaplan-Meier method, and RFS curves were compared using the log-rank test. The cox regression model performed univariate and multivariate analyses with a stepwise selection of variables. The time-dependent area under the receiver operating characteristic curve ROC). Based on the selected independent predictors, nomograms were constructed, and assessment calibration curves were plotted to assess the calibration of the model. Statistical analysis was performed using IBM SPSS 23.0 software (IBM, Armonk, NY, United States), GraphPad Prism 6.0 software (GraphPad Software, La Jolla, CA, United States), and the R statistical programming version 3.3.1 (Vienna, Austria, http://www.r-project.org). A value of P < 0.05 was considered statistically significant.
A total of 283 patients were recruited into the training and validation cohorts, including 241 men (85.2%) and 42 women (14.8%), with a mean age of 52.71 ± 11.15 years. The follow-up results showed that 144 (50.9%) patients developed recurrence within 2 years after hepatectomy, and the median RFS was 7.67 months (95%CI: 6.59-8.75), The baseline characteristics of the training and validation cohorts are presented in Table 1. All baseline characteristics were comparable between the two cohorts (P > 0.05). Among the 189 patients enrolled in the training cohort, 104 (55%) patients experienced HCC recurrence with the median RFS of 6 months (95%CI: 7.18-11.05), and the remaining 85 (45%) patients were assigned to the non-recurrence group. Further, as shown in Table 2, the recurrence rate was higher in patients with preoperative sarcopenia (66.7% vs 50.3%, P < 0.05) and HBV-DNA ≥ 2000 IU/mL (71% vs 47.2%, P < 0.05). There were significant differences in tumor differentiation, MVI and the maximum diameter of tumor between the recurrence group and the non-recurrence group (all P < 0.05). There was no significant difference in age, gender, liver capsular invasion, liver cirrhosis, ALT, albumin, total bilirubin, leukocyte, INR, PLT between the recurrence group and the non-recurrence group (P > 0.05).
| Characteristic | Total (n = 283) | Training cohort (n = 189) | Validation cohort (n = 94) | P value |
| Age (yr) | 52.71 ± 11.15 | 52.86 ± 11.65 | 52.43 ± 10.12 | 0.760 |
| Gender, n (%) | 0.467 | |||
| Male | 241 (85.2) | 163 (86.2) | 78 (83.0) | |
| Female | 42 (14.8) | 26 (13.8) | 16 (17.0) | |
| Sarcopenia, n (%) | 91 (32.2) | 54 (28.6) | 37 (39.4) | 0.067 |
| Liver cirrhosis, n (%) | 169 (59.7) | 105 (55.6) | 64 (68.1) | 0.053 |
| MVI, n (%) | 126 (44.5) | 89 (47.1) | 37 (39.6) | 0.218 |
| Tumor differentiation, n (%) | 0.530 | |||
| Poor | 56 (19.8) | 33 (17.5) | 23 (24.5) | |
| Moderate | 128 (45.2) | 81 (42.9) | 47 (50.0) | |
| Well | 99 (35.0) | 75 (39.6) | 24 (25.5) | |
| Liver capsular invasion | 160 (56.5) | 112 (59.3) | 45 (51.1) | 0.190 |
| Maximum diameter of tumor, n (%) | 0.062 | |||
| ≤ 3 cm | 72 (25.4) | 40 (21.2) | 32 (34.0) | |
| 3-5 cm | 62 (21.9) | 43 (22.8) | 19 (20.2) | |
| > 5 cm | 149 (52.7) | 106 (56.1) | 43 (45.7) | |
| HBV-DNA, n (%) | 0.130 | |||
| < 2000 IU/mL | 173 (61.1) | 127(67.2) | 46 (48.9) | |
| ≥ 2000 IU/mL | 110 (38.9) | 62 (32.8) | 48 (51.1) | |
| AFP ≥ 40 ng/mL | 158 (55.8) | 106 (56.1) | 52 (55.3) | 0.903 |
| ALT, U/L | 36 (26-58) | 36 (26-51.75) | 38.5 (26.75-63.5) | 0.191 |
| Total bilirubin (mmol/L) | 14.80 (11.15-22.02) | 14.80 (11.15-22.02) | 19.8 (11.95-30.4) | 0.051 |
| Albumin, g/L | 40.2 (35.80-43.70) | 40.52 (35.43-43.98) | 40.2 (36.32-43.02) | 0.796 |
| INR | 1.02 (0.95-1.09) | 1.01 (0.94-1.08) | 1.04 (0.96-1.12) | 0.146 |
| Leukocyte, 109/L | 5.49 (4.24-7.14) | 5.38 (4.37-6.49) | 6.13 (4.04-8.05) | 0.054 |
| PLT, 109/L | 154 (105-207) | 162 (109-220) | 137 (94-185) | 0.012 |
| Recurrence | 144 (50.9) | 104 (55) | 40 (42.6) | 0.221 |
| Characteristic | Total (n = 189) | Recurrence group (n = 104) | Non-recurrence group (n = 85) | P value |
| Age (yr) | 52.86 ± 11.65 | 51.92 ± 11.42 | 54 ± 11.88 | 0.751 |
| Gender, n (%) | 0.579 | |||
| Male | 163 (86.2) | 91 (87.5) | 72 (84.7) | |
| Female | 26 (13.8) | 13 (12.5) | 13 (15.3) | |
| Sarcopenia, n (%) | 54 (28.6) | 36 (66.7) | 18 (33.3) | 0.042 |
| Liver cirrhosis, n (%) | 105 (55.6) | 55 (52.4) | 50 (47.6) | 0.414 |
| MVI, n (%) | 89 (47.1) | 56 (62.9) | 33 (37.1) | 0.04 |
| Tumor differentiation, n (%) | 0.008 | |||
| Poor | 33 (17.5) | 22 (66.7) | 11 (33.3) | |
| Moderate | 81 (42.9) | 51 (63.0) | 30 (37.0) | |
| Well | 75 (39.6) | 31 (41.3) | 44 (58.7) | |
| Liver capsular invasion | 112 (59.3) | 67 (59.8) | 45 (40.2) | 0.11 |
| Maximum diameter of tumor, n (%) | 0.003 | |||
| ≤ 3 cm | 40 (21.2) | 16 (40.0) | 25 (60.0) | |
| 3-5 cm | 43 (22.8) | 18 (41.9) | 25 (58.1) | |
| > 5 cm | 106 (56.1) | 70 (66.0) | 38 (34.0) | |
| HBV-DNA, n (%) | 0.002 | |||
| < 2000 IU/mL | 127 (67.2) | 60 (47.2) | 67 (52.8) | |
| ≥ 2000 IU/mL | 62 (32.8) | 44 (71.0) | 20 (29.0) | |
| AFP ≥ 40 ng/mL | 106 (56.1) | 70 (66.0) | 36 (34.0) | 0.001 |
| ALT, U/L | 36 (26-51.75) | 36 (28-52.75) | 36 (22.25-51) | 0.481 |
| Total bilirubin (mmol/L) | 14.80 (11.15-22.02) | 15.90 (12.15-23.15) | 14.3 (10.55-19.70) | 0.051 |
| Albumin, g/L | 40.52 (35.43-43.98) | 39.05 (34.65-43.5) | 41 (36.62-44.48) | 0.096 |
| INR | 1.01 (0.94-1.08) | 1.0 (0.95-1.07) | 1.01 (0.93-1.09) | 0.846 |
| Leukocyte, 109/L | 5.38 (4.37-6.49) | 5.38 (4.24-6.79) | 5.39 (4.5-6.36) | 0.921 |
| PLT, 109/L | 162 (109-220) | 147 (100-200) | 176 (116-234) | 0.056 |
Univariate analysis showed that preoperative sarcopenia, tumor differentiation, the maximum diameter of tumor, HBV-DNA level, and AFP ≥ 40 ng/mL, tumor differentiation, MVI were related to HCC recurrence after hepatectomy (AFP cut-off value was analyzed by the ROC curve). Further multivariate analysis revealed that preoperative sarcopenia, AFP ≥ 40 ng/mL, the maximum diameter of tumor > 5 cm, and HBV-DNA level ≥ 2000 IU/mL were independent risk factors of HCC recurrence after hepatectomy, regardless of whether pathological factors were included in the multivariate analysis (Table 3).
| Variable | Univariate analysis | Multivariate analysis without pathological factors | Multivariate analysis with pathological factors | ||||||
| HR | 95%CI | P value | HR | 95%CI | P value | HR | 95%CI | P value | |
| Male | 1.038 | 0.58-1.858 | 0.9 | ||||||
| Age (yr) | 0.991 | 0.975-1.007 | 0.255 | ||||||
| Sarcopenia | 1.61 | 1.073-2.416 | 0.022 | 1.814 | 1.196-2.751 | 0.005 | 1.819 | 1.199-2.759 | 0.005 |
| Liver cirrhosis (yes, no) | 0.888 | 0.593-1.328 | 0.562 | ||||||
| Liver capsular invasion | 1.289 | 0.863-1.927 | 0.215 | ||||||
| ALT (U/L) | 1 | 0.997-1.002 | 0.746 | ||||||
| Total bilirubin (mmol/L) | 0.994 | 0.975-1.014 | 0.577 | ||||||
| Albumin (g/L) | 0.971 | 0.942-1.001 | 0.061 | ||||||
| INR | 1.055 | 0.951-1.171 | 0.312 | ||||||
| MVI | 1.663 | 1.13-2.449 | 0.01 | ||||||
| Tumor differentiation | |||||||||
| Well | Ref. | ||||||||
| Moderate | 1.764 | 1.127-2.76 | 0.013 | ||||||
| Poor | 1.93 | 1.115-3.34 | 0.019 | ||||||
| Maximum diameter of tumor | |||||||||
| ≤ 3 cm | Ref. | Ref. | Ref. | ||||||
| 3-5 cm | 1.063 | 0.542-2.087 | 0.858 | 1.134 | 0.576-2.233 | 0.715 | 1.151 | 0.585-2.268 | 0.684 |
| > 5 cm | 2.202 | 1.277-3.796 | 0.005 | 2.286 | 1.322-3.952 | 0.003 | 2.228 | 1.288-3.852 | 0.004 |
| HBV-DNA | |||||||||
| < 2000 IU/mL | Ref. | Ref. | Ref. | ||||||
| ≥ 2000 IU/mL | 2.007 | 1.358-2.966 | < 0.001 | 2.191 | 1.468-3.272 | < 0.001 | 2.162 | 1.45-3.223 | < 0.001 |
| AFP ≥ 40 ng/mL | 1.92 | 1.269-2.904 | 0.002 | 2.032 | 1.322-3.952 | < 0.001 | 1.823 | 1.198-2.773 | 0.005 |
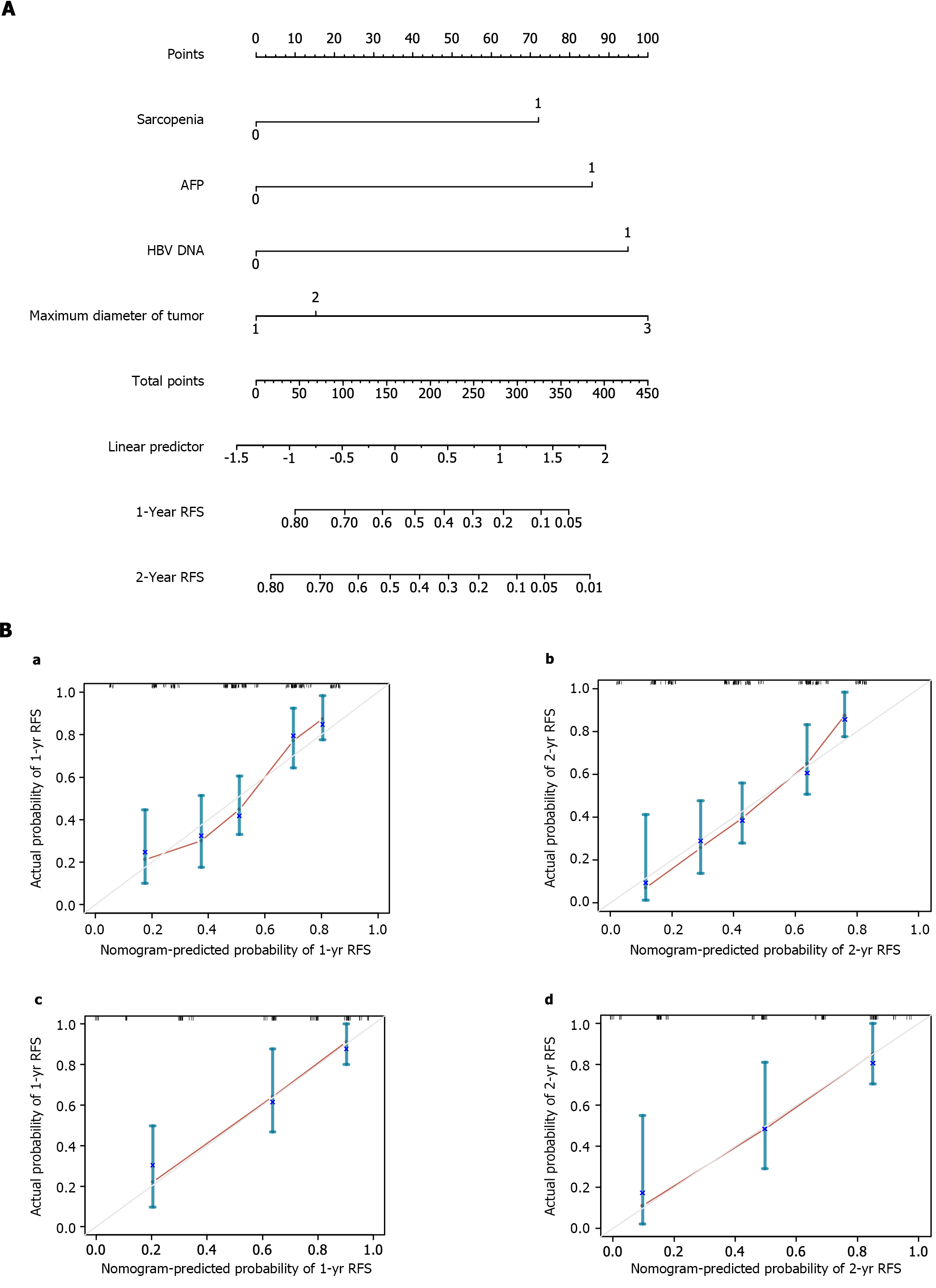
The four available risk factors found to be independent predictors of RFS after hepatectomy were used to develop a SAMD model, and a visual nomogram was established (Figure 2A). The total SAMD score was calculated using the corresponding score obtained from the vertical line of each risk factor coordinate axis (corresponding to the last coordinate axis) to get the 1- and 2-year RFS in each patient. To evaluate the SAMD model, we plotted a calibration curve based on the actual probability of postoperative recurrence and the predicted probability of postoperative recurrence. The calibration curves indicated a good consistency between the predicted and observed results in both the training cohort and validation cohort (Figure 2B). Meanwhile, an online calculator was developed to permit easy clinical application: https://yuchch.shinyapps.io/DynNomappRFS/. We can use Dynamic Nomogram to Predicted Survey at the Follow Up.
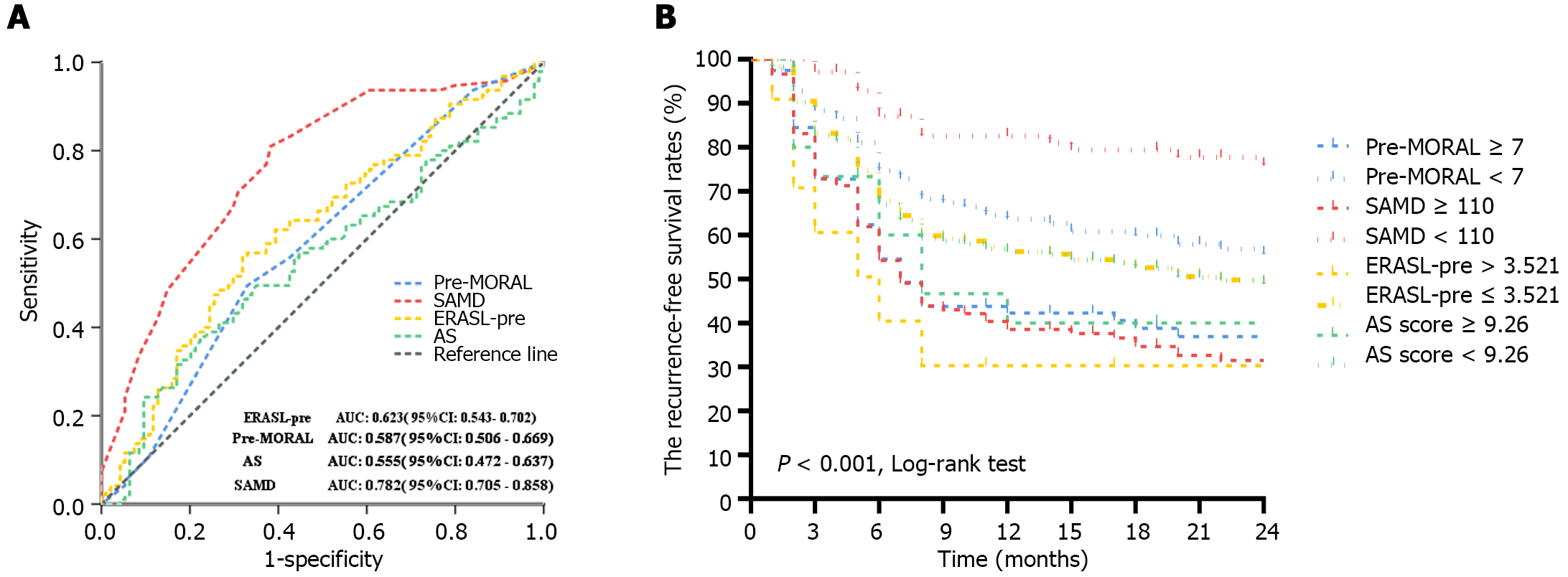
Furthermore, we compared the SAMD model with other preoperative models and assessed their prognostic values by analyzing the AUC (Figure 3A). The AUC of the SAMD model for predicting 2-year RFS was 0.782 (95%CI: 0.705-0.858) (P < 0.001). The sensitivity and specificity were 81% and 63%. The AUC of the verification cohort for predicting 2-year RFS was 0.773 (95%CI: 0.707-0.838), reflecting a good accuracy of the nomogram. For other models, the AUC of the AS score was 0.555 (95%CI: 0.472-0.637, P = 0.195), and the sensitivity and specificity were 37.9% and 77.7%, respectively. The AUC of the ERASL-pre model. and the pre-MORAL model was 0.623 (95%CI: 0.543-0.702, P = 0.004) and 0. 587 (95%CI: 0.506-0.669, P = 0.038), respectively. The sensitivity and specifcity were 55.8% and 68.1% for the ERASL-pre model and 81.1% and 61.7% for the pre-MORAL model, respectively. From all the above, our nomogram exhibits favorable clinical practicality and is a promising clinical decision-making tool. The SAMD model significantly outperforms other existing prediction systems.
Finally, the patients were divided into the high-risk and low-risk recurrence groups by the preselected cut-off point of SAMD score ≥ 110. Further, the RFS rates predicted by different models were compared by the kaplan-meier curve (Figure 3B). Meanwhile, according to hazard ratio (HR) by Cox regression, patients in the high-risk group with a SAMD score ≥ 110, ERASL-pre score > 3.521, pre-MORAL score > 7, and AS score ≥ 9.26 had higher likelihood of recurrence events than those patients in the low-risk group with the scores below cut-off points, and the HR were 4.228-fold, 2.053-fold, 1.802-fold, and 1.506-fold, respectively. It is proved that a SAMD score ≥ 110 can better distinguish the high-risk group for postoperative HCC recurrence.
Surgical resection is the best option for HCC patients to get a cure[15]. However, it cannot be ignored that a high recurrence rate after surgery. In our study, the recent recurrence rate is as high as 55%. Therefore, it is necessary to analyze the risk factors affecting the recurrence of early-stage HCC patients and establish a predictive model to make the prediction of recurrence risk. This study indicated that preoperative sarcopenic, AFP ≥ 40 ng/mL, the maximum diameter of tumor > 5 cm, and HBV-DNA level ≥ 2000 IU/mL were independent prognostic predictors for recurrence after hepatectomy. More importantly, in predicting the prognosis of HCC patients undergoing hepatectomy, the sarcopenia-based nomogram named SAMD model showed superior discrimination over other indicators, indicating that this nomogram may be helpful for clinical monitoring and timely intervention.
Previous studies have shown that recurrence after hepatectomy is associated with characteristics of tumors themselves, surgery-related factors, and patient's state including large tumor size, satellite lesions, poorly differentiated tumors, vascular invasion, age, male gender, and so on[16-19]. In this study, MVI, tumor differentiation degree, and tumor diameter were statistically different between the recurrence and non-recurrence groups. Since many risk factors of HCC recurrence can not be modified (such as age and pathological type), our focus should be on factors that can be intervened or improved preoperatively, which is even more attractive for clinical treatment guidance.
Our study found that 28.6% patients presented with sarcopenia, 66.7% of which experienced postoperative recurrence in the training cohort. Sarcopenia is accepted as one of the independent risk factors for postoperative recurrence[10]. Patients without sarcopenia can typically achieve a more prolonged RFS during follow-up. dietary supplementation may substantially improve muscle mass in cancer patients[20]. The mechanism underlying the association between sarcopenia and HCC recurrence is unclear but may be related to the tumor microenvironment (inflammation and immunity) and cytokine (myokines and adipokines)[21]. It seems promising to improve the prognosis through nutritional intervention. However, correcting the nutritional status takes a long time, and delaying surgical intervention in patients with cancer may worsen the prognosis and result in tumor progression. Therefore, we should strengthen the preoperative and postoperative monitoring of sarcopenia, supplement nutritional treatment, and implement long-term monitoring of nutritional status. Since every patient after hepatectomy of HCC requires an abdominal CT examination in follow-up. Determining sarcopenia by calculating abdominal CT SMI is feasible and objective, which shortens the patient's time and does not increase additional evaluation costs.
On the other hand, HBV infection is the leading cause of HCC worldwide, accounting for 33% of cases[22], which is also still one of the main factors causing HCC in China[5]. Many studies have shown that viral load plays a crucial role in the prognosis of HCC[23,24]. In this study, we observed a more significant recurrence rate in patients with high viral load. HBV DNA levels ≥ 2000 IU/mL were an independent prognostic factor for HCC recurrence after curative hepatectomy. At the same time, we also observed that although the recurrence rate is low in low-level viremia, 47.2% of patients still have a recurrence. Studies have shown that low-level viremia patients with a relatively low viral load can still benefit from effective antiviral therapy, significantly reducing HCC recurrence after hepatic resection[25]. Herein, it is recommended to use a highly sensitive real-time quantitative PCR method to detect HBV DNA, with a lower quantitative limit of 10-20 IU/mL or even lower, thereby initiating antiviral therapy or adjusting the treatment schedule promptly.
Several studies have proposed the prediction models of HCC recurrence, such as the preoperative (ERASL-pre) and postoperative (ERASL-post) risk models[5] based on gender, ALBI score, serum AFP, and tumor volume and quantity. The pre-MORAL and postoperative post-MORAL models[6] can predict recurrence after liver transplantation. The RETREAT scale can also assess the risk of recurrence after liver transplantation[5]. The AS score evaluates the risk of recurrence after liver resection and radiofrequency ablation[4]. However, the current prediction models didn't consider nutritional factors. Therefore, we compared the SAMD model with other preoperative prediction models and revealed that the SAMD model was more capable of predicting postoperative recurrence. Furthermore, compared for the different models prediction of RFS between the low- and high-risk groups, which revealed that the RFS rate in the high-risk patients with a SAMD model was lower than that in patients with an AS score and pre-MORAL. Patients in the high-risk group of the SAMD scores, the ERASL-pre scores, the Pre-MORAL scores and the AS scores had higher likelihood of recurrence events, and the hazard regression ratio are 4.228-fold, 2.053-fold, 1.802-fold and 1.506-fold, respectively. It is proved that a SAMD score was better to distinguish the high-risk group of postoperative recurrence of HCC than other model. Besides, a SAMD score < 110 can better distinguish the low-risk group of postoperative HCC recurrence, which is also significantly outperforms other prediction models. The RFS curve of the SAMD model indicates that the application of this model may extend the monitoring interval for low-risk patients, reduce patient economic costs, and improve social benefits.
However, the present study also has some limitations. Firstly, as a retrospective single-center study, this study has a limited sample size. This could limit the generalizability of the findings to a broader population. Extensive prospective studies with different geographical locations, ethnic backgrounds, or healthcare systems are needed to verify the model's efficacy. Secondly, the study does not seem to account for potential confounding factors that could influence the results, such as the patient's overall health status, lifestyle factors, or other comorbidities. Patients with liver cirrhosis and those without liver cirrhosis were included in this study. Although these groups were well-balanced, different grades of liver cirrhosis and varying degrees of portal hypertension might introduce selection bias and impact our results. On the other hand, limitations in statistical methods may also affect the effectiveness of the model. In addition, only the hepatitis B population was selected in this study, lacking the discussion of other causes.
In conclusion, we revealed that sarcopenia before hepatectomy is associated with recent recurrence after hepatectomy for hepatitis B-related HCC patients, and the prediction model based on liver nutrition was first established for postoperative recurrence of hepatitis B-related HCC. the SAMD model based on sarcopenia has favorable performance in predicting RFS in patients undergoing hepatectomy for hepatitis B-related HCC. It is helpful for the comprehensive clinical intervention in such patients. In the future, we need to further validate and apply this model, and conduct prospective studies to explore the impact of nutritional interventions on patient survival outcomes.
The recurrence of hepatocellular carcinoma (HCC) has a significant impact on the survival outcomes of patients, and early prediction and intervention can help improve patient survival outcomes. Nutritional factors have always been a hot topic of concern and are prone to intervention.
Sarcopenia is one of the effective indicators for evaluating nutritional status in chronic liver disease, which was reported that sarcopenia as a negative prognostic factor in patients with HCC. Hence, it is necessary to incorporate them into models for predicting early recurrence of HCC to screen out high-risk groups, as they may require more aggressive intervention.
This study aimed to construct a nutrition-based model to estimate recurrence-free survival (RFS) after hepatectomy for hepatitis B-related HCC based on sarcopenia.
According to the inclusion and exclusion criteria, 283 patients with hepatitis B-related HCC were eventually enrolled in this retrospective study: 189 patients in the training cohort and 94 patients in the validation cohort. Skeletal muscle index at the third lumbar spine was evaluated according to abdominal computed tomography scans before hepatectomy. Independent predictors of disease recrudescence were evaluated with univariate and multivariate Cox proportional hazard models in training cohort, and A nomogram model was developed to predict the RFS of HCC patients. Its predictive performance was validated in the validation cohort. Furthermore, we compared the predictive model with other preoperative models and assessed their prognostic values by analyzing the time-dependent area under the receiver operating characteristic curve (tdAUROC).
Our data demonstrated that among 144 (50.9%) patients developed recurrence within 2 years after hepatectomy, and the median RFS was 7.67 months (95%CI: 6.59-8.75). Multivariate analysis showed that sarcopenia, alpha-fetoprotein ≥ 40 ng/mL, the maximum diameter of tumor > 5 cm, and hepatitis B virus DNA level ≥ 2000 IU/ mL were independent risk factors associated with postoperative recurrence of HCC. The SAMD model predicting the RFS of HCC patients was established based on the above factors. The area under the curve of the SAMD model was 0.782 (95%CI: 0.705-0.858) in the training cohort (sensitivity 81%, specifcity 63%) and 0.773 (95%CI: 0.707-0.838) in the validation cohort. Besides, a SAMD score ≥ 110 was better to distinguish the high-risk group of postoperative recurrence of HCC compared to other models. Further multicenter studies are warranted to validate our findings.
Our study highlights the strong correlation between sarcopenia and recent recurrence after hepatectomy for hepatitis B-related HCC. A predictive model based on sarcopenia for assessing recent recurrence after liver resection for hepatitis B-associated hepatocellular carcinoma was developed for the first time.
The SAMD model based on sarcopenia has favorable performance in predicting RFS in patients undergoing hepatectomy for hepatitis B-related HCC. It is helpful for the comprehensive clinical intervention in such patients. In the future, we need to further validate and apply this model, and conduct prospective studies to explore the impact of nutritional interventions on patient survival outcomes.
The authors thank the volunteers who participated in the study and the reviewers for their helpful comments on this paper.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Virarkar M, United States S-Editor: Liu H L-Editor: A P-Editor: Chen YX
| 1. | Bureau of Medical Administration, National Health Commission of the People's Republic of China. [Standardization for diagnosis and treatment of hepatocellular carcinoma (2022 edition)]. Zhonghua Gan Zang Bing Za Zhi. 2022;30:367-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 26] [Reference Citation Analysis (0)] |
| 2. | Kanda T, Ogasawara S, Chiba T, Haga Y, Omata M, Yokosuka O. Current management of patients with hepatocellular carcinoma. World J Hepatol. 2015;7:1913-1920. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 38] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 3. | Tampaki M, Papatheodoridis GV, Cholongitas E. Intrahepatic recurrence of hepatocellular carcinoma after resection: an update. Clin J Gastroenterol. 2021;14:699-713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 50] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 4. | Lee M, Chang Y, Oh S, Cho YY, Jung DE, Kim HH, Nam JY, Cho H, Cho EJ, Lee JH, Yu SJ, Yi NJ, Lee KW, Lee DH, Lee JM, Yoon JH, Suh KS, Kim YJ. Assessment of the Surveillance Interval at 1 Year after Curative Treatment in Hepatocellular Carcinoma: Risk Stratification. Gut Liver. 2018;12:571-582. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 5. | Mehta N, Heimbach J, Harnois DM, Sapisochin G, Dodge JL, Lee D, Burns JM, Sanchez W, Greig PD, Grant DR, Roberts JP, Yao FY. Validation of a Risk Estimation of Tumor Recurrence After Transplant (RETREAT) Score for Hepatocellular Carcinoma Recurrence After Liver Transplant. JAMA Oncol. 2017;3:493-500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 291] [Article Influence: 36.4] [Reference Citation Analysis (0)] |
| 6. | Halazun KJ, Najjar M, Abdelmessih RM, Samstein B, Griesemer AD, Guarrera JV, Kato T, Verna EC, Emond JC, Brown RS Jr. Recurrence After Liver Transplantation for Hepatocellular Carcinoma: A New MORAL to the Story. Ann Surg. 2017;265:557-564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 218] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 7. | Simonsen C, de Heer P, Bjerre ED, Suetta C, Hojman P, Pedersen BK, Svendsen LB, Christensen JF. Sarcopenia and Postoperative Complication Risk in Gastrointestinal Surgical Oncology: A Meta-analysis. Ann Surg. 2018;268:58-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 253] [Article Influence: 42.2] [Reference Citation Analysis (0)] |
| 8. | Berardi G, Antonelli G, Colasanti M, Meniconi R, Guglielmo N, Laurenzi A, Ferretti S, Levi Sandri GB, Spagnoli A, Moschetta G, Schininà V, Antonini M, Marignani M, Ettorre GM. Association of Sarcopenia and Body Composition With Short-term Outcomes After Liver Resection for Malignant Tumors. JAMA Surg. 2020;155:e203336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 65] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 9. | Kamachi S, Mizuta T, Otsuka T, Nakashita S, Ide Y, Miyoshi A, Kitahara K, Eguchi Y, Ozaki I, Anzai K. Sarcopenia is a risk factor for the recurrence of hepatocellular carcinoma after curative treatment. Hepatol Res. 2016;46:201-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 73] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 10. | Kim YR, Park S, Han S, Ahn JH, Kim S, Sinn DH, Jeong WK, Ko JS, Gwak MS, Kim GS. Sarcopenia as a predictor of post-transplant tumor recurrence after living donor liver transplantation for hepatocellular carcinoma beyond the Milan criteria. Sci Rep. 2018;8:7157. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 11. | Lai JC, Tandon P, Bernal W, Tapper EB, Ekong U, Dasarathy S, Carey EJ. Malnutrition, Frailty, and Sarcopenia in Patients With Cirrhosis: 2021 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology. 2021;74:1611-1644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 325] [Cited by in RCA: 396] [Article Influence: 99.0] [Reference Citation Analysis (0)] |
| 12. | Chinese Society of Hepatology; Chinese Medical Association. [The consensus on tertiary prevention of primary liver cancer (2022 version)]. Zhonghua Gan Zang Bing Za Zhi. 2022;30:832-845. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 13. | Wen T, Jin C, Facciorusso A, Donadon M, Han HS, Mao Y, Dai C, Cheng S, Zhang B, Peng B, Du S, Jia C, Xu F, Shi J, Sun J, Zhu P, Nara S, Millis JM; MDT of West China Hospital*. Multidisciplinary management of recurrent and metastatic hepatocellular carcinoma after resection: an international expert consensus. Hepatobiliary Surg Nutr. 2018;7:353-371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 84] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 14. | Nishikawa H, Shiraki M, Hiramatsu A, Moriya K, Hino K, Nishiguchi S. Japan Society of Hepatology guidelines for sarcopenia in liver disease (1st edition): Recommendation from the working group for creation of sarcopenia assessment criteria. Hepatol Res. 2016;46:951-963. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 302] [Cited by in RCA: 479] [Article Influence: 53.2] [Reference Citation Analysis (0)] |
| 15. | Agopian VG, Petrowsky H, Kaldas FM, Zarrinpar A, Farmer DG, Yersiz H, Holt C, Harlander-Locke M, Hong JC, Rana AR, Venick R, McDiarmid SV, Goldstein LI, Durazo F, Saab S, Han S, Xia V, Hiatt JR, Busuttil RW. The evolution of liver transplantation during 3 decades: analysis of 5347 consecutive liver transplants at a single center. Ann Surg. 2013;258:409-421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 165] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 16. | Xu XF, Xing H, Han J, Li ZL, Lau WY, Zhou YH, Gu WM, Wang H, Chen TH, Zeng YY, Li C, Wu MC, Shen F, Yang T. Risk Factors, Patterns, and Outcomes of Late Recurrence After Liver Resection for Hepatocellular Carcinoma: A Multicenter Study From China. JAMA Surg. 2019;154:209-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 375] [Cited by in RCA: 396] [Article Influence: 66.0] [Reference Citation Analysis (0)] |
| 17. | Zheng J, Chou JF, Gönen M, Vachharajani N, Chapman WC, Majella Doyle MB, Turcotte S, Vandenbroucke-Menu F, Lapointe R, Buettner S, Groot Koerkamp B, Ijzermans JNM, Chan CY, Goh BKP, Teo JY, Kam JH, Jeyaraj PR, Cheow PC, Chung AYF, Chow PKH, Ooi LLPJ, Balachandran VP, Kingham TP, Allen PJ, D'Angelica MI, DeMatteo RP, Jarnagin WR, Lee SY. Prediction of Hepatocellular Carcinoma Recurrence Beyond Milan Criteria After Resection: Validation of a Clinical Risk Score in an International Cohort. Ann Surg. 2017;266:693-701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 94] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 18. | Zhao H, Chen C, Gu S, Yan X, Jia W, Mao L, Qiu Y. Anatomical versus non-anatomical resection for solitary hepatocellular carcinoma without macroscopic vascular invasion: A propensity score matching analysis. J Gastroenterol Hepatol. 2017;32:870-878. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 76] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 19. | Erstad DJ, Tanabe KK. Prognostic and Therapeutic Implications of Microvascular Invasion in Hepatocellular Carcinoma. Ann Surg Oncol. 2019;26:1474-1493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 288] [Article Influence: 48.0] [Reference Citation Analysis (0)] |
| 20. | Morley JE, Argiles JM, Evans WJ, Bhasin S, Cella D, Deutz NE, Doehner W, Fearon KC, Ferrucci L, Hellerstein MK, Kalantar-Zadeh K, Lochs H, MacDonald N, Mulligan K, Muscaritoli M, Ponikowski P, Posthauer ME, Rossi Fanelli F, Schambelan M, Schols AM, Schuster MW, Anker SD; Society for Sarcopenia, Cachexia, and Wasting Disease. Nutritional recommendations for the management of sarcopenia. J Am Med Dir Assoc. 2010;11:391-396. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 531] [Cited by in RCA: 446] [Article Influence: 29.7] [Reference Citation Analysis (0)] |
| 21. | Lutz CT, Quinn LS. Sarcopenia, obesity, and natural killer cell immune senescence in aging: altered cytokine levels as a common mechanism. Aging (Albany NY). 2012;4:535-546. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 179] [Cited by in RCA: 241] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 22. | Caines A, Selim R, Salgia R. The Changing Global Epidemiology of Hepatocellular Carcinoma. Clin Liver Dis. 2020;24:535-547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 23. | Kim TS, Sinn DH, Kang W, Gwak GY, Paik YH, Choi MS, Lee JH, Koh KC, Paik SW. Hepatitis B virus DNA levels and overall survival in hepatitis B-related hepatocellular carcinoma patients with low-level viremia. J Gastroenterol Hepatol. 2019;34:2028-2035. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 24. | Kubo S, Hirohashi K, Tanaka H, Tsukamoto T, Shuto T, Yamamoto T, Ikebe T, Wakasa K, Nishiguchi S, Kinoshita H. Effect of viral status on recurrence after liver resection for patients with hepatitis B virus-related hepatocellular carcinoma. Cancer. 2000;88:1016-1024. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 25. | Huang G, Li PP, Lau WY, Pan ZY, Zhao LH, Wang ZG, Wang MC, Zhou WP. Antiviral Therapy Reduces Hepatocellular Carcinoma Recurrence in Patients With Low HBV-DNA Levels: A Randomized Controlled Trial. Ann Surg. 2018;268:943-954. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 112] [Article Influence: 16.0] [Reference Citation Analysis (0)] |