Published online Mar 28, 2024. doi: 10.3748/wjg.v30.i12.1670
Peer-review started: December 28, 2023
First decision: January 19, 2024
Revised: January 29, 2024
Accepted: March 7, 2024
Article in press: March 7, 2024
Published online: March 28, 2024
Processing time: 90 Days and 17.9 Hours
This editorial highlights the remarkable advancements in medical treatment strategies for pancreatic neuroendocrine tumors (pan-NETs), emphasizing tailored approaches for specific subtypes. Cytoreductive surgery and somatostatin analogs (SSAs) play pivotal roles in managing tumors, while palliative options such as molecular targeted therapy, peptide receptor radionuclide therapy, and chemotherapy are reserved for SSA-refractory patients. Gastrinomas, insul
Core Tip: The evolving landscape of pancreatic neuroendocrine tumor treatment showcases tailored approaches based on tumor subtype. Cytoreductive surgery and somatostatin analogs are pivotal, whereas peptide receptor radionuclide therapy and molecular targeted agents are offering hope for refractory cases. Understanding genetic markers and exploring immunotherapies open promising avenues for future research.
- Citation: Giri S, Sahoo J. Advancements in medical treatment for pancreatic neuroendocrine tumors: A beacon of hope. World J Gastroenterol 2024; 30(12): 1670-1675
- URL: https://www.wjgnet.com/1007-9327/full/v30/i12/1670.htm
- DOI: https://dx.doi.org/10.3748/wjg.v30.i12.1670
Pancreatic neuroendocrine tumors (pan-NETs) present unique challenges in the field of oncology. These tumors account for 1%-2% of all pancreatic cancers and are mostly sporadic. Sometimes, pan-NETs are associated with various syndromes, such as multiple endocrine neoplasia 1 (MEN1), von Hippel-Lindau disease, tuberous sclerosis or neurofibromatosis. Often, these tumors are diagnosed at an advanced stage in the course of disease with metastasis to multiple organs, making curative surgery less successful. The role of medical therapy is as follows.
Most patients with advanced pan-NETs (50%-75%) have nonfunctioning tumors and do not have any associated hormonal syndrome. Somatostatin analogs (SSAs) provide a valuable palliative option by reducing hormonal secretion as well as the tumor burden. The European Neuroendocrine Tumor Society, North American Neuroendocrine Society, and National Comprehensive Cancer Network suggest the initiation of SSA in patients with unresectable, asymptomatic, well-differentiated pan-NETs and a high tumor burden, as SSAs have been shown to improve survival in the PROMID[1] and CLARINET trials[2]. Treatment of highly symptomatic patients may be initiated with short-acting octreotide with rapid transition to a long-acting formulation (LAR) and subsequent titration of the dose to optimize symptom control while the LAR formulation is starting to take effect (approximately 10-14 d). The depot preparation, e.g., octreotide LAR, has largely eliminated the need for daily octreotide injections. Patients may use additional short-acting octreotide for breakthrough symptoms while doses are being titrated. Lanreotide can be administered once monthly via deep subcutaneous injection and appears to have efficacy similar to that of octreotide.
Notably, molecular targeted therapies, peptide receptor radionuclide therapy (PRRT), and chemotherapy are typically reserved for patients refractory to SSAs[3]. According to recent European Society of Medical Oncology guidelines, the mammalian target of rapamycin (mTOR) inhibitor everolimus is recommended for the treatment of G1/G2 pan-NETs[4]. A good treatment effect of everolimus was also recorded irrespective of the volume of liver metastasis in the RADIANT-4 trial[5]. Everolimus in combination with SSAs in advanced and metastatic pan-NETs demonstrated greater benefit than everolimus monotherapy in the RADIANT-1 and RADIANT-3 trials. In contrast to these data, the combination of everolimus with pasireotide did not prove to exert any further benefit. Everolimus should be carefully co-administered with glucocorticoids since, in the RADIANT-3 cohort, their combination resulted in a fatal episode of acute respiratory distress, as everolimus can cause immunosuppression. The progression-free survival (PFS) associated with the com-bination of everolimus and metformin was better than that associated with everolimus alone[6]. The mechanisms affected by metformin include a reduction in blood glucose, insulin, and insulin like growth factor-1 levels; inhibition of mitochondrial oxidation; activation of AMP-activated protein kinase; and antibacterial cell autonomy via mTOR inhibition as well as oncogenic effects.
PRRT has emerged as a beacon of hope for patients with metastatic but low-grade pan-NETs where curative surgery is not possible. This targeted therapy utilizes radionuclides to deliver systemic treatment. In the NETTER-1 trial, patients who received 177Lu-DOTATATE with SSA experienced a 54.4% increase in estimated PFS at 20 months vs SSA alone[7].
On the other hand, neuroendocrine carcinoma (NEC) is genetically more similar to pancreatic cancer than to G1/G2 neuroendocrine tumors (NETs). Here comes the role of chemotherapy. Capecitabine plays synergistic role with temozolomide, perhaps by downregulating the DNA repair enzyme methylguanine methyltransferase. Although combined therapy is more effective for treating pan-NET, overall survival in patients with NEC is not rewarding (22 vs 4.6 months)[8]. In NEC patients, cisplatin-based therapy with etoposide or irinotecan remains the standard first-line chemotherapy option[9]. Distinguished clinical trials related to the management of pan-NETs are summarized in Table 1.
| Trial | Population | Study design | Drug | NET | Outcome |
| PROMID | 85 | RCT | Octreotide LAR | Metastatic Midgut NET | PFS 14.3 vs 6 m |
| CLARINET | 204 | RCT | Lanreotide | G1, G2 non-functioning NET | 32% increase in PFS at 24 m |
| RADIANT 4 | 302 | RCT | Everolimus | Non-functioning NET | PFS 11 vs 4 m; 52% reduction of death |
| Kurita et al[6] | 100 | Retrospective | Everolimus | Pan-NET | PFS is longer with G1, G2 than G3, NEC; Metformin increases PFS |
| NETTER 1 | 229 | RCT | Lu DOTATATE with Octreotide LAR | Metastatic Midgut NET | 54.4% increase in PFS at 20 m |
| Rogowski et al[8] | 32 | retrospective | capecitabine and temozolomide | G3 NET | PFS 15.3 m in G3 NET, 3.3 m in NEC |
| Morizane et al[9] | 170 | RCT | etoposide and cisplatin vs irinotecan and cisplatin | NEC | PFS 5.6 months in EP vs 5.1 months in IP |
| Bernard et al[13] | 12 | Retrospective | Everolimus | Malignant insulinoma | Hypoglycemia resolve in 11/12. Median recurrence at 6.5 m |
| TELECAST | 76 | RCT | Telotristat ethyl | Carcinoid syndrome | Sustained reductions in u5-HIAA and diarrhea |
In gastrinomas, where surgical intervention might pose significant risks, medical therapy with high-dose proton pump inhibitor therapy (PPI) with or without SSAs can effectively control symptoms and tumor growth. Patients without imageable pancreatic tumors in MEN1 exhibit promising survival rates, i.e., 5-year survival rates of approximately 90% and a 10-year survival rate of 54% in patients with disseminated distant metastasis, underscoring the importance of tailored approaches[10]. Long-acting PPIs such as omeprazole 60 mg a day or an equivalent dose of other PPIs once or twice a day are durable and effective in patients with sporadic gastrinoma without evidence of tachyphylaxis. The goal of PPI therapy is to restrict basal acid output to < 10 mEq/h during the hour before the next dose. Patients with MEN1-related gastrinoma may require a daily dose of 80-120 mg of omeprazole. Effective treatment of hypercalcemia in MEN1 patients is of paramount importance because it reduces gastric acid hypersecretion. It is advised to continue PPI therapy for at least 3-6 months after resection of the tumor due to the continued risk of gastroesophageal reflux disease complications caused by the increased parietal cell mass. Vigilance is required for potential side effects of long-term PPI therapy, such as vitamin B12 deficiency, hypomagnesemia and the risk of bone fracture. The role of SSA in PPI refractory patients was well documented in a retrospective study of 12 patients with gastrinoma[11]. In this study, all but one patient achieved complete clinical control with octreotide or lanreotide.
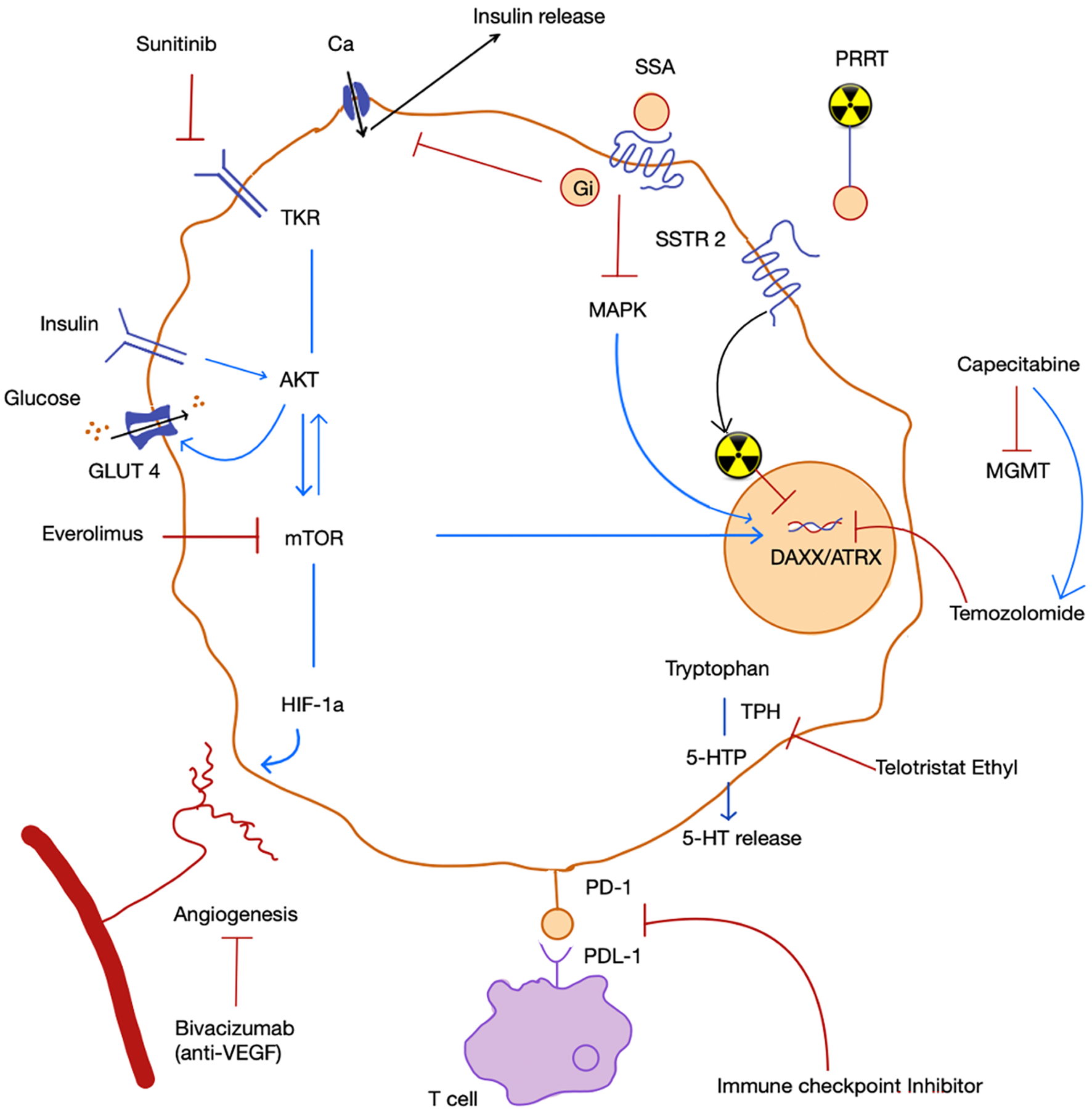
Insulinomas present a unique challenge, as patients develop hypoglycemia. Diazoxide (50-600 mg daily) is the primary medical therapy, and in refractory cases, glucocorticoids, verapamil, and diphenylhydantoin may be considered. SSAs can reduce insulin levels and are pivotal for the antiproliferative control of well-differentiated tumors. Since there is usually low expression of somatostatin receptor type (SSTR) 2 in benign insulinomas, SSA therapy may result in paradoxical worsening of hypoglycemia, as it also decreases glucagon secretion. In contrast, advanced insulinomas usually express SSTR2 and SSTR5. The pan-somatostatin receptor agonist pasireotide is a promising option for patients with refractory hypoglycemia, especially for those with metastatic insulinoma[12]. Akt/mTOR pathway is abnormally activated in NETs. Everolimus has both antineoplastic and antisecretory effects as shown in Figure 1 and has been successfully tested for refractory insulinoma[13]. However, exhaustion of its antineoplastic effect is observed after 2 years due to downregulation of mediators involved in the mTOR pathway instead of a true resistance[14]. Therefore, rechallenge with everolimus can be considered since this phenomenon appears to be transient.
For glucagonomas, SSAs are considered first-line treatments, as they have shown remarkable efficacy in controlling hormonal symptoms. In advanced cases, management must include SSAs to improve patient performance status, enabling surgical options to be considered[14]. Amino acid infusion and zinc therapy have shown promise in improving skin lesions associated with necrolytic migratory erythema.
VIPoma can cause severe and life-threatening diarrhea. Supportive care with intravenous fluids and electrolytes is crucial. SSAs have shown substantial promise in improving VIPoma-associated symptoms. Chemotherapy and sunitinib improved diarrhea in 10 out of 12 patients in a French study. Hypercalcemia associated with VIPoma is caused by the secretion of parathyroid hormone-related peptide and responds well to both zoledronate and denosumab[15].
Management of crisis due to carcinoid syndrome is a medical emergency that requires the infusion of octreotide along with serotonin antagonists such as ondansetron, cyproheptadine, and ranitidine. A bolus of octreotide 100-500 µg should be immediately administered, followed by a maintenance dose of 50-100 µg/h (maximum 500 µg/h). Sympathomimetics can precipitate hormonal release by the tumor, paradoxically leading to distributive shock, and should be used cautiously. However, if a patient needs to be on sympathomimetics, the selective alpha1-agonist phenylephrine and vasopressin are the preferred vasopressors in this context.
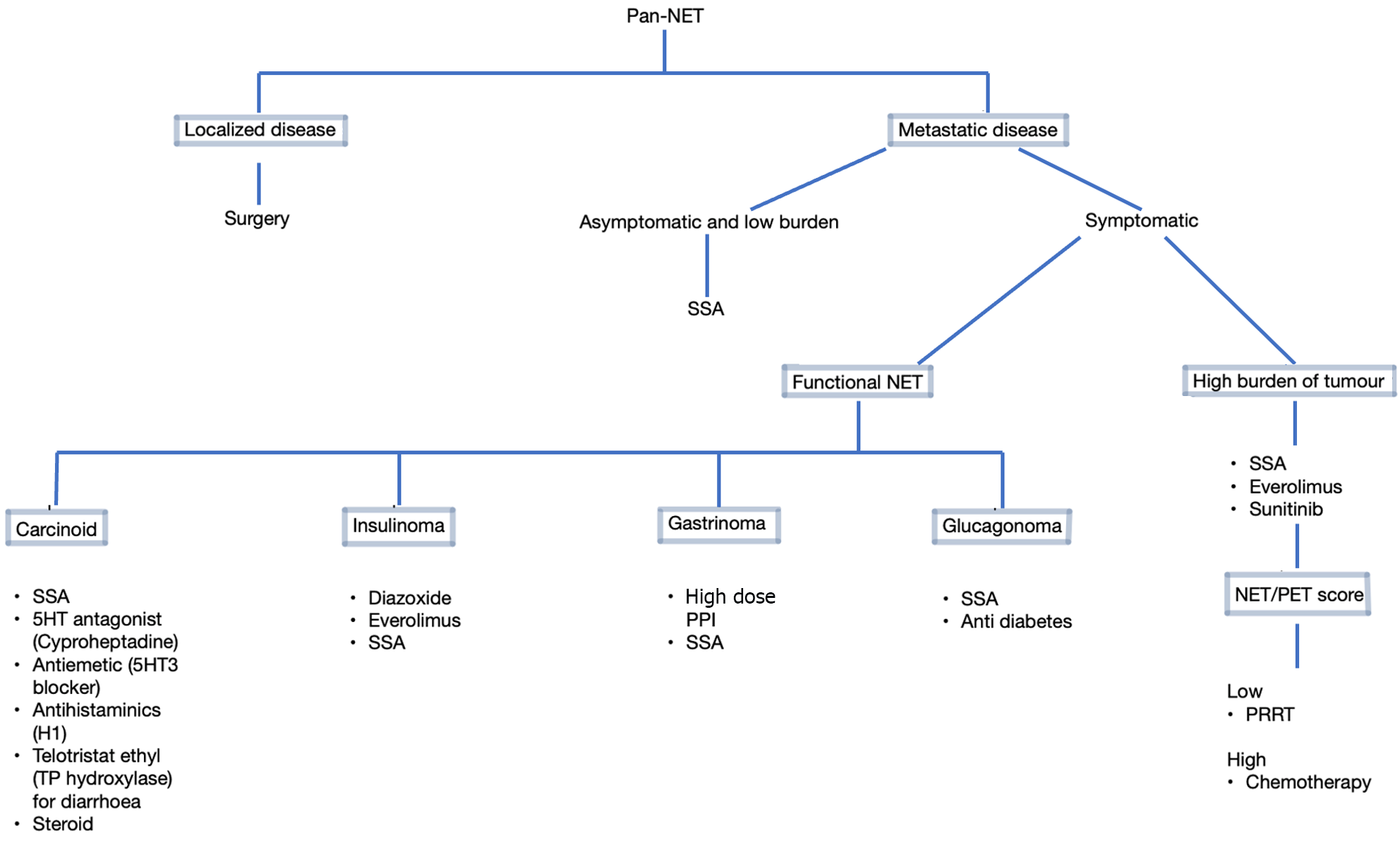
For the prophylaxis for carcinoid crisis, SSAs can be started before surgery or before PRRT. It is advised to keep SSA-free period before the start of PRRT as short as possible (8-24 h), with safe reintroduction of SSA 1 h after the infusion of 177Lu-DOTATATE in order to prevent functioning symptoms deterioration due to release of hormone following PRRT. Antidiarrheal agents such as loperamide are also useful. However, refractory diarrhea responds well to telotristat ethyl, a tryptophan hydroxylase inhibitor[16]. Patients with niacin deficiency or pellagra should be started on nicotinamide. A simplified flow diagram showing approach to medical therapy in pan-NETs is depicted in Figure 2.
Elucidating the genetic underpinnings of different pan-NET subtypes opens new avenues for tailored therapies. Mutations in genes such as DAXX/ATRX, EPHB4, ROS1, and KMT2A provide potential targets for future research[17]. Compared to NETs of extra-pancreatic origin, pan-NETs have higher expression levels of programmed cell death protein 1 (PD-1) and more tumor-infiltrating lymphocytes[18]. Immunotherapies, particularly PD-1 inhibitors, in combination with anti-vascular endothelial growth factor (bevacizumab) have shown potential in controlling pan-NETs.
The landscape of pan-NET treatment has evolved significantly, offering patients a more tailored and effective approach. From surgical interventions to targeted therapies and immunomodulatory agents, advancements in medical treatment for pan-NETs represent a beacon of hope for those facing this challenging disease. As research continues to uncover the intricacies of this condition, we can look forward to even more promising developments in the future.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: India
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Yin CH, China S-Editor: Qu XL L-Editor: A P-Editor: Cai YX
| 1. | Rinke A, Wittenberg M, Schade-Brittinger C, Aminossadati B, Ronicke E, Gress TM, Müller HH, Arnold R; PROMID Study Group. Placebo-Controlled, Double-Blind, Prospective, Randomized Study on the Effect of Octreotide LAR in the Control of Tumor Growth in Patients with Metastatic Neuroendocrine Midgut Tumors (PROMID): Results of Long-Term Survival. Neuroendocrinology. 2017;104:26-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 259] [Article Influence: 32.4] [Reference Citation Analysis (0)] |
| 2. | Caplin ME, Pavel M, Ruszniewski P. Lanreotide in metastatic enteropancreatic neuroendocrine tumors. N Engl J Med. 2014;371:1556-1557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 58] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 3. | Hofland J, Falconi M, Christ E, Castaño JP, Faggiano A, Lamarca A, Perren A, Petrucci S, Prasad V, Ruszniewski P, Thirlwell C, Vullierme MP, Welin S, Bartsch DK. European Neuroendocrine Tumor Society 2023 guidance paper for functioning pancreatic neuroendocrine tumour syndromes. J Neuroendocrinol. 2023;35:e13318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 104] [Reference Citation Analysis (0)] |
| 4. | Pavel M, Öberg K, Falconi M, Krenning EP, Sundin A, Perren A, Berruti A; ESMO Guidelines Committee. Gastroenteropancreatic neuroendocrine neoplasms: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2020;31:844-860. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 465] [Cited by in RCA: 694] [Article Influence: 138.8] [Reference Citation Analysis (0)] |
| 5. | Yao JC, Fazio N, Singh S, Buzzoni R, Carnaghi C, Wolin E, Tomasek J, Raderer M, Lahner H, Voi M, Pacaud LB, Rouyrre N, Sachs C, Valle JW, Fave GD, Van Cutsem E, Tesselaar M, Shimada Y, Oh DY, Strosberg J, Kulke MH, Pavel ME; RAD001 in Advanced Neuroendocrine Tumours, Fourth Trial (RADIANT-4) Study Group. Everolimus for the treatment of advanced, non-functional neuroendocrine tumours of the lung or gastrointestinal tract (RADIANT-4): a randomised, placebo-controlled, phase 3 study. Lancet. 2016;387:968-977. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 749] [Cited by in RCA: 901] [Article Influence: 100.1] [Reference Citation Analysis (0)] |
| 6. | Kurita Y, Kobayashi N, Hara K, Mizuno N, Kuwahara T, Okuno N, Haba S, Tokuhisa M, Hasegawa S, Sato T, Hosono K, Kato S, Kessoku T, Endo I, Shimizu Y, Kubota K, Nakajima A, Ichikawa Y, Niwa Y. Effectiveness and Prognostic Factors of Everolimus in Patients with Pancreatic Neuroendocrine Neoplasms. Intern Med. 2023;62:159-167. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 7. | Strosberg J, El-Haddad G, Wolin E, Hendifar A, Yao J, Chasen B, Mittra E, Kunz PL, Kulke MH, Jacene H, Bushnell D, O'Dorisio TM, Baum RP, Kulkarni HR, Caplin M, Lebtahi R, Hobday T, Delpassand E, Van Cutsem E, Benson A, Srirajaskanthan R, Pavel M, Mora J, Berlin J, Grande E, Reed N, Seregni E, Öberg K, Lopera Sierra M, Santoro P, Thevenet T, Erion JL, Ruszniewski P, Kwekkeboom D, Krenning E; NETTER-1 Trial Investigators. Phase 3 Trial of (177)Lu-Dotatate for Midgut Neuroendocrine Tumors. N Engl J Med. 2017;376:125-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1702] [Cited by in RCA: 2235] [Article Influence: 279.4] [Reference Citation Analysis (0)] |
| 8. | Rogowski W, Wachuła E, Gorzelak A, Lebiedzińska A, Sulżyc-Bielicka V, Iżycka-Świeszewska E, Żołnierek J, Kos-Kudła B. Capecitabine and temozolomide combination for treatment of high-grade, well-differentiated neuroendocrine tumour and poorly-differentiated neuroendocrine carcinoma - retrospective analysis. Endokrynol Pol. 2019;70:313-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 9. | Morizane C, Machida N, Honma Y, Okusaka T, Boku N, Kato K, Nomura S, Hiraoka N, Sekine S, Taniguchi H, Okano N, Yamaguchi K, Sato T, Ikeda M, Mizuno N, Ozaka M, Kataoka T, Ueno M, Kitagawa Y, Terashima M, Furuse J; Japan Clinical Oncology Group (JCOG). Effectiveness of Etoposide and Cisplatin vs Irinotecan and Cisplatin Therapy for Patients With Advanced Neuroendocrine Carcinoma of the Digestive System: The TOPIC-NEC Phase 3 Randomized Clinical Trial. JAMA Oncol. 2022;8:1447-1455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 61] [Reference Citation Analysis (0)] |
| 10. | Norton JA, Alexander HR, Fraker DL, Venzon DJ, Gibril F, Jensen RT. Comparison of surgical results in patients with advanced and limited disease with multiple endocrine neoplasia type 1 and Zollinger-Ellison syndrome. Ann Surg. 2001;234:495-505; discussion 505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 143] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 11. | Massironi S, Cavalcoli F, Elvevi A, Quatrini M, Invernizzi P. Somatostatin analogs in patients with Zollinger Ellison syndrome (ZES): an observational study. Endocrine. 2022;75:942-948. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 12. | Siddiqui M, Vora A, Ali S, Abramowitz J, Mirfakhraee S. Pasireotide: A Novel Treatment for Tumor-Induced Hypoglycemia Due to Insulinoma and Non-Islet Cell Tumor Hypoglycemia. J Endocr Soc. 2021;5:bvaa171. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 13. | Bernard V, Lombard-Bohas C, Taquet MC, Caroli-Bosc FX, Ruszniewski P, Niccoli P, Guimbaud R, Chougnet CN, Goichot B, Rohmer V, Borson-Chazot F, Baudin E; French Group of Endocrine Tumors. Efficacy of everolimus in patients with metastatic insulinoma and refractory hypoglycemia. Eur J Endocrinol. 2013;168:665-674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 71] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 14. | Alexandraki KI, Kaltsas GA, Grozinsky-Glasberg S. Emerging therapies for advanced insulinomas and glucagonomas. Endocr Relat Cancer. 2023;30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 15. | Giannetta E, Sesti F, Modica R, Grossrubatscher EM, Guarnotta V, Ragni A, Zanata I, Colao A, Faggiano A. Case Report: Unmasking Hypercalcemia in Patients With Neuroendocrine Neoplasms. Experience From Six Italian Referral Centers. Front Endocrinol (Lausanne). 2021;12:665698. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 16. | Pavel M, Gross DJ, Benavent M, Perros P, Srirajaskanthan R, Warner RRP, Kulke MH, Anthony LB, Kunz PL, Hörsch D, Weickert MO, Lapuerta P, Jiang W, Kassler-Taub K, Wason S, Fleming R, Fleming D, Garcia-Carbonero R. Telotristat ethyl in carcinoid syndrome: safety and efficacy in the TELECAST phase 3 trial. Endocr Relat Cancer. 2018;25:309-322. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 107] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 17. | Burns L, Naimi B, Ronan M, Xu H, Weber HC. Report of a Novel Molecular Profile in Malignant Insulinoma. J Clin Med. 2023;12. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 18. | Bösch F, Brüwer K, Altendorf-Hofmann A, Auernhammer CJ, Spitzweg C, Westphalen CB, Boeck S, Schubert-Fritschle G, Werner J, Heinemann V, Kirchner T, Angele M, Knösel T. Immune checkpoint markers in gastroenteropancreatic neuroendocrine neoplasia. Endocr Relat Cancer. 2019;26:293-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 65] [Article Influence: 10.8] [Reference Citation Analysis (0)] |