Published online Mar 28, 2024. doi: 10.3748/wjg.v30.i12.1663
Peer-review started: December 27, 2023
First decision: January 19, 2024
Revised: February 5, 2024
Accepted: March 12, 2024
Article in press: March 12, 2024
Published online: March 28, 2024
Processing time: 91 Days and 20.8 Hours
Liver disease accounts for approximately 2 million deaths per year worldwide. All chronic liver diseases (CLDs), whether of toxic, genetic, autoimmune, or infectious origin, undergo typical histological changes in the structure of the tissue. These changes may include the accumulation of extracellular matrix material, fats, triglycerides, or tissue scarring. Noninvasive methods for diagnosing CLD, such as conventional B-mode ultrasound (US), play a significant role in diagnosis. Doppler US, when coupled with B-mode US, can be helpful in evaluating the hemodynamics of hepatic vessels and detecting US findings associated with hepatic decompensation. US elastography can assess liver stiffness, serving as a surrogate marker for liver fibrosis. It is important to note that interpreting these values should not rely solely on a histological classification. Contrast-enhanced US (CEUS) provides valuable information on tissue perfusion and enables excellent differentiation between benign and malignant focal liver lesions. Clinical evaluation, the etiology of liver disease, and the patient current comorbidities all influence the interpretation of liver stiffness measurements. These measurements are most clinically relevant when interpreted as a probability of compensated advanced CLD. B-mode US offers a subjective estimation of fatty infiltration and has limited sensitivity for mild steatosis. The controlled attenuation parameter requires a dedicated device, and cutoff values are not clearly defined. Quan-titative US parameters for liver fat estimation include the attenuation coefficient, backscatter coefficient, and speed of sound. These parameters offer the advantage of providing fat quantification alongside B-mode evaluation and other US parameters. Multiparametric US (MPUS) of the liver introduces a new concept for complete noninvasive diagnosis. It encourages examiners to utilize the latest features of an US machine, including conventional B-mode, liver stiffness evaluation, fat quantification, dispersion imaging, Doppler US, and CEUS for focal liver lesion characterization. This comprehensive approach allows for diagnosis in a single examination, providing clinicians worldwide with a broader perspective and becoming a cornerstone in their diagnostic arsenal. MPUS, in the hands of skilled clinicians, becomes an invaluable predictive tool for diagnosing, staging, and monitoring CLD.
Core Tip: Multiparametric ultrasound (MPUS) of the liver introduces a new concept for complete liver evaluation. It encourages examiners to utilize the latest features of an ultrasound (US) machine, including conventional B-mode, liver stiffness evaluation, fat quantification, dispersion imaging, Doppler US, and contrast-enhanced US for focal liver lesion characterization. MPUS, in the hands of skilled clinicians, becomes an invaluable predictive tool for diagnosing, staging, and monitoring chronic liver disease.
- Citation: Peltec A, Sporea I. Multiparametric ultrasound as a new concept of assessment of liver tissue damage. World J Gastroenterol 2024; 30(12): 1663-1669
- URL: https://www.wjgnet.com/1007-9327/full/v30/i12/1663.htm
- DOI: https://dx.doi.org/10.3748/wjg.v30.i12.1663
Chronic liver disease (CLD) poses a global health challenge, contributing to approximately two million deaths annually worldwide[1]. The nature of these diseases, arising from diverse etiologies, present a complex array of structural and functional abnormalities. The assessment of liver tissue damage is a critical aspect of managing various liver diseases. Historically, liver tissue damage assessment relied heavily on invasive methods such as liver biopsy. Histological changes in liver tissue are characteristic of CLDs, encompassing toxic (alcoholic), genetic, autoimmune, and infectious etiologies[2]. Accumulation of extracellular matrix material, fats, triglycerides, or tissue scarring are common manifestations. The gold standard for evaluating CLDs is a liver biopsy. This is because examining the histologic specimen not only helps with fibrosis staging but also provides additional information about necroinflammation and other pathological changes. Offering direct insights into histopathological changes, it is an invasive procedure carrying potential complications and limitations such as sampling errors and interobserver variability. This underscores the necessity for noninvasive alternatives[3-5].
Traditional B-mode ultrasound (US) has been a cornerstone in diagnosing liver diseases, providing valuable insights into structural abnormalities[6]. Recent developments have expanded the diagnostic capabilities of US. Doppler US, when combined with B-mode imaging, offers a nuanced evaluation of hepatic vessel hemodynamics and identifies findings associated with hepatic decompensation. Contrast-enhanced US (CEUS) enhances tissue perfusion assessment, facilitating the differentiation between benign and malignant liver lesions[7]. US-based elastography, measuring liver stiffness, emerges as a pivotal tool for assessing liver fibrosis. However, its interpretation must consider clinical evaluation, the etiology of the liver disease, and the patient’s comorbidities. These measurements prove most clinically relevant when viewed as a probability of compensated advanced CLD (cACLD)[8]. Accurate diagnosis of liver disease is essential for effective management and timely intervention. Multiparametric US (MPUS) addresses this challenge by combining multiple imaging parameters to offer a detailed and nuanced assessment of liver health. The advent of MPUS marks a paradigm shift in liver disease diagnosis. By integrating various US features such as B-mode, liver stiffness, fat quantification, dispersion imaging, Doppler US, and CEUS, clinicians gain a comprehensive diagnostic perspective in a single examination. MPUS, when wielded by skilled clinicians, becomes an invaluable predictive tool for diagnosing, staging, and monitoring CLDs. The ability to provide a broader perspective enhances diagnostic accuracy, empowering clinicians worldwide with efficient diagnostic tools. The evolution of noninvasive methods, particularly MPUS, has revolutionized the landscape of liver disease diagnosis.
Traditional B-mode US remains a fundamental component, providing a structural overview of liver tissue. However, its limitations in detecting mild steatosis emphasize the need for a more comprehensive approach. When coupled with B-mode imaging, Doppler US enhances the evaluation of hepatic vessel hemodynamics. This addition aids in identifying early signs of hepatic decompensation, contributing to a more thorough diagnostic picture. Vascular thrombosis can be diagnosed very simply with standard US and with Doppler evaluation. CEUS provides valuable information on tissue perfusion, enabling accurate differentiation between benign and malignant focal liver lesions. The enhanced imaging capabilities contribute significantly to the diagnostic accuracy of MPUS. US-based elastography serves as a surrogate marker for liver fibrosis. However, the interpretation of these measurements requires a holistic consideration of clinical evaluation, the underlying etiology, confounding factors, and the patient comorbidities. The limitations of B-mode US in estimating fatty infiltration underscore the need for comprehensive approaches. The controlled attenuation parameter, though requiring a dedicated device, contributes valuable insights. Quantitative US parameters like attenuation coefficient, backscatter coefficient, and speed of sound offer a holistic evaluation of liver fat, complementing B-mode assessments. Interpreting results from noninvasive methods requires a nuanced understanding of the underlying liver disease, patient comorbidities, and the specific modality used. A comprehensive clinical evaluation is essential for accurate diagnosis. The availability of advanced diagnostic technologies varies globally, impacting the accessibility of these noninvasive methods. Efforts to enhance accessibility and reduce disparities are crucial for widespread adoption. Standardizing the interpretation of results and establishing cutoff values for different modalities remain ongoing challenges. Consistent guidelines are necessary to ensure uniformity in assessments across healthcare settings. Standardization efforts are essential to enhance reliability and comparability. The field of liver tissue damage assessment is rapidly evolving. Future advancements may involve the integration of artificial intelligence for enhanced diagnostic accuracy, the development of novel serum biomarkers, and the refinement of existing technologies to address current limitations. Introduction of these new modules of evaluation (stiffness, fatty quantification) to a middle-class US machine is essential for the future accessibility of these new developments of the method.
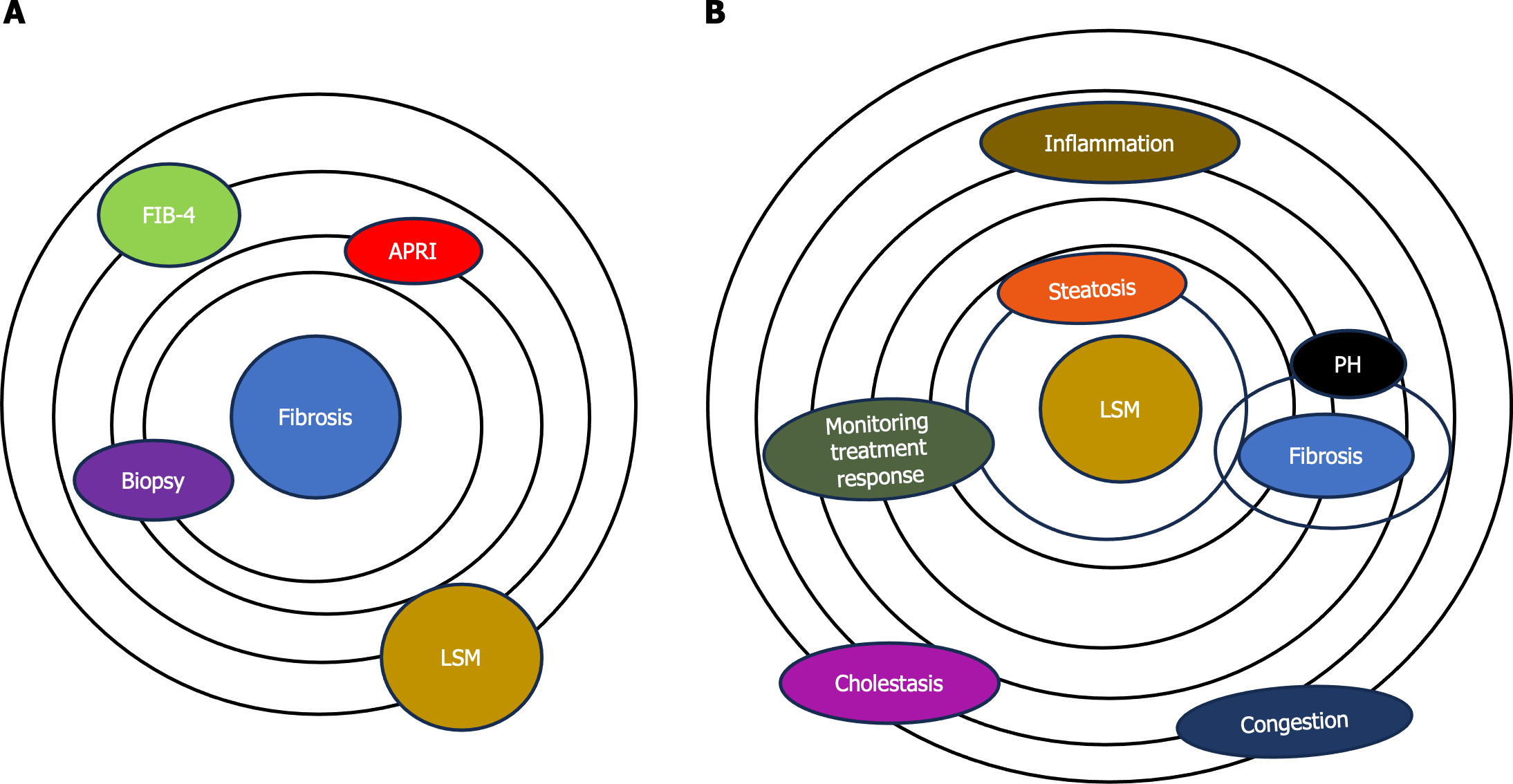
Liver fibrosis, a key feature of CLDs caused by various factors, can progress to liver cirrhosis along with its associated complications[3]. Evaluating the presence and extent of liver fibrosis is crucial in managing CLD patients as it can anticipate the prognosis and potentially impact treatment decision. Initially developed to estimate liver fibrosis by measuring tissue stiffness, elastography has transcended its original purpose. Elastography, once primarily associated with fibrosis assessment, has evolved into a versatile method offering insights into various aspects of liver tissue health (Figure 1).
Now, many experts explore the expanding role of elastography beyond fibrosis evaluation, highlighting its diverse applications in assessing the dynamic nature of liver tissues[8]. A model centered on applications of elastography beyond fibrosis offers several options including: (1) Liver steatosis assessment. Elastography has shown promise in quantifying liver steatosis, providing a noninvasive means to evaluate fat content. Identifying and quantifying fat infiltration contributes to a more comprehensive understanding of liver health; (2) inflammation detection. The dynamic nature of elastography allows for the detection of inflammatory changes within liver tissues. By assessing tissue stiffness alterations, elastography aids in identifying inflammation, a crucial factor in the progression of various liver diseases; (3) portal hypertension evaluation. Elastography provides valuable insights into portal hypertension by assessing liver stiffness. Monitoring changes in stiffness aids in understanding the impact of portal hypertension on liver tissues and guides appropriate interventions; and (4) monitoring treatment response. Elastography serves as a tool for monitoring responses to therapeutic interventions. Whether assessing the effectiveness of anti-inflammatory treatments or tracking changes in liver stiffness post-treatment, elastography offers real-time feedback on treatment outcomes (Figure 1).
However, there are some confounding factors that can increase the liver stiffness. These confounding factors can contribute to a false increase in liver stiffness. Cholestasis refers to the impaired flow of bile, leading to the accumulation of bile acids and other substances within the liver. The accumulation of bile acids and other components in liver tissue may lead to inflammation and fibrosis. Elastography measurements in cholestatic conditions may indicate increased liver stiffness, reflecting the fibrotic changes associated with chronic cholestasis.
Hepatic congestion, often seen in conditions such as congestive heart failure, can impact liver stiffness as well. Congestion in the liver causes increased pressure within the hepatic vasculature. This elevated pressure can affect the mechanical properties of liver tissue, leading to changes in stiffness. Elastography may detect increased liver stiffness in cases of hepatic congestion, indicating the mechanical alterations caused by elevated intrahepatic pressure (Figure 1). Assessing the severity of cholestasis, the degree of congestion, and other contributing factors is essential for accurate diagnosis and appropriate clinical management. However, the interpretation should be conducted in the broader clinical context, considering the underlying causes and potential coexisting factors influencing liver health.
Various techniques, such as shear wave elastography (SWE) and strain elastography, have demonstrated their efficacy in assessment of the mechanical properties of liver tissues. Various SWE techniques evaluate the speed of shear waves produced through mechanically induced stress. US SWE methods encompass vibration-controlled transient elastography (VCTE) and techniques based on acoustic radiation force impulse (ARFI). In VCTE, shear waves result from vibration controlled at the body surface, while in ARFI-based techniques, the waves stem from the push-pulse of a focused US beam. ARFI-based techniques comprise point SWE (pSWE), assessing stiffness in a specific and constant region, and two-dimensional SWE (2D-SWE), measuring stiffness across a broader area, accompanied by a color-coded parametric map of stiffness. The results of US SWE techniques are typically presented in meters per second (m/s), representing shear wave velocity. Alternatively, they can be converted to Young's modulus in kilopascals (kPa), although this conversion relies on assumptions that may not always be accurate[9].
Regular monitoring of liver stiffness can aid in assessing disease progression and the effectiveness of interventions in managing these conditions. It is crucial to interpret liver stiffness values in the context of the patient’s clinical history, including the underlying cause of cholestasis or congestion. The ongoing evolution of elastography suggests a promising future in liver tissue assessment. Advances in technology and research may lead to further refinements, increased standardization, and expanded applications, solidifying elastography as a cornerstone in liver health diagnostics. Elastography has transcended its initial role in fibrosis assessment, emerging as a powerful tool for comprehensive liver tissue evaluation. From steatosis to inflammation and portal hypertension, the diverse applications of elastography offer a nuanced understanding of liver health. As technology and standardization efforts progress, elastography is poised to play an increasingly central role in noninvasive liver assessments, shaping the future of liver disease diagnosis and management.
The acronym advanced CLD (ACLD) is employed for individuals in the advanced stages of CLD and serves as an alternative to the term "cirrhosis," which is based on histology[10,11]. This designation is intended to encompass a wide range of patients, including those with significant liver fibrosis (bridging fibrosis) as observed in histology and those with compensated cirrhosis[12].
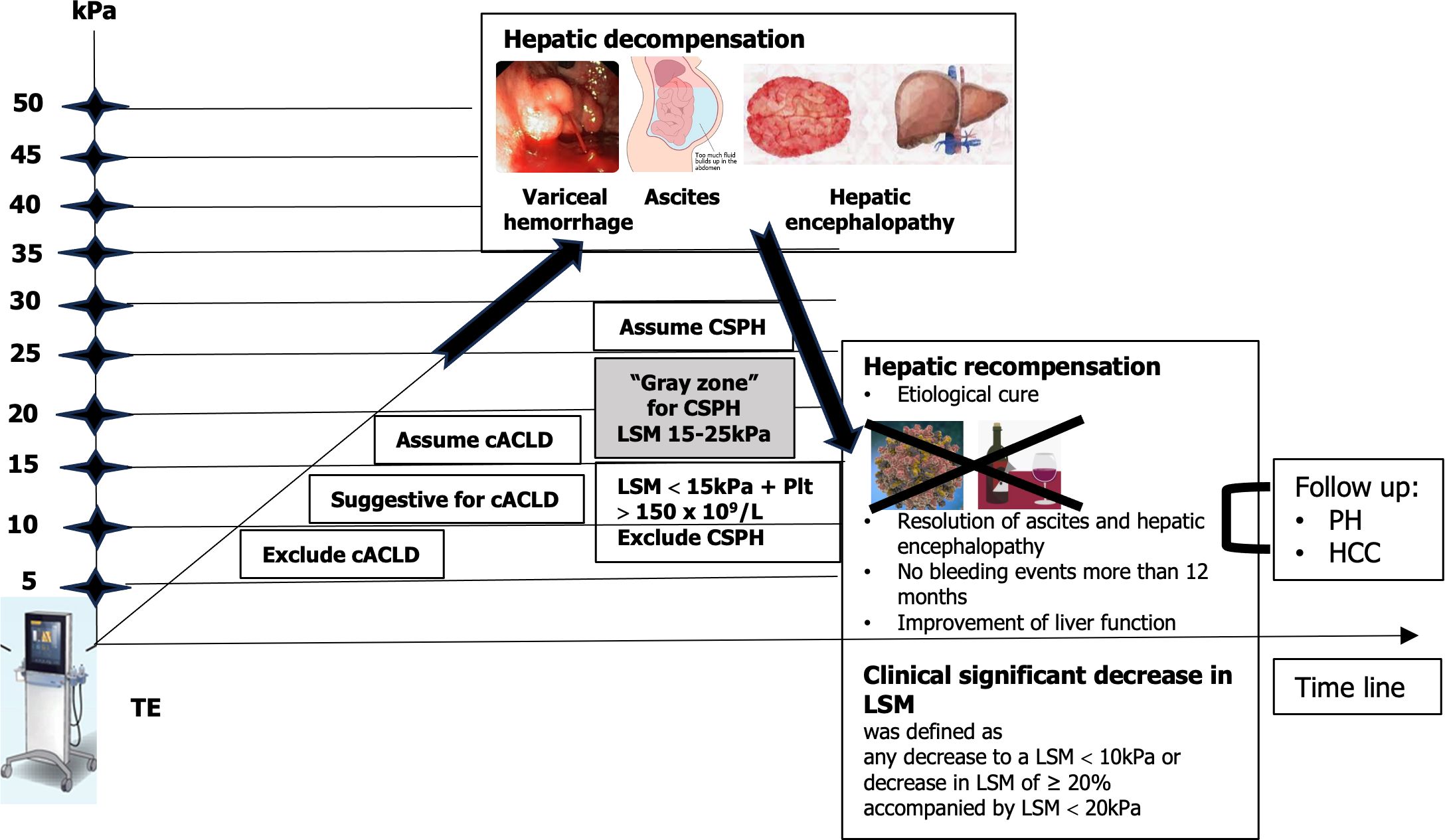
Many studies and meta-analyses proposed different cutoff values for liver stiffness evaluation with VCTE and in connection with different etiologies. In the Baveno VI and Baveno VII consensus[13] “rule of 5” was accepted. This is a very simple modality of stiffness value classification where < 5 kPa means normal liver, less than 10 kPa excludes cACLD, more than 15 kPa assumes cACLD, and more than 25 kPa assumes clinically significant portal hypertension (CSPH). This rule in daily practice can be used for a lot of purposes, like assessment of fibrosis and determining cACLD or CSPH (if liver stiffness is more than 25 kPa, the upper endoscopy can be avoided). Using the VCTE system in a patient and starting with the controlled attenuation parameter, we can stratify severity of steatosis and significant fibrosis can be determined in a very short time. It is important to note that while VCTE provides valuable information about liver stiffness, the interpretation should always be performed in conjunction with other clinical assessments, including medical history, laboratory tests, and potentially additional imaging studies and excluding confounding factors (including fasting, elevated aminotransferases, obstructive cholestasis, or right heart failure). As a prognostic tool, adopting the rule of 5 with cutoff values of liver stiffness measurement (LSM) using VCTE (10-15-20-25 kPa) is suggested. This approach enables a rapid estimation of the risk of decompensation and liver-related deaths, irrespective of the etiology of ACLD (Figure 2).
ARFI methods (pSWE and 2D-SWE) are implemented in a US system and can be used for standard US evaluation, Doppler examination, fatty quantification, stiffness measurement, and lesion discovery (focal liver lesion). Immediately, a CEUS examination can be performed. Then finally, this evaluation a MPUS method.
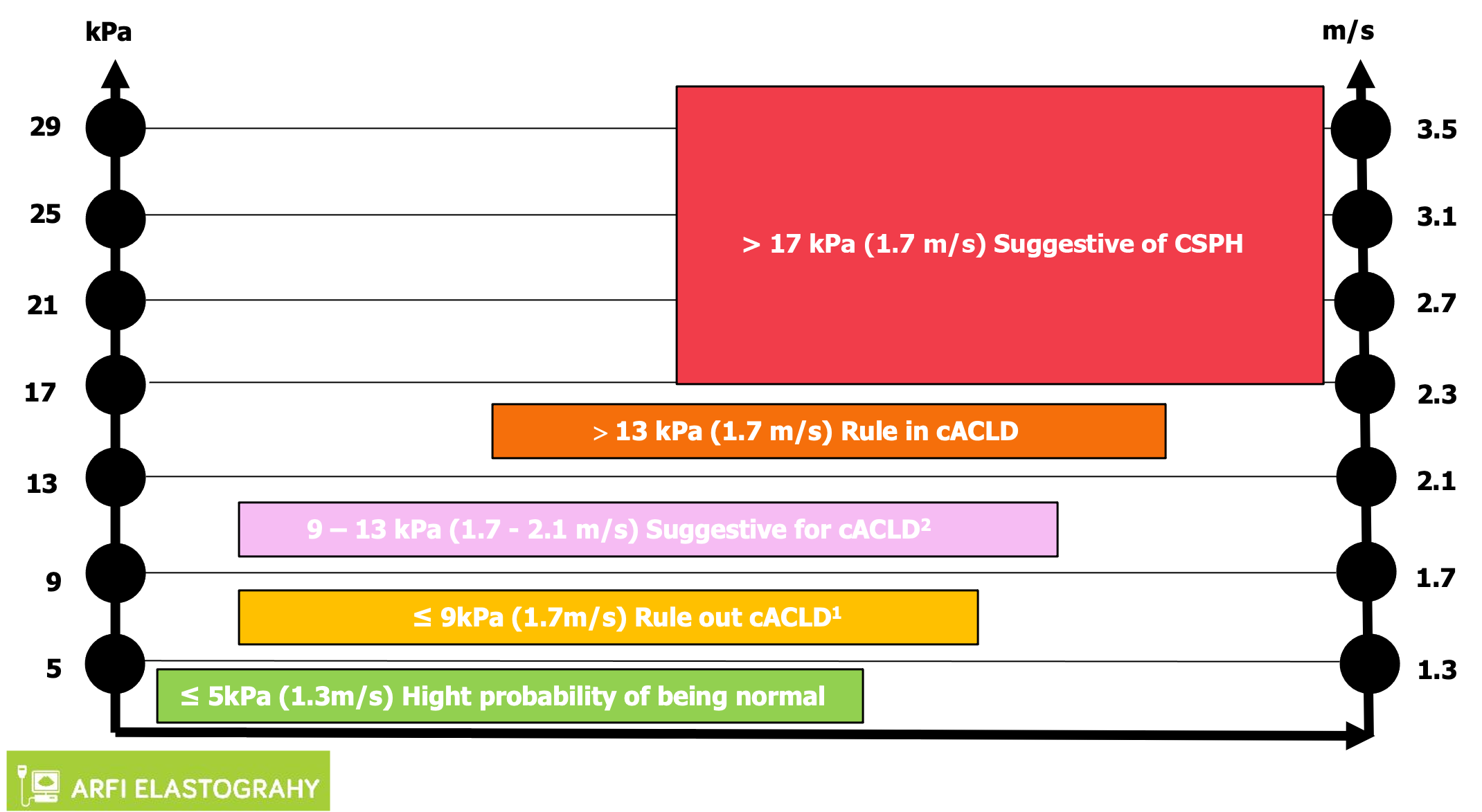
For many years, every company proposed their own cutoff values. Then in practice it was quite difficult to use these values. In 2020 a proposed algorithm, the “Rule of 4” for interpretation of liver stiffness (5-9-13-17 kPa), was presented[14]. In this system, it is quite easy to use the cutoffs for ARFI methods. If the values are < 5 kPa, the liver is normal, and below 9 kPa rules out cACLD. Values between 9-13 kPa are suggestive for cACLD and more than 13 kPa suggests the presence of cACLD. Values > 17 kPa are suggestive for CSPH (Figure 3). Concerning the practical value of SWE methods for liver stiffness evaluation, many published papers show the good results of these methods. There are meta-analyses and prospective studies (with most using liver biopsy as the gold standard). All these studies show that the area under the receiver operating characteristic curve of the methods increases with the severity of fibrosis, with more than 90% for liver cirrhosis[15-18].
Conventionally, cirrhosis progression was seen as a one-way street, transitioning inevitably from a compensated to a decompensated stage[19]. Yet, a growing body of evidence suggests that effective treatment or the elimination of the underlying liver disease etiology not only decelerates disease advancement but can even result in disease regression. The outlook is more optimistic than we once thought! The evolution in how we perceive things led to the development of the idea of hepatic recompensation[13]. This involves a significant improvement in hepatic function, along with a reduction in functional and structural factors like hepatic inflammation, fibrosis, and portal hypertension, all stemming from the successful treatment of the underlying cause. It emphasizes the encouraging potential for positive changes in liver health.
Numerous studies have investigated the significance of LSM in predicting liver-related events in individuals with liver diseases. However, a majority of these studies rely on one-time assessments. Precision in determining the long-term risk of liver complications based on a single LSM remains challenging. This is due to the fact that patients may encounter various situations over time, such as alterations in alcohol consumption, the emergence of metabolic disturbances, resolution of the underlying etiologic factor, or the introduction of new contributing factors, all of which can impact their prognosis. Repeated LSM offer an enhanced understanding of the liver disease's natural progression, potentially enabling personalized treatment decisions when integrated into clinical decision-making. However, certain aspects still require further exploration. Determining the optimal frequency of LSMs and the intervals between them must be established and proven to be cost-effective. Changes in LSM over time can be regarded as a dynamic prognostic biomarker. Repeated LSM holds the potential to refine predictions and individualize treatment strategies in clinical practice.
Prognostic biomarkers quantify the likelihood of clinical events, disease recurrence, or disease progression. As transitioning from a compensated to decompensated state is the single most important factor affecting survival in patients with cirrhosis, prediction of decompensation is a major prognostic target[20]. An LSM by transient elastography (TE) is the best validated prognostic marker for determining liver-related morbidity and mortality in patients with compensated liver disease. A study of 3028 patients with mixed etiologies found a cumulative incidence of decompensation of 3.7% after 5 years for patients with TE values < 15 kPa, increasing to 19% for patients with baseline TE values ≥ 25 kPa[21]. Other elastography techniques such as pSWE, 2D-SWE, and magnetic resonance elastography also exhibit comparable accuracy as prognostic markers of decompensation and mortality, but variation in published cutoffs and heterogeneity attributable to equipment from different manufacturers limit their generalizability. It is important to switch off assessment of fibrosis to evaluation of clinically important ACLD.
The assessment of liver tissue damage has witnessed a transformative shift from invasive to noninvasive methods, providing safer alternatives for patients. The continuous refinement of noninvasive diagnostic methods, particularly the MPUS approach, signifies a crucial stride in managing CLDs. As this technology becomes more accessible and its applications expand, it promises to reshape clinical practices, offering a holistic and efficient means of diagnosing, staging, and monitoring liver diseases on a global scale. Addressing current challenges and embracing emerging technologies will pave the way for more effective management and personalized treatment strategies for patients with liver diseases.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Moldova
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kumar R, India S-Editor: Qu XL L-Editor: A P-Editor: Chen YX
| 1. | Devarbhavi H, Asrani SK, Arab JP, Nartey YA, Pose E, Kamath PS. Global burden of liver disease: 2023 update. J Hepatol. 2023;79:516-537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 852] [Reference Citation Analysis (4)] |
| 2. | Mitten EK, Rutherford A. How Hepatologists Use Liver Biopsy in the Evaluation of Liver Disease? Surg Pathol Clin. 2023;16:443-456. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (1)] |
| 3. | Parola M, Pinzani M. Liver fibrosis: Pathophysiology, pathogenetic targets and clinical issues. Mol Aspects Med. 2019;65:37-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 286] [Cited by in RCA: 785] [Article Influence: 112.1] [Reference Citation Analysis (0)] |
| 4. | Davison BA, Harrison SA, Cotter G, Alkhouri N, Sanyal A, Edwards C, Colca JR, Iwashita J, Koch GG, Dittrich HC. Suboptimal reliability of liver biopsy evaluation has implications for randomized clinical trials. J Hepatol. 2020;73:1322-1332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 296] [Article Influence: 59.2] [Reference Citation Analysis (0)] |
| 5. | Bedossa P, Carrat F. Liver biopsy: the best, not the gold standard. J Hepatol. 2009;50:1-3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 249] [Article Influence: 15.6] [Reference Citation Analysis (1)] |
| 6. | Sporea I, Badea R, Martie A, Dumitru E, Ioaniţescu S, Şirli R, Socaciu M, Popescu A, Dănilă M, Voiculescu M. Contrast Enhanced Ultrasound for the evaluation of focal liver lesions in daily practice. A multicentre study. Med Ultrason. 2012;14:95-100. [PubMed] |
| 7. | Sporea I, Lupușoru R, Șirli R. Ultrasound Based Elastography Techniques for the Evaluation of Nonalcoholic Liver Disease. IntechOpen. 2022;. [DOI] [Full Text] |
| 8. | Sporea I, Gilja OH, Bota S, Şirli R, Popescu A. Liver elastography - an update. Med Ultrason. 2013;15:304-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 39] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 9. | Dietrich CF, Bamber J, Berzigotti A, Bota S, Cantisani V, Castera L, Cosgrove D, Ferraioli G, Friedrich-Rust M, Gilja OH, Goertz RS, Karlas T, de Knegt R, de Ledinghen V, Piscaglia F, Procopet B, Saftoiu A, Sidhu PS, Sporea I, Thiele M. EFSUMB Guidelines and Recommendations on the Clinical Use of Liver Ultrasound Elastography, Update 2017 (Long Version). Ultraschall Med. 2017;38:e16-e47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 601] [Cited by in RCA: 584] [Article Influence: 73.0] [Reference Citation Analysis (0)] |
| 10. | Berzigotti A. Advances and challenges in cirrhosis and portal hypertension. BMC Med. 2017;15:200. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 68] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 11. | Tsochatzis EA, Bosch J, Burroughs AK. Liver cirrhosis. Lancet. 2014;383:1749-1761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1139] [Cited by in RCA: 1315] [Article Influence: 119.5] [Reference Citation Analysis (0)] |
| 12. | de Franchis R; Baveno VI Faculty. Expanding consensus in portal hypertension: Report of the Baveno VI Consensus Workshop: Stratifying risk and individualizing care for portal hypertension. J Hepatol. 2015;63:743-752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2011] [Cited by in RCA: 2294] [Article Influence: 229.4] [Reference Citation Analysis (3)] |
| 13. | de Franchis R, Bosch J, Garcia-Tsao G, Reiberger T, Ripoll C; Baveno VII Faculty. Baveno VII - Renewing consensus in portal hypertension. J Hepatol. 2022;76:959-974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1537] [Cited by in RCA: 1506] [Article Influence: 502.0] [Reference Citation Analysis (2)] |
| 14. | Barr RG, Wilson SR, Rubens D, Garcia-Tsao G, Ferraioli G. Update to the Society of Radiologists in Ultrasound Liver Elastography Consensus Statement. Radiology. 2020;296:263-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 319] [Cited by in RCA: 276] [Article Influence: 55.2] [Reference Citation Analysis (1)] |
| 15. | Talwalkar JA, Kurtz DM, Schoenleber SJ, West CP, Montori VM. Ultrasound-based transient elastography for the detection of hepatic fibrosis: systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2007;5:1214-1220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 357] [Cited by in RCA: 364] [Article Influence: 20.2] [Reference Citation Analysis (1)] |
| 16. | Friedrich-Rust M, Ong MF, Martens S, Sarrazin C, Bojunga J, Zeuzem S, Herrmann E. Performance of transient elastography for the staging of liver fibrosis: a meta-analysis. Gastroenterology. 2008;134:960-974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1046] [Cited by in RCA: 1077] [Article Influence: 63.4] [Reference Citation Analysis (1)] |
| 17. | Tsochatzis EA, Gurusamy KS, Ntaoula S, Cholongitas E, Davidson BR, Burroughs AK. Elastography for the diagnosis of severity of fibrosis in chronic liver disease: a meta-analysis of diagnostic accuracy. J Hepatol. 2011;54:650-659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 490] [Cited by in RCA: 524] [Article Influence: 37.4] [Reference Citation Analysis (0)] |
| 18. | Bota S, Herkner H, Sporea I, Salzl P, Sirli R, Neghina AM, Peck-Radosavljevic M. Meta-analysis: ARFI elastography versus transient elastography for the evaluation of liver fibrosis. Liver Int. 2013;33:1138-1147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 351] [Cited by in RCA: 328] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 19. | D'Amico G, Morabito A, D'Amico M, Pasta L, Malizia G, Rebora P, Valsecchi MG. Clinical states of cirrhosis and competing risks. J Hepatol. 2018;68:563-576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 370] [Article Influence: 52.9] [Reference Citation Analysis (1)] |
| 20. | D'Amico G, Garcia-Tsao G, Pagliaro L. Natural history and prognostic indicators of survival in cirrhosis: a systematic review of 118 studies. J Hepatol. 2006;44:217-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1892] [Cited by in RCA: 2134] [Article Influence: 112.3] [Reference Citation Analysis (3)] |
| 21. | Shearer JE, Jones R, Parker R, Ferguson J, Rowe IA. The Natural History of Advanced Chronic Liver Disease Defined by Transient Elastography. Clin Gastroenterol Hepatol. 2023;21:694-703.e8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 17] [Article Influence: 8.5] [Reference Citation Analysis (0)] |