Published online Mar 21, 2024. doi: 10.3748/wjg.v30.i11.1621
Peer-review started: December 23, 2023
First decision: January 4, 2024
Revised: January 18, 2024
Accepted: March 4, 2024
Article in press: March 4, 2024
Published online: March 21, 2024
Processing time: 88 Days and 15.2 Hours
Neoadjuvant therapy is an essential modality for reducing the clinical stage of esophageal cancer; however, the superiority of neoadjuvant chemotherapy (nCT) or neoadjuvant chemoradiotherapy (nCRT) is unclear. Therefore, a discussion of these two modalities is necessary.
To investigate the benefits and complications of neoadjuvant modalities.
To address this concern, predefined criteria were established using the PICO protocol. Two independent authors performed comprehensive searches using predetermined keywords. Statistical analyses were performed to identify significant differences between groups. Potential publication bias was visualized using funnel plots. The quality of the data was evaluated using the Risk of Bias Tool 2 (RoB2) and the GRADE approach.
Ten articles, including 1928 patients, were included for the analysis. Significant difference was detected in pathological complete response (pCR) [P < 0.001; odds ratio (OR): 0.27; 95%CI: 0.16-0.46], 30-d mortality (P = 0.015; OR: 0.4; 95%CI: 0.22-0.71) favoring the nCRT, and renal failure (P = 0.039; OR: 1.04; 95%CI: 0.66-1.64) favoring the nCT. No significant differences were observed in terms of survival, local or distal recurrence, or other clinical or surgical complications. The result of RoB2 was moderate, and that of the GRADE approach was low or very low in almost all cases.
Although nCRT may have a higher pCR rate, it does not translate to greater long-term survival. Moreover, nCRT is associated with higher 30-d mortality, although the specific cause for postoperative complications could not be identified. In the case of nCT, toxic side effects are suspected, which can reduce the quality of life. Given the quality of available studies, further randomized trials are required.
Core Tip: Neoadjuvant chemoradiation increases pathological complete response and 30-d mortality in patients with esophageal adenocarcinoma; however, it has no effect on long-term survival. It may be associated with side effects that can reduce the quality of life.
- Citation: Csontos A, Fazekas A, Szakó L, Farkas N, Papp C, Ferenczi S, Bellyei S, Hegyi P, Papp A. Effects of neoadjuvant chemotherapy vs chemoradiotherapy in the treatment of esophageal adenocarcinoma: A systematic review and meta-analysis. World J Gastroenterol 2024; 30(11): 1621-1635
- URL: https://www.wjgnet.com/1007-9327/full/v30/i11/1621.htm
- DOI: https://dx.doi.org/10.3748/wjg.v30.i11.1621
Esophageal cancer (EC) is the eighth most prevalent cancer, with more than 500000 cases worldwide, and it is the sixth leading cause of tumor mortality. Squamous cell carcinoma (SCC) is still the leading subtype in the Asian EC Belt; however, in Western countries, such as North America, Oceania, and Western and Northern Europe, including Hungary, the incidence rate of adenocarcinoma (AC) has been increasing, surpassing that of SCC[1,2].
In the early stages, surgery can lead to full recovery; however, an advanced tumor stage at initial diagnosis can result in high morbidity and mortality rates[3]. Esophagectomy with radical lymphadenectomy is one of the most invasive gastrointestinal procedures. To improve treatment results, a multidisciplinary approach is important, including the application of the enhanced recovery after surgery protocol[4,5], the minimally invasive approach of esophagectomy[6], and neoadjuvant oncological therapy, which can decrease mortality by 25%-35% compared with that of surgery alone[7-9].
The superiority of neoadjuvant therapy has been proven in several meta-analyses[7-9]. Neoadjuvant chemotherapy (nCT) or preoperative neoadjuvant chemoradiotherapy (nCRT) can also improve oncologic endpoints[8-15], increase overall and progression-free surveillance, and pathological complete response (pCR); however, it may also be associated with numerous clinical or surgical side effects and impaired quality of life. Therefore, the cost-benefit balance of these modalities is still unclear, especially in cases of AC of the esophagus and esophagogastric junction (GEJ).
Previous meta-analyses have numerous limitations, including patients with SCC and AC as a homogenous population. Therefore, the results cannot be clearly applied to either subtype.
We performed a comprehensive, up-to-date investigation to determine whether nCT or nCRT yields more favorable results in the surgical treatment of AC of the esophagus and esophageal junction.
The objectives and methodologies of this meta-analysis were predefined in a protocol registered with PROSPERO[16]. The registration was accepted on November 01, 2023, under the number CRD42023478615.
To define the scope of this meta-analysis, we used the PICO protocol, focusing on patients with esophageal or cardiac AC, who received neoadjuvant therapy before surgery. Intervention assessed was preoperative nCT, which was compared to nCRT. We investigated the following outcomes: Survival, remission rate, mortality, short- and long-term clinical and surgical complications, and quality of life. First, we planned to investigate only randomized controlled trials (RCTs) to minimize the risk of bias; however, to achieve an adequate sample size and robust conclusions, propensity score matched and high-quality cohort studies were also included. Studies that did not strictly involve patients with AC were excluded.
We conducted a comprehensive search on September 15, 2023, using PubMed, Embase, Cochrane, Web of Science, and Scopus databases. We used previously defined search terms, including “neoadjuvant,” “chemotherapy,” “chemoradiotherapy,” ”esophageal cancer,” “esophagectomy,” and other random keywords, and their variants. The retrieved datasets were imported into the EndNote (ver. x9.3.3; Alfasoft AB, Göteborg, Sweden) library.
Two independent authors conducted the selection process using EndNote software. The Cohen’s kappa coefficients were calculated from these results. Discrepancies were resolved through consensus.
Data were extracted from text, figures, and tables of the included articles by two independent authors, with any discrepancies resolved through mutual agreement. Plot digitizer applications were used to collect data not provided in a numerical format[17]. Excel (Office 365, Microsoft, Redmond, WA, United States) datasheets were used to collect and organize the datasets. Descriptive data collected included study characteristics (author, year, type, and number of elements), patients demographics (age, sex, and performance), tumors (stage, location), and therapy (neoadjuvant regimen, surgical procedure). A meta-analysis was performed on outcomes with at least four homogeneous datasets. Outcomes, ineligible for statistical analysis, were qualitatively described. The outcomes assessed included pCR, surveillance (overall, progression-free, disease-free), mortality (30 or 90 d), tumor remission (local or distant), clinical complications (thromboembolism, respiratory and cardiac complications, renal failure, neutropenia) and surgical complication (anastomotic and chyle leakage, wound infection, bleeding, vocal cord paresis).
A random-effects meta-analysis was performed. Odds ratios (OR) with 95%CI were calculated to measure the effect size. To calculate the OR and pooled odds ratio, data for the total number of patients and those experiencing the event of interest in each group separately (referred as “raw data”) was extracted or calculated from the studies, where it was available. The results are presented as the odds of an event of interest in the experimental group vs the control group. The results were considered statistically significant if the pooled CI did not contain a null value. We also performed a supplementary analysis. Using the WebPlotDigitizer online tool, we digitalized the Kaplan-Meier (KM) curves published in the involved studies. Then, by applying the methodology of Guyot et al[18,19], we estimated the individual patient time-to-event data. Finally, we plotted all the available KM curves in the same figure. Using the estimated raw data, we calculated the hazard ratio (HR) within the studies and the pooled HR. A less than one HR suggests a smaller risk in the experimental group. The HR result was considered significant if it was not included in the confidence interval.
We visualized the findings in forest plots. Where applicable-the study number was large enough and not too heterogeneous-we also reported the prediction intervals (i.e., the expected range of effects of future studies) of the results. Additionally, between-study heterogeneity was described using Higgins and Thompson’s (I2) statistics (Higgins and Thompson[20], in 2002).
Publication bias was assessed by visual inspection of the funnel plots and calculation of the Harbord (modified Egger’s) test P value[21] for the OR effect size. We assumed the presence of a possible small study bias if the P value was < 10%. However, we kept in mind that the test has limited diagnostic assessment (below 10 studies). Potential outlier publications were explored using different influence measures and plots following the recommendations of Harrer et al[22]. All statistical analyses were performed with R (R Core Team 2023, v4.3.0)[23] using the meta (Schwarzer 2023, v6.2.1)[24] package for basic meta-analysis calculations and plots, IPDfromKM for raw data simulations, and the dmetar (v0.0.9000)[25] package for additional influential analysis calculations and plots. To pool the effect size, the pooled OR based on raw data was calculated using the Mantel-Haenszel method[26,27]. The Exact Mantel-Haenszel method (without continuity correction) was used to handle zero-cell counts[28,29]. We used the Hartung-Knapp adjustment[30,31] for the CIs. To estimate the heterogeneity variance measure for the raw data OR calculation, the Paule-Mandel method[32] (recommended by Veroniki et al[33]) was used with the Q profile method for the confidence interval. Prediction interval calculations were based on the t-distribution. In the case of 0 cell counts, individual study OR with 95%CI was calculated by adding 0.5, as continuity correction (it was used only for visualization on forest plot). The pooled HR was calculated using classical inverse-variance meta-analysis of log-transformed HR ratios using the REML heterogeneity variance estimator.
Descriptive analyses were performed by calculating the means, standard deviations, and percentages. The mean estimates from the median and range were calculated as follows[34]:
The Risk of Bias Tool 2 (RoB2) and GRADE approaches were used to assess the quality of the articles and our research.
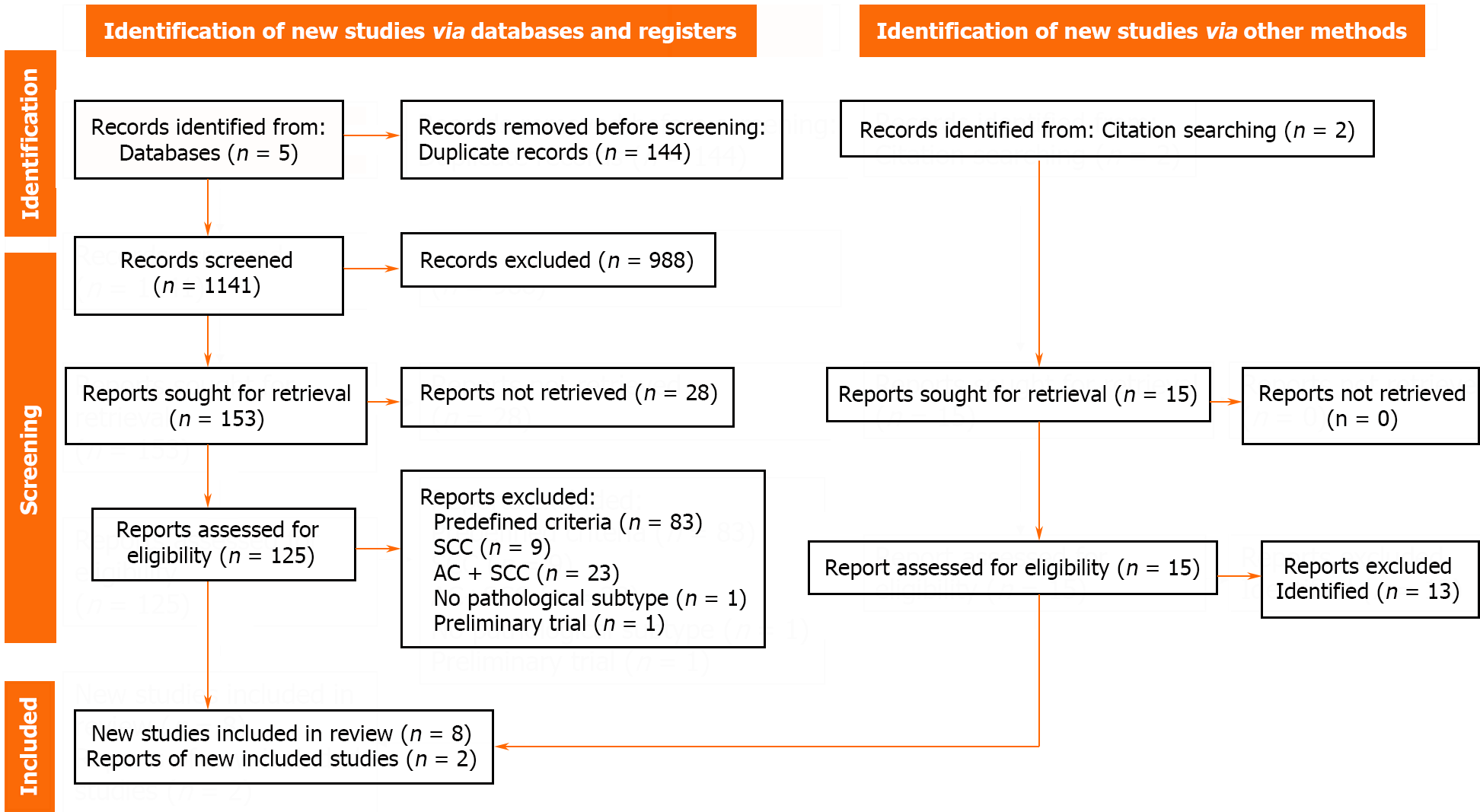
A total of 1285 articles were identified from the five databases. After removing duplicates, 1141 articles were screened, after which 485 and 153 articles were selected based on title and abstract screening, respectively. Subsequently, 125 full-text reports were examined, and eight studies were included in the quantitative synthesis. Cohen’s kappa indicated 99.74% substantial agreement (Cohen’s k: 0.77). Some reports could not be retrieved as they were conference abstracts[28]. Articles were excluded based on predefined criteria (83 articles), including those covering only SCCs (9 articles) or mixed group of ACs and SCCs (23 articles), mentioning no pathological subtype (1 article), and being a preliminary trial (1 article). Two additional articles were included during the screening of previous reviews. Overall, the analysis included ten articles. More information is provided in Figure 1.
Ten articles, published between 2011 and 2018, were included in this meta-analysis. Seven studies were conducted in Europe, two in Australia, and one in the United States[35-44]. Of the 10 studies, two were RCTs[43,44], four were propensity score-matched cohort study[39-42], and four were cohort study based on prospective institutional databases (clinical cancer registry)[35-38]. Six studies were single-center trials[36,39,40,42-44]. The articles collectively included data from 1928 patients, with 956 and 972 patients the nCT and nCRT groups, respectively. All included patients had esophageal ACs. Additional details are provided in Table 1.
| Ref. | Design | Center | Country | Year | Number of patients | nCT | nCRT | AC, % |
| Stahl et al[44], 2017 | RCT, Phase III | 1 | Germany | N/A | 119 | 59 | 60 | 100 |
| Burmeister et al[43], 2011 | RCT, Phase II | 1 | Australia | N/A | 75 | 36 | 39 | 100 |
| Visser et al[42], 2018 | PSM | 1 | Australia | 2000-2017 | 262 | 131 | 131 | 100 |
| Markar et al[41], 2017 | PSM | 10 | United Kingdom | 2001-2012 | 442 | 221 | 221 | 100 |
| Goense et al[40], 2017 | PSM | 1 | Netherlands | 2006-2015 | 172 | 86 | 86 | 100 |
| Favi et al[39], 2017 | PSM | 1 | Germany | 2011-2015 | 80 | 40 | 40 | 100 |
| Anderegg et al[35], 2017 | Cohort, PID | 3 | Netherlands | 2005-2011 | 313 | 137 | 176 | 100 |
| Spicer et al[38], 2016 | Cohort, PID | 3 | United States | 2002-2012 | 214 | 114 | 100 | 100 |
| Luc et al[36], 2015 | Cohort, PID | 1 | France | 2000-2012 | 116 | 61 | 55 | 100 |
| Münch et al[37], 2018 | Cohort, PCCR | 70 | Germany | 1998-2014 | 135 | 71 | 64 | 100 |
The estimated mean age of the patients in both the groups was 60 years. The age range was 12-84 and 19-83 years. The nCT and nCRT groups included 829 (91%) and 857 (94%) male patients, respectively. Based on the available data, 84%, 16% and < 0.1% of the patients had American Society of Anesthesiologists scores of I-II, III, and IV, respectively. The patients had coronary morbidity (18%), diabetes mellitus (16%), pulmonary morbidity, chronic obstructive pulmonary disease (9%), history of malignancy (6%), and history of smoking (42%). More detailed information is summarized in Supplementary Table 1.
Based on the available data, 99% of the tumors were diagnosed in the lower third of the esophagus or the GEJ. Clinical T-stages 1-4 accounted for 1%, 16%, 80%, and 3% of the cases, respectively. Nodal involvement was observed in 367 (61%) and 350 patients (59%) in the nCT and nCRT groups, respectively. Tumor differentiation was good in 2% and 1%, moderate in 36% and 31%, and poor in 57% and 64% of patients in the nCT and nCRT groups, respectively. Margin negative resection (R0) was performed in 696 (81%) and 800 (92%) patients in the nCT and nCRT groups. Pathological T-stage 1-4 accounted for 13%, 15%, 22%, and 47% of the cases, respectively, whereas N-stages 0-3 accounted for 45%, 31%, 14%, and 9% of the cases, respectively. Tumor regression grade (TRG, Mandard) stages 1-4/5 (in the nCT and nCRT groups) accounted for 14% (6%-22%), 17% (7%-26%), 24% (18%-29%), and 42% (62%-22%) of the cases, respectively additional details are provided in Supplementary Table 2.
Neoadjuvant regimens were administered to patients in both groups. The most frequently used neoadjuvant drugs were cisplatin, 5-fluorouracil, and docetaxel. The CROSS protocol was the most commonly used protocol in the chemoradiation group. Additional details are provided in Table 2[7,8,10,45-48].
| Ref. | nCT | nCRT | |
| Chemotherapy | Chemotherapy | Irradiation | |
| Stahl et al[44], 2017 | 15 × weekly CFFa | 15 × weekly CFFa followed by 3 wk course of CRT + 1 cycle CE | 30 Gy in 15 fractions of 2 Gy in 3 wk |
| Burmeister et al[43], 2011 | C (80 mg/m2) + iv 5-FU (1000 mg/m2/d) on days 1 and 21 | CF + RT, 5-FU reduced to 800 mg/m2/d (on day 21) | 35 Gy in 15 fractions in 3 wk (on day 21) |
| Visser et al[42], 2018 | OEO2 | OEO2 + RT | 35 Gy in 15 fractions or 45 Gy in 25 fractions |
| MAGIC | DCF (2 cycles pre-operatively) + RT | 45 Gy in 25 fractions | |
| DCF (2 cycles pre-operatively) | CROSS (since 2015) | 41.4 Gy in 23 fractions of 1.8 Gy in 5 wk | |
| Cisplatin + 5-FU: 92 (70) | Cisplatin + 5-FU: 94 (72) | 35 Gy: 69 (53) | |
| Epirubicin, cisplatin, 5-FU: 30 (23) | Epirubicin, cisplatin, 5-FU: 2 (2) | 41 Gy: 14 (11) | |
| Carboplatin + paclitaxel: 0 (0) | Carboplatin + paclitaxel: 20 (15) | 45 Gy: 40 (31) | |
| Other: 9 (7) | Other: 15 (11) | Other: 8 (6) | |
| Markar et al[41], 2017 | mainly MAGIC, OEO2 or OEO5 regimens[8,10,45] | CROSS regimen[7,46] | 41.4 Gy in 23 fractions of 1.8 Gy in 5 wk |
| Goense et al[40], 2017 | ECX | CROSS regimen[7,46] | 41.4 Gy in 23 fractions of 1.8 Gy in 5 wk |
| Favi et al[39] , 2017 | FLOT[47] | CROSS regimen[7,46] | 41.4 Gy in 23 fractions of 1.8 Gy in 5 wk |
| Anderegg et al[35], 2017 | ECX | CROSS regimen[7,46] | 41.4 Gy in 23 fractions of 1.8 Gy in 5 wk |
| Spicer et al[38], 2016 | Cornell: Platinum or taxane-based doublet, or both | concurrent ChT + RT | 50.4 Gy |
| McGill: DCF (3 cycles)[48] | |||
| Luc et al[36], 2015 | DCF (3 cycles pre- and postoperatively) | continuous iv 5-FU 750 mg/m2/d on days 1–5 by, C 20 mg/m2 on day 1 | 45 Gy for 5 d per week at 1.8 Gy/d (started on day 28 along with the second CT cycle) |
| Münch et al[37], 2018 | N/A | N/A | N/A |
Based on the available data, Ivor-Lewis (transthoracic), Orringer (transhiatal), McKeown (thoraco-abdomino-cervical) esophagectomies, and total gastrectomy were performed in 67%, 23%, 5%, and 4% of the patients, respectively. Minimally invasive or hybrid surgery techniques were performed in 27% and 51% of the patients, respectively, and open surgery was performed in only 23% of the patients. Two-field lymphadenectomy was the standard procedure in 74% of the patients, whereas three-field lymphadenectomy was performed in only 5% of the patients. Additional details are provided in Supplementary Table 3.
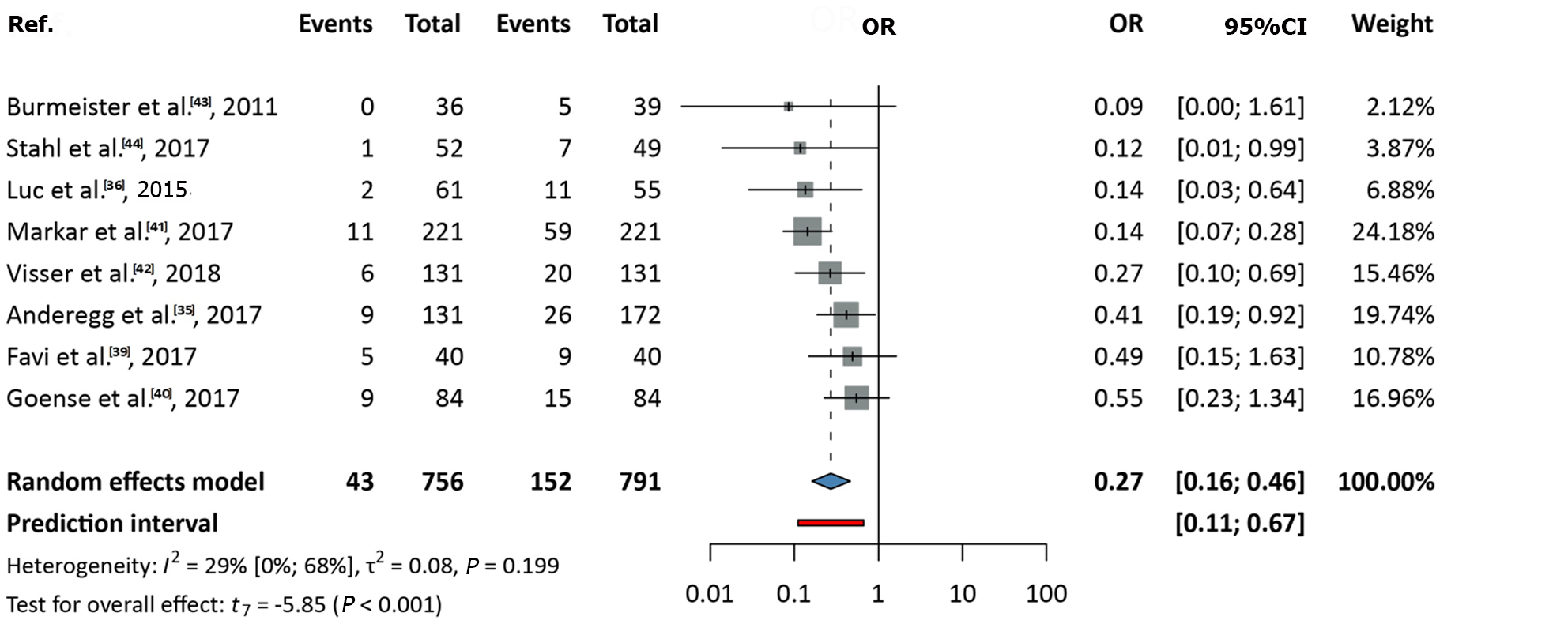
Data from eight studies, covering a total of 1547 patients, were analyzed[35,36,39-44]. The OR (pooled effect size) was 0.27 (95%CI: 0.16-0.46). A significant difference was observed, favoring nCRT over nCT (P < 0.001). Between-study heterogeneity, expressed as the I2 value, was 0.29 (95%CI: 0-0.68; Figure 2).
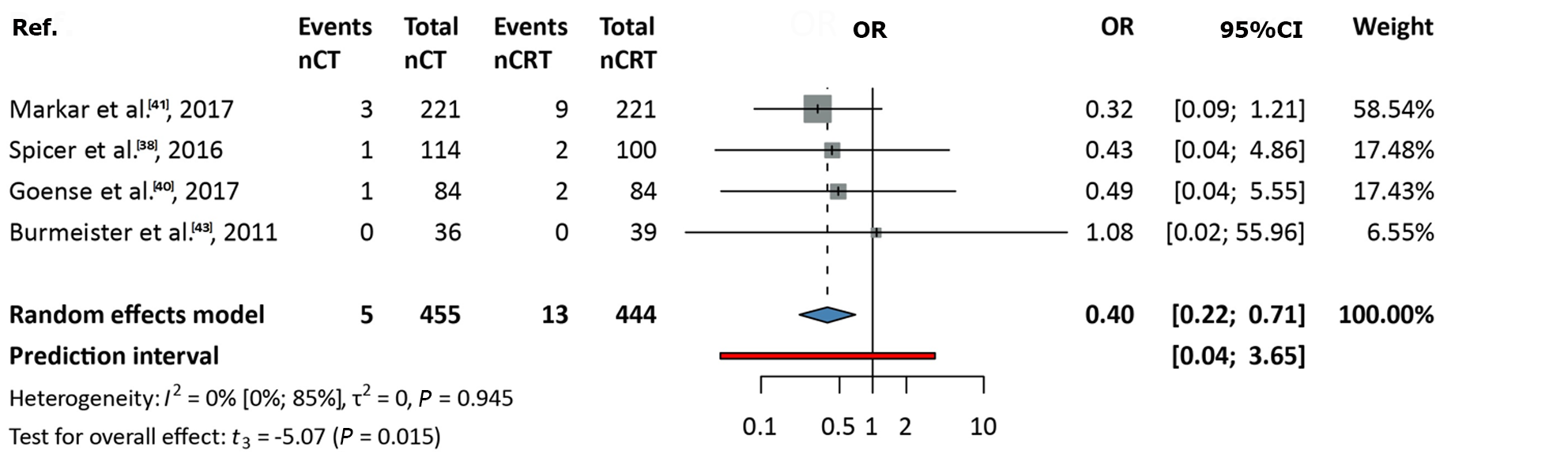
Data from four studies, including 899 patients, were analyzed[38,40,41,43]. The OR was 0.4 (95%CI: 0.22-0.71). A significant difference was observed, favoring nCRT over nCT (P = 0.015), with no between-study heterogeneity (I2 value: 0; 95%CI: 0-0.85; Figure 3).
Data from four studies, encompassing 108 patients, were analyzed[38,40-42]. The OR was 0.71 (95%CI: 0.28-1.84). No significant difference was observed between nCRT and nCT (P = 0.34), with no between-study heterogeneity (I2 value: 0; 95%CI: 0-0.85; Supplementary Figure 1).
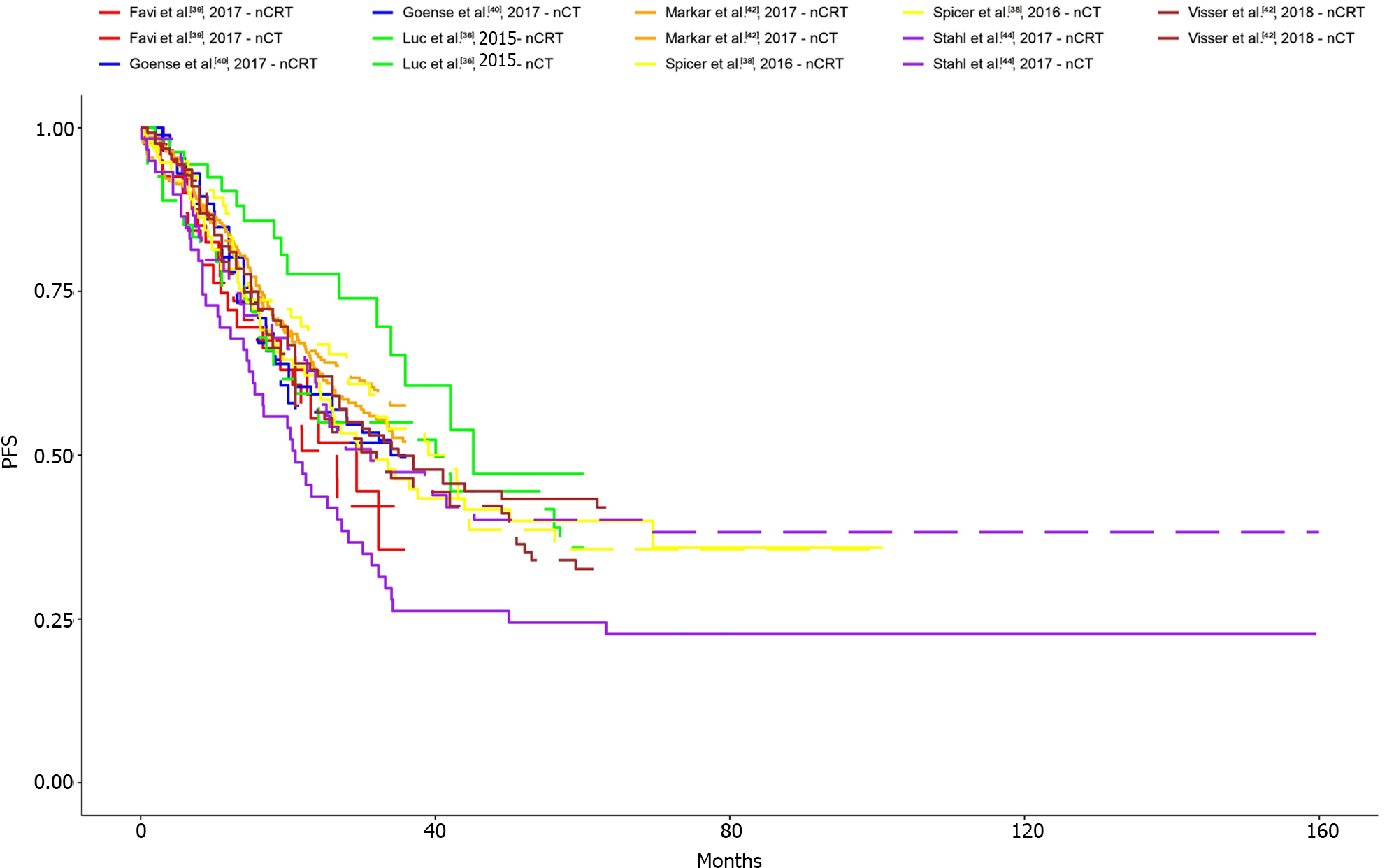
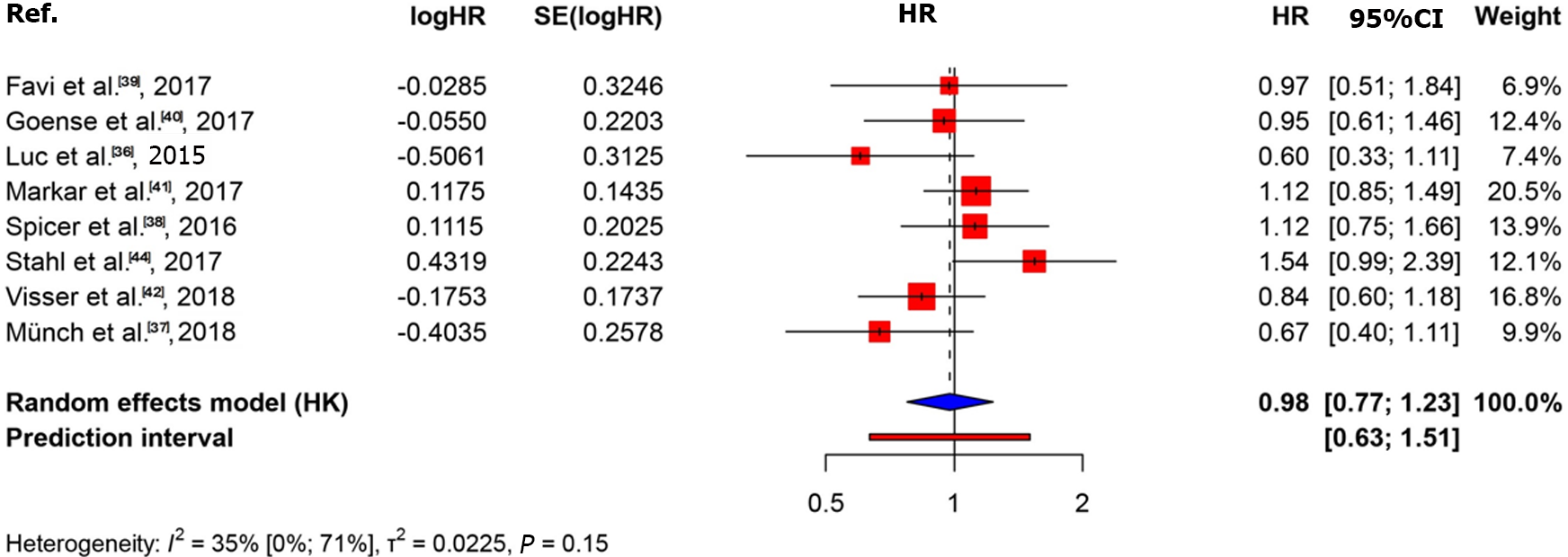
The KM curves and logHR analysis conducted for eight studies encompassing 1540 patients did not reveal significant differences between the two groups in terms of overall survival (P = 0.82)[36-42,44]. The OR was 0.98 (95%CI: 0.77-1.23) and between-study heterogeneity was 0.35 (95%CI: 0-0.71; Figures 4 and 5).
Considering the 12-month overall survival (OS), nine studies including 1588 patients were selected for analysis[36-44]. The OR was 1.08 (95%CI: 0.8-1.46). No significant difference was observed between the two groups (P = 0.551). The between-study heterogeneity was 0.05 (95%CI, 0-0.67; Supplementary Figure 2). The log HR analysis revealed no significant differences between the groups (Supplementary Figure 3).
For the 24-month OS, the OR was 1.03 (95%CI: 0.73-1.45)[36-44]. No significant difference was observed between the two groups (P = 0.858). The between-study heterogeneity was 0.42 (95%CI: 0-0.73; Supplementary Figure 4). The log HR analysis revealed no significant differences between the groups (Supplementary Figure 5).
Considering the 36-month OS, the OR was 0.93 (95%CI: 0.54-1.6)[36-44]. No significant difference was observed between the two groups (P = 0.754). The between-study heterogeneity was 0.73 (95%CI: 0.47-0.86; Supplementary Figure 6). The logHR analysis revealed no significant differences between the groups (Supplementary Figure 7).
Considering the 48-month OS, seven studies including 1066 patients were selected for analysis[36-38,40,42-44]. The OR was 0.67 (95%CI: 0.27-0.85). No significant difference was observed between the two groups (P = 0.616). The between-study heterogeneity was 0.67 (95%CI: 0.27-0.85; Supplementary Figure 8). The log HR analysis revealed no significant differences between the groups (Supplementary Figure 9).
Considering the 60-month OS, the OR was 1.15 (95%CI: 0.56-2.35)[36-38,40,42-44]. No significant difference was observed between the two groups (P = 0.658). The between-study heterogeneity was 0.67 (95%CI: 0.27-0.85; Supplementary Figure 10).
The KM curves and logHR analysis conducted for two studies including 578 patients did not reveal significant differences in overall survival between the two groups (P = 0.85)[36,38,42]. The OR was 1.04 (95%CI: 0.5-2.16). The between-study heterogeneity was 0.49 (95%CI: 0-0.85; Supplementary Figures 11 and 12).
Considering the 12-month Disease-free survival (DFS), the OR was 0.93 (95%CI: 0.44-1.97)[36,38,42]. No significant difference was observed between the two groups (P = 0.702). The between-study heterogeneity was 0.07 (95%CI: 0-0.9; Supplementary Figure 13). The logHR analysis revealed no significant differences between the groups (Supplementary Figure 14).
Considering the 24-month DFS, the OR was 0.95 (95%CI: 0.49-1.86)[36,38,42]. No significant difference was observed between the two groups (P = 0.789). The between-study heterogeneity was 0 (95%CI: 0-0.9; Supplementary Figure 15). The logHR analysis revealed no significant differences between the groups (Supplementary Figure 16).
Considering the 36-month DFS, the OR was 0.96 (95%CI: 0.4-2.28)[36,38,42]. No significant difference was observed between the two groups (P = 0.846). The between-study heterogeneity was 0.05 (95%CI: 0-0.9) was calculated (Supplementary Figure 17). The log HR analysis revealed no significant differences between the groups (Supplementary Figure 18).
Considering the 48-month DFS, the OR was 1.04 (95%CI: 0.31-3.51)[36,38,42]. No significant difference was observed between the two groups (P = 0.904). The between-study heterogeneity was 0.32 (95%CI: 0-0.93; Supplementary Figure 19). The log HR analysis revealed no significant differences between the groups (Supplementary Figure 20).
Considering the 60-month DFS, the OR was 1.04 (95%CI: 0.3-3.64)[36,38,42]. No significant difference was observed between the two groups (P = 0.913). The between-study heterogeneity was 0.32 (95%CI: 0-0.93) between the groups (Supplementary Figure 21).
For the 12-month progression-free survival (PFS), three studies including 340 patients were selected for analysis[40,43,44]. The OR was 0.73 (95%CI: 0.47-1.16). No statistically significant was observed difference between the two groups (P = 0.101). The between-study heterogeneity was 0 (95%CI: 0-0.9; Supplementary Figure 22).
Considering the 24-month PFS, the OR was 0.78 (95%CI: 0.1-6.18)[40,43,44]. No significant difference was observed between the two groups (P = 0.652). The between-study heterogeneity was 0.72 (95%CI: 0.04-0.92; Supplementary Figure 23).
Considering the 36-month PFS, the OR was 1.04 (95%CI: 0.1-11.05)[40,43,44]. No significant difference was observed between the two groups (P = 0.946). The between-study heterogeneity was 0.81 (95%CI: 0.39-0.94; Supplementary Figure 24).
Data from six studies including 1037 patients were analyzed, revealing locoregional recurrence in 12% of the patients[36,37,41-44]. The OR was 0.98 (95%CI: 0.35-2.77). No significant difference was observed between the two groups (P = 0.966). The between-study heterogeneity was 0.76 (95%CI: 0.47-0.89; Supplementary Figure 25).
Data from five studies including 910 patients were analyzed, revealing distal metastasis recurrence in 39% of the patients[37,41-44]. The OR was 1.12 (95%CI: 0.76-1.64). No significant difference was observed between the two groups (P = 0.462). The between-study heterogeneity was 0 (95%CI: 0-0.79; Supplementary Figure 26).
Data from four studies including 818 patients were analyzed for the occurrence of thromboembolism events[35,40,42,43]. The OR was 1.93 (95%CI: 0.1-38.65). No significant difference was observed between the two groups (P = 0.535). The between-study heterogeneity was 0.72 (95%CI: 0.22-0.90; Supplementary Figure 27).
Data from seven studies including 1580 patients were analyzed for the occurrence of cardiac complications[35,36,38,40-43]. The OR was 0.8 (95%CI: 0.42-1.52). No significant difference between the two groups (P = 0.425). The between-study heterogeneity was 0.46 (95%CI: 0-0.77; Supplementary Figure 28).
Data from seven studies including 1580 patients were analyzed for the occurrence of respiratory complications[35,36,38,40-43]. The OR was 1.04 (95%CI: 0.66-1.64). No significant difference was observed between the two groups (P = 0.835). The between-study heterogeneity was 0.59 (95%CI: 0.04-0.82; Supplementary Figure 29).
Data from three studies including 650 patients were analyzed for the occurrence of renal failure[35,42,43]. The OR was 2.43 (95%CI: 1.12-5.28). A statistically significant difference was observed, favoring nCT over nCRT (P = 0.039). The between-study heterogeneity was 0 (95%CI: 0-0.9; Supplementary Figure 30).
Data from three studies including 560 patients were analyzed for the occurrence of neutropenia[35,40,43]. The OR was 0.97 (95%CI: 0.09-10.29). No significant difference was observed between the two groups (P = 0.964). The between-study heterogeneity was 0.47 (95%CI: 0-0.84; Supplementary Figure 31).
Data from seven studies including 1580 patients were analyzed for the occurrence of anastomotic leakage[35,36,38,40-43]. The OR was 0.83 (95%CI: 0.41-1.68). No significant difference was observed between the two groups (P = 0.539). The between-study heterogeneity was 0.75 (95%CI: 0.48-0.88; Supplementary Figure 32).
Data from six studies including 1366 patients were analyzed for the occurrence of chyle leakage[35,36,40-43]. The OR was 0.99 (95%CI: 0.61-1.61). No significant difference was observed between the two groups (P = 0.961). The between-study heterogeneity was 0 (95%CI: 0.48-0.75; Supplementary Figure 33).
Data from five studies including 1022 patients were analyzed for the occurrence of wound infection[35,38,40,42,43]. The OR was 1.04 (95%CI: 0.36-3.02). No significant difference was observed between the two groups (P = 0.930). The between-study heterogeneity was 0.37 (95%CI: 0-0.76; Supplementary Figure 34).
Data from four studies including 849 patients were analyzed for the occurrence of bleeding[35,36,40,42]. The OR was 1.4 (95%CI: 0.425-7.79). No statistically significant difference was observed between the two groups (P = 0.581). The between-study heterogeneity was 0 (95%CI: 0-0.85; Supplementary Figure 35).
Data from three studies including 733 patients were analyzed for the occurrence of vocal cord paresis[35,40,42]. The OR was 1.21 (95%CI: 0.04-41.98). No significant difference was observed between the two groups (P = 0.537). The between-study heterogeneity was 0.5 (95%CI: 0-0.85; Supplementary Figure 36).
Two studies including 485 patients were selected for descriptive analyses[35,40]. Leukopenia occurred in 8% and 12% of the patients in the nCT and nCRT groups, respectively.
Two studies including 485 patients were selected for descriptive analyses[35,40]. Anemia occurred in 1% and 0.4% of the patients in the nCT and nCRT groups, respectively.
Three studies including 560 patients were selected for descriptive analyses[35,40,43]. Nausea or vomiting occurred in 9% and 3% of the patients in the nCT and nCRT groups, respectively.
Two studies including 485 patients were selected for descriptive analyses[35,40]. Diarrhea occurred in 7% in the nCT group, whereas no cases were noted in the nCRT group.
Two studies including 430 patients were selected for descriptive analyses[40,42]. The estimated mean hospital stay was 20 (range: 7-97) d in the nCT group and 18 (range: 7-75) d in the nCRT group.
As we expected, the two RCTs demonstrated a low risk of bias. However, for other included trials, ROB2 indicated some concerns, mainly due to the randomization process (D1). In one trial, concerns were noted regarding the measurement process due to the utilization of a plot digitizer[17]. No instance of high risk of bias was identified across the included studies. Additional information is presented in Table 3.
| Ref. | D1 | D2 | D3 | D4 | D5 | Overall |
| Stahl et al[44], 2017 | + | + | + | + | + | + |
| Burmeister et al[43], 2011 | + | + | + | + | + | + |
| Visser et al[42], 2018 | ! | + | + | + | + | ! |
| Markar et al[41], 2017 | ! | + | + | + | + | ! |
| Goense et al[40], 2017 | ! | + | + | + | + | ! |
| Favi et al[39], 2017 | ! | + | + | + | + | ! |
| Anderegg et al[35], 2017 | ! | + | + | + | + | ! |
| Spicer et al[38], 2016 | ! | + | + | + | + | ! |
| Luc et al[36], 2015 | ! | + | + | + | + | ! |
| Münch et al[37], 2018 | ! | + | + | ! | + | ! |
Employing the GRADE approach, our findings were determined to have low certainty for most outcomes; moderate certainty for 30-d mortality; very low certainty for 12-month OS, 36-month PFS, and the occurrence of thromboembolism events. The use of RoB2 indicated a moderate risk for all outcomes. High heterogeneity was reported for 36-month PFS and the occurrence of thromboembolic events. Imprecision was observed for pCR and 12-month OS. Additionally, a high variation in oncological treatments decreased the evidence quality, whereas a large effect size increased the quality of pCR and 30-d mortality[49] (Supplementary Table 4).
The benefits of neoadjuvant therapy have been previously reported[7-9]. Previous meta-analyses have examined the amplification of nCT and chemoradiotherapy in patients with AC or SCC. In the nCRT group, advantages were observed in terms of 3-year survival with R0 resection; however, the pCR rate had no effect on long-term survival. Perioperative mortality and cardiovascular complications are more common in patients with AC in the nCRT group[50]. A previous network meta-analysis showed that triplet-based chemotherapy increases overall survival and DFS in cases of AC of the stomach or GEJ[51].
pCR is defined as the lack of tumor in the resected specimen or lymph nodes (pT0 pN0 cM0)[15,36]. The 5-year survival rate is presumably 88% in patients with pCR compared to 39% in those without pCR[15,52]. According to a recent investigation comparing the long-term survival of the total population and patients with TRG grade 1-2 who underwent nCT or chemoradiotherapy before surgery revealed that tumor regression after neoadjuvant treatment is significantly associated with long-term survival, regardless of the treatment regimen[53]. Another retrospective cohort study revealed improved OS and DFS in patients who achieved pCR following nCT compared to those who achieved a lower rate of pCR following nCRT. The authors found a significant association between TRG and survival in both the groups. Additionally, patients who achieved pCR in the nCRT group did not have as good a survival rate as those in the nCT group, although their proportion was higher in the nCRT group. This finding suggests that esophageal AC should be considered a systemic disease and treated accordingly[53,54]. However, other trials have reported that a larger number of patients who achieved pCR do not have improved overall survival[55]. In this meta-analysis, we found a significantly higher pCR in the nCRT group; however, no differences were found in OS, DFS, or PFS, consistent with the findings of previous meta-analyses[50,55]. Based on this finding, we inferred that there is no association between pCR and OS; therefore, the use of pCR as a prognostic factor should be considered in cases of AC. These findings aligned with those of Gebauer et al’s study[56] reporting that high pCR after CROSS regimen is not clearly associated with longer overall survival[56]. Another study concluded that only clinically complete response without nodal metastasis is associated with long-term survival; therefore, the “watch-and-wait,” strategies should be considered carefully and applied only to patients who have achieved pCR[57]. The utility of pCR as a prognostic indicator of neoadjuvant therapy remains questionable, indicating the need for large number of randomized studies in the future.
Our analysis revealed that none of the investigated groups were superior considering local recurrence, which aligns with the findings of a previous meta-analysis[50]. This indicates that the higher local control provided by radiotherapy does not reduce the incidence of local recurrence. Additionally, we did not detect a significant difference in terms of metastases, which occurred in 39% of the cases compared to 12% of local recurrence cases, suggesting that AC should be treated as a systematic disease, and therefore, the “watch-and-wait” strategies should be considered critically.
Our findings revealed a significantly higher 30-d mortality risk in the chemoradiotherapy group. This can be attributed to complications arising in the postoperative period. However, differences in the outcomes of surgical complications were not noted, consistent with the findings of a previous meta-analysis, in which no difference was reported in anastomotic leakage[50]. Additionally, a previous meta-analysis reported a higher risk of mortality in the postoperative period among patients with AC. Therefore, further investigation into the effects of nCRT on postoperative complications is warranted[50]. We only performed descriptive analysis, which revealed a comparable duration of postoperative hospitalization in both the groups[40,42].
We observed no difference in any of the clinical complications in both the groups; however, a previous meta-analysis reported a higher risk of cardiovascular complications in the nRCT group than in the nCT group, which could be a toxic side effect of this modality.
nCT and radiotherapy are also associated with adverse events, including thromboembolic events, neutropenia, leukopenia, anemia, nausea or vomiting, and diarrhea[35]. Renal failure occurred more often in the nCT group than in the nCRT group, indicating a toxic side effect of nCT. However, no difference was reported in terms of cardiac failure, in contrast to a former meta-analysis[50]. According to previous investigations, neutropenia is not associated with either neoadjuvant treatment modality. In the descriptive analysis, leukopenia occurred 4% more frequently in the nCRT group than in the nCT group, making them more vulnerable to developing infections. Additionally, a low number of anemia cases was observed in both the groups. The quality of life can be assessed using the EORTC QLQ-C30 questionnaire[58], which includes encompasses side effects including nausea, vomiting, and diarrhea. Notably, these side effects occurred approximately 7% more frequently in the nCT group than in the nCRT group.
Our meta-analysis provides the most comprehensive and recent summary of the data, particularly focusing on patients with esophageal AC. In addition, various outcomes were analyzed in a sufficient number of patients. The data from this study accurately reflect the esophageal AC population. No significant differences in demographic characteristics were reported between patients of the two groups.
Nevertheless, our study has some limitations. Deviating from the protocol, we included propensity score-matched studies and cohort trials, which are less reliable than RCTs and have potentially significant biases. Additionally, all trials were conducted in Western countries, reflecting a characteristic of AC, thus limiting the generalizability of the results to the Asian population or other countries. The use of various neoadjuvant regimens and some the lack of separation between preoperative and perioperative therapies in some included studies also pose some limitations. Furthermore, the evidence for most outcomes was deemed low; therefore, the true effect may differ substantially from the estimate.
In summary, one might question the lack of impact of radiotherapy on overall survival, despite improvements in measures of pathological regression, known to correlate with survival. This discrepancy can be attributed to modification of these crucial measures by local therapy. In the context of modern surgical techniques, the systemic component of the disease is the primary determinant of survival in esophageal and gastroesophageal junction ACs. Hence, the incorporation of systemic chemotherapy, new immunotherapies, and targeted treatments capable of addressing distant diseases holds greater potential to enhance patient survival in the future.
In patients with esophageal AC, neoadjuvant chemoradiation increases pCR and 30-d mortality; however, it has no effect on long-term survival. nCT may be associated with side effects that can decrease the quality of life. Further randomized trials are required to address the limitations in the quality of the available studies.
The incidence of adenocarcinoma (AC) in the esophagus is increasing, especially in the Western countries, in contrast to the incidence of squamous cell carcinomas (SCC). Neoadjuvant therapy before surgery can improve patient survival in advanced stages. The superiority of neoadjuvant modalities, especially for ACs, remains unclear. Previous meta-analyses have numerous limitations, including the pooled populations of AC and SCC, which makes the application of their results specifically to either subtype difficult.
The superiority of neoadjuvant therapy has been proven previously; however, determining which modality has a greater benefit, especially for esophageal AC, remains uncertain. In this study, we performed a comprehensive, up-to-date investigation to compare the efficacy of neoadjuvant chemotherapy (nCT) and neoadjuvant chemoradiotherapy (nCRT) in the surgical treatment of AC of the esophagus and esophageal junction.
To address the questions of this meta-analysis, we used the PICO protocol to evaluate data from patients with esophageal or cardiac AC, who underwent neoadjuvant therapy before surgery. Intervention was preoperative nCT, which was compared with nCRT. We investigated the following outcomes: Survival, remission rate, mortality, short- and long-term clinical and surgical complications, and quality of life.
Following the PICO protocol, two authors independently performed a comprehensive search of multiple databases using the predefined criteria. Statistical analyses were performed by biostatisticians to calculate odds ratio and hazard ratio with the 95%CI. Results were visualized using forest plots and Kaplan-Meier curves. The Risk of Bias Tool 2 and GRADE approach were used to assess the quality of the results.
Ten articles were included after selection. After statistical analysis, we observed that 30-d mortality (P = 0.015) and pathological complete response (P < 0.001) were higher in the nCRT group than in the nCT group; however, no significant difference was observed for long-term survival. The risk of renal failure (P = 0.039) was higher in the nCT group, and the incidence of nausea or vomiting was 9% in the nCT group compared to 3% in the nCRT group. No significant difference was reported in other clinical or surgical complications.
Although the superiority of neoadjuvant therapy has been previously demonstrated, nCRT may increase pathological complete response and 30-d mortality, without improving long-term survival. Furthermore, nCT may lead to some adverse effects, which can decrease the quality of life.
The present study predominantly analyzed retrospective data, potentially introducing research bias; therefore, future randomized studies with more detailed data collection are warranted.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Hungary
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Haddadi S, Algeria S-Editor: Li L L-Editor: A P-Editor: Cai YX
| 1. | Liu CQ, Ma YL, Qin Q, Wang PH, Luo Y, Xu PF, Cui Y. Epidemiology of esophageal cancer in 2020 and projections to 2030 and 2040. Thorac Cancer. 2023;14:3-11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 154] [Reference Citation Analysis (0)] |
| 2. | Tinusz B, Szapáry LB, Paládi B, Papp A, Bogner B, Hegedűs I, Bellyei S, Vincze Á, Solt J, Micsik T, Dunás-Varga V, Pályu E, Vass T, Schnabel T, Farkas N, Hegyi P, Thrift AP, Erőss B. The Esophageal Adenocarcinoma Epidemic Has Reached Hungary: A Multicenter, Cross-Sectional Study. Front Oncol. 2020;10:541794. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Reference Citation Analysis (0)] |
| 3. | Rice TW, Patil DT, Blackstone EH. 8th edition AJCC/UICC staging of cancers of the esophagus and esophagogastric junction: application to clinical practice. Ann Cardiothorac Surg. 2017;6:119-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 303] [Cited by in RCA: 529] [Article Influence: 66.1] [Reference Citation Analysis (0)] |
| 4. | Liu F, Wang W, Wang C, Peng X. Enhanced recovery after surgery (ERAS) programs for esophagectomy protocol for a systematic review and meta-analysis. Medicine (Baltimore). 2018;97:e0016. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 5. | Sindler DL, Mátrai P, Szakó L, Berki D, Berke G, Csontos A, Papp C, Hegyi P, Papp A. Faster recovery and bowel movement after early oral feeding compared to late oral feeding after upper GI tumor resections: a meta-analysis. Front Surg. 2023;10:1092303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 6. | Szakó L, Németh D, Farkas N, Kiss S, Dömötör RZ, Engh MA, Hegyi P, Eross B, Papp A. Network meta-analysis of randomized controlled trials on esophagectomies in esophageal cancer: The superiority of minimally invasive surgery. World J Gastroenterol. 2022;28:4201-4210. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 15] [Cited by in RCA: 13] [Article Influence: 4.3] [Reference Citation Analysis (1)] |
| 7. | van Hagen P, Hulshof MC, van Lanschot JJ, Steyerberg EW, van Berge Henegouwen MI, Wijnhoven BP, Richel DJ, Nieuwenhuijzen GA, Hospers GA, Bonenkamp JJ, Cuesta MA, Blaisse RJ, Busch OR, ten Kate FJ, Creemers GJ, Punt CJ, Plukker JT, Verheul HM, Spillenaar Bilgen EJ, van Dekken H, van der Sangen MJ, Rozema T, Biermann K, Beukema JC, Piet AH, van Rij CM, Reinders JG, Tilanus HW, van der Gaast A; CROSS Group. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med. 2012;366:2074-2084. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3288] [Cited by in RCA: 4075] [Article Influence: 313.5] [Reference Citation Analysis (0)] |
| 8. | Cunningham D, Allum WH, Stenning SP, Thompson JN, Van de Velde CJ, Nicolson M, Scarffe JH, Lofts FJ, Falk SJ, Iveson TJ, Smith DB, Langley RE, Verma M, Weeden S, Chua YJ, MAGIC Trial Participants. Perioperative chemotherapy vs surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355:11-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4899] [Cited by in RCA: 4599] [Article Influence: 242.1] [Reference Citation Analysis (0)] |
| 9. | Ychou M, Boige V, Pignon JP, Conroy T, Bouché O, Lebreton G, Ducourtieux M, Bedenne L, Fabre JM, Saint-Aubert B, Genève J, Lasser P, Rougier P. Perioperative chemotherapy compared with surgery alone for resectable gastroesophageal adenocarcinoma: an FNCLCC and FFCD multicenter phase III trial. J Clin Oncol. 2011;29:1715-1721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1216] [Cited by in RCA: 1501] [Article Influence: 107.2] [Reference Citation Analysis (0)] |
| 10. | Allum WH, Stenning SP, Bancewicz J, Clark PI, Langley RE. Long-term results of a randomized trial of surgery with or without preoperative chemotherapy in esophageal cancer. J Clin Oncol. 2009;27:5062-5067. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 662] [Cited by in RCA: 753] [Article Influence: 47.1] [Reference Citation Analysis (0)] |
| 11. | Ronellenfitsch U, Schwarzbach M, Hofheinz R, Kienle P, Kieser M, Slanger TE, Burmeister B, Kelsen D, Niedzwiecki D, Schuhmacher C, Urba S, van de Velde C, Walsh TN, Ychou M, Jensen K. Preoperative chemo(radio)therapy vs primary surgery for gastroesophageal adenocarcinoma: systematic review with meta-analysis combining individual patient and aggregate data. Eur J Cancer. 2013;49:3149-3158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 123] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 12. | Ronellenfitsch U, Schwarzbach M, Hofheinz R, Kienle P, Kieser M, Slanger TE, Jensen K; GE Adenocarcinoma Meta‐analysis Group. Perioperative chemo(radio)therapy vs primary surgery for resectable adenocarcinoma of the stomach, gastroesophageal junction, and lower esophagus. Cochrane Database Syst Rev. 2013;CD008107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 83] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 13. | Schuhmacher C, Gretschel S, Lordick F, Reichardt P, Hohenberger W, Eisenberger CF, Haag C, Mauer ME, Hasan B, Welch J, Ott K, Hoelscher A, Schneider PM, Bechstein W, Wilke H, Lutz MP, Nordlinger B, Van Cutsem E, Siewert JR, Schlag PM. Neoadjuvant chemotherapy compared with surgery alone for locally advanced cancer of the stomach and cardia: European Organisation for Research and Treatment of Cancer randomized trial 40954. J Clin Oncol. 2010;28:5210-5218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 511] [Cited by in RCA: 532] [Article Influence: 35.5] [Reference Citation Analysis (0)] |
| 14. | Sjoquist KM, Burmeister BH, Smithers BM, Zalcberg JR, Simes RJ, Barbour A, Gebski V; Australasian Gastro-Intestinal Trials Group. Survival after neoadjuvant chemotherapy or chemoradiotherapy for resectable oesophageal carcinoma: an updated meta-analysis. Lancet Oncol. 2011;12:681-692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1141] [Cited by in RCA: 1265] [Article Influence: 90.4] [Reference Citation Analysis (0)] |
| 15. | Stahl M, Walz MK, Stuschke M, Lehmann N, Meyer HJ, Riera-Knorrenschild J, Langer P, Engenhart-Cabillic R, Bitzer M, Königsrainer A, Budach W, Wilke H. Phase III comparison of preoperative chemotherapy compared with chemoradiotherapy in patients with locally advanced adenocarcinoma of the esophagogastric junction. J Clin Oncol. 2009;27:851-856. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 664] [Cited by in RCA: 695] [Article Influence: 43.4] [Reference Citation Analysis (0)] |
| 16. | National Institute for Health and Care Research. PROSPERO is fast-tracking registration of protocols related to COVID-19. [cited 23 February 2024]. Available from: https://www.crd.york.ac.uk/prospero/. |
| 17. | Plotdigitizer. All-in-One Tool to Extract Data from Graphs, Plots & Images. [cited 23 February 2024]. Available from: https://plotdigitizer.com/app. |
| 18. | Guyot P, Ades AE, Ouwens MJ, Welton NJ. Enhanced secondary analysis of survival data: reconstructing the data from published Kaplan-Meier survival curves. BMC Med Res Methodol. 2012;12:9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 956] [Cited by in RCA: 1707] [Article Influence: 131.3] [Reference Citation Analysis (0)] |
| 19. | WebPlotDigitizer. Extract data from XY charts, Bar graphs, Polar diagrams and much more! [cited 23 February 2024]. Available from: https://automeris.io/WebPlotDigitizer/. |
| 20. | Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539-1558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21630] [Cited by in RCA: 25760] [Article Influence: 1120.0] [Reference Citation Analysis (0)] |
| 21. | Harbord RM, Harris RJ, Sterne JAC. Updated tests for small-study effects in meta-analyses. The Stata Journal: Promoting Communications on Statistics and Stata. 2009;9:197-210. [DOI] [Full Text] |
| 22. | Harrer M, Cuijpers P, Toshi F, Ebert DD. Doing meta-analysis with R: a hands-on guide. 1st ed. New York: Chapman and Hall/CRC Press, 2021. [DOI] [Full Text] |
| 23. | R Core Team. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. 2023. [cited 23 February 2024]. Available from: https://www.R-project.org/. |
| 24. | Schwarzer G. Meta-Analysis in R. In: Systematic Reviews in Health Research: Meta‐Analysis in Context, 3rd Ed. Egger M, Higgins JPT, Smith GD, editors. New York: John Wiley & Sons, 2022. [DOI] [Full Text] |
| 25. | Cuijpers P, Furukawa T, Ebert DD. Dmetar: companion R package for the guide doing meta-analysis in R. 2022. [cited 23 February 2024]. Available from: https://dmetar.protectlab.org. |
| 26. | Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959;22:719-748. [PubMed] |
| 27. | Robins J, Greenland S, Breslow NE. A general estimator for the variance of the Mantel-Haenszel odds ratio. Am J Epidemiol. 1986;124:719-723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 210] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 28. | Cooper HM, Hedges LV, Valentine JC. The handbook of research synthesis and meta-analysis. 2nd ed. New York: Russell Sage Foundation, 2009. |
| 29. | Sweeting MJ, Sutton AJ, Lambert PC. What to add to nothing? Use and avoidance of continuity corrections in meta-analysis of sparse data. Stat Med. 2004;23:1351-1375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1080] [Cited by in RCA: 1249] [Article Influence: 59.5] [Reference Citation Analysis (0)] |
| 30. | Knapp G, Hartung J. Improved tests for a random effects meta-regression with a single covariate. Stat Med. 2003;22:2693-2710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 928] [Cited by in RCA: 1218] [Article Influence: 55.4] [Reference Citation Analysis (0)] |
| 31. | IntHout J, Ioannidis JP, Borm GF. The Hartung-Knapp-Sidik-Jonkman method for random effects meta-analysis is straightforward and considerably outperforms the standard DerSimonian-Laird method. BMC Med Res Methodol. 2014;14:25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 670] [Cited by in RCA: 1273] [Article Influence: 115.7] [Reference Citation Analysis (0)] |
| 32. | Paule RC, Mandel J. Consensus Values and Weighting Factors. J Res Natl Bur Stand (1977). 1982;87:377-385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 256] [Cited by in RCA: 291] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 33. | Veroniki AA, Jackson D, Viechtbauer W, Bender R, Bowden J, Knapp G, Kuss O, Higgins JP, Langan D, Salanti G. Methods to estimate the between-study variance and its uncertainty in meta-analysis. Res Synth Methods. 2016;7:55-79. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 786] [Cited by in RCA: 905] [Article Influence: 100.6] [Reference Citation Analysis (0)] |
| 34. | Shinyapps. Estimating the sample mean and standard deviation. [cited 23 February 2024]. Available from: https://smcgrath.shinyapps.io/estmeansd. |
| 35. | Anderegg MCJ, van der Sluis PC, Ruurda JP, Gisbertz SS, Hulshof MCCM, van Vulpen M, Mohammed NH, van Laarhoven HWM, Wiezer MJ, Los M, van Berge Henegouwen MI, van Hillegersberg R. Preoperative Chemoradiotherapy Versus Perioperative Chemotherapy for Patients With Resectable Esophageal or Gastroesophageal Junction Adenocarcinoma. Ann Surg Oncol. 2017;24:2282-2290. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 35] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 36. | Luc G, Vendrely V, Terrebonne E, Chiche L, Collet D. Neoadjuvant chemoradiotherapy improves histological results compared with perioperative chemotherapy in locally advanced esophageal adenocarcinoma. Ann Surg Oncol. 2015;22:604-609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 37. | Münch S, Habermehl D, Agha A, Belka C, Combs SE, Eckel R, Friess H, Gerbes A, Nüssler NC, Schepp W, Schmid RM, Schmitt W, Schubert-Fritschle G, Weber B, Werner J, Engel J. Perioperative chemotherapy vs. neoadjuvant chemoradiation in gastroesophageal junction adenocarcinoma: A population-based evaluation of the Munich Cancer Registry. Strahlenther Onkol. 2018;194:125-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 38. | Spicer JD, Stiles BM, Sudarshan M, Correa AM, Ferri LE, Altorki NK, Hofstetter WL. Preoperative Chemoradiation Therapy Versus Chemotherapy in Patients Undergoing Modified En Bloc Esophagectomy for Locally Advanced Esophageal Adenocarcinoma: Is Radiotherapy Beneficial? Ann Thorac Surg. 2016;101:1262-9; discussion 1969. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 31] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 39. | Favi F, Bollschweiler E, Berlth F, Plum P, Hescheler DA, Alakus H, Semrau R, Celik E, Mönig SP, Drebber U, Hölscher AH. Neoadjuvant chemotherapy or chemoradiation for patients with advanced adenocarcinoma of the oesophagus? A propensity score-matched study. Eur J Surg Oncol. 2017;43:1572-1580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 40. | Goense L, van der Sluis PC, van Rossum PSN, van der Horst S, Meijer GJ, Haj Mohammad N, van Vulpen M, Mook S, Ruurda JP, van Hillegersberg R. Perioperative chemotherapy vs neoadjuvant chemoradiotherapy for esophageal or GEJ adenocarcinoma: A propensity score-matched analysis comparing toxicity, pathologic outcome, and survival. J Surg Oncol. 2017;115:812-820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 41. | Markar SR, Noordman BJ, Mackenzie H, Findlay JM, Boshier PR, Ni M, Steyerberg EW, van der Gaast A, Hulshof MCCM, Maynard N, van Berge Henegouwen MI, Wijnhoven BPL, Reynolds JV, Van Lanschot JJB, Hanna GB. Multimodality treatment for esophageal adenocarcinoma: multi-center propensity-score matched study. Ann Oncol. 2017;28:519-527. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 68] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 42. | Visser E, Edholm D, Smithers BM, Thomson IG, Burmeister BH, Walpole ET, Gotley DC, Joubert WL, Atkinson V, Mai T, Thomas JM, Barbour AP. Neoadjuvant chemotherapy or chemoradiotherapy for adenocarcinoma of the esophagus. J Surg Oncol. 2018;117:1687-1696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 43. | Burmeister BH, Thomas JM, Burmeister EA, Walpole ET, Harvey JA, Thomson DB, Barbour AP, Gotley DC, Smithers BM. Is concurrent radiation therapy required in patients receiving preoperative chemotherapy for adenocarcinoma of the oesophagus? A randomised phase II trial. Eur J Cancer. 2011;47:354-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 257] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 44. | Stahl M, Walz MK, Riera-Knorrenschild J, Stuschke M, Sandermann A, Bitzer M, Wilke H, Budach W. Preoperative chemotherapy vs chemoradiotherapy in locally advanced adenocarcinomas of the oesophagogastric junction (POET): Long-term results of a controlled randomised trial. Eur J Cancer. 2017;81:183-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 190] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 45. | Alderson D, Langley RE, Nankivell MG, Blazeby JM, Griffin M, Crellin A, Grabsch HI, Okines AFC, Goldstein C, Falk S, Thompson J, Krysztopik R, Coxon FY, Pritchard S, Langer R, Stenning SP, Cunningham D. Neoadjuvant chemotherapy for resectable oesophageal and junctional adenocarcinoma: results from the UK Medical Research Council randomised OEO5 trial (ISRCTN 01852072). J Clin Oncol. 2015;33:Abstr 4002. [RCA] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 51] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 46. | Shapiro J, van Lanschot JJB, Hulshof MCCM, van Hagen P, van Berge Henegouwen MI, Wijnhoven BPL, van Laarhoven HWM, Nieuwenhuijzen GAP, Hospers GAP, Bonenkamp JJ, Cuesta MA, Blaisse RJB, Busch ORC, Ten Kate FJW, Creemers GM, Punt CJA, Plukker JTM, Verheul HMW, Bilgen EJS, van Dekken H, van der Sangen MJC, Rozema T, Biermann K, Beukema JC, Piet AHM, van Rij CM, Reinders JG, Tilanus HW, Steyerberg EW, van der Gaast A; CROSS study group. Neoadjuvant chemoradiotherapy plus surgery vs surgery alone for oesophageal or junctional cancer (CROSS): long-term results of a randomised controlled trial. Lancet Oncol. 2015;16:1090-1098. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1292] [Cited by in RCA: 1822] [Article Influence: 182.2] [Reference Citation Analysis (0)] |
| 47. | Lorenzen S, Pauligk C, Homann N, Schmalenberg H, Jäger E, Al-Batran SE. Feasibility of perioperative chemotherapy with infusional 5-FU, leucovorin, and oxaliplatin with (FLOT) or without (FLO) docetaxel in elderly patients with locally advanced esophagogastric cancer. Br J Cancer. 2013;108:519-526. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 78] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 48. | Ferri LE, Ades S, Alcindor T, Chasen M, Marcus V, Hickeson M, Artho G, Thirlwell MP. Perioperative docetaxel, cisplatin, and 5-fluorouracil (DCF) for locally advanced esophageal and gastric adenocarcinoma: a multicenter phase II trial. Ann Oncol. 2012;23:1512-1517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 82] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 49. | Kirmayr M, Quilodrán C, Valente B, Loezar C, Garegnani L, Franco JVA. The GRADE approach, Part 1: how to assess the certainty of the evidence. Medwave. 2021;21:e8109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 57] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 50. | Han J, Wang Z, Liu C. Survival and complications after neoadjuvant chemotherapy or chemoradiotherapy for esophageal cancer: a meta-analysis. Future Oncol. 2021;17:2257-2274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 32] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 51. | Grizzi G, Petrelli F, Di Bartolomeo M, Viti M, Texeira Moraes M, Luciani A, Passalacqua R, Ghidini M, Tomasello G, Baiocchi GL, Celotti A. Preferred neoadjuvant therapy for gastric and gastroesophageal junction adenocarcinoma: a systematic review and network meta-analysis. Gastric Cancer. 2022;25:982-987. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 15] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 52. | Barbour AP, Jones M, Gonen M, Gotley DC, Thomas J, Thomson DB, Burmeister B, Smithers BM. Refining esophageal cancer staging after neoadjuvant therapy: importance of treatment response. Ann Surg Oncol. 2008;15:2894-2902. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 57] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 53. | Sciuto M, Capovilla G, Scarton A, Tagkalos E, Uzun E, Moletta L, Hadzijusufoviç Edin, Provenzano L, Salvador R, Pierobon E, Zanchettin G, Berlth F, Lang H, Grimminger P, Valmasoni M. 462. Major pathologic response in esophageal adenocarcinoma: should we adopt a new paradigm in defining response to treatments? Diseases of the Esophagus. 2003;36. [DOI] [Full Text] |
| 54. | Scarton A, Capovilla G, Tagkalos E, Uzun E, Moletta L, Hadzijusufoviç E, Provenzano L, Salvador R, Pierobon E, Zanchettin G, Berlth F, Grimminger P, Valmasoni M. 463. The impact of pathological tumor response following neoadjuvant chemotherapy and chemoradiotherapy for esophageal adenocarcinoma. A retrospective multicenter cohort study. Diseases of the Esophagus. 2003;36. [DOI] [Full Text] |
| 55. | Klevebro F, Alexandersson von Döbeln G, Wang N, Johnsen G, Jacobsen AB, Friesland S, Hatlevoll I, Glenjen NI, Lind P, Tsai JA, Lundell L, Nilsson M. A randomized clinical trial of neoadjuvant chemotherapy vs neoadjuvant chemoradiotherapy for cancer of the oesophagus or gastro-oesophageal junction. Ann Oncol. 2016;27:660-667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 302] [Article Influence: 33.6] [Reference Citation Analysis (0)] |
| 56. | Gebauer F, Plum PS, Damanakis A, Chon SH, Popp F, Zander T, Quaas A, Fuchs H, Schmidt T, Schröder W, Bruns CJ. Long-Term Postsurgical Outcomes of Neoadjuvant Chemoradiation (CROSS) Versus Chemotherapy (FLOT) for Multimodal Treatment of Adenocarcinoma of the Esophagus and the Esophagogastric Junction. Ann Surg Oncol. 2023;30:7422-7433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 57. | Schroeder W, Ghadimi MPH, Schloesser H, Loeser H, Schiller P, Zander T, Gebauer F, Fuchs H, Quaas A, Bruns CJ. Long-Term Outcome After Histopathological Complete Response with and Without Nodal Metastases Following Multimodal Treatment of Esophageal Cancer. Ann Surg Oncol. 2022;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 18] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 58. | Adenis A, Kulkarni AS, Girotto GC, de la Fouchardiere C, Senellart H, van Laarhoven HWM, Mansoor W, Al-Rajabi R, Norquist J, Amonkar M, Suryawanshi S, Bhagia P, Metges JP. Impact of Pembrolizumab Versus Chemotherapy as Second-Line Therapy for Advanced Esophageal Cancer on Health-Related Quality of Life in KEYNOTE-181. J Clin Oncol. 2022;40:382-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |