Published online Mar 21, 2024. doi: 10.3748/wjg.v30.i11.1545
Peer-review started: December 8, 2023
First decision: December 21, 2023
Revised: December 31, 2023
Accepted: February 18, 2024
Article in press: February 18, 2024
Published online: March 21, 2024
Processing time: 104 Days and 10.1 Hours
The gluten-free diet (GFD) has limitations, and there is intense research in the development of adjuvant therapies.
To examine the effects of orally administered Aspergillus niger prolyl endo
This was an exploratory, double-blind, randomized, placebo-controlled trial that enrolled CeD patients on a long-term GFD. After a 4-wk run-in period, patients were randomized to 4 wk of two AN-PEP capsules (GliadinX; AVI Research, LLC, United States) at each of three meals per day or placebo. Outcome endpoints were: (1) Average weekly stool gluten immunogenic peptides (GIP) between the run-in and end of treatments and between AN-PEP and placebo; (2) celiac symptom index (CSI); (3) CeD-specific serology; and (4) quality of life. Stool samples were collected for GIP testing by ELISA every Tuesday and Friday during run-ins and treatments.
Forty patients were randomized for the intention-to-treat analysis, and three were excluded from the per-protocol assessment. Overall, 628/640 (98.1%) stool samples were collected. GIP was undetectable (< 0.08 μg/g) in 65.6% of samples, and no differences between treatment arms were detected. Only 0.5% of samples had GIP concentrations sufficiently high (> 0.32 μg/g) to potentially cause mucosal damage. Median GIP concentration in the AN-PEP arm was 44.7% lower than in the run-in period. One-third of patients exhibiting GIP > 0.08 μg/g during run-in had lower or undetectable GIP after AN-PEP treatment. Compared with the run- in period, the proportion of symptomatic patients (CSI > 38) in the AN-PEP arm was significantly lower (P < 0.03). AN-PEP did not result in changes in specific serologies.
This exploratory study conducted in a real-life setting revealed high adherence to the GFD. The AN-PEP treatment did not significantly reduce the overall GIP stool concentration. However, given the observation of a significantly lower prevalence of patients with severe symptoms in the AN-PEP arm, further clinical research is warranted.
Core Tip: In treated celiac disease (CeD) patients, exposure to gluten due to both voluntary and involuntary dietary lapses is prevalent and often leads to persistent symptoms despite adherence to a gluten-free diet. The potential of oral administration of Aspergillus niger prolyl endopeptidase (AN-PEP) in preventing the effects of inadvertent gluten exposure, as confirmed by gluten immunogenic peptide (GIP) stool excretion, and reducing CeD-specific symptoms in adults remains uncertain. Our study findings indicate that while AN-PEP did not significantly reduce overall GIP stool excretion, it notably lowered the prevalence of severe symptoms compared to the placebo arm.
- Citation: Stefanolo JP, Segura V, Grizzuti M, Heredia A, Comino I, Costa AF, Puebla R, Temprano MP, Niveloni SI, de Diego G, Oregui ME, Smecuol EG, de Marzi MC, Verdú EF, Sousa C, Bai JC. Effect of Aspergillus niger prolyl endopeptidase in patients with celiac disease on a long-term gluten-free diet. World J Gastroenterol 2024; 30(11): 1545-1555
- URL: https://www.wjgnet.com/1007-9327/full/v30/i11/1545.htm
- DOI: https://dx.doi.org/10.3748/wjg.v30.i11.1545
Celiac disease (CeD) is a systemic autoimmune disorder that primarily affects the small intestine and is caused by gluten exposure in genetically susceptible people[1]. Currently, its only treatment is a strict gluten-free diet (GFD), which results in gradual improvement of symptoms and mucosal damage[2]. Gluten elimination is a difficult, time-consuming, and expensive process for patients[3]. Despite a GFD being notably effective in the long term for most patients, some may be considered non-responsive or experience symptom relapse and/or persistent enteropathy despite long-term adherence to a GFD[1,2]. Exposure to gluten caused by voluntary and involuntary dietary lapses is common and is thought to be responsible for most cases of persistent symptoms, low quality of life (QoL), intestinal mucosal damage, and the risk of complications[4-6]. Such dietary exposure to gluten has been objectively demonstrated by the excretion of gluten immunogenic peptides (GIP)[7,8].
The burden of following a GFD has led to the exploration of various potential therapies to be used alongside the GFD[2,9]. Mammalian enzymes cannot effectively break down proline and glutamine-rich protein sequences, resulting in incomplete degradation of gluten in the intestinal lumen. This exposes the intestinal mucosa to immunogenic gluten peptides, which in CeD will reactivate the disease and gluten-specific T cells[10,11]. Several microbial peptidases have been identified as potentially useful for detoxifying cereal prolamins[12-14]. The different proteases differ in affinities and hydrolysis specificity, as well as in their optimal pHs. One of the first peptidases studied in vitro and in vivo in pre-clinical studies was a prolyl endopeptidase from the Aspergillus niger (A. niger) fungus (AN-PEP)[10,15-17]. AN-PEP is optimally active at low pH values typically found in the stomach and is resistant to degradation by pepsin[13,17]. A study conducted in a dynamic system that resembles the human gastrointestinal tract revealed that AN-PEP can accelerate gluten degradation[18]. A study using a challenge meal with gluten plus AN-PEP found a high rate of hydrolysis with a reduction of gluten-immunogenic peptides in the stomach before reaching the small intestine[12]. A clinical challenge study conducted over a 2-wk period showed that AN-PEP was well tolerated but had no significant advantages over placebo on clinical features or biomarkers[19]. Given that AN-PEP has been approved for marketing as an alimentary supplement, no additional clinical studies have been conducted to demonstrate whether it could be efficacious in other clinical stages of the disease, such as symptomatic patients likely exposed to real-life contamination. Therefore, we explored whether oral administration of AN-PEP prevents the effects of the commonly occurring involuntary gluten exposure, as verified by GIP stool excretion, and the reduction of CeD-specific symptoms in adult CeD patients who continued their usual GFD.
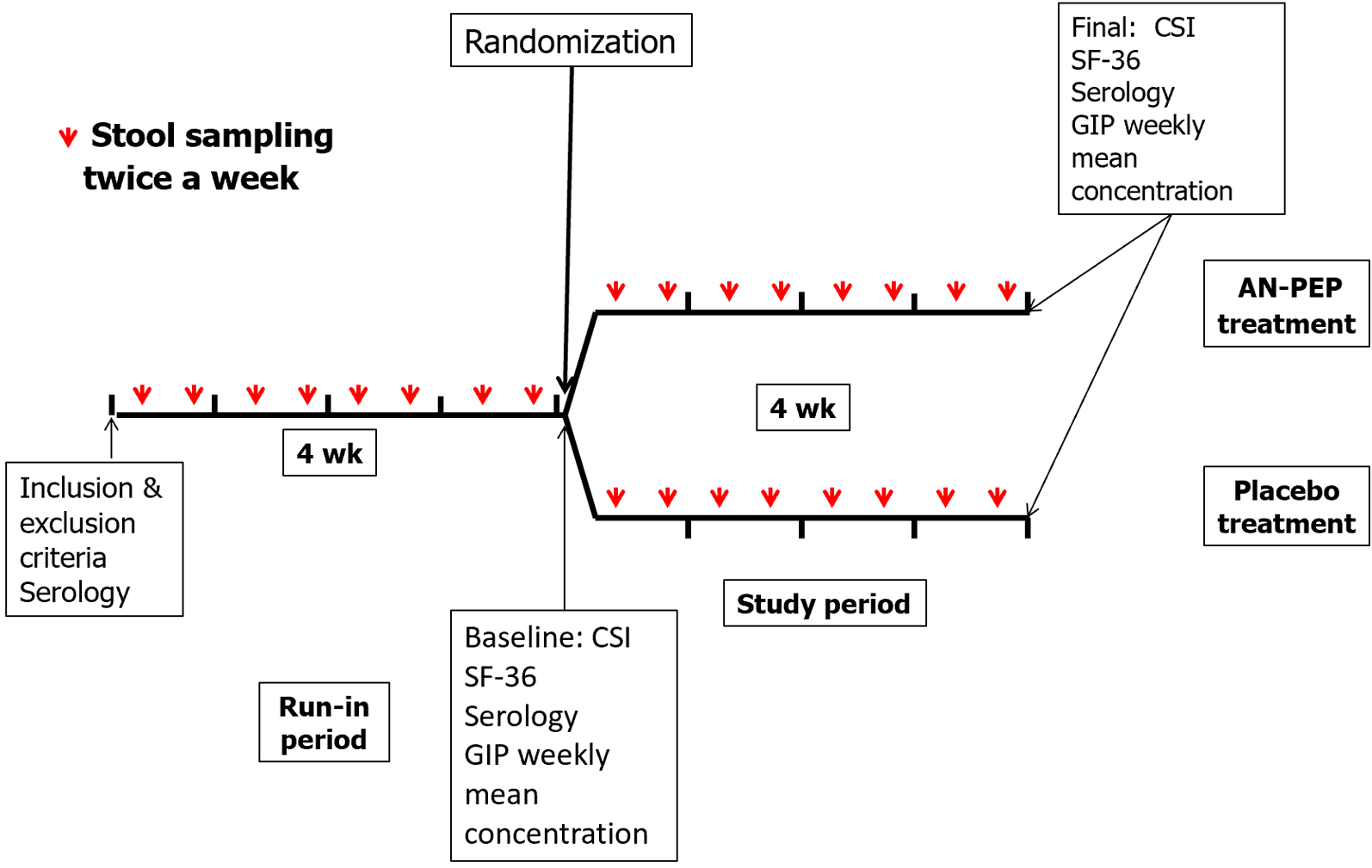
This was a real-life exploratory pilot study following a prospective, double-blind, randomized, placebo-controlled design that enrolled adult patients with CeD on a GFD for more than 2 years. Consecutive patients attending our Celiac Disease Clinic at the C. Bonorino Udaondo Gastroenterology Hospital were invited to be screened. CeD patients were enrolled if they met the following criteria: (1) A well-established histological (Marsh’s type 3) and serologic diagnosis of CeD; (2) self-considering to be adherent with the GFD for more than 2 years; (3) having at least one bowel movement/day or every other day; (4) being able to provide serum samples at enrollment and the end of the study period; (5) being able to collect and store samples frozen, and transport stool samples to our institution in specially provided containers; and (6) having the ability to respond to symptom and QoL questionnaires. Patients who were unwilling to participate, intentionally ingested gluten, had uncontrolled concomitant disorders (e.g., type I diabetes, hypothyroidism), had type II refractory CeD, or were taking drugs that could potentially affect stool GIP excretion (laxatives, probiotics, etc.) were excluded from the study. Patients who met the inclusion and exclusion criteria underwent clinical evaluation, completed the celiac symptom index (CSI) clinical questionnaire and the Short Form-36 (SF-36) QoL questionnaire, and provided blood samples for clinical biochemical and serological determinations at baseline and at the end of the study. After a 4-wk run-in period to achieve stable dietary adherence, patients were assigned to a 4-wk treatment period in which they consumed either two capsules during each of the three main meals/day of AN-PEP (325 mg/capsule containing 70% AN-PEP, 30% maltodextrin, and citric acid) (GliadinX; AVI Research, LLC, New York, NY, United States), or placebo (maltodextrin, citric acid, and microcrystalline cellulose) for 4 consecutive weeks (Figure 1). The protease is an enzyme preparation of prolyl-oligopeptidase produced with a genetically modified A. niger strain. This enzyme’s composition and production process have been previously reported[19,20]. The dose of AN-PEP administered was the highest dose employed in the study König et al[16], which provides 174000 protease picomol IU/meal, where the authors demonstrated that AN-PEP significantly degraded most gluten in the stomach before it entered the duodenum. Other studies have shown that degradation of gluten proteins in the insoluble fraction became evident after 30 min at pH 4, the optimum pH for AN-PEP activity, with the strongest effect observed at a concentration of 20 propyl peptidase units/g gluten[21,22].
The presence and intensity of symptoms were assessed at the time of randomization and at the end of the study using the CSI questionnaire. The CSI is a well-validated questionnaire of 36 items, developed in 2009 by an expert committee and, subsequently, internally and externally validated[23]. The CSI questionnaire includes 16 items, with 11 items evaluating “specific symptoms” related to CeD and five evaluating “general health” parameters. Participants rate each item on a Likert scale ranging from 1 to 5, where 1 represents no symptoms and 5 indicates the highest intensity for the given symptom. In previous comparative clinical studies, we have considered scores > 38 on the CSI to be indicative of a highly symptomatic clinical level of CeD[24]. By assessing symptom presence and intensity using the CSI, we were able to evaluate the impact of the enzyme treatment on symptom improvement and overall patient well-being throughout the study.
The impact of the enzyme treatment on patients’ QoL was evaluated by assessing parameters and gastrointestinal symptoms using the SF-36 health survey[24]. The SF-36 includes eight sub-dimension measures of functioning and wellness: Physical functioning, role limitations due to physical problems, bodily pain, general health, vitality, social functioning, role limitations due to emotional problems, and mental health. We measured these sub-dimensions at randomization and at the end of the trial and transformed the raw scores into a scale ranging from 0 to 100. A score of 0 indicates the poorest health and QoL, while a score of 100 represents optimal health and QoL. This assessment allowed us to evaluate the impact of the enzyme treatment on various aspects of the patient’s well-being and overall QoL.
During the study, patients were instructed to collect stool samples twice a week (on Tuesdays and Fridays) throughout the two 4-wk periods (run-in and treatment) in both arms. The samples were immediately placed in sealed containers and frozen at -10 °C until they were delivered to our laboratory. Upon arrival at the laboratory, the samples were stored at -20 °C until they were tested for GIP measurement. To quantify GIP in the stool samples, a commercial ELISA kit (iVYLISA GIP-Stool; Biomedal S.L., Sevilla, Spain) was used following the manufacturer’s instructions. This kit has been previously reported in the literature[8,25-27]. The detection limit of GIP is 0.08 μg/g of stool. Stool GIP testing was performed in duplicate, and the average concentration of eight measurements from both the run-in and treatment periods was reported. In the study, all analyses were conducted by operators who were deliberately blind to the clinical status of the patients and the type of treatment they were undergoing.
Serum samples were kept frozen at -20 °C until the assay was performed in only a single lab. The CeD-related tests and cut-offs were: (1) IgA tissue transglutaminase antibodies (tTG IgA) by ELISA (QUANTA LiteTM, h-tTG IgA; Inova Diagnostic Inc., San Diego, CA, United States); and (2) IgA antibodies reacting with deamidated gliadin-derived peptides (IgA DGP) (QUANTA LiteTM, IgA DGP; Inova Diagnostic Inc.) were performed at enrollment and at the end of the trial. Cut-off for both tests was 20 U/mL.
The study was approved by Dr. C. Bonorino Udaondo Gastroenterology Hospital’s institutional ethical committee and the local research committee (CODEI). All patients were required to provide written informed consent. Patients were enrolled between October 2020 and July 2022. The study’s results were blinded to the researchers performing clinical and biochemical analyses and collecting reports (CSI and SF-36 questionnaires). The study was registered at ClinicalTrials.gov under the number NCT04788797.
Based on the very limited availability of previous studies on AN-PEP and the evidence of gluten exposure in real life (determined by the excretion of stool GIP), this was an exploratory study. While we initially aimed at recruiting 80 patients, the long quarantine imposed in Argentina during the coronavirus disease 2019 (COVID-19) pandemic forced us to reset our aim to n = 40. Patients returning > 30% of capsules administered at the randomization were part of the per-protocol (PP) evaluation. After a 4-wk run-in period, patients were allocated to one of the two treatments (AN-PEP or placebo). A block randomization method (blocks of four subjects each) elaborated by an independent statistician was administered by an independent person who was not involved in the study analysis or an author of the study. The order of blocks was also randomized. Stata 14 (Stata Corp, College Station, TX, United States) was used for the statistical analysis. According to the data distribution, continuous variables were reported as median and 25%-75% percent interquartile range (IQR) or range. The comparison of results between treatment arms was conducted using the Mann-Whitney test. For assessing the differences within the same patients before and after treatment (paired samples), a Wilcoxon test was used. Proportions between groups were compared using the Chi-square test, while comparisons of proportions within the same patients before and after treatment (paired samples) were performed using the McNemar test. P values less than 0.05 were considered statistically significant. Outcome measurements were analyzed by intent-to-treat (ITT) analysis (all patients completing the run-in and treatment periods) and PP. For any safety concerns, all patients were in contact with expert monitors (AFC, MG, and JCB). If a safety issue was present, a self-reported form for safety concerns should have been completed.
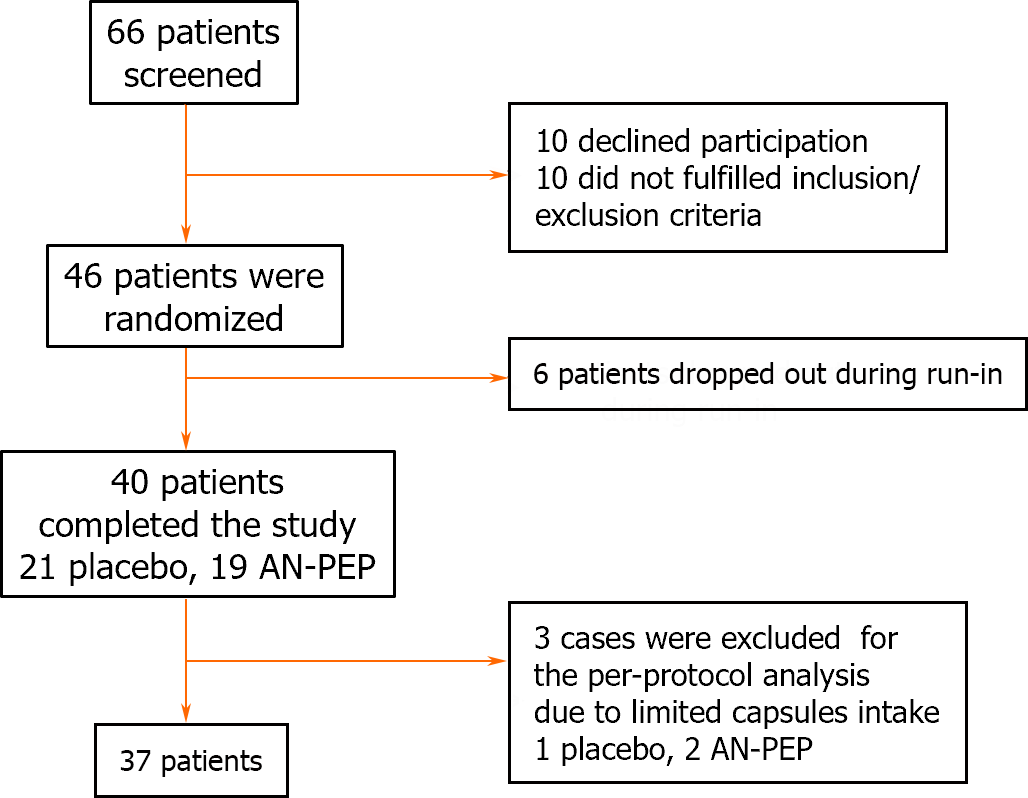
Figure 2 displays the flowchart illustrating trial progression. Due to limitations imposed by the COVID-19 pandemic on the activity of the ambulatory clinic for CeD patients, the enrollment period for the trial was from October 2020 to July 2022. Out of the initial 66 screened patients, 20 were excluded because they did not meet the inclusion and exclusion criteria. Additionally, six out of the remaining 46 enrolled patients did not initiate sample collection during the run-in period due to concerns about adhering to the protocol, leading to their exclusion from the study. Ultimately, 40patients completed the study for the ITT analysis. According to our pre-established guidelines, three patients were excluded from the final analysis because they returned more than 30% of the administered capsules. Table 1 presents the demographic information of the patients enrolled in the ITT analysis, while Table 2 provides the corresponding data for the PP analysis. No significant differences were observed between the AN-PEP and placebo-treated groups in terms of sex, age at enrollment, or duration of following a GFD. During and after the trial, no patients reported any abnormal symptom perception resulting from the use of AN-PEP or placebo.
| Item | Overall population | Placebo treatment | AN-PEP treatment |
| Number of patients | 40 | 21 | 19 |
| Age in yr, median (range) | 40.5 (29-47) | 42.0 (32-48) | 39.0 (28-46) |
| Females, n (%) | 33.0 (82.5) | 18.0 (85.7) | 15.0 (78.9) |
| CeD serology in U/mL, median (IQR) | |||
| IgA DGP at baseline | 3.0 (1.3-5.0) | 2.0 (1.0-5.0) | 4.0 (2.0-5.0) |
| End of study | 3 (2-6) | 3 (2-5) | 3 (2-6) |
| IgA tTG at baseline | 17 (7-44) | 10 (5-30) | 17 (8-49) |
| End of study | 12 (6-50) | 11 (6-15) | 17 (8-70) |
| SCI global score at randomization median score (IQR) | 36 (32-44) | 35 (30-44) | 40 (33-44) |
| End of the study median score (IQR) | 34 (28-40) | 32 (29-39) | 35 (28-41) |
| SCI scores > 38 at randomization, n (%) | 19 (47.5) | 8 (38.1) | 11 (57.9) |
| End of the study, n (%) | 12 (30.0) | 6 (28.6) | 6 (31.6)a |
| Stool GIP. Median and CI (IQR) of the average concentration for period (µg/g) | |||
| Run-in period | 0.37 (0-0.83)d | 0.34 (0-0.68)b | 0.42 (0-0.93)c |
| Treatment period | 0.21 (0-0.79)d | 0.19 (0.11-0.79)b | 0.22 (0-0.89)c |
| Item | Overall population | Placebo treatment | AN-PEP treatment |
| Number of patients | 37 | 20 | 17 |
| Age in yr, median (range) | 40 (29-46) | 41 (31-48) | 39 (28-45) |
| Females, n (%) | 31 (83.8) | 17 (85.0) | 14 (82.3) |
| CeD serology in AU/mL, median (IQR) | |||
| IgA DGP at baseline | 3 (1-5) | 2 (1-5) | 3 (1-5) |
| At end of study | 3 (2-6) | 3 (2-5) | 3 (2-6) |
| IgA tTG at baseline | 17 (7-43) | 13 (6-36) | 17 (11-48) |
| End of study | 12 (6-48) | 11 (5-20) | 17 (7-62) |
| SCI global score at randomization median score (IQR) | 36.0 (31.0-44.0) | 35.0 (30.0-45.5) | 40.0 (33.0-44.0) |
| End of the study median score (IQR) | 33.0 (28.0-39.0) | 32.0 (28.5-37.5) | 35.0 (28.0-41.0) |
| SCI scores > 38 at randomization, n (%) | 17 (45.9) | 7 (35.0) | 10 (58.8) |
| End of study, n (%) | 10 (27.0) | 5 (25.0) | 5 (29.4)a |
| Stool GIP median and CI (IQR) of the average concentration for period, µg/g | |||
| Run-in period | 0.40 (0-0.86) | 0.37 (0-0.73) | 0.55 (0-0.93) |
| Treatment period | 0.30 (0.10-0.80)b | 0.25 (0.11-0.79)c | 0.32 (0-0.89)d |
Throughout the trial, 628 (98.1%) samples were successfully collected. All analyses were conducted by investigators who were kept blind to the clinical status of the patients and the allocated treatment. A high proportion of samples from run-ins and treatment periods (65.6%) showed undetectable levels of gluten immunogenic peptides (GIP) (< 0.08 μg/g), indicating high adherence to the GFD. No significant differences in GIP levels were observed between the treatment arms in either the ITT or PP analyses. Three patients were excluded from the ITT analysis due to returning more than 30% of the capsules at the end of the trial, and none had a positive GIP. There were no differences in GIP between ITT and PP analyses. By using the information obtained in the PP, we observed that in the placebo arm, 52 out of 164 (31.7%) and 47 out of 165 (28.5%) stool samples collected tested positive for GIP during the run-in and placebo periods, respectively. Among these patients, 58.7% tested positive for GIP in both periods, 17.7% tested negative in both, 11.8% tested positive at the run-in and negative during the placebo, and 11.8% tested positive and negative, respectively. When comparing run-in and treatment periods in the placebo arm, 15% (3/20) of patients had GIP below 0.08 μg/g while 30% maintained high values at both time points and 25% (5/20) increased GIP during treatment with placebo. For the AN-PEP arm, 61 out of 151 (40.4%) samples tested positive for GIP during the run-in, while 54 out of 148 (36.5%) collected samples tested positive during the treatment period (data not shown). A more detailed analysis revealed that 17.7% (3/17) of patients exhibited no detectable stool GIP at both time points (run-in and AN-PEP administration), 29.4% (5/17) had reduced weekly GIP during AN-PEP and 17.7% (3/17) had unchanged GIP during both periods (data not shown). The average stool GIP per period for both arms is shown in Tables 1 and 2. During the run-in period, a noteworthy observation was that six patients (31.6%) in the AN-PEP arm exhibited an average stool GIP per period exceeding 0.08 μg/g. Among these cases, five patients had more than a 50% reduction or undetectable GIP. In contrast, among the four patients with high median concentrations (> 0.08 μg/g) at run-in in the placebo arm, only one patient experienced a decrease in GIP (data not shown). Overall, only 0.5% of all samples had average stool GIP concentrations greater than 0.32 μg/g, a threshold considered capable of causing mucosal damage. No patients were found to have a weekly average stool GIP concentration exceeding 0.64 μg/g.
The effects of AN-PEP on symptoms were analyzed based on the median CSI scores between the run-in periods and both treatments, as well as between the arms. Additionally, the proportion of cases with the highest CSI scores (> 38 points) was also examined. At randomization, no significant differences in median scores were observed between the placebo and AN-PEP arms, although patient scores in the AN-PEP arm were slightly higher than those in the placebo arm (Tables 1 and 2). Following treatment, there was a greater reduction in the median CSI score in the AN-PEP arm (12.5%) compared to the placebo arm (8.5%), although this difference was not statistically significant. The most notable finding was the decrease in the number of patients with the highest CSI scores (> 38 points) in the AN-PEP arm compared to those receiving placebo, which was statistically significant (P < 0.03) (Tables 1 and 2).
We also examined the impact of treatment on the concentrations of IgA tTG and IgA DGP autoantibodies before and after the intervention. In the placebo group, seven patients tested positive for IgA tTG at enrollment, and this number decreased to five after treatment. In the AN-PEP group, eight patients tested positive at enrollment, and this slightly decreased to seven after treatment. Regarding IgA DGP antibodies, the positivity rate was lower, with two patients in both the placebo and AN-PEP arms having high concentrations at enrollment, which increased to three and two patients, respectively, after treatment. However, as expected because of the short duration of the study, there were no significant differences observed when comparing the median serum concentrations of both antibodies between baseline and final measurements within the same treatment arm or between the placebo and AN-PEP arms (Tables 1 and 2).
The study also examined the impact of treatments on the QoL of patients using the SF-36 questionnaire, considering eight dimensions. Data for the ITT are reported in Table 3. At baseline, there were no significant differences in scores between arms across all dimensions for the ITT and PP analyses. After the treatment period, the placebo arm showed a significant improvement in general health, pain, and emotional limitations both in the ITT and PP analyses (P < 0.05 for all dimensions). In contrast, compared to the baseline assessment, patients in the AN-PEP arm had higher scores for pain (P < 0.05), but a higher score for vitality (P < 0.005), as assessed by both ITT and PP analyses. Neither AN-PEP nor placebo elicited any reported adverse events or concerns from the patients, indicating that the treatment doses were well-tolerated. Additionally, all three cases that were excluded from the ITT analysis tested negative for GIP. In these cases, the omissions in adhering to the trial were attributed to the individuals’ intense working activity outside of their homes rather than any issues related to the treatment itself.
| Item | Placebo arm | AN-PEP arm | ||
| At baseline | Final | At baseline | Final | |
| Number of patients | 21 | 19 | ||
| General health | ||||
| Median score (IQR) | 65 (50-72) | 70 (50-80)b | 50 (35-75) | 50 (40-80) |
| Pain | ||||
| Median score (IQR) | 80 (42-90) | 90 (50-80)b | 80 (45-90) | 67.5 (47.5-90.0)a |
| Vitality | ||||
| Median score (IQR) | 60 (45-65) | 60 (50-70) | 40 (30-60) | 55 (40-65)c |
| Mental health | ||||
| Median score (IQR) | 68 (52-72) | 68 (56-80) | 68 (40-72) | 64 (48-80) |
| Physical function | ||||
| Median score (IQR) | 95 (75-100) | 95 (75-100) | 95 (85-100) | 100 (90-100) |
| Social function | ||||
| Median score (IQR) | 75 (50-100) | 100 (62.5-100.0) | 50 (37-100) | 75 (50-100) |
| Physical limitations | ||||
| Median score (IQR) | 100 (50-100) | 100 (75-100) | 100 (25-100) | 75 (25-100) |
| Emotional limitations | ||||
| Median score (IQR) | 50 (0-100) | 100 (66-100)b | 66.7 (0-100) | 66.7 (0-100) |
The primary objective of this exploratory study was to investigate the effects of AN-PEP, a proline-specific endoprotease, at a dose of 650 mg per meal, three times a day (during breakfast, lunch, and dinner) for 4 consecutive weeks, on involuntary gluten exposure in patients following a long-term GFD. A placebo arm was included for comparison. By replicating a real-life situation, the study expands on previous challenge studies to address the common issue of dietary contamination occurring in diagnosed patients on a long-term GFD[8,25-28]. In the AN-PEP arm, there was an observable reduction in the average stool GIP concentration during the treatment period compared with the 4-wk run-in phase. Patients in the placebo arm also exhibited a decrease in GIP. While the magnitude of reduction in GIP tended to be higher in patients receiving AN-PEP (47.6%) vs placebo (44.1%), this difference did not reach statistical significance. Interestingly, during the run-in period, approximately 35.3% of patients in the AN-PEP arm had GIP levels exceeding 0.08 μg/g. After undergoing protease treatment, five out of these six patients experienced a 50% reduction or more in GIP after AN-PEP, while only one out of four patients in the placebo group experienced such a reduction.
Neither AN-PEP nor placebo elicited any reported adverse events or concerns from the patients, indicating that the treatment doses were well-tolerated. Additionally, all three cases that were excluded from the ITT analysis tested negative for GIP. In these cases, the omissions in adhering to the trial were attributed to the individuals’ intense working schedules rather than issues related to the treatment itself. Although CSI scores did not show a significant reduction between active treatment and placebo, the proportion of patients with the highest CSI score, which is considered indicative of the most symptomatic clinical course, was significantly reduced by AN-PEP. No changes were detected in specific CeD serology between treatment arms. Compared with the run-in period, patients in the placebo arm reported improved QoL scores in the areas of general health, while those in the AN-PEP arm had better scores for vitality and severe abdominal pain. These differences could be attributed to inherent limitations associated with questionnaires, such as subjectivity, and other external factors influencing QoL. Before this study, AN-PEP had demonstrated promising potential for degrading gluten in various in vitro and ex vivo laboratory models, as well as in gluten challenge trials.
However, testing AN-PEP in patients on long-term GFD who could be exposed to real-life dietary contamination has never been investigated[17-20]. While important in the daily management of CeD, a “real-life” design is not devoid of limitations, as real-life variability and trial effects cannot be controlled[8,29,30]. Indeed, this could explain our finding of an overall lower GIP when compared with the run-in period. Thus, and in contrast to findings from our previous studies[8], the level of GIP never reached one that could be associated with mucosal damage[27]. This discrepancy could be attributed to trial timing, which primarily took place during the COVID-19 pandemic when our country was characterized by high viral infection rates and prolonged isolation and lockdown. Consequently, the enforced stay-at-home measures during this time may have contributed to improved adherence to the GFD, as has been suggested by a recent study[31].
The relationship between the dose of AN-PEP, its enzymatic activity, the ratio of the protease administered per the total gluten intake, and the kinetics of substrate cleavage is a crucial aspect to consider. Previous studies have emphasized the importance of the dose-substrate relationship, contact time, and pH conditions in achieving optimal cleavage of gluten by peptidases[32]. König et al[16] conducted a study demonstrating the effective cleavage of gliadin peptides by AN-PEP shortly after its co-administration with gluten in a challenging study, even under varying caloric densities and acidic conditions. In our study, we opted for an AN-PEP dose that was twice the highest concentration previously demonstrated to effectively cleave gliadin in healthy individuals subjected to gluten challenges[21]. In essence, our assessment indicates that the observed gluten intake levels suggest a predominant trend of relatively low gluten exposure among the trial participants. However, it is important to underline that these individuals were concurrently receiving substantial doses of AN-PEP treatment. The study also investigated the impact of enzyme treatment on symptom intensity, as assessed by CSI score. Various factors have been associated with persistent symptoms in patients following a GFD, including involuntary and voluntary gluten exposure, irritable bowel syndrome, bacterial overgrowth syndrome, and a high intake of FODMAPs[1-5]. The most relevant and clinically interesting finding in the study was the significant reduction in the number of patients with the highest CSI scores (> 38 points) in the AN-PEP arm. This reduction could have been caused by a decrease in gluten exposure or through the action of the protease, and this cannot be identified with our design. While in a small number of patients, the observation that those with higher GIP experienced a > 50% reduction or non-detectable GIP after AN-PEP treatment supports the hypothesis of improved gluten degradation, as we did not observe a similar reduction in the placebo arm. Whether minor amounts of gluten exposure can contribute to symptom persistence is unclear, but hypersensitive patients have been reported[1]. Further research is needed to better understand the mechanisms underlying symptom persistence and the potential effects of AN-PEP treatment in larger trials with longer follow-up periods. Finally, while some parameters of QoL improved during AN-PEP vs placebo, we did not detect changes in CeD-specific serological tests.
One advantage of the present study was the attempt to mimic real-life conditions typically encountered by patients on lifelong GFD who are exposed to unpredictable gluten contamination. As previously discussed, this design is also susceptible to limitations; thus, while real-life studies are necessary for better long-term management of CeD, results need to be interpreted with caution as they lack the same level of control as gluten challenge studies[29,30]. Together, both designs can provide complementary and powerful clinical and pathophysiological insights. However, we believe that the duration of treatment (4 wk) might not suffice to detect significant modifications in outcome parameters, particularly in specific-serology testing.
In summary, AN-PEP treatment did not significantly reduce the overall GIP stool concentration. However, our results support the idea that AN-PEP has a positive impact on symptom intensity and on some aspects of QoL during a long-term GFD. More research is needed to unravel the underlying mechanisms this, as well as in larger populations with longer follow-ups to confirm these findings. These studies will help us better implement adjunct therapies for the GFD in CeD.
The impact of Aspergillus niger proline-specific endoprotease (AN-PEP) on gluten exposure resulting from inadvertent dietary lapses in celiac disease (CeD) patients adhering to a gluten-free diet (GFD) in real-life scenarios remains unknown.
Early-stage research has hinted at AN-PEP’s potential therapeutic role in breaking down gluten before it reaches the intestinal mucosa, potentially serving as an adjunct for managing gluten exposure. Despite AN-PEP’s approval as a dietary supplement, there is a lack of appropriate clinical studies to confirm its efficacy in detoxifying gluten immunogenic peptides (GIP).
To examine the effects of orally administered AN-PEP on inadvertent gluten exposure and symptom prevention in adult CeD patients following their usual GFD in a real-life scenario.
This exploratory trial employed a double-blind, randomized, placebo-controlled design involving CeD participants on a long-term GFD. After a 4-wk run-in phase, individuals were randomly assigned to receive either two AN-PEP capsules per meal for 4 wk or a placebo. The study main outcome endpoints were to compare the average weekly stool GIP and the celiac symptom index between run-in and treatment phases and between AN-PEP and placebo arms.
In this exploratory study, participants showed strong adherence to the GFD. While AN-PEP treatment did not decrease overall stool GIP concentration, the group receiving AN-PEP exhibited a significantly lower prevalence of severe symptoms compared to the placebo group.
Despite the lack of reduction in stool GIP due to AN-PEP administration in this real-life setting, the significant decrease in the number of symptomatic patients suggests the need for further investigation and more extensive studies.
Subsequent studies could delve into whether AN-PEP administration showcases a protective effect against gluten exposure using different research models. Additionally, these studies could aim to elucidate the reasons behind the observed reduction in symptomatic patients to better understand the potential mechanisms at play.
The authors thank Dr. Daniel Flores and Mrs. Karina Canguillen for their assistance with the independent design and administration of the randomization.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Argentina
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: de Moraes JB, Brazil S-Editor: Chen YL L-Editor: Filipodia P-Editor: Cai YX
| 1. | Catassi C, Verdu EF, Bai JC, Lionetti E. Coeliac Disease, Seminar. The Lancet. 2022;399:2413-2426. [RCA] [DOI] [Full Text] [Cited by in Crossref: 274] [Cited by in RCA: 229] [Article Influence: 76.3] [Reference Citation Analysis (1)] |
| 2. | Makharia GK, Singh P, Catassi C, Sanders DS, Leffler D, Ali RAR, Bai JC. The global burden of coeliac disease: opportunities and challenges. Nat Rev Gastroenterol Hepatol. 2022;19:313-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 77] [Article Influence: 25.7] [Reference Citation Analysis (1)] |
| 3. | Al-Toma A, Volta U, Auricchio R, Castillejo G, Sanders DS, Cellier C, Mulder CJ, Lundin KEA. European Society for the Study of Coeliac Disease (ESsCD) guideline for coeliac disease and other gluten-related disorders. United European Gastroenterol J. 2019;7:583-613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 293] [Cited by in RCA: 598] [Article Influence: 99.7] [Reference Citation Analysis (1)] |
| 4. | Kelly CP, Bai JC, Liu E, Leffler DA. Advances in diagnosis and management of celiac disease. Gastroenterology. 2015;148:1175-1186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 184] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 5. | Rubio-Tapia A, Hill ID, Semrad C, Kelly CP, Greer KB, Limketkai BN, Lebwohl B. American College of Gastroenterology Guidelines Update: Diagnosis and Management of Celiac Disease. Am J Gastroenterol. 2023;118:59-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 185] [Article Influence: 92.5] [Reference Citation Analysis (0)] |
| 6. | Husby S, Murray JA, Katzka DA. AGA Clinical Practice Update on Diagnosis and Monitoring of Celiac Disease-Changing Utility of Serology and Histologic Measures: Expert Review. Gastroenterology. 2019;156:885-889. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 156] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 7. | Coto L, Mendia I, Sousa C, Bai JC, Cebolla A. Determination of gluten immunogenic peptides for the management of the treatment adherence of celiac disease: A systematic review. World J Gastroenterol. 2021;27:6306-6321. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 17] [Cited by in RCA: 28] [Article Influence: 7.0] [Reference Citation Analysis (5)] |
| 8. | Stefanolo JP, Tálamo M, Dodds S, de la Paz Temprano M, Costa AF, Moreno ML, Pinto-Sánchez MI, Smecuol E, Vázquez H, Gonzalez A, Niveloni SI, Mauriño E, Verdu EF, Bai JC. Real-World Gluten Exposure in Patients With Celiac Disease on Gluten-Free Diets, Determined From Gliadin Immunogenic Peptides in Urine and Fecal Samples. Clin Gastroenterol Hepatol. 2021;19:484-491.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 55] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 9. | Kivelä L, Caminero A, Leffler DA, Pinto-Sanchez MI, Tye-Din JA, Lindfors K. Current and emerging therapies for coeliac disease. Nat Rev Gastroenterol Hepatol. 2021;18:181-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 79] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 10. | Piper JL, Gray GM, Khosla C. Effect of prolyl endopeptidase on digestive-resistant gliadin peptides in vivo. J Pharmacol Exp Ther. 2004;311:213-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 83] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 11. | Shan L, Marti T, Sollid LM, Gray M, Khosla C. Comparative biochemical analysis of three bacterial prolyl endopeptidases: implications for coeliac sprue. Biochem J. 2004;383:311-318. [RCA] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 177] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 12. | Salden BN, Monserrat V, Troost FJ, Bruins MJ, Edens L, Bartholomé R, Haenen GR, Winkens B, Koning F, Masclee AA. Randomised clinical study: Aspergillus niger-derived enzyme digests gluten in the stomach of healthy volunteers. Aliment Pharmacol Ther. 2015;42:273-285. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 55] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 13. | Gass J, Khosla C. Prolyl endopeptidases. Cell Mol Life Sci. 2007;64:345-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 105] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 14. | Wei G, Helmerhorst EJ, Darwish G, Blumenkranz G, Schuppan D. Gluten Degrading Enzymes for Treatment of Celiac Disease. Nutrients. 2020;12:2095. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 51] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 15. | Edens L, Dekker P, van der Hoeven R, Deen F, de Roos A, Floris R. Extracellular prolyl endoprotease from Aspergillus niger and its use in the debittering of protein hydrolysates. J Agric Food Chem. 2005;53:7950-7957. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 88] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 16. | König J, Holster S, Bruins MJ, Brummer RJ. Randomized clinical trial: Effective gluten degradation by Aspergillus niger-derived enzyme in a complex meal setting. Sci Rep. 2017;7:13100. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 47] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 17. | Montserrat V, Bruins MJ, Edens L, Koning F. Influence of dietary components on Aspergillus niger prolyl endoprotease mediated gluten degradation. Food Chem. 2015;174:440-445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 18. | Mitea C, Havenaar R, Drijfhout JW, Edens L, Dekking L, Koning F. Efficient degradation of gluten by a prolyl endoprotease in a gastrointestinal model: implications for coeliac disease. Gut. 2008;57:25-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 182] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 19. | Tack GJ, van de Water JM, Bruins MJ, Kooy-Winkelaar EM, van Bergen J, Bonnet P, Vreugdenhil AC, Korponay-Szabo I, Edens L, von Blomberg BM, Schreurs MW, Mulder CJ, Koning F. Consumption of gluten with gluten-degrading enzyme by celiac patients: a pilot-study. World J Gastroenterol. 2013;19:5837-5847. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 91] [Cited by in RCA: 103] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 20. | EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA), Turck D, Bresson JL, Burlingame B, Dean T, Fairweather-Tait S, Heinonen M, Hirsch-Ernst KI, Mangelsdorf I, McArdle HJ, Naska A, Neuhäuser-Berthold M, Nowicka G, Pentieva K, Sanz Y, Siani A, Sjödin A, Stern M, Tomé D, Vinceti M, Willatts P, Engel KH, Marchelli R, Pöting A, Poulsen M, Schlatter J, Gelbmann W, Van Loveren H. Safety of proline-specific oligopeptidase as a novel food pursuant to Regulation (EC) No 258/97. EFSA J. 2017;15:e04681. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 21. | Stepniak D, Spaenij-Dekking L, Mitea C, Moester M, de Ru A, Baak-Pablo R, van Veelen P, Edens L, Koning F. Highly efficient gluten degradation with a newly identified prolyl endoprotease: implications for celiac disease. Am J Physiol Gastrointest Liver Physiol. 2006;291:G621-G629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 186] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 22. | Janssen G, Christis C, Kooy-Winkelaar Y, Edens L, Smith D, van Veelen P, Koning F. Ineffective degradation of immunogenic gluten epitopes by currently available digestive enzyme supplements. PLoS One. 2015;10:e0128065. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 37] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 23. | Leffler DA, Dennis M, Edwards George J, Jamma S, Cook EF, Schuppan D, Kelly CP. A validated disease-specific symptom index for adults with celiac disease. Clin Gastroenterol Hepatol. 2009;7:1328-1334, 1334.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 91] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 24. | Nachman F, del Campo MP, González A, Corzo L, Vázquez H, Sfoggia C, Smecuol E, Sánchez MI, Niveloni S, Sugai E, Mauriño E, Bai JC. Long-term deterioration of quality of life in adult patients with celiac disease is associated with treatment noncompliance. Dig Liver Dis. 2010;42:685-691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 94] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 25. | Comino I, Fernández-Bañares F, Esteve M, Ortigosa L, Castillejo G, Fambuena B, Ribes-Koninckx C, Sierra C, Rodríguez-Herrera A, Salazar JC, Caunedo Á, Marugán-Miguelsanz JM, Garrote JA, Vivas S, Lo Iacono O, Nuñez A, Vaquero L, Vegas AM, Crespo L, Fernández-Salazar L, Arranz E, Jiménez-García VA, Antonio Montes-Cano M, Espín B, Galera A, Valverde J, Girón FJ, Bolonio M, Millán A, Cerezo FM, Guajardo C, Alberto JR, Rosinach M, Segura V, León F, Marinich J, Muñoz-Suano A, Romero-Gómez M, Cebolla Á, Sousa C. Fecal Gluten Peptides Reveal Limitations of Serological Tests and Food Questionnaires for Monitoring Gluten-Free Diet in Celiac Disease Patients. Am J Gastroenterol. 2016;111:1456-1465. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 128] [Cited by in RCA: 150] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 26. | Cebolla Á, Moreno ML, Coto L, Sousa C. Gluten Immunogenic Peptides as Standard for the Evaluation of Potential Harmful Prolamin Content in Food and Human Specimen. Nutrients. 2018;10:E1927. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 46] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 27. | Costa AF, Sugai E, Temprano MP, Niveloni SI, Vázquez H, Moreno ML, Domínguez-Flores MR, Muñoz-Suano A, Smecuol E, Stefanolo JP, González AF, Cebolla-Ramirez A, Mauriño E, Verdú EF, Bai JC. Gluten immunogenic peptide excretion detects dietary transgressions in treated celiac disease patients. World J Gastroenterol. 2019;25:1409-1420. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 58] [Cited by in RCA: 63] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 28. | Fernández-Bañares F, Beltrán B, Salas A, Comino I, Ballester-Clau R, Ferrer C, Molina-Infante J, Rosinach M, Modolell I, Rodríguez-Moranta F, Arau B, Segura V, Fernández-Salazar L, Santolaria S, Esteve M, Sousa C; CADER study group. Persistent Villous Atrophy in De Novo Adult Patients With Celiac Disease and Strict Control of Gluten-Free Diet Adherence: A Multicenter Prospective Study (CADER Study). Am J Gastroenterol. 2021;116:1036-1043. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 45] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 29. | Skodje GI, van Megen F, Stendahl M, Henriksen C, Lundin KEA, Veierød MB. Detection of gluten immunogenic peptides and the Celiac Disease Adherence Test to monitor gluten-free diet: a pilot study. Eur J Clin Nutr. 2022;76:902-903. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 30. | Camm AJ, Fox KAA. Strengths and weaknesses of 'real-world' studies involving non-vitamin K antagonist oral anticoagulants. Open Heart. 2018;5:e000788. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 100] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 31. | Azoulay L. Rationale, Strengths, and Limitations of Real-World Evidence in Oncology: A Canadian Review and Perspective. Oncologist. 2022;27:e731-e738. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 27] [Reference Citation Analysis (0)] |
| 32. | Monzani A, Lionetti E, Felici E, Fransos L, Azzolina D, Rabbone I, Catassi C. Adherence to the Gluten-Free Diet during the Lockdown for COVID-19 Pandemic: A Web-Based Survey of Italian Subjects with Celiac Disease. Nutrients. 2020;12:3467. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |