Published online Mar 14, 2024. doi: 10.3748/wjg.v30.i10.1431
Peer-review started: November 27, 2023
First decision: December 12, 2023
Revised: December 29, 2023
Accepted: February 6, 2024
Article in press: February 6, 2024
Published online: March 14, 2024
Processing time: 108 Days and 4.1 Hours
Serotonin receptor 2B (5-HT2B receptor) plays a critical role in many chronic pain conditions. The possible involvement of the 5-HT2B receptor in the altered gut sensation of irritable bowel syndrome with diarrhea (IBS-D) was investigated in the present study.
To investigate the possible involvement of 5-HT2B receptor in the altered gut sensation in rat model and patients with IBS-D.
Rectosigmoid biopsies were collected from 18 patients with IBS-D and 10 patients with irritable bowel syndrome with constipation who fulfilled the Rome IV criteria and 15 healthy controls. The expression level of the 5-HT2B receptor in colon tissue was measured using an enzyme-linked immunosorbent assay and correlated with abdominal pain scores. The IBS-D rat model was induced by intracolonic instillation of acetic acid and wrap restraint. Alterations in visceral sensitivity and 5-HT2B receptor and transient receptor potential vanilloid type 1 (TRPV1) expression were examined following 5-HT2B receptor antagonist administration. Changes in visceral sensitivity after administration of the TRPV1 antago
Here, we observed greater expression of the 5-HT2B receptor in the colonic mucosa of patients with IBS-D than in that of controls, which was correlated with abdominal pain scores. Intracolonic instillation of acetic acid and wrap restraint induced obvious chronic visceral hypersensitivity and increased fecal weight and fecal water content. Exogenous 5-HT2B receptor agonist administration increased visceral hypersensitivity, which was alleviated by successive administration of a TRPV1 antagonist. IBS-D rats receiving the 5-HT2B receptor antagonist exhibited inhibited visceral hyperalgesia.
Moreover, the percentage of 5-HT2B receptor-immunoreactive (IR) cells surrounded by TRPV1-positive cells (5-HT2B receptor I+) and total 5-HT2B receptor IR cells (5-HT2B receptor IT) in IBS-D rats was significantly reduced by the administration of a 5-HT2B receptor antagonist.
Our finding that increased expression of the 5-HT2B receptor contributes to visceral hyperalgesia by inducing TRPV1 expression in IBS-D patients provides important insights into the potential mechanisms underlying IBS-D-associated visceral hyperalgesia.
Core Tip: Higher expression of the serotonin receptor 2B (5-HT2B receptor) was found in patients with irritable bowel syndrome with diarrhea (IBS-D) than in that of controls, which was correlated with abdominal pain scores. Exogenous 5-HT2B receptor agonist administration increased visceral hypersensitivity, which was alleviated by successive administration of a transient receptor potential vanilloid type 1 (TRPV1) antagonist. IBS-D rats receiving the 5-HT2B receptor antagonist exhibited inhibited visceral hyperalgesia. Hence, 5-HT2B receptor-induced visceral hyperalgesia may be mediated by TRPV1 channels, and the analgesic effect of 5-HT2B receptor antagonist could be used as a novel treatment for IBS-D.
- Citation: Li ZY, Mao YQ, Hua Q, Sun YH, Wang HY, Ye XG, Hu JX, Wang YJ, Jiang M. Serotonin receptor 2B induces visceral hyperalgesia in rat model and patients with diarrhea-predominant irritable bowel syndrome. World J Gastroenterol 2024; 30(10): 1431-1449
- URL: https://www.wjgnet.com/1007-9327/full/v30/i10/1431.htm
- DOI: https://dx.doi.org/10.3748/wjg.v30.i10.1431
Irritable bowel syndrome (IBS) is a chronic functional bowel disorder characterized by recurrent abdominal pain with altered bowel habits that affects approximately 15% of the population worldwide[1]. IBS significantly impacts the quality of life of patients. Although the pathogenesis of IBS is not completely understood, the role of abnormal visceral sensitivity in IBS has recently emerged[2,3]. 5-Hydroxytryptamine (5-HT) is known to play a key role in the physiological states of the gastrointestinal tract. Plasma 5-HT levels in IBS with diarrhea (IBS-D) patients were greater than those in healthy controls[4], suggesting a possible role of 5-HT in the pathogenesis of IBS-D.
The serotonin receptor 2 (5-HT2 receptor) family comprises three subtypes: 5-HT2A, 5-HT2B, and 5-HT2c. All 5-HT2 receptors exhibit 46%-50% overall sequence identity, and all of these receptors preferentially bind to Gq/11 to increase inositol phosphates and intracellular calcium mobilization[5]. 5-HT2B receptors are widely expressed throughout the gut, and experimental evidence suggests that the primary function of 5-HT2B receptors is to mediate contractile responses to 5-HT through its action on smooth muscle[6]. The 5-HT2B receptor is localized to both neurons of the myenteric nerve plexus and smooth muscle in the human colon. The 5-HT2B receptor mediates 5-HT-evoked contraction of longitudinal smooth muscle[6]. These findings suggest that the 5-HT2B receptor could play an important role in modulating colonic motility, which could affect sensory signaling in the gut. Other laboratories have shown that the 5-HT2B receptor participates in the development of mechanical and formalin-induced hyperalgesia[7,8]. A 5-HT2B receptor antagonist reduced 2,4,6-trinitrobenzene sulfonic acid (TNBS) and stress-induced visceral hyperalgesia in rats[9,10]. However, the role of the 5-HT2B receptor in IBS-D patients and in acetic acid- and wrap restraint-induced IBS-D rat models was not investigated.
Transient receptor potential vanilloid type 1 (TRPV1) is a receptor that responds to heat, acidosis, capsaicin, and endovanilloids and is present on peripheral nerve endings, where its activation and signaling to the brain result in nociception[11]. Upregulation and/or sensitization of TRPV1 is considered an important mechanism of visceral hyperalgesia both in preclinical rat models and in patients with IBS[11,12]. The 5-HT2B receptor mediates 5-HT-induced mechanical hyperalgesia by regulating TRPV1 function[13]. TRPV1 function is enhanced by 5-HT2B receptor activation in mouse colon sensory neurons[14]. However, whether TRPV1 is involved in 5-HT2B receptor-induced visceral hyperalgesia in IBS-D patients is unknown.
In this study, we demonstrated that 5-HT2B receptor-induced visceral hyperalgesia in IBS-D patients occurs via the TRPV1 channel. 5-HT2B receptor expression was increased in the colonic mucosa of IBS-D patients and correlated with abdominal pain scores. Administration of a 5-HT2B receptor agonist significantly enhanced visceral sensitivity, and this increase was attenuated upon treatment with a TRPV1 antagonist. The number of 5-HT2B receptor- and TRPV1-positive cells was greater in IBS-D rats than in control rats. Interestingly, a 5-HT2B receptor antagonist not only alleviated visceral hypersensitivity but also decreased TRPV1 expression in IBS-D rats.
A total of 15 healthy volunteers (9 women and 6 men; 51.1 ± 10.3 years), 10 patients with IBS with constipation (IBS-C) (6 women and 4 men; 51.0 ± 11.5 years), and 18 patients with IBS-D (10 women and 8 men; 51.4 ± 9.8 years) meeting the Rome IV criteria were consecutively enrolled[15]. Patients who underwent colonoscopy for polyps and cancer surveillance had negative results. Patients who were subjected to major abdominal surgery, who received nonsteroidal or other anti-inflammatory drugs, or who had organic diseases, including celiac disease, psychiatric disorders, or allergic diseases, were excluded. All participants had macroscopically normal bowel mucosa upon examination. In total, four biopsy specimens were obtained from each participant from the rectosigmoid junction to standardize the site of sampling. In all the patients, one biopsy sample was sent for hematoxylin and eosin (HE) histological analysis and immunohistochemical staining. One biopsy sample was subjected to tissue immunofluorescence. Another two biopsy samples were immediately placed into a prepared Eppendorf tube, frozen immediately on ice and stored at -80 °C for enzyme-linked immunosorbent assay (ELISA) analysis.
The use of human tissue samples and clinical data was approved by the ethics committee of Dalian Friendship Hospital. All donors were informed of the aim of the study and gave consent to donate their samples.
All patients and controls were asked to score abdominal pain over 2 wk using a previously described validated questionnaire[16] since recollection was poor beyond this limit. Shorter periods may not have appropriately reflected the usual clinical picture, and this timeframe has previously been shown to be a reliable predictor of average pain intensity[17]. The severity of abdominal pain was graded as 0-4 based on the influence on daily activities of patients: 0, absent; 1, mild (no influence on activity); 2, relevant (diverting from but not urgently necessitating modification of activity); 3, severe (influencing activity to a marked extent, consequently requiring modifications); and 4, extremely severe (precluding daily activity). The frequency of abdominal pain was graded as 0–4 according to the frequency of symptoms per week: 0, absent; 1, up to 1 day/week; 2, 2 or 3 days/week; 3, 4-6 days/week; and 4, daily.
The 5-HT2B receptor agonist (BW723C86; Sigma–Aldrich, St. Louis, MO, United States) was diluted with saline. The 5-HT2B receptor antagonist 2-amino-4-(4-fluoronaphth-1-yl)-6-isopropylpyridine (RS-127445; Tocris Bioscience, Ellisville, Missouri, United States) was dissolved in 20% dimethylsulfoxide in physiological saline. The TRPV1 antagonist N-(3-methoxyphenyl)-4-chlorocinnamide (SB366791; Sigma–Aldrich, St. Louis, MO, United States) was dissolved in 50% dimethylsulfoxide in physiological saline. A human anti-5-HT2B receptor polyclonal antibody (MAB10322) was purchased from R&D Systems (Minneapolis, MN, United States). Mouse anti-5-HT2B receptor (AB194333), anti-alpha smooth muscle actin (AB5694) and rabbit anti-TRPV1 polyclonal antibodies (AB10296) were purchased from Abcam (Cambridge, MA, United States). Rabbit anti-Ecadherin antibody (CST 3195) was purchased from Cell Biological Technology, Inc. (Boston, United States). The rabbit anti-protein-encoding gene product (PGP) 9.5 (14730-1-AP) polyclonal antibody, rabbit anti-pankeratin (26411-1-AP) polyclonal antibody, and mouse anti-TRPV1 polyclonal antibody (66983-1-Ig) were obtained from Proteintech Group, Inc. (Chicago, United States). A 5-HT2B receptor ELISA kit was purchased from Jianglai Bio (Shanghai, China). A RNA polymerase chain reaction (PCR) kit was purchased from Takara Biotechnology (Dalian, China).
Adult male Sprague-Dawley rats (180-200 g) were obtained from Shanghai SIPPR-BK Laboratory Animal Co., Ltd. (Shanghai, China). Animals were housed with ad libitum access to food and water in standard rodent cages at 23 °C in a temperature- and light-controlled room. IBS-D rats were established by intracolonic instillation of acetic acid and wrap restraint as described by Williams et al[18] and Chen et al[19], with slight modifications. In brief, on the first day, after an overnight fast, the rats were anesthetized using ether, 1 mL of 4% acetic acid was instilled 8 cm proximal to the anus for 30 s, and then 1 mL of phosphate-buffered saline (PBS) was used to dilute the acetic acid and wash the colon. The rats were not subjected to any treatment for the following 3 d. From Days 5 to 18, the rats were subjected to wrap restraint stress for 2 wk (1 h per day).
The colorectal distension (CRD) test was carried out on Day 19 to evaluate visceral sensitivity, which was quantified using abdominal withdrawal reflex (AWR) scores. The threshold intensity of CRD was measured in rats on the same day, and the threshold intensity was determined as the pressure inducing the first abdominal contraction. Fecal weight and fecal water content were recorded on the same day. Rats that met the criteria for visceral hypersensitivity and had increased fecal weight and fecal water content were considered IBS-D rats.
The rats were divided into 6 groups, with 10 rats per group: (1) The normal control group included normal rats without any treatment; (2) In the BW723C86 group, normal control rats were injected intraperitoneally with BW723C86 (25 μg/kg/d, 100 μL/injection) daily for seven days; (3) In the BW723C86 + SB366791 group, normal control rats were injected intraperitoneally with SB366791 (10 mg/kg/d, 100 μL/injection) 30 min before BW723C86 was administered daily for seven days; (4) IBS-D rats without any treatment; (5) In the IBS-D + Vehicle group, IBS-D rats were injected intraperitoneally with 20% dimethylsulfoxide in physiological saline (100 μL/injection) daily for seven days; and (6) In the RS-127445 group, IBS-D rats were injected intraperitoneally with RS-127445 (10 mg/kg/d, 100 μL/injection) daily for seven days.
The doses and timings used in this study were chosen from previous studies[7,20]. At the end of the experiment, visceral sensitivity was evaluated by measuring the behavioral responses of the AWR to CRD in every animal group. The threshold intensity of CRD was measured in all the animal groups. Then, the animals were sacrificed, and distal colon tissue was collected. The colon was divided into 3 parts: One sample was used for ELISA analysis, and one sample was fixed in 4% paraformaldehyde for routine HE histological analysis, immunohistochemical staining and immunofluorescence. Under a stereomicroscope, another colon sample was gently peeled off, and the intestinal wall was peeled off into the mucosal layer and the intestinal muscle layer. Then, the tissue was stored at -80 °C for quantitative real-time reverse transcriptase polymerase chain reaction (RT-PCR). This study was approved by the Fudan University School of Medicine Animal Care and Use Committee and was performed in accordance with the guidelines of the International Association for the Study of Pain.
Briefly, rats were sedated with isoflurane for 1 min in a sealed cage connected to an animal anesthesia machine, and a flexible balloon guide wire (received as a gift from the Vascular Intervention Department of Jinshan Hospital of Fudan University) was then quickly inserted approximately 2.0 cm into the descending colon via the anus and firmly fixed with adhesive tape. The rats were placed in a restraint chamber that prevented them from escaping or turning around and allowed to adapt for 15 min. CRD was generated by slowly inflating the balloon to a constant pressure using a balloon vasodilation catheter to control inflation. The balloon was inflated to 15, 30, 45 and 60 mmHg for 20 s, followed by a resting interval of 5 min, and this process was repeated 3 times to achieve an accurate result. The responses of the animals to CRD were carefully observed until pain-related behaviors were observed. AWR scores were graded on a scale of 0–4[21] (0, normal behavior; 2, contraction of abdominal muscles; 3, lifting of the abdominal wall; 4, body arching and lifting of pelvic structures). The final score was calculated from the mean scores of three data points.
Fecal weight and fecal water content were recorded on Day 19 to evaluate the establishment of the IBS-D rat model. To measure the fecal weight and fecal water content, the feces expelled by each rat were collected and recorded after 3 h. The feces were weighed again after drying in an oven. Fecal water content = [wet fecal weight (g)-dried fecal weight (g)]/wet fecal weight (g) × 100%[19].
For histological experiments, distal colon tissue from each rat was fixed in 4% paraformaldehyde, embedded in paraffin, and cut into 4 μm sections. Sections were stained with HE and scored for histopathological structures by a pathologist.
Mucosal samples and muscular samples were homogenized in a prepared ice-cold 100 nM Tris mixture containing protease inhibitors (Beyotime, Shanghai, China) supplemented with 1 mmol/L phenylmethanesulfonyl fluoride. The mucosal and muscular samples were centrifuged at 12000 × g for 15 min at 4 °C. The homogenates were centrifuged at low temperature for 20 min at 3000 rpm. The protein concentration in the supernatant was detected on a Nanodrop 2000 (Thermo Fisher Scientific, Waltham, MA, United States). The concentrations of the 5-HT2B receptor in the supernatants were detected via ELISA using specific kits (Jianglai bio, Shanghai, China). The detection range for this assay was 2.5–40 ng/mL. Each sample was measured in duplicate. Reactivity was assessed at 450 nm.
Total RNA was extracted from colon samples with TRIzol reagent (Tiangen Biotech, Beijing, China). SYBR Green quantitative RT-PCR was performed to determine the expression of the 5-HT2B receptor and TRPV1 genes with a 7900HT Fast real-time PCR system (Applied Biosystems, Foster City, CA, United States) according to the instructions of the SYBR Premix EX Taq Kit (Takara Biotechnology, Dalian, China). Control quantitative reactions were performed in the absence of cDNA template. β-Actin was used as the reference gene. The primer sequences are shown in Table 1.
| Gene | Primer sequences (5’-3’) | |
| ACTB | Forward | GGCTGTATTCCCCTCCATCG |
| Reverse | CCAGTTGGTAACAATGCCATGT | |
| 5-HT2B receptor | Forward | AGAACCAGGGGAATACAG |
| Reverse | GGGAAATGGCACAGAGA | |
| TRPV1 | Forward | TGAAGCCGTTGCTCAGAATAACTG |
| Reverse | CTCAGGGTCTTTGAACTCGTT | |
Biopsy samples from participants and colon tissues from rats were fixed in 4% formalin, and 4 μm thick sections were used for immunohistological analyses. Following routine deparaffinization, rehydration and antigen retrieval, the sections were incubated with a rabbit anti-TRPV1 polyclonal antibody (1:300, AB1029) and mouse anti-5-HT2B receptor (1:500, AB194333) overnight at 4 °C. Next, the sections were incubated at room temperature for 2 h with a horseradish peroxidase-conjugated secondary antibody (Jingmei Biological Engineering Co., Shenzhen, China). Visualization involved the use of diaminobenzidine as a chromogen. Subsequently, the slides were counterstained with hematoxylin and examined under a light microscope (Olympus, Tokyo, Japan).
Colonic tissue samples collected from rats and biopsy samples from participants were perfused with saline followed by fixative containing 4% paraformaldehyde. Sections of colonic tissues were incubated with a rabbit anti-TRPV1 polyclonal antibody (1:300, AB1029), mouse anti-5-HT2B receptor (1:200, AB194333) or human anti-5-HT2B receptor polyclonal antibody (1:200, MAB10322) overnight at 4 °C, followed by donkey anti-rabbit and anti-mouse secondary antibodies at a dilution of 1:200 (Sigma). Sections of colonic tissues from rats were costained with mouse anti-5-HT2B receptor (1:100, AB194333), mouse anti-TRPV1 (1:200, Proteintech), rabbit anti-E-cadherin (1:400, CST 3195), rabbit anti-pankeratin (1:1500, Proteintech), anti-alpha smooth muscle actin (1:100, AB5694), and rabbit anti-PGP9.5 (1:1000, Proteintech) primary antibodies; FITC-conjugated goat-anti-rabbit-immunoglobulin G (IgG) antibody (1:200, Sigma); FITC-conjugated goat-anti-mouse-IgG antibody (1:200, Sigma); and FITC-conjugated goat-anti-guinea pig-IgG (1:200, Jackson ImmuoResearh). All the antibodies were diluted in PBS containing 1% BSA. The specimens were identified via a confocal microscope (Carl Zeiss AG, Jena, Germany). The digitized images were captured by using an automated acquisition system (TissueFAXS Plus, TissueGnostics GmbH, Austria). Then, 5-HT2B receptor-immunoreactive (IR) cells surrounded by TRPV1-positive in one-third or more of the 5-HT2B receptor-immunoreactive circumference were counted (5-HT2B receptor I+) and expressed as a percentage of total 5-HT2B receptor IR cells (5-HT2B receptor IT) in the fields analyzed (5-HT2B receptor I+/5-HT2B receptor IT). The data for each group were collected from 10 slides. A t test for two proportions was used to test the level of significance.
Depending on the distribution of the variables, Student's t-test, Chi-square test, or Wilcoxon rank sum was used to test for differences in parameters between groups. Continuous variables are presented as arithmetic mean ± SD. Categorical variables are presented as numbers (percentages). Associations between two parameters were analyzed via Spearman's rank correlation. All statistical analyses were performed using R software (version 4.1.0) and GraphPad Prism (version 6.0). The statistical significance level was set at P < 0.05 (both sides).
The clinical characteristics of the IBS-D patients, IBS-C patients and control subjects are described in Table 2. No significant differences in age or sex were evident among the three groups. A total of 18 patients with IBS-D were examined, and the mean scores of severity of abdominal symptoms were significantly greater in IBS-D patients than in controls (P < 0.001). The mean frequency of abdominal symptoms was significantly greater in IBS-D patients than in controls (P < 0.001). The mean scores of severity of abdominal symptoms in the IBS-C patients were significantly greater than that in the controls (P < 0.001). The mean frequency of abdominal symptoms in IBS-C patients was markedly greater than that in control patients (P < 0.001). We observed no significant differences between the IBS-D and IBS-C groups.
| Clinical characteristics | Subjects (n = 43) | IBS subtype (n = 28) | ||||
| IBS (n = 28) | Control (n = 15) | P value | IBS-D (n = 18) | IBS-C (n = 18) | P value | |
| Age | 51.2 (10.2) | 51.1 (10.3) | 0.96 | 51.4 (9.8) | 51.0 (11.5) | 0.93 |
| Sex | 0.86 | |||||
| Male | 12 (42.9) | 6 (40.0) | 8 (44.4) | 4 (40.0) | 1.00 | |
| Female | 16 (51.7) | 9 (60.0) | 10 (55.6) | 6 (60.0) | ||
| Severity of abdominal pain | < 0.001 | 0.49 | ||||
| Absent (0) | 0 (0.0) | 8 (53.4) | / | / | ||
| Mild (1) | 10 (35.7) | 7 (46.7) | 7 (38.9) | 3 (30.0) | ||
| Relevant (2) | 12 (42.9) | 0 (0.0%) | 8 (44.4) | 4 (40.0) | ||
| Severe (3) | 6 (21.4) | 0 (0.0) | 3 (16.7) | 3 (30.0) | ||
| Frequency of abdominal pain | < 0.001 | 0.88 | ||||
| Absent (0) | 0 (0.0) | 9 (60.0) | / | / | ||
| Up to 1 day/week (1) | 9 (32.1) | 6 (40.0) | 6 (33.3) | 3 (30.0) | ||
| 2 or 3 days/week (2) | 14 (50.0) | 0 (0.0) | 9 (50.0) | 5 (50.0) | ||
| 4-6 days/week (3) | 4 (14.3) | 0 (0.0) | 2 (11.1) | 2 (20.0) | ||
| Daily (4) | 1 (3.57) | 0 (0.0) | 1 (5.6) | 0 (0.0) | ||
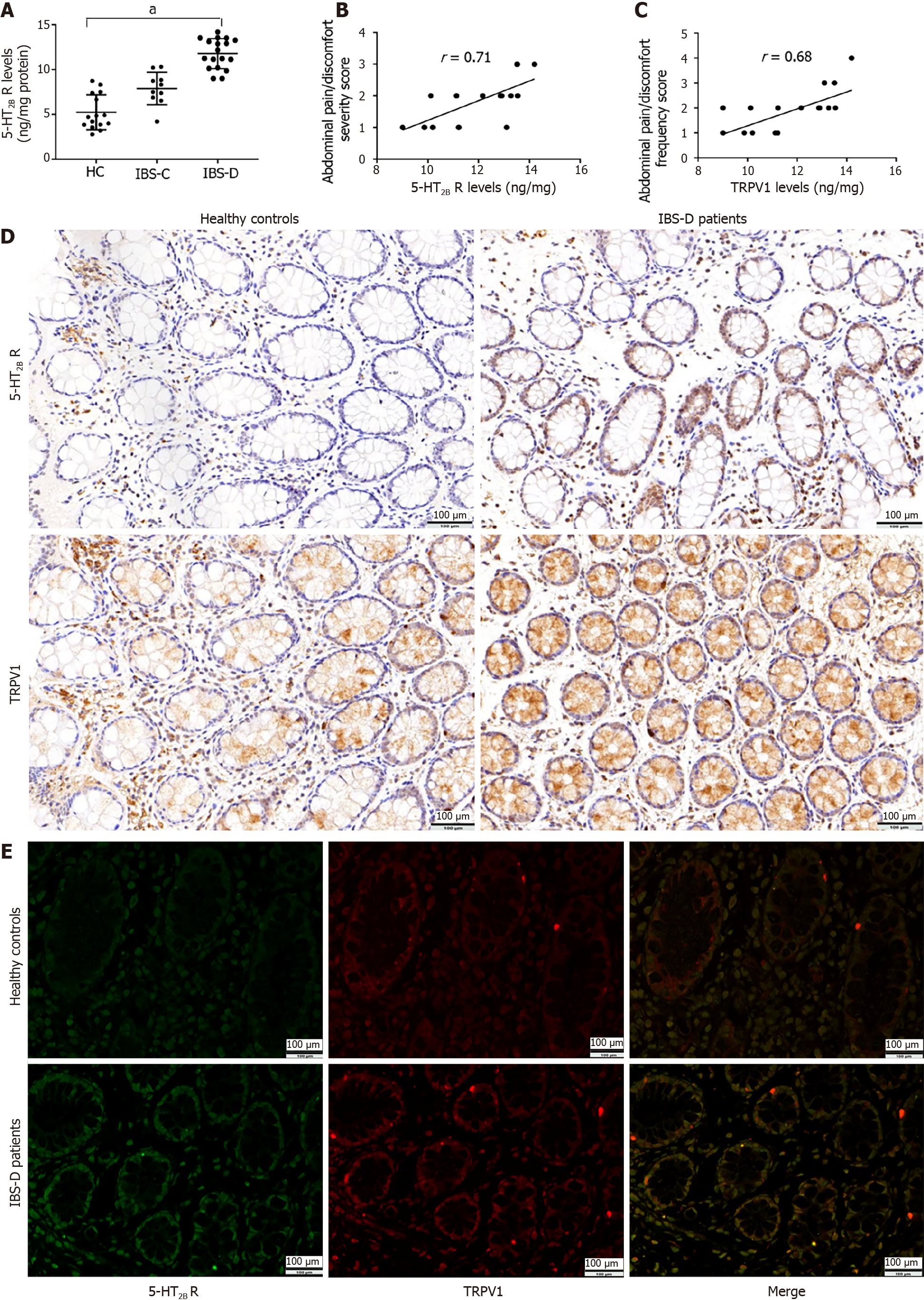
Compared with healthy controls, IBS-D patients exhibited significant upregulation of the 5-HT2B receptor in the intestinal mucosa (IBS-D: 11.78 ± 1.67 ng/mg protein vs healthy controls: 5.22 ± 1.95 ng/mg protein, P < 0.001) (Figure 1A). Compared with healthy controls, IBS-C patients also exhibited slight upregulation of the 5-HT2B receptor in the intestinal mucosa, but there was no significant difference between the IBS-C group and healthy controls (IBS-C: 6.08 ± 1.75 ng/mg protein vs. healthy controls: 5.22 ± 1.95 ng/mg protein, P = 0.42) (Figure 1A). Therefore, we selected the IBS-D group as our research group.
In patients with IBS-D, there was a significant correlation between mucosal 5-HT2B receptor levels and both the severity and frequency of abdominal pain (r = 0.71, P = 0.001 and r = 0.68, P = 0.001, respectively) (Figure 1B and C); however, this correlation was not observed in the control group.
As shown in Figure 1D, the immunohistochemical data confirmed the abundant expression of the 5-HT2B receptor in human colonic mucosal epithelial cells. TRPV1 was also expressed in human colonic mucosal epithelial cells (Figure 1D). The 5-HT2B receptor and TRPV1 were coexpressed in human colonic mucosal epithelial cells (Figure 1E).
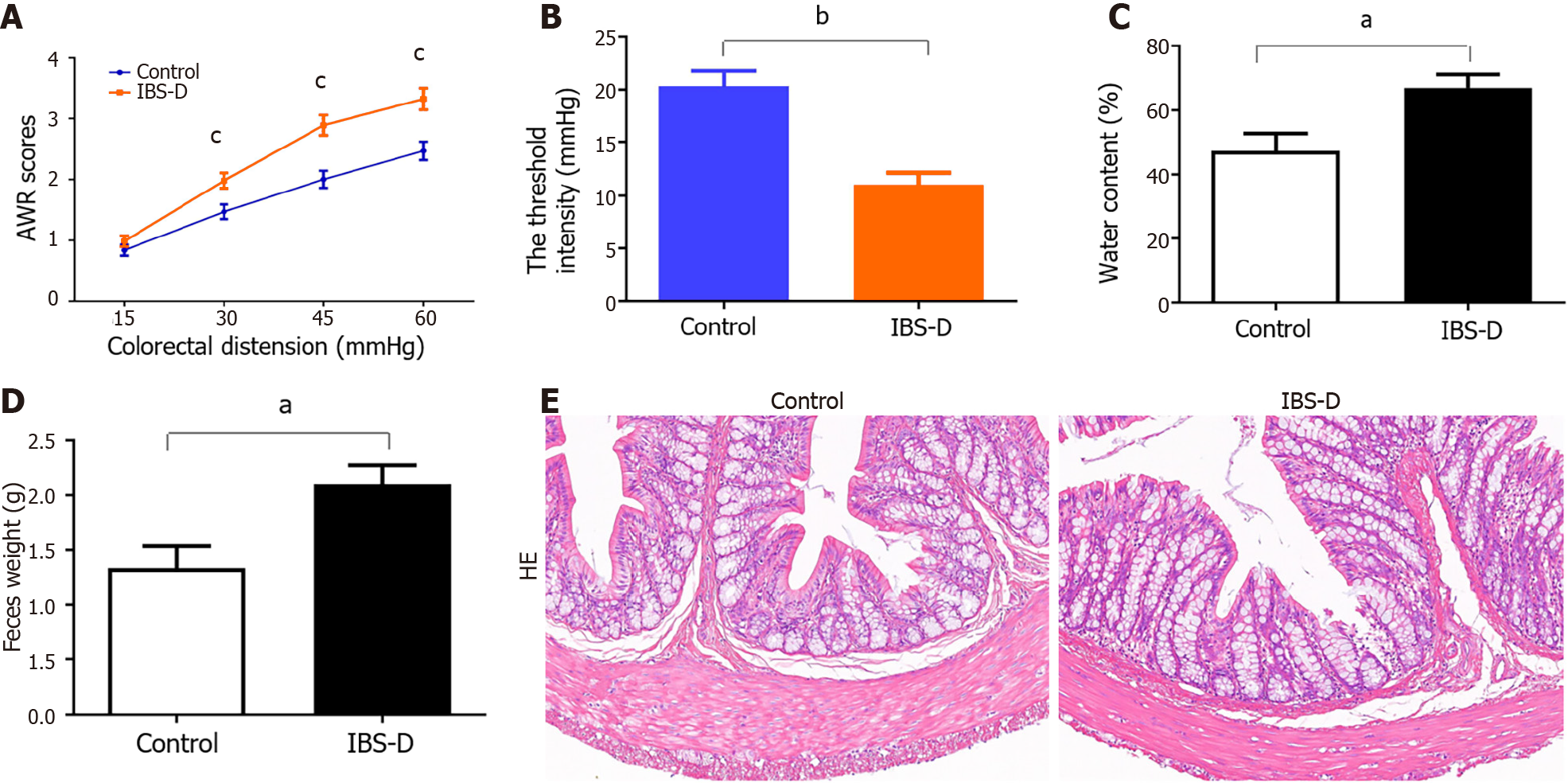
Visceral sensitivity was determined in animals on Day 19 by measuring the AWR scores in response to CRD. IBS-D rats had higher mean AWR scores at pressures of 30, 45 and 60 mmHg but not at 15 mmHg (Figure 2A). Compared with those in the normal control group, the threshold pressure in the IBS-D group also decreased (Figure 2B). Compared with that in the normal control group, the water content of the feces in the IBS-D group was significantly greater (Figure 2C). Additionally, the weight of the fecal pellets in the IBS-D group was greater than that in the normal control group (Figure 2D). HE-stained sections showed no obvious differences in histopathological structure between the IBS-D rats and normal control rats (Figure 2E). These results suggested that the IBS-D model was established successfully.
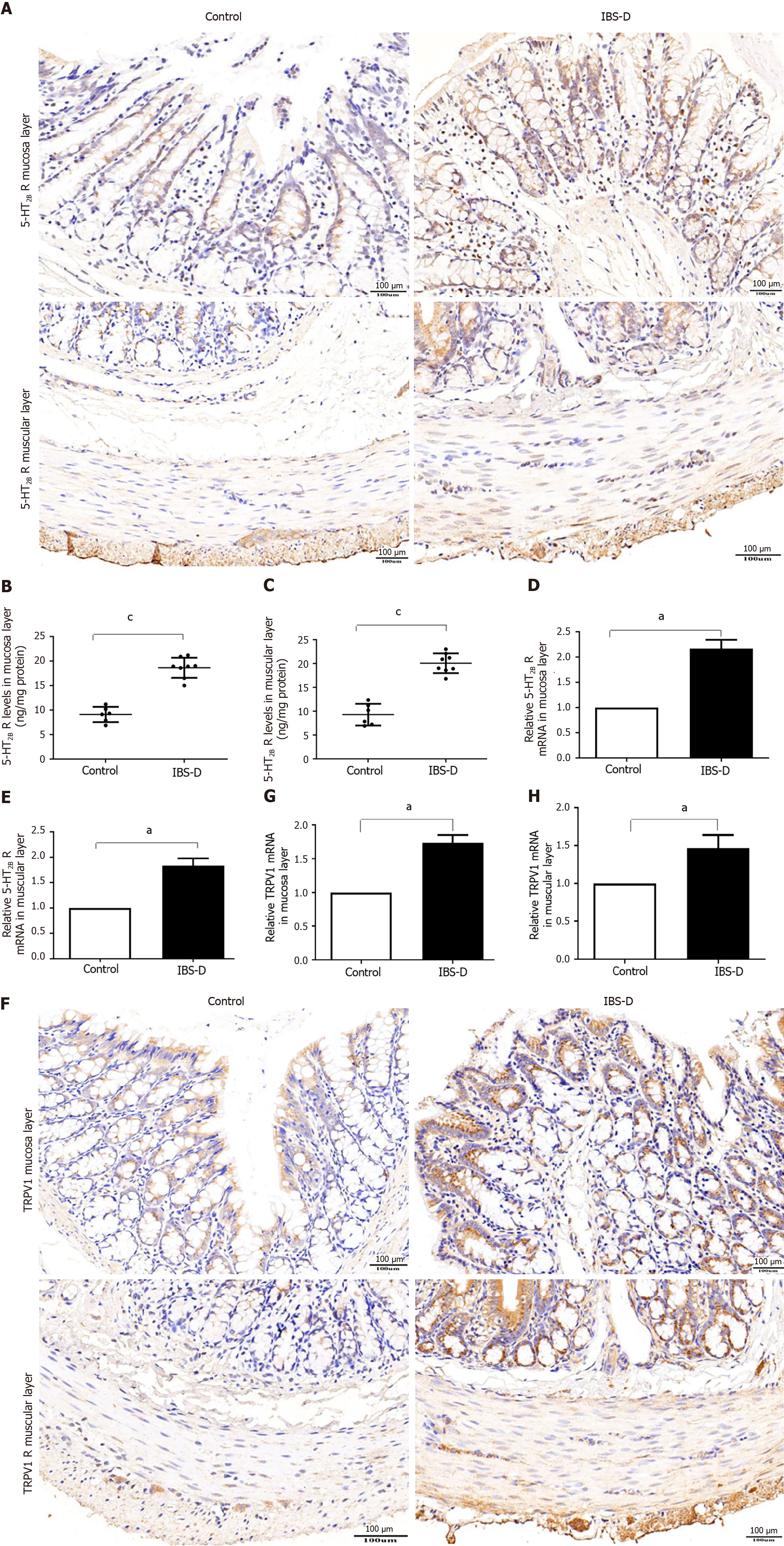
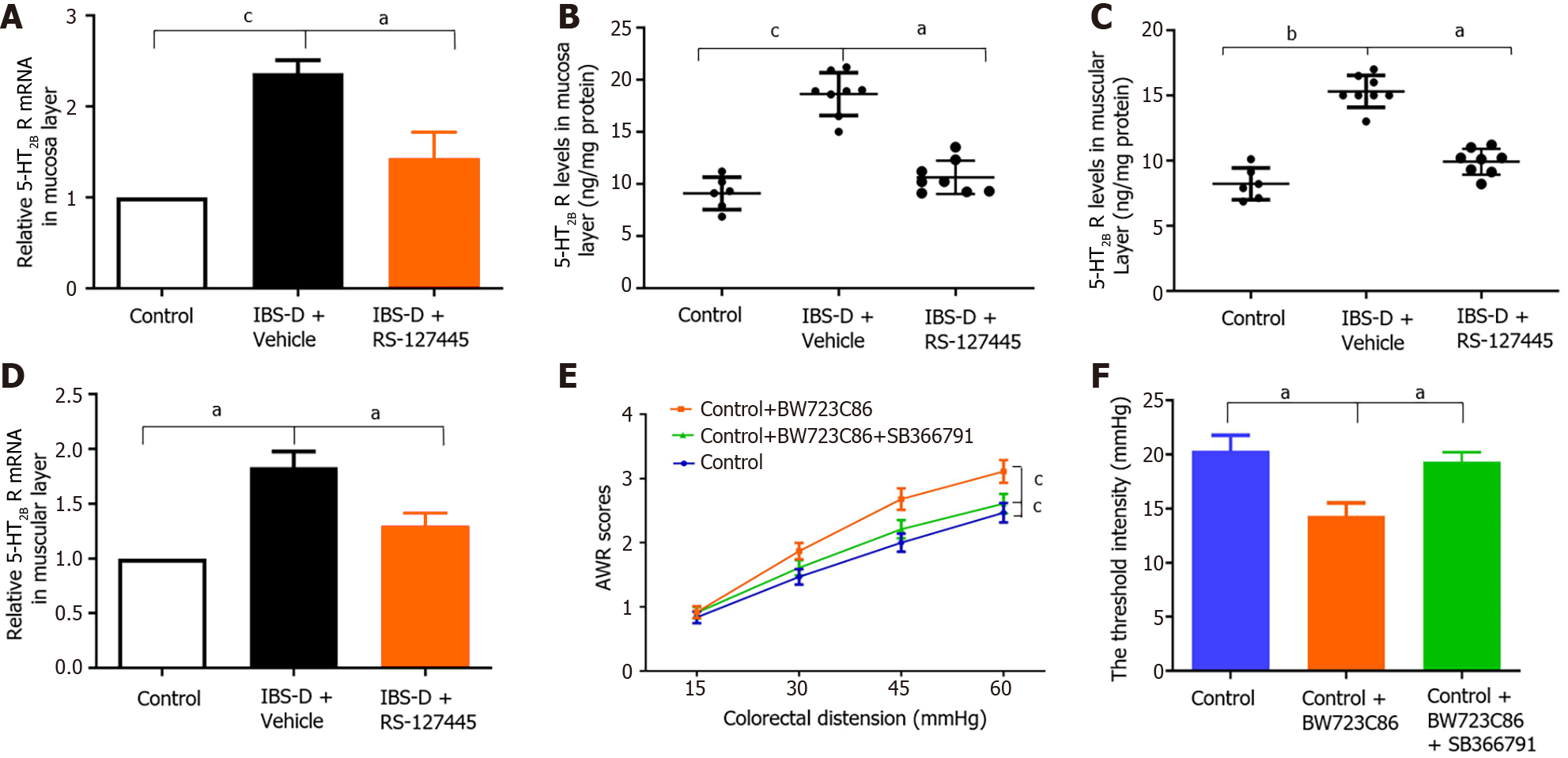
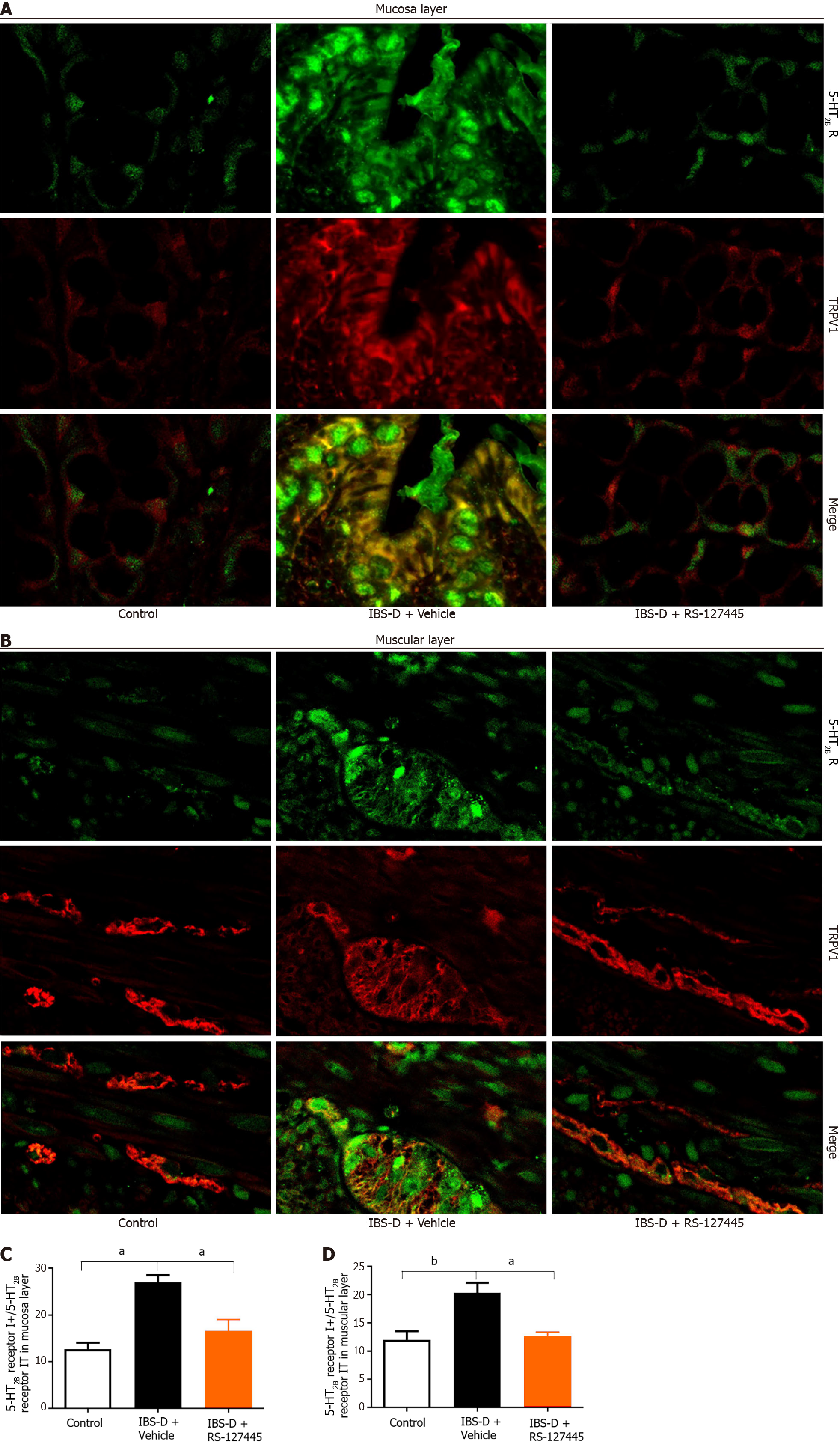
We investigated the changes in 5-HT2B receptor expression in the IBS-D rat group. A previous study showed that the 5-HT2B receptor is located mainly in smooth muscle layers and the myenteric nerve plexus[6]. Our results showed that 5-HT2B receptor immunoreactivity not only localized to the smooth muscle layer and myenteric nerve plexus but also to the colonic mucosa layer (Figure 3A). Notably, the expression of the 5-HT2B receptor in the IBS-D group was greater than that in the normal control group. The intestinal tissue of the 5-HT2B receptor in the IBS-D group was significantly upregulated compared with that in the normal control group (Figure 3B and C). qRT-PCR revealed that the expression of 5-HT2B receptor mRNA in the IBS-D group was significantly greater than that in the normal control group (Figure 3D and E). Immunohistochemistry showed that TRPV1 was localized in the colonic mucosa layer, smooth muscle layer, and myenteric nerve plexus (Figure 3F). qRT-PCR revealed that the expression of TRPV1 mRNA in the IBS-D group was greater than that in the normal control group (Figure 3G and H). These results indicated that upregulated 5-HT2B receptor and TRPV1 expression were involved in the pathogenesis of IBS-D.
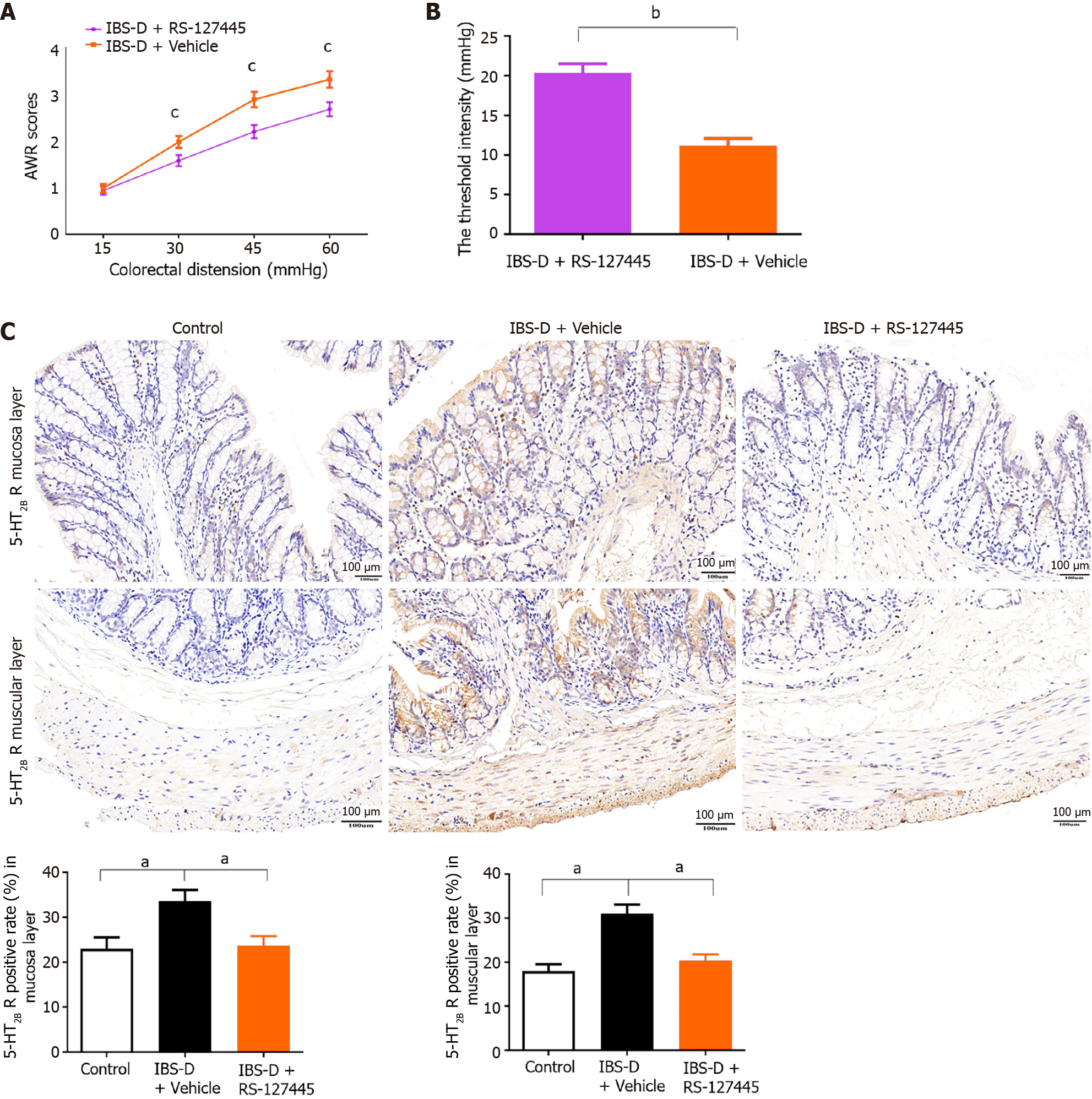
The 5-HT2B receptor was reported to participate in the pathophysiology of peripheral hyperalgesia[8]. The role of the 5-HT2B receptor in visceral hyperalgesia in IBS-D rats was investigated in our study. The 5-HT2B receptor antagonist RS-127445 was injected intraperitoneally into IBS-D rats. The results showed that, compared with vehicle, RS-127445 treatment induced a marked decrease in AWR and increased the threshold pressure (Figure 4A and B). Immunohistochemistry, qRT-PCR, and ELISA results demonstrated that 5-HT2B receptor protein and mRNA expression was downregulated in RS-127445-treated IBS-D rats (Figures 4C and 5A-D). Moreover, the 5-HT2B receptor agonist BW723C86 was injected intraperitoneally into healthy control rats once daily for 7 consecutive days. Interestingly, the results showed that the application of the 5-HT2B receptor agonist BW723C86 induced a marked increase in AWR scores and a decrease in threshold pressure in healthy rats compared with those in normal control rats (Figure 5E and F). These results demonstrated that the 5-HT2B receptor participated in the pathophysiology of visceral hyperalgesia in IBS-D rats.
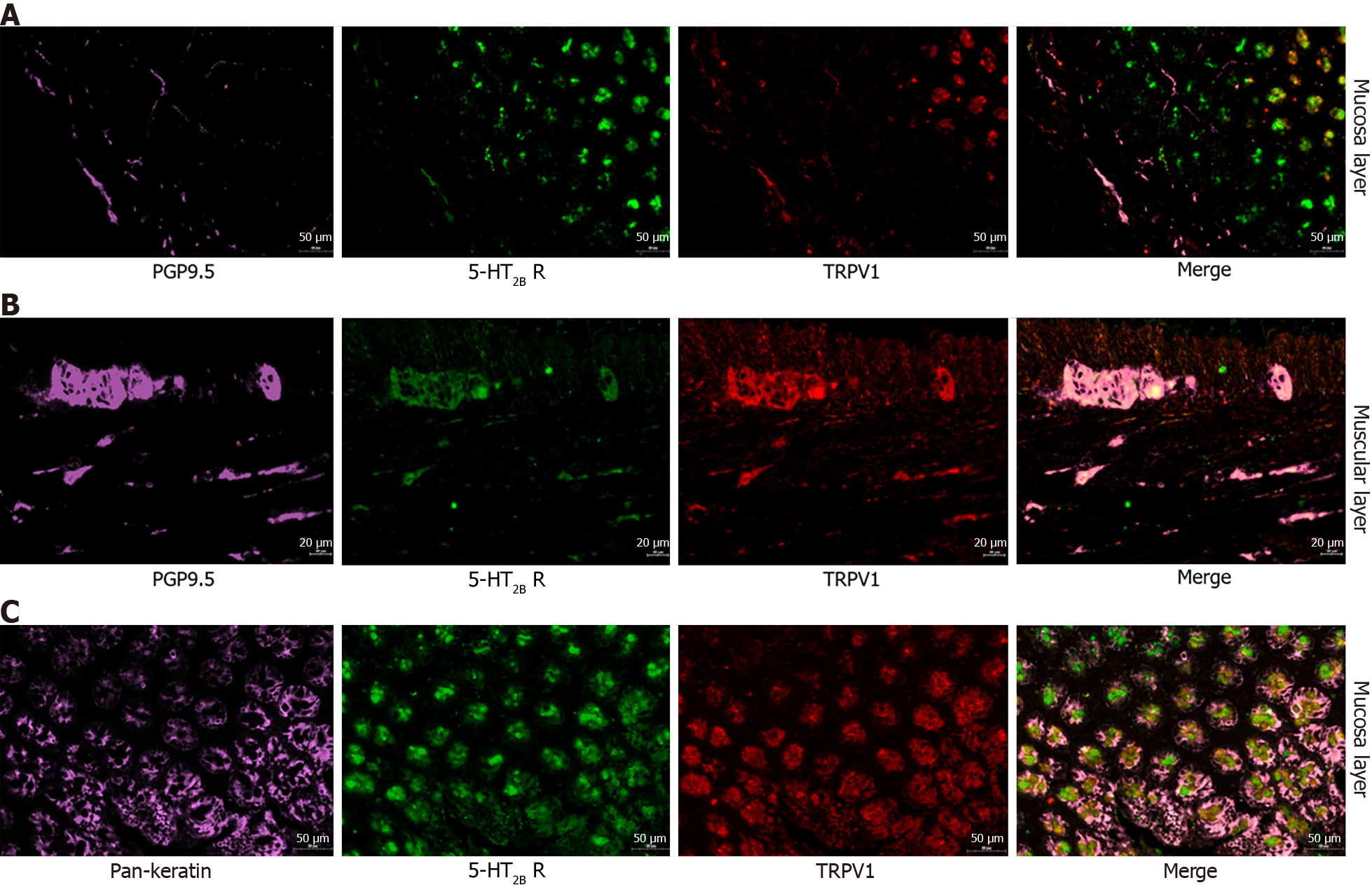
A previous study reported that a 5-HT2B receptor agonist induces mechanical hyperalgesia by regulating TRPV1 function[13]. In mouse colon sensory neurons, 5-HT2B receptor activation enhances TRPV1 function[14]. We hypothesized that 5-HT2B receptor-induced visceral hyperalgesia in IBS-D patients is mediated via the TRPV1 channel. First, we detected the coexpression of TRPV1 and the 5-HT2B receptor in colonic tissues. Immunofluorescence revealed that TRPV1 and the 5-HT2B receptor were coexpressed not only in smooth muscle layers and the myenteric nerve plexus but also in the colonic mucosa layer in humans and rats (Figures 1E, 6A and B). The percentage of 5-HT2B IR cells surrounded by TRPV1-positive cells (5-HT2B receptor I+) and total 5-HT2B receptor IR cells (5-HT2B receptor IT) in the IBS-D group was greater than that in the normal control group (Figure 6C and D).
Second, we aimed to identify in which layer the receptor of 5-HT2B and TRPV1 mediates visceral hyperalgesia in the IBS-D model. Then, 5-HT2B and TRPV1 were labeled with molecular markers of epithelial cells, peripheral nerve fibers and smooth muscle cells via immunofluorescence to determine the functions of these receptors in visceral pain sensation. Immunofluorescence staining demonstrated that 5-HT2B and TRPV1 were colocalized mainly in peripheral nerve fibers and colon epithelial cells (Figure 7). The 5-HT2B receptor and TRPV1 were not colocalized in smooth muscle cells. Visceral hypersensitivity is the most important pathophysiological alteration associated with visceral pain in IBS patients. The expression of 5-HT2B and TRPV1 in peripheral nerve fibers and the colonic mucosa layer may be involved in the gut sensation and visceral hyperalgesia observed in IBS-D patients.
Third, the TRPV1 antagonist SB366791 was administered to normal control rats 30 min before BW723C86, and the results showed that the BW723C86-induced visceral hyperalgesia was alleviated by SB366791 (Figure 5E and F).
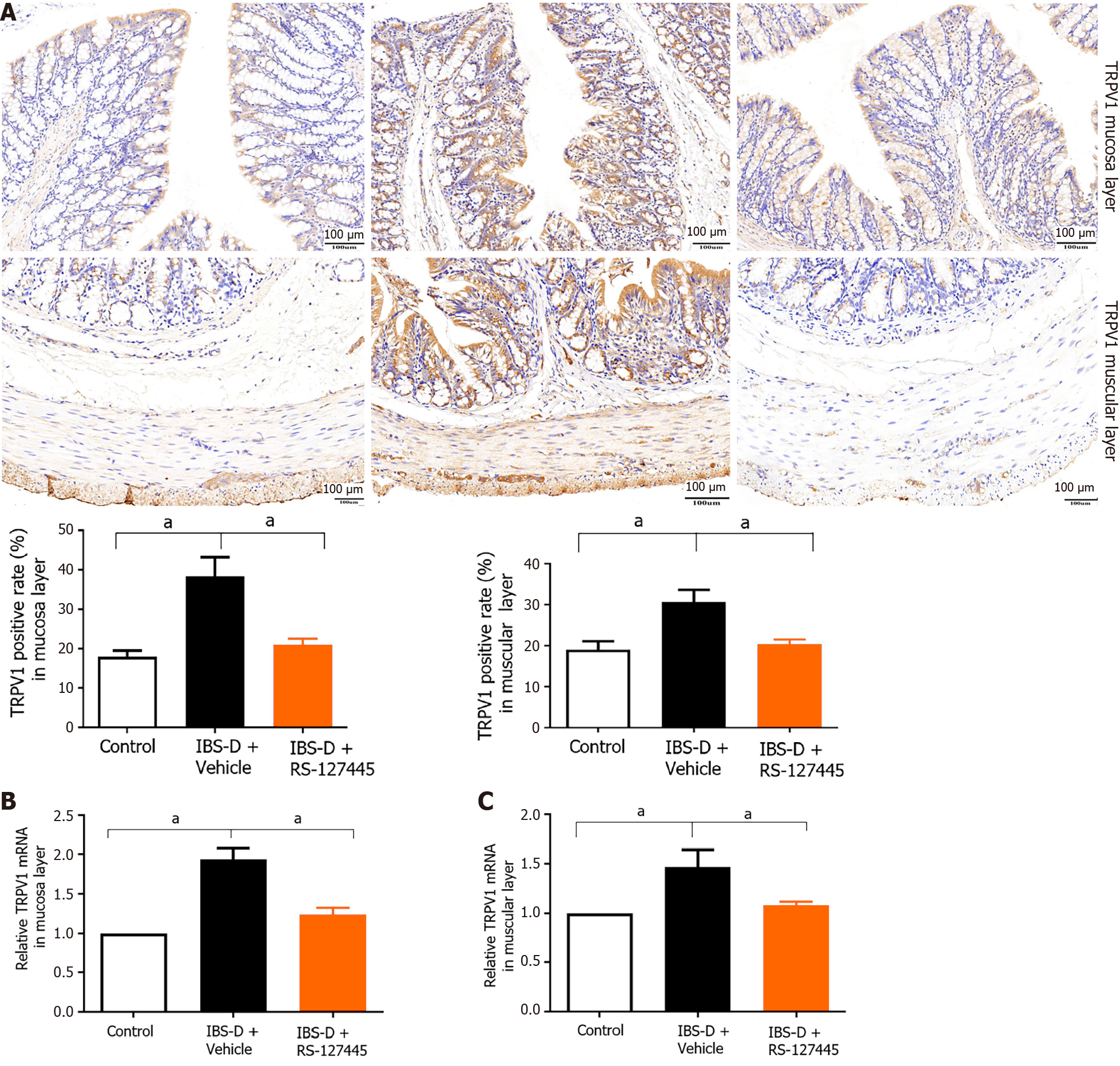
Fourth, qRT-PCR and immunohistochemistry were used to detect the expression of TRPV1 in RS-127445-treated IBS-D rats. The results showed that injection of RS-127445 significantly decreased the expression of TRPV1 in the colonic tissues of IBS-D rats compared with those of control IBS-D rats (Figure 8).
The 5-HT2B receptor has generated considerable interest in the research community owing to its critical role in the generation of hyperalgesic diseases. Previous studies have shown that peripheral and spinal 5-HT2B receptors have pronociceptive effects and participate in the development and maintenance of formalin-induced hyperalgesia[22]. The 5-HT2B receptor mediates 5-HT-induced mechanical hyperalgesia in mice[8]. Activation of the 5-HT2B receptor in meningeal nociception causes neurogenetic inflammation and the generation of migraine pain[23].
In the present study, we investigated the potential involvement of the 5-HT2B receptor in the visceral pain of IBS-D patients. Compared with those from control participants, colonic biopsies from patients with IBS-D revealed significant upregulation of the 5-HT2B receptor. Moreover, enhanced expression of the 5-HT2B receptor was positively correlated with the severity and frequency of abdominal pain symptoms. We instilled 1 mL of 4% acetic acid intracolonically and applied wrap restraint stress to induce an IBS-D rat model. Compared with normal control rats, this rat model showed visceral hypersensitivity and increased fecal weight and fecal water content, which was consistent with the clinical symptoms of IBS-D patients. Our findings showed that the 5-HT2B receptor was expressed not only in the colonic smooth muscle layers and myenteric nerve plexus but also in the colonic mucosa layer in rats. Increased expression of the 5-HT2B receptor in colon tissue was found in IBS-D rats compared with that in normal control rats. Previous studies demonstrated that the 5-HT2B receptor was expressed in smooth muscle layers and the myenteric nerve plexus of human and mouse intestines[6]. To our knowledge, this is the first study to explore the expression of the 5-HT2B receptor in the colon mucosa layer in IBS-D patients and rats. The upregulation of the 5-HT2B receptor in the colon mucosa layer may be involved in the gut sensation and visceral hyperalgesia of IBS-D patients.
Considering that visceral hypersensitivity is the most important pathophysiological alteration associated with visceral pain in IBS-D patients, we used a 5-HT2B receptor agonist (BW723C86). The administration of BW723C86 to normal control rats significantly increased visceral hypersensitivity. A 5-HT2B receptor antagonist (RS-127445) significantly decreased visceral hypersensitivity in IBS-D rats. In RS-127445-treated IBS-D rats, decreased mRNA expression of the 5-HT2B receptor was found in colon tissues. These results suggested the involvement of the 5-HT2B receptor in visceral hypersensitivity in rats. Our findings were consistent with previous work showing that RS-127445 inhibited visceral hyperalgesia in both TNBS-induced and stress-sensitive rat models of visceral hypersensitivity[9,10]. Tegaserod, which is effective at alleviating abdominal pain symptoms in IBS patients, is a potent 5-HT2B receptor antagonist[24]. Taken together, these data support the use of 5-HT2B receptor antagonists as novel treatments for visceral hyperalgesia in IBS-D patients.
Previous research has shown that 5-HT-induced excitatory effects in the human colon in vitro are mediated by the 5-HT2B receptor. RS-127445 inhibited 5-HT-induced excitatory effects[6]. Other research revealed that RS-127445 concentration-dependently reduced fecal output and peristaltic frequency in healthy mice[25]. A new study showed that the 5-HT2B receptor on interstitial cells of Cajal in diabetic mice was decreased and associated with constipation. Activation of the 5-HT2B receptor improved colonic motility and constipation in diabetic mice[20]. Taken together, these results indicated that 5-HT2B receptor activation was related to gut motility. Considering that the 5-HT2B receptor induces colonic motility, the upregulation of the 5-HT2B receptor may be related to the development of diarrhea in patients with IBS-D. Additionally, it is conceivable that the upregulation of the 5-HT2B receptor may be relevant to the visceral hyperalgesia that we observed in this study because disorders of muscle tone and colonic motility have been postulated to be related to visceral hyperalgesia.
The 5-HT2B antagonist RS-127445 effectively alleviated pain in the IBS-D model. However, it is not clear in which kind of cell these two receptors are located. It is also unclear in which layer of the colon the receptor of 5-HT2B and TRPV1 mediates visceral hyperalgesia in the IBS-D model. Because the same receptor plays different roles in different cells, 5-HT2B and TRPV1 were labeled with molecular markers of epithelial cells, peripheral nerve fibers and smooth muscle cells via immunofluorescence to determine the functions of these receptors in visceral pain sensation. The results demonstrated that 5-HT2B and TRPV1 were colocalized mainly in peripheral nerve fibers and colon epithelial cells. Visceral hypersensitivity is the most important pathophysiological alteration associated with visceral pain in IBS patients. The expression of 5-HT2B and TRPV1 in peripheral nerve fibers and the colonic mucosa layer may be involved in the gut sensation and visceral hyperalgesia observed in IBS-D patients.
TRPV1 channels are critical contributors to normal and pathological pain and are likely to be activated by inflammatory products in IBS[26]. Upregulation and/or sensitization of TRPV1 is considered an important mechanism of visceral hyperalgesia both in preclinical rat models and in patients with IBS[27]. Our results indicated that TRPV1 was upregulated in IBS-D patients and rats, which was consistent with previous findings[27]. A previous report showed that TRPV1 activity is regulated by inflammatory mediators, including prostaglandins and bradykinin, likely through protein kinase C (PKC)- or cAMP-dependent protein kinase-mediated phosphorylation of the receptor. Generally, protein kinase-mediated phosphorylation of the TRPV1 receptor results in peripheral sensitization[28]. Data from a mouse model suggested that 5-HT2B receptor activation may enhance the responsiveness of the TRPV1 receptor to temperature and acid and thereby contribute to peripheral sensitization[14]. Serotonin signaling alterations in IBS may occur in part through TRPV1 sensitization[29].
A previous study demonstrated that the administration of antagonists of 5-HT2B, PLCβ, PKCε, and TRPV1 inhibited 5-HT-induced mechanical hyperalgesia. In DRG neurons, 5-HT injection increased capsaicin- or 5-HT-induced calcium signals, which were regulated by the 5-HT2B-PLCβ-PKCε pathway. The possible mechanism is as follows: Injection of 5-HT induces activation of 5-HT2B-PLCβ-PKCε in the peripheral nociceptors, after which TRPV1 is relieved from PIP2 inhibition to produce peripheral sensitivity, which increases the number of neurons responding to 5-HT[13]. Thus, 5-HT2B mediates 5-HT-induced mechanical hyperalgesia by regulating TRPV1 function[13]. In mouse colon sensory neurons, 5-HT2B receptor activation enhances TRPV1 function[14]. Our present study demonstrated that 5-HT2B and TRPV1 are located mainly in peripheral nerve fibers and that the number of 5-HT2B receptor- and TRPV1-positive cells was significantly greater in IBS-D rats than in normal control rats. The modulation of TRPV1 function by the 5-HT2B receptor may increase afferent input from the colon and provide a peripheral mechanism for the development of visceral pain symptoms in IBS-D patients. 5-HT2B receptor agonist-induced visceral hyperalgesia in normal rats was alleviated by a TRPV1 antagonist. RS-127445 not only inhibited visceral hyperalgesia but also decreased the expression of TRPV1. These results indicated that increased 5-HT2B receptor expression may participate in visceral hyperalgesia in IBS-D rats via TRPV1 channels. However, the exact underlying mechanism requires further research.
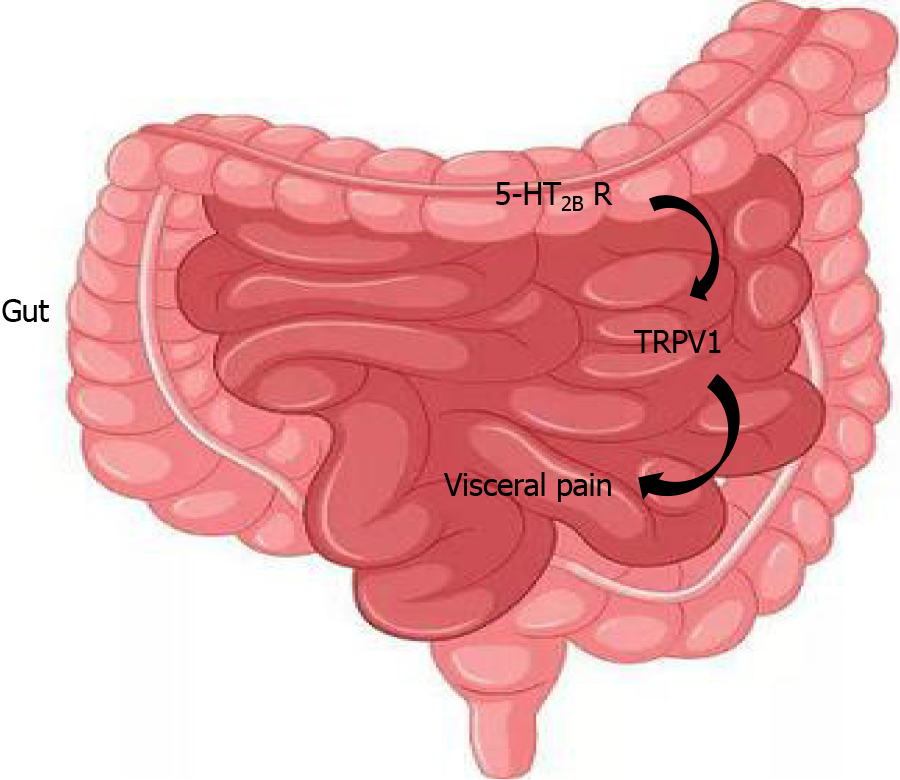
In conclusion, our present study showed that the 5-HT2B receptor may participate in visceral hyperalgesia in IBS-D patients. In addition, 5-HT2B receptor-induced visceral hyperalgesia may be mediated by TRPV1 channels (Figure 9). The analgesic effect of RS-127445 in IBS-D rats could be used as a novel treatment for IBS-D.
Patients with irritable bowel syndrome with diarrhea (IBS-D) experience a significant reduction in their quality of life. While the exact pathogenesis of IBS-D remains incompletely understood, research indicates that serotonin receptor 2B (5-HT2B receptor) plays a critical role in many chronic pain conditions. The role of 5-HT2B receptor in the altered gut sensation of IBS-D was not investigated.
This study is to identify the role of 5-HT2B receptor in the altered gut sensation via transient receptor potential vanilloid type 1 (TRPV1) channels in rat model and patients with diarrhea-predominant IBS.
This study aims to elucidate the role of the 5-HT2B receptor in both IBS-D patients and rat models induced by acetic acid and wrap restraint. The findings are anticipated to offer novel insights into potential avenues for IBS-D treatment.
Rectosigmoid biopsies were collected from IBS-D patients and healthy controls. The expression level of 5-HT2B receptor in colon tissue was measured and correlated with abdominal pain scores in IBS-D patients. The IBS-D rat model was induced by intracolonic instillation of acetic acid and wrap restraint. Alterations in visceral sensitivity, 5-HT2B receptor and TRPV1 expression were examined following 5-HT2B receptor antagonist administration. Changes in visceral sensitivity after the administration of the TRPV1 antagonist were recorded.
A higher expression of 5-HT2B receptor was observed in the colonic mucosa of patients with IBS-D compared to controls, correlating with abdominal pain scores. The IBS-D rats was successfully established through intracolonic instillation of acetic acid and wrap restraint. Administration of the exogenous 5-HT2B receptor agonist increased visceral hypersensitivity, which was subsequently alleviated by successive administration of TRPV1 antagonist. IBS-D rats receiving the 5-HT2B receptor antagonist displayed inhibition of visceral hyperalgesia. Additionally, the percentage of 5-HT2B receptor-immunoreactive (IR) cells surrounded by TRPV1-positive cells (5-HT2B receptor I+) and total 5-HT2B receptor IR cells (5-HT2B receptor IT) in IBS-D rats significantly decreased with the administration of 5-HT2B receptor antagonist.
The increased expression of 5-HT2B receptor contributing to visceral hyperalgesia through the induction of TRPV1 expression in IBS-D, providing important insights into the potential mechanisms underlying IBS-D-associated visceral hyperalgesia.
The analgesic effect of RS-127445 in IBS-D rats suggests its potential as a novel treatment for IBS-D.
The authors would like to acknowledge Prof. Yuan-Yuan Ruan (School of Basic Medical Sciences, Fudan University, Shanghai, China), Prof. Wei-Wei Zheng (School of Public Health, Fudan University, Shanghai, China) and Prof. Qian-Li (School of Basic Medical Sciences, Fudan University, Shanghai, China) for skillful technical assistance.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ghannam WM, Egypt S-Editor: Fan JR L-Editor: A P-Editor: Yu HG
| 1. | Lacy BE, Pimentel M, Brenner DM, Chey WD, Keefer LA, Long MD, Moshiree B. ACG Clinical Guideline: Management of Irritable Bowel Syndrome. Am J Gastroenterol. 2021;116:17-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 462] [Article Influence: 115.5] [Reference Citation Analysis (0)] |
| 2. | Lee YJ, Park KS. Irritable bowel syndrome: emerging paradigm in pathophysiology. World J Gastroenterol. 2014;20:2456-2469. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 102] [Cited by in RCA: 108] [Article Influence: 9.8] [Reference Citation Analysis (2)] |
| 3. | Mamieva Z, Poluektova E, Svistushkin V, Sobolev V, Shifrin O, Guarner F, Ivashkin V. Antibiotics, gut microbiota, and irritable bowel syndrome: What are the relations? World J Gastroenterol. 2022;28:1204-1219. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 11] [Cited by in RCA: 32] [Article Influence: 10.7] [Reference Citation Analysis (6)] |
| 4. | Cangemi DJ, Lacy BE. Management of irritable bowel syndrome with diarrhea: a review of nonpharmacological and pharmacological interventions. Therap Adv Gastroenterol. 2019;12:1756284819878950. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 52] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 5. | Cussac D, Boutet-Robinet E, Ailhaud MC, Newman-Tancredi A, Martel JC, Danty N, Rauly-Lestienne I. Agonist-directed trafficking of signalling at serotonin 5-HT2A, 5-HT2B and 5-HT2C-VSV receptors mediated Gq/11 activation and calcium mobilisation in CHO cells. Eur J Pharmacol. 2008;594:32-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 74] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 6. | Borman RA, Tilford NS, Harmer DW, Day N, Ellis ES, Sheldrick RL, Carey J, Coleman RA, Baxter GS. 5-HT(2B) receptors play a key role in mediating the excitatory effects of 5-HT in human colon in vitro. Br J Pharmacol. 2002;135:1144-1151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 79] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 7. | Cervantes-Durán C, Vidal-Cantú GC, Barragán-Iglesias P, Pineda-Farias JB, Bravo-Hernández M, Murbartián J, Granados-Soto V. Role of peripheral and spinal 5-HT2B receptors in formalin-induced nociception. Pharmacol Biochem Behav. 2012;102:30-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 8. | Lin SY, Chang WJ, Lin CS, Huang CY, Wang HF, Sun WH. Serotonin receptor 5-HT2B mediates serotonin-induced mechanical hyperalgesia. J Neurosci. 2011;31:1410-1418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 56] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 9. | Ohashi-Doi K, Himaki D, Nagao K, Kawai M, Gale JD, Furness JB, Kurebayashi Y. A selective, high affinity 5-HT 2B receptor antagonist inhibits visceral hypersensitivity in rats. Neurogastroenterol Motil. 2010;22:e69-e76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 10. | O'Mahony SM, Bulmer DC, Coelho AM, Fitzgerald P, Bongiovanni C, Lee K, Winchester W, Dinan TG, Cryan JF. 5-HT(2B) receptors modulate visceral hypersensitivity in a stress-sensitive animal model of brain-gut axis dysfunction. Neurogastroenterol Motil. 2010;22:573-578, e124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 63] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 11. | Zhou Q, Yang L, Larson S, Basra S, Merwat S, Tan A, Croce C, Verne GN. Decreased miR-199 augments visceral pain in patients with IBS through translational upregulation of TRPV1. Gut. 2016;65:797-805. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 100] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 12. | Grover M, Berumen A, Peters S, Wei T, Breen-Lyles M, Harmsen WS, Busciglio I, Burton D, Vazquez Roque M, DeVault KR, Camilleri M, Wallace M, Dasari S, Neumann H, Houghton LA. Intestinal chemosensitivity in irritable bowel syndrome associates with small intestinal TRPV channel expression. Aliment Pharmacol Ther. 2021;54:1179-1192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 13. | Su YS, Chiu YY, Lin SY, Chen CC, Sun WH. Serotonin Receptor 2B Mediates Mechanical Hyperalgesia by Regulating Transient Receptor Potential Vanilloid 1. J Mol Neurosci. 2016;59:113-125. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 14. | Sugiuar T, Bielefeldt K, Gebhart GF. TRPV1 function in mouse colon sensory neurons is enhanced by metabotropic 5-hydroxytryptamine receptor activation. J Neurosci. 2004;24:9521-9530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 105] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 15. | Ford AC, Lacy BE, Talley NJ. Irritable Bowel Syndrome. N Engl J Med. 2017;376:2566-2578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 344] [Cited by in RCA: 413] [Article Influence: 51.6] [Reference Citation Analysis (0)] |
| 16. | Yu YB, Zuo XL, Zhao QJ, Chen FX, Yang J, Dong YY, Wang P, Li YQ. Brain-derived neurotrophic factor contributes to abdominal pain in irritable bowel syndrome. Gut. 2012;61:685-694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 118] [Article Influence: 9.1] [Reference Citation Analysis (1)] |
| 17. | Barbara G, Stanghellini V, De Giorgio R, Cremon C, Cottrell GS, Santini D, Pasquinelli G, Morselli-Labate AM, Grady EF, Bunnett NW, Collins SM, Corinaldesi R. Activated mast cells in proximity to colonic nerves correlate with abdominal pain in irritable bowel syndrome. Gastroenterology. 2004;126:693-702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1004] [Cited by in RCA: 1024] [Article Influence: 48.8] [Reference Citation Analysis (0)] |
| 18. | Williams CL, Villar RG, Peterson JM, Burks TF. Stress-induced changes in intestinal transit in the rat: a model for irritable bowel syndrome. Gastroenterology. 1988;94:611-621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 182] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 19. | Chen Y, Li Z, Yang Y, Lin L, Zhang H. Role of glucagon-like peptide-1 in the pathogenesis of experimental irritable bowel syndrome rat models. Int J Mol Med. 2013;31:607-613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 20. | Jin B, Ha SE, Wei L, Singh R, Zogg H, Clemmensen B, Heredia DJ, Gould TW, Sanders KM, Ro S. Colonic Motility Is Improved by the Activation of 5-HT(2B) Receptors on Interstitial Cells of Cajal in Diabetic Mice. Gastroenterology. 2021;161:608-622.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 35] [Article Influence: 8.8] [Reference Citation Analysis (1)] |
| 21. | Su Z, Miao B, Xu MQ, Yang MJ, Fei SJ, Zhang JF. Protective effect of microinjection of glutamate into hypothalamus paraventricular nucleus on chronic visceral hypersensitivity in rats. Brain Res. 2020;1747:147048. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 22. | Cervantes-Durán C, Rocha-González HI, Granados-Soto V. Peripheral and spinal 5-HT receptors participate in the pronociceptive and antinociceptive effects of fluoxetine in rats. Neuroscience. 2013;252:396-409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 34] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 23. | Segelcke D, Messlinger K. Putative role of 5-HT(2B) receptors in migraine pathophysiology. Cephalalgia. 2017;37:365-371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 24. | Beattie DT, Smith JA, Marquess D, Vickery RG, Armstrong SR, Pulido-Rios T, McCullough JL, Sandlund C, Richardson C, Mai N, Humphrey PP. The 5-HT4 receptor agonist, tegaserod, is a potent 5-HT2B receptor antagonist in vitro and in vivo. Br J Pharmacol. 2004;143:549-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 91] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 25. | Bassil AK, Taylor CM, Bolton VJ, Gray KM, Brown JD, Cutler L, Summerfield SG, Bruton G, Winchester WJ, Lee K, Sanger GJ. Inhibition of colonic motility and defecation by RS-127445 suggests an involvement of the 5-HT2B receptor in rodent large bowel physiology. Br J Pharmacol. 2009;158:252-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 26. | Iftinca M, Defaye M, Altier C. TRPV1-Targeted Drugs in Development for Human Pain Conditions. Drugs. 2021;81:7-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 133] [Article Influence: 26.6] [Reference Citation Analysis (0)] |
| 27. | Perna E, Aguilera-Lizarraga J, Florens MV, Jain P, Theofanous SA, Hanning N, De Man JG, Berg M, De Winter B, Alpizar YA, Talavera K, Vanden Berghe P, Wouters M, Boeckxstaens G. Effect of resolvins on sensitisation of TRPV1 and visceral hypersensitivity in IBS. Gut. 2021;70:1275-1286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 52] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 28. | Premkumar LS, Ahern GP. Induction of vanilloid receptor channel activity by protein kinase C. Nature. 2000;408:985-990. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 602] [Cited by in RCA: 638] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 29. | Atkinson W, Lockhart S, Whorwell PJ, Keevil B, Houghton LA. Altered 5-hydroxytryptamine signaling in patients with constipation- and diarrhea-predominant irritable bowel syndrome. Gastroenterology. 2006;130:34-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 265] [Article Influence: 13.9] [Reference Citation Analysis (0)] |