Published online Mar 14, 2024. doi: 10.3748/wjg.v30.i10.1393
Peer-review started: December 24, 2023
First decision: January 4, 2024
Revised: January 16, 2024
Accepted: February 18, 2024
Article in press: February 18, 2024
Published online: March 14, 2024
Processing time: 81 Days and 8.7 Hours
Non-alcoholic fatty liver disease (NAFLD) is the most common liver disease worldwide, affecting about 1/4th of the global population and causing a huge global economic burden. To date, no drugs have been approved for the treatment of NAFLD, making the correction of unhealthy lifestyles the principle method of treatment. Identifying patients with poor adherence to lifestyle correction and attempting to improve their adherence are therefore very important.
To develop and validate a scale that can rapidly assess the adherence of patients with NAFLD to lifestyle interventions.
The Exercise and Diet Adherence Scale (EDAS) was designed based on com
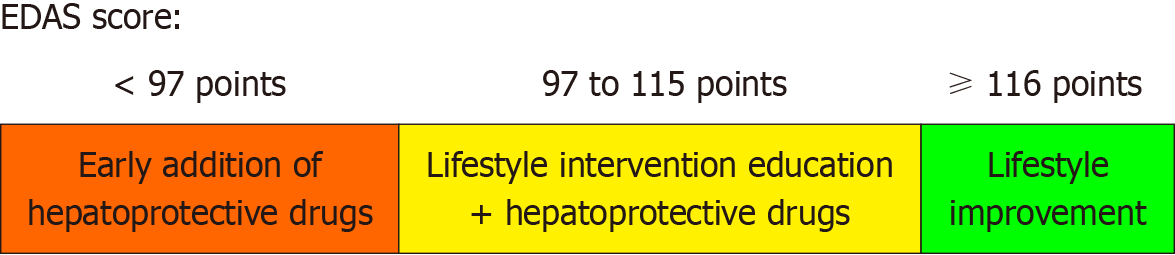
The EDAS consisted of 33 items in six dimensions, with a total of 165 points. Total EDAS score correlated significantly with daily number of exercise and daily reduction in calorie intake (P < 0.05 each), but not with overall weight loss. A total score of 116 was excellent in predicting adherence to daily reduction in calorie intake (> 500 kacl/d), (sensitivity/specificity was 100.0%/75.8%), while patients score below 97 could nearly rule out the possibility of daily exercise (sensitivity/specificity was 89.5%/44.4%). Total EDAS scores ≥ 116, 97-115, and < 97 points were indicative of good, average, and poor adherence, respectively, to diet and exercise recommendations.
The EDAS can reliably assess the adherence of patients with NAFLD to lifestyle interventions and have clinical application in this population.
Core Tip: This study developed and validated an Exercise and Diet Adherence Scale (EDAS) to rapidly assess adherence to lifestyle interventions in patients with non-alcoholic fatty liver disease (NAFLD). Patients can be grouped based on their EDAS scores and receive personalized treatments accordingly. The EDAS demonstrated reliability and effectiveness in predicting adherence to lifestyle changes and served as a vital tool in the clinical management of patients with NAFLD.
- Citation: Zeng MH, Shi QY, Xu L, Mi YQ. Establishment and validation of an adherence prediction system for lifestyle interventions in non-alcoholic fatty liver disease. World J Gastroenterol 2024; 30(10): 1393-1404
- URL: https://www.wjgnet.com/1007-9327/full/v30/i10/1393.htm
- DOI: https://dx.doi.org/10.3748/wjg.v30.i10.1393
The prevalence of non-alcoholic fatty liver disease (NAFLD) has been increasing over the past few decades, with this disease estimated to affect more than 30% of adults worldwide[1]. NAFLD is a progressive disease that can give rise to complications, such as hepatocellular carcinoma (HCC) and cardiovascular disease, which place a burden on the health care system and economy[2]. Additionally, the United Network for Organ Sharing has reported that NAFLD is currently the second leading indication for all liver transplants and will become the primary indication for liver transplantation in patients with HCC[3,4].
To date, no drugs have been approved for the treatment of NAFLD, with correction of unhealthy lifestyles remains a fundamental means of curing it. Therapeutic lifestyle changes can have a direct or significant effect on patients with NAFLD and contribute to a high rate of “placebo response”[5-8]. Because exercises and diets vary greatly, however, it has been difficult to quantify and evaluate patient adherence with these changes. Lifestyle interventions rely on patients’ “conscious” adherence to recommendations, with patient “self-reporting” required to evaluate adherence. Thus, patients must be intrinsically motivated to change their lifestyles. Some patients, however, are unable or unwilling to adhere to recommendations about diet and exercise. Approximately 3%-4% of healthy people are diagnosed with NAFLD each year, with lifestyle changes resulting in improvements in only 60% of these patients[9]. Additionally, the high rates of cardiovascular diseases, osteoarthritis and rheumatism in patients with NAFLD make exercise interventions difficult, with other conditions, including depression and anxiety, limiting the persistence of dietary interventions[10]. The adherence to lifestyle interventions for NAFLD remains largely unexplored. A questionnaire assessing adherence with lifestyle interventions is therefore urgently needed. This questionnaire can be used in the clinical and scientific assessment of patients with NAFLD, especially in assessing their responses to lifestyle changes (Figure 1).
Data from NAFLD patients aged 18-70 years who were admitted to the Second People’s Hospital of Tianjin from August 2013 to January 2014 were used to design the Exercise and Diet Adherence Scale (EDAS). The practice guidelines of the American Gastroenterological Association, the American Association for the Study of Liver Diseases, and the American College of Gastroenterology have defined NAFLD as an imaging or pathological diagnosis of hepatic steatosis in the absence of other known secondary causes of hepatic steatosis[11]. Patients with NAFLD combined with viral hepatitis, autoimmune liver disease and other types of hepatitis, those suspected of having cirrhosis or liver cancer; and men and women who consumed > 140 g and > 70 g, respectively, of alcohol per week were excluded. Also excluded were patients with serum creatinine concentrations > 1.5 times the upper limit of normal, and those with other serious systemic or infectious diseases, such as malignant tumors and severe cardiopulmonary diseases. Patients unable to control their diets or perform aerobic exercises due to illness or other reasons were also excluded. The validation cohort consisted of patients with NAFLD who were admitted between October 2022 and June 2023, using the same inclusion and exclusion criteria.
Sample size was calculated using a factor analysis approach, with eight times the number of items in the largest dimension of the EDAS, which has seven items. Based on a 20% dropout rate, the target enrollment was 67 participants. All patients enrolled in this study after providing informed consent.
Professional medical workers conducted face-to-face conversations with 20 patients with typical NAFLD. The reasons mentioned by the patients that affected their exercise and diet adherence were recorded in detail. Subsequently, the scale was divided into the following five dimensions and 36 items: Understanding and valuing (eight items), belief (six items), self-control (12 items), conditional restrictions (eight items), and mental stress in life and work (two items). To assess the validity of these items, the scale was analyzed using the Delphi method.
Five professors with NAFLD as their research field and one professor of psychology were selected for consultation. Some of these experts believed that “mental stress in life and work” should be incorporated into the condition “conditional restriction”; that “self-control” should be divided into “self-control of diet” and “strengthen exercise self-control”; and that “conditional restriction” should be divided into “control dietary conditions”, and “strengthen conditions for exercise”. After modification, the experts were again consulted and the importance of these items were scored. Each item was rated on a scale of 1-9 points, with higher score indicating greater importance. Feedback was received from all six experts, with the average score of each item being greater than 7; moreover, the coefficients of variation were less than 0.25, and the expert opinions tended to be consistent. The EDAS questionnaire was divided into the following six dimensions and 36 items: understanding and valuing (eight items), belief (six items), self-control of diet (seven items), strengthen exercise self-control (five items), control dietary conditions (three items), and strengthen conditions for exercise (seven items).
Based on the inclusion and exclusion criteria, 30 NAFLD patients were selected. Analysis of their completed questionnaires showed that the item “I believe that taking medicine can control fatty liver” in the dimension of “understanding and valuing” had a low degree of discrimination, and that, after deleting the items “I will measure my weight” and “I will review it regularly” in the dimension of “belief” improved the internal consistencies of the dimension “belief” and the total scale. Thus, these three items are deleted. Analysis of exploratory factors found six common factors, which can correspond to six dimensions, indicating that the EDAS scale had good construct validity. The final version of the EDAS consisted of 33 items across six dimensions, with 15 items of these items being reverse scored. The lowest score on this scale was 33, and the highest was 165, with higher scores indicating better patient adherence with lifestyle interventions.
Lifestyle interventions: The enrolled patients were subjected to exercise and dietary interventions for 6 months. Mode
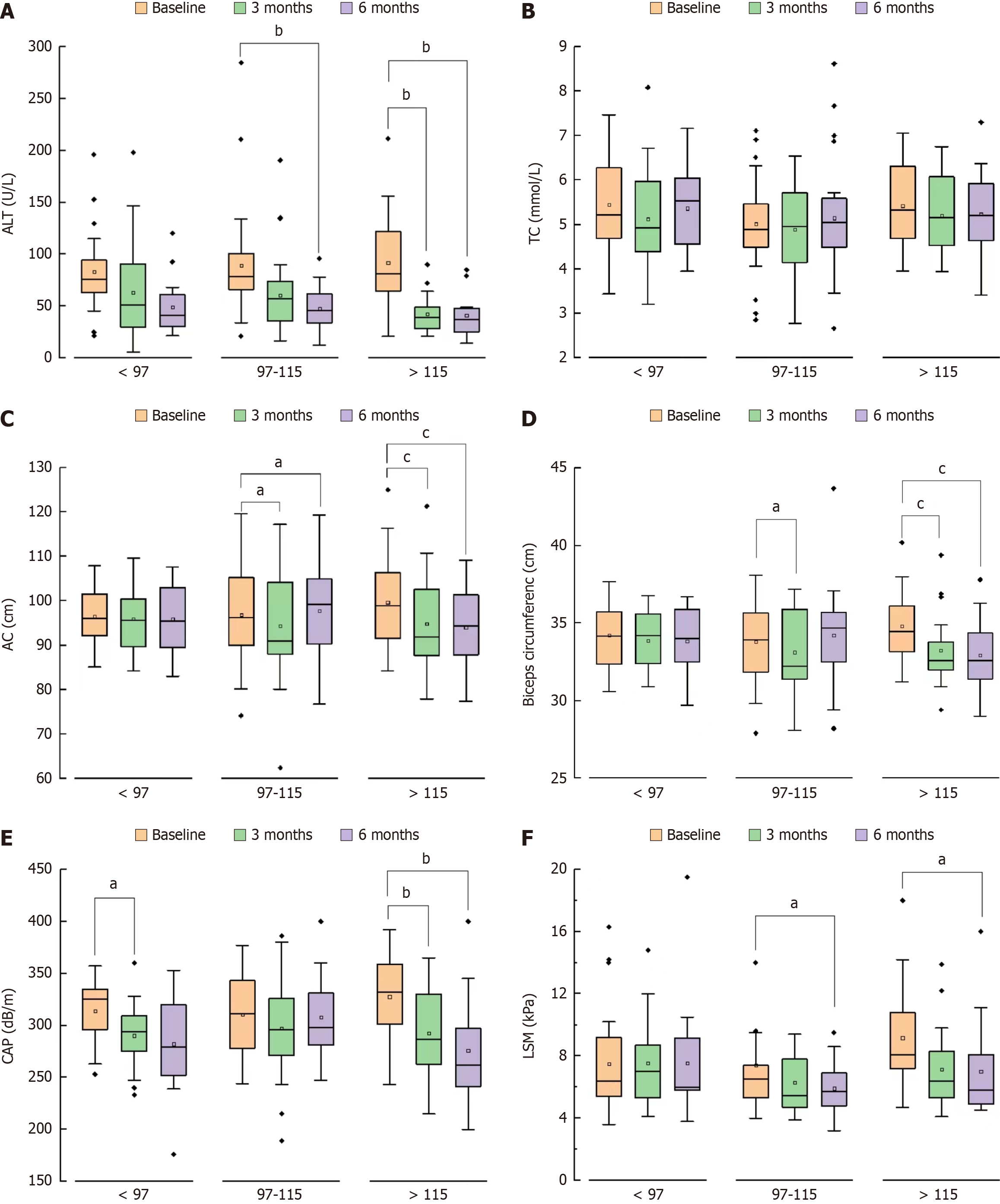
Demographic characteristics and laboratory indices: Body composition indices of patients were analyzed at baseline and after 3 and 6 months using InBodyS10 (Biospace, Seoul, South Korea). Parameters evaluated included weight, waist circumference, upper arm circumference and abdominal fat area. Laboratory variables, including serum concentrations of alanine aminotransferase (ALT), aspartate aminotransferase (AST), gamma-glutamyl transferase (GGT), alkaline phosphatase (ALP), total bilirubin (TBIL), triglyceride (TG), total cholesterol (TC), fasting blood glucose (FBG), and umbilical artery, were measured by a chemiluminescence method an Hitachi automatic biochemical instrument-7180 and reagents purchased from Guang Co., Ltd. Controlled attenuation parameter (CAP) and liver stiffness measurement (LSM) were determined using a the FibroScan 502 Touch device (Echosens, Paris, France). A pedometer was given to each subject to record the number of days per week of exercise, the type of exercise and the number of steps walked by fast walking patients, and the type and time of daily exercise in non-fast walking patients.
Reliability was determined by measuring Cronbach’s α coefficient and test-retest reliability. Validity analysis included content, criterion, and construct. Content validity was evaluated by experts, criterion validity was assessed to select a recognized criterion to test the correlation between the criterion and the scale. Construct validity was tested by confirmatory factor analysis.
Normally distributed continuous data were reported as the mean ± SD and compared by t-tests, abnormally distributed continuous data were reported as median and interquartile intervals and compared by rank sum tests, and categorical variables were reported as number (%) and compared by chi-squared tests. Reliability was analyzed by determining internal consistency and test-retest reliability. Internal consistency was expressed as Cronbach’s α coefficient, which ranged from 0-1, with values of 0.8-0.9, 0.7-0.8, and 0.6-0.7 indicating very good, good, and minimally acceptable values.
The retest interval was one week, with test-retest reliability determined by analyzing the correlation coefficient of two scores, with a retest reliability > 0.7 considered good. Correlations of normally distributed data were determined using Pearson correlation coefficients, whereas correlations of non-normally distributed data were determined using Spearman correlation coefficients.
Validity analysis included content validity, criterion validity and construct validity. Content validity was evaluated by experts, with the content validity index of each item scored as 1 (irrelevant), 2 (weakly relevant), 3 (strongly relevant), or 4 (very relevant). The proportion of experts providing scores of 3 and 4 was defined as the content validity index of each item. Construct validity was evaluated by confirmatory factor analysis.
The efficacy of the EDAS score in judging exercise steps and reducing calorie intake was evaluated by determining the areas under the receiver operating characteristics curves (AUROC). The optimal critical value for adherence, as well as the sensitivity, specificity, positive predictive value, negative predictive value positive likelihood ratio and negative likelihood ratio, were determined based on the maximum value of the Jordan index. AUROCs of 0.9-1.0, 0.8-0.9, 0.7-0.8, and < 0.7 were indicative of excellent, good, average, and poor effectiveness of judgment, respectively.
Statistical analyses were performed using SPSS 27.0 (SPSS Inc., Chicago, IL, United States) and MedCalc 9.3 (MedCalc Software, Mariakerke, Belgium) software and OriginPro 9.0 (OriginPro, Northampton, United States) was used for mapping. A P value < 0.05 was considered statistically significant.
This study included a total of 81 patients with NAFLD, with 66 completed the follow-up. The average amount of daily exercise completed was 4519 steps/d, and the caloric intake was reduced to 68 kcal/d. Of the 66 subjects, 37 (56.1%) lost weight. The average weight loss of these 37 subjects was 4.2 kg ± 2.9 kg, with the maximum weight loss being 15 kg (Table 1).
| Variable | Numerical value |
| Male, n (%) | 49 (74.2) |
| Age (yr), mean ± SD | 39 ± 12 |
| Fatty liver disease course (month) (M, Q) | 36 (9.0) |
| Smoking, n (%) | 15 (22.7) |
| Likes fried food, n (%) | 20 (30.3) |
| BMI (kg/m2), mean ± SD | 28.4 ± 3.3 |
| Waist-hip ratio, mean ± SD | 0.9 ± 0.0 |
| Abnormal blood pressure, n (%) | 20 (30.3) |
| ALT (U/L) (M, Q) | 64.5 (60.8) |
| AST (U/L) (M, Q) | 36.0 (25.4) |
| GGT (U/L) (M, Q) | 48.0 (44.0) |
| ALP (U/L) (M, Q) | 78.0 (29.5) |
| TBIL (µmol/L) (M, Q) | 14.7 (7.3) |
| FBG (mmol/L) (M, Q) | 6.0 (0.9) |
| TG (mmol/L) (M, Q) | 2.0 (1.3) |
| CHO (mmol/L), mean ± SD | 5.0 ± 1.1 |
| FINS (µU/L) (M, Q) | 13.9 (8.2) |
| UA (µmol/L) (M, Q) | 421.5 (116.5) |
| CAP (dB/m), mean ± SD | 331.4 ± 33.0 |
| LSM (kPa) (M, Q) | 6.6 (2.9) |
| Walking (number of steps) (M, Q) | 4519.0 (4564.5) |
| Reduction in caloric intake (kcal) (M, Q) | 68.0 (127.8) |
A comparison of the 27% of patients with the highest scores and the 27% of patients with the lowest scores showed that each item differed significantly (P < 0.05). The test-retest reliability after one week was 0.82. The internal consistency reliabilities of the seven dimensions were 0.739, 0.747, 0.771, 0.813, 0.791, 0.776, and 0.874, respectively, with each being above 0.7, and the Cronbach’s α coefficient of the total scale being 0.874 (Table 2). The inter-dimension correlation of EDAS ranged from 0.050 (understanding and valuing and strengthening conditions for exercise) to 0.624 (controlling diet and exercise conditions). The correlations between pairs of dimensions were not strong, indicating that the contents of these items were less repetitive (Table 3).
| EDAS | Number of entries | Score | Mean score | Standard deviation | Lowest score | Highest score | Cronbach’s α |
| Understanding and valuing | 7 | 35 | 24.65 | 4.64 | 13 | 35 | 0.739 |
| Belief | 4 | 20 | 16.23 | 2.39 | 7 | 20 | 0.747 |
| Self-control of diet | 7 | 35 | 22.39 | 3.89 | 10 | 33 | 0.771 |
| Strengthen exercise self-control | 5 | 25 | 15.00 | 3.65 | 7 | 25 | 0.813 |
| Control dietary conditions | 3 | 15 | 10.35 | 2.58 | 3 | 15 | 0.791 |
| Strengthen exercise conditions | 7 | 35 | 21.80 | 5.07 | 9 | 33 | 0.776 |
| Total scale | 33 | 165 | 110.42 | 14.49 | 67 | 149 | 0.874 |
| EDAS | Understanding and valuing | Belief | Self-control of diet | Strengthen exercise self-control | Control dietary conditions | Strengthen exercise conditions |
| Understanding and valuing | 1.000 | |||||
| Belief | 0.280 | 1.000 | ||||
| Self-control of diet | 0.056 | 0.335 | 1.000 | |||
| Strengthen exercise self-control | 0.096 | 0.324 | 0.455 | 1.000 | ||
| Control dietary conditions | 0.057 | 0.137 | 0.583 | 0.398 | 1.000 | |
| Strengthen exercise conditions | 0.050 | 0.322 | 0.473 | 0.494 | 0.624 | 1.000 |
Evaluation by experts showed that the content validity index of the EDAS items was 1, indicating good content validity. The total score of the scale correlated significantly with daily walking or other exercises and daily reduction in calorie intake, but not with weight loss. The number of exercise steps per day correlated significantly with belief (r = 0.29, P = 0.020), strengthening exercise self-control (r = 0.40, P = 0.001) and strengthening exercise conditions (r = 0.33, P = 0.007), whereas reduced daily calorie intake correlated significantly with belief (r = 0.34, 0.006), self-control of diet (r = 0.64, P < 0.001), control of dietary conditions (r = 0.56, P < 0.001) and strengthening exercise conditions (r = 0.26, P = 0.035) (Table 4).
| EDAS | r (P value) | ||
| Daily exercise steps | Daily calorie intake reduction (kcal) | Weight loss (kg) | |
| Understanding and valuing | 0.03 (0.842) | 0.11 (0.373) | 0.05 (0.712) |
| Belief | 0.29 (0.020)a | 0.34 (0.006)a | 0.24 (0.054) |
| Self-control of diet | 0.23 (0.064) | 0.64 (< 0.001)a | 0.14 (0.262) |
| Strengthen exercise self-control | 0.40 (0.001)a | 0.17 (0.183) | 0.14 (0.279) |
| Control dietary conditions | 0.20 (0.104) | 0.56 (< 0.001)a | 0.26 (0.037)a |
| Strengthen exercise conditions | 0.33 (0.007)a | 0.26 (0.035)a | 0.19 (0.133) |
| Total scale | 0.37 (0.002)a | 0.50 (< 0.001)a | 0.24 (0.056) |
Confirmatory factor analysis of the EDAS showed that the KMO coefficient was 0.675 (P < 0.001 on the Bartlett spherical test), with the spherical hypothesis being rejected. Variance maximization orthogonal rotation in factor analysis identified six common factors. These six factors explained 66.2% of the total table, with factor 1 accounting for 25.4% of the variation in interpretation. After the second dimensionality reduction of the six dimensions, the KMO coefficient was 0.710 (P < 0.001 on the Bartlett spherical test). Two common factors accounted for 64.7% of the total table; the first factor can be explained by external conditions and the second factor by internal motives.
Exercise conditions were divided into five categories: ≤ 3000, 3000-5000, 5000-8000, 8000-10000, and ≥ 10000 steps per day. The EDAS in patients with NAFLD was highly sensitive in determining exercise conditions, but its specificity was low. EDAS scores < 97 were therefore indicative of a lack of daily exercise (Figures 2A and 3).
The average daily calorie intake of patients was also divided into five categories: reductions of ≤ 50 kcal/d or an increase, and reductions of 50-100 kcal/d, 100-200 kcal/d, 200-500 kcal/d, and ≥ 500 kcal/d. The EDAS was highly sensitive and specific in determining large daily reductions in diet (> 500 kcal/d). EDAS scores > 116 were therefore indicative of a greater control of diet than scores below (Figures 2B and 3).
Characteristics of the enrolled population: 121 NAFLD patients admitted to our hospital for fatty liver treatment from January 2022 to June 2023, with 103 of these patients’ completing follow-up. After excluding 22 patients who were not at the first visit and 10 who were not followed up after 3 or 6 month, 84 patients were included, including 62 who completed the 3-month follow-up and 57 who completed the 6-month follow-up. The average age of these 84 patients was 38 years. They had a mean ± SD body mass index of 28.19 ± 2.99 kg/m2, ALT of 87.64 ± 44.80 U/L, AST of 40.2 U/L, GGT of 52.0 U/L, ALP of 72.0 U/L, and TBIL of 14.9 µmol/L. They had a mean ± SD FBG of 5.96 ± 0.76 mmol/L, TG of 1.8 mmol/L, FINS of 18.16 µU/L, CAP of 315.81 ± 35.16 dB/m, and LSM of 6.8 kPa. The EDAS questionnaire survey showed that 26 patients (31.0%) had poor compliance, 37 (44.0%) had moderate compliance, and 21 (25.0%) had good compliance.
Results of verification: NAFLD patients with better adherence had a greater proportion of weight, abdominal circumference, LSM reduction and ALT return to normal, but this difference decreased with the extension of follow-up months (Figure 4). The worse the compliance, the lower the proportion of blood glucose returning to normal in 6 months, in
Therapeutic changes in patient lifestyle remains the treatment of choice in NAFLD[12]. Because many of these patients are at high risk of cardiovascular disease, a healthy lifestyle can reduce its incidence[13]. Although adherence with therapeutic recommendations is important in managing chronic diseases[14], most patients have difficulty changing their long-standing dietary habits[15]. In addition, regular exercise decreases as patients age, with more than 50% of individuals stopping routine exercise and treatment within 1 year[16-18]. Early identification of patients with poor adherence can result in efforts to improve their adherence[19].
Most assessments of adherence are in relation to medication, but these studies have generally shown poor adherence[20-23]. For example, a retrospective study of initial treatment of patients with type 2 diabetes found that 48% stopped their medication within the first year, with most discontinuations occurring within the first 3 months after starting treatment[24]. Moreover, only about 50% of patients with myocardial infarction show adherence with the long-term use of antihypertensive and lipid-lowering drugs[25]. Fewer studies to date have assessed adherence with lifestyle interventions than those on drugs. with physicians paying no attention to lifestyle modifications. Therefore, patients were less able to recognize the importance of lifestyle interventions.
NAFLD is a progressive liver disease, with histology ranging from steatosis to fibrosis and cirrhosis. NAFLD is the eighth most common cause of death worldwide, being responsible for 1.2 million annual deaths. To date, however, there is currently no comprehensive scale to evaluate adherence with lifestyle interventions for NAFLD at home or abroad. The EDAS scale described in the present study was based on standardized scale preparation requirements and is, to our knowledge, the first scale to measure adherence in patients with NAFLD.
The internal consistency reliability of each dimension of the EDAS was above 0.7, and the Cronbach’s α coefficient of the total volume table was 0.874. No strong correlation was observed among the dimensions, indicating that the item content was less repetitive. The test-retest reliability at one-week was 0.820, indicating that the EDAS has high stability, consistency, and reliability. Experts rated each item of the EDAS as level 3 or 4, making the item content validity index of the EDAS 1, indicating that content validity was good.
Daily number of exercise steps was directly proportional to three dimensions on the EDAS: Belief, exercise self-control and strengthen conditions for exercise. In addition, daily calorie intake reduction was proportional to three dimensions: Belief, self-control of diet and control dietary conditions. These findings indicate that the EDAS reflects the actual adherence of NAFLD patients before exercise and diet intervention. Belief was significantly and positively correlated with exercise enhancement and diet control, suggesting that physician encouragement and a good doctor-patient relationship can establish a belief in patients that they can cure or control NAFLD. Strengthening exercise self-control and conditions were related to exercise, whereas dietary self-control and conditions were related to diet, indicating that the EDAS can independently reflect the exercise and diet conditions of patients. In contrast, weight loss was only significantly related to the control of diet, possibly because a controlled diet is more likely to lead to weight loss than exercise.
Confirmatory factor analysis showed that the KMO coefficient was 0.710, with Bartlett’s spherical test showing a P value < 0.001. Two common factors were identified, with the most frequent variation being the control of dietary conditions. Thus, the importance of improving diet control conditions should be emphasized in patients with poor ad
Clinically, patients with EDAS scores ≥ 116 should be regarded as having good adherence. If abnormalities in the liver function are not evident, lifestyle interventions alone can be administered. Adherence is considered general for patients with EDAS scores ranging from 97 to 115. The importance of lifestyle improvement should be emphasized in these patients, including improved adherence with exercise and diet recommendations, as well as treatment with hepatoprotective drugs when necessary. Patients with EDAS scores < 97 have poor adherence and should receive early administration of anti-inflammatory agents and psychotherapy (Figure 6).
To the best of our knowledge, this is the first study to use a questionnaire to assess adherence with lifestyle inter
Lifestyle intervention adherence scale developed in this study for patients with NAFLD was effective in determining the adherence of these patients with exercise and diet. This scale, which was relatively comprehensive in content, underwent appropriate verification in an independent patient cohort. The EDAS scale can be used as a tool to measure adherence with lifestyle interventions in patients with NAFLD and guide clinical interventions.
Non-alcoholic fatty liver disease (NAFLD) is a progressive disease that can lead to complications such as liver fibrosis, cirrhosis, hepatocellular carcinoma, cardiovascular diseases, and metabolic disorders such as type 2 diabetes. However, to date, no medications have been approved for treating NAFLD, and lifestyle modifications remain the cornerstone of treatment.
Changing an unhealthy lifestyle can be useful for alleviating hepatic steatosis in patients with NAFLD. However, not everyone is able or willing to adhere to the dietary and exercise guidelines. The variety of exercise and dietary controls makes it challenging to quantify and evaluate patient’s adherence.
To evaluate adherence effectively and swiftly with the recommendations for lifestyle changes in patients with NAFLD, implementing various intervention strategies based on adherence levels to prevent disease progression is crucial.
First, we identified factors affecting exercise and dietary adherence in patients with NAFLD. The Delphi method was used to analyze and modify the Exercise and Diet Adherence Scale (EDAS). After a preliminary small-scale survey and further adjustments, the EDAS was established. Enrolled patients with NAFLD followed exercise and diet interventions, filled the EDAS at the beginning, and were followed up for 6 months. Finally, we evaluated and validated the reliability of the EDAS.
The EDAS demonstrated good item discrimination; internal consistency reliability; test-retest reliability; and content, construct, and criterion validity. It can reliably measure the adherence of patients with NAFLD to exercise and dietary interventions.
The EDAS has been established to assess the adherence of patients objectively, directly, and rapidly with NAFLD to changing unhealthy lifestyles. This reliable tool supports early intervention in NAFLD, aims to prevent disease progression, and reduces the healthcare burden.
EDAS plays an important clinical role in the assessment, treatment, and management of NAFLD. However, its widespread application requires multicenter prospective studies. Additionally, the participants in this study did not undergo a liver biopsy. Thus, future research should explore the impact of EDAS on liver pathology.
The authors thank You-Fei Zhao and Lin Chen for inputting some of data.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Abbas Z, Pakistan S-Editor: Chen YL L-Editor: A P-Editor: Zheng XM
| 1. | Younossi ZM, Golabi P, Paik JM, Henry A, Van Dongen C, Henry L. The global epidemiology of nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH): a systematic review. Hepatology. 2023;77:1335-1347. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 270] [Cited by in RCA: 1417] [Article Influence: 708.5] [Reference Citation Analysis (2)] |
| 2. | Ekstedt M, Hagström H, Nasr P, Fredrikson M, Stål P, Kechagias S, Hultcrantz R. Fibrosis stage is the strongest predictor for disease-specific mortality in NAFLD after up to 33 years of follow-up. Hepatology. 2015;61:1547-1554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1353] [Cited by in RCA: 1701] [Article Influence: 170.1] [Reference Citation Analysis (1)] |
| 3. | Younossi ZM, Stepanova M, Ong J, Trimble G, AlQahtani S, Younossi I, Ahmed A, Racila A, Henry L. Nonalcoholic Steatohepatitis Is the Most Rapidly Increasing Indication for Liver Transplantation in the United States. Clin Gastroenterol Hepatol. 2021;19:580-589.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 375] [Cited by in RCA: 362] [Article Influence: 90.5] [Reference Citation Analysis (1)] |
| 4. | Doycheva I, Issa D, Watt KD, Lopez R, Rifai G, Alkhouri N. Nonalcoholic Steatohepatitis is the Most Rapidly Increasing Indication for Liver Transplantation in Young Adults in the United States. J Clin Gastroenterol. 2018;52:339-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 105] [Article Influence: 15.0] [Reference Citation Analysis (1)] |
| 5. | Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, Harrison SA, Brunt EM, Sanyal AJ. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67:328-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3544] [Cited by in RCA: 4939] [Article Influence: 705.6] [Reference Citation Analysis (9)] |
| 6. | Han MAT, Altayar O, Hamdeh S, Takyar V, Rotman Y, Etzion O, Lefebvre E, Safadi R, Ratziu V, Prokop LJ, Murad MH, Noureddin M. Rates of and Factors Associated With Placebo Response in Trials of Pharmacotherapies for Nonalcoholic Steatohepatitis: Systematic Review and Meta-analysis. Clin Gastroenterol Hepatol. 2019;17:616-629.e26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 98] [Article Influence: 16.3] [Reference Citation Analysis (1)] |
| 7. | Michel M, Schattenberg JM. Effectiveness of lifestyle interventions in NAFLD (nonalcoholic fatty liver disease) - how are clinical trials affected? Expert Opin Investig Drugs. 2020;29:93-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (1)] |
| 8. | Plauth M, Bernal W, Dasarathy S, Merli M, Plank LD, Schütz T, Bischoff SC. ESPEN guideline on clinical nutrition in liver disease. Clin Nutr. 2019;38:485-521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 402] [Article Influence: 67.0] [Reference Citation Analysis (3)] |
| 9. | Fan JG, Kim SU, Wong VW. New trends on obesity and NAFLD in Asia. J Hepatol. 2017;67:862-873. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 848] [Cited by in RCA: 800] [Article Influence: 100.0] [Reference Citation Analysis (2)] |
| 10. | Barritt AS, Watkins S, Gitlin N, Klein S, Lok AS, Loomba R, Schoen C, Reddy KR, Trinh HN, Mospan AR, Vos MB, Weiss LM, Cusi K, Neuschwander-Tetri BA, Sanyal AJ. Patient Determinants for Histologic Diagnosis of NAFLD in the Real World: A TARGET-NASH Study. Hepatol Commun. 2021;5:938-946. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 27] [Article Influence: 6.8] [Reference Citation Analysis (1)] |
| 11. | Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, Cusi K, Charlton M, Sanyal AJ. The diagnosis and management of non-alcoholic fatty liver disease: practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology. 2012;55:2005-2023. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2413] [Cited by in RCA: 2611] [Article Influence: 200.8] [Reference Citation Analysis (1)] |
| 12. | Chen Y, Feng R, Yang X, Dai J, Huang M, Ji X, Li Y, Okekunle AP, Gao G, Onwuka JU, Pang X, Wang C, Li C, Sun C. Yogurt improves insulin resistance and liver fat in obese women with nonalcoholic fatty liver disease and metabolic syndrome: a randomized controlled trial. Am J Clin Nutr. 2019;109:1611-1619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 82] [Article Influence: 13.7] [Reference Citation Analysis (1)] |
| 13. | Kasper P, Martin A, Lang S, Kütting F, Goeser T, Demir M, Steffen HM. NAFLD and cardiovascular diseases: a clinical review. Clin Res Cardiol. 2021;110:921-937. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 103] [Cited by in RCA: 382] [Article Influence: 76.4] [Reference Citation Analysis (1)] |
| 14. | De Geest S, Sabaté E. Adherence to long-term therapies: evidence for action. Eur J Cardiovasc Nurs. 2003;2:323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 303] [Cited by in RCA: 354] [Article Influence: 16.9] [Reference Citation Analysis (1)] |
| 15. | Shim JS, Heo JE, Kim HC. Factors associated with dietary adherence to the guidelines for prevention and treatment of hypertension among Korean adults with and without hypertension. Clin Hypertens. 2020;26:5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (1)] |
| 16. | Resurrección DM, Motrico E, Rigabert A, Rubio-Valera M, Conejo-Cerón S, Pastor L, Moreno-Peral P. Barriers for Nonparticipation and Dropout of Women in Cardiac Rehabilitation Programs: A Systematic Review. J Womens Health (Larchmt). 2017;26:849-859. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 84] [Article Influence: 10.5] [Reference Citation Analysis (1)] |
| 17. | Saida TGRH, Juul Sørensen T, Langberg H. Long-term exercise adherence after public health training in at-risk adults. Ann Phys Rehabil Med. 2017;60:237-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (1)] |
| 18. | Lopes S, Félix G, Mesquita-Bastos J, Figueiredo D, Oliveira J, Ribeiro F. Determinants of exercise adherence and maintenance among patients with hypertension: a narrative review. Rev Cardiovasc Med. 2021;22:1271-1278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (1)] |
| 19. | Lee YM, Kim RB, Lee HJ, Kim K, Shin MH, Park HK, Ahn SK, Kim SY, Lee YH, Kim BG, Lee H, Lee WK, Lee KS, Kim MJ, Park KS. Relationships among medication adherence, lifestyle modification, and health-related quality of life in patients with acute myocardial infarction: a cross-sectional study. Health Qual Life Outcomes. 2018;16:100. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 67] [Article Influence: 9.6] [Reference Citation Analysis (1)] |
| 20. | Chan AHY, Horne R, Hankins M, Chisari C. The Medication Adherence Report Scale: A measurement tool for eliciting patients' reports of nonadherence. Br J Clin Pharmacol. 2020;86:1281-1288. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 257] [Article Influence: 51.4] [Reference Citation Analysis (1)] |
| 21. | Hammad M, Bakry H. Satisfaction and Adherence to Biological Treatment in Patients with Rheumatic Diseases. Curr Rheumatol Rev. 2022;18:250-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (1)] |
| 22. | Huang Z, Tan E, Lum E, Sloot P, Boehm BO, Car J. A Smartphone App to Improve Medication Adherence in Patients With Type 2 Diabetes in Asia: Feasibility Randomized Controlled Trial. JMIR Mhealth Uhealth. 2019;7:e14914. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 57] [Article Influence: 9.5] [Reference Citation Analysis (1)] |
| 23. | Chan AHY, Vervloet M, Lycett H, Brabers A, van Dijk L, Horne R. Development and validation of a self-report measure of practical barriers to medication adherence: The medication practical barriers to adherence questionnaire (MPRAQ). Br J Clin Pharmacol. 2021;87:4197-4211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (1)] |
| 24. | Horsburgh S, Sharples K, Barson D, Zeng J, Parkin L. Patterns of metformin monotherapy discontinuation and reinitiation in people with type 2 diabetes mellitus in New Zealand. PLoS One. 2021;16:e0250289. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (1)] |
| 25. | Choudhry NK, Avorn J, Glynn RJ, Antman EM, Schneeweiss S, Toscano M, Reisman L, Fernandes J, Spettell C, Lee JL, Levin R, Brennan T, Shrank WH; Post-Myocardial Infarction Free Rx Event and Economic Evaluation (MI FREEE) Trial. Full coverage for preventive medications after myocardial infarction. N Engl J Med. 2011;365:2088-2097. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 485] [Cited by in RCA: 519] [Article Influence: 37.1] [Reference Citation Analysis (1)] |