Published online Jan 7, 2024. doi: 10.3748/wjg.v30.i1.108
Peer-review started: October 8, 2023
First decision: November 24, 2023
Revised: December 4, 2023
Accepted: December 18, 2023
Article in press: December 18, 2023
Published online: January 7, 2024
Processing time: 89 Days and 17.9 Hours
The radiological differential diagnosis of acute pancreatitis includes diffuse pancreatic lymphoma, diffuse autoimmune pancreatitis and groove located mass lesions that may mimic groove pancreatitis. Dual energy computed tomography and diffusion weighted magnetic resonance imaging are useful in the early diagnosis of acute pancreatitis, and dual energy computed tomography is also useful in severity assessment and prognosis prediction. Walled off necrosis is an important complication in terms of prognosis, and it is important to know its radiological findings and distinguish it from pseudocyst.
Core Tip: Radiological methods play a key role in diagnosing acute pancreatitis, assessing its severity and predicting its prognosis. This letter adds to the literature with radiological differential diagnoses of pancreatitis and additional imaging techniques that can be used in acute pancreatitis. In addition, we described the imaging features of walled off necrosis, which is a complication that negatively affects prognosis.
- Citation: Ozturk MO, Aydin S. Complementary comments on diagnosis, severity and prognosis prediction of acute pancreatitis. World J Gastroenterol 2024; 30(1): 108-111
- URL: https://www.wjgnet.com/1007-9327/full/v30/i1/108.htm
- DOI: https://dx.doi.org/10.3748/wjg.v30.i1.108
Hu et al[1] recently published a study that reviewed the diagnosis, severity prediction and prognosis assessment of acute pancreatitis. In their article details were provided regarding the utility and certain restrictions of magnetic resonance imaging (MRI), computed tomography (CT) and ultrasonography in the diagnosis, severity assessment, and the prognostic evaluation of acute pancreatitis. This letter aimed to contribute to the study by describing conditions that based on their radiological appearance can be mistaken for acute pancreatitis. This letter also discussed the usefulness of dual energy CT (DECT) and diffusion weighted MRI for diagnosis, severity assessment and prognosis prediction. This letter also covered the imaging methods that characterize walled off necrosis, as it is a serious complication of acute pancreatitis that impacts prognosis.
In the section of the article devoted to imaging, Hu et al[1] provided detailed imaging findings of acute pancreatitis. On the other hand, there are some diseases that, both clinically and radiologically, can be mistaken for acute pancreatitis. For instance, primary or secondary lymphomas may affect the pancreas. Amylase and lipase levels are frequently high, and the clinical symptoms frequently resemble acute pancreatitis. Involvement of pancreatic lymphoma can be focal or diffuse. Diffuse type shows an enlarged pancreas with irregular peripancreatic fat infiltration, mimicking acute pancreatitis[2]. Autoimmune pancreatitis, a form of chronic pancreatitis, is also a mimicker of acute pancreatitis with diffuse pancreatic enlargement and mild peripancreatic fat stranding[3]. Additionally, mass lesions in the groove between the pancreatic head, duodenum and common bile duct may be mistaken for groove pancreatitis[4].
As mentioned in the article by Hu et al[1], imaging methods, especially CT and MRI, play an important role in determining the severity and predicting the prognosis of acute pancreatitis. CT is frequently used to determine the presence and extent of pancreatic necrosis as well as to identify complications, thus showing the severity of the acute pancreatitis. In addition to being crucial in the diagnosis of acute pancreatitis, MRI can also be used to assess the severity and predict the prognosis of acute pancreatitis by identifying and characterizing extrapancreatic necrosis and inflammation. Our clinical experience also suggests that appropriately timed CT scans can be used effectively to diagnose acute pancreatitis, determine its severity and predict its prognosis. In our practice, MRI is used in acute pancreatitis in the presence of equivocal findings on CT and to better understand the nature (necrotic or non-necrotic) of extrapancreatic collections.
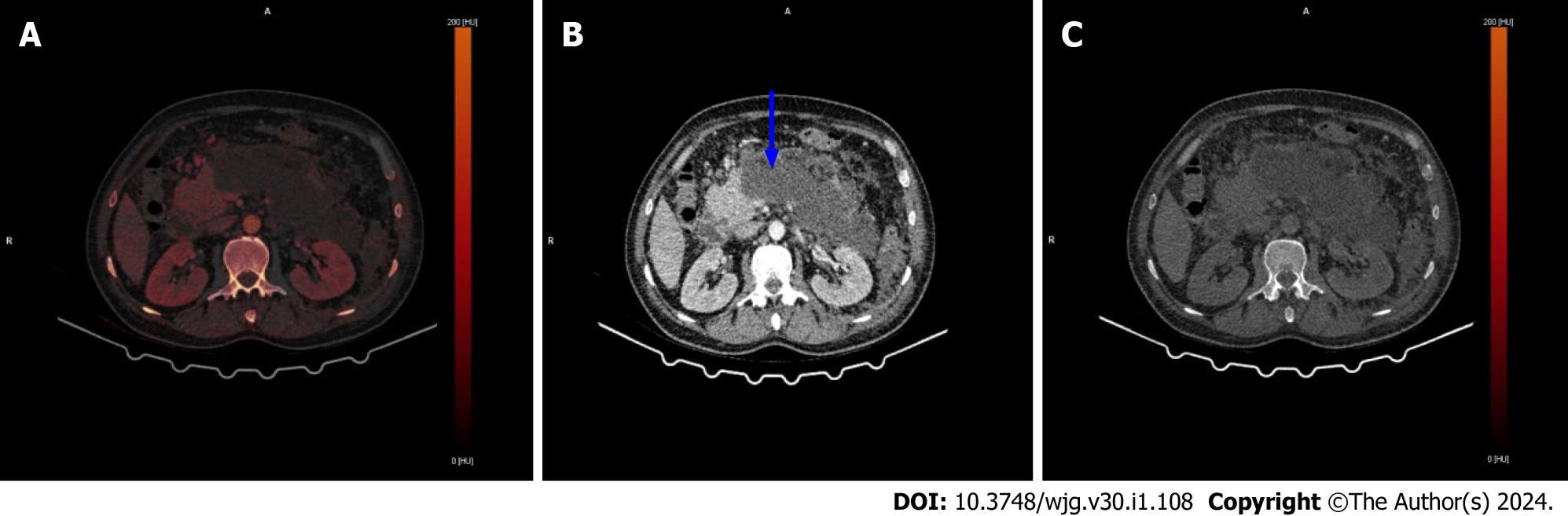
In addition to the imaging techniques listed in the article from Hu et al[1], DECT is another technique that can be used in diagnosis, severity assessment and prognosis prediction. When compared to standard CT, DECT has a better sensitivity for early acute pancreatitis[5]. While necrosis is a late finding on standard CT in patients with acute pancreatitis, DECT may be helpful for early diagnosis and prognosis prediction[6]. Additionally, Hamada et al[7] found in their study that determining iodine concentration using DECT is useful for determining the severity of acute pancreatitis. Figure 1 shows severe necrotic pancreatitis on DECT.
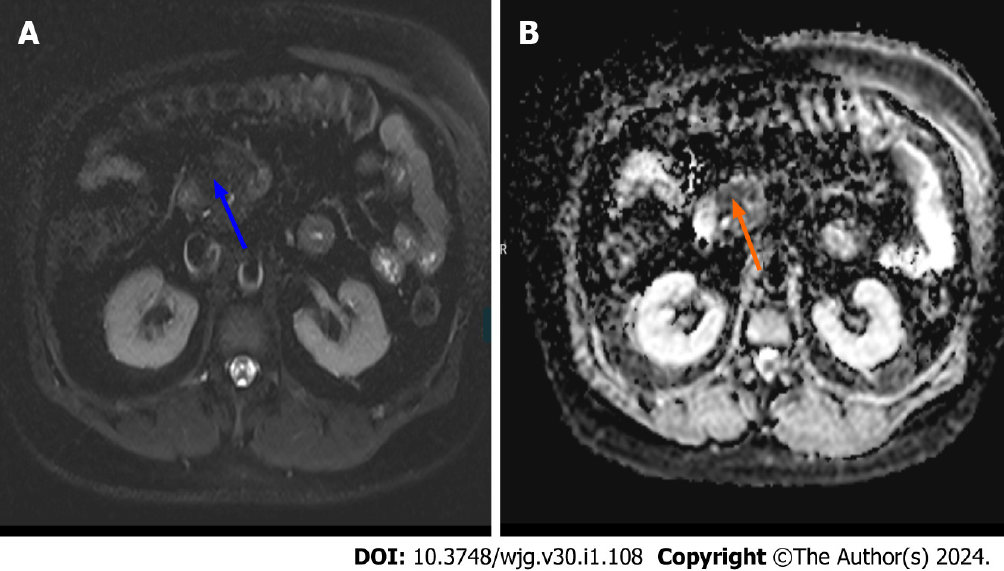
Acute pancreatitis findings can be successfully shown on diffusion weighted MRI at an earlier stage. Yencilek et al[8] reported that apparent diffusion coefficient values decrease with increasing pancreatitis severity. Figure 2 illustrates early acute pancreatitis with low apparent diffusion coefficient values that indicate diffusion restriction.
While imaging is essential to the diagnosis and the management of acute pancreatitis, its ability to diagnose, estimate severity and predict prognosis is not without limitations. Because of its limited sensitivity in detecting the necrotic debris in the early stage, it is challenging to differentiate between acute necrotic collection and acute periprancreatic fluid collection on CT. For that reason, the ideal time to have an initial CT assessment is between 72 h and 96 h after the onset of symptoms, according to the recommendations from the American Pancreatic Association and the International Association of Pancreatology. The limitations of MRI include the need for a greater degree of patient cooperation, limited field of view, increased cost and longer scanning times[1,9].
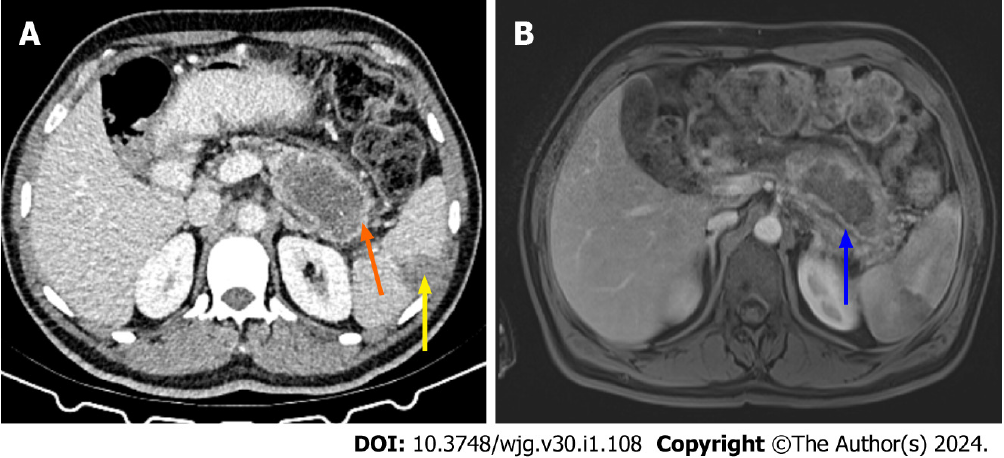
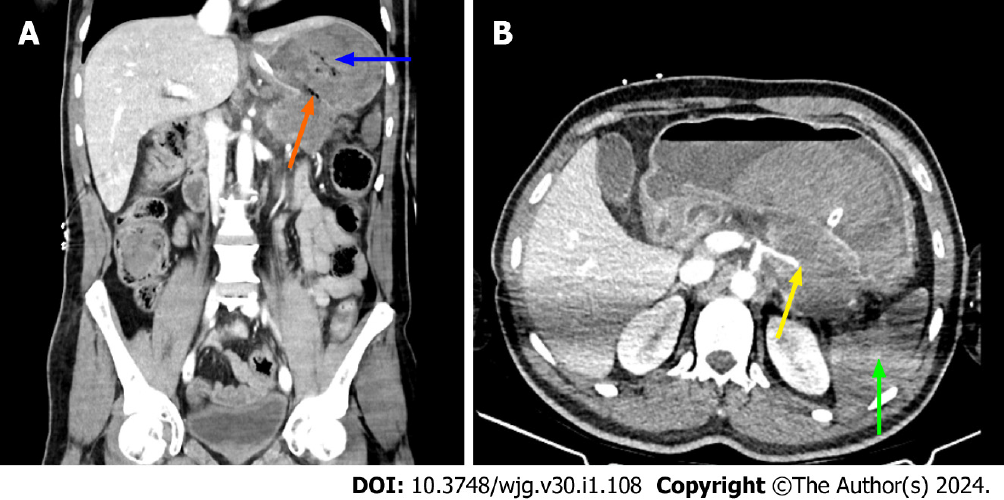
As stated in the article from Hu et al[1], necrosis can be mistaken for pseudocysts on a CT scan, which could lead to an underestimation of the severity of the disease. Walled off necrosis is a late complication of necrotizing pancreatitis, and it is a collection with solid luminal content that is partially liquified. The walled off necrosis seen on CT and MRI is a fluid collection that forms within the pancreatic necrosis and extends into the peripancreatic region[10]. MRI and DECT are superior to standard CT in discriminating walled off necrosis from pseudocyst[6,7,10]. Figure 3 shows CT and MRI images of walled off necrosis, and Figure 4 shows a complication caused by walled off necrosis in the same patient.
In this letter, we aimed to contribute to the literature by discussing radiological differential diagnosis, new imaging techniques and complications of acute pancreatitis with original images of cases in our daily practice. All authors are in complete agreement with the information stated. The content of this manuscript is our original work and has not been published, in whole or in part, before or simultaneously with this submission.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Turkey
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Mao EQ, China; Pan L, China S-Editor: Qu XL L-Editor: Filipodia P-Editor: Yuan YY
| 1. | Hu JX, Zhao CF, Wang SL, Tu XY, Huang WB, Chen JN, Xie Y, Chen CR. Acute pancreatitis: A review of diagnosis, severity prediction and prognosis assessment from imaging technology, scoring system and artificial intelligence. World J Gastroenterol. 2023;29:5268-5291. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 29] [Reference Citation Analysis (4)] |
| 2. | Merkle EM, Bender GN, Brambs HJ. Imaging findings in pancreatic lymphoma: differential aspects. AJR Am J Roentgenol. 2000;174:671-675. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 143] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 3. | Carbognin G, Girardi V, Biasiutti C, Camera L, Manfredi R, Frulloni L, Hermans JJ, Mucelli RP. Autoimmune pancreatitis: imaging findings on contrast-enhanced MR, MRCP and dynamic secretin-enhanced MRCP. Radiol Med. 2009;114:1214-1231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 49] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 4. | Al-Hawary MM, Kaza RK, Azar SF, Ruma JA, Francis IR. Mimics of pancreatic ductal adenocarcinoma. Cancer Imaging. 2013;13:342-349. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 39] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 5. | Martin SS, Trapp F, Wichmann JL, Albrecht MH, Lenga L, Durden J, Booz C, Vogl TJ, D'Angelo T. Dual-energy CT in early acute pancreatitis: improved detection using iodine quantification. Eur Radiol. 2019;29:2226-2232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 46] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 6. | George E, Wortman JR, Fulwadhva UP, Uyeda JW, Sodickson AD. Dual energy CT applications in pancreatic pathologies. Br J Radiol. 2017;90:20170411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 51] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 7. | Hamada H, Fujii T, Kittaka D, Nakai Y, Sato H, Kato K. Dual Energy CT for determining the severity of acute pancreatitis. Showa Univer J Med Sci. 2023;35:11-18. [DOI] [Full Text] |
| 8. | Yencilek E, Telli S, Tekesin K, Ozgür A, Cakır O, Türkoğlu O, Meriç K, Simşek M. The efficacy of diffusion weighted imaging for detection of acute pancreatitis and comparison of subgroups according to Balthazar classification. Turk J Gastroenterol. 2014;25:553-557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 9. | Xiao B, Xu HB, Jiang ZQ, Zhang J, Zhang XM. Current concepts for the diagnosis of acute pancreatitis by multiparametric magnetic resonance imaging. Quant Imaging Med Surg. 2019;9:1973-1985. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 10. | Takahashi N, Papachristou GI, Schmit GD, Chahal P, LeRoy AJ, Sarr MG, Vege SS, Mandrekar JN, Baron TH. CT findings of walled-off pancreatic necrosis (WOPN): differentiation from pseudocyst and prediction of outcome after endoscopic therapy. Eur Radiol. 2008;18:2522-2529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 51] [Article Influence: 3.0] [Reference Citation Analysis (0)] |