Published online Jun 15, 1997. doi: 10.3748/wjg.v3.i2.75
Revised: January 31, 1997
Accepted: March 1, 1997
Published online: June 15, 1997
AIM: To investigate the effects of DNA repair induced by DNA polymerase β in hepatoma cells after γ-ray irradiation.
METHODS: Cell nuclei were prepared from mouse model (SMMC LTNM), in which human hepatoma cells are transplanted on nude mice. The nuclei were then irradiated with 60Co-γ rays at different dose levels or dose rates. A selective inhibitor test was then used to detect the effects of the radiation on DNA repair using N-ethylmaleimide (NEM) and ddTTP as selective inhibitors to DNA polymerases γ and β respectively.
RESULTS: 3H-TTP incorporation into irradiated nuclei or calf thymus DNA was significantly higher than that the rate at which it is incorporated into non-irradiated nuclei when either DNA polymerase β or γ was inhibited. When both NEM and ddTTP are present, the 3H-TTP incorporation in irradiated DNA was not significantly different from the non-irradiated nuclei. Furthermore, 3H-TTP incorporation into DNA of SMMC-LTNM hepatoma nuclei was higher than that of normal hepatocyte nuclei (P < 0.01). This suggests that DNA repair induced by DNA polymerase β was more active in hepatoma cell nuclei than in normal hepatocyte nuclei.
CONCLUSION: DNA polymerase β may be more responsive to DNA damage in some tumor cells than that in normal cells, which may facilitate the cells to repair DNA damages from radiation more efficiently.
- Citation: Cai JM, Zheng XL, Luo C, Gao JG, Cheng TM. Characteristics of DNA repair induced by DNA polymerase β in hepatoma cells after γ-ray irradiation. World J Gastroenterol 1997; 3(2): 75-77
- URL: https://www.wjgnet.com/1007-9327/full/v3/i2/75.htm
- DOI: https://dx.doi.org/10.3748/wjg.v3.i2.75
DNA polymerase β (pol β ), which is the smallest and simplest polymerase, exists in most vertebrate cells. The exact cellular roles of pol β are still obscure, especially in the repair of DNA damaged from γ-ray exposure[1-3]. However, recent studies showed that pol β could participate in DNA repair synthesis after 60Co-γ ray irradiation. In the present study, the differential characteristics of pol β in radiation-induced DNA damage in DNA repair between hepatoma cells and normal liver cells was investigated.
BALB/c or NC nude mice bearing SMMC-LTNM hepatomas were killed and the livers and SMMC-LTNM hepatomas were harvested. The tissues were washed with Hanks solution, cut into pieces, homogenized and filtered through a nylon net. The cells were collected by centrifuge and the nuclei were isolated as described previously[4].
Cell nuclei suspensions or calf thymus DNA solutions (for the controls; Sigma Co.) were put in 1.5 mL sterile Eppendorf tubes in an ice bath before being transferred into a field of 60Co-γ rays (1.85 × 1015 Bq) and irradiated with 1-40 Gy at 1-10 Gy/min dose rates. The samples were maintained in an ice bath throughout the procedures. The DNA repair synthesis test was carried out immediately after irradiation.
DNA repair synthesis was measured in the following reaction mixture. Each reaction mixture contained 0.1 mL sample solution (nuclei or calf thymus DNA) and 0.1 mL DNA repair synthesis reaction solution. The reaction solution contained, at a final concentration, 18 mmol/L Tris-HCl (pH8.5), 2 mmol/L dithiothretol, 40 mmol/L MgCl2, 4 mmol/L ATP, 2 mmol/L dCTP, dGTP, dATP, and 3.7 × 10.4 Bq 3H-TTP. Selective inhibitors (NEN or ddTTP) were used as required. For the calf thymus DNA samples, rat DNA polymerase β (a gift from Dr. Akio Matsukage) was also put in the reaction mixtures. After incubation at 37 °C for 120 min with shaking, 1 mL reaction stopping solution containing 1 mol/L perchloric acid and 20 mmol/L sodium pyrophosphate was added to each reaction mixture. The radioactive DNA product was collected on a piece of cellulose paper, and the incorporation of 3H-TTP was measured using a scintillation counter.
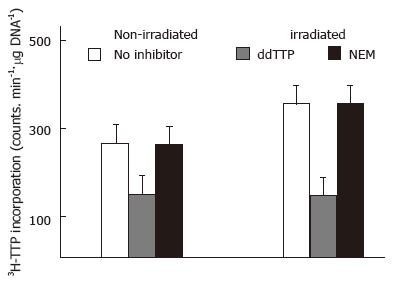
In order to evaluate the role of pol β in DNA radiation damage repair, we first examined the effects of radiation on DNA repair by comparing the amount of 3H-TTP incorporation in irradiated and non-irradiated calf thymus DNA (10 Gy, 5 Gy/min). The 3H-TTP incorporation was much higher (P < 0.01) in irradiated DNA than in non irradiated DNA (Figure 1). This increase was also present in DNA treated with NEM. However, when ddTTP was added to the reaction mixture to inhibit pol β , the amount of 3H-TTP incorporation was similar in the irradiated and non-irradiated DNA (Figure 1).
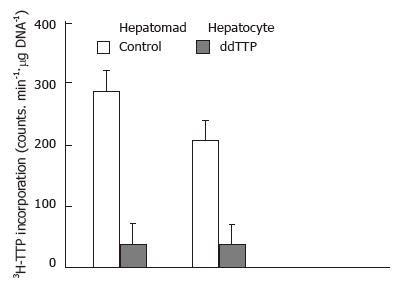
Next, we examined DNA repair in hepatoma and normal liver nuclei. As seen with the calf-thymus DNA, the incorporation of 3H-TTP into both irradiated SMMC-LTNM hepatoma nuclei and normal liver nuclei was higher (P < 0.05) than that in non irradiated nuclei (Table 1). To investigate whether pol β is responsible for the DNA repair synthesis in irradiated nuclei, a subset of irradiated hepatoma and normal liver nuclei were treated with the pol β selective inhibitor ddTTP. As illustrated in Figure 2, the incorporation of 3H-TTP did not significantly increase in nuclei treated with ddTTP; however, nuclei from both heptoma and normal liver that were not treated with ddTTP had a significant increase in the incorporation of 3H-TTP (P < 0.01).
The characteristics of DNA repair synthesis induced by pol β in SMMC-LTNM hepatoma nuclei were compared with that in normal hepatocyte nuclei after different doses of γ-ray exposure. We found that the increment of 3H-TTP incorporation (3H-TTP incorporation of irradiated nuclei-3H-TTP incorporation of non irradiated nuclei) of hepatoma nuclei were much higher than that in normal hepatocyte nuclei (P < 0.01) at all dose rate groups under the same reaction conditions and same absorbed dose (10 Gy) (Table 2).
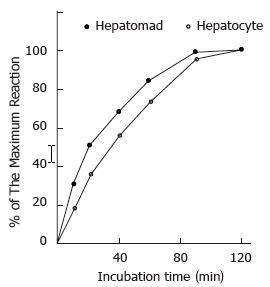
Further investigation revealed that there was a significant difference in the reaction speed of DNA repair synthesis induced by pol β between the two kinds of nuclei. The reaction induced by pol β in hepatoma nuclei was faster than that in hepatocyte nuclei. The incubation time, which the 3H-TTP incorporation increased to 50% of the maximum, was about 18 min in hepatoma nuclei and about 32 min in normal hepatocyte nuclei (Figure 3).
DNA damage caused by ionization can be partly repaired under proper conditions[5]. However, the mechanism of DNA repair is not clearly understood[6,7]. The role of pol β in DNA repair after ionization radiation exposure is one of the problems remaining to be solved. For example, which polymerase is responsible for DNA repair synthesis after γ-ray exposure has been a controversy in radiobiology. The evidence obtained in this study further demonstrates that pol β does participate in DNA repair synthesis and it is an important enzyme in the process of DNA repair after γ-ray exposure.
This study also found some intriguing differences in the DNA repair induced by pol β between SMMC-LTNM hepatoma cells and normal hepatocytes. We found that SMMC-LTNM hepatoma cells had a faster DNA repair synthesis reaction, a higher incorporation of 3H-TTP after radiation damage, and a lower dose of radiation required to reach the maximum 3H-TTP incorporation. All these characteristics suggest that the DNA repair synthesis induced by pol β in hepatoma cells was strong. In other experiments we found that the gene of pol β was overexpressed and the enzyme activity was high in hepatomas. These findings are meaningful in the radiotherapy of tumors. Cell death in radiotherapy is closely related to the extent of DNA damage and DNA repair. Cells in which their pol β gene is overexpressed and which have high enzymatic activity have a stronger ability for DNA repair synthesis, and therefore, more cells could theoretically survive the killing effects of radiation. The resistance to ionization radiation of some tumor cells may be related with the effects of pol β . It is highly necessary to investigate the radiobiologic characteristics of pol β in more tumors, especially in those tumors which are resistant to radiation, and to study the radiosensitizing effects by the way of selectively inhibiting the pol β in tumor cells.
Original title:
S- Editor: Filipodia L- Editor: Jennifer E- Editor: Liu WX
| 1. | Davies JF, Almassy RJ, Hostomska Z, Ferre RA, Hostomsky Z. 2.3 A crystal structure of the catalytic domain of DNA polymerase beta. Cell. 1994;76:1123-1133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 137] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 2. | Nowak R, Siedlecki JA. Effect of busulphan treatment and elevated temperature on the expression of the beta-pol gene in rat testis. Mol Biol Rep. 1991;15:25-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 3. | Cai JM, Zheng XL, Luo CJ, Gao JG, Chen TM, Yang RJ. The roles of DNA polymerase Y on repair of DNA irradiated by Y-rays. J Med Coll PLA. 1995;10:75-78. |
| 4. | Jiang YF, Zheng XL. Effect of DNA polymerase-Y on repair of Y-ray irradiated hepatoma (Ha22) cell nuclei. J Radiant Res Radiant Process. 1990;8:150-154. |
| 5. | Price A. The repair of ionising radiation-induced damage to DNA. Semin Cancer Biol. 1993;4:61-71. [PubMed] |
| 6. | Friedberg EC. DNA repair. San Francisco: W.H. Freeman and Company 1985; 75-220. |
| 7. | Ohnishi T, Yuba S, Date T, Utsumi H, Matsukage A. Rat DNA polymerase beta gene can join in excision repair of Escherichia coli. Nucleic Acids Res. 1990;18:5673-5676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.3] [Reference Citation Analysis (0)] |