Published online Jun 15, 1997. doi: 10.3748/wjg.v3.i2.64
Revised: January 31, 1997
Accepted: March 1, 1997
Published online: June 15, 1997
AIM: To explore the pathomorphological characteristics of large hepatocellular carcinoma (LHCC) with low serum alpha-fetoprotein (AFP) level.
METHODS: Specimens obtained from surgically resected LHCC with undetectable or low levels of serum AFP were fixed in formalin, embedded in paraffin, and prepared as serial sections. Routine hematoxylin and eosin as well as immunohistochemical stains (LSAB method) were used to test for expression of AFP, alpha-1-antitrypsin, epithelial membrane antigen, and vimentin. Some characteristics of the histopathological changes and immunohistochemical reactions of the cancerous tissues were observed under the light microscope.
RESULTS: The majority of the cases (19/30) of LHCC with undetectable or low levels of serum AFP were of the clear-cell-type HCC, with 2 being positive for AFP expression at the periphery of the cytoplasm.
CONCLUSION: The clear cell is the morphological manifestation of disturbance in glycogen and/or lipid metabolism of hepatoma cells. Such changes might be one of the factors hindering the synthesis of AFP and resulting in negative or low level serum AFP of the patient.
- Citation: Wu QM, Hu MH, Tan YS. Histopathology and immunohistochemistry of large hepatocellular carcinoma with undetectable or low serum levels of alpha-fetoprotein. World J Gastroenterol 1997; 3(2): 64-66
- URL: https://www.wjgnet.com/1007-9327/full/v3/i2/64.htm
- DOI: https://dx.doi.org/10.3748/wjg.v3.i2.64
Serum alpha-fetoprotein (AFP) is generally detected and often elevated in patients with hepatocellular carcinoma (HCC); however, some patients with small HCC (SHCC, diameter ≤ 3 cm) in early stage or large HCC (LHCC; Diameter, ≥ 3 cm) in the mid and late stages show absence or low levels of serum AFP. In the present study, 30 cases of LHCC with undetectable or low levels serum AFP were assessed histopathologically and immunohistochemically, and the relationship between some pathomorphological characteristics and the level of serum AFP was evaluated.
Specimens were obtained from the surgical resection of 30 LHCC with diameter of > 3 cm and undetectable or low levels of serum AFP (counter immunoelectrophoresis, negative; Rocket electrophoresis, ≤ 399 μg/L). Eight specimens from cases positive for serum AFP with high serum AFP levels (rocket electrophoresis ≥ 400 μg/L) were used as controls.
All specimens were fixed in 10% formalin normal saline, embedded in paraffin, prepared as serial sections, stained by routine hematoxylin-eosin and immunohistochemical technique with labeled streptavidin biotin (LSAB), and observed under the light microscope.
Immunohistochemistry was performed to detect the following: AFP, alpha-1-antitrypsin (AAT), epithelial membrane antigen (EMA), and vimentin. All antibodies and agents were products from DAKO Company.
Classification of the 30 cases of LHCC with undetectable or low levels of serum AFP according to the Edmondson 4-grade method[1] revealed that cell differentiation was of grade I in 1 case, grade II in 1 case, grade III in 18 cases, and grade IV in 10 cases (Table 1).
| Serum alpha-fetoprotein level (µg/L) | Cases | Degree of differentiation | |||
| I | II | III | IV | ||
| ≤ 20 | 18 | 1 | 9 | 7 | 1 |
| 21-299 | 12 | 0 | 9 | 3 | 0 |
| Total | 30 | 1 | 18 | 10 | 1 |
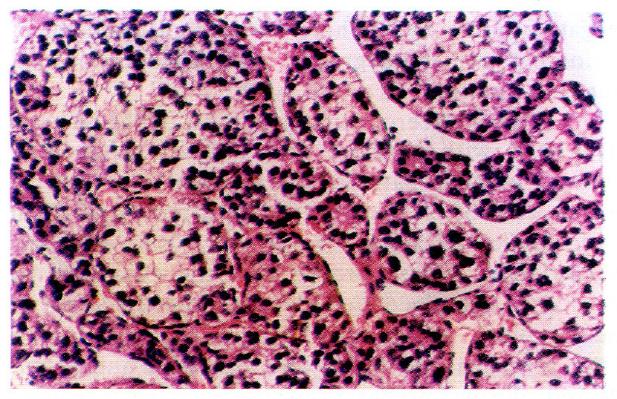
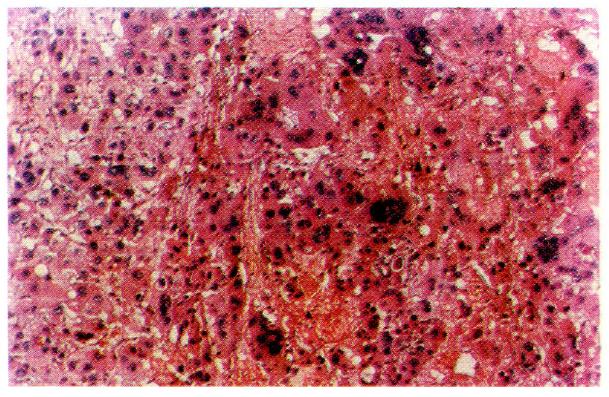
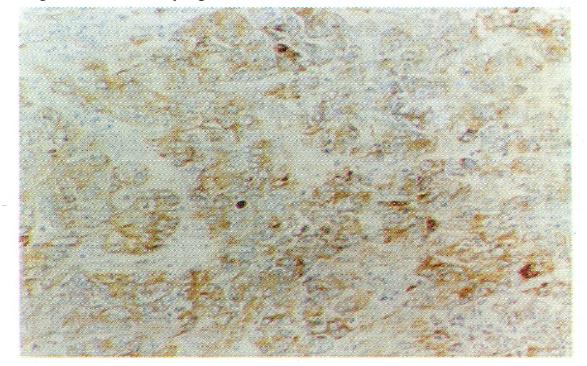
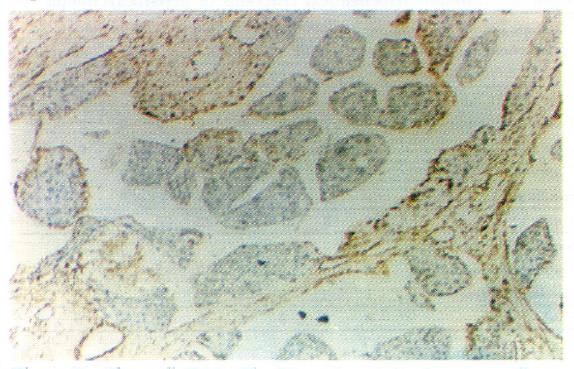
According to the WHO classification system[1], cells of carcinomatous tissues in the 30 cases of LHCC with undetectable or low levels of serum AFP showed peculiar morphological types. Among them, 19 were of the clear-cell type (clear cells occupied 30%-90% of total cancer cells), (Figure 1), 1 was of the giant-cell type (Figure 2), and 1 was of the spindle-cell type (Figure 3, Table 2).
| Serum alpha-fetoprotein level (µg/L) | Number of cases | Morphological types | |||
| Trabecular compact | Clear cell | Giant cell | Spindle cell | ||
| ≤ 20 | 18 | 6 | 10 | 1 | 1 |
| 21-299 | 12 | 3 | 9 | 0 | 0 |
| Total | 30 | 9 | 19 | 1 | 1 |
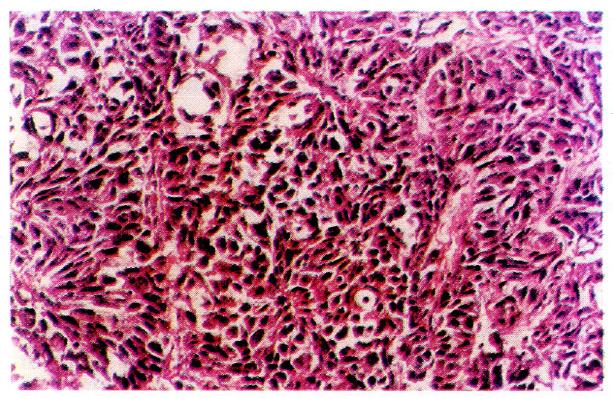
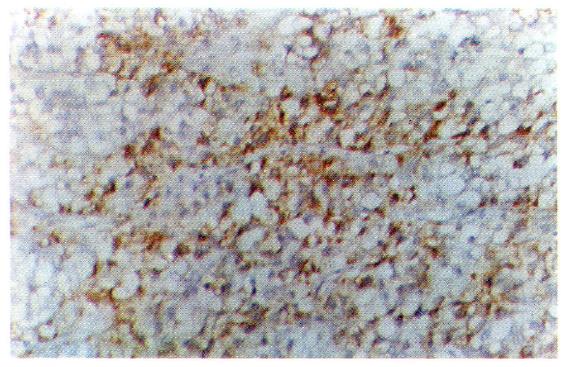
In histological sections of the 30 cases of LHCC with undetectable or low levels of serum AFP, only 3 were found to be positive for serum AFP in cells, 2 were of the clear-cell type, and 1 was of the non-clear-cell type. AFP-positive cancer cells were focally distributed and the AFP-positive portion in the clear cells was expressed in the periphery of the cytoplasm (Figure 4). AAT-positive cancer cells were found in 8 cases (Figure 5) and EMA-positive cancer cells in 28 cases (Figure 6). Tests for vimentin expression in cancer cells of all the 30 cases were negative, while those in the interstitial tissues (including endothelial cells of blood sinuses and blood vessels, smooth muscle cells of the vascular wall, and fibroblasts) were positive (Figure 7).
In paraffin sections of 8 cases of LHCC with positive and high expression levels of serum AFP observed immunohistochemically, various concentrations of AFP-positive, AAT-positive, and EMA-positive cancer cells were found in all cases; tests for vimentin expression in the 8 cases yielded the same results as those obtained in the cases with undetectable serum AFP levels, i.e. negative results for cancer cells and positive results for interstitial tissues (Table 3).
| Serum alpha-fetoprotein level (µg/L) | Number of cases | Morphological types | |||
| Trabecular compact | Clear cell | Giant cell | Spindle cell | ||
| ≤ 20 | 18 | 6 | 10 | 1 | 1 |
| 21-299 | 12 | 3 | 9 | 0 | 0 |
| Total | 30 | 9 | 19 | 1 | 1 |
Different degrees of cell differentiation were observed in the 30 cases investigated in this study, with Grade III ranking the first and Grade IV the next. Grade III and IV constituted the great majority of the cases, but this grade distribution was not only peculiar for those cases with undetectable or low levels of serum AFP. Two massive case reports from our country[2,3] indicated that Grades III and IV occupied the great majority of HCC, including both cases positive and negative for serum AFP expression. The distribution of Grades III and IV was also predominant in this study of 30 cases of LHCC that had undetectable or low levels of serum AFP, without any significant difference from that observed in HCC that were positive or negative for serum AFP expression. There was no significant correlation between the degree of differentiation of cancer cells and serum AFP level.
Cells of cancerous tissues in most of the 30 cases of LHCC with undetectable or low levels of serum AFP showed peculiar pathological morphology, most of which were of the clear-cell type, with a few belonging to the giant- and spindle-cell types. Wu et al[4] used periodic acid-Schiff and Sudan black stains and proved that the cytoplasm of clear cells were rich in glycogen and lipid, partly as ordinary cytoplasmic constituents (the glycogen and lipid dissolved and disappeared during the preparation of histological slides, which were left clear). On electron microscopy, cell organelles decreased, the rough surfaced endoplasmic reticulum (RER), free ribosomes and polyribosomes seemed to have been pushed aside by glycogen and lipid. Zhang et al[5] observed by immune-electronmicroscopy that AFP was mainly located in the membrane of RER and ribosomes. Our immunohistochemical study on 19 cases of clear-cell carcinoma showed positive results for AFP expression in only 2 cases, wherein AFP was focally distributed with positive staining of the peripheral cytoplasm. The latter findings coincided with the above mentioned phenomenon, i.e., the pushing of RER and ribosomes aside. It is suggested that clear cells are morphological manifestations of glycometabolic and lipometabolic disturbances in hepatocarcinomatous cells. It is a type of degeneration of the carcinoma cells that possibly hinders the synthesis of AFP. This might be one of the factors responsible for the undetectable or low levels of serum AFP in patients with HCC.
Thus, samples testing negative and positive for serum AFP expression did not show any significant differences in the results of the immunohistochemical studies such as those for EMA and vimentin. The positive results for EMA expression and negative results for vimentin expression in the cancer cells are indicative of their epithelial origin. Further, AAT-positive cases among those testing negative for serum AFP expression were apparently fewer than those testing positive for serum AFP for control. The reasons for these findings remain obscure and further studies are required to elucidate them.
Original title:
S- Editor: Filipodia L- Editor: Jennifer E- Editor: Liu WX
| 1. | Kojiro M. Pathology of hepatocellular carcinoma. Tokyo: Springer Verlag 1987; . [DOI] [Full Text] |
| 2. | National Coorperation Group of Pathology of Hepatoma. Pathology of primary hepatocellular carcinoma: an analysis of 500 autopsies. Jiangsu Yiyao Zazhi (in Chinese). 1985;1:2-7. |
| 3. | Wu MC, Gong WM, Zhang XH, Chen H, Zhang XZ. Clinico-pathological study of 1000 cases of hepatocellular carcinoma. Aizheng Fangzhi Yu Zhiliao. 1993;20:137-139. |
| 4. | Wu PC, Lai CL, Lam KC, Lok AS, Lin HJ. Clear cell carcinoma of liver. An ultrastructural study. Cancer. 1983;52:504-507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 5. | Zhang JS, Xu YD, Zhang XR, Yin YY. Pathological basis of serum AFP elevation during carcinomatous change in hepatoma of rats. Zhonghua Binglixue Zazhi. 1987;16:278-280. |