Published online Mar 15, 1997. doi: 10.3748/wjg.v3.i1.50
Revised: November 5, 1996
Accepted: December 14, 1996
Published online: March 15, 1997
AIM: To evaluate the role of cAMP/PKA and DAG/PKC pathways of MGc80-3 cells treated with a traditional Chinese medicine compound, bailong preparation (bailong).
METHODS: cAMP level, DAG content and activities of PKA and PKC were measured in different groups: control; 1.8 g/L bailong; 1.8 g/L bailong + 20 mg/L PKA inhibitor; and 5 μmol/L PKC inhibitor.
RESULTS: When MGc80-3 cells were treated with bailong for 3 h, cAMP level and PKA activity were 113% and 19.7% higher than those of the control, while DAG content and PKC activity were 47.0% and 64.2% lower than those of the control. When the PKC pathway was blocked by PKC inhibitor GF-109203 X, cAMP level and PKA activity were increased by 78.8% and 33.5% compared to inhibitor GF-109203 X, and cAMP level and PKA activity were increased by 78.8% and 33.5% compared to the control, while the DAG content and PKC activity were decreased by 40.3% and 56.3%. When MGc80-3 cells were treated with bailong and PKA inhibitor blocked PKA pathways at the same time, cAMP level and PKA activity were decreased by 46.0% and 28.9%. On the other hand, DAG content and PKC activity were increased by 50.7% and 51.6% compared to the bailong group.
CONCLUSION: There is a relationship of cause and effect between differentiation of MGc80-3 cells and the signal pathways. The results of this study are similar to that of hexamethylenebisacetamide (HMBA), suggesting that the two signal systems are the foundation of proliferative regulation of MGc80-3 cells treated with Chinese medicine bailong or HMBA.
- Citation: Gu SQ, Liang YY, Fan LR, Li BY, Wang DS. Co-regulative effects of the cAMP/PKA and DAG/PKC signal pathways on human gastric cancer cells during differentiation induced by traditional Chinese medicines. World J Gastroenterol 1997; 3(1): 50-53
- URL: https://www.wjgnet.com/1007-9327/full/v3/i1/50.htm
- DOI: https://dx.doi.org/10.3748/wjg.v3.i1.50
Most extracellular agonists exert their effects on cells by activating or inhibiting transmembrane signaling systems that control the production of second messengers. Recently, the biochemistry of cellular signaling systems have come to the fore as potential targets for the discovery and development of new anticancer drugs[1,2]. In general, cAMP plays a negative role in cell proliferation and a positive role in differentiation of cancer cells. On the other hand, the role of two second messengers, DAG and IP3, act against cAMP. As we know, malignant phenotypes of some cancer cells can be changed by cAMP and its derivatives through the regulation of the PKA system. Actually, it is the result of the combined regulation of several signal systems. Furthermore, an increasing body of literature suggests that PKC may play a role in the signal transduction pathways mediating hexamethylenebisacetamide (HMBA) induced changes in cellular growth and differentiation[3]. Nishizuka pointed out that there were crosstalk between the cAMP/PKA and DAG/PKC pathways and provided two models for their interaction[4]. Tumor therapy by TCM has been reported[5,6], but its mechanism of function is not clear and there are no papers on the co-regulative effects on different signal systems. Previous research showed that bailong responded well in tumor therapy and that its function was related to a signaling system. Further investigations on signal pathways of MGc80-3 cells treated with bailong were carried out in this study in order to evaluate its positive and negative regulation compared to that of HMBA.
Bailong, a traditional Chinese medical formula, is composed of 8 Chinese medicinal herbs, including Solanum lyratum thumb and Solanum nigrum L. The end product is produced by Tianjin Pharmaceutical Factory of Chinese Medicine. 300 g/L powder of bailong (its efficacy is 6 times higher than the crude drugs) was dissolved in 100 mL distilled water, then sterilized at 0.07MP for 20 min and centrifuged at 3000 r/min for 15 min after cooling down. The supernatant was collected as stock buffer and its concentration was 18 g/L, while the working buffer was 1.8 g/L. Normal medium was the same as the bailong medium.
[3H] glycerol was purchased from Amersham, [3H] cAMP and [r-32P] ATP were obtained from the Institute of Nuclear Energy of China, PKA inhibitor (type III) was purchased from Sigma and PKC inhibitor (GF-109203 X) was obtained from Boehringer Mannheim.
MGe80-3 cells were maintained at 37 °C in the presence of 5% CO2 in RPMI-1640 (Gibco) medium containing 15% bovine serum.
To take advantage of the competitive combination between cAMP and 3H-cAMP into specific protein kinase, intracellular cAMP level was measured using a radioimmunoassay kit, as described by the manufacturer, which was supplied by the Chinese Academy of Science. Samples were extracted according to Jakobsen[7]. cAMP level was expressed as pmol/10.6 cells.
To label MGc80-3 cells with 3H-glycerol for 15 h according to Wolfman et al[7], cells were collected and extracted, then extended on Silica gel G, followed by scintillation counting in Pico-fluor (Packard).
Cells were washed and scraped into cold phosphate buffered saline (PBS). After being centrifuged at 1000 r/min for 5 min, sediment was dissolved in cell lysis buffer (20 mmol/L, Tris HCI, 4 mmol/L EDTA, 1 mmol/L EGTA, pH7.5) at 0 °C, lysed by sonication without breaking the nuclear membrane, and then added to the same volume sucrose Tris buffer (40 mmol/L Tris HCI, pH7.5, 0.66 mol/L sucrose, 0.1 mol/L β-mercaptoethanol, 2 mmol/L PMSF). Centrifuged at 4 °C for 5 min in order to precipitate the nucleus, partial supernatant was used to measure the activity of PKA. The remaining sediment was re-suspended in the surplus, sonicated nuclear membrane at 0 °C, 10% Trito X 100 was added to final concentration of 1%, shaken on ice for 1 h and centrifuged again at 34000 r/min for 1 h. Supernatant was purified by DEAE-52 (Whatman) and the column was balanced with buffer (5 mmol/L Tris HCI, pH7.5, 1 mmol/L EGTA, 50 mmol/L β-mercaptoethanol) previously. The column was washed with 10 mL balance buffer after samples were added, then washed out with balance buffer containing 0.15 mol/L NaCl so that the washed buffer could be used for a PKC activity assay.
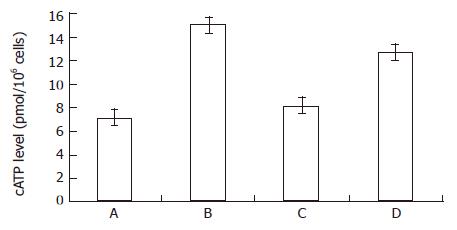
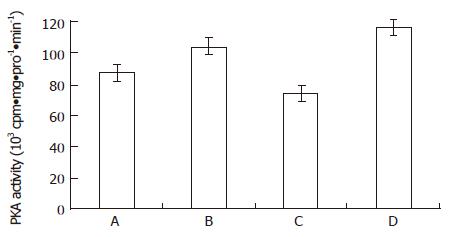
From Figure 1 and Figure 2, both the bailong and PKC inhibitor could enforce the cAMP-PKA pathway in MGc80-3 cells when treated for 3 h. When treated with bailong, cAMP level and PKA activity increased by 112.7% and 19.7%, while when treated with PKC inhibitor, it increased by 78.9% and 33.5% compared to the control. When treated with the bailong and PKA inhibitor, cAMP level and PKA activity decreased by 46.4% and 28.9% compared to the bailong group.
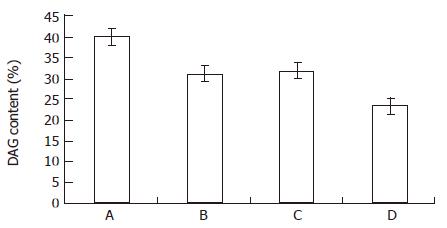
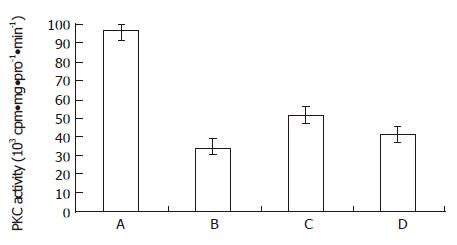
From Figure 3 and Figure 4, both the bailong and PKC inhibitor could block the DAG-PKC pathway in MGc80-3 cells treated with bailong for 3 h. When treated with bailong, DAG and PKA activity decreased by 47.0% and 64.2%, while when treated with PKC inhibitor it decreased by 40.3% and 56.3% compared to the control. When treated with bailong and PKA inhibitor, DAG and PKA activity increased by 50.7% and 51.6%.
According to the theory of traditional Chinese medicine (TCM), tumorigenesis and development always follow a process of blood stasis due to stagnation of Qi, causing accumulation of phlegm-dampness, stagnation of heat, disorder of the viscera and their interrelations, and deficiency of both blood and Qi. Upon this, bailong, a prescription of TCM, with 8 herbs including Solanum lyratum thumb and Solanum nigrum L. as ingredients, functions on fostering the original essence, strengthening the body defense, promoting blood circulation so as to remove the blood stasis and clear away pathogenic heat and toxic materials. After several modifications of its composition and much research on the production line, bailong was not made into a medicinal preparation until 1991. Clinical and experimental studies suggested that bailong could inhibit tumor growth in vivo and in vitro. Based on previous works, further investigations on the co-regulation and their interaction between cAMP/PKA and DAG/PKC signal systems of MGc80-3 cells treated with bailong was carried out in order to ascertain the malignancy reduction of cancer cells by regulating the expression of signal systems, which helps to integrate the theory of TCM and Western medicine to regulate tumor growth and differentiation at the molecular level.
In measuring the efficacy of bailong, remarkable growth inhibition of tumor cells occurred at the concentration of 1.0 g/L. At the concentration of 1.8 g/L, growth inhibition of MGc80-3 cells was 52.9% on the 3rd day and 75.8% on the 4th day. MGc80-3 cells were still treated with bailong at the concentration of 1.8 g/L. Three hours later, we found that cAMP level and PKA activity increased while the DAG content and PKC activity decreased. This suggests that: 1. the two signal systems were closely related to growth inhibition of MGc80-3 cells resulting from bailong. Picus thought that activation of PKC could be the common pathway in process of tumor promotion[12] and the role of differentiation of PKA II has been universally acknowledged. High expression of PKA related to differentiation and low expression of PKC related to proliferation are the reasons for growth inhibition of MGc80-3 cells. It is worth pointing out that bailong could translocate PKA II from cytoplasm to the nucleus in EAC cells, which is very similar to that of HMBA. This is the first time the role of TCM in regulating cell proliferation and differentiation of cancer cells through the cell signaling systems has been reported; 2. When MGc80-3 cells were cultured with bailong for 3 h, remarkable changes of the two signal systems were observed, but the growth inhibition was not obvious until the 3rd or 4th day, indicating that the changes of messenger molecules appeared earlier than that of cell proliferation by several cell cycles[13]. Regulation of bailong was similar to that of HMBA in signal transduction of MGc80-3 cells[14], which suggested both of them may have the same mechanism. To ascertain if the relationship of cause and effect between regulation of bailong and two signal systems existed in MGc80-3 cells, different blockers of signal pathways were used to inhibit signal transduction. When the cAMP/PKA pathway of MGc80-3 cells was blocked by PKA inhibitor (type III), the efficacy of bailong was obvious, that is to say, cAMP level and PKA activity did not rise compared to the bailong group and the DAG/PKC pathway returned to the level of the control. If the PKC inhibitor GF-109203 X was used as positive standard instead of treatment with bailong, it was found that the role of blocking the DAG/PKC pathway was similar to that of bailong. Thus it proved that cAMP/PKA and DAG/PKC signal pathways were related to the anticancer action of bailong. In view of recent research[15,16], cAMP and its derivatives (8-CI-cAMP, db-cAMP, etc.) and PKC inhibitor (such as staurosporine analogues: CGP41 251 and UCN-01) conspicuously reduce malignancy of cancer cells. Cellular signaling systems have been regarded as one of the potential targets for the discovery and development of new anticancer drugs, which suggested that the analysis of bailong should conform to international studies on cellular signaling systems, especially to the results of HMBA. So the promotion of differentiation of MGc80-3 cells induced by bailong might be the positive and negative result of the two signal pathways. Tony Hunter pointed out that the interaction of protein kinases and phosphatases conformed to Nishizuka’s[4] model, in which the interaction of protein kinases and phosphatases were suited to the TCM theory of Yin Yang[17]. The positive and negative regulation of cAMP/PKA and DAG/PKC in MGc80-3 cells could also be explained by the Yin Yang theory. All of these suggest that the same targets are shared by Chinese and Western medicine and further investigations are needed.
Original title:
Presented at The International Conference of Gastroenterology in Shanghai in November, 1996; at The 7th Chinese Symposium on Oncology in Integrated Chinese and Western Medicine in Hangzhou in September 1996; and at the 8th Chinese Symposium on Digestive Diseases in Integrated Chinese and Western Medicine in Xi’an in October 1996
S- Editor: Yang RC L- Editor: Ma JY E- Editor: Hu S
| 1. | Powis G. Signalling targets for anticancer drug development. Trends Pharmacol Sci. 1991;12:188-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 82] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 2. | Tritton TR, Hickman JA. How to kill cancer cells: membranes and cell signaling as targets in cancer chemotherapy. Cancer Cells. 1990;2:95-105. [PubMed] |
| 3. | Melloni E, Pontremoli S, Michetti M, Sacco O, Cakiroglu AG, Jackson JF, Rifkind RA, Marks PA. Protein kinase C activity and hexamethylenebisacetamide-induced erythroleukemia cell differentiation. Proc Natl Acad Sci USA. 1987;84:5282-5286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 76] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 4. | Nishizuka Y. Studies and perspectives of protein kinase C. Science. 1986;233:305-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3896] [Cited by in RCA: 3956] [Article Influence: 101.4] [Reference Citation Analysis (0)] |
| 5. | Yu QS, Zhang FZ, Tang XR. [Clinical study on early use of Chinese medicinal herbs and chemotherapy after operation of gastric cancer]. Zhongguo Zhongxiyi Jiehe Zazhi. 1995;15:459-461. [PubMed] |
| 6. | Wang GM, Chen CH, Sun GZ. [Clinical and experimental study in treating gastric cancer with replenishing qi and invigorating spleen oral liquid combined with chemotherapy]. Zhongguo Zhongxiyi Jiehe Zazhi. 1994;14:661-663. [PubMed] |
| 7. | Jakobsen A. Cellular cyclic AMP content independent of ascites tumour growth rate in vivo. Eur J Cancer. 1975;11:203-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 8. | Wolfman A, Macara IG. Elevated levels of diacylglycerol and decreased phorbol ester sensitivity in ras-transformed fibroblasts. Nature. 1987;325:359-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 254] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 9. | Plet A, Evain-Brion D, Gerbaud P, Anderson WB. Retinoic acid-induced rapid loss of nuclear cyclic AMP-dependent protein kinase in teratocarcinoma cells. Cancer Res. 1987;47:5831-5834. [PubMed] |
| 10. | Darbon JM, Issandou M, Delassus F, Bayard F. Phorbol esters induce both intracellular translocation and down-regulation of protein kinase C in MCF-7 cells. Biochem Biophys Res Commun. 1986;137:1159-1166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 43] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 11. | Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265-275. [PubMed] |
| 12. | Pincus SM, Beckman BS, George WJ. Inhibition of dimethylsulfoxide-induced differentiation in Friend erythroleukemic cells by diacylglycerols and phospholipase C. Biochem Biophys Res Commun. 1984;125:491-499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 13. | Shen DW, Real FX, DeLeo AB, Old LJ, Marks PA, Rifkind RA. Protein p53 and inducer-mediated erythroleukemia cell commitment to terminal cell division. Proc Natl Acad Sci USA. 1983;80:5919-5922. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 42] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 14. | Peng J, Liang YY, Fang JC, Shi YJ, Wang DS. [Study on the relationship between two second-messenger pathways on the regulation of proliferation and differentiation in MGc 80-3 cells]. Shiyan Shengwu Xuebao. 1993;26:187-195. [PubMed] |
| 15. | Meyer T, Regenass U, Fabbro D, Alteri E, Rösel J, Müller M, Caravatti G, Matter A. A derivative of staurosporine (CGP 41 251) shows selectivity for protein kinase C inhibition and in vitro anti-proliferative as well as in vivo anti-tumor activity. Int J Cancer. 1989;43:851-856. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 313] [Cited by in RCA: 319] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 16. | Akinaga S, Gomi K, Morimoto M, Tamaoki T, Okabe M. Antitumor activity of UCN-01, a selective inhibitor of protein kinase C, in murine and human tumor models. Cancer Res. 1991;51:4888-4892. [PubMed] |
| 17. | Hunter T. Protein kinases and phosphatases: the yin and yang of protein phosphorylation and signaling. Cell. 1995;80:225-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2197] [Cited by in RCA: 2146] [Article Influence: 71.5] [Reference Citation Analysis (0)] |