Published online Mar 15, 1997. doi: 10.3748/wjg.v3.i1.35
Revised: October 1, 1996
Accepted: January 1, 1997
Published online: March 15, 1997
AIM: To study the properties and factors of Helicobacter pylori (H. pylori) adherence to human epithelial cells.
METHODS: The adherent properties of human epithelial cells were studied using a group of isolated H. pylori strains, anti-H. pylori monoclonal antibodies and varied pH environment in in vitro adherence model with HEp2 cells.
RESULTS: H. pylori YC 11A was able to adhere to HEp2 cells specifically and its adherence efficiency reached the highest (81%) within 3 h after incubation with HEp2 cells. There was no significant difference between adherence in air and in 5% oxygen. The monoclonal antibodies specific to H. pylori predominant antigens did not inhibit activities on adherence of H. pylori to HEp2 cells. The pH value significantly affected the adherence process and the optimal pH was 3.0-4.6.
CONCLUSION: H. pylori specifically adheres to HEp2 cells, and pH value significantly affects this process. A high level of anti-H. pylori predominant antibodies in serum may have no protective activities against H. pylori infection.
- Citation: Wang ZX, Shen HF, Chen HJ. Adherent properties of Helicobacter pylori to human epithelial cells. World J Gastroenterol 1997; 3(1): 35-37
- URL: https://www.wjgnet.com/1007-9327/full/v3/i1/35.htm
- DOI: https://dx.doi.org/10.3748/wjg.v3.i1.35
Helicobacter pylori (H. pylori) is a pathogen of nearly all duodenal ulcers and most gastric ulcers and is associated with an increased risk of gastric adenocarcinoma[1,2]. H. pylori has been found in intercellular junctions as well as on the surface of natural cells in vivo, but never inside the cells for its poor invasive properties, yet its adherent properties are rarely identical and could generate the characteristic histopathological lesions. This study aims to develop an in vitro model of adherence of H. pylori and analyze the properties and the factors of adherence of H. pylori to human epithelial cells.
The H. pylori strains used were isolated initially from patients with chronic active gastritis or digestive ulcers and stored at 70 °C[3,4]. HEp2, an epithelial cell line, was obtained from the Chinese Academy of Preventive Medicine and has passed 23 generations in culture.
HEp2 cells were grown in 24 well microplates (Nunc, Roskilde, Denmark) with cover slips in 1.5 mL of Delbacco's modified Eagle’s medium with 10% fetal calf serum without antibiotics to obtain a subconfluent monolayer. The bacteria were cultured for 48-72 h on Skirrow’s blood medium at 35 °C under 5% O2, 10% CO2 and 85% N2 and were gently harvested in brucella broth to give a cell density of 10.7/mL. The HEp2 cell slips were washed three times with Hank’s solution, one time with 0.2 mol/L (pH3.6) citrate buffer, followed by addition of 0.9 mL of 0.2 mol/L (pH3.6) citrate buffer and 0.1 mL of the bacteria suspension. The microplates were then reincubated under microaerobic condition for 8 h and subsequently washed 5 times with strong agitation with 0.9% saline solution to remove nonadherent bacteria and fixed with 2.5% glutaraldehyde solution for 15 min at room temperature. The slides were stained and examined under light microscope.
To estimate the factors affecting the adherence, the adherence tests were carried out in air, in varied pH or in the system containing 0.1 mL of 1:10 monoclonal antibodies specific to H. pylori predominant antigens[5].
The results obtained for H. pylori YC 11A adherence to HEp2 are shown in Table 1. The adherence of H. pylori to HEp2 began 5 min after coincubation and peaked at the 3rd hour. There was no significant difference between adherence in air or in microaerobic atmosphere (P > 0.01).
| Time | Adherence efficiency (%) | |
| In microaerobic conditions | In air | |
| 5 min | 3 ± 2 | 4 ± 2 |
| 40 min | 16.5 ± 4.0 | 14.0 ± 3.5 |
| 1.5 h | 38 ± 5 | 34 ± 6 |
| 3 h | 81 ± 3 | 78 ± 4 |
| 4 h | 84 ± 5 | 76 ± 3 |
| 5 h | 82 ± 4 | 81 ± 4 |
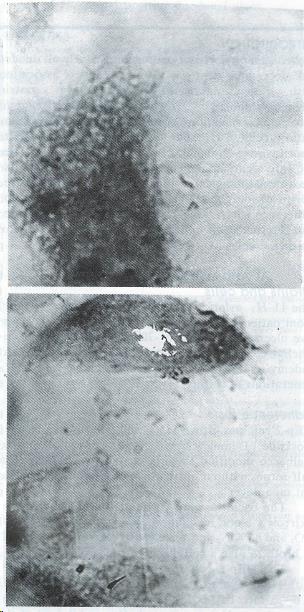
H. pylori YC-11A started to adhere to HEp2 with its terminal portion, and after a long time of incubation, it could adhere to every part of the surface of HEp2, yet adherence to apicals of HEp2 cells was more frequent (Figure 1).
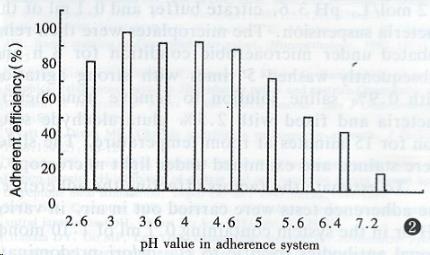
The adherence efficiency obtained with 11 strains of H. pylori isolates is listed in Table 2. The pH of adherence environment remarkably affected the adherence of H. pylori YC-11A to HEp2 cell (Figure 2). The optimal adherent pH was 2.6-4.6 and the maximum adherence efficiency was obtained with pH at 3.0. The results of inhibition of monoclonal antibodies specific to H. pylori on adherence are listed in Table 3 and there was no inhibited activity at pH3.6 in microaerobic atmosphere.
| Strains | Adherence efficiency (%) |
| H. pylori YC-1 | 74 |
| H. pylori YC-2 | 76 |
| H. pylori YC-3 | 52 |
| H. pylori YC-4 | 64 |
| H. pylori YC-5 | 61 |
| H. pylori YC-6 | 58 |
| H. pylori YC-7 | 71 |
| H. pylori YC-8 | 85 |
| H. pylori YC-9 | 80 |
| H. pylori YC-11A | 81 |
| H. pylori YC-11B | 79 |
| Monoclonal antibody | Adherence efficiency (%) |
| 21A5-3 | 81 |
| 22C6-3 | 84 |
| 23C2-2 | 78 |
| 31A10-1 | 83 |
| 31A11-3 | 74 |
| 31B1-1 | 76 |
| 31B1-2 | 80 |
| 31D12-2 | 78 |
| Control | 81 |
To colonize luminal mucus, H. pylori adheres to the apical plasma membrane of the epithelial cell surface in the antrum in vivo by the specific compounds on its surface. These specific structures include flagella and adhesins. All the eleven strains of H. pylori isolates showed different adherent efficiency, indicating that the expression level of adhesin and mobility by various isolates differed.
Current evidence suggested that there are a number of adhesins on the surface of H. pylori. These include fibrillar hemagglutinin[6] and M (microbial) selectins[7]. Fibrillar hemagglutinin specifically binds sialyllactose[6]. M selectin is similar to exoenzyme S from Pseudomonas aeruginosa in structure, and immunogenity and monoclonal antibodies against this adhesin prevent the attachment of H. pylori in vitro to its lipid receptors—gangliotetraosyceramide, gangliotriaosylceramide and phosphatidylethanolamine[8]. Yet, the gastric acidic environment has not been considered. Adherence of H. pylori to HEp2 cell was pH restricted and the low pH benefited the adherence, suggesting that the binding properties of adhesins of H. pylori to its receptor and the natural properties of the adhesins and their receptors possibly possess specificities, which differ greatly from those of other enteropathogens, such as enterotoxingenic Escherichia coli, whose virulence can be easily neutralized by antibodies specific to its adhesin[9]. H. pylori infection stimulates immune response, leading to a much higher level of antibodies in sera[4]. The monoclonal antibodies used were a cluster of antibodies specific to the predominant antigens of H. pylori[5], but they all had no inhibitory actions on adherence of H. pylori and even more promoted adherence of H. pylori. These results further indicated that a high level of antibodies in human serum against H. pylori predominant antigens might not benefit the clearance of H. pylori in gastric mucus and may be a factor for persistence of H. pylori infection.
Original title:
S- Editor: Yang RC L- Editor: Ma JY E- Editor: Liu WX
| 1. | Marshall BJ, Warren JR. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet. 1984;1:1311-1315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3302] [Cited by in RCA: 3264] [Article Influence: 79.6] [Reference Citation Analysis (1)] |
| 2. | Parsonnet J, Friedman GD, Vandersteen DP, Chang Y, Vogelman JH, Orentreich N, Sibley RK. Helicobacter pylori infection and the risk of gastric carcinoma. N Engl J Med. 1991;325:1127-1131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2805] [Cited by in RCA: 2739] [Article Influence: 80.6] [Reference Citation Analysis (0)] |
| 3. | Wang ZX, Wang XL and Wu Y. A singular procedure for culture isolation of Campylobacter pylori. Yangzhou Yixueyuan Xuebao. 1990;2:148-149. |
| 5. | Wang ZX, Shen HF, Chen HJ and Tong K. Establishment and preliminary characterization of the hybridoma cell lines secreting anti-Helicobacter pylori monoclonal antibodies. J Monoclonal Antibody. 1994;19:56-57. |
| 6. | Evans DG, Karjalainen TK, Evans DJ, Graham DY, Lee CH. Cloning, nucleotide sequence, and expression of a gene encoding an adhesin subunit protein of Helicobacter pylori. J Bacteriol. 1993;175:674-683. [PubMed] |
| 7. | Lingwood CA, Wasfy G, Han H, Huesca M. Receptor affinity purification of a lipid-binding adhesin from Helicobacter pylori. Infect Immun. 1993;61:2474-2478. [PubMed] |
| 8. | Gold BD, Huesca M, Sherman PM, Lingwood CA. Helicobacter mustelae and Helicobacter pylori bind to common lipid receptors in vitro. Infect Immun. 1993;61:2632-2638. [PubMed] |
| 9. | Tacket CO, Losonsky G, Link H, Hoang Y, Guesry P, Hilpert H, Levine MM. Protection by milk immunoglobulin concentrate against oral challenge with enterotoxigenic Escherichia coli. N Engl J Med. 1988;318:1240-1243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 200] [Article Influence: 5.4] [Reference Citation Analysis (0)] |