Published online Feb 28, 2023. doi: 10.3748/wjg.v29.i8.1374
Peer-review started: November 12, 2022
First decision: January 2, 2023
Revised: January 15, 2023
Accepted: February 15, 2023
Article in press: February 15, 2023
Published online: February 28, 2023
Processing time: 107 Days and 18.8 Hours
Bone disease is an under-recognized cause of morbidity in chronic pancreatitis (CP). Over the past decade, publications of original studies on bone disease in CP has warranted synthesis of the evidence to ascertain the true burden of the problem.
To quantify the prevalence of osteopenia, osteoporosis, and fragility fractures in CP patients and investigate the associated clinical features and outcomes.
A systematic search identified studies investigating bone disease in CP patients from Cochrane Library, Embase, Google Scholar, Ovid Medline, PubMed, Scopus, and Web of Science, from inception until October 2022. The outcomes included prevalence of osteopenia, osteoporosis, and fragility fractures, which were meta-analyzed using a random-effects model and underwent metaregression to delineate association with baseline clinical features.
Twenty-one studies were included for systematic review and 18 studies were included for meta-analysis. The pooled prevalence of osteopenia and osteoporosis in CP patients was 41.2% (95%CI: 35.2%-47.3%) and 20.9% (95%CI: 14.9%-27.6%), respectively. The pooled prevalence of fragility fractures described among CP was 5.9% (95%CI: 3.9%-8.4%). Meta-regression revealed significant association of pancreatic enzyme replacement therapy (PERT) use with prevalence of osteoporosis [coefficient: 1.7 (95%CI: 0.6-2.8); P < 0.0001]. We observed no associations with mean age, sex distribution, body mass index, alcohol or smoking exposure, diabetes with prevalence of osteopenia, osteoporosis or fragility fractures. Paucity of data on systemic inflammation, CP severity, and bone mineralization parameters precluded a formal meta-analysis.
This meta-analysis confirms significant bone disease in patients with CP. Other than PERT use, we observed no patient or study-specific factor to be significantly associated with CP-related bone disease. Further studies are needed to identify confounders, at-risk population, and to understand the mechanisms of CP-related bone disease and the implications of treatment response.
Core Tip: Bone disease is an under recognized cause of morbidity in chronic pancreatitis (CP). This systematic review and meta-analysis demonstrate substantial burden of bone disease (osteopenia and osteoporosis) and fragility fractures in CP patients. In addition, metaregression has demonstrated a significant association of osteoporosis with pancreatic enzyme replacement. The study is powered by high-quality studies with large sample size and clearly defined study population and outcome measures.
- Citation: Chhoda A, Hernandez-Woodbine MJ, Addo NAA, Nasir SA, Grimshaw A, Gunderson C, Ahmed A, Freedman SD, Sheth SG. Burden of bone disease in chronic pancreatitis: A systematic review and meta-analysis. World J Gastroenterol 2023; 29(8): 1374-1394
- URL: https://www.wjgnet.com/1007-9327/full/v29/i8/1374.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i8.1374
Chronic pancreatitis (CP) is a progressive, multifactorial fibro-inflammatory syndrome, which arises from persistent pathological response to noxious stimuli[1]. It is characterized by chronic abdominal pain, exocrine and endocrine insufficiency. Over time, nutritional deficiencies, systemic inflammation, and etiological factors like alcohol or smoking may disrupt the balance between bone formation and resorption[2]. These maladaptive alterations may subsequently cause bone disease i.e., osteopenia and osteoporosis, leading to fractures which result from low energy trauma, called fragility fractures, and have significant implications on quality of life[3].
Osteoporosis is a public health hazard with a substantial economic burden[4]. Recent data from the National Health and Nutrition Examination Survey found its age-adjusted prevalence among adults older than 50 years to be 12.6% affecting more women than men (19.6% vs 4.4%). In view of the burden of bone disease and subsequent fracture risk in individuals affected by CP, various consensus guidelines have recommended baseline bone health assessment[5,6]. The adherence to guidelines has been low and studies have demonstrated that less than a quarter of CP patients are screened for bone disease, suggesting that bone disease is an underappreciated source of morbidity in CP[7,8].
A previous systematic review by Duggan et al[9] estimated the prevalence of osteopenia and osteoporosis in patients with CP to be 39.8% and 23.4%, respectively. The analysis was based on a limited number of studies (n = 11) and did not quantify fragility fractures which are complications of CP-related bone disease. Over the last decade, multiple large-sized studies including those from multicenter cohorts have investigated bone disease in CP as well as the nutritional, anthropometric, and inflammatory parameters which may impact bone health outcomes. Thus, with quantitative data on covariates and fragility fractures available, an updated synthesis of evidence is pertinent. This systematic review and meta-analysis aims to quantify the prevalence of osteopenia, osteoporosis, and fragility fractures in CP patients and to delineate clinical parameters which impact their occurrence.
This systematic review was performed in accordance with PRISMA guidelines[10]. The review methodology was pre-registered at open-source forum[11].
A systematic search of the literature was conducted by a medical librarian in the following databases: Cochrane Library, Ovid Embase, Google Scholar, Ovid Medline, PubMed, Scopus, and Web of Science Core Collection to find articles published from the inception of the database to October 20, 2022. The search was performed using a combination of controlled and free text terms for CP and bone diseases (strategy outlined in Supplementary Table 1). The search was not limited to publication type or date. the search was peer-reviewed by a second librarian using peer review for electronic search strategies[12]. Citations were imported into an Endnote 20 Library (Version 20.2, Clarivate Analytics, Philadelphia), and after removal of duplicates, the remaining studies were imported into Covidence (Melbourne, Australia) for screening and data extraction.
Two co-authors (MJ, AC) independently used the inclusion and exclusion criteria to assess titles and abstracts. Eligibility decisions and disagreements were reconciled through discussion with the senior author (SS). Full-text articles included by two reviewers underwent data extraction and quality assessment.
Inclusion criteria: This systematic review included original cross-sectional and cohort studies, which were of prospective and retrospective design. CP patients were diagnosed based on specific codes related to CP per the International Classification of Diseases (ICD-9/10) and through predefined clinical, radiologic and/or histologic findings (described in Table 1).
| Ref. | Design | CP patients | CP severity, n (%) | CP etiology, n (%) | Study population | Race | Female patients, n (%) | Age, mean ± SD | BMI, mean ± SD |
| Morán et al[17], 1997 | Cross sectional | Clinicoradiological | All severe | Alcohol: 10 (71.4) | CP: 14 | CP: 0 | CP: 561 (-) | CP: 22.64 | |
| Idiopathic 4 (28.6) | Controls: - | Controls: - | Controls: - | Controls: - | |||||
| Haaber et al[18], 2000 | Cross sectional | Clinicoradiological | Alcohol: 46 (79) | CP: 58 | CP: - | CP: 26 (44.8) | CP: 53 (9) | CP: 23 (5) | |
| Controls: - | Controls: - | Controls: - | Controls: - | Controls: - | |||||
| Dujsikova et al[19], 2008 | Cross sectional | EUS based criteria | Wiersema classification: Mild: 41 (56.2), moderate: 12 (16.4), severe: 20 (27.4) | CP: 73 | CP: - | CP:17 (23.28) | CP: 46.61 (13.23) | CP: - | |
| Controls: - | Controls: - | Controls: - | Controls: - | Controls: - | |||||
| Tignor et al[20], 2010 | Retrospective cohort | ICD-9 code 577.1 | CP: 3192 | CP: White: 2091 (65.5), black: 419 (13.1), hispanic: 222 (6.9), others: 532 (16.7) | CP: 1636 (51.25) | CP: - | CP: - | ||
| Controls: 1436699 | Control: White: 860190 (59.9), black: 115199 (8.0), hispanic: 102000, other: 451110 | Controls: 907328 (63.15) | Controls: - | Controls: - | |||||
| Sudeep et al[22], 2011 | Cross sectional | Not defined | Tropical pancreatitis: 20 (65) | CP: 31 | CP: - | CP: 0 | CP: 35.8 (9) | CP: 18.46 (2.86) | |
| Idiopathic: 11 (35) | Controls: 35 | Controls: - | Controls: 0 | Controls: 38.6 (5.2) | Controls: 22.6 (3.1) | ||||
| Joshi et al[21], 2011 | Cross sectional | Clinicoradiological | All patients with tropical calcific pancreatitis | CP: 72 | CP: - | CP: 34 (47.2) | CP: 31.1 (10.3) | CP: 19 (3.1) | |
| Controls: 100 | Controls: - | Controls: 50 (50) | Controls: 32.6 (9.6) | Controls: 23.6 (3.2) | |||||
| Duggan et al[23], 2012 | Cross sectional | Clinicoradiological | Cambridge classification: Mild (37.1), severe (27.4) | Alcohol: 24 (38.7) | CP: 62 | CP: - | CP: 17 (27.41) | CP: 47.9 (12.5) | CP: 25.6 (5) |
| Idiopathic: 38 (61.3) | Controls: 66 | Controls: - | Controls: 18 (27.27) | Controls: 47.74 (11) | Controls: 28.0 (4.1) | ||||
| Sikkens et al[25], 2013 | Prospective cohort | Clinicoradiological | Alcohol: 20 (50) | CP: 40 | CP: - | CP: 17 (42.5) | CP: 52 (11) | CP: 24 (5) | |
| Idiopathic: 17 (43) | Controls: - | Controls: - | Controls: - | Controls: - | Controls: - | ||||
| Other: 3 (7) | |||||||||
| Prabhakaran et al[28], 2014 | Cross sectional | Clinicoradiological. | Cambridge classification: Mild (13.1), moderate (5.05), marked: (81.8) | Alcohol: 72 (70) | CP: 103 | CP: - | CP: 0 | CP: 38.6 (20.64) | CP: 19.7 |
| Idiopathic: 31 (29.1) | Controls: - | Controls: - | Controls: 0 | Controls: 36.7 (20.70) | Controls: - | ||||
| Bang et al[26], 2014 | Prospective cohort | ICD-10: K86.0 (alcohol induced CP), K86.1 (other CP) | CP: 11972 | CP: - | CP: 4011 (33.5) | CP: 54.5 (14) | CP: - | ||
| Controls: 119720 | Controls: - | Controls: 40106 (33.49) | Controls: 54.5 (14) | Controls: - | |||||
| Duggan et al[27], 2015 | Cross sectional | Clinicoradiological | Cambridge classification (unspecified number in each category) | Alcohol: 18 (62.1) | CP: 29 | CP: - | CP: 12 (41.37) | CP: 44.3 (12.3) | CP: 25.2 (5.1) |
| Idiopathic: 8 (27.6) | Controls: 29 | Controls: - | Controls: 12 (41.37) | Controls: 45.8 (9.8) | Controls: 27.3 (3.7) | ||||
| Other: 3 (10.3) | |||||||||
| Munigala et al[24], 2016 | Cross sectional | ICD-9 code 577.1 | CP: 3257 | CP: White 2120 (65), black 1012 (31), others 125 (4) | CP: 178 (5.46) | CP: 54.2 (11.1) | CP: - | ||
| Controls: 450655 | Controls: White: 325132 (72), black: 76031 (17), others: 49492 (11) | Controls: 53108 (11.78) | Controls: 53.6 (13.9) | Controls: - | |||||
| Kumar et al[29], 2017 | Cross sectional | Clinicoradiological | CP: 102 | CP: - | CP: 17 (16.7) | CP: 40.8 (12.6) | CP: 22.5 (3.2) | ||
| Controls: - | Controls: - | Controls: - | Controls: - | Controls: - | |||||
| Stigliano et al[32], 2018 | Cross sectional | M-ANNHEIM criteria | M-ANNHEIM scoring system: Minor: 74 (35), Increased: 99 (47), advanced: 32 (15), marked: 6 (3) | Alcoholic: 91 (43) | CP: 211 | CP: - | CP: 69 (32.7) | CP: 60 (-) | CP: 24 (4) |
| Idiopathic: 40 (19) | Controls: - | Controls: - | Controls: - | Controls: - | Controls: - | ||||
| Hereditary: 8 (4) | |||||||||
| Obstructive: 12 (5.7) | |||||||||
| Kuhlmann et al[30], 2018 | Cross sectional | Score ≥ 4 points based on Lüneburg criteria | CP: 67 | CP: - | CP: 27 (40.29) | CP: 601 (-) | CP: 22.7 (15-37.9) | ||
| Controls: - | Controls: - | Controls: - | Controls: - | Controls: - | |||||
| Min et al[31], 2018 | Prospective cohort | EUS criteria and/or | EUS criteria (unspecified number in each category) | Toxic/metabolic: 54 (59.3) | CP: 91 | CP: - | CP: 34 (37.36) | CP: 48.6 (10.4) | CP: 26.1 (7.8) |
| secretin stimulation testing | Idiopathic: 17 (18.6) | Controls: - | Controls: - | Controls: - | Controls: - | Controls: - | |||
| Hereditary: 13 (14.3) | |||||||||
| Autoimmune: 5 (5.5) | |||||||||
| Gupta et al[33], 2019 | Prospective cohort | Clinicoradiological and EUS | CP: 38 | CP: White 35 (92), black 3 (8) | CP: 19 (50) | CP: 44 (10.7) | CP: 26.7 (5.9) | ||
| Controls: - | Controls: - | Controls: - | Controls: - | Controls: - | |||||
| Kanakis et al[7], 2020 | Retrospective cohort | Clinicoradiological | CP: 239 | CP: White 43 (88), minorities: 6 (12) | CP: 37 (15.48) | CP: 561 (-) | CP: 23 (8) | ||
| Controls: - | Controls: - | Controls: - | Controls: - | Controls: - | |||||
| Hart et al[34], 2021 | Cross sectional | Clinicoradiological | Cambridge classification (unspecified number in each category) | - | CP: 282 | CP: White race (87.2), minorities (12.8) | CP: 145 (51.41) | CP: 561 (-) | CP: - |
| Controls: - | Controls: - | Controls: - | Controls: - | Controls: - | |||||
| Vujasinovic et al[8], 2021 | Retrospective cohort | 2002 Asia-Pacifc consensus report | Alcohol and smoking: 40 (33.9) | CP: 118 | CP: - | CP: 49 (41.52) | CP: 53.1 (16.3) | CP: 23.9 (4.4) | |
| Smoking only: 12 (11) | Controls: - | Controls: - | Controls: - | Controls: - | Controls: - | ||||
| Alcohol only: 7 (5.9) | |||||||||
| Hereditary: 21 (11.8) | |||||||||
| Immunological: 23 (14.4) | |||||||||
| Efferent duct factors: 11 (9.3) | |||||||||
| Tang et al[35], 2021 | Cross sectional | ICD-9 based codes | M-ANNHEIM clinical stage 0: 6 (5.8), I: 59 (56.7), II: 26 (25.0), III: 8 (7.7), IV: 5 (4.8) | CP: 104 | CP: - | CP: 31 (29.8) | CP: 46.08 (14.43) | CP: 21.43 (2.85) | |
| Controls: - | Controls: - | Controls: - | Controls: - | Controls: - |
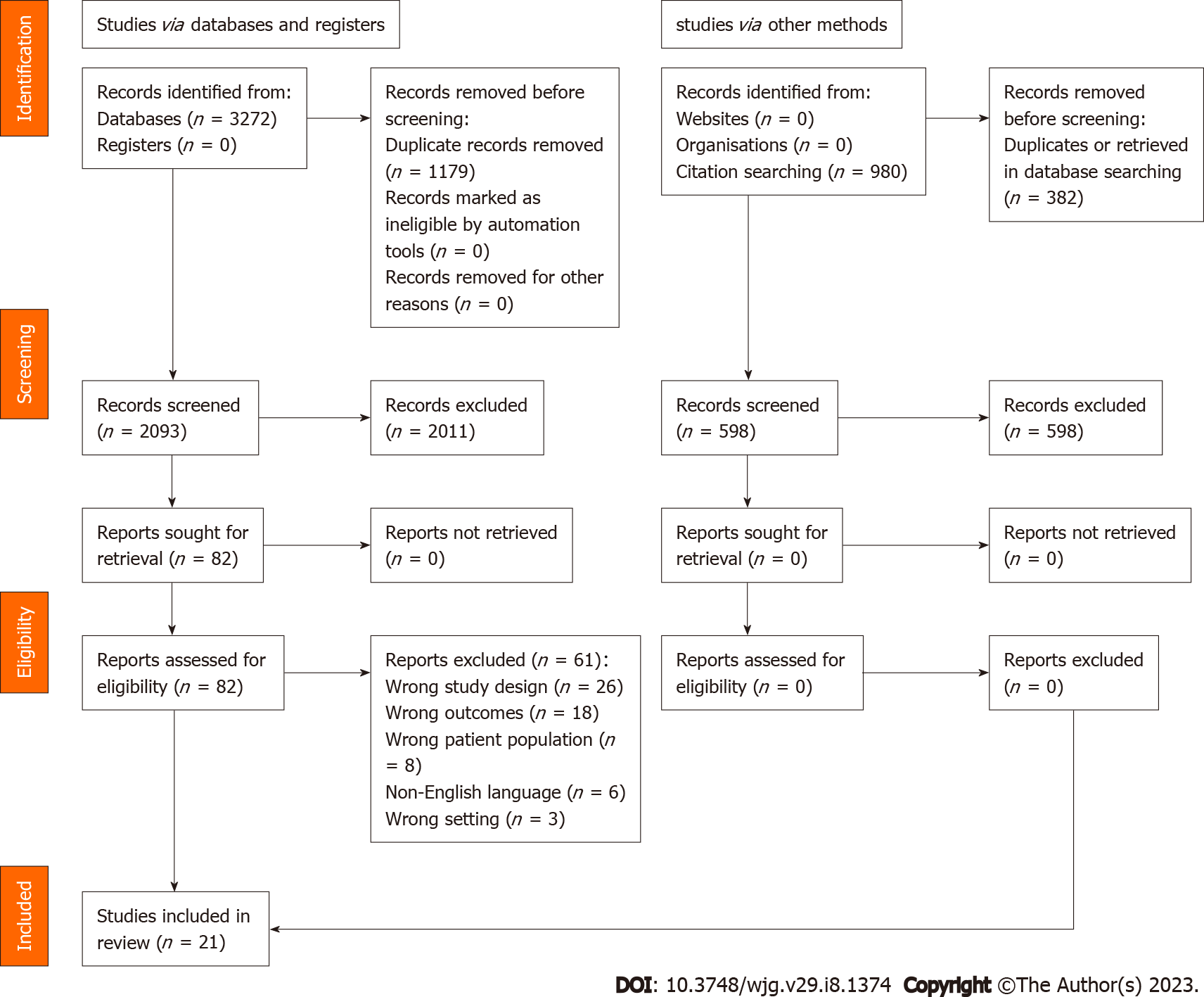
Exclusion criteria: The studies which did not report bone diseases in CP patients were ineligible for the systematic review. We also excluded studies published in foreign languages, associated with animal research, reviews, abstracts, letters, case reports, and series with a sample size of less than 10 patients were excluded (Figure 1).
We noted data on study design, population characteristics such as age, gender, method of CP diagnosis (ICD-code based vs systematic clinical-radiologic features), body mass index (BMI), and exposure to alcohol and smoking. These data were independently extracted by two authors (MJ and AC). The outcome of interest included the prevalence of osteopenia, osteoporosis, and fragility fractures among the CP patients. The definitions of these bone outcome measures were also noted and have been outlined in Table 2.
| Ref. | Population | Outcome definition | Osteoporosis | Osteopenia | Pathologic fracture |
| Morán et al[17], 1997 | CP: 14 | T score < -2.5 | CP: 3 (21.4) | CP: 10 (71.4) | CP: - |
| Controls: - | T score -1 to -2.5 | Controls: - | Controls: - | Controls: - | |
| Unavailable | |||||
| Haaber et al[18], 2000 | CP: 58 | Z score < -2 | CP: 13 (22.4) | CP: 36 (62) | CP: - |
| Controls: - | Z score < -1.0 | Controls: - | Controls: - | Controls: - | |
| Unavailable | |||||
| Dujsikova et al[19], 2008 | CP: 73 | T score < -2.5 | CP: 4 (5.5) | CP: 19 (26) | CP: 1 (1.3) |
| Controls: - | T score -1 to -2.5 | Controls: - | Controls: - | Controls: - | |
| Undefined | |||||
| Tignor et al[20], 2010 | CP: 3192 | Not studied | CP: - | CP: - | CP: 154 (4.8) |
| Controls: 1436699 | Not studied | Controls: - | Controls: - | Controls: - | |
| Vertebral, hip, and wrist fractures using ICD-9 codes | |||||
| Sudeep et al[22], 2011 | CP: 31 | T score < -2.5 | CP: 9 (29) | CP: - | CP: - |
| Controls: 35 | T score -1 to -2.5 | Controls: 3 (8.5) | Controls: - | Controls: - | |
| Unavailable | |||||
| Joshi et al[21], 2011 | CP: 72 | Z score < -2 | CP: 22 (30.5) | CP: - | CP: 0 |
| Controls: 100 | Unavailable | Controls: - | Controls: - | Controls: - | |
| Undefined | |||||
| Duggan et al[23], 2012 | CP: 62 | T score < -2.5 | CP: 18 (33) | CP: 21 (39.6) | CP: - |
| Controls: 66 | T score -1 to -2.5 | Controls: 6 (10.1) | Controls: 20 (33.8) | Controls: - | |
| Unavailable | |||||
| Sikkens et al[25], 2013 | CP: 40 | T score < -2.5 | CP: 4 (10) | CP: 18 (45) | CP: - |
| Controls: - | T score -1 to -2.5 | Controls: - | Controls: - | Controls: - | |
| Unavailable | |||||
| Prabhakaran et al[28], 2014 | CP: 103 | T score < -2.5 | CP: 25 (30.1) | CP: 38 (45.7) | CP: - |
| Controls: - | T score -1 to -2.5 | Controls: - | Controls: - | Controls: - | |
| Unavailable | |||||
| Bang et al[26], 2014 | CP: 11972 | M80.0-M81.9 based on ICD-10 code | CP: 898 (7.5) | CP: - | CP: 1055 (8.8) |
| Controls: 119720 | Unavailable | Controls: 4070 (3.3) | Controls: - | Controls: 8485 (7) | |
| Spine, humerus, distal forearm, and proximal femur based on ICD-10 codes | |||||
| Duggan et al[27], 2015 | CP: 29 | T score < -2.5 | CP: 9 (31) | CP: 13 (44.8) | CP: - |
| Controls: 29 | T score -1 to -2.5 | Controls: 2 (6.8) | Controls: 15 (51.7) | Controls: - | |
| Unavailable | |||||
| Munigala et al[24], 2016 | CP: 3257 | Unspecified ICD-9 codes | CP: - | CP: - | CP: 153 (4.6) |
| Controls: 450655 | Unavailable | Controls: - | Controls: - | Controls: 9325 (2) | |
| ICD-9 codes: vertebral (805.2, 805.3, 805.4,805.5, 805.6, 805.7), hip (820.0, 820.1, 820.2, 820.3, 820.8, 820.9), or wrist fractures (814.0, 814.1, 813.4, 813.5) | |||||
| Kumar et al[29], 2017 | CP: 102 | Z score < -2 | CP: 6 (5.8) | CP: 21 (20.5) | CP: - |
| Controls: - | Unavailable | Controls: - | Controls: - | Controls: - | |
| Unavailable | |||||
| Stigliano et al[32], 2018 | CP: 211 | T score < -2.5 | CP: 46 (21.8) | CP: 89 (42.1) | CP: 13 (6.1) |
| Controls: - | T score: -1 to -2.5 | Controls: - | Controls: - | Controls: - | |
| Occurring at the spine, hip and distal radius, and not associated with traumatic events | |||||
| Kuhlmann et al[30], 2018 | CP: 67 | T score < -2.5 | CP: 18 (26.8) | CP: 34 (50.7) | CP: - |
| Controls: - | T score: -1 to -2.5 | Controls: - | Controls: - | Controls: - | |
| Unavailable | |||||
| Min et al[31], 2018 | CP: 91 | T score < -2.5 | CP: 10 (22.2) | CP: 21 (46.6) | CP: - |
| Controls: - | T score: -1 to -2.5 | Controls: - | Controls: - | Controls: - | |
| Unavailable | |||||
| Gupta et al[33], 2019 | CP: 38 | T score < -2.5 | CP: 21 (55.2) | CP: - | CP: - |
| Controls: - | T score: -1 to -2.5 | Controls: - | Controls: - | Controls: - | |
| A fall from standing height or less that resulted in a fracture | |||||
| Kanakis et al[7], 2020 | CP: 239 | T score < -2.5 or history of fragility fracture | CP: 15 (30.6) | CP: 27 (55.1) | CP: 22 (9) |
| Controls: - | T score: -1 to -2.5 | Controls: - | Controls: - | Controls: - | |
| Hip or vertebral fracture not due to excess trauma | |||||
| Hart et al[34], 2021 | CP: 282 | T score < -2.5 | CP: 48 (17) | CP: 110 (39) | CP: 6 (2.1) |
| Controls: - | T score: -1 to -2.5 | Controls: - | Controls: - | Controls: - | |
| Spontaneous fractures | |||||
| Vujasinovic et al[8], 2021 | CP: 118 | T score < -2.5 | CP: 30 (25.4) | CP: 33 (27.9) | CP: 33 (27.9) |
| Controls: - | T score: -1 to -2.5 | Controls: - | Controls: - | Controls: - | |
| Unavailable | |||||
| Tang et al[35], 2021 | CP: 104 | T score < -2.5 | CP: 6 (5.7) | CP: 32 (30.7) | CP: - |
| Controls: - | T score: -1 to -2.5 | Controls: - | Controls: - | Controls: - | |
| Occurring in the hip, spine, or wrist |
Prevalence rates were pooled using random-effects meta-analysis and calculated using the score method[13]. Between-study heterogeneity was estimated using I² statistics wherein 25%, 50%, and 75% were considered to indicate low, moderate, and high heterogeneity, respectively. Sensitivity analysis was conducted by sequentially removing individual studies. Statistical analysis was performed using Stata/IC, version 17 (StataCorp, College Station, TX). Metaregression, subgroup analysis and publication bias assessment could not be performed for analyses including < 10 studies[14].
Metaregression using the restricted maximum likelihood method was also performed using variables of mean age, sex, BMI, smoking or alcohol exposure, diabetes, serum parathyroid hormone (PTH), percentage population with vitamin D deficiency, and pancreatic enzyme replacement therapy (PERT).
The quality of observational studies was independently assessed using the Newcastle-Ottawa Scale by two investigators (SN and AA) and recorded in a Microsoft Excel spreadsheet (XP Professional Edition; Microsoft Corp, Redmond, WA, United States)[15]. Any discrepancy was resolved by the senior author (SS). Publication bias were assessed by inspection of a funnel plot and Egger’s test[16].
The literature search yielded 2081 results and after the removal of 20 duplicates, 2061 citations underwent title and abstract screening. The exclusion of 1979 citations due to lack of relevance to the research question resulted in 82 citations for full-text review. The review of their full texts enabled exclusion of 61 citations and finally 21 studies were included[7,8,17-35] (Supplementary Table 2). The excluded studies had incorrect study design (n = 27), wrong outcomes (n = 17), ineligible patient population (n = 8), non-English language (n = 6), wrong setting (n = 3), and duplicate center or overlapping populations (n = 1) (Supplementary Table 3). The search and selection processes are summarized in Figure 1.
All the eligible studies were observational studies and included 14 cross-sectional and 7 cohort studies (described in Table 1). Among the cohort studies, four were performed prospectively[25,26,31,33], and remaining three had retrospective design[7,8,35]. Most of the studies originated from European countries (n = 9), while the remaining were from the United States (n = 6), India (n = 4), China (n = 1), and Argentina (n = 1). Multicenter collaboration was noted in two eligible studies whereas the remaining 19 were performed at single centers. The controls of matched patients were also analyzed in seven studies which were from a healthcare system (n = 3)[20,22,24]; healthy community population (n = 3)[21,26,27]; or were not defined (n = 1)[28].
The qualitative review of 21 studies included 20155 CP patients and 2007278 control patients (from seven controlled studies). The patients with CP were identified based on the combination of clinical, radiologic, endoscopic and/or histologic findings in 17 studies[7,8,17-19,21-23,25,27-34], and diagnosed through ICD-9/10 codes in four studies[20,24,26,35] (Table 1).
We performed meta-analysis of 21 eligible studies which included 20155 CP patients evaluated for bone disease.
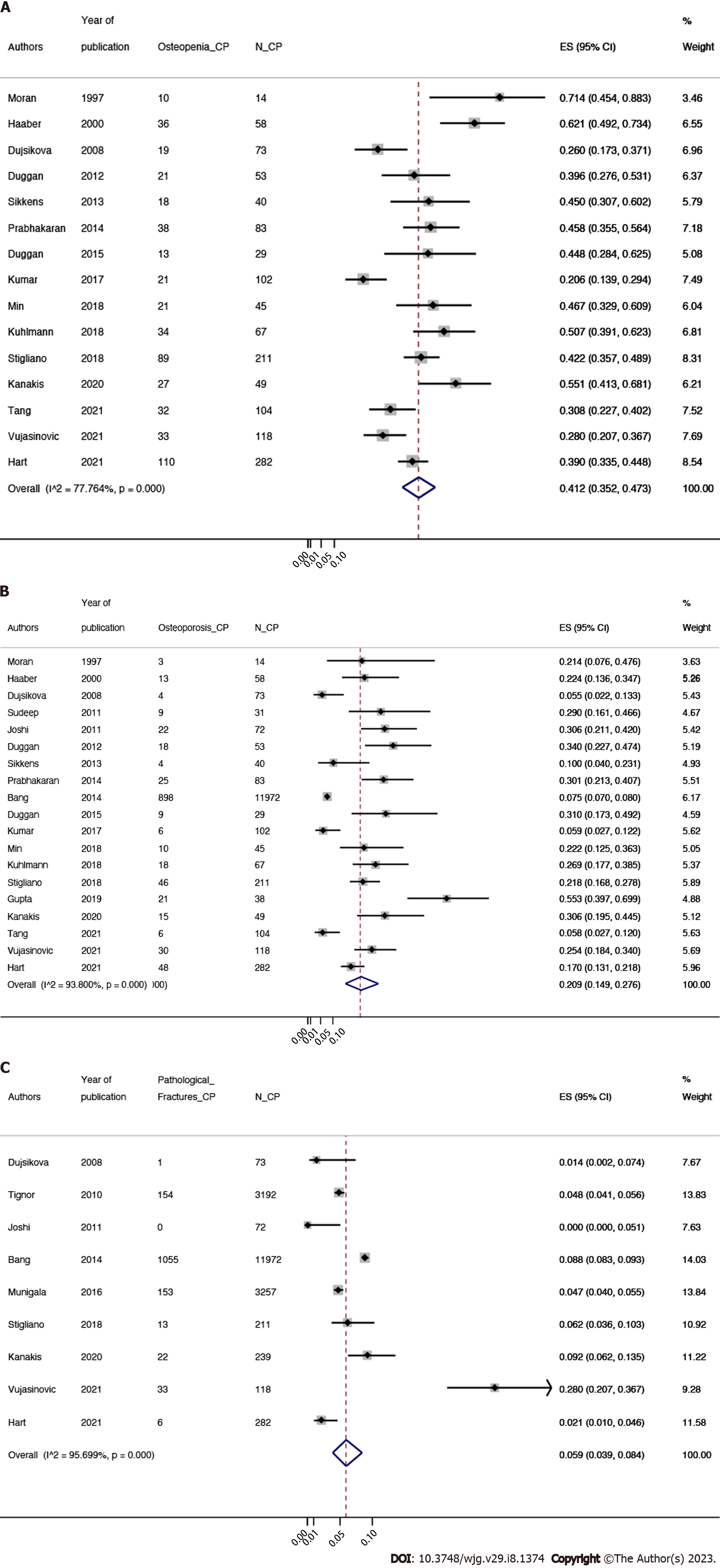
Prevalence of osteopenia in CP patients: In adult CP patients, the pooled prevalence of osteopenia was 41.2% (95%CI: 35.2%-47.3%, P < 0.0001; χ2 = 63.0, df = 14, P < 0.0001; I2 = 77.8%, τ2= 0.04)[7,8,17-19,23,25,27-32,34,35] (Figure 2A). All of the studies prevalence estimates had high heterogeneity since I2 > 75%.
Prevalence of osteoporosis among CP patients: The pooled prevalence of osteoporosis in CP patients was 20.9% (95%CI: 14.9%-27.6%; P < 0.0001; χ2 = 290.33, df = 18, P < 0.0001; I2 = 93.8%, τ2 = 0.1)[7,8,17-19,21-23,25-35] (Figure 2B). The pooled prevalence of osteoporosis in CP patients had high heterogeneity since I2 > 75%.
Prevalence of fragility fracture among CP patients: The pooled prevalence of fragility fracture was 5.9% (95%CI: 3.9%-8.4%, P < 0.0001; χ2 = 186.0, df = 8, P < 0.0001; I2 = 95.7%, τ2 = 0.02)[7,19-21,24,26,32,34,35] (Figure 2C). All of the studies prevalence estimates had high heterogeneity since I2 > 75%.
Factors impacting bone disease in CP patients: Various covariates underwent qualitative assessment for their association with CP related bone disease (Table 3).
| Ref. | PERT use, n (%) | Inflammatory markers: CRP/IL-6, mean ± SD | Vitamin D deficiency, n (%) | Serum PTH, mean ± SD | Alcohol exposure, n (%) | Smokers, n (%) | Diabetes, n (%) | Nutritional parameters | Relevant covariates findings |
| Morán et al[17], 1997 | CP: 4 (28.57) | CP: - | CP: 7 (50) | CP: - | CP: 0 | CP: - | CP: - | Mean serum albumin 3.8 g/dL, 4 (28.6) had BMI < 20. Non-significant associations between osteopathy and BMI | Non-significant associations between osteopathy and (1) CP severity (as per fecal fat or bicarbonate secretion assessments); (2) CP etiology; (3) Age; and (4) Vitamin D, PTH or calcium |
| Controls: - | Controls: - | Controls: - | Controls: - | Controls: - | Controls: - | Controls: - | |||
| Haaber et al[18], 2000 | CP: 26 (44.82) | CP: - | CP: - | CP: 401 (31) | CP: - | CP: - | CP: - | Non-significant associations between osteopathy and (1) Duration of CP; and (2) Vitamin D and PTH | |
| Controls: - | Controls: - | Controls: - | Controls: - | Controls: - | Controls: - | Controls: - | |||
| Dujsikova et al[19], 2008 | CP: - | CP: - | CP: 63 (86.3) | CP: - | CP: 8 (10.95) | CP: - | CP: - | Non-significant associations between osteopathy and severity of disease | |
| Controls: - | Controls: - | Controls: - | Controls: - | Controls: - | Controls: - | Controls: - | |||
| Tignor et al[20], 2010 | CP: - | CP: - | CP: - | CP: - | CP: - | CP: - | CP: - | No descriptions of regression analysis or covariate adjustment | |
| Controls: - | Controls: - | Controls: - | Controls: - | Controls: - | Controls: - | Controls: - | |||
| Sudeep et al[22], 2011 | CP: - | CP: - | CP: 16 (51.6) | CP: - | CP: - | CP: - | CP: - | BMI correlated significantly with BMC (r = 0.426; P = 0.017). There was an inverse correlation between stool fat and BMC (r = -0.47; P = 0.03) | Non-significant associations between osteopathy and (1) EPI (as per 72-hour fecal fat); and (2) Vitamin D |
| Controls: - | Controls: - | Controls: - | Controls: - | Controls: - | Controls: - | Controls: - | |||
| Joshi et al[21], 2011 | CP: 33 (45.83) | CP: CRP < 0.32 (-) | CP: 62 (86.11) | CP: 43.381 (-) | CP: - | CP: 7 (9.7) | CP: 52 (72.2) | Lumbar Z score was associated with BMI (beta: 0.276; P = 0.04), serum albumin was significantly lower in patients compared with controls [4.0 (0.6) vs 4.6 (0.7) g/dL, P < 0.001] | |
| Controls: - | Controls: CRP < 0.32 (-) | Controls: 85 (85) | Controls: 84.871 (-) | Controls: - | Controls: - | Controls: - | |||
| Significant association of Lumbar Z score with log vitamin D (beta: 0.274; P = 0.04) | |||||||||
| Duggan et al[23], 2012 | CP: - | CP: - | CP: - | CP: - | CP: 58 (93.5) | CP: 46 (74.19) | CP: - | BMI < 20: low BMD: 15 (23.8) vs normal BMD 10 (1.1) | Higher T scores for the lowest age tertile (P = 0.003). Lower T-score for smokers (P = 0.002). Non-significant associations between T scores at any area and (1) CP severity; (2) EPI; and (3) Ssex |
| Controls: - | Controls: - | Controls: - | Controls: - | Controls: 62 (93.9) | Controls: 40 (60.6) | Controls: - | |||
| Sikkens et al[25], 2013 | CP: 19 (47.5) | CP: - | CP: - | CP: - | CP: 1 (2.5) | CP: 27 (67.5) | CP: - | A high BMI is predictive of a ‘‘higher’’ lowest T-score [Coeff: 0.58 (0.2); P = 0.003] | Significant association between osteopathy and untreated EPI (P = 0.013) |
| Controls: - | Controls: - | Controls: - | Controls: - | Controls: - | Controls: - | Controls: - | |||
| Prabhakaran et al[28], 2014 | CP: - | CP: - | CP: 20 (19.41) | CP: 27.6 (39.8) | CP: 72 (69.9) | CP: - | CP: 39 (37.86) | - | Non-significant associations between osteopathy and (1) EPI (as per steatorrhea assessment); (2) CP severity; and (3) CP etiology |
| Controls: - | Controls: - | Controls: - | Controls: - | Controls: - | Controls: - | Controls: - | |||
| Non-significant associations between osteopathy and vitamin D, PTH and alkaline phosphatase | |||||||||
| Bang et al[26], 2014 | CP: 3545 (29.61) | CP: - | CP: - | CP: - | CP: 3651 (30.49) | CP: - | CP: - | Increased risk of fracture among smokers (HR, 1.8; 95%CI, 1.7-1.8) and alcohol related CP (HR, 2.0 vs 1.5; P < 0.0001) | |
| Controls: - | Controls: - | Controls: - | Controls: - | Controls: 2753 (2.29) | Controls: - | Controls: - | |||
| Reduced fracture risk among PERT treated CP patients (HR, 0.8; 95%CI, 0.7-0.9) | |||||||||
| Duggan et al[27], 2015 | CP: - | CP: CRP: 3.15 (-), IL-6: 5.61 (-) | CP: 20 | CP: 47.1 (19.4) | CP: 27 (93.10) | CP: 23 (79.3) | CP: - | Lower T scores were associated with BMI (P = 0.04) | Lower T scores were associated with age (P = 0.006). Non-significant association with carboxy-terminal telopeptide of type I collagen; osteocalcin; Procollagen 1 amino-terminal propeptide |
| Controls: - | Controls: CRP: 0.9 (-), IL-6: 3.58 (1.82) | Controls: 18 | Controls: 46.3 (14) | Controls: 28 (96.55) | Controls: 10 (34.4) | Controls: - | |||
| Non-significant association with IL-6 and CRP | Lower T scores were associated with serum vitamin D (P = 0.002). No association with PTH | ||||||||
| Munigala et al[24], 2016 | CP: - | CP: - | CP: - | CP: - | CP: 494 (15.16) | CP: 505 (15.5) | CP: - | A significant association of BMD in the columnar spine with vitamin D level (coefficient 0.13 g/cm2; P = 0.017) and BMI (coefficient 0.14 g/cm2; P = 0.007) were observed on univariate analysis | Increased fracture risk among males (adjusted OR, 1.73% (95%CI: 1.46%-2.05%); P < 0.0001), alcohol consumers (OR, 2.30), and smokers (OR, 1.97). Non-significant associations between osteopathy and age |
| Controls: - | Controls: - | Controls: - | Controls: - | Controls: 37146 (8.24) | Controls: 77926 (17.29) | Controls: - | |||
| Kumar et al[29], 2017 | CP: - | CP: - | CP: 69 (67.64) | CP: - | CP: - | CP: - | CP: 54 (52.94) | A MUST score (malnutrition score) of 1 or higher was associated with an increased risk for osteopenia and osteoporosis on Fisher’s exact test (P = 0.0037) | Non-significant association between osteopathy and duration of CP |
| Controls: - | Controls: - | Controls: - | Controls: - | Controls: - | Controls: - | Controls: - | |||
| Non-significant association between osteopathy and vitamin D | |||||||||
| Stigliano et al[32], 2018 | CP: 116 (54.97) | CP: - | CP: 119 (56.39) | CP: - | CP: 127 (60.18) | CP: 145 (68.72) | CP: 77 (36.49) | Observed significant association of BMI with osteopathy (OR 0.89; 95%CI: 0.83-0.96; P = 0.003) | Osteopathy more prevalent with increasing age (OR 1.06; P = 0.0002), female sex (OR: 3.44; P = 0.0005). Non-significant association between osteopathy and (1) CP severity; (2) EPI (as assessed by fecal elastase); (3) Smoking; (4) Duration of CP; and (5) Alcohol exposure |
| Controls: - | Controls: - | Controls: - | Controls: - | Controls: - | Controls: - | Controls: - | |||
| Non-significant association between osteopathy and PERT usage | Non-significant association between osteopathy and IL-6/CRP | Non-significant association between osteopathy, vitamin D and PTH | |||||||
| Kuhlmann et al[30], 2018 | CP: 28 (41.79) | CP: - | CP: - | CP: - | CP: 42 (62.68) | CP: 42 (62.68) | CP: 22 (32.83) | The underweight BMI category, had significant higher odds of osteopathy (OR: 7.40; 95%CI: 1.56-34.99; P < 0.001) | Lower Z scores associated with (1) EPI (P = 0.01); (2) Smoking (P = 0.02). Non-significant association with alcohol exposure |
| Controls: - | Controls: - | Controls: - | Controls: - | Controls: - | Controls: - | Controls: - | |||
| Lower Z scores associated with vitamin D (P = < 0.001) | |||||||||
| Min et al[31], 2018 | CP: - | CP: - | CP: - | CP: - | CP: - | CP: - | CP: - | Non-significant association with BMI | Non-significant association with (1) CP severity; (2) PERT usage |
| Controls: - | Controls: - | Controls: - | Controls: - | Controls: - | Controls: - | Controls: - | |||
| Gupta et al[33], 2019 | CP: - | CP: - | CP: - | CP: - | CP: 13 (34.21) | CP: 18 (47.36) | CP: 12 (31.57) | Low bone mass was associated with lower BMI. Non-significant association with CP duration | |
| Controls: - | Controls: - | Controls: - | Controls: - | Controls: - | Controls: - | Controls: - | |||
| Kanakis et al[7], 2020 | CP: - | CP: - | CP: - | CP: - | CP: 130 (54.39) | CP: 132 (55.23) | CP: - | For patients, there was no association between total hip BMD and BMI (P = 0.753) | No descriptions of regression analysis or covariate adjustment |
| Controls: - | Controls: - | Controls: - | Controls: - | Controls: - | Controls: - | Controls: - | |||
| Hart et al[34], 2021 | CP: 161 (57.09) | CP: - | CP: - | CP: - | CP: - | CP: 191 (67.7) | CP: 111 (39.36) | Higher osteopathy risk associated with low BMI (P ≤ 0.001) | Increased risk of osteopathy with white race (P = 0.017), age (P ≤ 0.001), female sex (P ≤ 0.01) and past or present smoking (P ≤ 0.01). No associations with (1) CP severity (per atrophy assessment); and (2) Duration of CP |
| Controls: - | Controls: - | Controls: - | Controls: - | Controls: - | Controls: - | Controls: - | |||
| Reduced osteopathy among PERT users (P = 0.02) | |||||||||
| Vujasinovic et al[8], 2021 | CP: 104 (88.13) | CP: - | CP: - | CP: - | CP: 53 (44.91) | CP: 76 (64.4) | CP: 28 (23.72) | ||
| Controls: - | Controls: - | Controls: - | Controls: - | Controls: - | Controls: - | Controls: - | |||
| Reduced time to first fracture in PERT-treated patients | |||||||||
| Tang et al[35], 2021 | CP: 51 | CP: CRP: 0.75 (-), IL-6: 4.51 (-) | CP: 76 (73.07) | CP: 40.86 | CP: 52 (50) | CP: 45 (43.26) | CP: 28 (26.92) | Independent predictors of osteopathy: BMI (OR, 0.72; 95%CI, 0.58-0.89; P = 0.003) | Non-significant association between osteopathy and (1) Age; and (2) Duration of CP |
| Controls: - | Controls: - | Controls: - | Controls: - | Controls: - | Controls: - | Controls: - | |||
| Non-significant association between osteopathy and IL-6/CRP | Non-significant association between osteopathy and PTH |
Baseline patient characteristics: Female population was excluded in three studies[17,22,28] whereas 6376 (31.6%) female subjects were incorporated in the remaining 18 studies. In addition, three studies excluded post-menopausal females[19,25,33]. The race of CP patients was described in only five studies[7,20,24,33,34]. Its association with bone disease was investigated only by Hart and colleagues and they observed significantly higher burden among Caucasian population[34].
CP activity: The impact of CP severity on the bone outcomes was investigated in only one third of the eligible studies[17,19,27,28,31,32,34]. These studies observed no correlation with CP severity and alteration in bone mineral density. Moreover, the association of bone disease with duration of CP was also studied and lacked statistically significance[18,29,32,33,34,35]. The studies by Sikkens et al[25], and Kuhlman et al[30], observed a significant association of bone disease with exocrine insufficiency. Improved bone disease outcomes were observed in patients receiving PERT based on four large sized studies[8,26,30,34]. PERT usage and CP-related bone disease lacked statistical significance in five observational studies[17,22,23,28,32].
Inflammatory factors: Inflammation-based biomarkers such as C-reactive protein and interleukin (IL)-6 levels also yielded non-significant results in the investigation by two groups[27,32].
Endocrine factors: Among the eligible studies, hormones which regulate calcium metabolism have been widely studied. Serum 25-OH cholecalciferol had a positive correlation with bone density in three studies[21,27,30], whereas the remaining reported no significant associations[17,18,22,28,29,32]. Similarly, alkaline phosphatase and calcium had non-significant correlation with bone mineral density in two studies[17,28]. Serum PTH level did not correlate with bone disease in three studies[17,18,28], in contrast to the significant association found by Stigliano et al[32], Duggan et al[27], and Tang et al[35]. Novel bone turnover-based biomarkers i.e., carboxy-terminal telopeptide of type I collagen, osteocalcin, procollagen 1 amino-terminal propeptide also had non-significant results in limited observational studies[27,35]. Thyroid-stimulating hormones and Insulin-like growth factors 1 were considered by Munigala et al[24], but no correlation was found with bone mineral density (BMD). Although hypogonadism was higher in subjects with low BMD in the study by Gupta et al[33], no statistically significant difference was found. Exogenous hormone uses or replacement therapy was not investigated in any of the included studies.
Nutritional factors: The evidence linking BMI with bone disease outcomes has been conflicting. Whereas six studies described higher BMI as a protective factor[19,21-23,30,32,35], others reported an increased risk or non-significant findings[17,31,33,34]. Serum albumin was also explored in two studies with no mention of outcome association[17,21]. Min and colleagues studied the Malnutrition Universal Screening Tool, a validated nutritional assessment tool and observed that a score of 1 or more had significant association with osteopenia and osteoporosis (P = 0.004)[31]. Three studies described calcium supplement intake but did not study its relationship with bone disease[21,22,30].
Lifestyle factors: The definitions for exposure to alcohol and/or smoking were heterogeneous with limited evidence on their impact on bone outcomes[7,8,17,19,21,23-28,30,32-35]. Outdoor activity and sunlight exposure was only investigated by Joshi et al[21], and although correlated positively with vitamin D levels, their impact on osteoporosis was not studied.
Meta-regression revealed significant association of PERT use with prevalence of osteoporosis [coefficient: 1.7 (95%CI: 0.6-2.8); P < 0.0001] but not with osteopenia or fragility fractures. No associations with mean age, sex distribution, BMI, alcohol or smoking exposure, vitamin D deficiency, PTH levels, and diabetes with prevalence of osteopenia, osteoporosis or fragility fractures (Table 4).
| Covariates | Osteopenia | Osteoporosis | Fragility fracture | |||
| 95%CI | P value | 95%CI | P value | 95%CI | P value | |
| Sex distribution | 0.2 (-1.2-1.5) | P = 0.8 | 0.5 (-1.9-2.9) | P = 0.7 | 0.3 (-3.2-3.7) | P = 0.9 |
| Age | 0.0 (0-1) | P = 0.6 | 0.0 (-0.1-0.0) | P = 0.3 | 0.0 (-0.5-0.4) | P = 0.7 |
| DM | 0.3 (-5.5-6.0) | P = 0.9 | 0.0 (-3.9-3.8) | P = 0.9 | -13.8 (-107.4-79.8) | P = 0.3 |
| Alcohol use | 0.0 (-1.5-1.3) | P = 0.1 | 0.8 (-8.5-10.0) | P = 0.7 | -0.5 (-5.4-4.4) | P = 0.3 |
| Vitamin D | 0.0 (-0.02-0.0012) | P = 0.4 | -0.002 (-0.004-0.001) | P = 0.2 | 0.0 (-0.003-0.005) | P = 0.5 |
| PTH levels | 0.002 (-0.02-0.02) | P = 0.5 | 0.0 (-0.02-0.03) | P = 0.4 | - | - |
| PERT | 1.7 (0.6 -2.8) | P = 0.2 | 1.7 (0.6-2.8) | P < 0.0001 | 1.0 (-4.3-6.2) | P = 0.5 |
| Smoking | 0.0 (-3.6-3.6) | P = 0.9 | -0.5 (-2.9-1.9) | P = 0.6 | 2.0 (-3.4-7.9) | P = 0.3 |
| Mean BMI | NA | P = 0.9 | 0.0 (-0.12-0.21) | P = 0.6 | 0.5 (-17.2-18.2) | P = 0.8 |
Sensitivity analysis through forest plot calculation was performed using the “remove one study” method as described above. Confidence intervals and heterogeneity was measured through I2 and no significant alterations were observed after removal of one particular study (Supplementary Table 4).
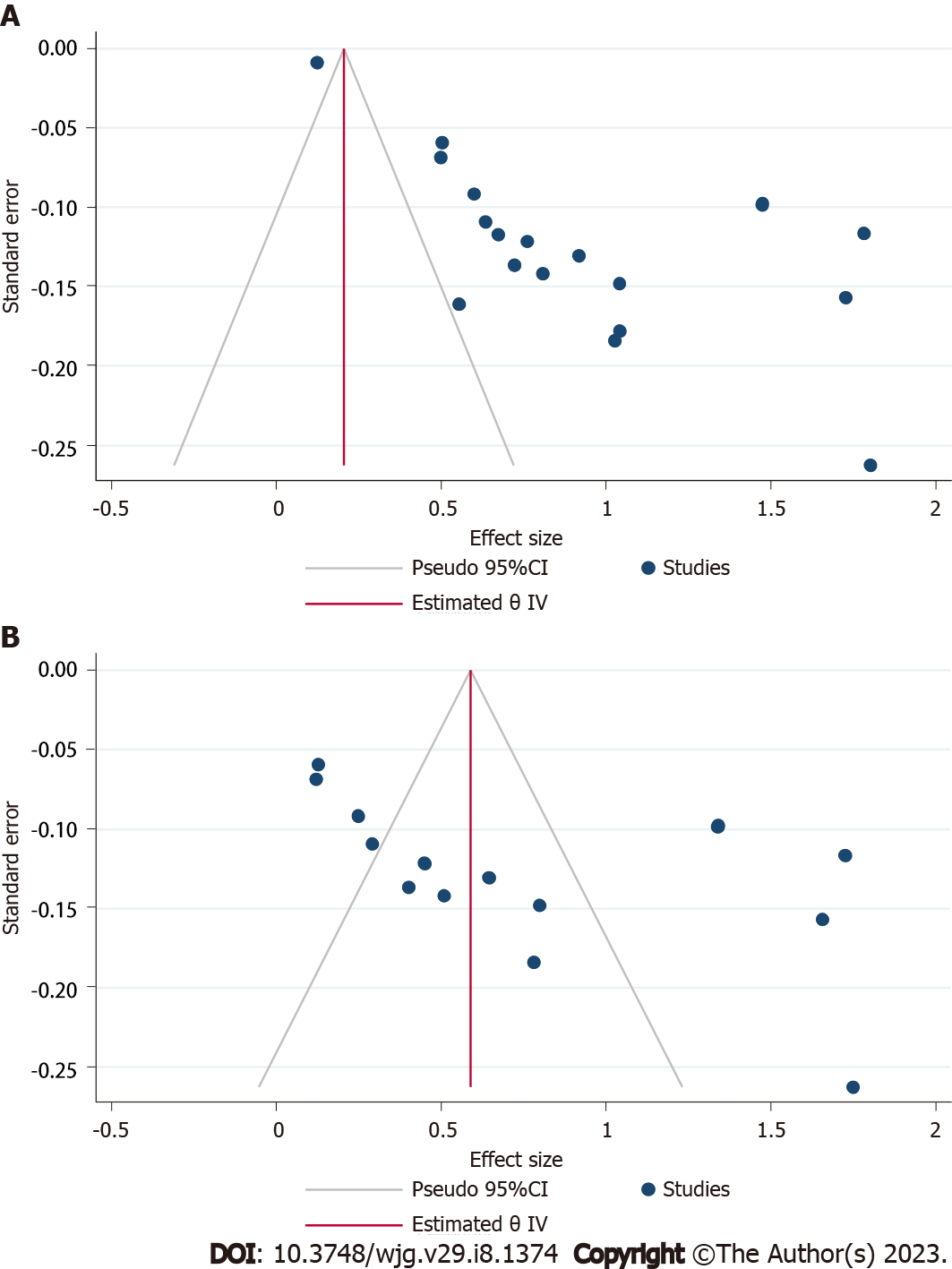
All the observational studies were assessed for quality in selection, comparability, and out-come/exposure domain and their scores ranged from 5-8 and were in good agreement (93% agreement, Cohen’s κ = 0.87) (Supplementary Table 5). The comparability domain was variable based upon the presence or absence of controls. Visual inspection of funnel plot and Egger’s regression for studies describing osteopenia (P = 0.003), and osteoporosis (P < 0.001) demonstrated significant publication bias (Figure 3).
Through this systematic review we present the burden of osteopenia, osteoporosis, and fragility fractures in CP patients. The majority of the studies used bone mineral density to quantitate bone disease, which is a validated tool for use in the general population[36]. Our meta-analyses revealed osteopenia and osteoporosis to be prevalent in 41.2% and 20.9% of the CP population, respectively. We also observed a fracture risk of 5.9% among CP patients, which includes low trauma fractures predominantly in the hip, vertebrae and distal radius. These may cause pain, falls, and hospitalizations which may significantly impair the lives of CP patients along with pre-existing morbidities like malabsorption and chronic abdominal pain[37].
Although dedicated studies among CP patients are lacking, reduced bone mass and resultant fractures cause significant pain, reduced functionality, and quality of life in both men and women[38]. Chronic inflammatory conditions affecting the gastrointestinal tract, such as inflammatory bowel disease, celiac disease and chronic liver disease have been known to disrupt the balance between bone resorption and formation and cause bone disease. Histomorphometric analysis among alcohol related CP patients has revealed low cortical and trabecular bone thickness and endocortical apposition and growth rate as compared to controls[39]. Similarly, increased bone turnover and mineralization defects due to malnutrition have been observed in CP[27,40]. Although recent CP guidelines in the United States and Europe have suggested surveillance of bone-related disorders, studies report poor adherence to these guidelines, suggesting CP-related bone disease is an underappreciated clinical entity[7,8].
CP-related bone disease has been quantified previously by Duggan et al[9] in 2014 who observed similar prevalence of osteopenia and osteoporosis i.e., 39.8% and 23.4%, respectively. Since this publication, over the past decade, several additional observational studies have investigated bone mineral density and metabolism in CP patients[7,8,24,25,27-31,33,34], and therefore, provide a unique opportunity to further investigate the evidence in this clinical domain. Hence, we appraised all the available literature, including the most recent publications, in an effort to contribute evidence for future by also uniquely quantifying fragility fractures which are significant complications of CP-related bone disease.
The current systematic review is powered by high-quality studies with large sample size and clearly defined study population and outcome measures. In addition, strengths of the study include a clearly articulated a priori analysis plan, a thorough search strategy and a conservative analysis. However, our study is limited by variability in the CP definitions from centers worldwide and spanning over 2 decades. Besides inconsistencies in definitions, high heterogeneity in the effect sizes is evident from I2 mandated performance of metaregression and sensitivity analysis. The heterogeneity was not attributable to any specific study as demonstrated by sensitivity analysis. We observed significant association of osteoporosis with patients with PERT use on metaregression analysis. Although PERT use signifies severe disease, this association doesn’t not establish causality and this in conjunction with limited information on the dosage of PERT or nutritional outcomes prevent substantial inferences to be drawn from this association[41]. We acknowledge significant publication bias, and a priori exclusion of studies in foreign language and conference abstracts[42]. These limitations prevent making categorical recommendations to patients.
Various mechanisms have been hypothesized to cause CP-mediated bone disease. Risk factors for CP such as cigarette smoking and alcohol exposure have been proven to alter the PTH-vitamin D axis and gonadal hormones and cause oxidative stress[39,43-46]. This clinical entity is also hypothesized to be driven by RANK ligand-induced osteoclastogenesis typically stimulated by inflammation-mediated nuclear factor-kappa B ligand[47]. Prior studies have also evaluated the relation of CP with inflammatory markers, such as IL-6, IL-1, and tumor necrosis factor-alpha[48]. Protein malnutrition lowers bone mass whereas deficiency of fat-soluble vitamins contributes to defects in mineralization and thus causes osteoporosis and resultant stress fracture[49,50]. CP is also characterized by low skeletal muscle, weight loss, and low mobility, all of which negatively impacts bone mass[51-53].
CP-related bone disease warrants further investigation to answer a few clinical queries. While CP patients are at risk, the impact of disease severity and duration on bone outcomes are unknown. Additionally, poor correlation between clinical symptomatology, severity and imaging findings in CP patients presents challenges for research[54]. We also systematically assessed various confounders including baseline clinical features and impact of bone turnover and inflammatory markers, albeit our results were unremarkable. The evidence on calcium supplements, hormone levels and outdoor activity among included studies was lacking. The studies that investigated mechanisms of systemic inflammation, bone turn over and malabsorption were underpowered, whereas those pertaining to vitamin D had conflicted evidence[17,18,21,22,27-30,32]. Among this at-risk group, efficacy of preventative therapy for osteoporotic fracture, drug interactions, and adverse effect also remain elusive.
In summary, this meta-analysis confirms significant bone disease in patients with CP. We observed significant association of PERT with CP related osteoporosis. Our study calls for improved methodology dedicated at delineation of confounders and studies targeting identification of at-risk CP patients, deeper understanding of mechanisms of CP related bone disease and their implications of treatment response. Fragility fractures are an important consequence of bone disease, which we have found to be increased in patients with CP. Screening strategies in this at-risk population with CP are needed as well as evaluating quality of life due to consequences of bone disease.
Chronic pancreatitis (CP) is a multifactorial fibro-inflammatory syndrome is characterized by nutritional deficiencies, systemic inflammation, and etiological factors like alcohol or smoking may disrupt the balance between bone formation and resorption. These maladaptive alterations may cause osteopenia and osteoporosis, resulting in fragility fractures which result from low energy trauma and have significant implications on quality of life.
Multiple large-sized studies on CP patients including those from multicenter cohorts have investigated the burden of bone disease in CP as well as their association with nutritional, anthropometric, and inflammatory parameters. Thus, with quantitative data on covariates and fragility fractures available, a synthesis of evidence is pertinent.
This systematic review and meta-analysis sought to quantify the prevalence of osteopenia, osteoporosis, and fragility fractures in CP patients and delineate clinical parameters which impact their occurrence.
The study included systematic review and then metanalysis of studies describing bone disease in CP patients. A preregistered systematic search enabled identification of original studies from Cochrane Library, Embase, Google Scholar, Ovid Medline, PubMed, Scopus, and Web of Science, from inception until October 2022. The metanalysis was performed using random effect model and the outcomes of interest included prevalence of osteopenia, osteoporosis, and fragility fractures. To assess the association of these outcomes with covariates metaregression using random effect model was performed.
Twenty-one studies were included for systematic review and 18 studies were included for meta-analysis. The pooled prevalence of osteopenia and osteoporosis in CP patients was 41.2% (95%CI: 35.2%-47.3%) and 20.9% (95%CI: 14.9%-27.6%), respectively. The pooled prevalence of fragility fractures described among CP was 5.9% (95%CI: 3.9%-8.4%). Meta-regression showed no associations of bone outcomes in CP patients with mean age, sex distribution, body mass index, alcohol or smoking exposure, diabetes, serum parathyroid levels, and vitamin D deficiency with prevalence of osteopenia, osteoporosis or fragility fractures. A significant association of pancreatic enzyme replacement therapy use with prevalence of osteoporosis [coefficient: 1.7 (95%CI: 0.6-2.8); P < 0.0001]. Due to sparse data on systemic inflammation, CP severity, and bone mineralization parameters a formal meta-analysis was not feasible.
This meta-analysis confirms significant bone disease: Osteopenia, osteoporosis, and fragility fractures in CP patients. Although pancreatic enzyme use had significant association with osteoporosis, the link between osteopathy and various patient or study-specific factors remains unclear. Further investigation is needed for delineation of at-risk population, to understand the mechanisms of CP-related bone disease, and assess the therapeutic response to treatment modalities.
Our study calls for dedicated studies targeting delineation of confounders and identification of at-risk features in CP patients. There is also a knowledge gap in therapeutic response among CP patients with bone disease.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Liu L, China; Salimi M, Iran S-Editor: Zhang H L-Editor: A P-Editor: Zhang H
| 1. | Beyer G, Habtezion A, Werner J, Lerch MM, Mayerle J. Chronic pancreatitis. Lancet. 2020;396:499-512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 561] [Reference Citation Analysis (1)] |
| 2. | Ahmed A, Deep A, Kothari DJ, Sheth SG. Bone disease in chronic pancreatitis. World J Clin Cases. 2020;8:1574-1579. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 9] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 3. | Veronese N, Kolk H, Maggi S. Epidemiology of Fragility Fractures and Social Impact. 2020 Aug 21. In: Falaschi P, Marsh D. Orthogeriatrics: The Management of Older Patients with Fragility Fractures [Internet]. 2nd ed. Cham (CH): Springer; 2021. [PubMed] |
| 4. | Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King A, Tosteson A. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005-2025. J Bone Miner Res. 2007;22:465-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2687] [Cited by in RCA: 2932] [Article Influence: 162.9] [Reference Citation Analysis (0)] |
| 5. | Löhr JM, Dominguez-Munoz E, Rosendahl J, Besselink M, Mayerle J, Lerch MM, Haas S, Akisik F, Kartalis N, Iglesias-Garcia J, Keller J, Boermeester M, Werner J, Dumonceau JM, Fockens P, Drewes A, Ceyhan G, Lindkvist B, Drenth J, Ewald N, Hardt P, de Madaria E, Witt H, Schneider A, Manfredi R, Brøndum FJ, Rudolf S, Bollen T, Bruno M; HaPanEU/UEG Working Group. United European Gastroenterology evidence-based guidelines for the diagnosis and therapy of chronic pancreatitis (HaPanEU). United European Gastroenterol J. 2017;5:153-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 521] [Cited by in RCA: 425] [Article Influence: 53.1] [Reference Citation Analysis (0)] |
| 6. | Gardner TB, Adler DG, Forsmark CE, Sauer BG, Taylor JR, Whitcomb DC. ACG Clinical Guideline: Chronic Pancreatitis. Am J Gastroenterol. 2020;115:322-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 203] [Article Influence: 40.6] [Reference Citation Analysis (0)] |
| 7. | Kanakis A, Vipperla K, Papachristou GI, Brand RE, Slivka A, Whitcomb DC, Yadav D. Bone health assessment in clinical practice is infrequenty performed in patients with chronic pancreatitis. Pancreatology. 2020;20:1109-1114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 8. | Vujasinovic M, Nezirevic Dobrijevic L, Asplund E, Rutkowski W, Dugic A, Kahn M, Dahlman I, Sääf M, Hagström H, Löhr JM. Low Bone Mineral Density and Risk for Osteoporotic Fractures in Patients with Chronic Pancreatitis. Nutrients. 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 9. | Duggan SN, Smyth ND, Murphy A, Macnaughton D, O'Keefe SJ, Conlon KC. High prevalence of osteoporosis in patients with chronic pancreatitis: a systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2014;12:219-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 125] [Article Influence: 11.4] [Reference Citation Analysis (1)] |
| 10. | Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Rev Esp Cardiol (Engl Ed). 2021;74:790-799. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 230] [Article Influence: 57.5] [Reference Citation Analysis (0)] |
| 11. | Chhoda A, Woodbine MJH. Bone outcomes in patients with chronic pancreatitis. [cited 8 November 2022]. Available from: https: //osf.io/fgvkq/. |
| 12. | McGowan J, Sampson M, Salzwedel DM, Cogo E, Foerster V, Lefebvre C. PRESS Peer Review of Electronic Search Strategies: 2015 Guideline Statement. J Clin Epidemiol. 2016;75:40-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1398] [Cited by in RCA: 2987] [Article Influence: 331.9] [Reference Citation Analysis (0)] |
| 13. | Nyaga VN, Arbyn M, Aerts M. Metaprop: a Stata command to perform meta-analysis of binomial data. Arch Public Health. 2014;72:39. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1058] [Cited by in RCA: 1618] [Article Influence: 147.1] [Reference Citation Analysis (0)] |
| 14. | Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA. Cochrane handbook for systematic reviews of interventions. New York: John Wiley & Sons, 2019. |
| 15. | Wells GA, Wells G, Shea B, O'Connell D, Peterson J, Welch, Losos M, Tugwell P, Ga SW, Zello GA, Petersen JA. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. 2014. |
| 16. | Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629-634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34245] [Cited by in RCA: 40537] [Article Influence: 1447.8] [Reference Citation Analysis (2)] |
| 17. | Morán CE, Sosa EG, Martinez SM, Geldern P, Messina D, Russo A, Boerr L, Bai JC. Bone mineral density in patients with pancreatic insufficiency and steatorrhea. Am J Gastroenterol. 1997;92:867-871. [PubMed] |
| 18. | Haaber AB, Rosenfalck AM, Hansen B, Hilsted J, Larsen S. Bone mineral metabolism, bone mineral density, and body composition in patients with chronic pancreatitis and pancreatic exocrine insufficiency. Int J Pancreatol. 2000;27:21-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 86] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 19. | Dujsikova H, Dite P, Tomandl J, Sevcikova A, Precechtelova M. Occurrence of metabolic osteopathy in patients with chronic pancreatitis. Pancreatology. 2008;8:583-586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 47] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 20. | Tignor AS, Wu BU, Whitlock TL, Lopez R, Repas K, Banks PA, Conwell D. High prevalence of low-trauma fracture in chronic pancreatitis. Am J Gastroenterol. 2010;105:2680-2686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 87] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 21. | Joshi A, Reddy SV, Bhatia V, Choudhuri G, Singh RK, Singh N, Bhatia E. High prevalence of low bone mineral density in patients with tropical calcific pancreatitis. Pancreas. 2011;40:762-767. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 22. | Sudeep K, Chacko A, Thomas N, Selvakumar R, George B, Paul TV, Seshadri MS. Predictors of osteodystrophy in patients with chronic nonalcoholic pancreatitis with or without diabetes. Endocr Pract. 2011;17:897-905. [PubMed] [DOI] [Full Text] |
| 23. | Duggan SN, O'Sullivan M, Hamilton S, Feehan SM, Ridgway PF, Conlon KC. Patients with chronic pancreatitis are at increased risk for osteoporosis. Pancreas. 2012;41:1119-1124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 51] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 24. | Munigala S, Agarwal B, Gelrud A, Conwell DL. Chronic Pancreatitis and Fracture: A Retrospective, Population-Based Veterans Administration Study. Pancreas. 2016;45:355-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 25. | Sikkens EC, Cahen DL, Koch AD, Braat H, Poley JW, Kuipers EJ, Bruno MJ. The prevalence of fat-soluble vitamin deficiencies and a decreased bone mass in patients with chronic pancreatitis. Pancreatology. 2013;13:238-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 121] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 26. | Bang UC, Benfield T, Bendtsen F, Hyldstrup L, Beck Jensen JE. The risk of fractures among patients with cirrhosis or chronic pancreatitis. Clin Gastroenterol Hepatol. 2014;12:320-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 55] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 27. | Duggan SN, Purcell C, Kilbane M, O'Keane M, McKenna M, Gaffney P, Ridgway PF, Boran G, Conlon KC. An association between abnormal bone turnover, systemic inflammation, and osteoporosis in patients with chronic pancreatitis: a case-matched study. Am J Gastroenterol. 2015;110:336-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 67] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 28. | Prabhakaran A, Bhasin DK, Rana SS, Bhadada SK, Bhansali A, Rao C, Gupta R, Khandelwal N. Bone mineral metabolism and bone mineral density in alcohol related and idiopathic chronic pancreatitis. Trop Gastroenterol. 2014;35:107-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 29. | Kumar KH, Sood AK, Manrai M. Occult metabolic bone disease in chronic pancreatitis. Niger J Clin Pract. 2017;20:1122-1126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 30. | Kuhlmann L, Poulsen JL, Kohler M, Rasmussen HH, Vestergaard P, Drewes AM, Olesen SS. Osteoporosis in Chronic Pancreatitis Outpatients Associates with Several Risk Factors. J Pancreas. 2018;19:183-189. [DOI] [Full Text] |
| 31. | Min M, Patel B, Han S, Bocelli L, Kheder J, Vaze A, Wassef W. Exocrine Pancreatic Insufficiency and Malnutrition in Chronic Pancreatitis: Identification, Treatment, and Consequences. Pancreas. 2018;47:1015-1018. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 58] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 32. | Stigliano S, Waldthaler A, Martinez-Moneo E, Lionetto L, Robinson S, Malvik M, Hedstrom A, Kaczka A, Scholdei M, Haas S, Simmaco M, Delle Fave G, Lohr M, Simon P, Capurso G. Vitamins D and K as Factors Associated with Osteopathy in Chronic Pancreatitis: A Prospective Multicentre Study (P-BONE Study). Clin Transl Gastroenterol. 2018;9:197. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 46] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 33. | Gupta N, Singh S, Vargas L, Moore TE, Shostrom VK, Boerner BP. Prevalence of Low Bone Density and Comorbid Hypogonadism in Patients With Chronic Pancreatitis. Pancreas. 2019;48:387-395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 34. | Hart PA, Yadav D, Li L, Appana S, Fisher W, Fogel E, Forsmark CE, Park WG, Pandol S, Topazian MD, Van Den Eden SK, Vege SS, Bradley D, Serrano J, Conwell DL; Consortium for the Study of Chronic Pancreatitis, Diabetes, and Pancreatic Cancer (CPDPC). High Prevalence of Osteopathy in Chronic Pancreatitis: A Cross-sectional Analysis From the PROCEED Study. Clin Gastroenterol Hepatol. 2022;20:2005-2013. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 35. | Tang XY, Ru N, Li Q, Qian YY, Sun H, Zhu JH, He L, Wang YC, Hu LH, Li ZS, Zou WB, Liao Z. Prevalence and Risk Factors for Osteopathy in Chronic Pancreatitis. Dig Dis Sci. 2021;66:4008-4016. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 36. | Humadi A, Alhadithi RH, Alkudiari SI. Validity of the DEXA diagnosis of involutional osteoporosis in patients with femoral neck fractures. Indian J Orthop. 2010;44:73-78. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 37. | Machicado JD, Amann ST, Anderson MA, Abberbock J, Sherman S, Conwell DL, Cote GA, Singh VK, Lewis MD, Alkaade S, Sandhu BS, Guda NM, Muniraj T, Tang G, Baillie J, Brand RE, Gardner TB, Gelrud A, Forsmark CE, Banks PA, Slivka A, Wilcox CM, Whitcomb DC, Yadav D. Quality of Life in Chronic Pancreatitis is Determined by Constant Pain, Disability/Unemployment, Current Smoking, and Associated Co-Morbidities. Am J Gastroenterol. 2017;112:633-642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 145] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 38. | Borhan S, Papaioannou A, Gajic-Veljanoski O, Kennedy C, Ioannidis G, Berger C, Goltzman D, Josse R, Kovacs CS, Hanley DA, Prior JC, Morin SN, Kaiser SM, Cheung AM, Thabane L, Adachi J; CaMos Research Group. Incident Fragility Fractures Have a Long-Term Negative Impact on Health-Related Quality of Life of Older People: The Canadian Multicentre Osteoporosis Study. J Bone Miner Res. 2019;34:838-848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 39. | Schnitzler CM, Mesquita JM, Shires R. Cortical and trabecular bone microarchitecture and turnover in alcohol-induced chronic pancreatitis: a histomorphometric study. J Bone Miner Metab. 2010;28:456-467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 22] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 40. | Rasmussen HH, Irtun O, Olesen SS, Drewes AM, Holst M. Nutrition in chronic pancreatitis. World J Gastroenterol. 2013;19:7267-7275. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 81] [Cited by in RCA: 78] [Article Influence: 6.5] [Reference Citation Analysis (2)] |
| 41. | Thompson SG, Higgins JP. How should meta-regression analyses be undertaken and interpreted? Stat Med. 2002;21:1559-1573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1956] [Cited by in RCA: 2129] [Article Influence: 92.6] [Reference Citation Analysis (0)] |
| 42. | Scherer RW, Saldanha IJ. How should systematic reviewers handle conference abstracts? Syst Rev. 2019;8:264. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 111] [Cited by in RCA: 186] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 43. | Segal SJ, Burgos M, Lyttle CR. Progesterone-dependent messenger RNA: identification of the products of heterospecific activity in vivo. Ann N Y Acad Sci. 1977;286:125-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 44. | Al-Bashaireh AM, Haddad LG, Weaver M, Chengguo X, Kelly DL, Yoon S. The Effect of Tobacco Smoking on Bone Mass: An Overview of Pathophysiologic Mechanisms. J Osteoporos. 2018;2018:1206235. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 104] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 45. | Godos J, Giampieri F, Chisari E, Micek A, Paladino N, Forbes-Hernández TY, Quiles JL, Battino M, La Vignera S, Musumeci G, Grosso G. Alcohol Consumption, Bone Mineral Density, and Risk of Osteoporotic Fractures: A Dose-Response Meta-Analysis. Int J Environ Res Public Health. 2022;19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 64] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 46. | Cheraghi Z, Doosti-Irani A, Almasi-Hashiani A, Baigi V, Mansournia N, Etminan M, Mansournia MA. The effect of alcohol on osteoporosis: A systematic review and meta-analysis. Drug Alcohol Depend. 2019;197:197-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 67] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 47. | Abu-Amer Y. NF-κB signaling and bone resorption. Osteoporos Int. 2013;24:2377-2386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 277] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 48. | Komar HM, Hart PA, Cruz-Monserrate Z, Conwell DL, Lesinski GB. Local and Systemic Expression of Immunomodulatory Factors in Chronic Pancreatitis. Pancreas. 2017;46:986-993. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 49. | Haas S, Krins S, Knauerhase A, Löhr M. Altered bone metabolism and bone density in patients with chronic pancreatitis and pancreatic exocrine insufficiency. JOP. 2015;16:58-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 18] [Reference Citation Analysis (0)] |
| 50. | Martínez-Moneo E, Stigliano S, Hedström A, Kaczka A, Malvik M, Waldthaler A, Maisonneuve P, Simon P, Capurso G. Deficiency of fat-soluble vitamins in chronic pancreatitis: A systematic review and meta-analysis. Pancreatology. 2016;16:988-994. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 63] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 51. | Wiese ML, Gärtner S, von Essen N, Doller J, Frost F, Tran QT, Weiss FU, Meyer F, Valentini L, Garbe LA, Metges CC, Bannert K, Sautter LF, Ehlers L, Jaster R, Lamprecht G, Steveling A, Lerch MM, Aghdassi AA. Malnutrition Is Highly Prevalent in Patients With Chronic Pancreatitis and Characterized by Loss of Skeletal Muscle Mass but Absence of Impaired Physical Function. Front Nutr. 2022;9:889489. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 52. | Olesen SS, Frandsen LK, Poulsen JL, Vestergaard P, Rasmussen HH, Drewes AM. The prevalence of underweight is increased in chronic pancreatitis outpatients and associates with reduced life quality. Nutrition. 2017;43-44:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 34] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 53. | Asomaning K, Bertone-Johnson ER, Nasca PC, Hooven F, Pekow PS. The association between body mass index and osteoporosis in patients referred for a bone mineral density examination. J Womens Health (Larchmt). 2006;15:1028-1034. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 108] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 54. | Wilcox CM, Yadav D, Ye T, Gardner TB, Gelrud A, Sandhu BS, Lewis MD, Al-Kaade S, Cote GA, Forsmark CE, Guda NM, Conwell DL, Banks PA, Muniraj T, Romagnuolo J, Brand RE, Slivka A, Sherman S, Wisniewski SR, Whitcomb DC, Anderson MA. Chronic pancreatitis pain pattern and severity are independent of abdominal imaging findings. Clin Gastroenterol Hepatol. 2015;13:552-60; quiz e28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 137] [Article Influence: 13.7] [Reference Citation Analysis (0)] |