Published online Feb 28, 2023. doi: 10.3748/wjg.v29.i8.1359
Peer-review started: November 16, 2022
First decision: December 11, 2022
Revised: December 30, 2022
Accepted: February 16, 2023
Article in press: February 16, 2023
Published online: February 28, 2023
Processing time: 103 Days and 19.8 Hours
Serum protein induced by vitamin K absence or antagonist-II (PIVKA-II) is a promising biomarker for hepatocellular carcinoma (HCC) surveillance.
To identify the contributing factors related to the abnormal elevation of PIVKA-II level and assess their potential influence on the performance of PIVKA-II in detecting HCC.
This study retrospectively enrolled in 784 chronic liver disease (CLD) patients and 267 HCC patients in Mengchao Hepatobiliary Hospital of Fujian Medical University from April 2016 to December 2019. Logistic regression and the area under the receiver operating characteristic curve (AUC) were used to evaluate the influencing factors and diagnostic performance of PIVKA-II for HCC, respectively.
Elevated PIVKA-II levels were independently positively associated with alcohol-related liver disease, serum alkaline phosphatase (ALP), and total bilirubin (TBIL) for CLD patients and aspartate aminotransferase (AST) and tumor size for HCC patients (all P < 0.05). Serum PIVKA-II were significantly lower in patients with viral etiology, ALP ≤ 1 × upper limit of normal (ULN), TBIL ≤ 1 × ULN, and AST ≤ 1 × ULN than in those with nonviral disease and abnormal ALP, TBIL, or AST (all P < 0.05), but the differences disappeared in patients with early-stage HCC. For patients with TBIL ≤ 1 × ULN, the AUC of PIVKA-II was significantly higher compared to that in patients with TBIL > 1 × ULN (0.817 vs 0.669, P = 0.015), while the difference between ALP ≤ 1 × ULN and ALP > 1 × ULN was not statistically significant (0.783 vs 0.729, P = 0.398). These trends were then more prominently perceived in subgroups of patients with viral etiology and HBV alone.
Serum PIVKA-II has better performance in detecting HCC at an early stage for CLD patients with normal serum TBIL.
Core Tip: This study demonstrated that elevated serum protein induced by vitamin K absence or antagonist-II (PIVKA-II) were positively associated with serum total bilirubin (TBIL) in patients with chronic liver disease (CLD), and the levels of PIVKA-II in CLD patients with normal serum TBIL were significantly lower than those in CLD patients with abnormal serum TBIL. Serum PIVKA-II has better performance in detecting hepatocellular carcinoma (HCC) at an early stage for CLD patients with normal serum TBIL, which was more prominently perceived in patients with viral etiology and hepatitis B virus alone. These findings may be important for surveillance counseling of early-stage HCC.
- Citation: Qian XJ, Wen ZM, Huang XM, Feng HJ, Lin SS, Liu YN, Li SC, Zhang Y, Peng WG, Yang JR, Zheng ZY, Zhang L, Zhang DW, Lu FM, Liu LJ, Pan WD. Better performance of PIVKA-II for detecting hepatocellular carcinoma in patients with chronic liver disease with normal total bilirubin. World J Gastroenterol 2023; 29(8): 1359-1373
- URL: https://www.wjgnet.com/1007-9327/full/v29/i8/1359.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i8.1359
Hepatocellular carcinoma (HCC), a serious issue for global health, is still the sixth most prevalently diagnosed cancer and the third highest cause of cancer death worldwide[1,2]. Chronic liver disease (CLD) induced by viral and nonviral factors has been identified as the main cause of HCC, including chronic infection with hepatitis B virus (HBV) or hepatitis C virus (HCV), alcohol-related liver disease (ALD), nonalcoholic fatty liver disease (NAFLD), autoimmune liver disease (AILD), and so on, with the major risk factors varying from region to region[1-3].
In recent years, although great progress has been achieved on cancer treatment with the development by surgical resection, molecular targeted therapy, and immunotherapy[2,4-6], 5-year survival rate of HCC remains not satisfactory, with only 15%-17% as reported[7,8]. The main cause of this extremely poor prognosis is that more than half of HCC patients present with advanced stages once at diagnosis[7,8]. Detecting HCC at an early stage is an efficacious way for the improvement of long term survival of HCC patients[2,9,10].
Serum HCC biomarkers as surveillance tools remain controversial because the diagnostic accuracy of the traditional biomarker for HCC, serum alpha-fetoprotein (AFP), is unsatisfactory[8,11,12]. Serum AFP is frequently influenced by many noncancerous factors and is falsely raised in non-HCC patients who have active chronic viral and advanced liver disease[13-15]. Hence, protein induced by vitamin K absence or antagonist-II (PIVKA-II), has gained increasing attention in recent years since it was first reported for the diagnosis of HCC in 1984[2,8,9,16]. PIVKA-II is poorly related to AFP, and the diagnostic performance of HCC by combining the two biomarkers was higher compared to AFP alone[8,17]. In addition, the guidelines of the Japan Society of Hepatology has recommended serum PIVKA-II as one of the HCC surveillance markers in at-risk populations[18,19].
PIVKA-II could also be employed to monitor HCC prognosis, treatment response, and recurrence because of its possible associations with the tumor size, HCC progression and recurrence[20,21]. Serum PIVKA-II levels have also been reported to be influenced by several noncancerous factors, such as vitamin K deficiency[22], ALD[23-26], acute hepatic failure[27], and the use of warfarin or antibiotics[26,28] by leading to serum PIVKA-II elevation in non-HCC patients.
However, it remains unclear what the factors are regarding different liver disease etiologies, the liver injury severity, and disease activity degree associated with abnormal PIVKA-II levels in patients with CLD and HCC. Therefore, we aimed to identify the potential contributing factors and evaluate their influence on serum PIVKA-II levels and the performance of PIVKA-II for the diagnosis of HCC in different populations.
This study eventually retrospectively registered 1051 patients after excluding 80 patients from 1131 registered patients. The mean age of the total cohort was 48.93 years ± 13.70 years, and 73.6% of them were male (n = 773). Supplementary Figure 1 shows the selection flowchart and the analysis process. All patients were recruited in Mengchao Hepatobiliary Hospital of Fujian Medical University from April 2016 to December 2019.
The patients included following the criteria as: (1) With CLD or HCC of clear etiology diagnosed by clinical or histological evidence, including chronic hepatitis B (CHB), chronic hepatitis C (CHC), ALD, NAFLD, and AILD; (2) CHB and CHC were defined as patients with positive hepatitis B surface antigen (HBsAg) and positive hepatitis C virus (HCV) antibodies and/or HCV RNA for at least 6 months; and (3) complete and detailed laboratory and clinical information, including serum PIVKA-II, relevant laboratory tests, and other clearly clinical characteristics and records. The patients excluded following the criteria as: (1) Patients diagnosed with CHB and ALD simultaneously; (2) patients with liver failure, esophageal and gastric variceal bleeding, or drug-induced liver disease; (3) patients without sufficient information on relevant laboratory tests and clinical records; (4) patients without a certain clinical diagnosis of HCC; (5) patients accepting warfarin or long-term treatment with antibiotics at the time of enrollment; and (6) patients with other malignant tumors or a prior history of antitumor treatment in HCC.
According to the Milan criteria, early-stage HCC is defined as a nodule with the diameter less than 5 cm or two to three nodules with the diameter each less than 3 cm and do not have significant vascular invasion or extrahepatic metastases[2,15,29], and beyond the Milan criteria was considered as late-stage HCC. A positive lesion discovered by the suggested imaging techniques and contrast agents or histopathological confirmation were used to determine the diagnosis of HCC[2,3,9,15,30]. For CLD patients at enrollment, the lack of an HCC was determined through that there were no any suspicious hepatic masses in clinical and imaging evidence, and if with an abnormal PIVKA-II level, a continuous imaging surveillance within the subsequent months was appropriate.
The existence of cirrhosis was generally determined via imaging, laboratory and clinical characteristics, or liver biopsy without a routine examination.
The ethics committee of Peking University Health Science Center granted this study approval (IRB00001052-19081). All procedures carried out in this study had followed the Helsinki declaration or equivalent ethical principles.
Serum PIVKA-II level was quantitatively detected through the chemiluminescence enzyme immunoassay (LUMIPULSE® G1200, FUJIREBIO INC, Japan) by using Lumipulse® G PIVKA-II reaction cartridges in the clinical laboratory of Mengchao Hepatobiliary Hospital of Fujian Medical University, according to manufacturer’s instructions. The lower limit of detection is > 0 mAU/mL, and the upper is 75000 mAU/mL. The upper limit of normal (ULN) was 40 mAU/mL[9]. Furthermore, abnormal elevation of serum PIVKA-II was defined as its values elevated above 40 mAU/mL (ULN).
Liver-related laboratory tests, including liver enzyme and function and routine blood, were performed in the clinical laboratory using commercially available kits by manufacturers’ instructions. Test values would be reviewed if judged as unusual in order to ensure their accuracy.
SPSS 24.0 software (New York, NK, United States) was applied to finish the basic statistical analyses. The mean ± SD or median and interquartile range (IQR), as the continuous variables, were analyzed by t or Mann-Whitney test between two groups or Kruskal-Wallis test among three or more groups; χ2 test for categorical variables. Univariate and multivariate (forward) analyses of logistic regression were constructed to investigate factors associated with the abnormal elevation of serum PIVKA-II for non-HCC and HCC patients, respectively. Then, GraphPad Prism 7 (California, CA, United States) was used to plot the distributions of the levels of PIVKA-II in different subgroups and further compare them. The area under receiver operating characteristic (ROC) curve (AUC) and 95% confidence interval (CI), which reflecting the PIVKA-II’s performance for HCC, were conducted and calculated by MedCalc version 18.2.1 (Ostend, Belgium). The best cutoff value, sensitivity, specificity, positive likelihood ratio (LR+), and negative likelihood ratio (LR-) of PIVKA-II levels were also calculated and shown. P < 0.05 was deemed statistically significant, and all tests and power analyses of significance were two-tailed.
The comprehensive clinical and laboratory characteristics of the study population are listed in Table 1. A total of 595 viral liver disease patients, 189 nonviral liver disease patients, 127 early-stage HCC patients, and 140 late-stage HCC patients were enrolled in. Compared to viral liver disease patients, patients with nonviral liver disease had significantly older mean age and higher median serum platelet (PLT) and GGT levels (P < 0.05); however, their male proportion and median serum ALT, AST and TBIL levels were significantly lower (P < 0.05). Interestingly, the abnormally elevated proportion of PIVKA-II > 40 mAU/mL (ULN) in patients with nonviral liver disease was significantly higher than that in patients with viral liver disease (20.6% vs 13.9%, P < 0.050), as well as the substantially higher median levels of serum PIVKA-II (27.0 vs 23.0, P < 0.001).
| Variable | Non-HCC | HCC | ||||
| Viral liver disease (n = 595) | Nonviral liver disease (n = 189) | P value | Early stage HCC (n = 127) | Late stage HCC (n = 140) | P value | |
| Age (yr) | 46.2 ± 13.4 | 49.6 ± 13.5 | 0.003 | 56.5 ± 10.8 | 55.5 ± 12.1 | 0.488 |
| Male, n (%) | 418 (70.3) | 117 (61.9) | 0.032 | 113 (89.0) | 125 (89.3) | 0.935 |
| Etiology | - | 0.333 | ||||
| HBV | 561 (94.3) | - | 115 (90.6) | 130 (92.9) | ||
| HCV | 34 (5.7) | - | 2 (1.6) | 2 (1.4) | ||
| NAFLD | - | 56 (29.6) | 2 (1.6) | - | ||
| ALD | - | 82 (43.4) | 6 (4.7) | 8 (5.7) | ||
| AILD | - | 51 (27.0) | 2 (1.6) | - | ||
| Cirrhosis, n (%) | 274 (46.1) | 75 (39.7) | 0.125 | 112 (88.2) | 120 (85.7) | 0.550 |
| ALT (U/L) | 48 (25, 171) | 40 (24, 74) | < 0.001 | 31 (22, 42) | 40 (25, 67) | 0.003 |
| AST (U/L) | 44 (27, 101) | 38 (25, 60) | 0.007 | 32 (24, 47) | 60 (35, 95) | < 0.001 |
| ALP (U/L) | 92 (75, 122) | 97 (72, 141) | 0.153 | 90 (70, 120) | 112 (88, 177) | < 0.001 |
| GGT (U/L) | 50 (28, 113) | 94 (39, 241) | < 0.001 | 44 (25, 78) | 113 (57, 208) | < 0.001 |
| TBIL (μmol/L) | 19.8 (13.2, 31.6) | 16.1 (10.5, 35.0) | 0.006 | 18.4 (11.6, 29.4) | 19.3 (13.6, 30.2) | 0.277 |
| ALB (g/L) | 38.8 ± 6.3 | 38.7 ± 7.7 | 0.834 | 38.3 ± 7.0 | 36.6 ± 6.0 | 0.036 |
| PLT (109/L) | 161 (104, 199) | 206 (124, 273) | < 0.001 | 145 (87, 178) | 188 (132, 242) | < 0.001 |
| Tumor size (cm) | - | - | - | 2.2 (1.5, 2.9) | 9.3 (5.8, 13.2) | < 0.001 |
| Number of tumors (1/2-3/> 3) | - | - | - | 115/12/0 | 62/33/45 | < 0.001 |
| Vascular invasion | - | - | - | - | 53 (37.9) | - |
| Extrahepatic metastases | - | - | - | - | 13 (9.3) | - |
| PIVKA II > 40 mAU/mL, n (%) | 83 (13.9) | 39 (20.6) | 0.027 | 77 (60.6) | 131 (93.6) | < 0.001 |
| PIVKA II (mAU/mL) | 23.0 (18.0, 31.0) | 27.0 (20.0, 38.0) | < 0.001 | 58.0 (25.0, 228.0) | 5124 (691.0, 36245.0) | < 0.001 |
The median serum ALT, AST, ALP, GGT, and PLT levels in patients with early-stage HCC were significantly lower compared to those in patients with late-stage HCC (P < 0.01); and the significantly smaller tumor size and proportion of the number of tumors ≥ 2 were also observed in early-stage HCC patients (both P < 0.001), but they had a higher median level of serum ALB (P < 0.05). Furthermore, patients with early-stage HCC had a significantly lower abnormal proportion of PIVKA-II > 40 mAU/mL (ULN) than patients with late-stage HCC (60.6% vs 93.6%, P < 0.001), as well as significantly lower median serum PIVKA-II level (58 vs 5124, P < 0.001).
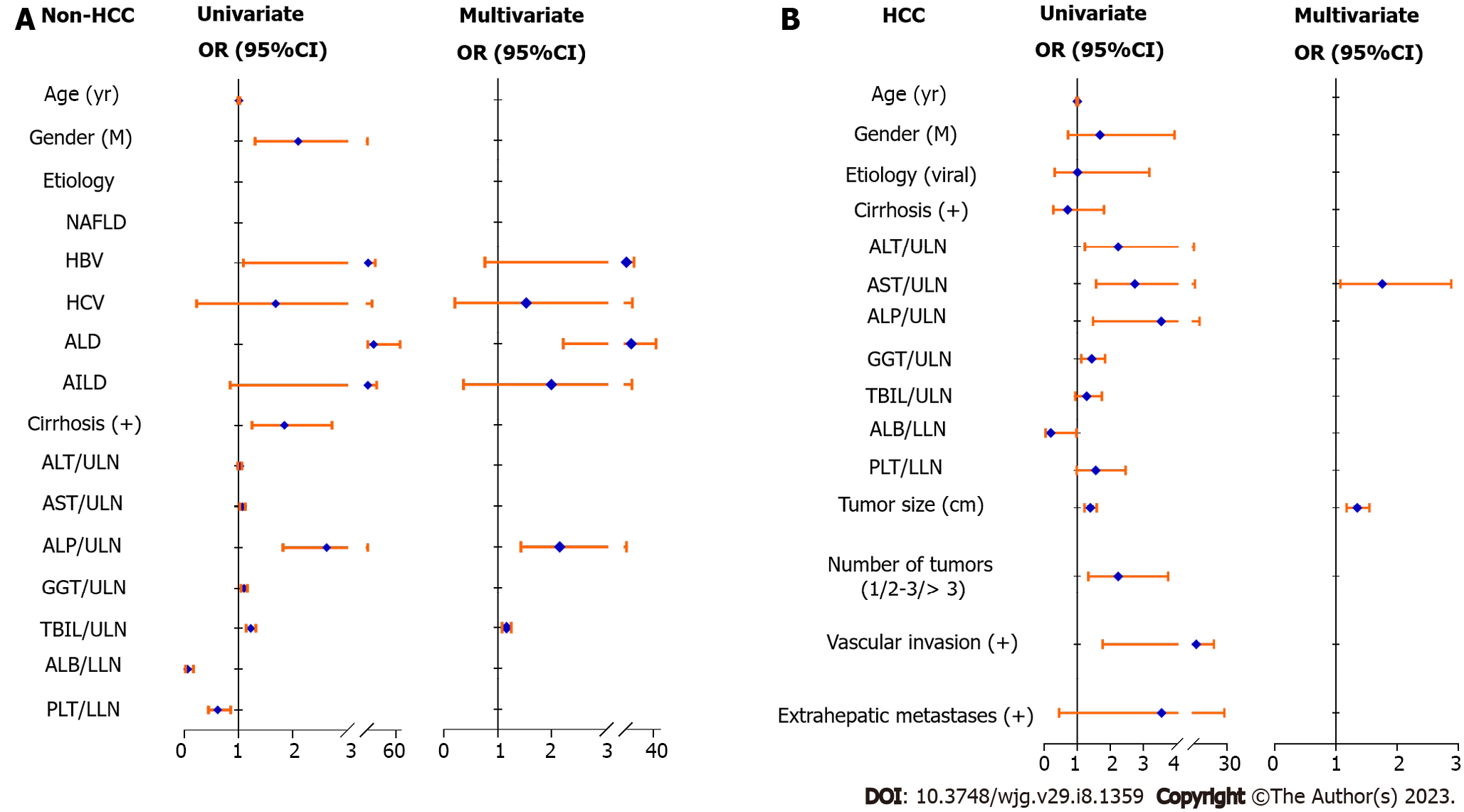
Then, we further analyzed and identified the independent factors related to the abnormal elevation of PIVKA-II level in non-HCC and HCC patients through logistic regression analyses, respectively. Table 2 and Figure 1A summarize the results of non-HCC patients. ALD etiology (OR: 9.883, 95%CI: 2.216-44.086, P < 0.001) was an independent positive factor associated with the abnormal elevation of serum PIVKA-II in non-HCC patients, as well as the factors of ALP/ULN (OR: 2.146, 95%CI: 1.429-3.221, P < 0.001) and TBIL/ULN (OR: 1.162, 95%CI: 1.080-1.250, P < 0.001). Table 3 and Figure 1B summarize the results of HCC patients. AST/ULN (OR: 1.759, 95%CI: 1.072-2.887, P = 0.025) was an independent positive factor associated with the abnormal elevation of serum PIVKA-II in HCC patients, as well as the factor of tumor size (OR: 1.349, 95%CI: 1.175-1.549, P < 0.001). These findings implied that there were obvious differences in the factors associated with the abnormal elevation of PIVKA-II level between non-HCC and HCC patients.
| Non-HCC | Univariate | Multivariate | ||
| OR (95%CI) | P value | OR (95%CI) | P value | |
| Age (yr) | 1.009 (0.995, 1.024) | 0.195 | ||
| Gender (M) | 2.102 (1.308, 3.379) | 0.002 | ||
| Etiology | < 0.001 | < 0.001 | ||
| NAFLD | - | - | ||
| HBV | 4.556 (1.089, 19.055) | 3.214 (0.762, 13.548) | ||
| HCV | 1.687 (0.227, 12.571) | 1.528 (0.204, 11.442) | ||
| ALD | 15.577 (3.542, 68.507) | 9.883 (2.216, 44.086) | ||
| AILD | 4.295 (0.849, 21.729) | 1.993 (0.368, 10.792) | ||
| Cirrhosis (+) | 1.848 (1.250, 2.731) | 0.002 | ||
| ALT/ULN | 1.026 (0.991, 1.063) | 0.150 | ||
| AST/ULN | 1.076 (1.029, 1.126) | 0.001 | ||
| ALP/ULN | 2.630 (1.823, 3.795) | < 0.001 | 2.146 (1.429, 3.221) | < 0.001 |
| GGT/ULN | 1.105 (1.050, 1.163) | < 0.001 | ||
| TBIL/ULN | 1.228 (1.142, 1.321) | < 0.001 | 1.162 (1.080, 1.250) | < 0.001 |
| ALB/LLN | 0.063 (0.024, 0.166) | < 0.001 | ||
| PLT/LLN | 0.618 (0.445, 0.857) | 0.004 | ||
| HCC | Univariate | Multivariate | ||
| OR (95%CI) | P value | OR (95%CI) | P value | |
| Age (yr) | 1.002 (0.977, 1.027) | 0.895 | ||
| Gender (M) | 1.692 (0.726, 3.944) | 0.223 | ||
| Etiology (viral) | 1.008 (0.319, 3.185) | 0.989 | ||
| Cirrhosis (+) | 0.712 (0.280, 1.810) | 0.475 | ||
| ALT/ULN | 2.242 (1.235, 4.068) | 0.008 | ||
| AST/ULN | 2.745 (1.573, 4.788) | < 0.001 | 1.759 (1.072, 2.887) | 0.025 |
| ALP/ULN | 3.542 (1.478, 8.490) | 0.005 | ||
| GGT/ULN | 1.436 (1.120, 1.843) | 0.004 | ||
| TBIL/ULN | 1.287 (0.947, 1.749) | 0.108 | ||
| ALB/LLN | 0.202 (0.042, 0.974) | 0.046 | ||
| PLT/LLN | 1.558 (0.985, 2.464) | 0.058 | ||
| Tumor size (cm) | 1.394 (1.221, 1.592) | < 0.001 | 1.349 (1.175, 1.549) | < 0.001 |
| Number of tumors (1/2-3/> 3) | 2.242 (1.340, 3.752) | 0.002 | ||
| Vascular invasion (+) | 5.907 (1.772, 19.696) | 0.004 | ||
| Extrahepatic metastases (+) | 3.551 (0.452, 27.887) | 0.228 | ||
The distributions and comparisons of serum PIVKA-II were further investigated according to the above results of independent factors associated with the abnormal elevation of PIVKA-II level.
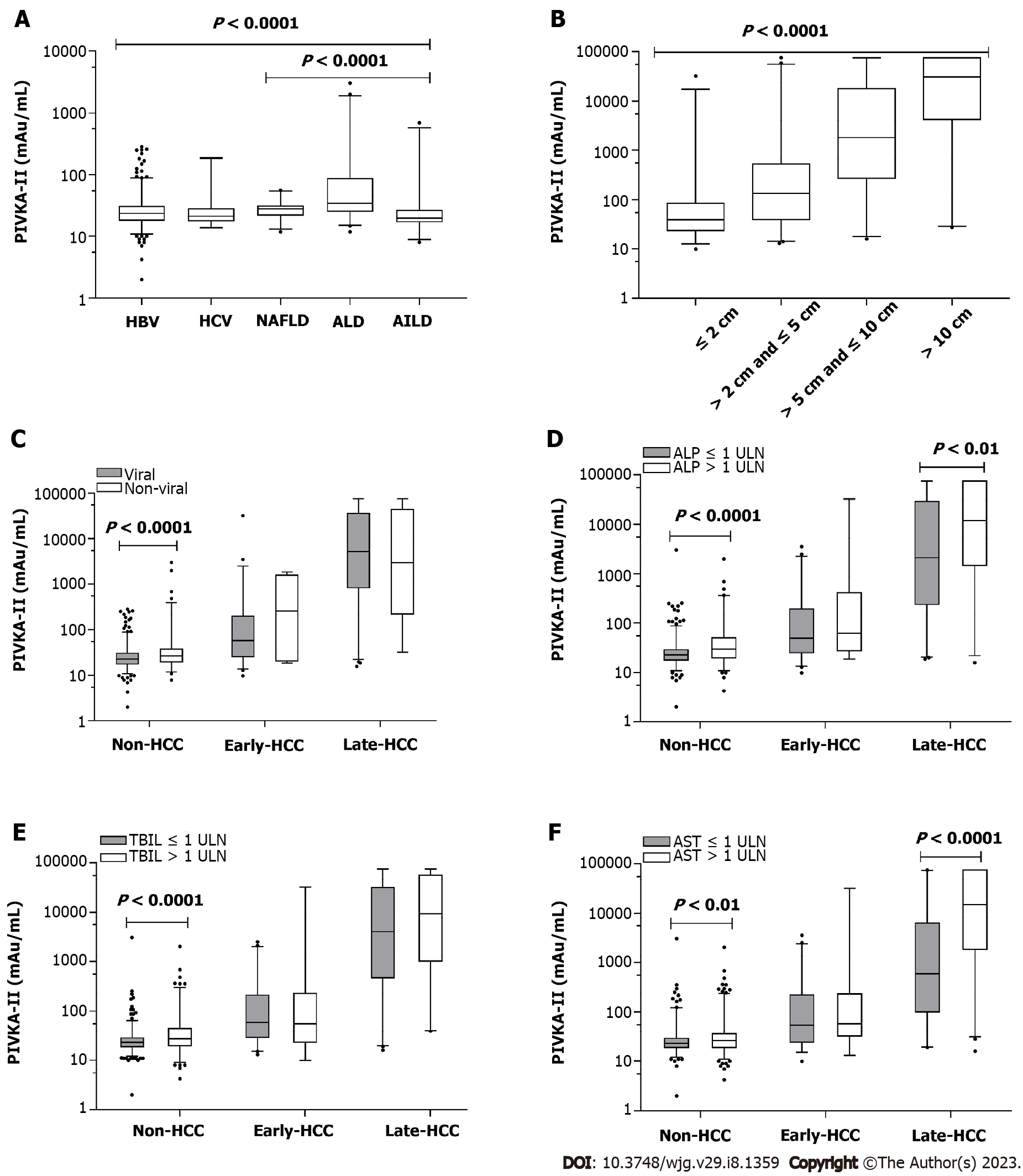
According to Figure 2A, the median PIVKA-II levels among subgroups of different etiologies were significantly different (P < 0.0001); for viral liver disease without HCC, no difference was observed between HBV and HCV (23.8 vs 21.5, P > 0.05), but among nonviral liver diseases of NAFLD, ALD and AILD (28.0 vs 34.5 vs 20.0, P < 0.0001), the differences were significant. Further analysis showed that compared to CLD patients with HBV or HCV, the median PIVKA-II level in ALD was substantially higher (both P < 0.0001), and also in NAFLD (both P < 0.05). Meanwhile, AILD patients had significantly lower PIVKA-II levels than HBV patients (P < 0.05), whereas no difference was observed between HCV and AILD (P > 0.05). For patients with HCC (Figure 2B), the median PIVKA-II levels gradually significantly increased as the tumor size changed by ≤ 2 cm, > 2 cm and ≤ 5 cm, > 5 cm and ≤ 10 cm to > 10 cm (39.5 vs 135.5 vs 1811.0 vs 30987.0, P < 0.0001).
Further analysis revealed that viral liver disease patients had a significantly lower median level of PIVKA-II than patients with nonviral liver disease (23.0 vs 27.0, P < 0.0001) in non-HCC (Figure 2C), and there were also similar trends between ALP ≤ 1 × ULN and ALP > 1 × ULN (23.0 vs 30.0, P < 0.0001) (Figure 2D), TBIL ≤ 1 × ULN and TBIL > 1 × ULN (23.0 vs 27.0, P < 0.0001) (Figure 2E), AST ≤ 1 × ULN and AST > 1 × ULN (23.0 vs 26.0, P < 0.01) (Figure 2F) in non-HCC. However, similar tendencies were not observed in early-stage HCC patients, and the PIVKA-II levels between the above subgroups did not differ significantly. Furthermore, for late-stage HCC, the median level of PIVKA-II of ALP ≤ 1 × ULN was significantly lower compared to that of ALP > 1 × ULN (2129 vs 11992, P < 0.01), and also between AST ≤ 1 × ULN and AST > 1 × ULN (593.0 vs 15164, P < 0.0001); however, between viral and nonviral liver disease, a significant difference was not observed, nor between TBIL ≤ 1 × ULN and TBIL > 1 × ULN.
Additionally, Supplementary Table 1 shows that irrespective of the subgroups of etiology (viral and nonviral liver disease), ALP (≤ 1 × ULN and > 1 × ULN), TBIL (≤ 1 × ULN and > 1 × ULN), AST (≤ 1 × ULN and > 1 × ULN), serum PIVKA-II levels differed significantly among non-HCC, early-stage and late-stage HCC.
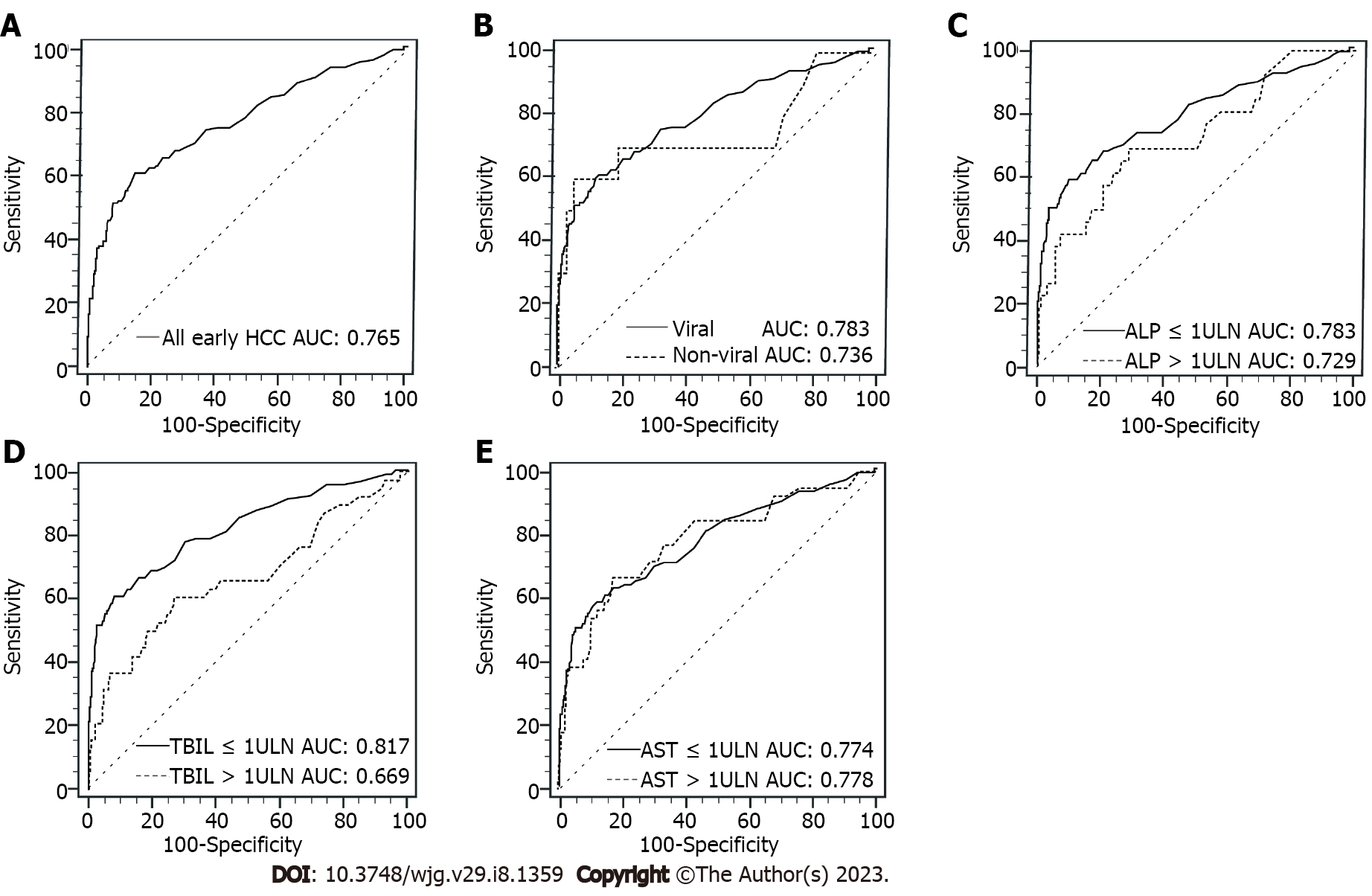
The above findings suggested that abnormally elevated PIVKA-II level was independently positively associated with etiology, ALP, and TBIL for non-HCC patients and AST and tumor size for HCC patients. Then, the AUCs of serum PIVKA-II for the diagnosis of early-stage HCC and late-stage HCC were further analyzed for each subgroup of patients by etiology, ALP, TBIL, and AST.
In Table 4, serum PIVKA-II had the best performance for diagnosing early-stage HCC in TBIL ≤ 1 × ULN subgroup, and the AUC of PIVKA-II in TBIL ≤ 1 × ULN subgroup was significantly higher than that in TBIL > 1 × ULN subgroup (0.817 vs 0.669, P = 0.015). When compared with the subgroup of nonviral liver disease, the AUC of PIVKA-II in the subgroup of viral liver disease for diagnosing early-stage HCC was only marginally higher (0.783 vs 0.736, P = 0.678), the difference was not significant, nor was the difference between ALP ≤ 1 × ULN and ALP > 1 × ULN (0.783 vs 0.729, P = 0.398). However, the AUC of PIVKA-II in AST ≤ 1 × ULN subgroup was almost the same to AST > 1 × ULN subgroup (0.774 vs 0.778, P = 0.941) in diagnosing early-stage HCC. Figure 3 shows the corresponding ROC curves of PIVKA-II for diagnosing early-stage HCC in different subgroups.
| Early HCC | AUC (95%CI) | Cutoff value (mAU/mL) | Se (%) | Sp (%) | LR+ | LR- | P value |
| All early | 0.765 (0.714, 0.815) | 41.0 | 60.63 | 85.08 | 4.06 | 0.46 | |
| Etiology | 0.678 | ||||||
| Viral | 0.783 (0.732, 0.835) | 42.8 | 58.97 | 87.73 | 4.81 | 0.47 | |
| Nonviral | 0.736 (0.519, 0.952) | 177.0 | 60.00 | 94.18 | 10.31 | 0.42 | |
| ALP | 0.398 | ||||||
| ≤ 1 ULN | 0.783 (0.725, 0.840) | 41.0 | 58.42 | 89.95 | 5.81 | 0.46 | |
| > 1 ULN | 0.729 (0.618, 0.841) | 42.0 | 69.23 | 71.07 | 2.39 | 0.43 | |
| TBIL | 0.015 | ||||||
| ≤ 1 ULN | 0.817 (0.762, 0.872) | 41.0 | 60.67 | 91.73 | 7.34 | 0.43 | |
| > 1 ULN | 0.669 (0.563, 0.775) | 42.0 | 60.53 | 73.19 | 2.26 | 0.54 | |
| AST | 0.941 | ||||||
| ≤ 1 ULN | 0.774 (0.712, 0.836) | 41.0 | 57.95 | 88.08 | 4.86 | 0.48 | |
| > 1 ULN | 0.778 (0.691, 0.864) | 41.0 | 66.67 | 82.41 | 3.79 | 0.40 |
However, subsequent analysis of Supplementary Table 2 showed that the above factors, including etiology, ALP, TBIL, and AST, had almost no influence on the performance of PIVKA-II for the diagnosis of late-stage HCC. The AUCs of PIVKA-II did not differ significantly between subgroups in etiology (viral vs nonviral), ALP (≤ 1 × ULN vs > 1 × ULN), TBIL (≤ 1 × ULN vs > 1 × ULN) and AST (≤ 1 × ULN vs > 1 × ULN) (P all > 0.05). Supplementary Figure 2 also shows the corresponding ROC curves of PIVKA-II for diagnosing late-stage HCC in different subgroups.
Furthermore, we also analyzed the AUCs of PIVKA-II for HCC between viral and ALD etiologies. Supplementary Figure 3 shows that compared to the subgroup of viral etiology, the AUC of PIVKA-II for early-stage HCC was lower in ALD etiology (0.783 vs 0.655, P = 0.470), and also for late-stage HCC (0.970 vs 0.909, P = 0.361).
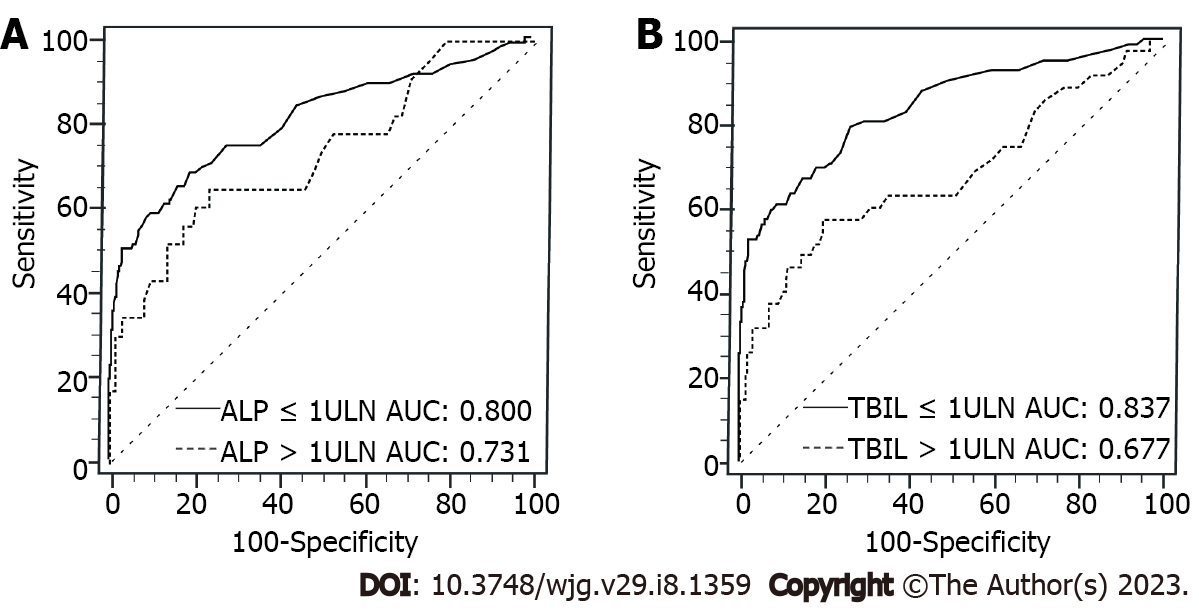
Different etiologies of liver disease might induce diverse progression of liver injuries and changes in indicators of liver function, such as serum ALP and TBIL. Then, we further analyzed the AUCs of ROC curves of PIVKA-II by each subgroup of ALP and TBIL in patients of viral etiology and HBV alone, respectively, but not in patients with nonviral etiology on account of the limited cases of HCC.
Figure 4 shows that patients with TBIL ≤ 1 × ULN of viral etiology had substantially higher AUC of PIVKA-II for diagnosing early-stage HCC (0.837 vs 0.677, P = 0.012) than patients with TBIL > 1ULN. Also, for patients with viral etiology, the AUC of PIVKA-II in ALP ≤ 1 × ULN was higher (0.800 vs 0.731, P = 0.318) than that in ALP > 1 × ULN, but no statistical significance was found. Supplementary Table 3 further shows the detailed value of AUC, best cutoff value, sensitivity, specificity, LR+, and LR- in each subgroup.
Furthermore, similar tendencies were also validated in patients with HBV alone. As shown in Supplementary Table 4, for patients with HBV alone, the AUCs of PIVKA-II were 0.832 and 0.676 (P = 0.015) in TBIL ≤ 1 × ULN and TBIL > 1 × ULN, and 0.794 and 0.732 (P = 0.368) in ALP ≤ 1 × ULN and ALP > 1 × ULN, respectively.
When the cutoff value of PIVKA-II for detecting early-stage HCC was set at 40 mAU/mL (ULN), the sensitivity, specificity, LR+, and LR- were further analyzed in patients with viral etiology and HBV alone. As shown in Table 5, compared to TBIL > 1 × ULN of viral etiology, the sensitivity of PIVKA-II in TBIL ≤ 1 × ULN of viral etiology increased from 30.30% to 57.47%, and the LR+ increased from 3.80 to 6.66, without compromising the corresponding specificity (92.02% vs 91.37%). Similarly, for patients with HBV alone, the sensitivity of PIVKA-II in TBIL ≤ 1 × ULN was also significantly higher than that in TBIL > 1 × ULN (57.14% vs 30.77%), as well as LR+ (6.16 vs 3.73), with similar specificity (90.72% vs 91.76%). Noticeably, the sensitivities in TBIL ≤ 1 × ULN have consistently been greater than those in ALP≤ 1 × ULN, both for patients with viral etiology (57.47% vs 52.88%) and HBV alone (57.14% vs 53.00%), and the corresponding specificities were almost the same. However, this tendency disappeared, and the sensitivities tended to be the same between TBIL > 1 × ULN and ALP > 1 × ULN, regardless of viral etiology (30.30% vs 30.61%) and HBV alone (30.77% vs 30.61%).
| Cutoff value (mAU/mL) | Viral etiology | HBV | |||||||
| Se (%) | Sp (%) | +LR | -LR | Se (%) | Sp (%) | +LR | -LR | ||
| ALP | |||||||||
| ≤ 1ULN | 40 | 52.88 | 91.39 | 6.14 | 0.52 | 53.00 | 90.80 | 5.76 | 0.52 |
| 1ULN | 40 | 30.61 | 92.45 | 4.06 | 0.75 | 30.61 | 92.23 | 3.94 | 0.75 |
| TBIL | |||||||||
| ≤ 1ULN | 40 | 57.47 | 91.37 | 6.66 | 0.47 | 57.14 | 90.72 | 6.16 | 0.47 |
| 1ULN | 40 | 30.30 | 92.02 | 3.80 | 0.76 | 30.77 | 91.76 | 3.73 | 0.75 |
In the current study, 1051 patients in total were analyzed, and we determined the independent variables linked to elevated serum PIVKA-II levels in CLD and HCC patients, with the purpose of expounding their influence on the PIVKA-II performance for early-stage HCC and late-stage HCC detection. For patients with CLD, abnormally increased PIVKA-II levels were independently positively associated with ALD etiology, serum ALP and TBIL, but serum AST and tumor size for HCC patients. Compared to subgroups of nonviral etiology, ALP > 1 × ULN, TBIL > 1 × ULN and AST > 1 × ULN, serum PIVKA-II levels were significantly lower in subgroups of viral etiology, ALP ≤ 1 × ULN, TBIL ≤ 1 × ULN, and AST ≤ 1 × ULN in CLD patients; however, these differences disappeared in early-stage HCC. Furthermore, serum PIVKA-II in a subgroup of TBIL ≤ 1 × ULN always had the highest AUCs and the best performance in detecting early-stage HCC than TBIL > 1 × ULN, irrespective of all patients, viral etiology or HBV alone. However, the above factors, including etiology, ALP, TBIL, and AST, had almost no influence on PIVKA-II performance for late-stage HCC detection.
Four previous studies found that ALD patients had higher levels of PIVKA-II compared to viral hepatitis-related CLD patients[23-26], and ALD was also verified to be a significant factor related to positive serum PIVKA-II by a retrospective case-control study[26]. Consistently, in this study, patients with CLD with ALD etiology had the highest median level of PIVKA-II compared to those with other etiologies of HBV, HCV, NAFLD, and AILD, and was demonstrated to be independently associated with abnormally elevated PIVKA-II levels. A recent study enrolled 130 cases and showed that patients with CHB, CHC, and nonviral CLD had no significant differences in PIVKA-II levels (32 mAU/mL vs 35 mAU/mL vs 35 mAU/mL, any two P > 0.05)[31]. In the current study, however, a significant difference was observed in PIVKA-II levels between viral and nonviral CLD patients; meanwhile, PIVKA-II levels in NAFLD and ALD patients were significantly higher compared to those in HBV and HCV patients, and AILD patients had the lowest PIVKA-II levels than those of other CLD patients.
In this study, tumor size was an independent factor relevant to abnormally elevated PIVKA-II levels in HCC patients, and the median levels of PIVKA-II gradually significantly increased as the tumor size changed by ≤ 2 cm, > 2 cm and ≤ 5 cm, > 5 cm and ≤ 10 cm to > 10 cm, which was consistent with the results of previous studies[32,33]. Moreover, the number of tumors and vascular invasion were associated with the abnormal elevation of PIVKA-II level which was only perceived in the univariate analysis. The results imply that tumor size had more influence on the PIVKA-II level than the number of tumors and vascular invasion.
Prior researches had reported that PIVKA-II serum levels are not elevated in hepatic flares and are influenced by liver regeneration triggered by necroinflammation in patients with active CLD, and have paid little attention to the confounding factors of liver injury and function that influence the levels of PIVKA-II[12,31]. Nonetheless, this is the first study to our knowledge to demonstrate that the abnormal PIVKA-II levels are significantly associated with serum ALP and TBIL in CLD patients and serum AST in HCC patients. Furthermore, in patients with CLD, the median PIVKA-II levels differed significantly between the subgroups of ALP ≤ 1 × ULN and ALP > 1 × ULN, as well as between TBIL ≤ 1 × ULN and TBIL > 1 × ULN; and in patients with late-stage HCC, the differences in PIVKA-II median levels were also observed between the subgroups of ALP and AST classified by 1 × ULN. However, these changes in PIVKA-II serum levels disappeared in early-stage HCC patients in the same situation.
Regretfully, the underlying mechanism behind the associations of ALD, ALP, and TBIL with elevated PIVKA-II levels in CLD patients remains unclear. Although vitamin K insufficiency may arise in chronic alcoholics[34], prior studies[23,25] found there was no direct correlation between PIVKA-II serum levels and vitamin K serum concentration. For ALP and TBIL, one likely explanation is that abnormalities in serum ALP and TBIL were always seen in all types of liver disorders and cholestasis. Vitamin K deficiency, as one of the fat-soluble vitamin deficiencies, is also a typical complication in chronic cholestasis patients, and vitamin levels were inversely correlated with serum TBIL levels[35-37]. Interestingly, one recent study also reported that hepatitis E patients in the raised PIVKA-II subgroup had significantly greater TBIL levels than those in the normal PIVKA-II subgroup (P < 0.05), and the trend of changes in serum PIVKA and TBIL were similar and related to the disease course[38].
It has been reported that the PIVKA-II’s AUC for HCC in the CHB group was the highest compared to those in CHC and nonviral CLD patients (0.833 vs 0.732 vs 0.806)[31]. Similarly, in the current study, the AUC of PIVKA-II was slightly higher in patients with viral etiology compared to that in patients with nonviral etiology, irrespective of whether the HCC is in its early or late stages. Patients with ALD etiology had much lower AUCs of PIVKA-II for detecting HCC than patients with viral etiology, especially for early-stage HCC. However, in previous literature, the influences of serum ALP and TBIL on the performance of PIVKA-II for the detection of HCC have not been evaluated. In this study, we provided evidence that patients in TBIL ≤ 1 × ULN subgroup had the best performance of PIVKA-II for detecting early-stage HCC, and the AUC of PIVKA-II in TBIL ≤ 1 × ULN subgroup was significantly higher compared to that in TBIL > 1 × ULN subgroup. Between ALP ≤ 1 × ULN and ALP > 1 × ULN, a similar trend was also observed, although the difference was not significant. However, no significant influences of serum ALP and TBIL were observed on the performance of PIVKA-II for late-stage HCC.
Further analysis also showed that the above changes and differences in the performance of PIVKA-II for the detection of early-stage HCC still existed between different subgroups of ALP and TBIL in patients with viral etiology and HBV alone. Moreover, when 40 mAU/mL (ULN) was set as the cutoff value of PIVKA-II, for patients with TBIL ≤ 1 × ULN, serum PIVKA-II had the highest sensitivities in detecting early-stage HCC than other subgroups and enough high specificities at the same time, irrespective of viral etiology or HBV alone. These results strongly suggest that serum PIVKA-II would have better performance in detecting HCC at an early-stage for patients with normal serum TBIL.
The current study had a number of limitations. Firstly, it was a retrospective design, and some unidentified potential biases might exist, although we completely ruled out the possible interferences on serum PIVKA-II by the potential confounding factors in the exclusion criteria. Then, the limited sample size, including HCC patients with CHC, ALD, NAFLD, and AILD, does not allow us to independently evaluate the influences of these factors on the performance of PIVKA-II in HCC patients with CHC, NAFLD, and AILD, and decrease the reliability of that in HCC patients with ALD. Finally, further multicenter research with more participants are required to validate the above findings.
The present study suggests that abnormally elevated PIVKA-II levels were independently positively associated with ALD etiology, serum ALP and TBIL for non-HCC CLD patients and serum AST and tumor size for HCC patients. Better performance of PIVKA-II for discriminating HCC at an early stage from patients with CLD would be achieved in patients with normal TBIL, and more attention should be given to the availability of PIVKA-II in HCC surveillance for at-risk patients with elevated serum TBIL.
Serum protein induced by vitamin K absence or antagonist-II (PIVKA-II) is a promising biomarker for hepatocellular carcinoma (HCC) surveillance.
Investigate those unclear factors regarding different liver disease etiologies, the liver injury severity, and disease activity associated with the abnormal levels of serum PIVKA-II in chronic liver disease (CLD) and HCC patients.
Identify the potential contributing factors and evaluate their influence on serum PIVKA-II levels and the performance of PIVKA-II for the diagnosis of HCC in different populations.
This study retrospectively enrolled in 784 CLD patients and 267 HCC patients in Mengchao Hepatobiliary Hospital of Fujian Medical University from April 2016 to December 2019. Logistic regression and the area under the receiver operating characteristic curve (AUC) were used to evaluate the influencing factors and diagnostic performance of PIVKA-II for HCC, respectively.
Elevated PIVKA-II levels were independently positively associated with alcohol-related liver disease (ALD), serum alkaline phosphatase (ALP), and total bilirubin (TBIL) for CLD patients and aspartate aminotransferase (AST) and tumor size for HCC patients (all P < 0.05). Serum PIVKA-II were significantly lower in patients with viral etiology, ALP ≤ 1 × upper limit of normal (ULN), TBIL ≤ 1 × ULN, and AST ≤ 1 × ULN than in those with nonviral disease and abnormal ALP, TBIL, or AST (all P < 0.05), but the differences disappeared in patients with early-stage HCC. For patients with TBIL ≤ 1 × ULN, the AUC of PIVKA-II was significantly higher compared to that in patients with TBIL > 1 × ULN (0.817 vs 0.669, P = 0.015), while the difference between ALP ≤ 1 × ULN and ALP > 1 × ULN was not statistically significant found between ALP ≤ 1 × ULN and ALP > 1 × ULN (0.783 vs 0.729, P = 0.398). These trends were then more prominently perceived in subgroups of patients with viral etiology and HBV alone.
Abnormally elevated PIVKA-II levels were independently positively associated with ALD etiology, serum ALP and TBIL for non-HCC CLD patients and serum AST and tumor size for HCC patients. Better performance of PIVKA-II for discriminating HCC at an early stage from patients with CLD would be achieved in patients with normal TBIL.
More attention should be given to the availability of PIVKA-II in HCC surveillance for at-risk patients with elevated serum TBIL, which may be important for surveillance counseling of early-stage HCC.
We thank all the colleagues who assisted in laboratory analyses and clinical information collection.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Bredt LC, Brazil; Mao Y, China; Sripongpun P, Thailand S-Editor: Chen YL L-Editor: A P-Editor: Chen YL
| 1. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 64636] [Article Influence: 16159.0] [Reference Citation Analysis (176)] |
| 2. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol. 2018;69:182-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5593] [Cited by in RCA: 6059] [Article Influence: 865.6] [Reference Citation Analysis (3)] |
| 3. | Bureau of Medical Administration, National Health and Family Planning Comission of the People's Republic of China. [Diagnosis, management, and treatment of hepatocellular carcinoma (V2017)]. Zhonghua Gan Zang Bing Za Zhi. 2017;25:886-895. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 4. | Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A, Schwartz M, Porta C, Zeuzem S, Bolondi L, Greten TF, Galle PR, Seitz JF, Borbath I, Häussinger D, Giannaris T, Shan M, Moscovici M, Voliotis D, Bruix J; SHARP Investigators Study Group. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9016] [Cited by in RCA: 10268] [Article Influence: 604.0] [Reference Citation Analysis (2)] |
| 5. | Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, Luo R, Feng J, Ye S, Yang TS, Xu J, Sun Y, Liang H, Liu J, Wang J, Tak WY, Pan H, Burock K, Zou J, Voliotis D, Guan Z. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3854] [Cited by in RCA: 4650] [Article Influence: 273.5] [Reference Citation Analysis (0)] |
| 6. | Kudo M, Finn RS, Qin S, Han KH, Ikeda K, Piscaglia F, Baron A, Park JW, Han G, Jassem J, Blanc JF, Vogel A, Komov D, Evans TRJ, Lopez C, Dutcus C, Guo M, Saito K, Kraljevic S, Tamai T, Ren M, Cheng AL. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018;391:1163-1173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3128] [Cited by in RCA: 3826] [Article Influence: 546.6] [Reference Citation Analysis (1)] |
| 7. | Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet. 2018;391:1301-1314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2800] [Cited by in RCA: 4105] [Article Influence: 586.4] [Reference Citation Analysis (6)] |
| 8. | Lok AS, Sterling RK, Everhart JE, Wright EC, Hoefs JC, Di Bisceglie AM, Morgan TR, Kim HY, Lee WM, Bonkovsky HL, Dienstag JL; HALT-C Trial Group. Des-gamma-carboxy prothrombin and alpha-fetoprotein as biomarkers for the early detection of hepatocellular carcinoma. Gastroenterology. 2010;138:493-502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 376] [Cited by in RCA: 450] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 9. | Omata M, Cheng AL, Kokudo N, Kudo M, Lee JM, Jia J, Tateishi R, Han KH, Chawla YK, Shiina S, Jafri W, Payawal DA, Ohki T, Ogasawara S, Chen PJ, Lesmana CRA, Lesmana LA, Gani RA, Obi S, Dokmeci AK, Sarin SK. Asia-Pacific clinical practice guidelines on the management of hepatocellular carcinoma: a 2017 update. Hepatol Int. 2017;11:317-370. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1628] [Cited by in RCA: 1644] [Article Influence: 205.5] [Reference Citation Analysis (0)] |
| 10. | Heimbach JK, Kulik LM, Finn RS, Sirlin CB, Abecassis MM, Roberts LR, Zhu AX, Murad MH, Marrero JA. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. 2018;67:358-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2107] [Cited by in RCA: 3029] [Article Influence: 432.7] [Reference Citation Analysis (3)] |
| 11. | Kanwal F, Singal AG. Surveillance for Hepatocellular Carcinoma: Current Best Practice and Future Direction. Gastroenterology. 2019;157:54-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 306] [Article Influence: 51.0] [Reference Citation Analysis (0)] |
| 12. | Loglio A, Iavarone M, Facchetti F, Di Paolo D, Perbellini R, Lunghi G, Ceriotti F, Galli C, Sandri MT, Viganò M, Sangiovanni A, Colombo M, Lampertico P. The combination of PIVKA-II and AFP improves the detection accuracy for HCC in HBV caucasian cirrhotics on long-term oral therapy. Liver Int. 2020;40:1987-1996. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 52] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 13. | Qian X, Liu S, Long H, Zhang S, Yan X, Yao M, Zhou J, Gong J, Wang J, Wen X, Zhou T, Zhai X, Xu Q, Zhang T, Chen X, Hu G, Gao Z, Nan Y, Chen J, Hu B, Zhao J, Lu F. Reappraisal of the diagnostic value of alpha-fetoprotein for surveillance of HBV-related hepatocellular carcinoma in the era of antiviral therapy. J Viral Hepat. 2021;28:20-29. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 14. | Yang JD, Dai J, Singal AG, Gopal P, Addissie BD, Nguyen MH, Befeler AS, Reddy KR, Schwartz M, Harnois DM, Yamada H, Gores GJ, Feng Z, Marrero JA, Roberts LR. Improved Performance of Serum Alpha-Fetoprotein for Hepatocellular Carcinoma Diagnosis in HCV Cirrhosis with Normal Alanine Transaminase. Cancer Epidemiol Biomarkers Prev. 2017;26:1085-1092. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 51] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 15. | Qian X, Liu Y, Wu F, Zhang S, Gong J, Nan Y, Hu B, Chen J, Zhao J, Chen X, Pan W, Dang S, Lu F. The Performance of Serum Alpha-Fetoprotein for Detecting Early-Stage Hepatocellular Carcinoma Is Influenced by Antiviral Therapy and Serum Aspartate Aminotransferase: A Study in a Large Cohort of Hepatitis B Virus-Infected Patients. Viruses. 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 16. | Liebman HA, Furie BC, Tong MJ, Blanchard RA, Lo KJ, Lee SD, Coleman MS, Furie B. Des-gamma-carboxy (abnormal) prothrombin as a serum marker of primary hepatocellular carcinoma. N Engl J Med. 1984;310:1427-1431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 395] [Cited by in RCA: 421] [Article Influence: 10.3] [Reference Citation Analysis (16)] |
| 17. | Li C, Zhang Z, Zhang P, Liu J. Diagnostic accuracy of des-gamma-carboxy prothrombin versus α-fetoprotein for hepatocellular carcinoma: A systematic review. Hepatol Res. 2014;44:E11-E25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 56] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 18. | Izumi N. Diagnostic and treatment algorithm of the Japanese society of hepatology: a consensus-based practice guideline. Oncology. 2010;78 Suppl 1:78-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 38] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 19. | Kudo M, Izumi N, Kokudo N, Matsui O, Sakamoto M, Nakashima O, Kojiro M, Makuuchi M; HCC Expert Panel of Japan Society of Hepatology. Management of hepatocellular carcinoma in Japan: Consensus-Based Clinical Practice Guidelines proposed by the Japan Society of Hepatology (JSH) 2010 updated version. Dig Dis. 2011;29:339-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 549] [Cited by in RCA: 664] [Article Influence: 47.4] [Reference Citation Analysis (0)] |
| 20. | Poté N, Cauchy F, Albuquerque M, Voitot H, Belghiti J, Castera L, Puy H, Bedossa P, Paradis V. Performance of PIVKA-II for early hepatocellular carcinoma diagnosis and prediction of microvascular invasion. J Hepatol. 2015;62:848-854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 244] [Article Influence: 24.4] [Reference Citation Analysis (4)] |
| 21. | Ueno M, Hayami S, Shigekawa Y, Kawai M, Hirono S, Okada K, Tamai H, Shingaki N, Mori Y, Ichinose M, Yamaue H. Prognostic impact of surgery and radiofrequency ablation on single nodular HCC ⩽5 cm: Cohort study based on serum HCC markers. J Hepatol. 2015;63:1352-1359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 59] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 22. | Suttie JW. Recent advances in hepatic vitamin K metabolism and function. Hepatology. 1987;7:367-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 44] [Article Influence: 1.2] [Reference Citation Analysis (1)] |
| 23. | Ohhira M, Ohtake T, Saito H, Ikuta K, Tanaka K, Tanabe H, Kawashima T, Fujimoto Y, Naraki T, Ono M, Kohgo Y. Increase of serum des-gamma-carboxy prothrombin in alcoholic liver disease without hepatocellular carcinoma. Alcohol Clin Exp Res. 1999;23:67S-70S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 24. | Ohhira M, Saito H, Suzuki Y, Naraki T, Sakurai S, Ohtake T, Suzuki M, Ohhira M, Fujimoto And Y, Kohgo Y. A variant of des-gamma-carboxy prothrombin was increased in alcoholic liver disease without hepatocellular carcinoma. Alcohol Clin Exp Res. 2001;25:46S-50S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 25. | Sakizono K, Oita T, Eto M, Bito S, Takegawa H, Kasakura S. [Studies on the mechanism of elevation of serum PIVKA-II levels in alcoholic liver cirrhosis]. Rinsho Byori. 2002;50:289-295. [PubMed] |
| 26. | Kang KH, Kim JH, Kang SH, Lee BJ, Seo YS, Yim HJ, Yeon JE, Park JJ, Kim JS, Bak YT, Byun KS. The influence of alcoholic liver disease on serum PIVKA-II levels in patients without hepatocellular carcinoma. Gut Liver. 2015;9:224-230. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 27. | Takikawa Y. [Abnormal prothrombin in acute hepatic failure: the characterization and clinical evaluation]. Nihon Shokakibyo Gakkai Zasshi. 1991;88:1074-1082. [PubMed] |
| 28. | Umeki S, Umeki Y. Levels of acarboxy prothrombin (PIVKA-II) and coagulation factors in warfarin-treated patients. Med Lab Sci. 1990;47:103-107. [PubMed] |
| 29. | Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F, Montalto F, Ammatuna M, Morabito A, Gennari L. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334:693-699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5110] [Cited by in RCA: 5311] [Article Influence: 183.1] [Reference Citation Analysis (0)] |
| 30. | Ministry of Health of the People's Republic of China. [Updated standards for the diagnosis and treatment of primary liver cancer]. Zhonghua Gan Zang Bing Za Zhi. 2012;20:419-426. [PubMed] |
| 31. | Ricco G, Cavallone D, Cosma C, Caviglia GP, Oliveri F, Biasiolo A, Abate ML, Plebani M, Smedile A, Bonino F, Pontisso P, Brunetto MR. Impact of etiology of chronic liver disease on hepatocellular carcinoma biomarkers. Cancer Biomark. 2018;21:603-612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 32. | Xu F, Zhang L, He W, Song D, Ji X, Shao J. The Diagnostic Value of Serum PIVKA-II Alone or in Combination with AFP in Chinese Hepatocellular Carcinoma Patients. Dis Markers. 2021;2021:8868370. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 33. | Feng H, Li B, Li Z, Wei Q, Ren L. PIVKA-II serves as a potential biomarker that complements AFP for the diagnosis of hepatocellular carcinoma. BMC Cancer. 2021;21:401. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 102] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 34. | Iber FL, Shamszad M, Miller PA, Jacob R. Vitamin K deficiency in chronic alcoholic males. Alcohol Clin Exp Res. 1986;10:679-681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 16] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 35. | Poupon R. Liver alkaline phosphatase: a missing link between choleresis and biliary inflammation. Hepatology. 2015;61:2080-2090. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 78] [Article Influence: 7.8] [Reference Citation Analysis (1)] |
| 36. | Shen YM, Wu JF, Hsu HY, Ni YH, Chang MH, Liu YW, Lai HS, Hsu WM, Weng HL, Chen HL. Oral absorbable fat-soluble vitamin formulation in pediatric patients with cholestasis. J Pediatr Gastroenterol Nutr. 2012;55:587-591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 37. | Mancell S, Islam M, Dhawan A, Whelan K. Fat-soluble vitamin assessment, deficiency and supplementation in infants with cholestasis. J Hum Nutr Diet. 2022;35:273-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 38. | Chen Y, Yang Y, Li S, Lin M, Xie X, Shi H, Jiang Y, Zheng S, Shao H, Yang N, Lu M. Changes and Clinical Significance of PIVKA-II in Hepatitis E Patients. Front Public Health. 2021;9:784718. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Reference Citation Analysis (0)] |