Published online Feb 28, 2023. doi: 10.3748/wjg.v29.i8.1315
Peer-review started: November 3, 2022
First decision: November 14, 2022
Revised: November 22, 2022
Accepted: February 16, 2023
Article in press: February 16, 2023
Published online: February 28, 2023
Processing time: 117 Days and 6.7 Hours
Stress granules (SGs) could be formed under different stimulation to inhibit cell injury.
To investigate whether SGs could protect hepatocytes from hypoxia-induced damage during acute liver failure (ALF) by reducing endoplasmic reticulum stress (ERS) mediated apoptosis.
The agonist of SGs, arsenite (Ars) was used to intervene hypoxia-induced hepatocyte injury cellular model and ALF mice models. Further, the siRNA of activating transcription factor 4 (ATF4) and SGs inhibitor anisomycin was then used to intervene in cell models.
With the increase of hypoxia time from 4 h to 12 h, the levels of HIF-1α, ERS and apoptosis gradually increased, and the expression of SGs marker G3BP1 and TIA-1 was increased and then decreased. Compared with the hypoxia cell model group and ALF mice model, the levels of HIF-1α, apoptosis and ERS were increased in the Ars intervention group. After siRNA-ATF4 intervention, the level of SGs in cells increased, and the levels of HIF-1α, ERS and apoptosis decreased. Compared with the siRNA-ATF4 group, the levels of G3BP1 in the siRNA-ATF4+anisomycin group were decreased, and the levels of HIF-1α, ERS and apoptosis were increased. Moreover, compared with the ALF group, the degree of liver injury and liver function, the levels of HIF-1α, ERS and apoptosis in the Ars intervention group were decreased, the level of SGs was increased.
SGs could protect hepatocytes from hypoxia-induced damage during ALF by reducing ERS-mediated apoptosis.
Core Tip: Hepatocytes were damaged by hypoxia and ischemia injury in the process of acute liver failure. At this time, the content of HIF-1α in cells increased, which inhibited the formation of stress granules (SGs) mediated by G3BP1 and promoted the expression of endoplasmic reticulum stress (ERS) marker molecules activating transcription factor 4 (ATF4) and CCAAT/enhancer-binding protein-homologous protein. The activated ERS pathway further promotes hepatocyte apoptosis. Promoting SGs synthesis can inhibit the level of hepatocyte apoptosis by inhibiting the ATF4-mediated ERS pathway.
- Citation: Li WY, Yang F, Li X, Wang LW, Wang Y. Stress granules inhibit endoplasmic reticulum stress-mediated apoptosis during hypoxia-induced injury in acute liver failure. World J Gastroenterol 2023; 29(8): 1315-1329
- URL: https://www.wjgnet.com/1007-9327/full/v29/i8/1315.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i8.1315
Acute liver failure (ALF) is a common clinical syndrome in intensive care units, with a high mortality due to various causes, such as drugs, toxic exposure, viral hepatitis, etc[1]. ALF can be preliminarily diagnosed in patients with no history of basic liver disease, with rapid deepening of jaundice in a short period of time. It has the typical symptom, such as poor appetite, vomiting, diarrhea, abdominal distension and hepatic encephalopathy. Advanced high bilirubin or aminotransferase, and prothrombin activity of coagulation item ≤ 40% can be the basic diagnosis[2]. For such patients, it is extremely important to be transferred to intensive care unit as soon as possible for mechanical ventilation to improve oxygenation. The comprehensive medical treatment, blood purification (artificial liver) and maintenance of homeostasis in human body should be carried out throughout the treatment. For patients with ALF, liver transplantation is the only completely effective treatment option. However, the development of liver transplantation is limited by shortage of liver source, strict transplantation conditions, huge treatment cost and rejection after transplantation[3].
Endoplasmic reticulum stress (ERS) plays an important role in the regulation of inflammatory response and apoptosis, and severe ERS promotes the occurrence and development of ALF[4]. When cells undergo ERS, they can be induced by type-1 ER transmembrane protein kinase (IRE1), double-stranded RNA-dependent protein kinase-like ER kinase (PERK) and activating transcription factor 6 (ATF6), induce unfolded protein response (UPR)[5]. Unfolded proteins can alter cellular transcription and translation programs to regulate protein synthesis, secretion, and degradation to relieve endoplasmic reticulum stress, thereby protecting cells[6]. When ERS exceeds the controllable range of the body, cells will undergo apoptosis. CCAAT/enhancer-binding protein-homologous protein (CHOP) is a transcription factor that controls gene-encoded components in apoptosis[7]. The activation of ERS of the three pathways will eventually induce the expression of the apoptotic factor CHOP[7]. Among them, PERK can activate eIF2a and further induce the expression of ATF4. ATF4 can promote the activation of CHOP, and the highly expressed CHOP triggers the bax/bad system to activate caspase9 and caspase3, which in turn leads to apoptosis[8].
Stress granules (SGs) refer to the fact that when eukaryotic cells are subjected to various environmental stimuli, such as oxidative stress, heat shock, ultraviolet radiation, and viral infection. Aids in the transport of dense granular material formed into the cytoplasm, which is an adaptive regulation mechanism of cells[9]. SGs are a protective mechanism of cells, which is the product of the host's response to external stress. The production mechanism of SGs is related to neurological diseases[10], viral infections[11], and cancers[12], etc. There is no study on the relationship between SGs, ERS and hepatocyte apoptosis in ALF. This experiment investigates the effect and mechanism of SGs on the apoptosis process induced by ERS during the hypoxic injury of hepatocytes in ALF.
The normal human liver L02 cell line was purchased from the China Center for Type Culture Collection (Wuhan University). DMEM medium and fetal bovine serum (FBS) were purchased from GIBCO (NY, United States). Lipopolysaccharide (LPS), D-galactosamine (D-Gal), arsenite (Ars) and anisomycin were purchased from Sigma (St. Louis, United States). Rabbit anti-human/ mouse G3BP1, T-cell restricted intracellular antigen-1 (TIA-1), ATF4, CHOP, BAX, BCL2, GAPDH antibodies, Cy3-labeled goat anti-rabbit secondary antibody were purchased from Proteintech (Wuhan, China). Rabbit anti-human/ mouse cleaved caspase3 (c-cas3), c-cas9 antibodies were purchased from Cell Signaling Technology (Boston, United States). Goat anti-rabbit fluorescent secondary antibody IRDye800 was purchased from LI-COR Biosciences (Lincoln, United States). Human and mouse lactate dehydrogenase (LDH) and HIF-1α enzyme-linked immunosorbent assay kits were purchased from Elabscience (Wuhan, China). RNAiso Plus, PrimeScript™, RT reagent and SYBR Premix Ex Taq kit were purchased from TaKaRa (Dalian, China). The Annexin V-phycoerythrin/7-aminoactinomycin D (Annexin V-PE/7AAD) apoptosis kit Apoptosis kit was purchased from BD (San Diego, United States). The transfection reagent Lipo200 was purchased from Invitrogen (CA, United States).
After receiving the L02 cells, they were centrifuged to remove the cell cryopreservation solution. The cells were then added DMEM complete medium (containing 10% FBS), and cultured in a 37 °C 5% CO2 constant temperature incubator in a suitable humidity environment. The adherence and growth of the cells were observed under a microscope, and the cells were in the logarithmic growth phase for experiments. The hypoxia-treated cell group was placed in a three-gas incubator at 37 °C with a volume fraction of 95% N2 + a volume fraction of 5% CO2 for hypoxia treatment for 4 h, 8 h, and 12 h, respectively. Hepatocytes treated with hypoxia for 12 h were used as model group. The Hypoxia + Ars group was first treated with Hypoxia for 12 h, followed by hypoxia treatment for 12 h. Hypoxia + anisomycin + siRNA-ATF4 group cells were first transfected with siRNA, then intervened with anisomycin for 12 h, and finally treated with hypoxia for 12 h. The sequence of siRNA-ATF4 was 5'-TCC CTC AGT GCA TAA AGG A-3'. And the sequence of non-targeting control siRNA was 5'-UUC UCC GAA CGU GUC ACG A-3'. The siRNA was transfected into cells using Lipo2000 according to the manufacturer's instructions.
All animal experimental procedures were evaluated and approved by the Laboratory Animal Care and Committee of Renmin Hospital of Wuhan University (WDRM20181018) and followed institutional guidelines as well as ARRIVE guidelines. Thirty male C57BL/6 rats were randomly divided into 3 groups, namely the normal group, the model group, and the Ars group, with 10 rats in each group. All mice were acclimated to feeding for 1 wk before the experiment. Referring to the previous modeling method[13,14], the mice in the model group and Ars group were injected with D-Gal 400 mg/kg and LPS 100 μg/kg intraperitoneally. The mice in Ars group were given Ars (1 mg/kg) intraperitoneal treatment 3 d before modeling, once a day, until 24 h after modeling. The rats in the normal group and the model group were given normal saline by gavage every day. 12 h after modeling, the survival of mice in each group was observed and recorded. Body weights and health of the animals were monitored every other day. If the mice were unable to eat or drink, showed any abnormal behavior, or signs of toxicity, pain, or distress, they would be removed from the study. At the end of the experiment, the mice were euthanized by sodium pentobarbital, and death was confirmed by the absence of a heartbeat. About 1 mL of ocular venous blood was collected. Liver tissue was taken, washed with normal saline, and then stored in -80 °C freezer for tissue homogenate, protein and mRNA extraction. The rest of the liver tissue specimens were fixed with 4% paraformaldehyde, embedded in paraffin, and used for hematoxylin-eosin (HE) and transferase-mediated deoxyuridine triphosphate-biotin nick end labeling (TUNEL) staining.
Referring to the previous method[15], the apoptosis level of L02 cells in each group was detected by flow cytometry using Annexin V-PE/7AAD apoptosis kit. After intervention in each group, 1.0 × 105 cells were added to 400 μL buffer solution for staining. 5 μL of Annexin V-PE and 5 μL of 7AAD were then added to the cell suspension. The cell was incubated in the dark for 15 min at 37 °C. The apoptosis rate of early and late cells was detected by flow cytometry (BD, United States). According to the description in the previous experiments[16], the cell supernatant, homogenates of cells and liver tissues in each group were taken. Then the HIF-1α and LDH detection kits were used to evaluate the content of HIF-1α in cell or tissue homogenates, LDH in cell supernatant or serum according to the kit instructions.
The cells in each group were modeled and intervened by the above method. After 24 h of incubation, they were fixed with cell fixative for 25 min, and then washed with PBS for 3 times. 0.2% Triton was permeabilized for 20 min, and 0.5% BSA was blocked for 30 min. G3BP1 primary antibody (1:1000) was incubated overnight. The next day, the secondary antibody was incubated for 1 h, nuclei were stained with DAPI for 3 min, washed with PBS for 5 times. The anti-fluorescence quencher was added dropwise, and the slides were mounted and observed under an upright microscope.
The total RNA of each group of cells was extracted by RNAiso Plus kit. The cDNA was further amplified using Prime-Script RT reagent kit. The amplified cDNA was subjected to quantitative polymerase chain reaction (qPCR) by real-time PCR (RT-PCR) using SYBR Premix Ex Taq kit. The PCR program was set as: initial 95 °C for 10 s; 95 °C for 5 s and 60 °C for 20 s, 40 cycles. ATF4 primer sequences: forward 5'-TCAAACCTCATGGGTTCTCC-3' and reverse 5'-GTGTCATCCAACGTGGTCAG-3'. GAPDH primer sequences: forward 5'-ACCACAGTCCATGCCATCAC-3', reverse 5'-TCCACCACCCTGTTGCTGTA-3'. The relative gene expression of ATF4 was calculated by 2−ΔΔCT. The above methods refer to previous reports[17].
Referring to the previous experimental method[18], the cells or liver tissues of each group were collected into the centrifuge tube, and were broken under ice bath by ultrasound. After centrifugation at 4 °C, the supernatant was taken. Total protein was determined by bicinchoninic acid method and protein concentration was adjusted. It was then added protein loading buffer, mixed well, and boiled for 10 min. Electrophoresis was performed with 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis. 10 μL sample to each well was then add. Electrophoresis was performed at 80 V and then at a constant current of 200 mA. PVDF membranes were sealed with 5% skim milk powder at room temperature for 1h. Then primary antibody (G3BP1, 1:1,000; TIA-1, 1:1000; ATF4, 1:1000; CHOP, 1:1000; BAX, 1:1000; BCL2, 1:1000; c-cas3, 1:1,000; c-cas9, 1:1000; GAPDH, 1:3000) was used to incubated the membranes overnight at 4 °C. After the membranes were washed, fluorescent secondary antibody (1:10000) was used to incubate at room temperature without light for 1 h. Finally, the membranes were scanned with Odyssey system to detect and compare the relative protein expression levels of each group.
The levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST) and total bilirubin (TBIL) in serum in each group were detected by automatic biochemical analyzer. Liver tissues fixed by formaldehyde and embedded in paraffin were taken from each group. The paraffin blocks were sectioned. Hematoxylin and eosin were used to stain the slices. The slices were dehydrated and dried in turn. The histopathological changes of liver in each group were observed under inverted microscope. The slices were stained with TUNEL and incubated away from light. After washing with PBS, DAPI was dropped and incubated away from light. Cell morphology was observed under fluorescence microscope, and apoptosis index in each tissue was calculated according to the method described previously[19].
SPSS 19.0 statistical software was used for statistical analysis. All data were expressed as mean ± SD. t-test was used for comparison between two groups, and analysis of variance test was used for comparison between three groups and more than three groups. P < 0.05 means the difference was statistically significant.
The histopathological examination of liver failure revealed massive necrosis and extensive intrahepatic inflammatory reaction, resulting in the embolization of micro vessels and the structural destruction of hepatic sinuses. The microcirculation disturbance could lead to obvious hepatic perfusion obstruction, reflecting ischemic hypoxic injury[20]. The ischemia-hypoxia and ischemia-reperfusion (IR) could further activate local inflammatory response or aggravate local perfusion disorders through platelet activation pathway[21].
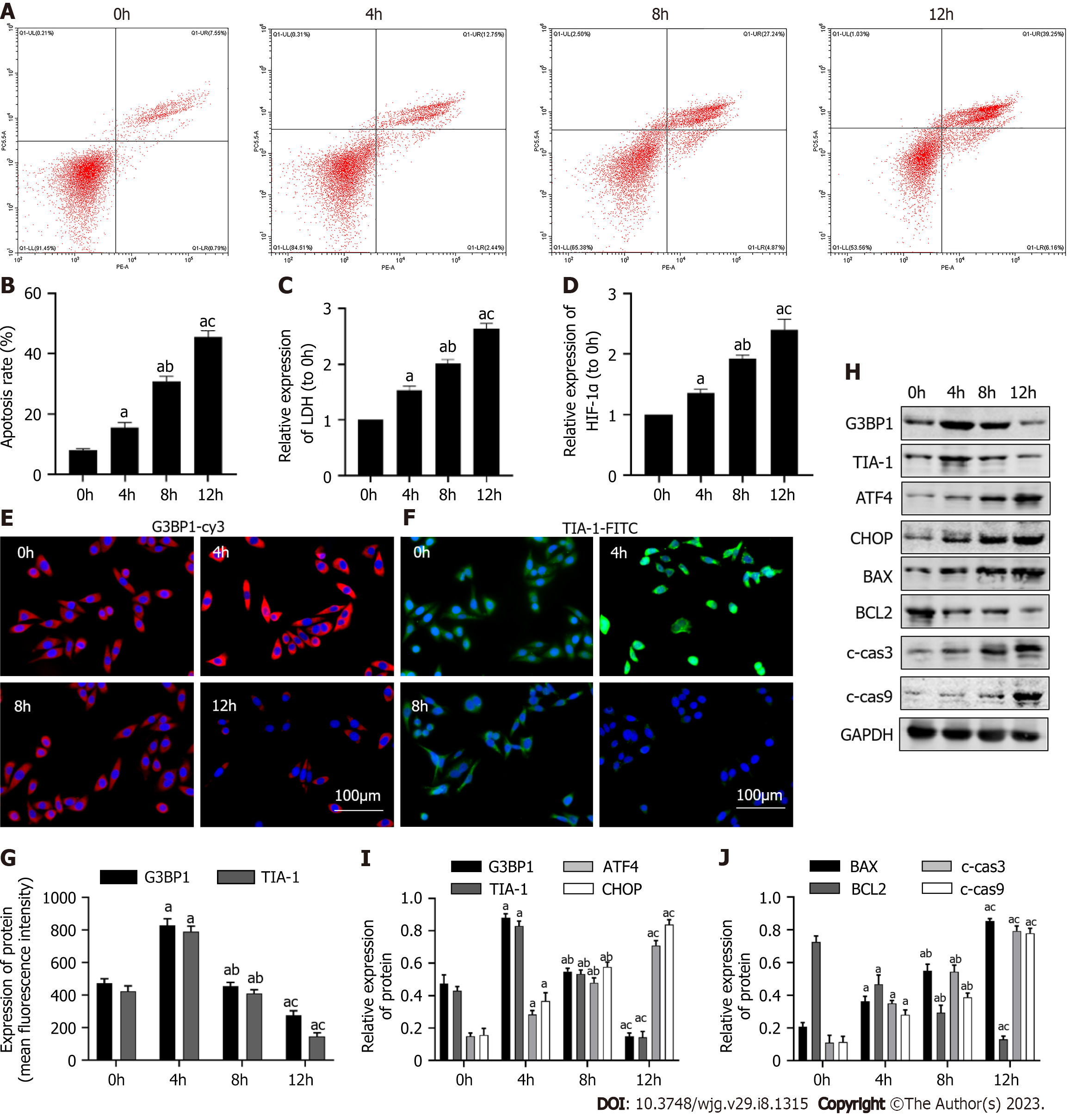
In this part, the model of hepatocyte hypoxia in vitro was used to simulate the ischemic hypoxia injury during ALF. It was firstly observed how the levels of SGs, ERS and apoptosis changed over time. As shown in Figure 1A-D, compared with the normal group, the apoptosis rate, HIF-1α and LDH contents in the hypoxia group for 4 h were increased (P < 0.05). Compared with the 4 h group, the apoptosis rate and the contents of HIF-1α and LDH in hypoxia group for 8 h and 12 h was furtherly increased (P < 0.05). As shown in Figure 1E-G, compared with the normal group, the expression levels of G3BP1 and TIA-1, the marker molecules of SGs, were increased in the hypoxia group for 4 h (P < 0.05). The expression of G3BP1 decreased in the hypoxia groups of 8 h and 12 h compared with the hypoxia group of 4 h (P < 0.05). As shown in Figure 1H-J, compared with the normal group, the protein expression levels of ATF4, CHOP, BAX, c-cas3 and c-cas9 in the hypoxia group for 4 h increased (P < 0.05), the expression of G3BP1, TIA-1, BCL2 was decreased (P < 0.05). After 8 h and 12 h hypoxia, the protein expression levels of ATF4, CHOP, BAX, c-cas3 and c-cas9 were further increased (P < 0.05), the expression of G3BP1, TIA-1, BCL2 was further decreased (P < 0.05). In conclusion, after 12 h hypoxia treatment, ERS and apoptosis levels of L02 cells were the highest, while SGs content was the lowest. Therefore, the cells treated with hypoxia for 12 h were used as cell models for subsequent research, which was consistent with reported studies[22].
Arsenite can induce SGs formation by promoting e-IF2α phosphorylation[23], but arsenite has certain hepatotoxicity[24,25]. Refer to the reported studies, the 10 μmol/L intervention dose was used[25,26]. In this part, the Ars intervention group was set to observe that Ars could induce the formation of SGs without causing hepatocyte damage and related molecular changes.
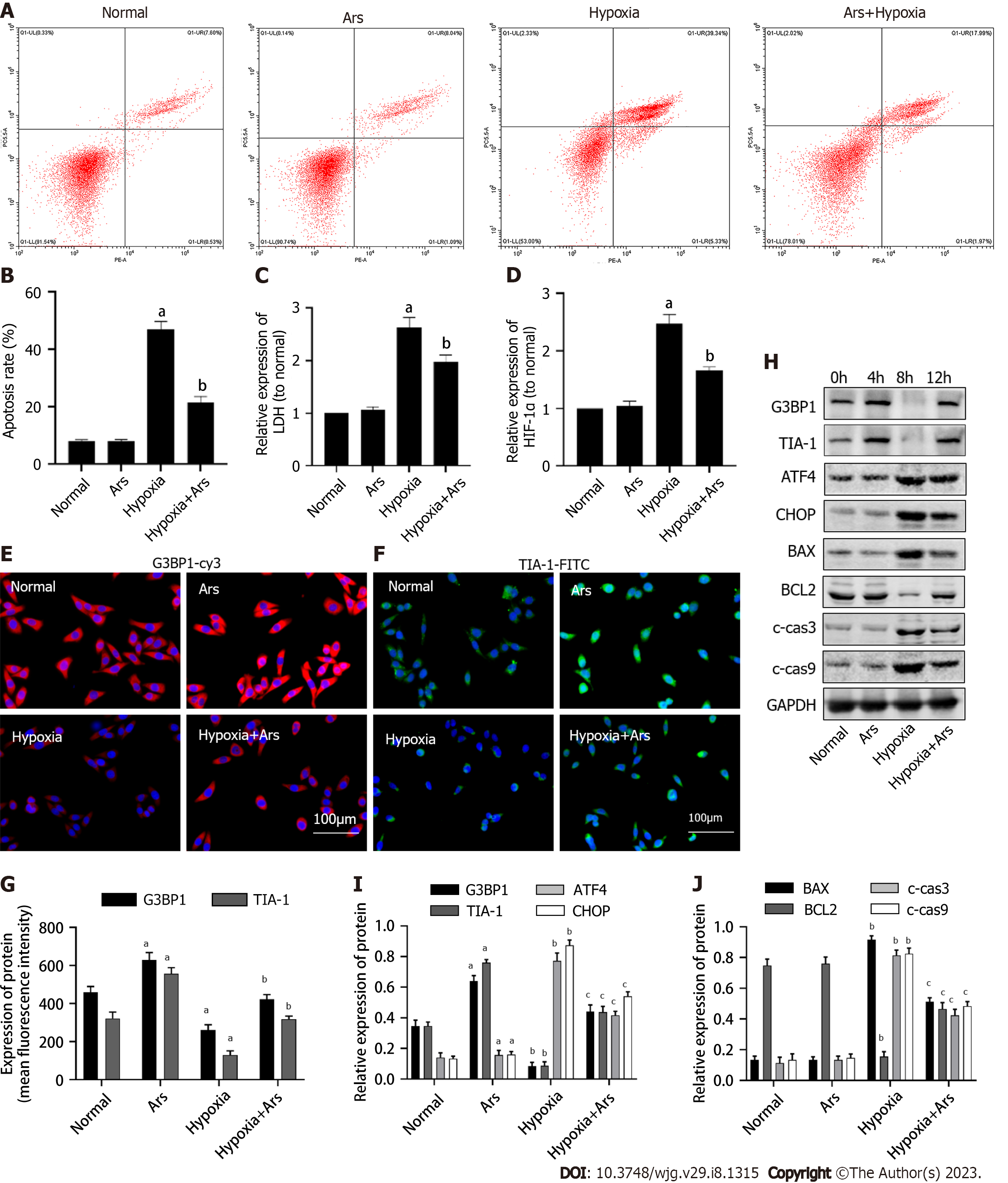
As shown in Figure 2, we first verified the effect of SGs agonist Ars on normal L02 cells. Compared with the normal group, the expression level of G3BP1 was increased in the Ars group, while the level of ERS and apoptosis did not change significantly. Subsequently, we used Ars to intervene the L02 hepatocyte model treated with 12 h hypoxia. As shown in Figure 2A-D, compared with the Hypoxia group, the apoptosis rate, HIF-1α and LDH contents in cells in the Hypoxia + Ars group were decreased (P < 0.05). As shown in Figure 2E-G, compared with the Hypoxia group, the expression levels of G3BP1 and TIA-1 were increased in the Hypoxia + Ars group (P < 0.05). As shown in Figure 2H-J, compared with the Hypoxia group, the protein expression levels of ATF4, CHOP, BAX, c-cas3 and c-cas9 in the Hypoxia + Ars group was decreased (P < 0.05), the expression of G3BP1, TIA-1, BCL2 was increased (P < 0.05).
ATF4 plays a key role in the process of ERS, which negatively regulates Bcl-2 by promoting the expression of CHOP, leading to the transport of Bax from cytoplasm to mitochondria and the initiation of mitochondrial apoptosis pathway[27]. When excessive ERS occurs, PERK, IRE1, and ATF4 activate and activate the corresponding downstream factors, and up-regulate the expression of ATF4 at the transcriptional and translational levels, resulting in the destruction of the endoplasmic reticulum membrane, the efflux of Ca2+, and the occurrence of apoptosis[28]. The knockdown of ATF4 and the SGs inhibitor anisomycin showed an antagonistic effect on the protective cells. In this part, the groups were set to show that SGs can affect hepatocyte injury through the ATF4-mediated ERS pathway.
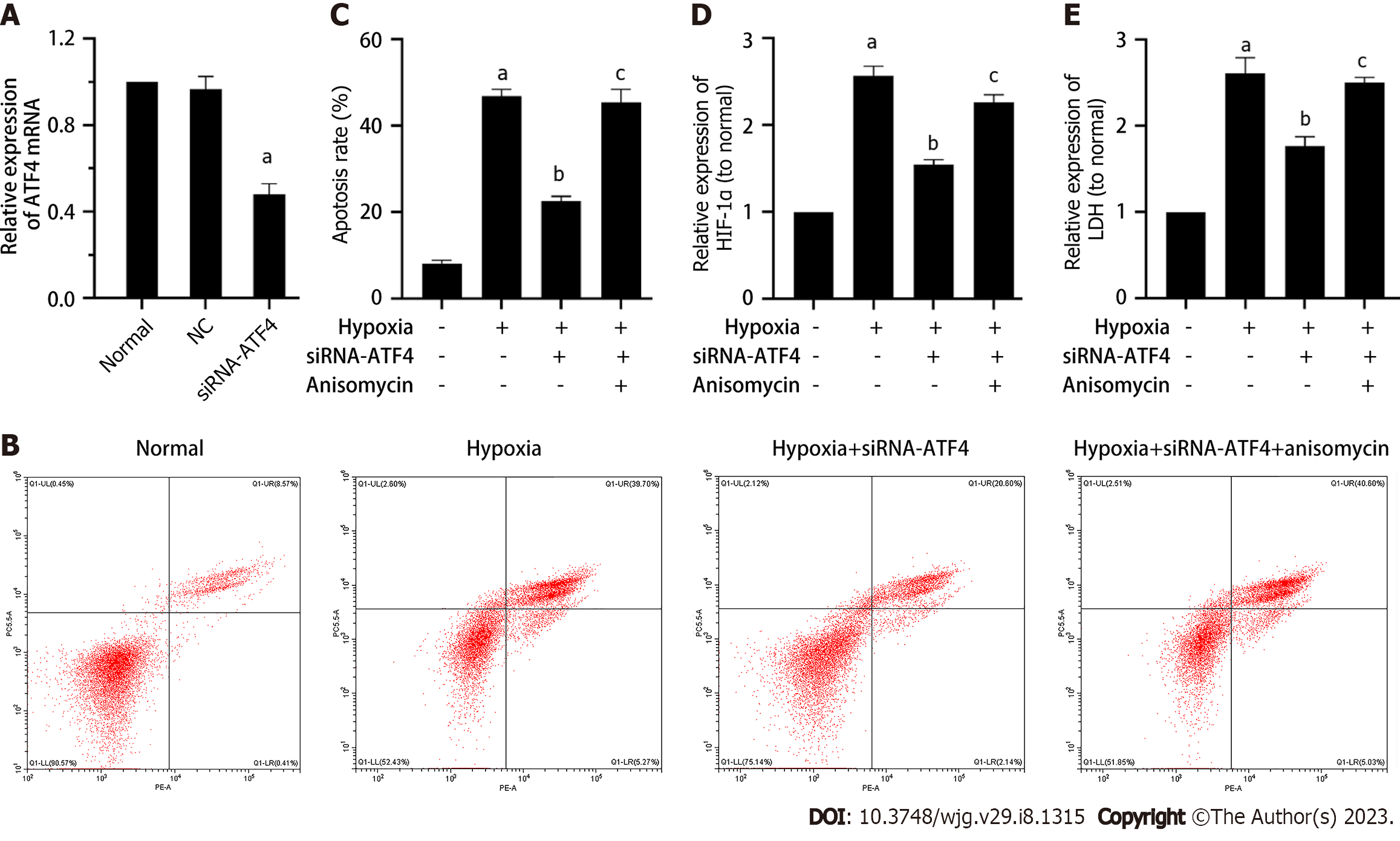
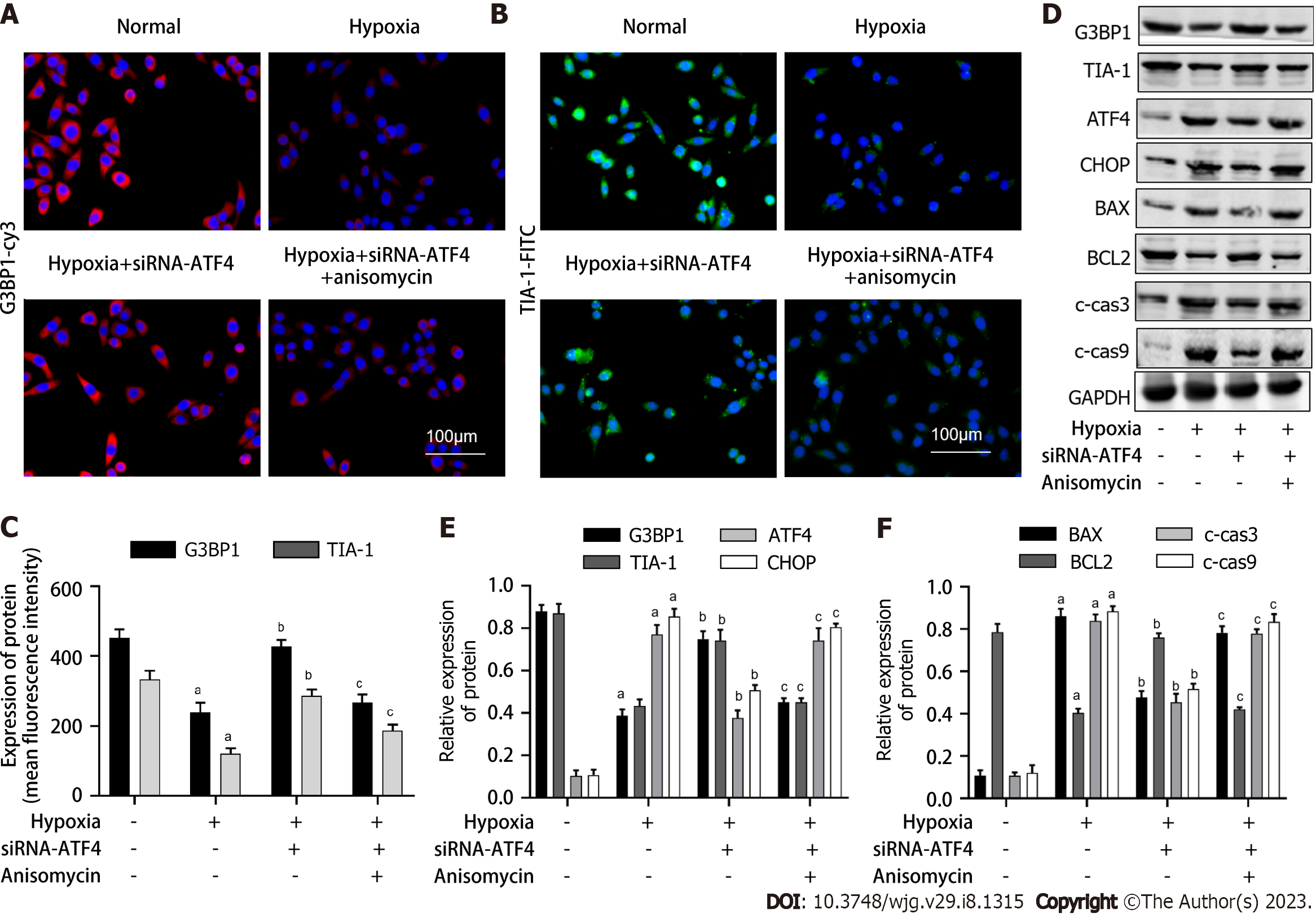
We first verified the intervention effect of siRNA-ATF4. As shown in Figure 3A, compared with the normal group, the level of ATF4 mRNA in the normal control group did not change, while the level of ATF4 mRNA in the siRNA-ATF4 group decreased significantly (P < 0.05). As shown in Figure 3B-E, compared with the Hypoxia group, the apoptosis rate, HIF-1α and LDH contents in cells of the Hypoxia + siRNA-ATF4 group were decreased (P < 0.05). Compared with the Hypoxia + siRNA-ATF4, the apoptosis rate, HIF-1 α and LDH contents in cells of the Hypoxia + siRNA-ATF4 + anisomycin group were increased (P < 0.05). As shown in Figure 4A-C, compared with the Hypoxia group, the expression of G3BP1 and TIA-1 in Hypoxia + siRNA-ATF4 group was increased (P < 0.05). Compared with the Hypoxia + siRNA-ATF4, the expression of G3BP1 and TIA-1 in Hypoxia + siRNA-ATF4 + anisomycin group was decreased (P < 0.05). As shown in Figure 4C-F, compared with the Hypoxia group, the protein expression levels of ATF4, CHOP, BAX, c-cas3 and c-cas9 in the Hypoxia + siRNA-ATF4 group was decreased (P < 0.05), the expression of G3BP1, TIA-1, BCL2 was increased (P < 0.05). Compared with the Hypoxia + siRNA-ATF4 group, the protein expression levels of ATF4, CHOP, BAX, c-cas3 and c-cas9 in the Hypoxia + siRNA-ATF4 + anisomycin group was increased (P < 0.05), the expression of BCL2 was decreased (P < 0.05).
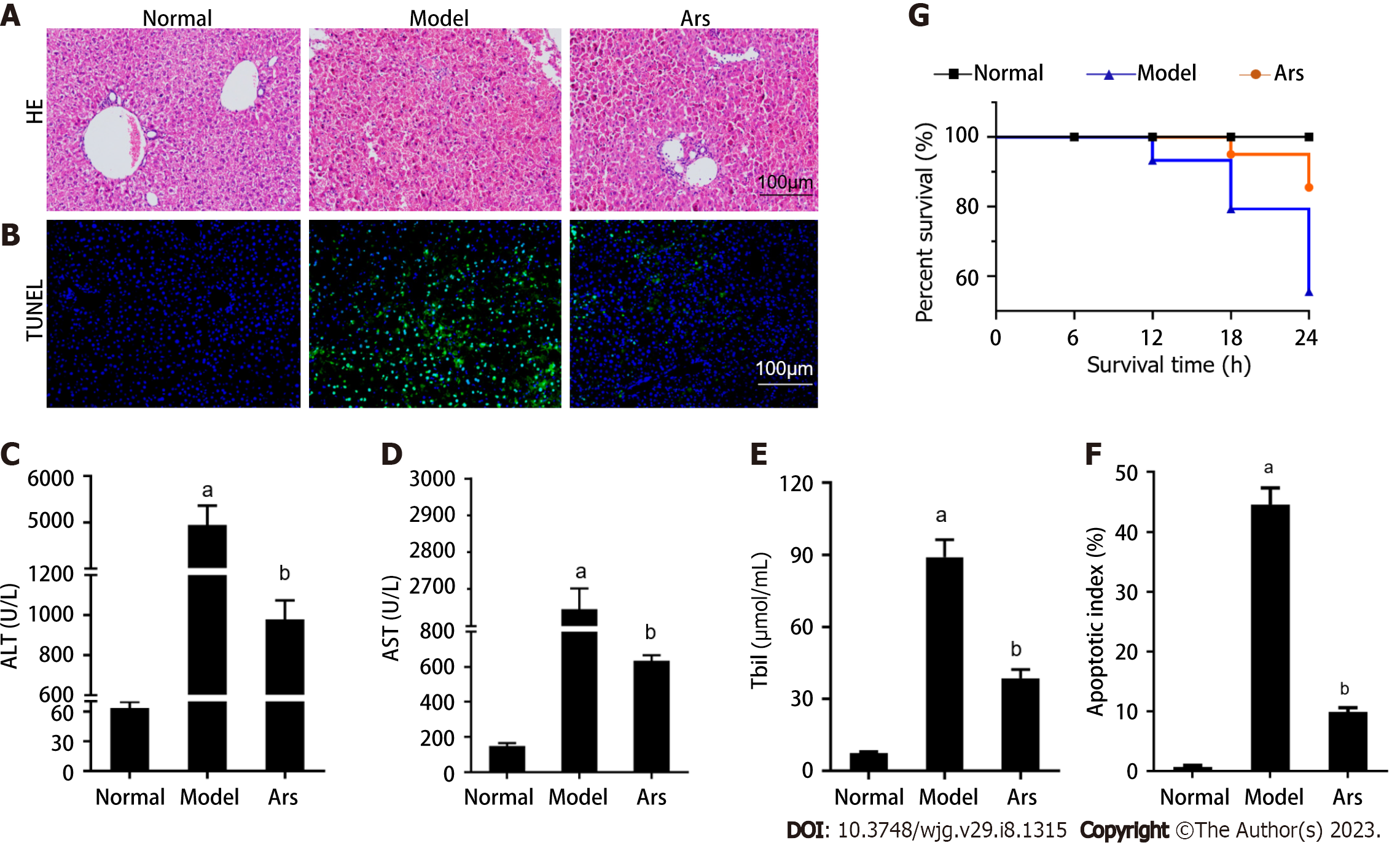
In the in vivo experiment, we first detected whether the ALF mouse model was successfully established, and then detected the effects of Ars on liver pathological changes and serum biochemical indexes. As shown in Figure 5A, the liver lobules in the normal group were clearly structured and the hepatocytes were neatly arranged. The lobule structure of liver tissue in ALF group was not clear, hepatocyte was necrotic around with inflammatory cells infiltrate. Compared with ALF model group, the hepatic lobule structure in Ars group was clearer and the infiltration of inflammatory cells was reduced. As shown in Figure 5B and F, compared with the normal group, the apoptosis level in liver tissues of mice in model group was significantly increased (P < 0.05). After Ars intervention, apoptosis level of liver tissue was significantly reduced (P < 0.05). As shown in Figure 5C-E, the serum levels of ALT, AST and TBIL in model group were higher than those in normal group (P < 0.05). Compared with model group, ALT, AST and TBIL levels in Ars group were significantly decreased (P < 0.05). In addition, as shown in Figure 5G, the 24 h survival rate of mice was observed in each group. The results showed that 90% of mice survived in Ars group, whereas only 70.0% in model group.
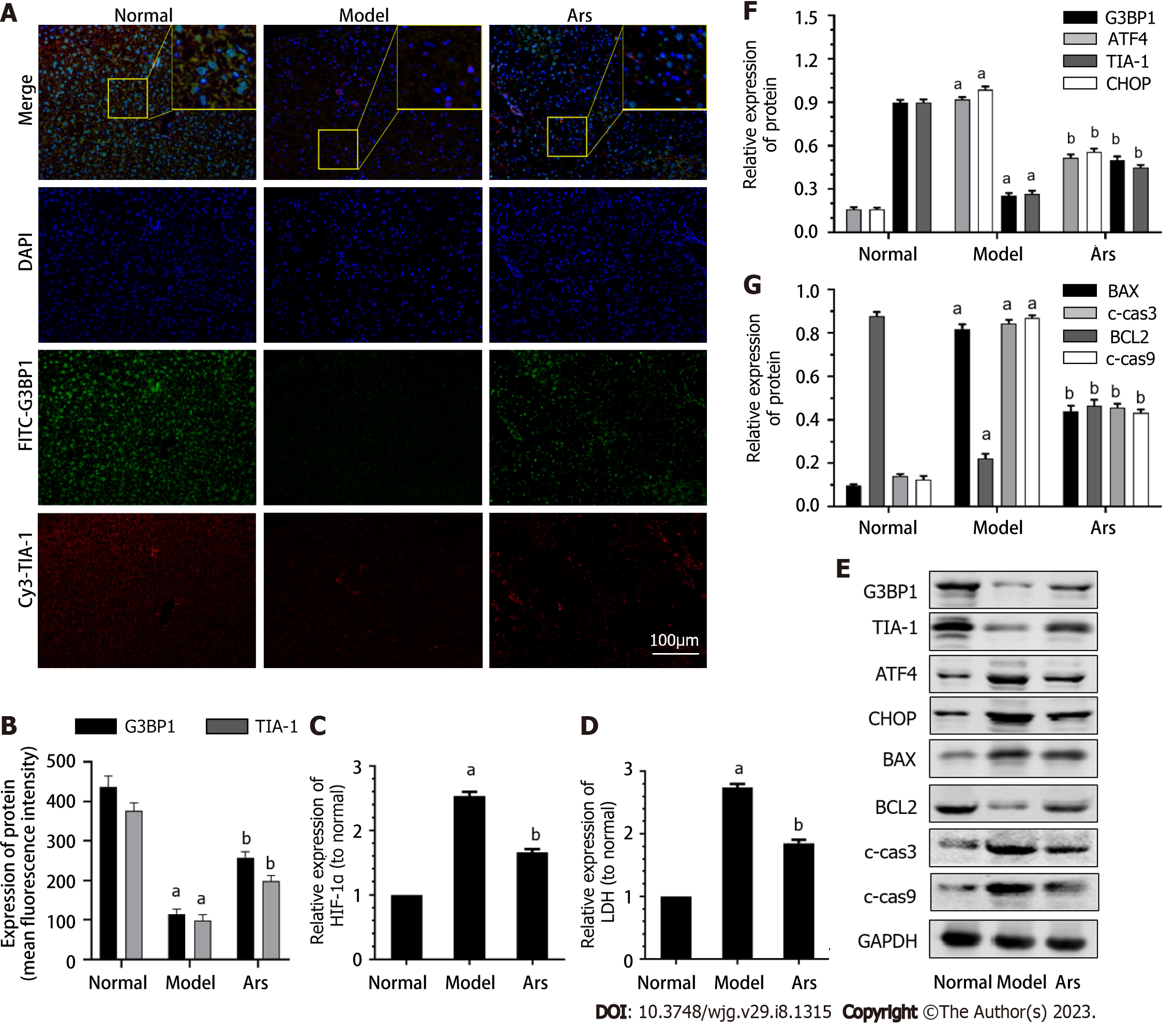
As shown in Figure 6A and B, compared with the normal group, the expression of G3BP1 and TIA-1 in model group was decreased (P < 0.05). Compared with the model group, the expression of G3BP1 and TIA-1 in Ars group was increased (P < 0.05). As shown in Figure 6C-G, compared with the normal group, the level of HIF-1α and LDH, the protein expression of ATF4, CHOP, BAX, c-cas3 and c-cas9 in the model was increased (P < 0.05), the expression of G3BP1, TIA-1, BCL2 was decreased (P < 0.05). Compared with the model group, the level of HIF-1α and LDH, the protein expression of protein expression levels of ATF4, CHOP, BAX, c-cas3 and c-cas9 in the Ars group was decreased (P < 0.05), the expression of BCL2 was increased (P < 0.05).
Liver is very sensitive to ischemic hypoxic injury because of its high aerobic tissue and metabolism. Ischemia-hypoxia and IR injury induce a large number of ERS and mitochondrial apoptosis pathway. ERS can be induced under ischemia and hypoxia through HIF-1α[29,30]. The complex functions of ERS in hepatocytes can regulate inflammation, oxidative stress, etc., which are closely related to the pathogenesis of liver failure. A large number of experimental studies have confirmed that ERS occurs in the liver of various types of ALF animal models. In the liver of ALF mice/rats induced by N-acetaminophen (APAP)[31], LPS combined with D-Gal[32], and carbon tetrachloride[33] induced liver, p-eIF2α, ATF4 and CHOP GRP78 markedly increased. The ERS inhibitors 4-phenylbutyric acid[34] and TUDCA[35] can alleviate the up-regulation of endoplasmic reticulum stress markers CHOP, XBP1, p-eIF2α, and reduce inflammation in liver tissue. damage, alleviate the up-regulation of serum transaminases. At the same time, inhibiting of ERS had a protective effect on APAP-induced liver injury, can reduce liver necrosis, and improve mouse survival[36]. It can be seen that ERS-related molecules can be used as new targets, and the development of drugs targeting ERS provides new ideas for the treatment of ALF.
ERS is an important homeostatic device during liver disease progression, which can assist cells to resist stress, but excessive stress can also lead to cell damage. In the early stage of stress, the endoplasmic reticulum reduces the cell load caused by the accumulation of wrong proteins by inhibiting protein synthesis and accelerating protein transport and degradation, and maintains cell stability[37]. However, when the stress intensity continues to increase, ERS will initiate cell apoptosis pathway. Among them, ATF4 induces the production of endoplasmic reticulum oxide protein and activates the inositol triphosphate receptor, which is a key way to induce apoptosis[38].
SGs are formed by RNA and protein aggregates that control RNA metabolism, signaling, and cell survival under stress. When translation is inhibited, polysomes lose their mRNAs, and "naked" mRNAs assemble with SG nucleating proteins for liquid-liquid phase separation[39]. SGs are dynamically complex and variable biomolecular condensates whose composition and structure undergo dramatic changes under different types of stress. The ability of cells to respond rapidly to various environmental stresses[40,41]. Therefore, the dynamic process of SGs and their regulation are crucial for cells to cope with stress. Studies have shown that the process of SGs formation is stalled by the accumulation of translation initiation complexes in response to various stresses[42]. G3BP1 bind mRNA and aggregate to form SGs. While large SGs aggregates are formed by smaller aggregates through post-translational modification and microtubule transport[43]. Another typical SGs maker, TIA-1 RNA-binding protein packet TIA-1 binds to mRNA, causing mRNA to stop translation and aggregate to form SGs[44,45]. Therefore, G3BP1 and TIA-1 were selected as markers for SGs in this study. Therefore, G3BP1 and TIA-1 were selected as markers for SGs in this study.
There is no relevant report on the relationship between SGs, ERS and hepatocyte apoptosis in the process of hepatocyte ischemia and hypoxia for ALF. In the in vitro study, we firstly observed how the levels of SGs, ERS and apoptosis changed over time. With the prolongation of hypoxia time, the levels of ERS and apoptosis in hepatocytes increased. The level of SGs increased at 4h and then decreased. Therefore, hepatocyte treated with hypoxia for 12 h were used as cell models for subsequent research. This suggested that SGs were stress-increased in the early stage of hepatocyte hypoxia to protect cells from damage. However, with the prolongation of hypoxia time, the production of SGs would decrease, and the effect of protecting hepatocytes will be weakened. Then the effects of SGs on ERS and apoptosis in hepatocyte hypoxia model was observed. Compared with the Hypoxia group, the apoptosis rate and ERS level decreased in SGs hepatocyte Ars treated group. However, Ars could elevate the level of SGs. In the next, it was verified the effect of SGs on apoptosis level of hepatocyte hypoxia model through ERS. It was verified the intervention effect of siRNA-ATF4. Compared with the normal group, the level of ATF4 mRNA in the siRNA-ATF4 group was decreased significantly. Compared with the Hypoxia group, the apoptosis rate, HIF-1α and LDH contents in cells, the level of ERS was decreased in the siRNA-ATF4 treatment group. Moreover, on the basis of siRNA-ATF4 intervention group, it was found that the apoptosis rate, HIF-1α and LDH contents in cells, the level of ERS was decreased.
According to previous studies, the criteria for success in animal modeling of ALF include liver histopathological damage, serological changes, especially elevated levels of transaminase and bilirubin[13,16,46]. In this study, LPS injection combined with D-Gal simulates acute inflammatory liver injury model, which is widely accepted and used to explore and develop new liver protective reagents for inflammatory liver injury[47]. LPS is in the outer membrane of the gram-negative bacteria[48], and can combine with myeloid differentiation factor 2 and cluster of differentiation 14 to form a complex. This complex is recognized by the toll-like receptor 4 on the membrane of Kupffer cells (KCs) in the liver. KCs can produce the inflammatory mediators, such as TNF-α, IL-1β, IL-6, etc., leading to liver cell damage[49,50]. However, LPS has a low specificity for liver injury, so it is often combined with DGal to establish an animal model of acute inflammatory liver injury to simulate human hepatitis[48]. Studies have shown that D-Gal can inhibit protein synthesis by depletion of uridine triphosphate through the galactose pathway, and reactive oxygen species production can induce liver injury. Therefore, D-Gal can be used as a sensitizer in LPS-induced liver injury[51]. In the course of occurrence and development of liver failure, a large number of liver cell death is the most core event.
So, In the in vivo experiment, it was first detected whether the ALF mouse model was successfully established. HE staining showed that the liver tissue structure of mice in the normal group was regular, and the liver cells were neatly arranged in the hepatic lobules. No necrosis of liver cells and inflammatory cell infiltration. The liver lobule structure of the ALF model group was destroyed by LPS combined with D-Gal, accompanied by a large number of necrotic liver cells and infiltrating inflammatory cells. Compared with normal group, ALT, AST and TBIL levels in serum of model group were increased. The above studies indicate that the mouse model of ALF has been successfully constructed in this study, which is consistent with previous reports[13,16]. After Ars intervention, the degree of infiltration of necrotic liver cells and inflammatory cells in mouse liver tissue was reduced, and the levels of ALT, AST and TBIL in serum were decreased.
Moreover, compared with the normal group, the apoptosis level in liver tissues of mice in model group was significantly increased. The expression of G3BP1 in model group was decreased. The expression of G3BP1 in model group was decreased. Moreover, compared with the normal group, the level of HIF-1α and LDH, the protein expression of ATF4, CHOP, BAX, c-cas3 and c-cas9 in the model was increased, the expression of BCL2 was decreased. Compared with the model group, the level of HIF-1α and LDH, the protein expression of protein expression levels of ATF4, CHOP, BAX, c-cas3 and c-cas9 in the Ars group was decreased, the expression of BCL2 was increased.
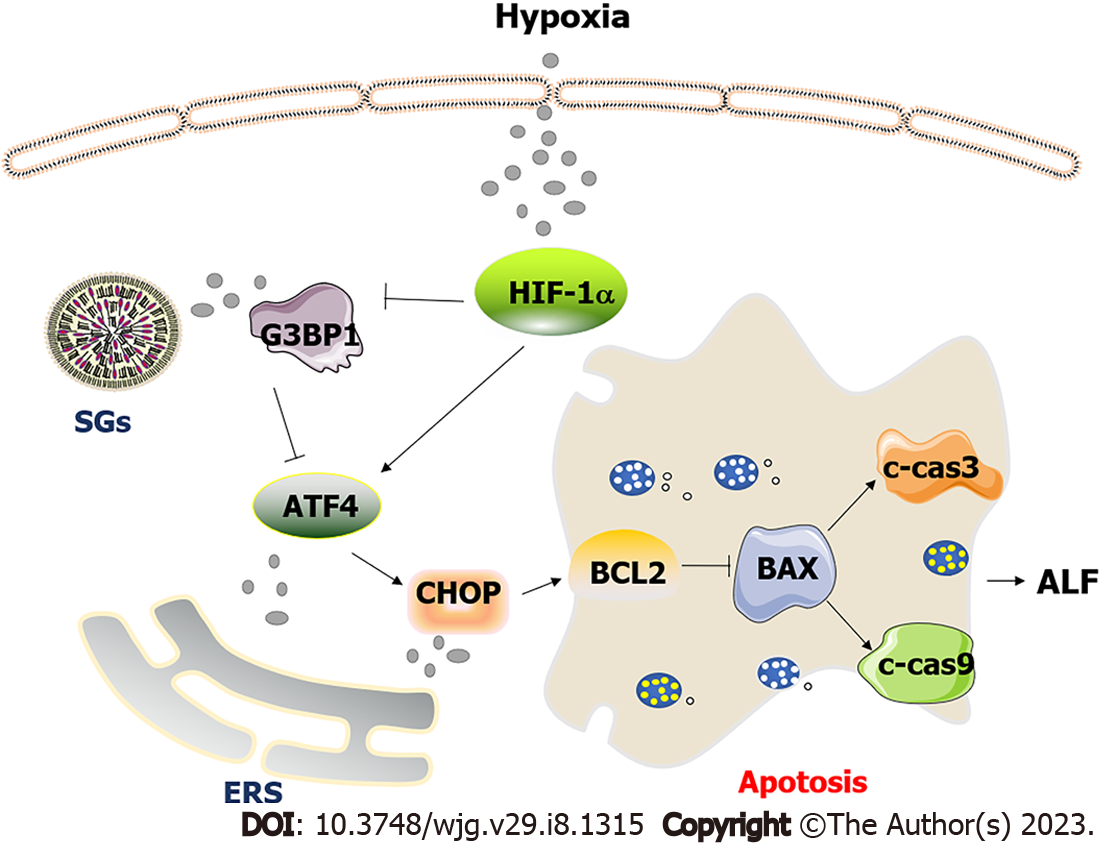
In conclusion, as shown in Figure 7, hepatocytes were damaged by hypoxia and ischemia injury in the process of ALF. At this time, the content of HIF-1α in cells increased, which inhibited the formation of SGs mediated by G3BP1 and promoted the expression of ERS marker molecules ATF4 and CHOP. The activated ERS pathway further promotes hepatocyte apoptosis. Promoting SGs synthesis can inhibit the level of hepatocyte apoptosis by inhibiting the ATF4-mediated ERS pathway. However, the inhibition of ERS-mediated hepatocyte apoptosis pathway by SGs still needs to be further studied. Despite the use of Ars and anisomycin in the intervention of SGs, there is still no direct evidence to prove the influence of SGs on hepatocyte apoptosis. In the next studies, we will use more appropriate methods to directly detect the influence of SGs on hepatocyte. At the same time, this paper provides potential targets and ideas for clinical treatment of ALF.
There is no relevant report on the relationship between stress granules (SGs), endoplasmic reticulum stress (ERS) and hepatocyte apoptosis in the process of hepatocyte ischemia and hypoxia for acute liver failure (ALF).
This paper provides potential targets and ideas for clinical treatment of ALF.
This study was to investigate whether SGs could protect hepatocytes from hypoxia-induced damage during ALF by reducing ERS mediated apoptosis.
The agonist of SGs, arsenite (Ars) was used to intervene hypoxia-induced hepatocyte injury cellular model and ALF mice models. Further, the siRNA of ATF4 and SGs inhibitor anisomycin was then used to intervene in cell models.
In the in vitro study, we firstly observed how the levels of SGs, ERS and apoptosis changed over time. With the prolongation of hypoxia time, the levels of ERS and apoptosis in hepatocytes increased. The level of SGs increased at 4h and then decreased. Therefore, hepatocyte treated with hypoxia for 12 h were used as cell models for subsequent research. This suggested that SGs were stress-increased in the early stage of hepatocyte hypoxia to protect cells from damage. However, with the prolongation of hypoxia time, the production of SGs would decrease, and the effect of protecting hepatocytes will be weakened. Then the effects of SGs on ERS and apoptosis in hepatocyte hypoxia model was observed. Compared with the Hypoxia group, the apoptosis rate and ERS level decreased in SGs hepatocyte Ars treated group. However, Ars could elevate the level of SGs. In the next, it was verified the effect of SGs on apoptosis level of hepatocyte hypoxia model through ERS. It was verified the intervention effect of siRNA-ATF4. Compared with the normal group, the level of ATF4 mRNA in the siRNA-ATF4 group was decreased significantly. Compared with the Hypoxia group, the apoptosis rate, HIF-1α and lactate dehydrogenase (LDH) contents in cells, the level of ERS was decreased in the siRNA-ATF4 treatment group. Moreover, on the basis of siRNA-ATF4 intervention group, it was found that the apoptosis rate, HIF-1α and LDH contents in cells, the level of ERS was decreased. In the in vivo experiment, it was first detected whether the ALF mouse model was successfully established. HE staining showed that the liver tissue structure of mice in the normal group was regular, and the liver cells were neatly arranged in the hepatic lobules. No necrosis of liver cells and inflammatory cell infiltration. The liver lobule structure of the ALF model group was destroyed by LPS combined with D-Gal, accompanied by a large number of necrotic liver cells and infiltrating inflammatory cells. Compared with normal group, alanine aminotransferase (ALT), aspartate aminotransferase (AST) and total bilirubin (TBIL) levels in serum of model group were increased. The above studies indicate that the mouse model of ALF has been successfully constructed in this study, which is consistent with previous reports. After Ars intervention, the degree of infiltration of necrotic liver cells and inflammatory cells in mouse liver tissue was reduced, and the levels of ALT, AST and TBIL in serum were decreased.
Hepatocytes were damaged by hypoxia and ischemia injury in the process of ALF. At this time, the content of HIF-1α in cells increased, which inhibited the formation of SGs mediated by G3BP1 and promoted the expression of ERS marker molecules ATF4 and CHOP. The activated ERS pathway further promotes hepatocyte apoptosis. Promoting SGs synthesis can inhibit the level of hepatocyte apoptosis by inhibiting the ATF4-mediated ERS pathway.
SGs could protect hepatocytes from hypoxia-induced damage during ALF by reducing ERS-mediated apoptosis.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: He YH, China; Xie Y, China S-Editor: Gong ZM L-Editor: A P-Editor: Chen YX
| 1. | Nanchal R, Subramanian R, Karvellas CJ, Hollenberg SM, Peppard WJ, Singbartl K, Truwit J, Al-Khafaji AH, Killian AJ, Alquraini M, Alshammari K, Alshamsi F, Belley-Cote E, Cartin-Ceba R, Dionne JC, Galusca DM, Huang DT, Hyzy RC, Junek M, Kandiah P, Kumar G, Morgan RL, Morris PE, Olson JC, Sieracki R, Steadman R, Taylor B, Alhazzani W. Guidelines for the Management of Adult Acute and Acute-on-Chronic Liver Failure in the ICU: Cardiovascular, Endocrine, Hematologic, Pulmonary, and Renal Considerations. Crit Care Med. 2020;48:e173-e191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 74] [Article Influence: 14.8] [Reference Citation Analysis (1)] |
| 2. | European Association for the Study of the Liver; Clinical Practice Guidelines Panel; Julia Wendon; Panel Members; Cordoba J, Dhawan A, Larsen FS, Manns M, Samuel D, Simpson KJ, Yaron I; EASL Governing Board representative, Bernardi M. EASL Clinical Practical Guidelines on the management of acute (fulminant) liver failure. J Hepatol. 2017;66:1047-1081. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 417] [Cited by in RCA: 621] [Article Influence: 77.6] [Reference Citation Analysis (1)] |
| 3. | Trovato FM, Rabinowich L, McPhail MJW. Update on the management of acute liver failure. Curr Opin Crit Care. 2019;25:157-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 4. | Liu Y, Pan X, Li S, Yu Y, Chen J, Yin J, Li G. Endoplasmic reticulum stress restrains hepatocyte growth factor expression in hepatic stellate cells and rat acute liver failure model. Chem Biol Interact. 2017;277:43-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 5. | Marciniak SJ, Chambers JE, Ron D. Pharmacological targeting of endoplasmic reticulum stress in disease. Nat Rev Drug Discov. 2022;21:115-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 303] [Article Influence: 75.8] [Reference Citation Analysis (0)] |
| 6. | Hetz C, Zhang K, Kaufman RJ. Mechanisms, regulation and functions of the unfolded protein response. Nat Rev Mol Cell Biol. 2020;21:421-438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 499] [Cited by in RCA: 1608] [Article Influence: 321.6] [Reference Citation Analysis (0)] |
| 7. | Hu H, Tian M, Ding C, Yu S. The C/EBP Homologous Protein (CHOP) Transcription Factor Functions in Endoplasmic Reticulum Stress-Induced Apoptosis and Microbial Infection. Front Immunol. 2018;9:3083. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 330] [Cited by in RCA: 789] [Article Influence: 131.5] [Reference Citation Analysis (0)] |
| 8. | Rana SVS. Endoplasmic Reticulum Stress Induced by Toxic Elements-a Review of Recent Developments. Biol Trace Elem Res. 2020;196:10-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 94] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 9. | Anderson P, Kedersha N. RNA granules: post-transcriptional and epigenetic modulators of gene expression. Nat Rev Mol Cell Biol. 2009;10:430-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 621] [Cited by in RCA: 697] [Article Influence: 43.6] [Reference Citation Analysis (0)] |
| 10. | Wolozin B, Ivanov P. Stress granules and neurodegeneration. Nat Rev Neurosci. 2019;20:649-666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 380] [Cited by in RCA: 499] [Article Influence: 83.2] [Reference Citation Analysis (0)] |
| 11. | Eiermann N, Haneke K, Sun Z, Stoecklin G, Ruggieri A. Dance with the Devil: Stress Granules and Signaling in Antiviral Responses. Viruses. 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 90] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 12. | Asadi MR, Rahmanpour D, Moslehian MS, Sabaie H, Hassani M, Ghafouri-Fard S, Taheri M, Rezazadeh M. Stress Granules Involved in Formation, Progression and Metastasis of Cancer: A Scoping Review. Front Cell Dev Biol. 2021;9:745394. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 13. | Wang Y, Li X, Chen Q, Jiao F, Shi C, Pei M, Wang L, Gong Z. Histone Deacetylase 6 Regulates the Activation of M1 Macrophages by the Glycolytic Pathway During Acute Liver Failure. J Inflamm Res. 2021;14:1473-1485. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 14. | Wang Y, Zhang H, Chen Q, Jiao F, Shi C, Pei M, Lv J, Wang L, Gong Z. TNF-α/HMGB1 inflammation signalling pathway regulates pyroptosis during liver failure and acute kidney injury. Cell Prolif. 2020;53:e12829. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 153] [Article Influence: 30.6] [Reference Citation Analysis (0)] |
| 15. | Wang Y, Yang F, Jiao FZ, Chen Q, Zhang WB, Wang LW, Gong ZJ. Modulations of Histone Deacetylase 2 Offer a Protective Effect through the Mitochondrial Apoptosis Pathway in Acute Liver Failure. Oxid Med Cell Longev. 2019;2019:8173016. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 16. | Wang Y, Chen Q, Jiao F, Shi C, Pei M, Wang L, Gong Z. Histone deacetylase 2 regulates ULK1 mediated pyroptosis during acute liver failure by the K68 acetylation site. Cell Death Dis. 2021;12:55. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 40] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 17. | Wang Y, Chen H, Chen Q, Jiao FZ, Zhang WB, Gong ZJ. The Protective Mechanism of CAY10683 on Intestinal Mucosal Barrier in Acute Liver Failure through LPS/TLR4/MyD88 Pathway. Mediators Inflamm. 2018;2018:7859601. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 18. | Wang Y, Chen Q, Shi C, Jiao F, Gong Z. Mechanism of glycyrrhizin on ferroptosis during acute liver failure by inhibiting oxidative stress. Mol Med Rep. 2019;20:4081-4090. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 58] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 19. | Liu Y, Wang Y, Chen Q, Jiao F, Wang L, Gong Z. HDAC2 inhibitor CAY10683 reduces intestinal epithelial cell apoptosis by inhibiting mitochondrial apoptosis pathway in acute liver failure. Histol Histopathol. 2019;34:1173-1184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 20. | Khandoga A, Mende K, Iskandarov E, Rosentreter D, Schelcher C, Reifart J, Jauch KW, Thasler WE. Augmenter of liver regeneration attenuates inflammatory response in the postischemic mouse liver in vivo. J Surg Res. 2014;192:187-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 21. | Nakano Y, Kondo T, Matsuo R, Hashimoto I, Kawasaki T, Kohno K, Myronovych A, Tadano S, Hisakura K, Ikeda O, Watanabe M, Murata S, Fukunaga K, Ohkohchi N. Platelet dynamics in the early phase of postischemic liver in vivo. J Surg Res. 2008;149:192-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 39] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 22. | Xin L, Fan W, Tingting D, Zuoming S, Qiang Z. 4-phenylbutyric acid attenuates endoplasmic reticulum stress-mediated apoptosis and protects the hepatocytes from intermittent hypoxia-induced injury. Sleep Breath. 2019;23:711-717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 23. | Farny NG, Kedersha NL, Silver PA. Metazoan stress granule assembly is mediated by P-eIF2alpha-dependent and -independent mechanisms. RNA. 2009;15:1814-1821. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 109] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 24. | Torres-Avila M, Leal-Galicia P, Sánchez-Peña LC, Del Razo LM, Gonsebatt ME. Arsenite induces aquaglyceroporin 9 expression in murine livers. Environ Res. 2010;110:443-447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 15] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 25. | Bi D, Shi M, Zheng D, Hu Q, Wang H, Peng L, Lou D, Zhang A, Hu Y. Mechanism underlying the targeted regulation of the SOD1 3'UTR by the AUF1/Dicer1/miR-155/SOD1 pathway in sodium arsenite-induced liver injury. Ecotoxicol Environ Saf. 2022;243:113990. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 26. | Samir P, Kesavardhana S, Patmore DM, Gingras S, Malireddi RKS, Karki R, Guy CS, Briard B, Place DE, Bhattacharya A, Sharma BR, Nourse A, King SV, Pitre A, Burton AR, Pelletier S, Gilbertson RJ, Kanneganti TD. DDX3X acts as a live-or-die checkpoint in stressed cells by regulating NLRP3 inflammasome. Nature. 2019;573:590-594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 249] [Cited by in RCA: 296] [Article Influence: 49.3] [Reference Citation Analysis (0)] |
| 27. | Thon M, Hosoi T, Ozawa K. Dehydroascorbic acid-induced endoplasmic reticulum stress and leptin resistance in neuronal cells. Biochem Biophys Res Commun. 2016;478:716-720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 28. | Nie J, Liu A, Tan Q, Zhao K, Hu K, Li Y, Yan B, Zhou L. AICAR activates ER stress-dependent apoptosis in gallbladder cancer cells. Biochem Biophys Res Commun. 2017;482:246-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 29. | Wu W, Li W, Wei J, Wang C, Yao Y, Zhu W, He W, Zhou W, Liu J. Chronic intermittent hypoxia accelerates liver fibrosis in rats with combined hypoxia and nonalcoholic steatohepatitis via angiogenesis rather than endoplasmic reticulum stress. Acta Biochim Biophys Sin (Shanghai). 2019;51:159-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 30. | Zhang X, Du P, Luo K, Li Y, Liu Z, Wang W, Zeng C, Ye Q, Xiao Q. Hypoxia-inducible factor-1alpha protects the liver against ischemia-reperfusion injury by regulating the A2B adenosine receptor. Bioengineered. 2021;12:3737-3752. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 31. | Yan M, Huo Y, Yin S, Hu H. Mechanisms of acetaminophen-induced liver injury and its implications for therapeutic interventions. Redox Biol. 2018;17:274-283. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 468] [Cited by in RCA: 433] [Article Influence: 61.9] [Reference Citation Analysis (0)] |
| 32. | Wang H, Chen L, Zhang X, Xu L, Xie B, Shi H, Duan Z, Zhang H, Ren F. Kaempferol protects mice from d-GalN/LPS-induced acute liver failure by regulating the ER stress-Grp78-CHOP signaling pathway. Biomed Pharmacother. 2019;111:468-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 65] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 33. | Yang J, Zhu D, Wen L, Xiang X, Hu J. Gentianella turkestanerum Showed Protective Effects on Hepatic Injury by Modulating the Endoplasmic Reticulum Stress and NF-κB Signaling Pathway. Curr Mol Med. 2019;19:452-460. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 34. | Urano Y, Oda S, Tsuneyama K, Yokoi T. Comparative hepatic transcriptome analyses revealed possible pathogenic mechanisms of fasiglifam (TAK-875)-induced acute liver injury in mice. Chem Biol Interact. 2018;296:185-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 35. | Torres S, Baulies A, Insausti-Urkia N, Alarcón-Vila C, Fucho R, Solsona-Vilarrasa E, Núñez S, Robles D, Ribas V, Wakefield L, Grompe M, Lucena MI, Andrade RJ, Win S, Aung TA, Kaplowitz N, García-Ruiz C, Fernández-Checa JC. Endoplasmic Reticulum Stress-Induced Upregulation of STARD1 Promotes Acetaminophen-Induced Acute Liver Failure. Gastroenterology. 2019;157:552-568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 93] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 36. | Uzi D, Barda L, Scaiewicz V, Mills M, Mueller T, Gonzalez-Rodriguez A, Valverde AM, Iwawaki T, Nahmias Y, Xavier R, Chung RT, Tirosh B, Shibolet O. CHOP is a critical regulator of acetaminophen-induced hepatotoxicity. J Hepatol. 2013;59:495-503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 156] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 37. | Sozen E, Ozer NK. Impact of high cholesterol and endoplasmic reticulum stress on metabolic diseases: An updated mini-review. Redox Biol. 2017;12:456-461. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 133] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 38. | Malhi H, Kaufman RJ. Endoplasmic reticulum stress in liver disease. J Hepatol. 2011;54:795-809. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 980] [Cited by in RCA: 952] [Article Influence: 68.0] [Reference Citation Analysis (0)] |
| 39. | Rehbein U, Prentzell MT, Cadena Sandoval M, Heberle AM, Henske EP, Opitz CA, Thedieck K. The TSC Complex-mTORC1 Axis: From Lysosomes to Stress Granules and Back. Front Cell Dev Biol. 2021;9:751892. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 32] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 40. | Sidibé H, Vande Velde C. RNA Granules and Their Role in Neurodegenerative Diseases. Adv Exp Med Biol. 2019;1203:195-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 41. | Buchan JR, Parker R. Eukaryotic stress granules: the ins and outs of translation. Mol Cell. 2009;36:932-941. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1001] [Cited by in RCA: 1144] [Article Influence: 76.3] [Reference Citation Analysis (0)] |
| 42. | Panas MD, Ivanov P, Anderson P. Mechanistic insights into mammalian stress granule dynamics. J Cell Biol. 2016;215:313-323. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 242] [Cited by in RCA: 285] [Article Influence: 35.6] [Reference Citation Analysis (0)] |
| 43. | McCormick C, Khaperskyy DA. Translation inhibition and stress granules in the antiviral immune response. Nat Rev Immunol. 2017;17:647-660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 270] [Article Influence: 33.8] [Reference Citation Analysis (0)] |
| 44. | Khong A, Matheny T, Jain S, Mitchell SF, Wheeler JR, Parker R. The Stress Granule Transcriptome Reveals Principles of mRNA Accumulation in Stress Granules. Mol Cell. 2017;68:808-820.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 588] [Cited by in RCA: 536] [Article Influence: 67.0] [Reference Citation Analysis (0)] |
| 45. | Ripin N, Parker R. Are stress granules the RNA analogs of misfolded protein aggregates? RNA. 2022;28:67-75. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 40] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 46. | Jaeschke H, Akakpo JY, Umbaugh DS, Ramachandran A. Novel Therapeutic Approaches Against Acetaminophen-induced Liver Injury and Acute Liver Failure. Toxicol Sci. 2020;174:159-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 137] [Article Influence: 34.3] [Reference Citation Analysis (0)] |
| 47. | Shang Y, Liu Y, Du L, Wang Y, Cheng X, Xiao W, Wang X, Jin H, Yang X, Liu S, Chen Q. Targeted expression of uncoupling protein 2 to mouse liver increases the susceptibility to lipopolysaccharide/galactosamine-induced acute liver injury. Hepatology. 2009;50:1204-1216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 40] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 48. | Hu X, Yang C, Wang PG, Zhang GL. ADP-heptose: A new innate immune modulator. Carbohydr Res. 2019;473:123-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 49. | Zhang J, Li N, Yang L, Xie H, Yang Y, Wang H, Wu C, Shen T, Zhu Q. Bradykinin contributes to immune liver injury via B2R receptor-mediated pathways in trichloroethylene sensitized mice: A role in Kupffer cell activation. Toxicology. 2019;415:37-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 50. | Li X, Wang J, Song X, Wu H, Guo P, Jin Z, Wang C, Tang C, Wang Y, Zhang Z. Ketamine ameliorates ischemia-reperfusion injury after liver autotransplantation by suppressing activation of Kupffer cells in rats. Can J Physiol Pharmacol. 2018;96:886-892. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 51. | Maes M, Vinken M, Jaeschke H. Experimental models of hepatotoxicity related to acute liver failure. Toxicol Appl Pharmacol. 2016;290:86-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 161] [Article Influence: 17.9] [Reference Citation Analysis (0)] |