Published online Feb 21, 2023. doi: 10.3748/wjg.v29.i7.1157
Peer-review started: October 21, 2022
First decision: November 30, 2022
Revised: December 16, 2022
Accepted: February 14, 2023
Article in press: February 14, 2023
Published online: February 21, 2023
Processing time: 122 Days and 10.1 Hours
Mucosal healing (MH) is vital in maintaining homeostasis within the gut and protecting against injury and infections. Multiple factors and signaling pathways contribute in a dynamic and coordinated manner to maintain intestinal home
Core Tip: Mucosal healing (MH) is vital in maintaining intestinal homeostasis and protecting against infection and injury. MH has emerged as an important clinical criterion in effective treatment of inflammatory bowel disease (IBD). However, there remains a relative lack of therapeutics that can restore MH due to the complexity of the disease and healing processes. Through increased understanding of the molecular mechanisms of MH, tissue damage from IBD may be ameliorated by developing novel therapeutics. Here, we introduce the concept of MH and its relevance in IBD, and discuss the mechanisms of IBD and potential strategies for altering these processes for inducing MH.
- Citation: Otte ML, Lama Tamang R, Papapanagiotou J, Ahmad R, Dhawan P, Singh AB. Mucosal healing and inflammatory bowel disease: Therapeutic implications and new targets. World J Gastroenterol 2023; 29(7): 1157-1172
- URL: https://www.wjgnet.com/1007-9327/full/v29/i7/1157.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i7.1157
Mucosal healing (MH) is a process of wound repair that restores the integrity of damaged epithelial barrier and homeostatic function after an injury compromises barrier integrity[1]. It is a complex process regulated by multiple cell types, through distinct mechanisms in response to highly specific stimuli within multiple signaling and cytokine pathways[1]. For simplification, the MH process is considered to have three phases: Epithelial restitution, proliferation, and differentiation and maturation[1]. Restitution consists of epithelial cells migrating into a wound within hours, followed by proliferation of epithelial cells in hours to days, and finally differentiation of intestinal stem cells into all mature intestinal cell types[1-3]. Each phase is induced and regulated by multiple cytokines, growth factors, and cell types reviewed in[1] and can be influenced by many factors that enhance or prevent wound healing as well as by the source of barrier injury. When the homeostatic process of wound healing is slowed or delayed by external or genetic factors, chronic inflammation may develop because repair of the intestinal epithelial barrier (IEB) and subsequent reduction of inflammation will not occur unless wound healing mechanisms are present[3]. Due to inflammation and chronic immune response, the consequences of impaired MH are chronicity of autoimmune diseases, including Inflammatory Bowel Disease, (IBD) and its progression to colorectal cancer.
Inflammatory Bowel Disease (IBD) is a term that represents autoimmune inflammatory diseases of the gut, with ulcerative colitis (UC) and Crohn’s Disease (CD) being the major disease types; however, the etiology of IBD remains unclear. IBD is known to have genetic and environmental risk factors, but the mechanisms by which these factors induce IBD are not well-understood[4]. Common symptoms include abdominal pain, diarrhea, weight loss, malnutrition, and particularly in CD, nausea, vomiting, intestinal blockages, fistulae, and abscesses[4,5]. IBD is an increasingly common and often debilitating disease, affecting up to 200 individuals per 100000 people in the United States[4]. Onset of IBD often occurs before the age of 30, and patients experience poor quality of life along with high risk of developing colorectal cancer due to the chronic and progressive symptoms and persistent inflammatory state[1,4]. Though multiple treatment mechanisms exist, including corticosteroids, anti-inflammatory medications, monoclonal antibodies, stem cell treatments, and surgery, IBD cannot currently be cured[4]. Therefore, continued research into the mechanisms of pathogenesis and development of new pharmaceuticals and treatment methods is vital to decrease mortality and improve quality of life for IBD patients.
Autoimmune disease pathogenesis is difficult to study due to the multifactorial causes of disease and complex molecular mechanisms, as well as the predicament of patients not presenting to the clinic until late in the disease development process. Although the disease complexity and difficulty in identifying the initial molecular instigators has thus far precluded full understanding of the specific molecular mechanisms of IBD pathogenesis, the general overarching processes have been elucidated. During IBD pathogenesis, the IEB and mucosal layers become damaged and inflamed due to injury and/or infection, which can develop into a state of chronic inflammation and reduced IEB integrity. Genetic factors can also play a role in pathogenesis, particularly in CD through genes such as a variant that impairs autophagy and dysregulates the IEB and gut microbiota[6,7]. The damage to the IEB results in microbial and antigen exposure in the intestinal lumen, leading to an inflammatory cascade and disturbed homeostasis[8,9]. Multiple cytokines and immune cell types are also thought to contribute to the pathogenesis of or protection against IBD; therefore, further study of the complex interactions governing the maintenance or breakdown of gut barrier homeostasis is imperative to deeper understanding of IBD pathogenesis and the development of effective treatments.
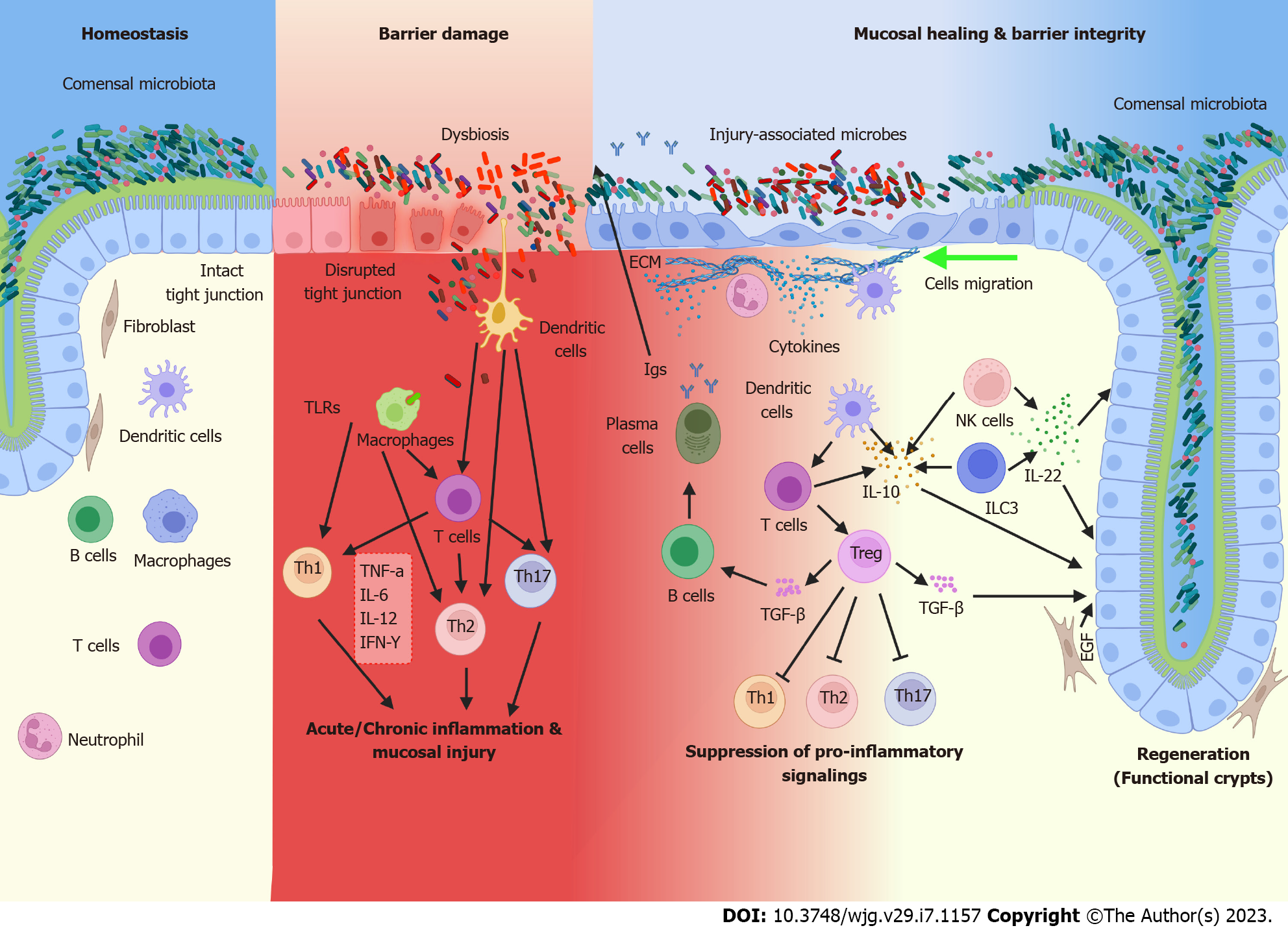
The main goals of IBD treatment are two-pronged: Reducing symptoms and preventing new inflammation and intestinal injury through traditional treatment methods, and recently a new, ambitious goal of inducing wound healing of existing inflammation and damage[8]. Some current treatments may contribute to both treatment goals; however, many existing clinical treatments are more targeted toward the traditional goal of preventing inflammation and damage. Even with the advent of some newer MH-focused treatments, additional avenues of further enhancing MH should be explored. Although clinical remission or preventing new inflammation is possible, patients may still have residual disease symptoms during remission due to the defective wound healing process leaving previous intestinal damage unrepaired. Additionally, up to half of IBD patients experience non-response or loss of response to standard therapeutics, leading to relapse[10]. Hence, MH induction presents an attractive goal in effective long-term treatment of IBD and prevention of relapse, and thus also prevention of progression to colitis-associated colon cancer. In this review, we will summarize the main current and prospective treatments of IBD, as described in Table 1, and their benefits and limitations towards the goal of reducing inflammation and achieving MH. More detailed analysis of the mechanisms of action, safety, and efficacy profiles of current IBD therapies and clinical trials can be found in Neurath et al[11]. However, in this review, we will emphasize areas that have not yet been as extensively clinically explored, including the temporal control of gut immune function, which presents novel potential for fine-tuning of the immune system in MH and restoration of gut homeostasis. We will examine three major factors contributing to the pathogenesis and tissue damage of IBD, as shown in Figure 1: Gut barrier dysfunction, gut dysbiosis, and inflammatory cytokine responses. Specifically, we will focus on the prospect of altering these factors and associated pathways summarized in Table 2 for both the reduction of inflammation and induction of MH.
| Treatment type | Available therapeutics | Mucosal healing relevance/ Success |
| Corticosteroids | Prednisone/ Prednisolone/ Methylprednisone | Prednisone treatment for 14 d (20 mg/day) decreased mucosal inflammation indicating a possible role in developing short-term MH[139]. 29% of patients in one study displayed endoscopic remission after steroid treatment[140]. |
| Nutritional therapy | Enteral nutrition (EN)/ Partial enternal nutrition (PEN) | EN/PEN induce MH in both adults and children[14]. |
| Aminosalicylates (5-ASA) | Sulfasalazine/ Mesalamine/ Olsalazine/ Balsalazide | On average induce MH in 43.7% of patients[141]. |
| Immunomodulators | Azathioprine/ 6-mercaptopurine | Azathioprine alone has achieved MH in 16.5% of cases and in 43.9% when used in combination with antibody therapies[18]. After 16 wk of mercaptopurine treatment, patients in remission showed a 47.1% rate of MH[142]. |
| Cyclosporine | Shown to induce MH when used in conjunction with Vendolizumab[143]. | |
| Tacrolimus | Shown to induce MH when used in conjunction with Vendolizumab[143]. | |
| Methotrexate | After 36 wk, methotrexate treatment had a MH rate of 47.4%[142]. | |
| Monoclonal antibody/ Biologic therapies | Adalimumab | Induced MH in 24% of patients treated[24]. |
| Certolizumab | Clinical response rate at weeks 2 and 12 was 29.7% and 52.8% (respectively) in CD[25]. | |
| Infliximab | Treatment induced MH in up to 60.3% of patients in phase 2 clinical trials[23]. | |
| Natalizumab | MH achieved by 42.3% of patients after 14.1 mo of treatment[144]. | |
| Risankizumab-rzaa | Endoscopic response and deep remission observed in 55% and 29% of patients (respectively), indicating MH[27]. | |
| Ustekinumab | Treatment of individuals with moderate to severe CD showed MH via a reduced disease score after 8 wk[19]. | |
| Vedolizumab | Has shown to induce MH in up to 50% of UC patients and 29% of CD patients in clinical trials[26,27]. |
| Pathways/ Mechanism of action | Associated models studied | Ref. |
| EGFR signaling | In vitro, colorectal cancer mice, EGFR mutant mice | [43,116] |
| Hippo/YAP signaling | In vitro, YAP-1 transgenic mice | [36,59] |
| Notch signaling | Villin-Claudin-1 transgenic mice | [41,42] |
| Wnt/β-catenin signaling | In vitro and In vivo models of injury/repair | [44,60,61] |
| Vitamin D receptor (VDR) signaling | In vitro, VDR knockout mice | [45] |
| Src/focal adhesion kinase | In vitro, Mechanical colonic wound in mice, Nox1 and AnxA1 knockout mice, oral gavage in mice | [76-78] |
| Autophagy/ATG16L1 | Patient biopsies; ATG16L1 T300A knock-in mice; Atg5-manipulated mice | [6,7,104] |
| SCFA-mediated signaling [acetate, propionate, butyrate, etc.] | In vitro, Patient biopsies, oral gavage in mice. T-cell induced colitis, trinitrobenzenesulphonic acid (TNBS) colitis | [83-85,91,93,100,101,114] |
| TLR-mediated signaling | DSS colitis | [109,110,112] |
| MyD88 mediated bacterial sensing | Mechanical colonic wound, MyD88 knockout mice | [111] |
| Prostaglandin-endoperoxidase synthase 2 enzyme (PGE2) | In vitro, mechanical colonic wound, Ptgs2 knockout mice, Ptger4 knockout mice | [111,112] |
| Mucin 2 signaling | In vitro, DSS colitis, EGFR mutant mice | [80,116] |
| IL-6/IL-22/IL-23/STAT3 signaling | DSS colitis, Th2-mediated colitis, cytokine deficient mice, bone marrow transplant mice, T-cell induced colitis, human and mouse intestinal organoid culture | [94,97,98,136-138] |
| TGF-β signaling | In vitro, DSS colitis, TGF-β transgenic mice | [50,130,131] |
| IL-10 signaling | In vitro, mechanical colonic wound in mice, IL-10-deficient mice | [132,133] |
Historically, there has been a disconnect between the expectation of IBD treatment promoting MH and real treatment outcomes, as many therapies for UC and CD primarily target symptom relief and reduction of chronic inflammation[12]. Corticosteroids, Methotrexate, and surgery are typically utilized to achieve these goals, but they do not promote MH as the primary therapeutic endpoint[13,14]. Because UC and CD are progressive diseases, patients may still experience intestinal damage even during periods without physical symptoms, and disease progression is typically only slowed by these treatments, not stopped[12,15]. However, achieving MH may help stop disease progression as well as decrease symptom severity. New treatment plans broaden the therapeutic focus to include inducing MH through a variety of mechanisms, such as by altering the gut microbiome and altering inflammatory and anti-inflammatory cytokines with antibodies or exogenous cytokine therapies.
One such mechanism for emphasizing MH includes suppressing specific parts of the patient’s immune system to decrease the main contributors of chronic inflammation. When new inflammation is reduced, it may then be possible for the wound healing process to begin to keep up with the rate of tissue damage. Methods of achieving this goal include enteral nutrition (EN), partial EN (PEN), 5-aminosalicylates (5-ASA), and Azathioprine treatments, which focus on decreasing IBD-associated inflammation by suppressing the host immune system in a less global manner than corticosteroids or preventing the production of inflammatory molecules. EN is a dietary treatment for IBD patients that can either totally (with EN) or partially (with PEN) replace solid food intake with specialized formula[16]. Although the mechanism by which EN induces MH is currently unknown, microbiome changes are implicated and are hypothesized to aid in decreasing chronic inflammation[17]. While typically a pediatric treatment, a pilot study shows MH in adults following EN and PEN treatment as well[16]. Currently, the largest drawback of EN treatment is low patient compliance, especially in adults[16]. 5-ASA drugs, which are used to induce remission in early IBD, are shown to induce MH in 43.7% of patients[18]. Azathioprine is a purine analog inhibiting purine metabolism and blocking T cell activation and co-stimulation, therefore functioning by suppressing the immune system and decreasing inflammation in IBD[11,19-21]. It is shown to achieve MH independently in some cases (30.1%) alone but is more successful when used in conjunction with anti-TNF-α antibodies such as Infliximab (44%)[22]. Other immunomodulating drugs shown to induce MH when given with monoclonal antibodies include Cylosporine and Tacrolimus[23].
Monoclonal antibodies targeting inflammatory cytokines are an emerging class of IBD therapies that more directly focus on permitting or inducing MH and are an attractive method of IBD treatment. Monoclonal antibody drugs targeting multiple cytokines are approved or in trials, and function by removing inflammatory cytokines. Anti-tumor necrosis factor-alpha (TNF-α) antibody-based drugs such as Infliximab, Adalimumab, and Certolizumab are biologic therapies that have achieved significant clinical success and are now widely used as front-line treatments for IBD, with the goal of reducing inflammation and promoting MH. The target of anti-TNF-α therapies is a pro-inflammatory cytokine contributing to the chronic and severe inflammatory response observed in UC and CD, and the monoclonal antibody functions by downregulating pro-inflammatory molecules and restoring IEB integrity, allowing the body to begin to heal[8,24-26]. Infliximab is an Immunoglobulin G1 anti-TNF-α antibody that is shown to induce MH. Active phase 1 and 2 clinical trials of Infliximab in UC patients find that, respectively, 45.5% of patients and 60.3% of patients exhibit MH[27]. Adalimumab is another anti-TNF-α antibody presenting some indication of MH effects. In the double blind, randomized, placebo controlled clinical trial, 24% of patients display MH at week 52 compared to 0% in the placebo group[28]. Certolizumab pegol is an anti-TNF-α antibody fragment targeting soluble and trans
Although inflammation reduction and monoclonal antibody treatments have greatly enhanced the quality of IBD treatment in recent years, lack or loss of response occurs with concerning frequency, and even in patients who respond well to treatments, complete resolution of the disease has not been achieved10]. This is due to the complex nature of disease development through many aberrant proteins and signaling pathways, as well as the multi-faceted process of MH that must be approached from many angles to restore complete IEB homeostasis. Overall, while current therapies offer evidence of permitting MH in IBD, some of these therapies do not directly promote MH through enhancing the processes of restitution, proliferation, or differentiation, but rather by simply inducing immune suppression to decrease inflammation and associated injury. Subsequently, there is a critical need to better understand the processes of inflammation and MH in IBD to aid in the development of actively MH-inducing IBD therapies.
A major role of the intestinal epithelium is its function as a barrier against the luminal environment and antigens; a role that is critical in maintaining normal mammalian homeostasis[35]. Accordingly, IEB dysregulation is a major factor in gut inflammation; thus, reinforcement of IEB integrity is a key consideration when developing more effective treatments for gut inflammatory diseases, including IBD. The MH process has been shown to help reinforce barrier integrity[36]. A complex and dynamic coordination between epithelial and immune factors facilitates MH, however, the ‘paradoxical’ role of the barrier-integral proteins in promoting MH remains ill-understood. Here, we summarize the key components of IEB regulation to illustrate the dynamic causal relationship between MH and the proteins comprising the structural and functional units of the gut barrier.
The physical component of the IEB consists of a single layer of epithelium, with cells linked by junctional complexes along the apicobasal axis. Excellent reviews have described the types and roles of junctional complex proteins in barrier integrity, thus here we focus on mechanisms by which regulation of these proteins influences inflammation and MH[35-39]. Tight junctions, the most apical cell-cell adhesions are considered the “gate” of the IEB and consist of multiple proteins, including the Claudin family of proteins, Occludin, junctional adhesion molecule (JAM) and the zonula occludens (ZO)-proteins[40]. Studies in cell and mouse models demonstrate through genetic manipulation that tight junction proteins are not merely static structural entities of the IEB; they perform additional non-canonical roles of regulating epithelial cell proliferation, survival, differentiation, and migration, which are the same processes integral to MH. In this regard, ZO-1 regulates expression and nuclear localization of the transcription factor ZO-1-associated nucleic-acid-binding protein to influence cell proliferation in a density dependent manner[41,42]. Recent studies also show that the ZO-proteins modulate Hippo/Yes-associated protein 1 signaling, a critical regulator of crypt growth and MH[43]. A similar role of JAM in regulating intestinal epithelial cell (IEC) proliferation has been reported[44,45]. Occludin, on the other hand, is shown to regulate IEC apoptosis and survival[46,47]. Recent studies demonstrate that the Claudin family of proteins is integral to tight junction structure and function and plays a complex role in gut inflammation and regenerative processes. For example, Claudin-1 overexpression in the intestinal epithelium of mouse models of IBD induces significant dysregulation of Notch/Wnt signaling and severe colitis, resulting in delayed recovery from colitis-associated injury[48,49]. Claudin-2 is unique among the Claudin proteins expressed in the intestine, as it is primarily expressed at the crypt base among proliferative undifferentiated cells and associates inversely with the differentiation state of IECs[48]. Of note, Claudin-2 is a direct target of Epidermal Growth Factor Receptor (EGFR), Wnt/β-catenin, and Vitamin D receptor signaling, all of which promote intestinal MH[50-52]. Claudin-3 on the other hand, which functions as a receptor for Clostridium perfringens, is sharply downregulated in the biopsies of the IBD patients, and loss of its function in the mouse gut promotes colitis-associated cancer[53-55]. We have also previously reported that Claudin-3 Loss enhances gp130/IL-6/STAT3 (signal transducer and activator of transcription 3) signaling, which promotes colitis-associated injury/repair[50]. Interestingly, the loss of intestinal Claudin-7 expression results in spontaneous inflammation due to dysregulation of epithelial-extracellular matrix interactions[56,57]. Claudin-7 also regulates intestinal stem cell function in association with the Epithelial Cellular Adhesion Molecule protein[58,59]. Conversely, Claudin-15 Loss in the IEB results in a mega-intestine[60]. Hence, dysregulation of tight junction protein expression in the IEB results in IBD, indicating that restoration of tight junction homeostasis is a vital component of MH and a promising target for treatment development.
Like tight junction component proteins, adherens junction proteins contribute significantly to gut inflammation and MH. Here, E-cadherin, a protein whose expression indicates an epithelial phenotype, contributes to inflammation-associated epithelial repair by regulating the epithelial-to-mesenchymal transition, a process associated with cell proliferation and migration[58,61,62]. Specifically, E-cadherin expression inhibits migration of IECs and wound healing[63]. Contrastingly, complete E-cadherin loss causes a severe inflammatory phenotype characterized by villus blunting, which is a marker of premature epithelial death, and incomplete brush border development[64]. The stabilization of E-cadherin expression is facilitated partly by binding with the cytoplasmic domain of p120-cadherin. Accordingly, p120 knockout mice displayed disrupted intestinal integrity and early death from intestinal injury[65]. Other heterodimeric adherens junction proteins, α- and β-catenin, are key players in the regulation of Hippo and Wnt signaling[66,67]. Although α- and β-catenin expression dictates IEC proliferation and differentiation during injury repair, their expression counter-acts each other. Moreover, ubiquitination of β-catenin by α-catenin aids the degradation of β-catenin, balancing the Wnt signaling pathway[68]. Taken together, these findings support a complex and dynamic interdependence between gut barrier regulation and MH, which should be considered for therapeutic potential.
Aberrant microbiome-immune interactions can lead to improper immune activation and are potentially responsible for the clinical and endoscopic observations in IBD patients. Mechanisms of microbial involvement in IBD include production of short-chain fatty acids (SCFAs), interaction with autophagy pathways, activation of immune cells, TLR signaling, and prostaglandin pathways[69-73]. Excellent reviews have covered the details of such interactions and the dynamic association with gut inflammatory processes[71-75]. Thus, here we focus primarily on how gut microbiota may contribute to MH processes under normal and/or inflammatory conditions.
Gut dysbiosis is mediated by pathogenic microbes harboring genes encoding toxin proteins, which disrupt the IEB via disassembly or redistribution of tight junction proteins. For example, human epithelial cells treated with Escherichia coli or Salmonella typhimurium demonstrate downregulation of ZO-1 and Occludin proteins while by contrast, Claudin-2 is upregulated[76-78]. Shigella flexneri and Campylobacter jejuni are involved in deregulating E-cadherin, as well as activating IL-8 and nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB), thereby inducing barrier dysfunction and inflammation[79,80]. Overall, studies suggest that pathogenic gut bacteria promote chronic mucosal inflammation by dysregulating IEB integrity.
On the other hand, commensal gut bacteria seem to promote the initial stage of epithelial restitution, as studies in germ-free mice show impaired rates of epithelial cell migration, which is dependent on the formation of focal adhesions[81,82]. In this context, a commensal bacterium activates the focal adhesion kinase, thereby enhancing epithelial restitution and promoting mucosal wound repair in a redox-dependent manner[83-85]. In a mouse colonoscopy-based wound healing model, an abundance of anaerobic bacteria such as Akkermansia spp augmented early stages of MH[86]. The selection of mucin-producing bacteria from the mucin layer also helps close mucosal wounds[87,88]. These microbes help generate SCFAs such as acetate, propionate, and butyrate, which are considered the primary energy sources of gut colonocytes and are therefore critical supporters of IEB restoration and integrity following tissue damage[89-92]. The major producers of SCFAs include the genus Bacteroides, Clostridium clusters IV and XIVa, and Bifidobacterium, though they use diverse mechanisms to achieve homeostatic outcomes[89,93,94]. For example, Bacteroides ovatus decreases lipopolysaccharide-induced inflammation and produces indole-3-acetic acid that likely promotes IL-22 production by immune cells, yielding beneficial effects in epithelial regeneration[95,96]. SCFAs produced by fiber-fermenting commensal microbes are also linked to upregulation of Foxp3+ T regulatory (Tregs) cell development, which have a widely documented role in protection against epithelial injury and colitis [97]. Inhibition of histone deacetylases and/or activation of the latent form of transforming growth factor-β (TGF-β) to act as a potent inducer of Tregs are potential mechanisms of SCFAs[98,99]. SCFAs also mediate activation of STAT3 which plays a vital role in mucosal homeostasis[100,101]. Clostridia-related segmented filamentous bacteria promote IL-23 production by antigen-presenting cells, which activate type 3 innate lymphoid cells (ILCs) to initiate an IL-23R/IL-22/STAT3 loop, thereby producing serum amyloid A which promotes IL-17 production by Th17 cells[102-105]. The importance of the SCFA propionate is the augmentation of dendritic cell and macrophage hematopoiesis precursors that impact intestinal immunity to control the growth of invading mucosal pathogens[106,107]. A breach in this regulation is a central mechanism in triggering, maintaining, and exacerbating IBD. Supplementation with another important SCFA, butyrate, rescues deficiencies in mitochondrial respiration and increases autophagy in the colonocytes of germ-free mice compared to conventionally raised mice[108]. Due to the critical role in repair of gut dysbiosis, the regulation of specific gut bacteria and SCFAs may therefore possess significant potential for clinical treatment of IBD.
As introduced above, accumulating evidence suggests a causal interdependence between autophagic flux in the intestinal epithelium and gut microbiota colonization. In this regard, CD patients who are homozygous for the ATG16L1T300A gene variant exhibit higher abundance of Enterobacteriaceae, Bacteroidaceae and Fusobacteriaceae in the inflamed ileum compared with patients homozygous for the wild type ATG16L1 allele[6]. Similar findings have been obtained from mice where expression of the autophagy gene Atg7 is genetically knocked out in the gut epithelium, as these mice display altered microbial composition with enrichment in Clostridium septum, Eubacterium cylindroides, and Bacteroides fragilis compared to wild type mice[109]. ATG16L1T300A variant mice also show changes in fecal microbiota composition compared to wild type mice, displaying an increase in the order Bacteroidales, which is associated with increased Th17 and Th1 cells in the colon and ileum lamina propria without the development of intestinal inflammation[7]. However, Atg5 deficient mice display reduced bacterial diversity, as observed in IBD patients, and contain a low number of the Lachnospiraceae, Ruminococcaceae, and Akkermansia families that control inflammatory responses[110]. Of note, a role of autophagy in regulating intestinal stem cell function and mucosal injury/repair has been demonstrated by several recent studies[111,112]. Taken together, these studies highlight a complex causal integration between host cell autophagy processes and intestinal microbial communities in regulating intestinal homeostasis and injury/repair.
Additionally, infiltrating immune cells such as macrophages and neutrophils responding to gut dysbiosis comprise essential components of intestinal wound healing by altering aberrant physiological parameters of the local microenvironment, such as microbe-associated molecular patterns (MAMPs) and decreased oxygen levels from the formation of reactive oxygen species[86,113-115]. Of note, Toll-like receptors (TLRs) expressed on multiple immune cell lineages induce signaling pathways upon binding by MAMPs and improve outcomes in experimental mouse colitis models via the promotion of wound healing[116,117]. Specific microbes in proximity to the wound bed also activate host epithelial proliferative signaling through a formyl peptide receptor pathway[83,84]. Studies employing a mechanical colonic wound method further disclose a protective role for prostaglandin E2 (PGE2) in re-establishment of the IEB through a TLR2/MyD88-dependent manner[118,119]. A follow-up study from the same group shows that in the early repair phase, a TLR2/PGE2 axis is required for barrier establishment; however, PGE2 must then decrease to allow for epithelial proliferation and regeneration[118]. In this context, Jain et al[120] elegantly demonstrates that temporal regulation of the bacterial metabolite PGE2/deoxycholate during colonic repair is critical for crypt regeneration[120]. The highly specific and time-dependent switching of microbial colonization and signaling pathways can therefore act to promote MH in a localized manner.
Several therapeutic approaches have been examined by administration of prebiotics or probiotics to regulate the microbiota. For example, butyrate enemas are effective in treating experimental colitis and UC patients[121,122]. Also, p40, a protein produced by Lactobacillus rhamnosus GG (LGG), activates host epithelial EGFR signaling and mediates wound healing[123]. Of note, the mechanism of MH promotion by LGG is via a positive effect on epithelial barrier maturation by upregulation of Claudin-3[124]. Recently, genetically modified probiotic bacteria–based precision delivery of human EGF also appears to be a promising intervention against mucosal inflammation through crypt-derived MH and barrier restoration[125,126]. Firmicutes, such as Faecalibacterium prausnitzii play an essential role in mucosal barrier homeostasis by regulating NF-κB activation and IL-8 production[125,126]. In another study, oral gavage with Faecalibacterium prausnitzii during dextran sodium sulfate (DSS) colitis improves outcomes compared to mice treated with DSS alone, likely due to participation of Claudin-1 and Claudin-2[125]. The probiotic mixture known as VSL #3, containing 4 strains of Lactobacilli, 3 strains of Bifidobacteria, and one strain of Streptococcus is effective in preventing pouchitis and in treating UC flareups[127]. This probiotic functions by partially upregulating mucin production and restoring the IEB by stimulating ZO-1 and Occludin expression while decreasing Claudin-2[127]. Taken together, a complex interdependence exists between gut microbiota and MH processes in the promotion of barrier integrity that should be fully explored for its intriguing potential in improving clinical outcomes.
Due to dysregulation of many cytokines and growth factors in IBD and the regulatory importance of many of these same molecules in MH, we discuss the potential of altering immune signaling and inflammatory cascades in restoring proper intestinal homeostatic balance. Increasing knowledge of the coordination of these pathways will contribute to the development of more effective and targeted therapies to ameliorate disease while preserving essential immune system function.
Major cytokines and growth factors that are considered pro-inflammatory in IBD include IL-1β, interferon-gamma (IFN-γ), TNF-α, and IL-6. During an infection in the gut, IL-1β, TNF-α, and IFN-γ are shown to be produced by inflammatory monocytes, among which IL-1β and TNF-α are associated with increased IEB permeability[2]. TNF-α and IFN-γ are also produced by ILCs and serve to recruit and activate additional inflammatory cells[3]. TNF-α is particularly well-studied and often targeted in the treatment of autoimmune diseases as detailed in our discussion of monoclonal antibody therapies above. Although necessary for innate immune responses against acute pathogens and acute DSS colitis, when produced chronically by T cells TNF-α can be a major contributing factor to the loss of epithelial barrier and development of autoimmune disease[128-130]. IFN-γ has been shown to be regulated during wound healing of skin epithelium by Tregs, where lack of Tregs resulted in increased IFN-γ, accumulation of pro-inflammatory macrophages, and hindered wound healing[131]. Gut microbiota can also impact immune system activation through cytokine signaling. Kuhn et al.[132] demonstrates that intraepithelial lymphocytes (IELs) must interact with commensal Bacteroidales order microbes to produce IL-6 in response to acute C. rodentium colitis infection, aiding in repair of the IEB via increased Claudin-1 expression[132]. Despite the necessary effects of pro-inflammatory cytokines for immune system response and homeostasis, each also possesses drawbacks. When the location, amount, or duration of cytokine production becomes dysregulated, chronic inflammation and disease can result. IFN-γ, TNF-α, and IL-6 are upregulated after secretion by immune cells in the chronic inflammatory state associated with IBD[3,130,132]. These cytokines are known to increase gut permeability by altering tight junction protein expression[132-134]. In particular, increased IL-6 in the IECs and lamina propria mononuclear cells increases Claudin-2, which promotes intestinal permeability and is known to be upregulated in IBD[134,135]. Interestingly, it is IELs that produce IL-6 in a protective manner during acute infection through the c-Jun N-terminal Kinase pathway, rather than the Mitogen-activated protein kinase/extracellular signal-regulated kinase pathway, suggesting both duration and cell type-specific layers of complexity in the role of IL-6[132,134]. It is likely that similar multi-layer and highly context-specific pathways exist for other inflammatory cytokines as well. Therein lies the challenge of harnessing pro-inflammatory cytokines and signaling pathways in the immune system for MH: Targeting modifications toward the decrease of detrimental chronic effects without impairing their beneficial and homeostatic functions. Continuing in the discussion of IL-6, global reduction of IL-6 expression can decrease chronic inflammation in IBD and enhance MH, but may also increase susceptibility to infection[132,136]. It would therefore be ideal to develop a method of reducing IL-6 production in IECs while leaving expression in IELs intact to avoid increasing the risk of dangerous infections in patients. The potential in fine-tuning inflammatory cytokine expression to promote MH is great, but much work remains to ensure it can be accomplished safely without severe detrimental effects to other functions of the immune system.
Conversely, several other cytokines, growth factors, and cell types function primarily in an anti-inflammatory role in IBD and intestinal homeostasis and offer additional mechanisms to enhance MH by resolving chronic inflammation. Growth factors and cytokines considered to be anti-inflammatory include TGF-β, IL-10, IL-22, and IL-17. TGF-β is a well-studied growth factor that has been demonstrated by Beck et al[137]. to play a role in the restitution, or IEC migration, phase of MH, evidenced by the lack of IEC migration and impaired wound healing after TGF-β inhibition in DSS colitis[8,137]. Additionally, TGF-β when produced by a macrophage secretome called SuperMApo aids in removal of apoptotic cells and resolution of inflammation in IBD models[57,138]. Importantly, since this secretome produces TGF-β from a singular cell type, rather than globally, its administration can provide the context- and location-dependent production of beneficial TGF-β while avoiding potential opposing or off-target effects. Macrophages and Th2 cells also produce IL-10, which enhances the proliferation phase of MH, maintains immune tolerance to the many antigens encountered by the IEB, and promotes barrier integrity[139,140]. Similarly, the production of IL-17 by anti-inflammatory Tregs helps with IEC proliferation and blockage of detrimental microbiota in colitis models, providing protection against IBD[141]. One of the major players in protection against IBD is IL-22, which is produced by multiple cell types and promotes MH by more than one mechanism, as reviewed in[142]. Most importantly in IBD, IL-22 is produced by ILCs, CD4+ T cells, and NK cells, demonstrating activation of both the innate and adaptive immune system[143,144]. IL-22 primarily acts on intestinal stem cells (ISCs) and IECs and functions by activating STAT3 signaling, which induces IEC proliferation and therefore MH[101,142,143,145]. Regarding ISCs, IL-22 both protects them from depletion during intestinal inflammation and induces regeneration[101,143,145]. Though some of these cytokines and growth factors can be inflammatory under certain circumstances, in the context of IBD they function in an anti-inflammatory manner and are beneficial in promoting MH. Some cytokines even demonstrate potential to be produced or administered selectively in only beneficial locations or cell types. Therefore, anti-inflammatory cytokines and growth factors present promising options for treatment of IBD through selectively reducing inflammatory signals and inducing or enhancing the MH process.
In recent years, IBD has become increasingly common in the United States and abroad. As progressive and debilitating diseases, UC and CD have long-term negative implications on health. In the past, treatments have focused on reducing the clinical manifestations of the disease, often leaving underlying disease mechanisms active in the gut[12,15]. With increased understanding of the mechanisms and complex pathogenesis of IBD, further innovation in treatment approaches must occur to improve patients’ long-term outcomes. Subsequently, instead of merely hoping to reduce symptoms, doctors now desire therapies that actively aid in healing and regeneration of damaged tissue. MH has therefore emerged as the main goal for research and treatment end points to ensure long-term remission, survival, and a good quality of life for patients. Going forward, understanding the interactions regulating the breakdown and regeneration of the IEB, as well as overarching gut homeostasis processes, will be paramount to treating and curing patients with IBD. Monoclonal antibody therapies offer a promising start to revolutionizing treatment, aiming not only to reduce clinical manifestations, but also to interrupt disease activity on a cellular and molecular level. However, even newly developed antibody therapies cannot by themselves completely resolve IBD and restore total gut homeostasis. Therefore, the three approaches to targeting the molecular machinery governing IBD of restoring the IEB, regulating the gut microbiota, and altering the cytokine signaling-mediated immune response are all being studied as potential mechanisms for achieving MH. Optimal future treatment protocols for IBD will ideally include a combination of these approaches, with the intent of restoring intestinal homeostasis by balancing expression of multiple proteins and repairing several of the many dysregulated pathways involved in IBD pathogenesis.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: American Gastrointestinal Association (AGA), 992160.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Long P, China; Mohammadi S, Iran S-Editor: Liu GL L-Editor: A P-Editor: Liu GL
| 1. | Pariente B, Hu S, Bettenworth D, Speca S, Desreumaux P, Meuwis MA, Danese S, Rieder F, Louis E. Treatments for Crohn's Disease-Associated Bowel Damage: A Systematic Review. Clin Gastroenterol Hepatol. 2019;17:847-856. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 2. | de Sablet T, Potiron L, Marquis M, Bussière FI, Lacroix-Lamandé S, Laurent F. Cryptosporidium parvum increases intestinal permeability through interaction with epithelial cells and IL-1β and TNFα released by inflammatory monocytes. Cell Microbiol. 2016;18:1871-1880. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 34] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 3. | Bernink JH, Peters CP, Munneke M, te Velde AA, Meijer SL, Weijer K, Hreggvidsdottir HS, Heinsbroek SE, Legrand N, Buskens CJ, Bemelman WA, Mjösberg JM, Spits H. Human type 1 innate lymphoid cells accumulate in inflamed mucosal tissues. Nat Immunol. 2013;14:221-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 698] [Cited by in RCA: 818] [Article Influence: 68.2] [Reference Citation Analysis (0)] |
| 4. | McDowell C, Farooq U, Haseeb M. Inflammatory Bowel Disease. 2022 Jun 27. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan-. [PubMed] |
| 5. | Fakhoury M, Negrulj R, Mooranian A, Al-Salami H. Inflammatory bowel disease: clinical aspects and treatments. J Inflamm Res. 2014;7:113-120. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 221] [Cited by in RCA: 337] [Article Influence: 30.6] [Reference Citation Analysis (0)] |
| 6. | Sadaghian Sadabad M, Regeling A, de Goffau MC, Blokzijl T, Weersma RK, Penders J, Faber KN, Harmsen HJ, Dijkstra G. The ATG16L1-T300A allele impairs clearance of pathosymbionts in the inflamed ileal mucosa of Crohn's disease patients. Gut. 2015;64:1546-1552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 72] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 7. | Lavoie S, Conway KL, Lassen KG, Jijon HB, Pan H, Chun E, Michaud M, Lang JK, Gallini Comeau CA, Dreyfuss JM, Glickman JN, Vlamakis H, Ananthakrishnan A, Kostic A, Garrett WS, Xavier RJ. The Crohn's disease polymorphism, ATG16L1 T300A, alters the gut microbiota and enhances the local Th1/Th17 response. Elife. 2019;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 90] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 8. | Sommer K, Wiendl M, Müller TM, Heidbreder K, Voskens C, Neurath MF, Zundler S. Intestinal Mucosal Wound Healing and Barrier Integrity in IBD-Crosstalk and Trafficking of Cellular Players. Front Med (Lausanne). 2021;8:643973. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 66] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 9. | Neurath MF. Cytokines in inflammatory bowel disease. Nat Rev Immunol. 2014;14:329-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1545] [Cited by in RCA: 1973] [Article Influence: 179.4] [Reference Citation Analysis (1)] |
| 10. | Fine S, Papamichael K, Cheifetz AS. Etiology and Management of Lack or Loss of Response to Anti-Tumor Necrosis Factor Therapy in Patients With Inflammatory Bowel Disease. Gastroenterol Hepatol (N Y). 2019;15:656-665. [PubMed] |
| 11. | Neurath MF. New targets for mucosal healing and therapy in inflammatory bowel diseases. Mucosal Immunol. 2014;7:6-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 255] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 12. | Klenske E, Bojarski C, Waldner M, Rath T, Neurath MF, Atreya R. Targeting mucosal healing in Crohn's disease: what the clinician needs to know. Therap Adv Gastroenterol. 2019;12:1756284819856865. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 63] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 13. | Baars JE, Vogelaar L, Wolfhagen FH, Biermann K, Kuipers EJ, van der Woude CJ. A short course of corticosteroids prior to surveillance colonoscopy to decrease mucosal inflammation in inflammatory bowel disease patients: results from a randomized controlled trial. J Crohns Colitis. 2010;4:661-668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 14. | Modigliani R, Mary JY, Simon JF, Cortot A, Soule JC, Gendre JP, Rene E. Clinical, biological, and endoscopic picture of attacks of Crohn's disease. Evolution on prednisolone. Groupe d'Etude Thérapeutique des Affections Inflammatoires Digestives. Gastroenterology. 1990;98:811-818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 529] [Cited by in RCA: 476] [Article Influence: 13.6] [Reference Citation Analysis (1)] |
| 15. | Peyrin-Biroulet L, Sandborn W, Sands BE, Reinisch W, Bemelman W, Bryant RV, D'Haens G, Dotan I, Dubinsky M, Feagan B, Fiorino G, Gearry R, Krishnareddy S, Lakatos PL, Loftus EV Jr, Marteau P, Munkholm P, Murdoch TB, Ordás I, Panaccione R, Riddell RH, Ruel J, Rubin DT, Samaan M, Siegel CA, Silverberg MS, Stoker J, Schreiber S, Travis S, Van Assche G, Danese S, Panes J, Bouguen G, O'Donnell S, Pariente B, Winer S, Hanauer S, Colombel JF. Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE): Determining Therapeutic Goals for Treat-to-Target. Am J Gastroenterol. 2015;110:1324-1338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1487] [Cited by in RCA: 1408] [Article Influence: 140.8] [Reference Citation Analysis (115)] |
| 16. | Wall CL, Gearry RB, Day AS. Treatment of Active Crohn's Disease with Exclusive and Partial Enteral Nutrition: A Pilot Study in Adults. Inflamm Intest Dis. 2018;2:219-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 49] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 17. | Horwat P, Kopeć S, Garczyk A, Kaliciak I, Staręga Z, Drogowski K, Mardas M, Stelmach-Mardas M. Influence of Enteral Nutrition on Gut Microbiota Composition in Patients with Crohn's Disease: A Systematic Review. Nutrients. 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 18. | Römkens TE, Kampschreur MT, Drenth JP, van Oijen MG, de Jong DJ. High mucosal healing rates in 5-ASA-treated ulcerative colitis patients: results of a meta-analysis of clinical trials. Inflamm Bowel Dis. 2012;18:2190-2198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 42] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 19. | Pearson DC, May GR, Fick GH, Sutherland LR. Azathioprine and 6-mercaptopurine in Crohn disease. A meta-analysis. Ann Intern Med. 1995;123:132-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 721] [Cited by in RCA: 628] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 20. | Bunea MC, Diculescu VC, Enculescu M, Iovu H, Enache TA. Redox Mechanism of Azathioprine and Its Interaction with DNA. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 21. | Huang Z, Chao K, Li M, Zhi M, Tang J, Hu P, Gao X. Methotrexate for Refractory Crohn's Disease Compared with Thiopurines: A Retrospective Non-head-to-head Controlled Study. Inflamm Bowel Dis. 2017;23:440-447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 22. | Colombel JF, Sandborn WJ, Reinisch W, Mantzaris GJ, Kornbluth A, Rachmilewitz D, Lichtiger S, D'Haens G, Diamond RH, Broussard DL, Tang KL, van der Woude CJ, Rutgeerts P; SONIC Study Group. Infliximab, azathioprine, or combination therapy for Crohn's disease. N Engl J Med. 2010;362:1383-1395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2539] [Cited by in RCA: 2374] [Article Influence: 158.3] [Reference Citation Analysis (1)] |
| 23. | Christensen B, Gibson PR, Micic D, Colman RJ, Goeppinger SR, Kassim O, Yarur A, Weber CR, Cohen RD, Rubin DT. Safety and Efficacy of Combination Treatment With Calcineurin Inhibitors and Vedolizumab in Patients With Refractory Inflammatory Bowel Disease. Clin Gastroenterol Hepatol. 2019;17:486-493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 67] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 24. | Hanauer SB, Sandborn WJ, Rutgeerts P, Fedorak RN, Lukas M, MacIntosh D, Panaccione R, Wolf D, Pollack P. Human anti-tumor necrosis factor monoclonal antibody (adalimumab) in Crohn's disease: the CLASSIC-I trial. Gastroenterology. 2006;130:323-33; quiz 591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1153] [Cited by in RCA: 1194] [Article Influence: 62.8] [Reference Citation Analysis (0)] |
| 25. | Suenaert P, Bulteel V, Lemmens L, Noman M, Geypens B, Van Assche G, Geboes K, Ceuppens JL, Rutgeerts P. Anti-tumor necrosis factor treatment restores the gut barrier in Crohn's disease. Am J Gastroenterol. 2002;97:2000-2004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 304] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 26. | Baert FJ, D'Haens GR, Peeters M, Hiele MI, Schaible TF, Shealy D, Geboes K, Rutgeerts PJ. Tumor necrosis factor alpha antibody (infliximab) therapy profoundly down-regulates the inflammation in Crohn's ileocolitis. Gastroenterology. 1999;116:22-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 338] [Cited by in RCA: 339] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 27. | Rutgeerts P, Vermeire S, Van Assche G. Mucosal healing in inflammatory bowel disease: impossible ideal or therapeutic target? Gut. 2007;56:453-455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 224] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 28. | Rutgeerts P, Van Assche G, Sandborn WJ, Wolf DC, Geboes K, Colombel JF, Reinisch W; EXTEND Investigators, Kumar A, Lazar A, Camez A, Lomax KG, Pollack PF, D'Haens G. Adalimumab induces and maintains mucosal healing in patients with Crohn's disease: data from the EXTEND trial. Gastroenterology. 2012;142:1102-1111.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 399] [Cited by in RCA: 452] [Article Influence: 34.8] [Reference Citation Analysis (0)] |
| 29. | Schreiber S, Rutgeerts P, Fedorak RN, Khaliq-Kareemi M, Kamm MA, Boivin M, Bernstein CN, Staun M, Thomsen OØ, Innes A; CDP870 Crohn's Disease Study Group. A randomized, placebo-controlled trial of certolizumab pegol (CDP870) for treatment of Crohn's disease. Gastroenterology. 2005;129:807-818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 441] [Cited by in RCA: 424] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 30. | Rutgeerts P, Gasink C, Chan D, Lang Y, Pollack P, Colombel JF, Wolf DC, Jacobstein D, Johanns J, Szapary P, Adedokun OJ, Feagan BG, Sandborn WJ. Efficacy of Ustekinumab for Inducing Endoscopic Healing in Patients With Crohn's Disease. Gastroenterology. 2018;155:1045-1058. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 134] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 31. | D'Haens G, Panaccione R, Baert F, Bossuyt P, Colombel JF, Danese S, Dubinsky M, Feagan BG, Hisamatsu T, Lim A, Lindsay JO, Loftus EV Jr, Panés J, Peyrin-Biroulet L, Ran Z, Rubin DT, Sandborn WJ, Schreiber S, Neimark E, Song A, Kligys K, Pang Y, Pivorunas V, Berg S, Duan WR, Huang B, Kalabic J, Liao X, Robinson A, Wallace K, Ferrante M. Risankizumab as induction therapy for Crohn's disease: results from the phase 3 ADVANCE and MOTIVATE induction trials. Lancet. 2022;399:2015-2030. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 227] [Article Influence: 75.7] [Reference Citation Analysis (0)] |
| 32. | Sakuraba A, Annunziata ML, Cohen RD, Hanauer SB, Rubin DT. Mucosal healing is associated with improved long-term outcome of maintenance therapy with natalizumab in Crohn's disease. Inflamm Bowel Dis. 2013;19:2577-2583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 33. | Noman M, Ferrante M, Bisschops R, De Hertogh G, Van den Broeck K, Rans K, Rutgeerts P, Vermeire S, Van Assche G. Vedolizumab Induces Long-term Mucosal Healing in Patients With Crohn's Disease and Ulcerative Colitis. J Crohns Colitis. 2017;11:1085-1089. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 54] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 34. | Feagan BG, Rutgeerts P, Sands BE, Hanauer S, Colombel JF, Sandborn WJ, Van Assche G, Axler J, Kim HJ, Danese S, Fox I, Milch C, Sankoh S, Wyant T, Xu J, Parikh A; GEMINI 1 Study Group. Vedolizumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2013;369:699-710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1576] [Cited by in RCA: 1864] [Article Influence: 155.3] [Reference Citation Analysis (1)] |
| 35. | Groschwitz KR, Hogan SP. Intestinal barrier function: molecular regulation and disease pathogenesis. J Allergy Clin Immunol. 2009;124:3-20; quiz 21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1288] [Cited by in RCA: 1247] [Article Influence: 77.9] [Reference Citation Analysis (2)] |
| 36. | Blikslager AT, Moeser AJ, Gookin JL, Jones SL, Odle J. Restoration of barrier function in injured intestinal mucosa. Physiol Rev. 2007;87:545-564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 375] [Cited by in RCA: 422] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 37. | Rusu AD, Georgiou M. The multifarious regulation of the apical junctional complex. Open Biol. 2020;10:190278. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 51] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 38. | Chelakkot C, Ghim J, Ryu SH. Mechanisms regulating intestinal barrier integrity and its pathological implications. Exp Mol Med. 2018;50:1-9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 484] [Cited by in RCA: 1151] [Article Influence: 164.4] [Reference Citation Analysis (1)] |
| 39. | Buckley A, Turner JR. Cell Biology of Tight Junction Barrier Regulation and Mucosal Disease. Cold Spring Harb Perspect Biol. 2018;10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 265] [Cited by in RCA: 478] [Article Influence: 68.3] [Reference Citation Analysis (0)] |
| 40. | Anderson JM, Van Itallie CM. Physiology and function of the tight junction. Cold Spring Harb Perspect Biol. 2009;1:a002584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 652] [Cited by in RCA: 771] [Article Influence: 51.4] [Reference Citation Analysis (0)] |
| 41. | Spadaro D, Tapia R, Jond L, Sudol M, Fanning AS, Citi S. ZO proteins redundantly regulate the transcription factor DbpA/ZONAB. J Biol Chem. 2014;289:22500-22511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 34] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 42. | Balda MS, Garrett MD, Matter K. The ZO-1-associated Y-box factor ZONAB regulates epithelial cell proliferation and cell density. J Cell Biol. 2003;160:423-432. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 291] [Cited by in RCA: 301] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 43. | Oka T, Remue E, Meerschaert K, Vanloo B, Boucherie C, Gfeller D, Bader GD, Sidhu SS, Vandekerckhove J, Gettemans J, Sudol M. Functional complexes between YAP2 and ZO-2 are PDZ domain-dependent, and regulate YAP2 nuclear localization and signalling. Biochem J. 2010;432:461-472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 160] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 44. | Fan S, Smith MS, Keeney J, O'Leary MN, Nusrat A, Parkos CA. JAM-A signals through the Hippo pathway to regulate intestinal epithelial proliferation. iScience. 2022;25:104316. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 45. | Nava P, Capaldo CT, Koch S, Kolegraff K, Rankin CR, Farkas AE, Feasel ME, Li L, Addis C, Parkos CA, Nusrat A. JAM-A regulates epithelial proliferation through Akt/β-catenin signalling. EMBO Rep. 2011;12:314-320. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 72] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 46. | Kuo WT, Shen L, Zuo L, Shashikanth N, Ong MLDM, Wu L, Zha J, Edelblum KL, Wang Y, Nilsen SP, Turner JR. Inflammation-induced Occludin Downregulation Limits Epithelial Apoptosis by Suppressing Caspase-3 Expression. Gastroenterology. 2019;157:1323-1337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 167] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 47. | Chelakkot C, Ghim J, Rajasekaran N, Choi JS, Kim JH, Jang MH, Shin YK, Suh PG, Ryu SH. Intestinal Epithelial Cell-Specific Deletion of PLD2 Alleviates DSS-Induced Colitis by Regulating Occludin. Sci Rep. 2017;7:1573. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 48. | Pope JL, Ahmad R, Bhat AA, Washington MK, Singh AB, Dhawan P. Claudin-1 overexpression in intestinal epithelial cells enhances susceptibility to adenamatous polyposis coli-mediated colon tumorigenesis. Mol Cancer. 2014;13:167. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 74] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 49. | Gowrikumar S, Ahmad R, Uppada SB, Washington MK, Shi C, Singh AB, Dhawan P. Upregulated claudin-1 expression promotes colitis-associated cancer by promoting β-catenin phosphorylation and activation in Notch/p-AKT-dependent manner. Oncogene. 2019;38:5321-5337. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 50. | Ahmad R, Kumar B, Pan K, Dhawan P, Singh AB. HDAC-4 regulates claudin-2 expression in EGFR-ERK1/2 dependent manner to regulate colonic epithelial cell differentiation. Oncotarget. 2017;8:87718-87736. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 51. | Mankertz J, Hillenbrand B, Tavalali S, Huber O, Fromm M, Schulzke JD. Functional crosstalk between Wnt signaling and Cdx-related transcriptional activation in the regulation of the claudin-2 promoter activity. Biochem Biophys Res Commun. 2004;314:1001-1007. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 76] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 52. | Zhang YG, Wu S, Lu R, Zhou D, Zhou J, Carmeliet G, Petrof E, Claud EC, Sun J. Tight junction CLDN2 gene is a direct target of the vitamin D receptor. Sci Rep. 2015;5:10642. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 88] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 53. | Sonoda N, Furuse M, Sasaki H, Yonemura S, Katahira J, Horiguchi Y, Tsukita S. Clostridium perfringens enterotoxin fragment removes specific claudins from tight junction strands: Evidence for direct involvement of claudins in tight junction barrier. J Cell Biol. 1999;147:195-204. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 492] [Cited by in RCA: 480] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 54. | Prasad S, Mingrino R, Kaukinen K, Hayes KL, Powell RM, MacDonald TT, Collins JE. Inflammatory processes have differential effects on claudins 2, 3 and 4 in colonic epithelial cells. Lab Invest. 2005;85:1139-1162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 335] [Cited by in RCA: 379] [Article Influence: 19.0] [Reference Citation Analysis (1)] |
| 55. | Yuan B, Zhou S, Lu Y, Liu J, Jin X, Wan H, Wang F. Changes in the Expression and Distribution of Claudins, Increased Epithelial Apoptosis, and a Mannan-Binding Lectin-Associated Immune Response Lead to Barrier Dysfunction in Dextran Sodium Sulfate-Induced Rat Colitis. Gut Liver. 2015;9:734-740. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 56. | Wang K, Ding Y, Xu C, Hao M, Li H, Ding L. Cldn-7 deficiency promotes experimental colitis and associated carcinogenesis by regulating intestinal epithelial integrity. Oncoimmunology. 2021;10:1923910. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 33] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 57. | Martin-Rodriguez O, Gauthier T, Bonnefoy F, Couturier M, Daoui A, Chagué C, Valmary-Degano S, Gay C, Saas P, Perruche S. Pro-Resolving Factors Released by Macrophages After Efferocytosis Promote Mucosal Wound Healing in Inflammatory Bowel Disease. Front Immunol. 2021;12:754475. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 45] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 58. | Xing T, Benderman LJ, Sabu S, Parker J, Yang J, Lu Q, Ding L, Chen YH. Tight Junction Protein Claudin-7 Is Essential for Intestinal Epithelial Stem Cell Self-Renewal and Differentiation. Cell Mol Gastroenterol Hepatol. 2020;9:641-659. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 42] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 59. | Nübel T, Preobraschenski J, Tuncay H, Weiss T, Kuhn S, Ladwein M, Langbein L, Zöller M. Claudin-7 regulates EpCAM-mediated functions in tumor progression. Mol Cancer Res. 2009;7:285-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 100] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 60. | Tamura A, Kitano Y, Hata M, Katsuno T, Moriwaki K, Sasaki H, Hayashi H, Suzuki Y, Noda T, Furuse M, Tsukita S. Megaintestine in claudin-15-deficient mice. Gastroenterology. 2008;134:523-534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 165] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 61. | Wei M, Ma Y, Shen L, Xu Y, Liu L, Bu X, Guo Z, Qin H, Li Z, Wang Z, Wu K, Yao L, Li J, Zhang J. NDRG2 regulates adherens junction integrity to restrict colitis and tumourigenesis. EBioMedicine. 2020;61:103068. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 62. | Aban CE, Lombardi A, Neiman G, Biani MC, La Greca A, Waisman A, Moro LN, Sevlever G, Miriuka S, Luzzani C. Downregulation of E-cadherin in pluripotent stem cells triggers partial EMT. Sci Rep. 2021;11:2048. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 58] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 63. | Silvestre J, Kenis PJ, Leckband DE. Cadherin and integrin regulation of epithelial cell migration. Langmuir. 2009;25:10092-10099. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 64. | Bondow BJ, Faber ML, Wojta KJ, Walker EM, Battle MA. E-cadherin is required for intestinal morphogenesis in the mouse. Dev Biol. 2012;371:1-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 68] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 65. | Smalley-Freed WG, Efimov A, Burnett PE, Short SP, Davis MA, Gumucio DL, Washington MK, Coffey RJ, Reynolds AB. p120-catenin is essential for maintenance of barrier function and intestinal homeostasis in mice. J Clin Invest. 2010;120:1824-1835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 111] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 66. | Schlegelmilch K, Mohseni M, Kirak O, Pruszak J, Rodriguez JR, Zhou D, Kreger BT, Vasioukhin V, Avruch J, Brummelkamp TR, Camargo FD. Yap1 acts downstream of α-catenin to control epidermal proliferation. Cell. 2011;144:782-795. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 919] [Cited by in RCA: 865] [Article Influence: 61.8] [Reference Citation Analysis (0)] |
| 67. | Rima M, Daghsni M, Lopez A, Fajloun Z, Lefrancois L, Dunach M, Mori Y, Merle P, Brusés JL, De Waard M, Ronjat M. Down-regulation of the Wnt/β-catenin signaling pathway by Cacnb4. Mol Biol Cell. 2017;28:3699-3708. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 68. | Hwang SG, Yu SS, Ryu JH, Jeon HB, Yoo YJ, Eom SH, Chun JS. Regulation of beta-catenin signaling and maintenance of chondrocyte differentiation by ubiquitin-independent proteasomal degradation of alpha-catenin. J Biol Chem. 2005;280:12758-12765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 77] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 69. | Nishida A, Inoue R, Inatomi O, Bamba S, Naito Y, Andoh A. Gut microbiota in the pathogenesis of inflammatory bowel disease. Clin J Gastroenterol. 2018;11:1-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 533] [Cited by in RCA: 904] [Article Influence: 113.0] [Reference Citation Analysis (3)] |
| 70. | Lavelle A, Sokol H. Gut microbiota-derived metabolites as key actors in inflammatory bowel disease. Nat Rev Gastroenterol Hepatol. 2020;17:223-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 482] [Cited by in RCA: 1196] [Article Influence: 239.2] [Reference Citation Analysis (0)] |
| 71. | Pickard JM, Zeng MY, Caruso R, Núñez G. Gut microbiota: Role in pathogen colonization, immune responses, and inflammatory disease. Immunol Rev. 2017;279:70-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 604] [Cited by in RCA: 1104] [Article Influence: 157.7] [Reference Citation Analysis (0)] |
| 72. | Ni J, Wu GD, Albenberg L, Tomov VT. Gut microbiota and IBD: causation or correlation? Nat Rev Gastroenterol Hepatol. 2017;14:573-584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1021] [Cited by in RCA: 1179] [Article Influence: 147.4] [Reference Citation Analysis (0)] |
| 73. | Al Bander Z, Nitert MD, Mousa A, Naderpoor N. The Gut Microbiota and Inflammation: An Overview. Int J Environ Res Public Health. 2020;17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 99] [Cited by in RCA: 426] [Article Influence: 85.2] [Reference Citation Analysis (0)] |
| 74. | Sankarasubramanian J, Ahmad R, Avuthu N, Singh AB, Guda C. Gut Microbiota and Metabolic Specificity in Ulcerative Colitis and Crohn's Disease. Front Med (Lausanne). 2020;7:606298. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 68] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 75. | Lama Tamang R, Juritsch AF, Ahmad R, Salomon JD, Dhawan P, Ramer-Tait AE, Singh AB. The diet-microbiota axis: a key regulator of intestinal permeability in human health and disease. Tissue Barriers. 2022;2077069. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Reference Citation Analysis (0)] |
| 76. | Babbin BA, Sasaki M, Gerner-Schmidt KW, Nusrat A, Klapproth JM. The bacterial virulence factor lymphostatin compromises intestinal epithelial barrier function by modulating rho GTPases. Am J Pathol. 2009;174:1347-1357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 21] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 77. | Li Q, Zhang Q, Wang C, Li N, Li J. Invasion of enteropathogenic Escherichia coli into host cells through epithelial tight junctions. FEBS J. 2008;275:6022-6032. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 78. | Jepson MA, Schlecht HB, Collares-Buzato CB. Localization of dysfunctional tight junctions in Salmonella enterica serovar typhimurium-infected epithelial layers. Infect Immun. 2000;68:7202-7208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 42] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 79. | Chen ML, Ge Z, Fox JG, Schauer DB. Disruption of tight junctions and induction of proinflammatory cytokine responses in colonic epithelial cells by Campylobacter jejuni. Infect Immun. 2006;74:6581-6589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 152] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 80. | Wu S, Rhee KJ, Zhang M, Franco A, Sears CL. Bacteroides fragilis toxin stimulates intestinal epithelial cell shedding and gamma-secretase-dependent E-cadherin cleavage. J Cell Sci. 2007;120:1944-1952. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 213] [Cited by in RCA: 187] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 81. | Abrams GD, Bauer H, Sprinz H. Influence of the normal flora on mucosal morphology and cellular renewal in the ileum. A comparison of germ-free and conventional mice. Lab Invest. 1963;12:355-364. [PubMed] |
| 82. | Pull SL, Doherty JM, Mills JC, Gordon JI, Stappenbeck TS. Activated macrophages are an adaptive element of the colonic epithelial progenitor niche necessary for regenerative responses to injury. Proc Natl Acad Sci U S A. 2005;102:99-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 466] [Cited by in RCA: 482] [Article Influence: 24.1] [Reference Citation Analysis (0)] |
| 83. | Alam A, Leoni G, Wentworth CC, Kwal JM, Wu H, Ardita CS, Swanson PA, Lambeth JD, Jones RM, Nusrat A, Neish AS. Redox signaling regulates commensal-mediated mucosal homeostasis and restitution and requires formyl peptide receptor 1. Mucosal Immunol. 2014;7:645-655. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 117] [Cited by in RCA: 111] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 84. | Leoni G, Alam A, Neumann PA, Lambeth JD, Cheng G, McCoy J, Hilgarth RS, Kundu K, Murthy N, Kusters D, Reutelingsperger C, Perretti M, Parkos CA, Neish AS, Nusrat A. Annexin A1, formyl peptide receptor, and NOX1 orchestrate epithelial repair. J Clin Invest. 2013;123:443-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 242] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 85. | Swanson PA 2nd, Kumar A, Samarin S, Vijay-Kumar M, Kundu K, Murthy N, Hansen J, Nusrat A, Neish AS. Enteric commensal bacteria potentiate epithelial restitution via reactive oxygen species-mediated inactivation of focal adhesion kinase phosphatases. Proc Natl Acad Sci U S A. 2011;108:8803-8808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 131] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 86. | Alam A, Leoni G, Quiros M, Wu H, Desai C, Nishio H, Jones RM, Nusrat A, Neish AS. The microenvironment of injured murine gut elicits a local pro-restitutive microbiota. Nat Microbiol. 2016;1:15021. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 175] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 87. | Liang L, Liu L, Zhou W, Yang C, Mai G, Li H, Chen Y. Gut microbiota-derived butyrate regulates gut mucus barrier repair by activating the macrophage/WNT/ERK signaling pathway. Clin Sci (Lond). 2022;136:291-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 132] [Article Influence: 44.0] [Reference Citation Analysis (0)] |
| 88. | Lee JS, Wang RX, Goldberg MS, Clifford GP, Kao DJ, Colgan SP. Microbiota-Sourced Purines Support Wound Healing and Mucous Barrier Function. iScience. 2020;23:101226. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 60] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 89. | Cummings JH, Macfarlane GT. The control and consequences of bacterial fermentation in the human colon. J Appl Bacteriol. 1991;70:443-459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 950] [Cited by in RCA: 912] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 90. | Clausen MR, Mortensen PB. Kinetic studies on colonocyte metabolism of short chain fatty acids and glucose in ulcerative colitis. Gut. 1995;37:684-689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 161] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 91. | Jørgensen JR, Clausen MR, Mortensen PB. Oxidation of short and medium chain C2-C8 fatty acids in Sprague-Dawley rat colonocytes. Gut. 1997;40:400-405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 39] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 92. | Roediger WE. Utilization of nutrients by isolated epithelial cells of the rat colon. Gastroenterology. 1982;83:424-429. [PubMed] |
| 93. | Louis P, Young P, Holtrop G, Flint HJ. Diversity of human colonic butyrate-producing bacteria revealed by analysis of the butyryl-CoA:acetate CoA-transferase gene. Environ Microbiol. 2010;12:304-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 449] [Cited by in RCA: 564] [Article Influence: 35.3] [Reference Citation Analysis (0)] |
| 94. | Martens EC, Lowe EC, Chiang H, Pudlo NA, Wu M, McNulty NP, Abbott DW, Henrissat B, Gilbert HJ, Bolam DN, Gordon JI. Recognition and degradation of plant cell wall polysaccharides by two human gut symbionts. PLoS Biol. 2011;9:e1001221. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 515] [Cited by in RCA: 622] [Article Influence: 44.4] [Reference Citation Analysis (1)] |
| 95. | Tan H, Zhao J, Zhang H, Zhai Q, Chen W. Novel strains of Bacteroides fragilis and Bacteroides ovatus alleviate the LPS-induced inflammation in mice. Appl Microbiol Biotechnol. 2019;103:2353-2365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 111] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 96. | Ihekweazu FD, Engevik MA, Ruan W, Shi Z, Fultz R, Engevik KA, Chang-Graham AL, Freeborn J, Park ES, Venable S, Horvath TD, Haidacher SJ, Haag AM, Goodwin A, Schady DA, Hyser JM, Spinler JK, Liu Y, Versalovic J. Bacteroides ovatus Promotes IL-22 Production and Reduces Trinitrobenzene Sulfonic Acid-Driven Colonic Inflammation. Am J Pathol. 2021;191:704-719. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 57] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 97. | Negi S, Saini S, Tandel N, Sahu K, Mishra RPN, Tyagi RK. Translating Treg Therapy for Inflammatory Bowel Disease in Humanized Mice. Cells. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 36] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 98. | Park J, Kim M, Kang SG, Jannasch AH, Cooper B, Patterson J, Kim CH. Short-chain fatty acids induce both effector and regulatory T cells by suppression of histone deacetylases and regulation of the mTOR-S6K pathway. Mucosal Immunol. 2015;8:80-93. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 575] [Cited by in RCA: 870] [Article Influence: 87.0] [Reference Citation Analysis (0)] |
| 99. | Smith PM, Howitt MR, Panikov N, Michaud M, Gallini CA, Bohlooly-Y M, Glickman JN, Garrett WS. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science. 2013;341:569-573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2937] [Cited by in RCA: 3948] [Article Influence: 329.0] [Reference Citation Analysis (1)] |
| 100. | Sun M, Wu W, Chen L, Yang W, Huang X, Ma C, Chen F, Xiao Y, Zhao Y, Yao S, Carpio VH, Dann SM, Zhao Q, Liu Z, Cong Y. Microbiota-derived short-chain fatty acids promote Th1 cell IL-10 production to maintain intestinal homeostasis. Nat Commun. 2018;9:3555. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 221] [Cited by in RCA: 432] [Article Influence: 61.7] [Reference Citation Analysis (0)] |
| 101. | Pickert G, Neufert C, Leppkes M, Zheng Y, Wittkopf N, Warntjen M, Lehr HA, Hirth S, Weigmann B, Wirtz S, Ouyang W, Neurath MF, Becker C. STAT3 Links IL-22 signaling in intestinal epithelial cells to mucosal wound healing. J Exp Med. 2009;206:1465-1472. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 791] [Cited by in RCA: 858] [Article Influence: 53.6] [Reference Citation Analysis (0)] |
| 102. | Ivanov II, Atarashi K, Manel N, Brodie EL, Shima T, Karaoz U, Wei D, Goldfarb KC, Santee CA, Lynch SV, Tanoue T, Imaoka A, Itoh K, Takeda K, Umesaki Y, Honda K, Littman DR. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell. 2009;139:485-498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3529] [Cited by in RCA: 3522] [Article Influence: 220.1] [Reference Citation Analysis (0)] |
| 103. | Flannigan KL, Denning TL. Segmented filamentous bacteria-induced immune responses: a balancing act between host protection and autoimmunity. Immunology. 2018;154:537-546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 69] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 104. | Sugimoto K, Ogawa A, Mizoguchi E, Shimomura Y, Andoh A, Bhan AK, Blumberg RS, Xavier RJ, Mizoguchi A. IL-22 ameliorates intestinal inflammation in a mouse model of ulcerative colitis. J Clin Invest. 2008;118:534-544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 566] [Article Influence: 33.3] [Reference Citation Analysis (0)] |
| 105. | Shih VF, Cox J, Kljavin NM, Dengler HS, Reichelt M, Kumar P, Rangell L, Kolls JK, Diehl L, Ouyang W, Ghilardi N. Homeostatic IL-23 receptor signaling limits Th17 response through IL-22-mediated containment of commensal microbiota. Proc Natl Acad Sci U S A. 2014;111:13942-13947. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 87] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 106. | Nastasi C, Candela M, Bonefeld CM, Geisler C, Hansen M, Krejsgaard T, Biagi E, Andersen MH, Brigidi P, Ødum N, Litman T, Woetmann A. The effect of short-chain fatty acids on human monocyte-derived dendritic cells. Sci Rep. 2015;5:16148. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 188] [Cited by in RCA: 288] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 107. | Millard AL, Mertes PM, Ittelet D, Villard F, Jeannesson P, Bernard J. Butyrate affects differentiation, maturation and function of human monocyte-derived dendritic cells and macrophages. Clin Exp Immunol. 2002;130:245-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 159] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 108. | Donohoe DR, Garge N, Zhang X, Sun W, O'Connell TM, Bunger MK, Bultman SJ. The microbiome and butyrate regulate energy metabolism and autophagy in the mammalian colon. Cell Metab. 2011;13:517-526. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1507] [Cited by in RCA: 1351] [Article Influence: 96.5] [Reference Citation Analysis (0)] |
| 109. | Tsuboi K, Nishitani M, Takakura A, Imai Y, Komatsu M, Kawashima H. Autophagy Protects against Colitis by the Maintenance of Normal Gut Microflora and Secretion of Mucus. J Biol Chem. 2015;290:20511-20526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 95] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 110. | Yang L, Liu C, Zhao W, He C, Ding J, Dai R, Xu K, Xiao L, Luo L, Liu S, Li W, Meng H. Impaired Autophagy in Intestinal Epithelial Cells Alters Gut Microbiota and Host Immune Responses. Appl Environ Microbiol. 2018;84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 85] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 111. | Asano J, Sato T, Ichinose S, Kajita M, Onai N, Shimizu S, Ohteki T. Intrinsic Autophagy Is Required for the Maintenance of Intestinal Stem Cells and for Irradiation-Induced Intestinal Regeneration. Cell Rep. 2017;20:1050-1060. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 94] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 112. | Trentesaux C, Fraudeau M, Pitasi CL, Lemarchand J, Jacques S, Duche A, Letourneur F, Naser E, Bailly K, Schmitt A, Perret C, Romagnolo B. Essential role for autophagy protein ATG7 in the maintenance of intestinal stem cell integrity. Proc Natl Acad Sci U S A. 2020;117:11136-11146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 49] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 113. | Amulic B, Cazalet C, Hayes GL, Metzler KD, Zychlinsky A. Neutrophil function: from mechanisms to disease. Annu Rev Immunol. 2012;30:459-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1013] [Cited by in RCA: 1184] [Article Influence: 91.1] [Reference Citation Analysis (0)] |
| 114. | Zhang D, Frenette PS. Cross talk between neutrophils and the microbiota. Blood. 2019;133:2168-2177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 99] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 115. | Gorjifard S, Goldszmid RS. Microbiota-myeloid cell crosstalk beyond the gut. J Leukoc Biol. 2016;100:865-879. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 71] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 116. | Rose WA 2nd, Sakamoto K, Leifer CA. TLR9 is important for protection against intestinal damage and for intestinal repair. Sci Rep. 2012;2:574. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 66] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 117. | Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell. 2004;118:229-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3061] [Cited by in RCA: 3162] [Article Influence: 150.6] [Reference Citation Analysis (0)] |
| 118. | Manieri NA, Drylewicz MR, Miyoshi H, Stappenbeck TS. Igf2bp1 is required for full induction of Ptgs2 mRNA in colonic mesenchymal stem cells in mice. Gastroenterology. 2012;143:110-21.e10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 61] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 119. | Miyoshi H, VanDussen KL, Malvin NP, Ryu SH, Wang Y, Sonnek NM, Lai CW, Stappenbeck TS. Prostaglandin E2 promotes intestinal repair through an adaptive cellular response of the epithelium. EMBO J. 2017;36:5-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 180] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 120. | Jain U, Lai CW, Xiong S, Goodwin VM, Lu Q, Muegge BD, Christophi GP, VanDussen KL, Cummings BP, Young E, Hambor J, Stappenbeck TS. Temporal Regulation of the Bacterial Metabolite Deoxycholate during Colonic Repair Is Critical for Crypt Regeneration. Cell Host Microbe. 2018;24:353-363.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 55] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 121. | Butzner JD, Parmar R, Bell CJ, Dalal V. Butyrate enema therapy stimulates mucosal repair in experimental colitis in the rat. Gut. 1996;38:568-573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 155] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 122. | Oliva S, Di Nardo G, Ferrari F, Mallardo S, Rossi P, Patrizi G, Cucchiara S, Stronati L. Randomised clinical trial: the effectiveness of Lactobacillus reuteri ATCC 55730 rectal enema in children with active distal ulcerative colitis. Aliment Pharmacol Ther. 2012;35:327-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 204] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 123. | Wang L, Cao H, Liu L, Wang B, Walker WA, Acra SA, Yan F. Activation of epidermal growth factor receptor mediates mucin production stimulated by p40, a Lactobacillus rhamnosus GG-derived protein. J Biol Chem. 2014;289:20234-20244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 105] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 124. | Patel RM, Myers LS, Kurundkar AR, Maheshwari A, Nusrat A, Lin PW. Probiotic bacteria induce maturation of intestinal claudin 3 expression and barrier function. Am J Pathol. 2012;180:626-635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 266] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 125. | Carlsson AH, Yakymenko O, Olivier I, Håkansson F, Postma E, Keita AV, Söderholm JD. Faecalibacterium prausnitzii supernatant improves intestinal barrier function in mice DSS colitis. Scand J Gastroenterol. 2013;48:1136-1144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 148] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 126. | Sokol H, Pigneur B, Watterlot L, Lakhdari O, Bermúdez-Humarán LG, Gratadoux JJ, Blugeon S, Bridonneau C, Furet JP, Corthier G, Grangette C, Vasquez N, Pochart P, Trugnan G, Thomas G, Blottière HM, Doré J, Marteau P, Seksik P, Langella P. Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients. Proc Natl Acad Sci U S A. 2008;105:16731-16736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2747] [Cited by in RCA: 3199] [Article Influence: 188.2] [Reference Citation Analysis (0)] |
| 127. | Dai C, Zhao DH, Jiang M. VSL#3 probiotics regulate the intestinal epithelial barrier in vivo and in vitro via the p38 and ERK signaling pathways. Int J Mol Med. 2012;29:202-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 64] [Article Influence: 4.6] [Reference Citation Analysis (1)] |
| 128. | Grivennikov SI, Tumanov AV, Liepinsh DJ, Kruglov AA, Marakusha BI, Shakhov AN, Murakami T, Drutskaya LN, Förster I, Clausen BE, Tessarollo L, Ryffel B, Kuprash DV, Nedospasov SA. Distinct and nonredundant in vivo functions of TNF produced by t cells and macrophages/neutrophils: protective and deleterious effects. Immunity. 2005;22:93-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 161] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 129. | Kojouharoff G, Hans W, Obermeier F, Männel DN, Andus T, Schölmerich J, Gross V, Falk W. Neutralization of tumour necrosis factor (TNF) but not of IL-1 reduces inflammation in chronic dextran sulphate sodium-induced colitis in mice. Clin Exp Immunol. 1997;107:353-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 243] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 130. | Semin I, Ninnemann J, Bondareva M, Gimaev I, Kruglov AA. Interplay Between Microbiota, Toll-Like Receptors and Cytokines for the Maintenance of Epithelial Barrier Integrity. Front Med (Lausanne). 2021;8:644333. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 26] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 131. | Nosbaum A, Prevel N, Truong HA, Mehta P, Ettinger M, Scharschmidt TC, Ali NH, Pauli ML, Abbas AK, Rosenblum MD. Cutting Edge: Regulatory T Cells Facilitate Cutaneous Wound Healing. J Immunol. 2016;196:2010-2014. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 300] [Article Influence: 33.3] [Reference Citation Analysis (0)] |
| 132. | Kuhn KA, Schulz HM, Regner EH, Severs EL, Hendrickson JD, Mehta G, Whitney AK, Ir D, Ohri N, Robertson CE, Frank DN, Campbell EL, Colgan SP. Bacteroidales recruit IL-6-producing intraepithelial lymphocytes in the colon to promote barrier integrity. Mucosal Immunol. 2018;11:357-368. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 106] [Cited by in RCA: 119] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 133. | Madara JL, Stafford J. Interferon-gamma directly affects barrier function of cultured intestinal epithelial monolayers. J Clin Invest. 1989;83:724-727. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 577] [Cited by in RCA: 575] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 134. | Suzuki T, Yoshinaga N, Tanabe S. Interleukin-6 (IL-6) regulates claudin-2 expression and tight junction permeability in intestinal epithelium. J Biol Chem. 2011;286:31263-31271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 336] [Cited by in RCA: 430] [Article Influence: 30.7] [Reference Citation Analysis (0)] |
| 135. | Kusugami K, Fukatsu A, Tanimoto M, Shinoda M, Haruta J, Kuroiwa A, Ina K, Kanayama K, Ando T, Matsuura T. Elevation of interleukin-6 in inflammatory bowel disease is macrophage- and epithelial cell-dependent. Dig Dis Sci. 1995;40:949-959. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 109] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 136. | Danese S, Vermeire S, Hellstern P, Panaccione R, Rogler G, Fraser G, Kohn A, Desreumaux P, Leong RW, Comer GM, Cataldi F, Banerjee A, Maguire MK, Li C, Rath N, Beebe J, Schreiber S. Randomised trial and open-label extension study of an anti-interleukin-6 antibody in Crohn's disease (ANDANTE I and II). Gut. 2019;68:40-48. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 90] [Cited by in RCA: 136] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 137. | Beck PL, Rosenberg IM, Xavier RJ, Koh T, Wong JF, Podolsky DK. Transforming growth factor-beta mediates intestinal healing and susceptibility to injury in vitro and in vivo through epithelial cells. Am J Pathol. 2003;162:597-608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 152] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 138. | Bonnefoy F, Gauthier T, Vallion R, Martin-Rodriguez O, Missey A, Daoui A, Valmary-Degano S, Saas P, Couturier M, Perruche S. Factors Produced by Macrophages Eliminating Apoptotic Cells Demonstrate Pro-Resolutive Properties and Terminate Ongoing Inflammation. Front Immunol. 2018;9:2586. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 37] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 139. | Quiros M, Nishio H, Neumann PA, Siuda D, Brazil JC, Azcutia V, Hilgarth R, O'Leary MN, Garcia-Hernandez V, Leoni G, Feng M, Bernal G, Williams H, Dedhia PH, Gerner-Smidt C, Spence J, Parkos CA, Denning TL, Nusrat A. Macrophage-derived IL-10 mediates mucosal repair by epithelial WISP-1 signaling. J Clin Invest. 2017;127:3510-3520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 155] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 140. | Kühn R, Löhler J, Rennick D, Rajewsky K, Müller W. Interleukin-10-deficient mice develop chronic enterocolitis. Cell. 1993;75:263-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3189] [Cited by in RCA: 3226] [Article Influence: 100.8] [Reference Citation Analysis (0)] |
| 141. | Song X, Dai D, He X, Zhu S, Yao Y, Gao H, Wang J, Qu F, Qiu J, Wang H, Li X, Shen N, Qian Y. Growth Factor FGF2 Cooperates with Interleukin-17 to Repair Intestinal Epithelial Damage. Immunity. 2015;43:488-501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 181] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 142. | Rutz S, Eidenschenk C, Ouyang W. IL-22, not simply a Th17 cytokine. Immunol Rev. 2013;252:116-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 343] [Cited by in RCA: 362] [Article Influence: 30.2] [Reference Citation Analysis (0)] |
| 143. | Hanash AM, Dudakov JA, Hua G, O'Connor MH, Young LF, Singer NV, West ML, Jenq RR, Holland AM, Kappel LW, Ghosh A, Tsai JJ, Rao UK, Yim NL, Smith OM, Velardi E, Hawryluk EB, Murphy GF, Liu C, Fouser LA, Kolesnick R, Blazar BR, van den Brink MR. Interleukin-22 protects intestinal stem cells from immune-mediated tissue damage and regulates sensitivity to graft versus host disease. Immunity. 2012;37:339-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 459] [Cited by in RCA: 483] [Article Influence: 37.2] [Reference Citation Analysis (0)] |
| 144. | Zenewicz LA, Yancopoulos GD, Valenzuela DM, Murphy AJ, Stevens S, Flavell RA. Innate and adaptive interleukin-22 protects mice from inflammatory bowel disease. Immunity. 2008;29:947-957. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 690] [Cited by in RCA: 687] [Article Influence: 40.4] [Reference Citation Analysis (0)] |
| 145. | Lindemans CA, Calafiore M, Mertelsmann AM, O'Connor MH, Dudakov JA, Jenq RR, Velardi E, Young LF, Smith OM, Lawrence G, Ivanov JA, Fu YY, Takashima S, Hua G, Martin ML, O'Rourke KP, Lo YH, Mokry M, Romera-Hernandez M, Cupedo T, Dow L, Nieuwenhuis EE, Shroyer NF, Liu C, Kolesnick R, van den Brink MRM, Hanash AM. Interleukin-22 promotes intestinal-stem-cell-mediated epithelial regeneration. Nature. 2015;528:560-564. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 756] [Cited by in RCA: 850] [Article Influence: 85.0] [Reference Citation Analysis (1)] |