Published online Feb 14, 2023. doi: 10.3748/wjg.v29.i6.908
Peer-review started: September 12, 2022
First decision: December 12, 2022
Revised: December 18, 2022
Accepted: January 9, 2023
Article in press: January 9, 2023
Published online: February 14, 2023
Processing time: 150 Days and 16.4 Hours
Coronavirus disease 2019 is an infectious disease caused by the severe acute respiratory syndrome coronavirus 2 that manifests as a variety of clinical manifestations, including liver damage commonly detected by a hepatocellular pattern from liver function tests. Liver injury is associated with a worse prognosis overall. Conditions associated with the severity of the disease include obesity and cardiometabolic comorbidities, which are also associated with nonalcoholic fatty liver disease (NAFLD). The presence of NAFLD, similarly to obesity, is associated with an unfavourable impact on the coronavirus disease 2019 outcome. Individuals with these conditions could present with liver damage and elevated liver function tests due to direct viral cytotoxicity, systemic inflammation, ischemic or hypoxic liver damage or drug side effects. However, liver damage in the setting of NAFLD could also be attributed to a pre-existing chronic low-grade inflammation associated with surplus and dysfunctional adipose tissue in these individuals. Here we investigate the hypothesis that a pre-existing inflammatory status is exacerbated after severe acute respiratory syndrome coronavirus 2 infection, which embodies a second hit to the underestimated liver damage.
Core Tip: The severe acute respiratory syndrome coronavirus 2 that causes coronavirus disease 2019 has a variety of clinical manifestations, including liver damage. Obesity and other dysmetabolic diseases linked to nonalcoholic fatty liver disease are a few of the factors that contribute to the severity of the illness. Due to direct viral cytotoxicity, people with these illnesses may have liver damage and increased liver function tests. However, liver injury might also be related to pre-existing inflammation and the detrimental effects of excessive and dysfunctional adipose tissue in these people.
- Citation: Lempesis IG, Karlafti E, Papalexis P, Fotakopoulos G, Tarantinos K, Lekakis V, Papadakos SP, Cholongitas E, Georgakopoulou VE. COVID-19 and liver injury in individuals with obesity. World J Gastroenterol 2023; 29(6): 908-916
- URL: https://www.wjgnet.com/1007-9327/full/v29/i6/908.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i6.908
Coronavirus disease 2019 (COVID-19) is an infectious disease caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)[1,2], which was first identified in December 2019 in Wuhan, China, and has since spread globally, resulting in an ongoing pandemic[2]. COVID-19 represents a systemic disease that can cause a variety of clinical manifestations, which range from asymptomatic individuals to mild respiratory symptoms and severe critical illness[3-7]. A plethora of underlying pathways drive the pathophysiology, including activation or dysregulation of localized (e.g., vascular) and generalized inflammation, which leads to multiorgan failure and eventually death[8-12].
Among the clinical manifestations, liver involvement, indicated by abnormal liver function tests (LFT) in patients, was noted early in the pandemic’s development[13]. However, major liver damage is not common[14-18]. The liver may be affected by SARS-CoV-2 directly (i.e. translocation of the virus from the intestines to the liver) or via indirect ways (i.e. systemic inflammatory response, ischemic and hypoxic liver damage, drug-related liver injury and impacts on pre-existing liver diseases)[14,16,17]. Coexisting liver disease, including metabolic/obesity related nonalcoholic fatty liver disease (NAFLD), may also negatively impact the course of COVID-19[14,15].
Early observations of COVID-19 have highlighted obesity as one of the most common underlying conditions in patients hospitalized with COVID-19[19-21]. Obesity was further associated with hospital admission and as an independent predictor of severity, intensive medical intervention and mortality of COVID-19[15,19]. Obesity, determined by increased total fat mass (body mass index > 30 kg/m2), is a chronic multifactorial disease that predisposes patients to numerous comorbidities, including metabolic diseases (insulin resistance states, type 2 diabetes mellitus, NAFLD), cardiovascular diseases[15,22-24] and various types of cancer[25-28].
Patients with poorer clinical outcomes for COVID-19 are characteristically older (more than 60 years) and have several metabolic syndrome manifestations, obesity and type 2 diabetes mellitus, a profile similar to those at increased risk of NAFLD[13,29,30]. Coexisting hepatic diseases like NAFLD and metabolic dysregulations could theoretically impact the manifestations and progression of COVID-19, and the liver injury in that population could represent a combination of the underlying condition exacerbated by the virus and inflammatory responses.
Here, we presented the current evidence for hepatic involvement in COVID-19 in obese individuals and the impact of fatty liver disease. Then, we tested the idea that liver damage in people with obesity and/or NAFLD could be caused by a “second hit” from the virus on top of hepatic dysfunction that was already there. This is likely caused by metabolic dysregulation and a chronic inflammatory state that is linked to obesity.
Patients with COVID-19 have experienced varying degrees of liver damage, ranging from self-limited hepatitis to significant liver injury and liver failure[31,32]. Impaired LFT without other hepatic manifestations was shown to be present in almost 50% of patients with COVID-19 in various studies[31-33]. The spectrum of pathological LFT includes elevated liver enzyme concentrations of transaminases [aspartate transaminase (AST), alanine transaminase (ALT)], cholestatic enzymes including alkaline phosphatase (ALP) and gamma-glutamyl transferase (GGT), lactate dehydrogenase and bilirubin and impaired synthetic activity as seen with prothrombin time (PT) prolongation and decreased albumin concentrations[31,34]. Based on the pattern of the affected LFT, liver damage is categorized as parenchymal (AST, ALT), cholestatic (ALP, GGT) or mixed. The most common type of liver damage, in up to 75% of cases, is parenchymal, indicating hepatocellular damage as a major part of physiopathology[31,34,35].
Several studies have shown elevated AST or ALT during acute COVID-19 illness[36-39]. A higher proportion of patients with severe cases (46.2% vs 12.7% in mild cases) had elevated AST and ALT[36,40-42]. AST appeared to be elevated first, followed by ALT in patients with severe disease[33,34]. Accordingly, AST was found to be correlated with mortality and liver dysfunction[5], showing an increased odds ratio in severe cases in other studies[42,43]. Elevated LFT was also recorded in subclinical cases (AST in 8.7% and ALT in 8.9%)[39].
The cholestatic pattern of elevated LFT was typically accompanied by elevated parenchymal enzymes[31]. In both mild and severe cases, ALP and bilirubin levels were found to be moderately elevated[37]. In a systematic review and meta-analysis of 64 studies, it was shown that the prevalence of elevated total bilirubin (9.7%), GGT (15.0%) and ALP (4.0%) were lower compared to elevated levels of aminotransferases (AST 23.2% and ALT 21.1%)[44]. Interestingly, in a retrospective study where ALP and GGT concentration levels were usually within normal values, pathological levels of GGT were found in 37.6% of patients with NAFLD[45].
In addition, a meta-analysis of 24 studies showed that a significantly longer PT in patients with severe COVID-19 was associated with higher morbidity rates. Elshazli et al[46] also concluded in their systematic review that prolonged PT was associated with a higher risk of disease progression to severe COVID-19 and admission to an intensive care unit. Impaired synthetic liver function, as assessed by hypoalbuminemia, was present and associated with an increased risk in patients with COVID-19[47]. Similarly, hypoalbuminemia was correlated with the severity of the disease and morbidity[31].
Overall, irrespectively of which LFT was affected, once hepatic damage was involved, patients had an increased risk of mortality and severe disease, which was shown in a systematic review of 107 studies and 20874 patients[48]. Acute liver injury is an important predictor for disease development[49]. In patients with chronic liver disease, including NAFLD, liver damage may occur more often. Chronic liver disease from any cause was discovered to be a risk factor for extended hospitalization and the fatal course of COVID-19[31].
The occurrence of NAFLD in patients with SARS-CoV-2 infection is substantial[4,31,50]. This is the case as specific cardiometabolic chronic conditions intrinsically linked to NAFLD (hypertension, diabetes, obesity, coronary artery disease, and cerebrovascular disease) have been noted as important risk factors associated with an increased risk of severe COVID-19[4,45,51]. In a recent meta-analysis and systematic review of 16 studies, Hayat et al[29] examined NAFLD prevalence, COVID-19 and the outcomes among patients with NAFLD vs those without. The study findings showed that individuals with NAFLD, as compared to those without, had a higher probability of severe COVID-19 and intensive care unit admission[29]. Furthermore, NAFLD patients tended to have higher COVID-19-related mortality compared to non-NAFLD patients[6,29].
Assessment of LFT and clinical outcomes in patients with or without NAFLD showed that in the vast majority of patients, liver damage was minor and hepatocellular in type[13,45]. A higher probability of abnormal LFT from admission to discharge was recorded for patients with NAFLD[13,45]. A Chinese analysis found that patients with NAFLD had a higher incidence of severe COVID-19 than those who did not have NAFLD but only those under 60-years-old. This observation was observed even after adjusting for potentially confounding factors like obesity, diabetes and hypertension[13,52]. In contrast to that age group, there was no association between patients age 60 years and older and NAFLD and the severity of COVID-19[13,52]. It was suggested that NAFLD-induced hepatic and systemic immune responses may worsen the severity of the cytokine storm in COVID-19 patients who are younger. Aside from that, older people often have other cardiometabolic comorbidities, which may change any link to NAFLD[13,52].
Dysfunctional adipose tissue (AT), an organ critical for whole-body homeostasis, is a hallmark of obesity and obesity-related chronic cardiometabolic diseases[28,53-55]. Dysfunctional AT is distinguished by, among other things, hormonal/adipocytokine dysregulation, a state of chronic low-grade inflammation associated with adipocyte hypertrophy and lipid metabolism impairments (reduced capacity to buffer the daily influx of dietary lipids), resulting in ectopic fat accumulation[28,56-58]. Additionally, decreased AT blood flow, mitochondrial dysfunction and altered oxygenation are frequently present in obesity[28,59].
Several proinflammatory molecules are increased in obesity and are related to aspects of the metabolic syndrome and other metabolic dysfunctions, including tumour necrosis factor alpha, interleukin 6 (IL-6), plasminogen activator inhibitor-1, dipeptidyl peptidase 4 and monocyte chemoattractant protein-1[53,54,60-62]. IL-6 can activate macrophages and contribute to the cytokine storm seen in COVID-19[13,63]. The loss of muscle mass and its anti-inflammatory properties, which are common in sarcopenic obesity, may have a negative impact on the chronic inflammatory status, various comorbidities and COVID-19 outcomes[64].
Liver dysfunction associated with obesity and metabolic diseases, including the spectrum of NAFLD, is characterized by a multifactorial pathophysiological mechanism still under investigation involving multiple pathways[65]. It has been suggested that, in part, the inability to sufficiently increase subcutaneous AT triglyceride stores in response to excessive energy intake and weight gain causes lipids to be diverted to other organs, such as the liver and skeletal muscle, where they accumulate ectopically and cause cellular lipotoxicity. This results in insulin resistance and inflammation in these tissues[66-70].
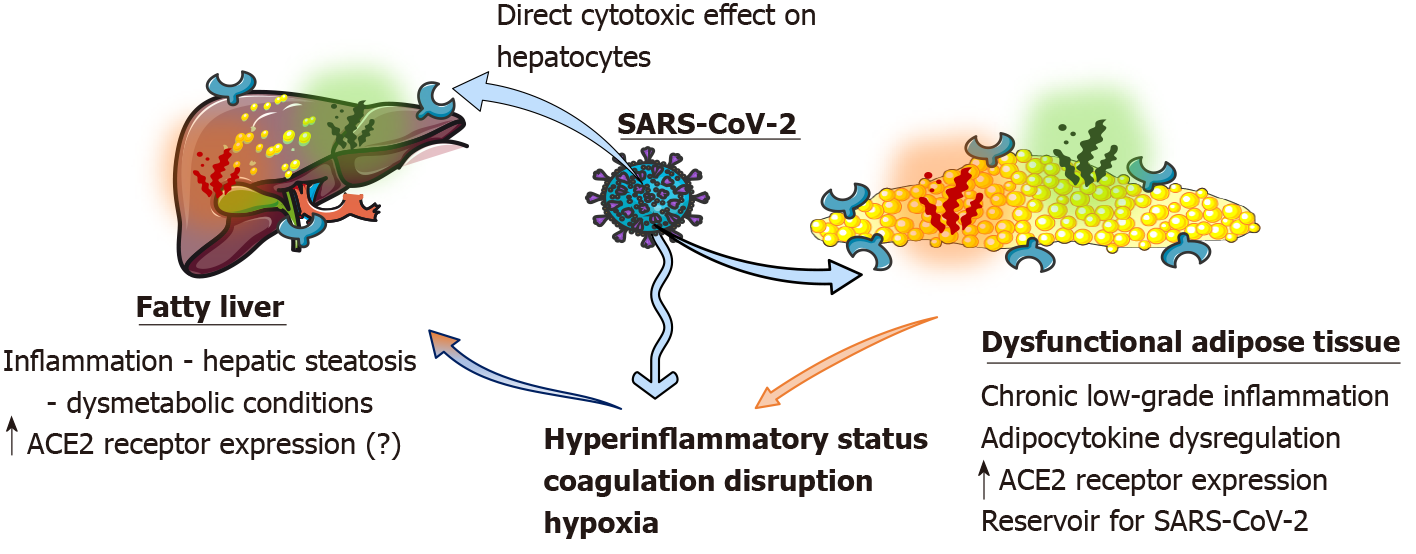
The COVID-19 systemic inflammatory state is linked with the angiotensin converting enzyme 2 (ACE2) receptor expressed in a variety of tissues[2,9,11]. Many organ systems, including the lungs, liver, heart, kidney and blood vessels, contain ACE2 receptors[31]. AT of patients with obesity is estimated to exhibit higher amounts of ACE2, perhaps acting as a SARS-CoV-2 reservoir with delayed viral shedding time[13,63]. The structural spike protein, characteristic of SARS-CoV-2, predominantly promotes the host’s immunological reaction[9]. The spike protein attaches to ACE2 receptor sites on the cell membrane, facilitating cell penetration[9].
In the liver, ACE2 receptor expression is significantly higher in cholangiocytes than in hepatocytes[31,41]. Direct virus cytopathogenic effects, inflammatory responses and intrahepatic immune cell stimulation, (micro)vascular thrombosis, hepatic obstruction, destabilized gut-liver axis, drug-induced toxicity and interfaces all play a part in liver damage during COVID-19 infection[31,35,71]. Interestingly, as demonstrated in a diet-induced nonalcoholic steatohepatitis (NASH) model in rodents, ACE2 hepatic expression was shown to be dramatically elevated, potentially enhancing hepatic vulnerability towards SARS-CoV-2 infection in individuals with NAFLD and/or NASH[13,72]. However, the link between NAFLD and liver-induced production of SARS-CoV-2 vital entry proteins, essentially ACE2, is still debatable[13]. A direct viral cytotoxic effect on hepatocytes appeared to induce mitochondrial and endoplasmic reticulum damage (swelling and distention accordingly), decreased glycogen granules and malfunction of the cell membrane in hepatocytes[31,33,34,42]. As in dysmetabolic conditions, the homeostasis of the liver and metabolic parameters are already impaired. This could have an additional effect on NAFLD. Finally, moderate microvesicular steatosis, along with other histopathological findings in deceased COVID-19 patients, may indicate additional pathways of metabolic derangement and liver damage[73].
In addition, the hyperinflammatory status in COVID-19 may act as a “second hit” to an inflamed fatty liver, causing “acute-on-chronic” steatohepatitis with increased aminotransferases[13,74]. Immune-mediated liver injury is caused by proinflammatory cytokines, chemokines and inflammatory cells produced against the SARS-CoV-2, which are also increased in obesity (IL-6, tumour necrosis factor)[32,34,75]. This may explain the finding that the C-reactive protein to albumin ratio was an independent risk factor for mortality in NAFLD patients with COVID-19 in one study[6]. Another significant immunological mediator of damage is viral-induced T cells (CD8)[31,32].
Another major cause of liver injury in COVID-19 appears to be coagulation disruption and endothelial damage[31,73]. Obesity is associated with hypercoagulation/thrombotic status dysregulation due to increased circulating concentrations of factor VII, von Willebrand factor and fibrinogen[13]. Ischemia-induced hypoxic liver injury most likely promotes the establishment of microvascular thrombosis[13,63]. Hypoxia caused by pneumonia is another major cause of liver damage[31]. This could be made worse in the case of COVID-19 and obesity, which is linked to obstructive sleep apnea and lower oxygen levels[28].
Ultimately, other factors may contribute to liver damage. It could be the end result of antivirals, anti-inflammatory medications, anticoagulants, antibiotics and other treatments used to treat underlying cardiometabolic chronic conditions and utilized during the COVID-19 infection, leading to liver injury[31]. Finally, dysbiosis and gut microbiota changes that usually occur in obesity could be exacerbated during COVID-19 and due to various medications[31,76]. A summary of the mechanisms can be found in Figure 1.
Future studies should focus on elucidating and comparing a variety of populations with various body mass indexes (including normal weight, overweight and various degrees of obesity) because to date few studies have examined the presence of liver steatosis in patients with overweight or obesity[77]. Furthermore, the extent of underlying liver damage (NASH, liver fibrosis, or even liver cancer) should be determined in people who have pre-existing NAFLD and to monitor the severity of the COVID-19 infection outcomes. Finally, it would be of great interest to examine the potential differential impact of COVID-19 infection due to different viral variants on individuals with NAFLD and other liver manifestations, as these studies are sparse and focus on cirrhosis of various aetiologies[78].
In conclusion, the poor clinical outcomes of COVID-19 and the greater risk of pathological LFTs seen in people with obesity and/or NAFLD are likely caused by immune activation and a tendency toward a proinflammatory state during obesity as well as by metabolic and immune-mediated parameters that place the liver at risk. Thus, obesity should be prevented, especially in the context of the COVID-19 pandemic.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author’s Membership in Professional Societies: Athens Medical Association, 072781; European Respiratory Society, 344658.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Greece
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Hernandez-Caballero A, Mexico; Zhang LL, China S-Editor: Wang JJ L-Editor: Filipodia P-Editor: Wang JJ
| 1. | Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, Wang B, Xiang H, Cheng Z, Xiong Y, Zhao Y, Li Y, Wang X, Peng Z. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA. 2020;323:1061-1069. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14113] [Cited by in RCA: 14764] [Article Influence: 2952.8] [Reference Citation Analysis (0)] |
| 2. | Georgakopoulou VE, Makrodimitri S, Triantafyllou M, Samara S, Voutsinas PM, Anastasopoulou A, Papageorgiou CV, Spandidos DA, Gkoufa A, Papalexis P, Xenou E, Chelidonis G, Sklapani P, Trakas N, Sipsas NV. Immature granulocytes: Innovative biomarker for SARSCoV2 infection. Mol Med Rep. 2022;26. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 14] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 3. | Yue H, Bai X, Wang J, Yu Q, Liu W, Pu J, Wang X, Hu J, Xu D, Li X, Kang N, Li L, Lu W, Feng T, Ding L, Qi X; Gansu Provincial Medical Treatment Expert Group of COVID-19. Clinical characteristics of coronavirus disease 2019 in Gansu province, China. Ann Palliat Med. 2020;9:1404-1412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 48] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 4. | Gracia-Ramos AE, Jaquez-Quintana JO, Contreras-Omaña R, Auron M. Liver dysfunction and SARS-CoV-2 infection. World J Gastroenterol. 2021;27:3951-3970. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 21] [Cited by in RCA: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 5. | Cholongitas E, Bali T, Georgakopoulou VE, Giannakodimos A, Gyftopoulos A, Georgilaki V, Gerogiannis D, Basoulis D, Eliadi I, Karamanakos G, Mimidis K, Sipsas NV, Samarkos M. Prevalence of abnormal liver biochemistry and its impact on COVID-19 patients' outcomes: a single-center Greek study. Ann Gastroenterol. 2022;35:290-296. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 6. | Cholongitas E, Bali T, Georgakopoulou VE, Kamiliou A, Vergos I, Makrodimitri S, Samara S, Triantafylou M, Basoulis D, Eliadi I, Karamanakos G, Sipsas NV, Samarkos M. Comparison of liver function test- and inflammation-based prognostic scores for coronavirus disease 2019: a single center study. Eur J Gastroenterol Hepatol. 2022;34:1165-1171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 7. | Georgakopoulou VE, Bali T, Adamantou M, Asimakopoulou S, Makrodimitri S, Samara S, Triantafyllou M, Voutsinas PM, Eliadi I, Karamanakos G, Basoulis D, Chatzipanagiotou O, Adamopoulou E, Alevizou A, Athanasiadis M, Spandidos DA, Papalexis P, Tarantinos K, Sipsas NV, Samarkos M, Cholongitas E. Acute hepatitis and liver injury in hospitalized patients with COVID19 infection. Exp Ther Med. 2022;24:691. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 8. | Gkoufa A, Maneta E, Ntoumas GN, Georgakopoulou VE, Mantelou A, Kokkoris S, Routsi C. Elderly adults with COVID-19 admitted to intensive care unit: A narrative review. World J Crit Care Med. 2021;10:278-289. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 13] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 9. | Georgakopoulou VE, Gkoufa A, Garmpis N, Makrodimitri S, Papageorgiou CV, Barlampa D, Garmpi A, Chiapoutakis S, Sklapani P, Trakas N, Damaskos C. COVID-19 and Acute Pancreatitis: A Systematic Review of Case Reports and Case Series. Ann Saudi Med. 2022;42:276-287. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 8] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 10. | Georgakopoulou VE, Gkoufa A, Damaskos C, Papalexis P, Pierrakou A, Makrodimitri S, Sypsa G, Apostolou A, Asimakopoulou S, Chlapoutakis S, Sklapani P, Trakas N, Spandidos DA. COVID-19-associated acute appendicitis in adults. A report of five cases and a review of the literature. Exp Ther Med. 2022;24:482. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 11. | Georgakopoulou VE, Lembessis P, Skarlis C, Gkoufa A, Sipsas NV, Mavragani CP. Hematological Abnormalities in COVID-19 Disease: Association With Type I Interferon Pathway Activation and Disease Outcomes. Front Med (Lausanne). 2022;9:850472. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 12. | Madjid M, Safavi-Naeini P, Solomon SD, Vardeny O. Potential Effects of Coronaviruses on the Cardiovascular System: A Review. JAMA Cardiol. 2020;5:831-840. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1095] [Cited by in RCA: 1249] [Article Influence: 249.8] [Reference Citation Analysis (2)] |
| 13. | Herta T, Berg T. COVID-19 and the liver - Lessons learned. Liver Int. 2021;41 Suppl 1:1-8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 14. | Méndez-Sánchez N, Valencia-Rodríguez A, Qi X, Yoshida EM, Romero-Gómez M, George J, Eslam M, Abenavoli L, Xie W, Teschke R, Carrion AF, Keaveny AP. What Has the COVID-19 Pandemic Taught Us so Far? J Clin Transl Hepatol. 2020;8:0024. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 34] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 15. | Cordeiro A, Ribamar A, Ramalho A. Adipose tissue dysfunction and MAFLD in obesity on the scene of COVID-19. Clin Res Hepatol Gastroenterol. 2022;46:101807. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 16. | Li J, Fan JG. Characteristics and Mechanism of Liver Injury in 2019 Coronavirus Disease. J Clin Transl Hepatol. 2020;8:13-17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 199] [Cited by in RCA: 206] [Article Influence: 41.2] [Reference Citation Analysis (2)] |
| 17. | Portincasa P, Krawczyk M, Smyk W, Lammert F, Di Ciaula A. COVID-19 and non-alcoholic fatty liver disease: Two intersecting pandemics. Eur J Clin Invest. 2020;50:e13338. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 108] [Cited by in RCA: 99] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 18. | Bangash MN, Patel J, Parekh D. COVID-19 and the liver: little cause for concern. Lancet Gastroenterol Hepatol. 2020;5:529-530. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 324] [Cited by in RCA: 357] [Article Influence: 71.4] [Reference Citation Analysis (0)] |
| 19. | Hu X, Pan X, Zhou W, Gu X, Shen F, Yang B, Hu Z. Clinical epidemiological analyses of overweight/obesity and abnormal liver function contributing to prolonged hospitalization in patients infected with COVID-19. Int J Obes (Lond). 2020;44:1784-1789. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 20. | Garg S, Kim L, Whitaker M, O'Halloran A, Cummings C, Holstein R, Prill M, Chai SJ, Kirley PD, Alden NB, Kawasaki B, Yousey-Hindes K, Niccolai L, Anderson EJ, Openo KP, Weigel A, Monroe ML, Ryan P, Henderson J, Kim S, Como-Sabetti K, Lynfield R, Sosin D, Torres S, Muse A, Bennett NM, Billing L, Sutton M, West N, Schaffner W, Talbot HK, Aquino C, George A, Budd A, Brammer L, Langley G, Hall AJ, Fry A. Hospitalization Rates and Characteristics of Patients Hospitalized with Laboratory-Confirmed Coronavirus Disease 2019 - COVID-NET, 14 States, March 1-30, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:458-464. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1712] [Cited by in RCA: 1699] [Article Influence: 339.8] [Reference Citation Analysis (0)] |
| 21. | Zheng Y, Ley SH, Hu FB. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat Rev Endocrinol. 2018;14:88-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2249] [Cited by in RCA: 3393] [Article Influence: 484.7] [Reference Citation Analysis (0)] |
| 22. | Heymsfield SB, Wadden TA. Mechanisms, Pathophysiology, and Management of Obesity. N Engl J Med. 2017;376:254-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 904] [Cited by in RCA: 1156] [Article Influence: 144.5] [Reference Citation Analysis (0)] |
| 23. | Bray GA, Heisel WE, Afshin A, Jensen MD, Dietz WH, Long M, Kushner RF, Daniels SR, Wadden TA, Tsai AG, Hu FB, Jakicic JM, Ryan DH, Wolfe BM, Inge TH. The Science of Obesity Management: An Endocrine Society Scientific Statement. Endocr Rev. 2018;39:79-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 550] [Cited by in RCA: 518] [Article Influence: 74.0] [Reference Citation Analysis (0)] |
| 24. | Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet. 2008;371:569-578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3505] [Cited by in RCA: 3677] [Article Influence: 216.3] [Reference Citation Analysis (1)] |
| 25. | Martin SD, McGee SL. Metabolic reprogramming in type 2 diabetes and the development of breast cancer. J Endocrinol. 2018;237:R35-R46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 26. | Renehan AG, Zwahlen M, Egger M. Adiposity and cancer risk: new mechanistic insights from epidemiology. Nat Rev Cancer. 2015;15:484-498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 381] [Cited by in RCA: 470] [Article Influence: 47.0] [Reference Citation Analysis (0)] |
| 27. | Frühbeck G, Busetto L, Dicker D, Yumuk V, Goossens GH, Hebebrand J, Halford JGC, Farpour-Lambert NJ, Blaak EE, Woodward E, Toplak H. The ABCD of Obesity: An EASO Position Statement on a Diagnostic Term with Clinical and Scientific Implications. Obes Facts. 2019;12:131-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 173] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 28. | Lempesis IG, van Meijel RLJ, Manolopoulos KN, Goossens GH. Oxygenation of adipose tissue: A human perspective. Acta Physiol (Oxf). 2020;228:e13298. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 88] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 29. | Hayat U, Ashfaq MZ, Johnson L, Ford R, Wuthnow C, Kadado K, El Jurdi K, Okut H, Kilgore WR, Assi M, Siddiqui AA. The Association of Metabolic-Associated Fatty Liver Disease with Clinical Outcomes of COVID-19: A Systematic Review and Meta-Analysis. Kans J Med. 2022;15:241-246. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 30. | Williamson EJ, Walker AJ, Bhaskaran K, Bacon S, Bates C, Morton CE, Curtis HJ, Mehrkar A, Evans D, Inglesby P, Cockburn J, McDonald HI, MacKenna B, Tomlinson L, Douglas IJ, Rentsch CT, Mathur R, Wong AYS, Grieve R, Harrison D, Forbes H, Schultze A, Croker R, Parry J, Hester F, Harper S, Perera R, Evans SJW, Smeeth L, Goldacre B. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584:430-436. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4343] [Cited by in RCA: 4205] [Article Influence: 841.0] [Reference Citation Analysis (0)] |
| 31. | Ozkurt Z, Çınar Tanrıverdi E. COVID-19: Gastrointestinal manifestations, liver injury and recommendations. World J Clin Cases. 2022;10:1140-1163. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 29] [Cited by in RCA: 26] [Article Influence: 8.7] [Reference Citation Analysis (7)] |
| 32. | Cichoż-Lach H, Michalak A. Liver injury in the era of COVID-19. World J Gastroenterol. 2021;27:377-390. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 53] [Cited by in RCA: 51] [Article Influence: 12.8] [Reference Citation Analysis (2)] |
| 33. | Metawea MI, Yousif WI, Moheb I. COVID 19 and liver: An A-Z literature review. Dig Liver Dis. 2021;53:146-152. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 60] [Article Influence: 15.0] [Reference Citation Analysis (2)] |
| 34. | Garrido I, Liberal R, Macedo G. Review article: COVID-19 and liver disease-what we know on 1st May 2020. Aliment Pharmacol Ther. 2020;52:267-275. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 125] [Cited by in RCA: 118] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 35. | Boettler T, Marjot T, Newsome PN, Mondelli MU, Maticic M, Cordero E, Jalan R, Moreau R, Cornberg M, Berg T. Impact of COVID-19 on the care of patients with liver disease: EASL-ESCMID position paper after 6 months of the pandemic. JHEP Rep. 2020;2:100169. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 103] [Cited by in RCA: 121] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 36. | Wang Q, Zhao H, Liu LG, Wang YB, Zhang T, Li MH, Xu YL, Gao GJ, Xiong HF, Fan Y, Cao Y, Ding R, Wang JJ, Cheng C, Xie W. Pattern of liver injury in adult patients with COVID-19: a retrospective analysis of 105 patients. Mil Med Res. 2020;7:28. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 50] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 37. | Lei F, Liu YM, Zhou F, Qin JJ, Zhang P, Zhu L, Zhang XJ, Cai J, Lin L, Ouyang S, Wang X, Yang C, Cheng X, Liu W, Li H, Xie J, Wu B, Luo H, Xiao F, Chen J, Tao L, Cheng G, She ZG, Zhou J, Wang H, Lin J, Luo P, Fu S, Ye P, Xiao B, Mao W, Liu L, Yan Y, Chen G, Huang X, Zhang BH, Yuan Y. Longitudinal Association Between Markers of Liver Injury and Mortality in COVID-19 in China. Hepatology. 2020;72:389-398. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 318] [Cited by in RCA: 309] [Article Influence: 61.8] [Reference Citation Analysis (0)] |
| 38. | Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, Davidson KW; the Northwell COVID-19 Research Consortium, Barnaby DP, Becker LB, Chelico JD, Cohen SL, Cookingham J, Coppa K, Diefenbach MA, Dominello AJ, Duer-Hefele J, Falzon L, Gitlin J, Hajizadeh N, Harvin TG, Hirschwerk DA, Kim EJ, Kozel ZM, Marrast LM, Mogavero JN, Osorio GA, Qiu M, Zanos TP. Presenting Characteristics, Comorbidities, and Outcomes Among 5700 Patients Hospitalized With COVID-19 in the New York City Area. JAMA. 2020;323:2052-2059. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6024] [Cited by in RCA: 6516] [Article Influence: 1303.2] [Reference Citation Analysis (0)] |
| 39. | Shi H, Han X, Jiang N, Cao Y, Alwalid O, Gu J, Fan Y, Zheng C. Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: a descriptive study. Lancet Infect Dis. 2020;20:425-434. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2493] [Cited by in RCA: 2310] [Article Influence: 462.0] [Reference Citation Analysis (0)] |
| 40. | Mao R, Qiu Y, He JS, Tan JY, Li XH, Liang J, Shen J, Zhu LR, Chen Y, Iacucci M, Ng SC, Ghosh S, Chen MH. Manifestations and prognosis of gastrointestinal and liver involvement in patients with COVID-19: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2020;5:667-678. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 741] [Cited by in RCA: 753] [Article Influence: 150.6] [Reference Citation Analysis (0)] |
| 41. | Yao N, Wang SN, Lian JQ, Sun YT, Zhang GF, Kang WZ, Kang W. [Clinical characteristics and influencing factors of patients with novel coronavirus pneumonia combined with liver injury in Shaanxi region]. Zhonghua Gan Zang Bing Za Zhi. 2020;28:234-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 39] [Reference Citation Analysis (0)] |
| 42. | Wang Y, Zhang D, Du G, Du R, Zhao J, Jin Y, Fu S, Gao L, Cheng Z, Lu Q, Hu Y, Luo G, Wang K, Lu Y, Li H, Wang S, Ruan S, Yang C, Mei C, Wang Y, Ding D, Wu F, Tang X, Ye X, Ye Y, Liu B, Yang J, Yin W, Wang A, Fan G, Zhou F, Liu Z, Gu X, Xu J, Shang L, Zhang Y, Cao L, Guo T, Wan Y, Qin H, Jiang Y, Jaki T, Hayden FG, Horby PW, Cao B, Wang C. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2020;395:1569-1578. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2337] [Cited by in RCA: 2487] [Article Influence: 497.4] [Reference Citation Analysis (0)] |
| 43. | Wu ZH, Yang DL. A meta-analysis of the impact of COVID-19 on liver dysfunction. Eur J Med Res. 2020;25:54. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 48] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 44. | Wijarnpreecha K, Ungprasert P, Panjawatanan P, Harnois DM, Zaver HB, Ahmed A, Kim D. COVID-19 and liver injury: a meta-analysis. Eur J Gastroenterol Hepatol. 2021;33:990-995. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 63] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 45. | Ji D, Qin E, Xu J, Zhang D, Cheng G, Wang Y, Lau G. Non-alcoholic fatty liver diseases in patients with COVID-19: A retrospective study. J Hepatol. 2020;73:451-453. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 336] [Cited by in RCA: 408] [Article Influence: 81.6] [Reference Citation Analysis (2)] |
| 46. | Elshazli RM, Toraih EA, Elgaml A, El-Mowafy M, El-Mesery M, Amin MN, Hussein MH, Killackey MT, Fawzy MS, Kandil E. Diagnostic and prognostic value of hematological and immunological markers in COVID-19 infection: A meta-analysis of 6320 patients. PLoS One. 2020;15:e0238160. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 162] [Cited by in RCA: 140] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 47. | Wong YJ, Tan M, Zheng Q, Li JW, Kumar R, Fock KM, Teo EK, Ang TL. A systematic review and meta-analysis of the COVID-19 associated liver injury. Ann Hepatol. 2020;19:627-634. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 36] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 48. | Kulkarni AV, Kumar P, Tevethia HV, Premkumar M, Arab JP, Candia R, Talukdar R, Sharma M, Qi X, Rao PN, Reddy DN. Systematic review with meta-analysis: liver manifestations and outcomes in COVID-19. Aliment Pharmacol Ther. 2020;52:584-599. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 199] [Cited by in RCA: 183] [Article Influence: 36.6] [Reference Citation Analysis (0)] |
| 49. | Figliozzi S, Masci PG, Ahmadi N, Tondi L, Koutli E, Aimo A, Stamatelopoulos K, Dimopoulos MA, Caforio ALP, Georgiopoulos G. Predictors of adverse prognosis in COVID-19: A systematic review and meta-analysis. Eur J Clin Invest. 2020;50:e13362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 225] [Article Influence: 45.0] [Reference Citation Analysis (0)] |
| 50. | Eslam M, Newsome PN, Sarin SK, Anstee QM, Targher G, Romero-Gomez M, Zelber-Sagi S, Wai-Sun Wong V, Dufour JF, Schattenberg JM, Kawaguchi T, Arrese M, Valenti L, Shiha G, Tiribelli C, Yki-Järvinen H, Fan JG, Grønbæk H, Yilmaz Y, Cortez-Pinto H, Oliveira CP, Bedossa P, Adams LA, Zheng MH, Fouad Y, Chan WK, Mendez-Sanchez N, Ahn SH, Castera L, Bugianesi E, Ratziu V, George J. A new definition for metabolic dysfunction-associated fatty liver disease: An international expert consensus statement. J Hepatol. 2020;73:202-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2883] [Cited by in RCA: 2827] [Article Influence: 565.4] [Reference Citation Analysis (1)] |
| 51. | Petrilli CM, Jones SA, Yang J, Rajagopalan H, O'Donnell L, Chernyak Y, Tobin KA, Cerfolio RJ, Francois F, Horwitz LI. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. BMJ. 2020;369:m1966. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1590] [Cited by in RCA: 1819] [Article Influence: 363.8] [Reference Citation Analysis (1)] |
| 52. | Zhou YJ, Zheng KI, Wang XB, Yan HD, Sun QF, Pan KH, Wang TY, Ma HL, Chen YP, George J, Zheng MH. Younger patients with MAFLD are at increased risk of severe COVID-19 illness: A multicenter preliminary analysis. J Hepatol. 2020;73:719-721. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 104] [Cited by in RCA: 106] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 53. | Goossens GH. The role of adipose tissue dysfunction in the pathogenesis of obesity-related insulin resistance. Physiol Behav. 2008;94:206-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 350] [Cited by in RCA: 376] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 54. | Rosen ED, Spiegelman BM. What we talk about when we talk about fat. Cell. 2014;156:20-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1414] [Cited by in RCA: 1778] [Article Influence: 161.6] [Reference Citation Analysis (0)] |
| 55. | Blüher M. Adipose tissue dysfunction contributes to obesity related metabolic diseases. Best Pract Res Clin Endocrinol Metab. 2013;27:163-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 274] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 56. | Shulman GI. Ectopic fat in insulin resistance, dyslipidemia, and cardiometabolic disease. N Engl J Med. 2014;371:2237-2238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 133] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 57. | Goossens GH, Blaak EE. Adipose tissue dysfunction and impaired metabolic health in human obesity: a matter of oxygen? Front Endocrinol (Lausanne). 2015;6:55. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 110] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 58. | Frayn KN, Karpe F. Regulation of human subcutaneous adipose tissue blood flow. Int J Obes (Lond). 2014;38:1019-1026. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 100] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 59. | Lempesis IG, Goossens GH, Manolopoulos KN. Measurement of human abdominal and femoral intravascular adipose tissue blood flow using percutaneous Doppler ultrasound. Adipocyte. 2021;10:119-123. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 60. | Fasshauer M, Blüher M. Adipokines in health and disease. Trends Pharmacol Sci. 2015;36:461-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 523] [Cited by in RCA: 750] [Article Influence: 75.0] [Reference Citation Analysis (0)] |
| 61. | Lamers D, Famulla S, Wronkowitz N, Hartwig S, Lehr S, Ouwens DM, Eckardt K, Kaufman JM, Ryden M, Müller S, Hanisch FG, Ruige J, Arner P, Sell H, Eckel J. Dipeptidyl peptidase 4 is a novel adipokine potentially linking obesity to the metabolic syndrome. Diabetes. 2011;60:1917-1925. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 431] [Cited by in RCA: 461] [Article Influence: 32.9] [Reference Citation Analysis (0)] |
| 62. | Kim CS, Park HS, Kawada T, Kim JH, Lim D, Hubbard NE, Kwon BS, Erickson KL, Yu R. Circulating levels of MCP-1 and IL-8 are elevated in human obese subjects and associated with obesity-related parameters. Int J Obes (Lond). 2006;30:1347-1355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 327] [Cited by in RCA: 377] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 63. | Pasquarelli-do-Nascimento G, Braz-de-Melo HA, Faria SS, Santos IO, Kobinger GP, Magalhães KG. Hypercoagulopathy and Adipose Tissue Exacerbated Inflammation May Explain Higher Mortality in COVID-19 Patients With Obesity. Front Endocrinol (Lausanne). 2020;11:530. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 68] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 64. | Pérez-Campos Mayoral L, Matias-Cervantes CA, Pérez-Campos E, Romero Díaz C, Laguna Barrios LÁ, Pina Canseco MDS, Martínez Cruz M, Pérez-Campos Mayoral E, Solórzano Mata CJ, Rodal Canales FJ, Martínez Ruíz H, Hernández-Huerta MT. Associations of Dynapenic Obesity and Sarcopenic Obesity with the Risk of Complications in COVID-19. Int J Mol Sci. 2022;23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 65. | Sarwar R, Pierce N, Koppe S. Obesity and nonalcoholic fatty liver disease: current perspectives. Diabetes Metab Syndr Obes. 2018;11:533-542. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 129] [Cited by in RCA: 164] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 66. | Beals JW, Smith GI, Shankaran M, Fuchs A, Schweitzer GG, Yoshino J, Field T, Matthews M, Nyangau E, Morozov D, Mittendorfer B, Hellerstein MK, Klein S. Increased Adipose Tissue Fibrogenesis, Not Impaired Expandability, Is Associated With Nonalcoholic Fatty Liver Disease. Hepatology. 2021;74:1287-1299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 39] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 67. | Ter Horst KW, Gilijamse PW, Versteeg RI, Ackermans MT, Nederveen AJ, la Fleur SE, Romijn JA, Nieuwdorp M, Zhang D, Samuel VT, Vatner DF, Petersen KF, Shulman GI, Serlie MJ. Hepatic Diacylglycerol-Associated Protein Kinase Cε Translocation Links Hepatic Steatosis to Hepatic Insulin Resistance in Humans. Cell Rep. 2017;19:1997-2004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 127] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 68. | Korenblat KM, Fabbrini E, Mohammed BS, Klein S. Liver, muscle, and adipose tissue insulin action is directly related to intrahepatic triglyceride content in obese subjects. Gastroenterology. 2008;134:1369-1375. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 484] [Cited by in RCA: 461] [Article Influence: 27.1] [Reference Citation Analysis (0)] |
| 69. | Sanyal AJ, Campbell-Sargent C, Mirshahi F, Rizzo WB, Contos MJ, Sterling RK, Luketic VA, Shiffman ML, Clore JN. Nonalcoholic steatohepatitis: association of insulin resistance and mitochondrial abnormalities. Gastroenterology. 2001;120:1183-1192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1458] [Cited by in RCA: 1510] [Article Influence: 62.9] [Reference Citation Analysis (0)] |
| 70. | Sharpton SR, Schnabl B, Knight R, Loomba R. Current Concepts, Opportunities, and Challenges of Gut Microbiome-Based Personalized Medicine in Nonalcoholic Fatty Liver Disease. Cell Metab. 2021;33:21-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 124] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 71. | Morgan K, Samuel K, Vandeputte M, Hayes PC, Plevris JN. SARS-CoV-2 Infection and the Liver. Pathogens. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 36] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 72. | Zhang W, Li C, Liu B, Wu R, Zou N, Xu YZ, Yang YY, Zhang F, Zhou HM, Wan KQ, Xiao XQ, Zhang X. Pioglitazone upregulates hepatic angiotensin converting enzyme 2 expression in rats with steatohepatitis. Ann Hepatol. 2013;12:892-900. [PubMed] |
| 73. | Lagana SM, Kudose S, Iuga AC, Lee MJ, Fazlollahi L, Remotti HE, Del Portillo A, De Michele S, de Gonzalez AK, Saqi A, Khairallah P, Chong AM, Park H, Uhlemann AC, Lefkowitch JH, Verna EC. Hepatic pathology in patients dying of COVID-19: a series of 40 cases including clinical, histologic, and virologic data. Mod Pathol. 2020;33:2147-2155. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 130] [Cited by in RCA: 181] [Article Influence: 36.2] [Reference Citation Analysis (0)] |
| 74. | Napodano C, Pocino K, Stefanile A, Marino M, Miele L, Gulli F, Basile V, Pandolfi F, Gasbarrini A, Rapaccini GL, Basile U. COVID-19 and hepatic involvement: The liver as a main actor of the pandemic novel. Scand J Immunol. 2021;93:e12977. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 75. | Fara A, Mitrev Z, Rosalia RA, Assas BM. Cytokine storm and COVID-19: a chronicle of pro-inflammatory cytokines. Open Biol. 2020;10:200160. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 205] [Cited by in RCA: 229] [Article Influence: 45.8] [Reference Citation Analysis (0)] |
| 76. | Pereira SS, Alvarez-Leite JI. Low-Grade Inflammation, Obesity, and Diabetes. Curr Obes Rep. 2014;3:422-431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 154] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 77. | Chen VL, Hawa F, Berinstein JA, Reddy CA, Kassab I, Platt KD, Hsu CY, Steiner CA, Louissaint J, Gunaratnam NT, Sharma P. Hepatic Steatosis Is Associated with Increased Disease Severity and Liver Injury in Coronavirus Disease-19. Dig Dis Sci. 2021;66:3192-3198. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 35] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 78. | Elhence A, Vaishnav M, Biswas S, Anand A, Gunjan D, Kedia S, Mahapatra SJ, Nayak B, Sheikh S, Soni KD, Trikha A, Goel A, Shalimar. Predictors of in-hospital Outcomes in Patients With Cirrhosis and Coronavirus Disease-2019. J Clin Exp Hepatol. 2022;12:876-886. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 4.3] [Reference Citation Analysis (0)] |