Published online Feb 14, 2023. doi: 10.3748/wjg.v29.i6.1090
Peer-review started: August 24, 2022
First decision: November 5, 2022
Revised: December 11, 2022
Accepted: January 5, 2023
Article in press: January 5, 2023
Published online: February 14, 2023
Processing time: 170 Days and 2.2 Hours
The impact of racial and regional disparity on younger patients with gastric cancer (GC) remains unclear.
To investigate the clinicopathological characteristics, prognostic nomogram, and biological analysis of younger GC patients in China and the United States.
From 2000 to 2018, GC patients aged less than 40 years were enrolled from the China National Cancer Center and the Surveillance Epidemiology and End Results database. Biological analysis was performed based on the Gene Expression Omnibus database. Survival analysis was conducted via Kaplan-Meier estimates and Cox proportional hazards models.
A total of 6098 younger GC patients were selected from 2000 to 2018, of which 1159 were enrolled in the China National Cancer Center, and 4939 were collected from the Surveillance Epidemiology and End Results database. Compared with the United States group, younger patients in China revealed better survival outcomes (P < 0.01). For race/ethnicity, younger Chinese cases also enjoyed a better prognosis than that in White and Black datasets (P < 0.01). After stratification by pathological Tumor-Node-Metastasis (pTNM) stage, a survival advantage was observed in China with pathological stage I, III, and IV (all P < 0.01), whereas younger GC patients with stage II showed no difference (P = 0.16). In multivariate analysis, predictors in China involved period of diagnosis, linitis plastica, and pTNM stage, while race, diagnostic period, sex, location, differentiation, linitis plastica, signet ring cell, pTNM stage, surgery, and chemotherapy were confirmed in the United States group. Prognostic nomograms for younger patients were established, with the area under the curve of 0.786 in the China group and of 0.842 in the United States group. Moreover, three gene expression profiles (GSE27342, GSE51105, and GSE38749) were enrolled in further biological analysis, and distinctive molecular characteristics were identified in younger GC patients among different regions.
Except for younger cases with pTNM stage II, a survival advantage was observed in the China group with pathological stage I, III, and IV compared to the United States group, which might be partly due to differences in surgical approaches and the improvement of the cancer screening in China. The nomogram model provided an insightful and applicable tool to evaluate the prognosis of younger patients in China and the United States. Furthermore, biological analysis of younger patients was performed among different regions, which might partly explain the histopathological behavior and survival disparity in the subpopulations.
Core Tip: The impact of racial and regional disparity on younger patients with gastric cancer (GC) is not clear. A total of 6098 younger GC patients were selected from 2000 to 2018, of which 1159 were enrolled in the China National Cancer Center, and 4939 were collected from the Surveillance Epidemiology and End Results database. Compared with the United States group, younger patients in China revealed better survival outcomes and a better prognosis. Three gene expression profiles from the Gene Expression Omnibus database were enrolled in further biological analysis, and distinctive molecular characteristics were identified in younger GC patients among different regions.
- Citation: Niu PH, Zhao LL, Wang WQ, Zhang XJ, Li ZF, Luan XY, Chen YT. Survival benefit of younger gastric cancer patients in China and the United States: A comparative study. World J Gastroenterol 2023; 29(6): 1090-1108
- URL: https://www.wjgnet.com/1007-9327/full/v29/i6/1090.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i6.1090
Gastric cancer (GC) is the fourth leading cause of cancer-related deaths worldwide[1], with the highest incidence among individuals aged 50-70 years. Only 2.0%-6.2% of documented cases are among patients younger than 40 years[2,3]. Although the incidence of GC has gradually declined, important age-specific details may be obscured by the overall trend[4]. During the last decades, the incidence of younger GC patients has remained stable or even increased in both Western and Eastern populations[5-7].
It was long thought that younger patients with GC had aggressive behavior and serious prognosis[8]. Further insight into which subpopulations were at the highest risk of dying remained a crucial requisite so that interventions could be initiated appropriately. Recently, several studies have demonstrated a wide survival discrepancy of GC in different regions or races[9,10]. Notably, GC patients in Asia had more favorable prognoses than patients in Western countries. The important difference has also been found in certain subtypes of GC, including sex and anatomic location[9,10]. For younger GC patients, however, knowledge concerning the regional and racial disparity is scarce. Chen et al[11] reported that younger patients in China have a longer survival time than younger patients in the United States, whereas Strong et al[12] showed no difference between the United States and Chinese cohorts. The inconsistent findings from these studies might derive from the small sample sizes, with the population records ranging from 336 to 1075.
As such, based on a unique combination of the Surveillance Epidemiology and End Results (SEER) database in the United States and a high-volume National Cancer Center Database in China, we sought to compare the clinicopathological features, survival outcomes, and prognostic nomograms in younger Chinese and United States patients. Moreover, biological analysis of younger GC patients was further evaluated, which might partly explain the histopathological behaviors and survival disparity among different regions and races.
The study queried clinical data from 2000 to 2018 based on the two large independent cohorts. The histologically confirmed GC cases in China were selected through the China National Cancer Center Gastric Cancer Database (NCCGCDB). As a single but high-volume cohort, NCCGCDB included more than 18000 patients from all regions of China over the past 20 years. Meanwhile, the United States group was identified from the SEER database. The SEER database, supported by the National Cancer Institute, constituted approximately 27.8% of the United States population[13]. Younger patients were defined as GC cases younger than 40 years of age, which remains consistent with the majority of previous studies[14,15]. Clinical data abstracted from the NCCGCDB and SEER databases included younger patients’ demographics, clinicopathological characteristics, and survival variables. The stage of GC was assessed according to the 8th edition of the American Joint Committee on Cancer Tumor-Node-Metastasis (TNM) staging system. The primary endpoint of the study was overall survival (OS). Moreover, the gene expression sets evaluated in the study were obtained from the Gene Expression Omnibus (GEO) database (https://www.ncbi.nlm.nih.gov/geo/). The study protocol was approved by the ethics committee of National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College (No. 17-156/1412), and all methods were carried out in accordance with relevant guidelines and regulations in the ethics approval and consent.
The line chart was plotted to analyze the changing ratios of younger GC patients from 2000 to 2018. Categorical variables were compared using the χ2 test. Comparisons were performed using the t-test for normally distributed continuous variables and the Mann-Whitney U test for variables not normally distributed. Survival curves for different regions and races were calculated with the Kaplan-Meier method, while the log-rank test estimated the relevant survival disparity. Univariate and multivariate Cox proportional hazards models were used to determine the prognostic factors for younger patients, while the corresponding hazard ratio and 95% confidence interval (CI) were generated. The covariates with a P value of < 0.10 in the univariate models were included in the multivariate analysis[16]. Statistical significance was set at a two-sided P value less than 0.05. The survival nomogram for China and the United States was formulated based on the multivariate analysis. Younger cases were randomized 7:3, which were adopted in the training set and the validation set, respectively. The area under the curves (AUC), the concordance index (C-index), and the calibration plots were performed to measure the effectiveness of nomograms. All statistical analyses in the study were conducted using R software v.3.6.3 (http://www.r-project.org/).
Based on the GEO database, the differentially expressed genes (DEGs) analysis was further performed to evaluate gene discrepancy for younger patients among different regions, while genes that met the cutoff criteria, adjusted P-value < 0.05 and |logFC| > 2.0, where FC represents fold change, were considered as DEGs. A heatmap was constructed with 50 differential genes from the DEGs analysis. The Search Tool for the Retrieval of Interacting Genes database (http://string-db.org/) was designed to analyze the protein-protein interaction (PPI) network, which was subsequently visualized by Cytoscape software (www.cytoscape.org/). As a plugin in Cytoscape, CytoHubba was used to calculate the degree of protein node, and the top 10 genes were identified as hub genes.
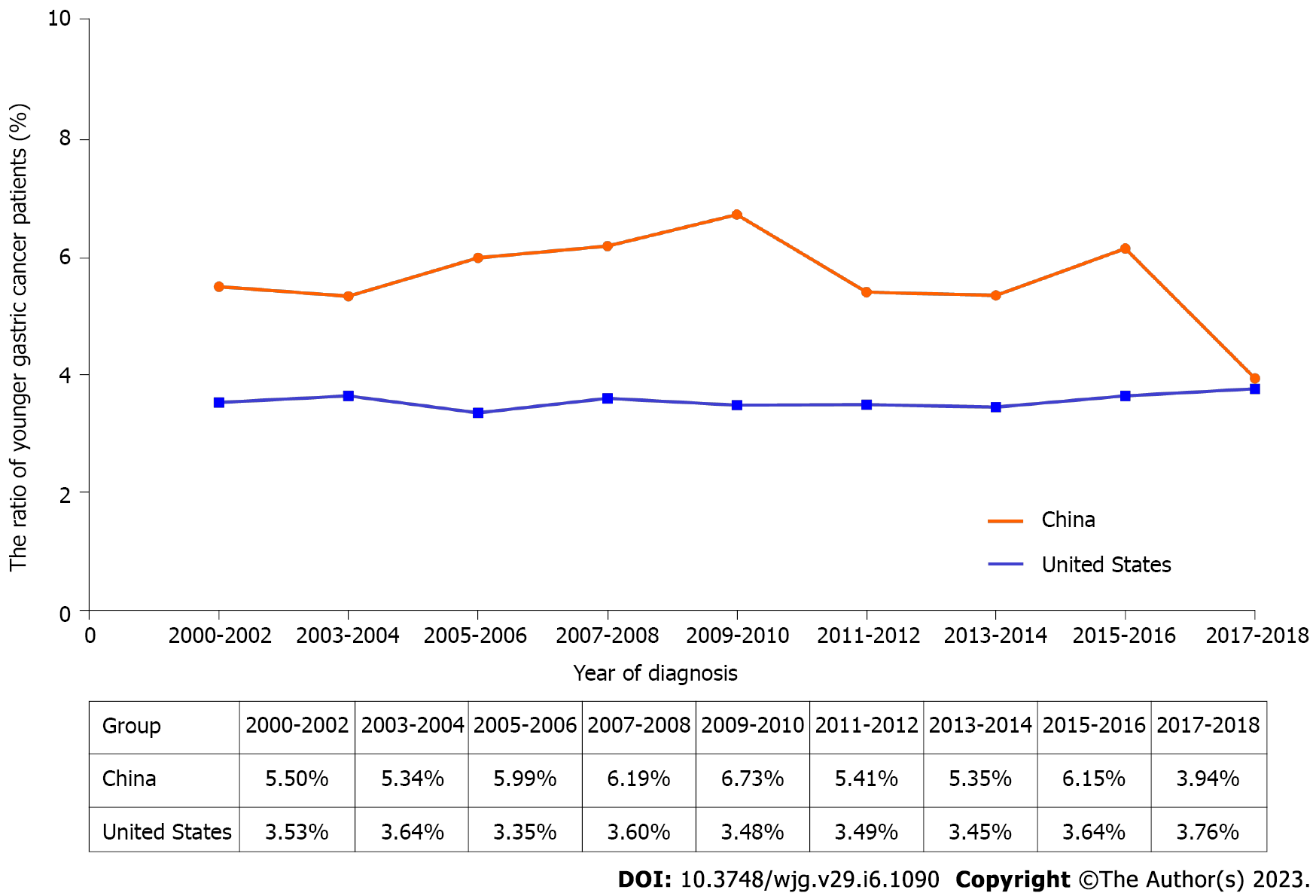
From 2000 to 2018, a total of 6098 younger GC patients were selected, of which 1159 were enrolled in NCCGCDB, and 4939 were collected from the SEER database. The mean age of younger patients was 33.77 ± 4.53 in China, and 32.91 ± 5.38 in the United States (P < 0.0001). As shown in Figure 1, time trends of younger patients from 2000 to 2018 have remained stable in both China and the United States sets (range 3.9%-6.7%, range 3.4%-3.8%, respectively). Compared to the United States group, the China group had a higher ratio of younger patients over periods.
Notably, compared to the United States, younger GC patients in China predominantly had distal tumor location (83.7% vs 66.7%, P < 0.0001), poor differentiation (82.5% vs 78.5%, P < 0.0001), and signet ring cell carcinoma (52.9% vs 28.9%, P < 0.0001). Conversely, relatively higher percentages of proximal location (33.3% vs 16.3%, P < 0.0001), well differentiation (7.5% vs 1.2%, P < 0.0001), and pTNM stage IV tumors (52.5% vs 21.4%, P < 0.0001) were revealed in the United States patients (Table 1). As for treatment, the percentage of surgery (75.1% vs 41.4%, P < 0.0001), lymphadenectomy (67.4% vs 19.1%, P < 0.0001), and lymphadenectomy with at least 15 examined lymph nodes (ELNs ≥ 15) (52.4% vs 10.6%, P < 0.0001) were higher in China than those in the United States. In addition, nearly 60.6% of the patients in the United States cohort received chemotherapy compared with only 36.9% in the Chinese cohort (P < 0.0001).
| China (n = 1159), n (%) | United States (n = 4939), n (%) | P value | |||
| Period of diagnosis | < 0.0001 | ||||
| 1 (2000-2003) | 194 | 16.7 | 972 | 19.7 | |
| 2 (2004-2008) | 237 | 20.5 | 1248 | 25.3 | |
| 3 (2009-2013) | 396 | 34.3 | 1280 | 25.9 | |
| 4 (2014-2018) | 332 | 28.6 | 1439 | 29.1 | |
| Race | < 0.0001 | ||||
| Chinese | 1159 | 100.0 | 126 | 2.6 | |
| White | 3405 | 69.9 | |||
| Black | 703 | 14.4 | |||
| Others | 640 | 13.1 | |||
| Gender | 0.135 | ||||
| Male | 581 | 50.1 | 2599 | 52.6 | |
| Female | 578 | 49.9 | 2340 | 47.4 | |
| Age (year), mean ± SD | 0.005 | ||||
| 34 | 32.91 | ||||
| 5 | 5.38 | ||||
| Primary tumor location | < 0.0001 | ||||
| Proximal | 176 | 16.3 | 1030 | 33.3 | |
| Distal | 903 | 83.7 | 2067 | 66.7 | |
| Differentiation | < 0.0001 | ||||
| Well | 9 | 1.2 | 211 | 7.5 | |
| Moderate | 124 | 16.3 | 394 | 14.0 | |
| Poor/Undifferentiated | 626 | 82.5 | 2214 | 78.5 | |
| Linitis plastica | 0.070 | ||||
| Yes | 29 | 2.6 | 57 | 1.7 | |
| No | 1101 | 97.4 | 3279 | 98.3 | |
| Signet ring cell carcinoma | < 0.0001 | ||||
| Yes | 426 | 52.9 | 1429 | 28.9 | |
| No | 380 | 47.1 | 3510 | 71.1 | |
| Pathologic T-stage (AJCC 8th) | < 0.0001 | ||||
| T1 | 197 | 22.2 | 498 | 24.3 | |
| T2 | 97 | 10.9 | 266 | 13.0 | |
| T3 | 143 | 16.1 | 509 | 24.9 | |
| T4 | 452 | 50.8 | 773 | 37.8 | |
| Pathologic N-stage (AJCC 8th) | < 0.0001 | ||||
| N0 | 412 | 41.8 | 1137 | 49.9 | |
| N1 | 119 | 12.1 | 724 | 31.7 | |
| N2 | 162 | 16.4 | 232 | 10.2 | |
| N3 | 292 | 29.6 | 188 | 8.2 | |
| Pathologic M-stage | < 0.0001 | ||||
| M0 | 869 | 79.4 | 1268 | 45.9 | |
| M1 | 225 | 20.6 | 1492 | 54.1 | |
| pTNM stage (AJCC TNM 8th) | < 0.0001 | ||||
| I | 222 | 21.2 | 649 | 20.3 | |
| II | 169 | 16.1 | 377 | 11.7 | |
| III | 433 | 41.3 | 498 | 15.5 | |
| IV | 225 | 21.4 | 1687 | 52.5 | |
| Surgery | < 0.0001 | ||||
| Yes | 869 | 75.1 | 2047 | 41.4 | |
| No | 288 | 24.9 | 2892 | 58.6 | |
| Chemotherapy | < 0.0001 | ||||
| Yes | 428 | 36.9 | 2991 | 60.6 | |
| No | 731 | 63.1 | 1948 | 39.4 | |
| Lymphadenectomy | < 0.0001 | ||||
| Yes | 780 | 67.4 | 941 | 19.1 | |
| No | 377 | 32.6 | 3398 | 80.9 | |
| Lymphadenectomy with at least 15 lymph nodes | < 0.0001 | ||||
| Yes | 606 | 52.4 | 524 | 10.6 | |
| No | 551 | 47.6 | 4415 | 89.4 | |
| Number nodes examined (n), mean ± SD | < 0.0001 | ||||
| 25 | 18.9 | ||||
| 13 | 15.0 | ||||
| Number positive nodes (n), mean ± SD | < 0.0001 | ||||
| 6 | 7.4 | ||||
| 8 | 7.4 | ||||
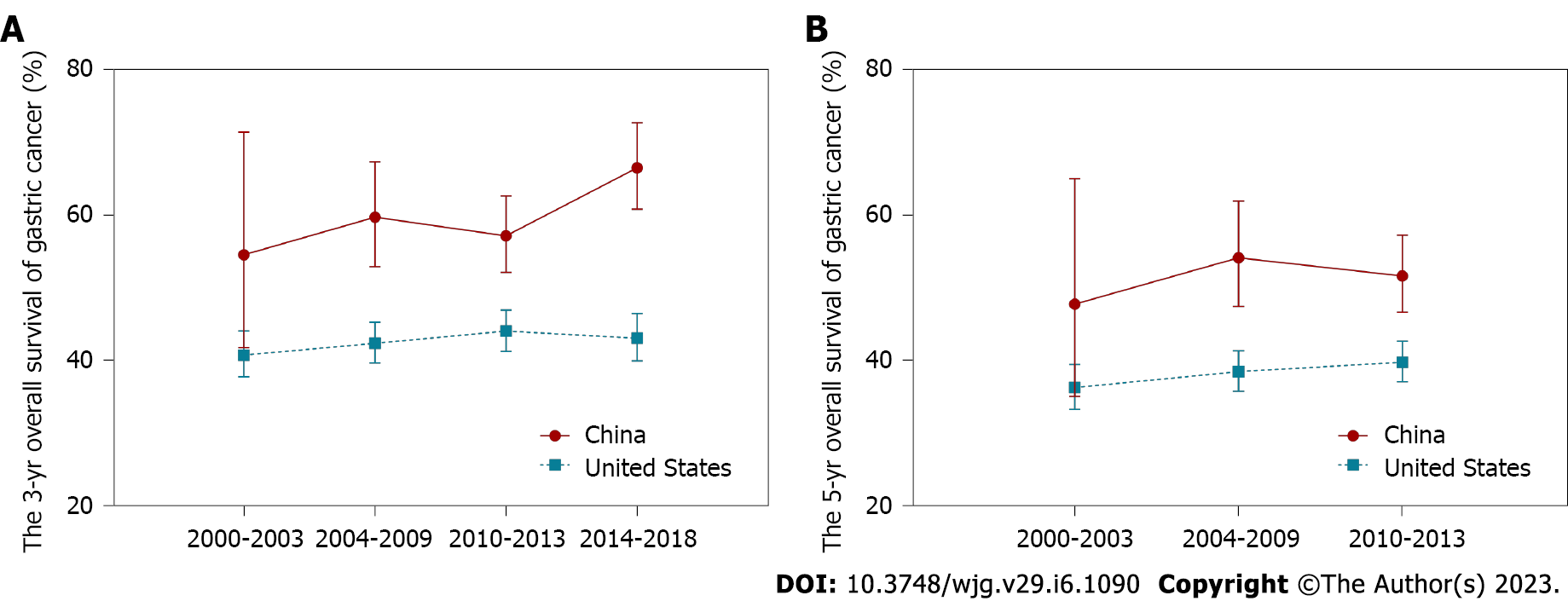
Survival trends of younger GC patients are summarized in Table 2 and Figure 2. Compared to the stable prognosis of the United States, a noticeable survival increment was shown in younger Chinese patients. The 3-year OS increased from 54.5% (95%CI: 41.7%-71.4%) in 2000-2003 to 66.5% (95%CI: 60.8%-72.7%) in 2014-2018, while the 5-year OS improved from 47.7% (95%CI: 35.0%-65.0%) in 2000-2003 to 51.6% (95%CI: 46.6%-57.2%) in 2009-2013.
| Characteristics | China | United States | ||||
| 1-yr, % (95%CI) | 3-yr, % (95%CI) | 5-yr, % (95%CI) | 1-yr, % (95%CI) | 3-yr, % (95%CI) | 5-yr, % (95%CI) | |
| Total | ||||||
| Period of diagnosis | ||||||
| 1 (2000-2003) | 75.0 (63.2-89.0) | 54.5 (41.7-71.4) | 47.7 (35.0-65.0) | 58.4 (55.3-61.7) | 40.7 (37.7-44.0) | 36.2 (33.2-39.4) |
| 2 (2004-2008) | 80.1 (74.5-86.1) | 59.7 (52.9-67.3) | 54.1 (47.4-61.9) | 58.1 (55.4-61.0) | 42.3 (39.6-45.2) | 38.4 (35.7-41.3) |
| 3 (2009-2013) | 76.7 (72.3-81.3) | 57.1 (52.1-62.6) | 51.6 (46.6-57.2) | 60.8 (58.1-63.6) | 44.0 (41.2-46.9) | 39.7 (37.0-42.6) |
| 4 (2014-2018) | 88.6 (85.1-92.3) | 66.5 (60.8-72.7) | 61.6(58.9-64.4) | 43.0 (39.9-46.4) | ||
| Race | ||||||
| Chinese | 81.4 (78.9-84.1) | 60.9 (57.6-64.3) | 52.3 (48.8-56.0) | 66.0 (58.1-75.1) | 52.9 (44.4-62.9) | 42.9 (34.4-53.5) |
| White | 59.7 (58.1-61.5) | 41.7 (40.0-43.6) | 37.8 (36.0-39.6) | |||
| Black | 58.0 (54.3-61.8) | 42.7 (39.0-46.7) | 38.2 (34.6-42.3) | |||
| Others | 59.4 (55.6-63.5) | 43.3 (39.4-47.6) | 37.2 (33.3-41.6) | |||
| Gender | ||||||
| Male | 82.2 (78.7-85.8) | 61.5 (57.1-66.2) | 53.6 (49.0-58.7) | 57.0 (55.0-59.0) | 39.6 (37.6-41.7) | 34.8 (32.8-36.9) |
| Female | 80.6 (76.9-84.5) | 60.2 (55.5-65.3) | 50.7 (45.7-56.2) | 63.0 (61.0-65.1) | 46.2 (44.1-48.4) | 42.2 (40.1-44.4) |
| Primary tumor location | ||||||
| Proximal | 76.7 (69.8-84.2) | 47.7 (39.8-57.2) | 42.7 (34.7-52.4) | 59.0 (56.0-62.2) | 35.8 (32.8-39.1) | 30.5 (27.5-33.7) |
| Distal | 84.5 (81.8-87.3) | 66.3 (62.8-70.1) | 57.4 (53.5-61.5) | 64.6 (62.5-66.7) | 48.0 (45.8-50.4) | 42.9 (40.6-45.3) |
| Differentiation | ||||||
| Well | 100.0 (100.0-100.0) | 100.0 (100.0-100.0) | 85.7 (63.3-100.0) | 92.5 (88.9-96.2) | 83.3 (78.0-89.0) | 78.8 (72.6-85.6) |
| Moderate | 90.4 (84.7-96.6) | 76.1 (67.6-85.6) | 66.3 (56.4-78.0) | 72.3 (67.9-76.9) | 50.1 (45.2-55.5) | 44.6 (39.6-50.1) |
| Poor/Undifferentiated | 87.7 (84.8-90.7) | 69.6 (65.4-74.0) | 61.6 (57.0-66.5) | 48.3 (46.3-50.5) | 25.7 (23.9-27.6) | 19.9 (18.2-21.8) |
| Linitis plastica | ||||||
| Yes | 57.1 (39.5-82.8) | 14.3 (5.0-40.7) | 4.8 (0.7-32.2) | 49.5 (37.8-65.0) | 14.8 (7.6-29.0) | 12.3 (5.8-26.4) |
| No | 83.9 (81.4-86.6) | 68.7 (65.4-72.2) | 63.8 (60.2-67.5) | 60.0 (58.6-61.4) | 43.1 (41.6-44.6) | 38.6 (37.1-40.1) |
| Signet ring cell carcinoma | ||||||
| Yes | 91.0 (87.9-94.2) | 80.3 (75.8-85.0) | 76.9 (72.1-82.1) | 49.5 (37.8-65.0) | 14.8 (7.6-29.0) | 12.3 (5.8-26.4) |
| No | 90.2 (86.7-93.8) | 75.1 (70.0-80.6) | 70.8 (65.2-76.8) | 60.0 (58.6-61.4) | 43.1 (41.6-44.6) | 38.6 (37.1-40.1) |
| pTNM stage (AJCC TNM 8th) | ||||||
| I | 100.0 (100.0-100.0) | 99.4 (98.4-100.0) | 99.4 (98.4-100.0) | 91.5 (89.3-93.8) | 86.4 (83.6-89.2) | 83.4 (80.2-86.6) |
| II | 97.1 (94.3-99.9) | 83.6 (77.5-90.3) | 69.8 (61.9-78.7) | 84.2 (80.4-88.2) | 68.4 (63.4-73.8) | 61.0 (55.5-67.0) |
| III | 83.5 (79.4-87.9) | 54.9 (49.3-61.0) | 42.6 (37.0-49.2) | 74.8 (70.9-78.8) | 41.0 (36.5-46.1) | 29.6 (25.3-34.7) |
| IV | 51.2 (43.8-59.7) | 17.4 (12.3-24.8) | 9.9 (6.0-16.3) | 29.7 (27.5-32.1) | 10.4 (8.8-12.1) | 7.1 (5.7-8.7) |
| Surgery | ||||||
| Yes | 89.6 (87.3-92.0) | 72.4 (69.0-76.0) | 63.4 (59.5-67.5) | 82.6 (80.9-84.3) | 61.2 (59.0-63.5) | 53.6 (51.3-56.1) |
| No | 58.4 (51.8-65.9) | 33.4 (26.9-41.5) | 28.7 (22.2-37.0) | 43.2 (41.3-45.1) | 29.1 (27.4-31.0) | 27.0 (25.3-28.9) |
| Chemotherapy | ||||||
| Yes | 89.1 (85.8-92.6) | 67.0 (61.9-72.6) | 52.7 (47.1-59.1) | 55.9 (54.1-57.8) | 32.7 (31.0-34.6) | 27.4 (25.7-29.2) |
| No | 76.8 (73.3-80.4) | 57.2 (53.1-61.6) | 51.9 (47.7-56.5) | 66.4 (64.2-68.6) | 59.2 (56.9-61.6) | 56.2 (53.9-58.7) |
| Lymphadenectomy | ||||||
| Yes | 90.8 (88.4-93.2) | 77.7 (74.3-81.3) | 73.7 (69.9-77.6) | 70.9 (67.9-73.9) | 39.4 (36.2-42.8) | 29.2 (26.1-32.6) |
| No | 64.0 (58.3-70.3) | 41.4 (35.4-48.5) | 35.1 (29.0-42.5) | 57.2 (55.6-58.8) | 43.7 (42.1-45.4) | 40.7 (39.1-42.4) |
| Lymphadenectomy with at least 15 lymph nodes | ||||||
| Yes | 91.8 (89.3-94.3) | 79.9 (76.2-83.3) | 76.2 (72.2-80.6) | 77.3 (73.7-81.1) | 43.4 (39.4-48.6) | 32.4 (28.1-37.2) |
| No | 86.5 (80.4-93.1) | 69.0 (60.9-78.3) | 64.1 (55.6-73.8) | 63.0 (58.4-67.9) | 34.0 (29.6-39.1) | 25.3 (21.2-30.2) |
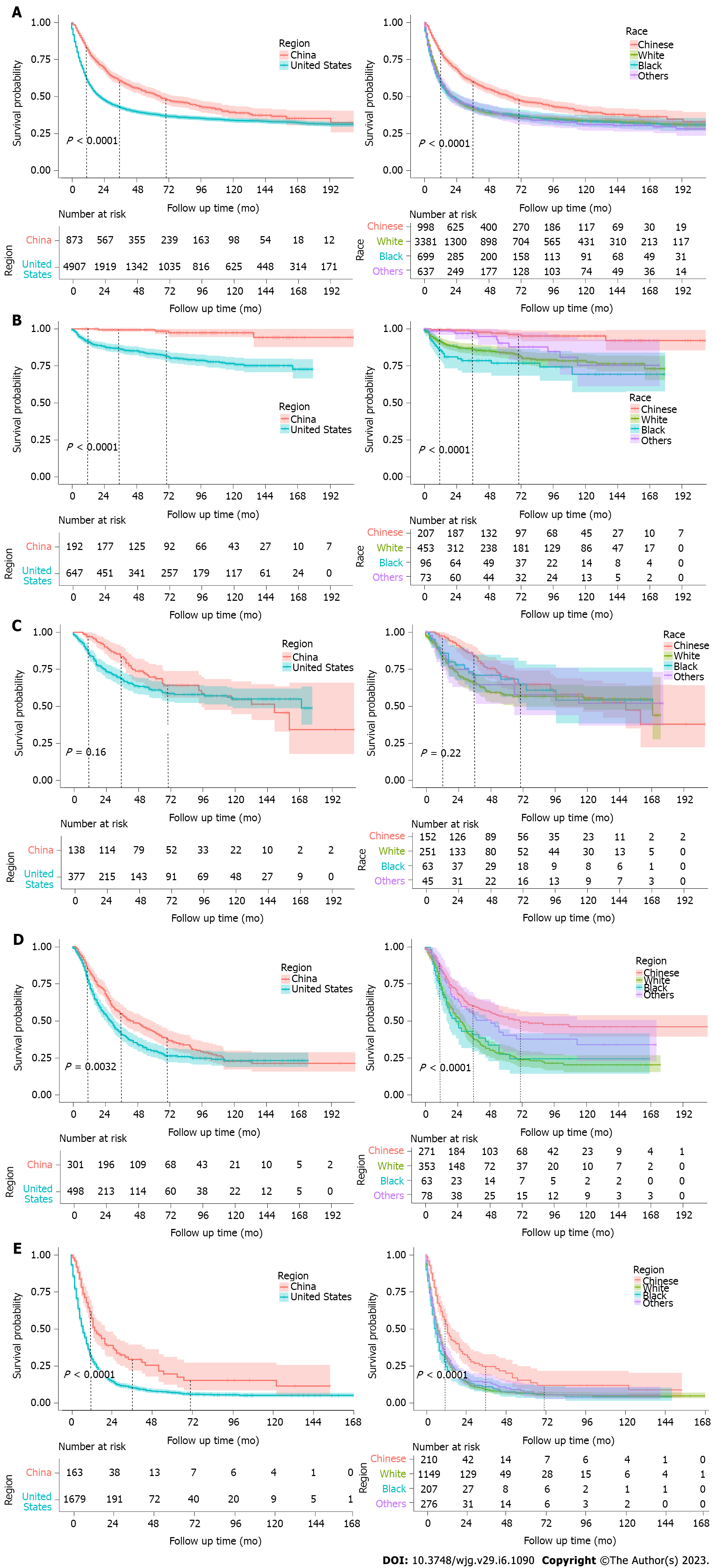
Figure 3 depicts the Kaplan-Meier curves for different regions and races. The survival outcomes were much better in Chinese patients or patients diagnosed in China, compared with other races (mentioned in SEER dataset) or the United States patients (all P < 0.0001). To avoid the bias of pathological stage, further analysis was performed to evaluate prognosis of younger cases divided by pTNM stage. The left column of Figure 3 shows the survival curves of younger patients in China and the United States diagnosed as all pTNM stages (Figure 3A), pTNM stage I (Figure 3B), pTNM stage II (Figure 3C), pTNM stage III (Figure 3D), and pTNM stage IV (Figure 3E), while the right column evaluated the OS among different races/ethnicities. Except for stage II GC patients (P = 0.16, P = 0.22, respectively), all other Kaplan-Meier curves revealed obvious survival advantages in patients who were Chinese or diagnosed in China (all P < 0.01).
Moreover, the univariate and multivariate analyses were performed to investigate the prognostic factors for younger GC patients in the China group and the United States group (Tables 3 and 4).
| Characteristics | Total | P value | China | P value | United States | P value | China vs United States | P value | |||||
| HR | 95%CI | HR | 95%CI | HR | 95%CI | HR | 95%CI | ||||||
| Region | China | 1 | |||||||||||
| United States | 2.06 | 1.84-2.31 | < 0.0001 | ||||||||||
| Race | Chinese | 1 | 1 | 1.24 | 1.00-1.54 | 0.11 | |||||||
| White | 2.08 | 1.85-2.33 | < 0.0001 | 1.22 | 0.95-1.56 | 0.12 | |||||||
| Black | 2.14 | 1.85-2.47 | < 0.0001 | 1.26 | 0.97-1.63 | 0.08 | |||||||
| Others | 2.07 | 1.79-2.38 | < 0.0001 | 1.26 | 0.97-1.64 | 0.08 | |||||||
| Period of diagnosis | Period 1 (2000-2003) | 1 | 1 | 1 | 1.12 | 0.83-1.49 | 0.55 | ||||||
| Period 2 (2004-2008) | 0.90 | 0.81-0.99 | 0.009 | 0.67 | 0.45-1.00 | 0.05 | 0.95 | 0.85-1.05 | 0.30 | 1.52 | 1.27-1.80 | < 0.0001 | |
| Period 3 (2009-2013) | 0.87 | 0.78-0.96 | 0.008 | 0.84 | 0.57-1.22 | 0.35 | 0.91 | 0.82-1.01 | 0.09 | 1.36 | 1.19-1.55 | < 0.0001 | |
| Period 4 (2014-2018) | 0.84 | 0.76-0.94 | 0.008 | 0.74 | 0.49-1.10 | 0.14 | 0.91 | 0.82-1.02 | 0.10 | 2.03 | 1.72-2.41 | < 0.0001 | |
| Gender | Male | 1 | 1 | 1 | 1.70 | 1.51-1.91 | < 0.0001 | ||||||
| Female | 0.85 | 0.80-0.91 | < 0.0001 | 1.05 | 0.87-1.26 | 0.64 | 0.82 | 0.76-0.88 | < 0.0001 | 1.52 | 1.27-1.80 | < 0.0001 | |
| Primary tumor location | Proximal | 1 | 1 | 1 | 1.30 | 1.08-1.57 | 0.02 | ||||||
| Distal | 0.68 | 0.62-0.75 | < 0.0001 | 0.59 | 0.47-0.75 | < 0.0001 | 0.77 | 0.70-0.85 | < 0.0001 | 1.56 | 1.40-1.74 | < 0.0001 | |
| Differentiation | Well | 1 | 1 | 1 | 1.10 | 0.33-3.62 | 0.90 | ||||||
| Moderate | 2.82 | 2.05-3.88 | < 0.0001 | 1.62 | 0.39-6.76 | 0.51 | 3.17 | 2.28-4.42 | < 0.0001 | 2.14 | 1.56-2.94 | < 0.0001 | |
| Poor/Undifferentiated | 5.27 | 3.91-7.11 | < 0.0001 | 2.19 | 0.54-8.83 | 0.27 | 6.15 | 4.53-8.35 | < 0.0001 | 3.20 | 2.83-3.62 | < 0.0001 | |
| Linitis plastica | Yes | 1.95 | 1.58-2.40 | < 0.0001 | 3.69 | 2.41-5.65 | < 0.0001 | 1.89 | 1.41-2.52 | < 0.0001 | 1.03 | 0.66-1.60 | 0.91 |
| No | 1 | 1 | 1 | 2.25 | 2.03-2.50 | < 0.0001 | |||||||
| Signet ring cell carcinoma | Yes | 1.61 | 1.52-1.72 | < 0.0001 | 0.80 | 0.61-1.05 | 0.18 | 2.01 | 1.86-2.17 | < 0.0001 | 6.53 | 5.33-7.99 | < 0.0001 |
| No | 1 | 1 | 1 | 2.45 | 2.02-2.96 | < 0.0001 | |||||||
| pTNM stage (AJCC TNM 8th) | 1 | 1 | 1 | 1 | 9.88 | 4.28-22.83 | < 0.0001 | ||||||
| 2.85 | 2.25-3.60 | < 0.0001 | 2.85 | 19.50 | 7.04-54.05 | < 0.0001 | 2.27 | 1.76-2.93 | < 0.0001 | 1.27 | 0.96-1.67 | 0.16 | |
| 6.18 | 5.04-7.58 | < 0.0001 | 6.18 | 46.77 | 17.36-125.96 | < 0.0001 | 4.88 | 3.93-6.07 | < 0.0001 | 1.31 | 1.13-1.53 | 0.003 | |
| 19.52 | 16.09-23.68 | < 0.0001 | 19.52 | 137.73 | 50.88-372.83 | < 0.0001 | 14.25 | 11.69-17.36 | < 0.0001 | 1.46 | 1.26-1.68 | < 0.0001 | |
| Surgery | Yes | 0.36 | 0.34-0.38 | < 0.0001 | 0.23 | 0.19-0.28 | < 0.0001 | 0.41 | 0.37-0.44 | < 0.0001 | 1.29 | 1.15-1.45 | 0.0002 |
| No | 1 | 1 | 1 | 1.25 | 1.08-1.25 | 0.01 | |||||||
| Chemotherapy | Yes | 1.71 | 1.59-1.84 | < 0.0001 | 0.93 | 0.77-1.13 | 0.45 | 1.80 | 1.66-1.95 | < 0.0001 | 2.10 | 1.84-2.40 | < 0.0001 |
| No | 1 | 1 | 1 | 0.97 | 0.87-1.09 | 0.69 | |||||||
| Lymphadenectomy with at least 15 lymph nodes | Yes | 0.56 | 0.49-0.63 | < 0.0001 | 0.64 | 0.47-0.87 | < 0.0001 | 0.73 | 0.63-0.86 | 0.0001 | 2.49 | 2.12-2.91 | < 0.0001 |
| No | 1 | 1 | 1 | 3.86 | 3.21-4.65 | < 0.0001 | |||||||
| Characteristics | Total1 | P value | China2 | P value | United States3 | P value | ||||
| HR | 95%CI | HR | 95%CI | HR | 95%CI | |||||
| Region | China | 1 | ||||||||
| United States | 2.42 | 1.35-4.33 | 0.01 | |||||||
| Race | Chinese | 1 | 1 | |||||||
| White | 0.80 | 0.45-1.41 | 0.52 | 1.21 | 0.94-1.55 | 0.13 | ||||
| Black | 0.84 | 0.46-1.53 | 0.63 | 1.48 | 1.14-1.92 | 0.003 | ||||
| Others | 0.76 | 0.42-1.38 | 0.45 | 1.19 | 0.91-1.55 | 0.20 | ||||
| Period of diagnosis | Period 1 (2000-2003) | 1 | 1 | 1 | ||||||
| Period 2 (2004-2008) | 0.62 | 0.27-0.78 | 0.02 | 0.51 | 0.29-0.89 | 0.047 | 0.55 | 0.47-0.64 | < 0.0001 | |
| Period 3 (2009-2013) | 0.54 | 0.25-0.73 | 0.01 | 0.39 | 0.22-0.67 | 0.004 | 0.50 | 0.43-0.59 | < 0.0001 | |
| Period 4 (2014-2018) | 0.48 | 0.16-0.49 | 0.0002 | 0.07 | 0.03-0.14 | < 0.0001 | 0.47 | 0.40-0.56 | < 0.0001 | |
| Gender | Male | 1 | 1 | |||||||
| Female | 0.92 | 0.80-1.07 | 0.35 | 0.85 | 0.79-0.92 | < 0.0001 | ||||
| Primary tumor location | Proximal | 1 | 1 | 1 | ||||||
| Distal | 0.92 | 0.75-1.12 | 0.47 | 0.88 | 0.60-1.29 | 0.58 | 0.88 | 0.80-0.98 | 0.02 | |
| Differentiation | Well | 1 | 1 | |||||||
| Moderate | 2.89 | 0.88-9.48 | 0.14 | 1.90 | 1.36-2.65 | 0.0002 | ||||
| Poor/Undifferentiated | 4.77 | 1.47-15.44 | 0.03 | 2.97 | 2.16-4.07 | < 0.0001 | ||||
| Linitis plastica | Yes | 1.73 | 1.12-2.68 | 0.04 | 6.70 | 2.72-16.50 | 0.0005 | 1.63 | 1.21-2.19 | 0.001 |
| No/unknown | 1 | 1 | 1 | |||||||
| Signet ring cell carcinoma | Yes | 1.07 | 0.92-1.24 | 0.47 | 1.48 | 1.36-1.61 | < 0.0001 | |||
| No/unknown | 1 | 1 | ||||||||
| pTNM stage (AJCC TNM 8th) | I | 1 | 1 | 1 | ||||||
| II | 10.96 | 5.05-23.75 | < 0.0001 | 18.69 | 5.55-62.96 | < 0.0001 | 2.35 | 1.81-3.04 | < 0.0001 | |
| III | 27.32 | 12.87-58.00 | < 0.0001 | 58.95 | 18.18-191.13 | < 0.0001 | 4.51 | 3.57-5.69 | < 0.0001 | |
| IV | 57.20 | 26.58-123.10 | < 0.0001 | 158.6 | 43.85-573.93 | < 0.0001 | 8.26 | 6.72-10.16 | < 0.0001 | |
| Surgery | Yes | 0.50 | 0.36-0.70 | 0.0005 | 0.46 | 0.20-1.03 | 0.11 | 0.42 | 0.37-0.46 | < 0.0001 |
| No | 1 | 1 | 1 | |||||||
| Chemotherapy | Yes | 0.69 | 0.58-0.84 | 0.001 | 0.82 | 0.75-0.90 | < 0.0001 | |||
| No | 1 | 1 | ||||||||
| Lymphadenectomy with at least 15 lymph nodes | Yes | 0.74 | 0.63-0.86 | 0.001 | 0.88 | 0.64-1.20 | 0.49 | 1.04 | 0.90-1.20 | 0.64 |
| No | 1 | 1 | 1 | |||||||
Significant variables on univariate analysis were enrolled in the multivariate modeling, followed by region, race, period of diagnosis, sex, location, differentiation, linitis plastica, signet ring cell carcinoma, pTNM, surgery, chemotherapy, ELNs ≥ 15 (all P < 0.05). For younger GC patients, the independent prognosis factors included region, period of diagnosis, poorly differentiated, linitis plastica, pTNM stage, surgery, chemotherapy, and ELNs ≥ 15 (all P < 0.05). However, significant predictors in China only involved period of diagnosis (all P < 0.05), linitis plastica (P = 0.0005), and pTNM stage (all P < 0.0001). For the United States patients, Black ethnicity, poorly differentiated, linitis plastica, signet ring cell, and later pathological stage were related to serious prognosis (all P < 0.01), whereas recent period, female, distal location, surgery, and chemotherapy emerged as protective factors (all P < 0.05).
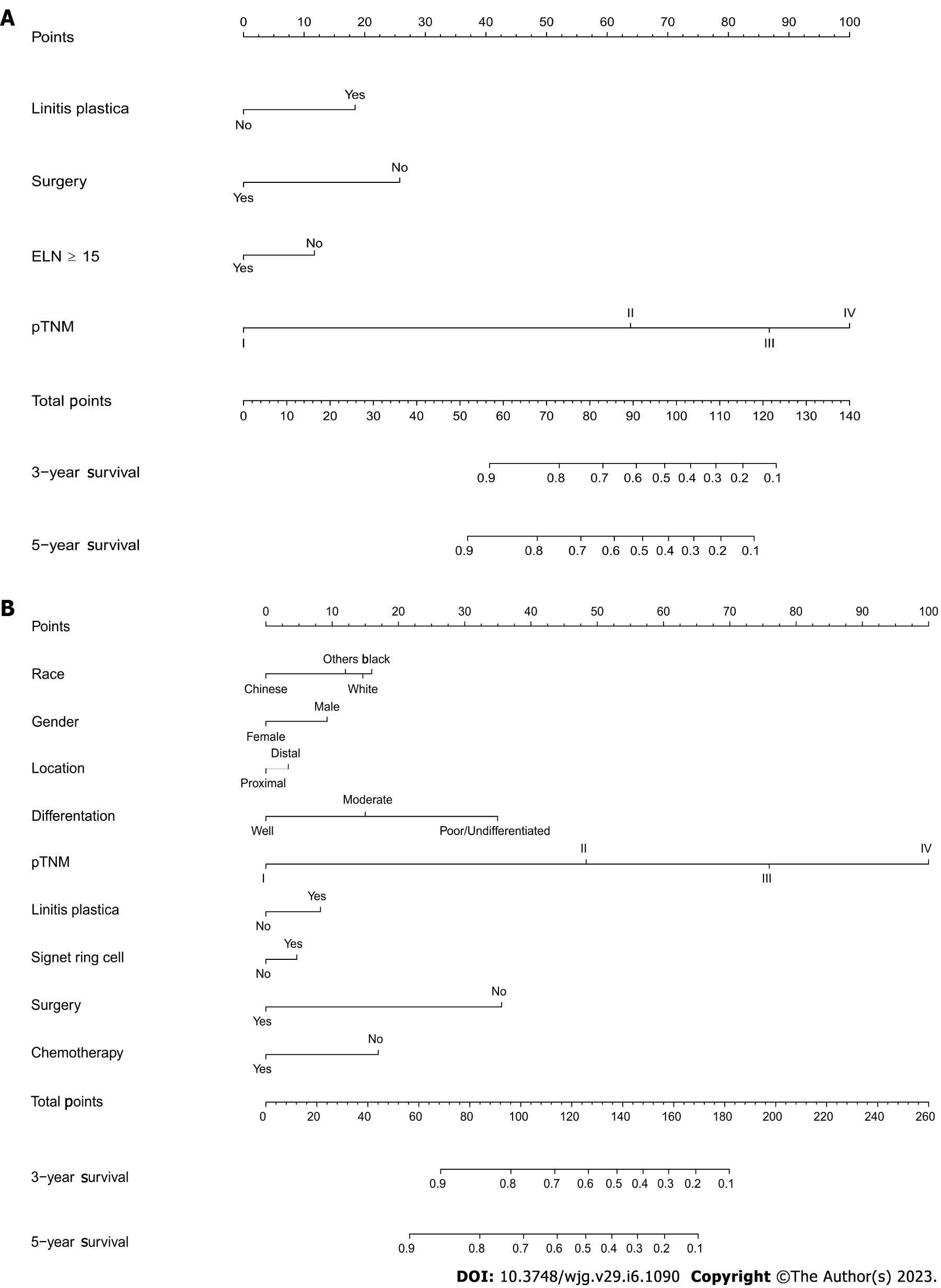
To predict OS of younger patients with GC, nomograms for China and the United States group were established separately based on the results of the Cox regression analysis. As illustrated in Figure 4, a total of four clinical parameters (including linitis plastica, surgery, ELNs ≥ 15, and pTNM stage) were included in the Chinese models, while the nomogram for the United States identified the following parameters: Race, sex, tumor location, differentiation, pTNM stage, linitis plastica, signet ring cell, surgery, and chemotherapy.
According to the results of the validation set, the C-index for the China and United States models was 0.814 and 0.787, respectively. The AUC that was applied to evaluate the discernment of the nomogram models was 0.786 in the China group and 0.842 in the United States group (Supplementary Figure 1). In addition, high-quality calibration plots in both China and the United States models had been demonstrated (Supplementary Figure 2).
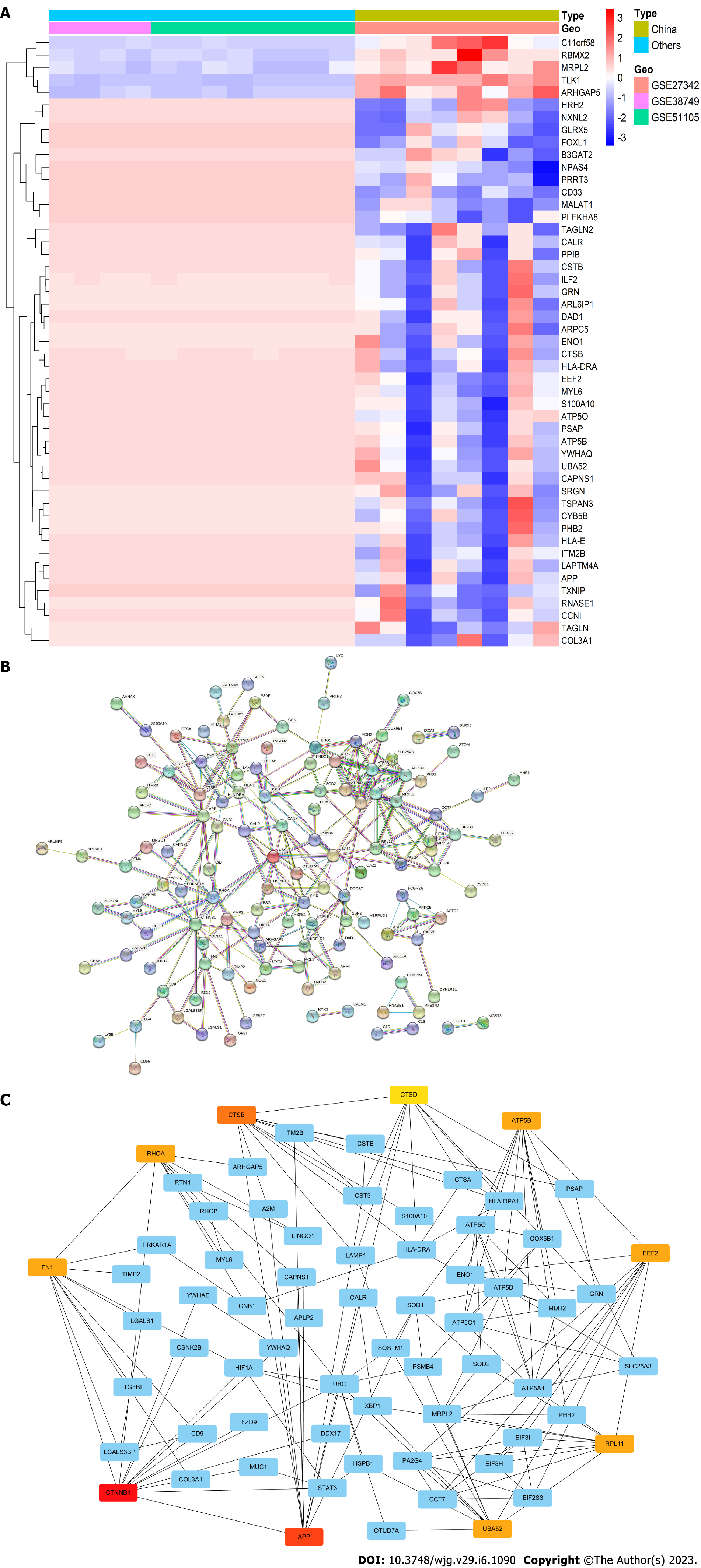
To evaluate gene disparities according to different races, we performed DEGs analysis for younger GC patients from the GEO database. Three gene expression profiles (GSE27342, GSE51105, and GSE38749), which contained 8 younger cases from China, and 12 samples from the Americas and Oceania, were included in this study. After DEGs analysis was performed among different regional sets, 50 significant DEGs were selected (Figure 5). Compared to other regions, certain genes were downregulated in China (Figure 5A), while C11orf58, TLK1, ARHGAP5, MRPL2, and RBMX2 were upregulated. Moreover, the PPIs among the DEGs were performed with the Search Tool for the Retrieval of Interacting Genes. A total of 200 nodes and 245 edges were involved in the PPI network (Figure 5B and C). The top 10 genes evaluated by connectivity degree in the PPI network were as follows: CTNNB1, APP, CTSB, UBA52, RHOA, ATP5B, EEF2, FN1, RPL11, and CTSD.
Our study compared younger GC patients in China and the United States at clinical and biological levels. Survival advantages of younger GC patients in China were demonstrated from two high-volume databases. These data were stronger than previous studies[17], and high-quality nomograms were further constructed for younger patients according to different regions. Compared to western regions, DEGs in China were confirmed, which might partly explain the histopathological behaviors and survival disparity.
Staying in line with the previous results[11,18], our conclusions showed that younger patients in China were more prominent in the distal location, while GC in the proximal location was more common in the United States. The above discrepancy was presumably related to certain predisposing factors[19-21]. In Western countries, obesity and gastroesophageal reflux were associated with proximal GC[20], whereas Helicobacter pylori infection in China may partially account for a high incidence of the distal location[21]. Additionally, Chinese cases were more likely diagnosed with an early pathological stage than those in the United States. This might partially be due to the improvement of cancer screening and early detection programs in China, which have expanded to 31 provinces since 2015[22,23]. When considering surgical patterns, it was thought that gastrectomy with D2 lymphadenectomy was the standard treatment for GC in Eastern Asia[24]. However, most patients in Western countries undergo D1 lymphadenectomy, and D2 lymphadenectomy was only recommended rather than the therapeutic norm[25,26]. These treatment differences might explain the higher percentage of ELNs ≥ 15 and the larger number of nodes examined for Chinese younger patients in our study.
With a direct comparison from a high-volume GC cohort in China and the SEER database, we reported significant survival differences for younger GC patients. Consistent with the previous studies[11,18], our findings revealed that younger cases had a better prognosis in China than in the United States. With the exception of pTNM stage II, significantly better OS was observed in the China group with stages I, III, and IV. Notably, the survival advantage of younger Chinese patients could partially be attributed to a remarkable improvement in the quality of clinical services, such as improved access to primary healthcare, early cancer screening, and individual multimodal therapies[22,23,27]. Moreover, it was well known that gastrectomy with D2 lymphadenectomy was common in Asian areas, while the vast majority of GC cases in Western countries undergo D1 lymphadenectomy[24]. The above type of surgical procedures might be associated with the survival disparity among different regions. In addition, the later tumor stage and some other factors including lifestyle and high body mass index in the United States patients might also in part explain the poor prognosis[28].
As a convenient statistical predictive tool, nomograms have been widely applied for physicians to clarify a diagnosis and predict survival[29,30]. To our best knowledge, the present study, which used the largest sample sizes, constructed survival nomograms for younger GC patients in China and the United States. Compared with previous models[18], nomograms not only avoid the bias of regions but also achieve reliable predictive performance, which was reflected by an AUC of 0.786 for China and 0.842 for the United States. In addition, the calibration plots of the training set and validation set (Supplementary Figure 1) illustrated great agreement between nomogram prediction and actual observation, thus further suggesting a robust predictive ability of nomograms in the present study.
Although specific gene expression among regions and races was not yet fully ascertained, several studies demonstrated a strong association between regional/racial-related genes and the prognosis of GC[31,32]. Loh et al[31] reviewed the genetic polymorphisms of GC among different races/ethnicities. The results showed that 37 polymorphisms across 27 genes were significantly related to GC in Asians, while 12 polymorphisms across 11 genes were found in Caucasians. Then, Li et al[32] found significant gene disparity among White, Black, and Asian patients. Four core genes, including GYG2P1, RPS4Y1, TXLNG, and EIF1AX, were demonstrated in White ethnicity, which were relevant for RNA binding and transcription pathways. Black ethnicity with GC was mainly enriched in cell structural changes, and DNAJC5, HDAC10, NEO1, and SMG5 were identified. For Asian patients, TMSB4Y, UTY, ZFY, and ZNF787 were screened based on the relationship between gene expression and DNA methylation. In our study, we first evaluated the race/ethnicity-associated genes for younger GC patients. After the construction of PPI networks and screening according to the degree of the protein node, the hub genes of younger GC patients were as follows: CTNNB1, APP, CTSB, UBA52, RHOA, ATP5B, EEF2, FN1, RPL11, and CTSD. Further mechanistic studies are warranted to confirm the related signaling and action mechanisms.
Our study has certain strengths. First, two high-volume databases based on the SEER and the NCCGCDB were comprehensively evaluated for the regional and racial disparity of younger GC patients. Second, our study, utilizing the largest sample sizes globally, constructed prognostic nomograms for younger GC patients in China and the United States, thus providing an intuitive accessible tool for physicians to predict survival and develop resurvey schedules. Moreover, to our best knowledge, it was the first study to perform biological analysis for younger patients among different regions, which provided a molecular interpretation for the survival discrepancy. Despite all this, several limitations need to be considered in the present study. Some of the key baseline prognostic factors, including body mass index, smoking, drinking, and the efficacy of neo/adjuvant therapy were absent in the SEER database. In addition, as a high-volume single center, some findings from NCCGCDB might not be strongly generalizable to China, which should be replicated in a larger multicenter experience. Lastly, even after correcting by batch effects (Supplementary Figure 3), there was also potential bias during encoding samples from GEO database. These factors might affect the accuracy of the results.
In conclusion, younger GC patients diagnosed in China had a better prognosis than those in the United States. Except for younger cases with pTNM stage II, a survival advantage was observed in the China group with pathological stages I, III, and IV. Moreover, utilizing the largest sample sizes globally, prognostic nomograms in China and the United States were constructed and showed robust predictive performance. Further large-scale studies are warranted to investigate more molecular characteristics and related mechanisms for younger GC patients among different regions and races.
The impact of racial and regional disparity on younger patients with gastric cancer (GC) remains unclear.
This study aimed to provide a national view of younger GC patients in China and the United States.
To investigate the clinicopathological characteristics, prognostic nomogram, and biological analysis of younger GC patients in China and the United States.
From 2000 to 2018, GC patients aged less than 40 years were selected from the China National Cancer Center and the Surveillance Epidemiology and End Results database. Biological analysis was enrolled from the Gene Expression Omnibus database.
A total of 6098 younger GC patients were selected from 2000 to 2018, of which 1159 were enrolled in the China National Cancer Center, and 4939 were collected from the Surveillance Epidemiology and End Results database. Compared with the United States group, younger patients in China revealed better survival outcomes (P < 0.01). For race/ethnicity, younger Chinese cases also enjoyed a better prognosis than that in White and Black subsets (P < 0.01). Prognostic nomograms for younger patients were established, with area under the curves of 0.786 for China and 0.842 for the United States. Moreover, three gene expression profiles (GSE27342, GSE51105, and GSE38749) were enrolled in further biological analysis, and distinctive molecular characteristics were identified in younger GC patients in different regions.
Except for younger cases with pathological Tumor-Node-Metastasis stage II, a survival advantage was observed in the China group with pathological stages I, III, and IV. Biological analysis of younger patients was performed among different regions, which might partly explain the histopathological behaviors and survival disparity in the subpopulations.
Further large-scale studies are warranted to investigate more molecular characteristics and related mechanisms for younger GC patients among different regions and races.
We thank Da-Wei Zhou (Qilu Hospital, Cheeloo College of Medicine, Shandong University) for assistance with data analysis.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Mishra TS, India; Senchukova M, Russia S-Editor: Fan JR L-Editor: Filipodia A P-Editor: Fan JR
| 1. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 64542] [Article Influence: 16135.5] [Reference Citation Analysis (176)] |
| 2. | Camargo MC, Anderson WF, King JB, Correa P, Thomas CC, Rosenberg PS, Eheman CR, Rabkin CS. Divergent trends for gastric cancer incidence by anatomical subsite in US adults. Gut. 2011;60:1644-1649. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 121] [Cited by in RCA: 119] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 3. | Kulig J, Popiela T, Kolodziejczyk P, Sierzega M, Jedrys J, Szczepanik AM; Polish Gastric Cancer Study Group. Clinicopathological profile and long-term outcome in young adults with gastric cancer: multicenter evaluation of 214 patients. Langenbecks Arch Surg. 2008;393:37-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 31] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 4. | Hoyert DL, Anderson RN. Age-adjusted death rates: trend data based on the year 2000 standard population. Natl Vital Stat Rep. 2001;49:1-6. [PubMed] |
| 5. | Anderson WF, Camargo MC, Fraumeni JF Jr, Correa P, Rosenberg PS, Rabkin CS. Age-specific trends in incidence of noncardia gastric cancer in US adults. JAMA. 2010;303:1723-1728. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 354] [Cited by in RCA: 356] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 6. | Takatsu Y, Hiki N, Nunobe S, Ohashi M, Honda M, Yamaguchi T, Nakajima T, Sano T. Clinicopathological features of gastric cancer in young patients. Gastric Cancer. 2016;19:472-478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 110] [Article Influence: 12.2] [Reference Citation Analysis (1)] |
| 7. | Yin J, Song JN, Bai ZG, Cai J, Zhang J, Zheng Z, Wu HW, Ye PP, Gao X, Zhang ZT. Gastric Cancer Mortality Trends in China (2006-2013) Reveal Increasing Mortality in Young Subjects. Anticancer Res. 2017;37:4671-4679. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 8. | Niu P, Huang H, Zhao L, Wang T, Zhang X, Wang W, Zhang Y, Guo C, Zhao D, Chen Y. Clinicopathological characteristics, survival outcomes, and genetic alterations of younger patients with gastric cancer: Results from the China National Cancer Center and cBioPortal datasets. Cancer Med. 2022;11:3057-3073. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (1)] |
| 9. | Strong VE, Song KY, Park CH, Jacks LM, Gonen M, Shah M, Coit DG, Brennan MF. Comparison of gastric cancer survival following R0 resection in the United States and Korea using an internationally validated nomogram. Ann Surg. 2010;251:640-646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 250] [Cited by in RCA: 285] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 10. | Zhao L, Niu P, Zhao D, Chen Y. Regional and racial disparity in proximal gastric cancer survival outcomes 1996-2016: Results from SEER and China National Cancer Center database. Cancer Med. 2021;10:4923-4938. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 11. | Chen QY, Zhong Q, Wang W, Chen S, Li P, Xie JW, Wang JB, Lin JX, Lu J, Cao LL, Lin M, Tu RH, Huang ZN, Lin JL, Zheng HL, Liu ZY, Zheng CH, Peng JS, Zhou ZW, Huang CM. Prognosis of Young Survivors of Gastric Cancer in China and the U.S.: Determining Long-Term Outcomes Based on Conditional Survival. Oncologist. 2019;24:e260-e274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 12. | Strong VE, Russo A, Yoon SS, Brennan MF, Coit DG, Zheng CH, Li P, Huang CM. Comparison of Young Patients with Gastric Cancer in the United States and China. Ann Surg Oncol. 2017;24:3964-3971. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 13. | Enewold L, Parsons H, Zhao L, Bott D, Rivera DR, Barrett MJ, Virnig BA, Warren JL. Updated Overview of the SEER-Medicare Data: Enhanced Content and Applications. J Natl Cancer Inst Monogr. 2020;2020:3-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 63] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 14. | Smith AW, Bellizzi KM, Keegan TH, Zebrack B, Chen VW, Neale AV, Hamilton AS, Shnorhavorian M, Lynch CF. Health-related quality of life of adolescent and young adult patients with cancer in the United States: the Adolescent and Young Adult Health Outcomes and Patient Experience study. J Clin Oncol. 2013;31:2136-2145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 262] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 15. | Adolescent; Group YAOPR. Closing the Gap: Research and Care Imperatives for Adolescents and Young Adults with Cancer (NIH Publication No. 06-6067), Department of Health and Human Services, National Institutes of Health, National Cancer Institute, and the LIVESTRONG Young Adult Alliance Bethesda, MD, 2006. |
| 16. | Smith-Bindman R, Lebda P, Feldstein VA, Sellami D, Goldstein RB, Brasic N, Jin C, Kornak J. Risk of thyroid cancer based on thyroid ultrasound imaging characteristics: results of a population-based study. JAMA Intern Med. 2013;173:1788-1796. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 194] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 17. | Yu C, Zhang Y. Development and validation of prognostic nomogram for young patients with gastric cancer. Ann Transl Med. 2019;7:641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 65] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 18. | Wu C, Wang N, Zhou H, Wang T, Zhao D. Development and validation of a nomogram to individually predict survival of young patients with nonmetastatic gastric cancer: A retrospective cohort study. Saudi J Gastroenterol. 2019;25:236-244. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 19. | Karimi P, Islami F, Anandasabapathy S, Freedman ND, Kamangar F. Gastric cancer: descriptive epidemiology, risk factors, screening, and prevention. Cancer Epidemiol Biomarkers Prev. 2014;23:700-713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1159] [Cited by in RCA: 1327] [Article Influence: 120.6] [Reference Citation Analysis (0)] |
| 20. | Derakhshan MH, Malekzadeh R, Watabe H, Yazdanbod A, Fyfe V, Kazemi A, Rakhshani N, Didevar R, Sotoudeh M, Zolfeghari AA, McColl KE. Combination of gastric atrophy, reflux symptoms and histological subtype indicates two distinct aetiologies of gastric cardia cancer. Gut. 2008;57:298-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 137] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 21. | Lee YC, Chiang TH, Chou CK, Tu YK, Liao WC, Wu MS, Graham DY. Association Between Helicobacter pylori Eradication and Gastric Cancer Incidence: A Systematic Review and Meta-analysis. Gastroenterology. 2016;150:1113-1124.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 737] [Cited by in RCA: 674] [Article Influence: 74.9] [Reference Citation Analysis (0)] |
| 22. | Zeng H, Chen W, Zheng R, Zhang S, Ji JS, Zou X, Xia C, Sun K, Yang Z, Li H, Wang N, Han R, Liu S, Mu H, He Y, Xu Y, Fu Z, Zhou Y, Jiang J, Yang Y, Chen J, Wei K, Fan D, Wang J, Fu F, Zhao D, Song G, Jiang C, Zhou X, Gu X, Jin F, Li Q, Li Y, Wu T, Yan C, Dong J, Hua Z, Baade P, Bray F, Jemal A, Yu XQ, He J. Changing cancer survival in China during 2003-15: a pooled analysis of 17 population-based cancer registries. Lancet Glob Health. 2018;6:e555-e567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 795] [Cited by in RCA: 973] [Article Influence: 139.0] [Reference Citation Analysis (2)] |
| 23. | Zou XN. Epidemic trend, screening, and early detection and treatment of cancer in Chinese population. Cancer Biol Med. 2017;14:50-59. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 35] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 24. | Verlato G, Giacopuzzi S, Bencivenga M, Morgagni P, De Manzoni G. Problems faced by evidence-based medicine in evaluating lymphadenectomy for gastric cancer. World J Gastroenterol. 2014;20:12883-12891. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 29] [Cited by in RCA: 36] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 25. | Degiuli M, Sasako M, Ponti A; Italian Gastric Cancer Study Group. Morbidity and mortality in the Italian Gastric Cancer Study Group randomized clinical trial of D1 vs D2 resection for gastric cancer. Br J Surg. 2010;97:643-649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 223] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 26. | Degiuli M, Sasako M, Ponti A, Vendrame A, Tomatis M, Mazza C, Borasi A, Capussotti L, Fronda G, Morino M; Italian Gastric Cancer Study Group. Randomized clinical trial comparing survival after D1 or D2 gastrectomy for gastric cancer. Br J Surg. 2014;101:23-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 200] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 27. | Liu K, Yang K, Zhang W, Chen X, Zhang B, Chen Z, Chen J, Zhao Y, Zhou Z, Chen L, Hu J. Changes of Esophagogastric Junctional Adenocarcinoma and Gastroesophageal Reflux Disease Among Surgical Patients During 1988-2012: A Single-institution, High-volume Experience in China. Ann Surg. 2016;263:88-95. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 131] [Cited by in RCA: 126] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 28. | Lin JX, Yi BC, Yoon C, Li P, Zheng CH, Huang CM, Yoon SS. Comparison of Outcomes for Elderly Gastric Cancer Patients at Least 80 Years of Age Following Gastrectomy in the United States and China. Ann Surg Oncol. 2018;25:3629-3638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 29. | Li M, Zhang J, Dan Y, Yao Y, Dai W, Cai G, Yang G, Tong T. A clinical-radiomics nomogram for the preoperative prediction of lymph node metastasis in colorectal cancer. J Transl Med. 2020;18:46. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 86] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 30. | Yang Z, Gao Y, Fan X, Zhao X, Zhu S, Guo M, Liu Z, Yang X, Han Y. A multivariate prediction model for high malignancy potential gastric GI stromal tumors before endoscopic resection. Gastrointest Endosc. 2020;91:813-822. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 31. | Loh M, Koh KX, Yeo BH, Song CM, Chia KS, Zhu F, Yeoh KG, Hill J, Iacopetta B, Soong R. Meta-analysis of genetic polymorphisms and gastric cancer risk: variability in associations according to race. Eur J Cancer. 2009;45:2562-2568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 85] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 32. | Li H, Wang C, Wei Z, Chen W, Guo Z, He Y, Zhang C. Differences in the prognosis of gastric cancer patients of different sexes and races and the molecular mechanisms involved. Int J Oncol. 2019;55:1049-1068. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |