Published online Feb 7, 2023. doi: 10.3748/wjg.v29.i5.890
Peer-review started: October 24, 2022
First decision: December 12, 2022
Revised: December 17, 2022
Accepted: January 16, 2023
Article in press: January 16, 2023
Published online: February 7, 2023
Processing time: 105 Days and 5.4 Hours
Conventional transarterial chemoembolization (cTACE) is the current standard treatment for intermediate-stage hepatocellular carcinoma (HCC). Post-embolization syndrome (PES) is complex clinical syndrome that presents as fever, abdominal pain, nausea, and vomiting. Either dexamethasone (DEXA) or N-acetylcysteine (NAC) is used to prevent PES; however, the synergistic effect of their combined therapy for preventing PES and liver decompensation has not been determined.
To evaluate the efficacy of DEXA and NAC combination in preventing PES and liver decompensation after cTACE.
Patients with Barcelona Clinic Liver Cancer stage A or B HCC who were scheduled for TACE were prospectively enrolled. All patients were randomly stratified to receive NAC and DEXA or placebo. The dual therapy (NAC + DEXA) group received intravenous administration of 10 mg DEXA every 12 h, NAC 24 h prior to cTACE (150 mg/kg/h for 1 h followed by 12.5 mg/kg/h for 4 h), and a continuous infusion of 6.25 mg/h NAC plus 4 mg DEXA every 12 h for 48 h after cTACE. The placebo group received an infusion of 5% glucose solution until 48 h after procedure. PES was defined by South West Oncology Group toxicity code grading of more than 2 that was calculated using incidence of fever, nausea, vomiting, and pain.
One-hundred patients were enrolled with 50 patients in each group. Incidence of PES was significantly lower in the NAC + DEXA group compared with in the placebo group (6% vs 80%; P < 0.001). Multivariate analysis showed that the dual treatment is a protective strategic therapy against PES development [odds ratio (OR) = 0.04; 95% confidence interval (CI): 0.01-0.20; P < 0.001). Seven (14%) patients in the placebo group, but none in the NAC + DEXA group, developed post-TACE liver decompensation. A dynamic change in Albumin-Bilirubin score of more than 0.5 point was found to be a risk factor for post-TACE liver decompensation (OR = 42.77; 95%CI: 1.01-1810; P = 0.049).
Intravenous NAC + DEXA administration ameliorated the occurrence of PES event after cTACE in patients with intermediate-stage HCC.
Core Tip: Conventional transarterial chemoembolization (TACE) is the current standard treatment for intermediate-stage hepatocellular carcinoma (HCC). A combination of N-acetylcysteine and dexamethasone ameliorated the occurrence of post-embolization syndrome event after TACE in patients with intermediate-stage HCC. A dynamic change in Albumin-Bilirubin score of more than 0.5 point was found to be a risk factor for post-TACE liver decompensation.
- Citation: Simasingha N, Tanasoontrarat W, Claimon T, Sethasine S. Efficacy of dexamethasone and N-acetylcysteine combination in preventing post-embolization syndrome after transarterial chemoembolization in hepatocellular carcinoma. World J Gastroenterol 2023; 29(5): 890-903
- URL: https://www.wjgnet.com/1007-9327/full/v29/i5/890.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i5.890
Hepatocellular carcinoma (HCC) is a major-public health concern and the fourth common cause of cancer-related deaths worldwide[1]. In Thailand, HCC is the second most common tumor type and the most common cause of cancer-related death. Without treatment, patients with HCC have a one-year overall survival rate of less than 20%. Conventional transarterial chemoembolization (cTACE) has been established as a standard treatment for HCC with Barcelona Clinic Liver Cancer (BCLC) stage B. Systemic reviews and meta-analyses have demonstrated that cTACE therapy improved the survival of patient at this stage. HCC patients who underwent super-selective TACE had a 5-year survival rate of 40%-48%. cTACE involves embolization of vessel supply to tumors, causing ischemia in not only tumor cells but also normal hepatocytes, along with targeted chemotherapy; the systemic effects of chemotherapeutic agents result in the occurrence of post-embolization syndrome (PES)[2-5].
PES is a complex clinical syndrome manifested as fever, abdominal pain, nausea, and vomiting[6,7] with a South West Oncology Group (SWOG) score > 2[7]. PES is self-limited and either resolves within 24 h or exhibits sustained symptoms for up to two weeks based on various factors such as tumor size, tumor numbers, dosage of chemotherapy, and performance status of the patient[6,8,9]. Management of PES mainly includes supportive treatment such as with analgesic, antiemetic, and antipyretic administration[9,10]. Depending on its pathogenesis, PES may be related to systemic inflammation, resulting in toxic and ischemic effects on tumor cells and hepatocytes[9]. Steroids and antioxidants may play an important role in the prevention and treatment of PES. Dexamethasone (DEXA) and prednisolone are recommended by the National Comprehensive Cancer Network as effective medications for preventing chemotherapy-induced nausea/vomiting. Moreover, a few randomized control trials have demonstra
A randomized, double blind, placebo-controlled trial was conducted at the Gastroenterology and Liver Unit, Vajira Hospital, Navamindradhiraj University, Bangkok, Thailand from November 2020 to January 2022.
Eligible patients were those aged 18-80 years with diagnosed early- or intermediate-stage HCC, according to BCLC classification, and had a good performance status, defined by the Eastern Cooperative Oncology Group. Diagnosis of HCC was based on either histological or radiological typical hallmark criteria according to the American Association for the Study of Liver Disease[18] and European Association for the Study of the Liver[19]. The exclusion criteria were as follows: (1) Decompensated liver cirrhosis (Child-Pugh score ≥ 9) or cirrhosis with main portal vein invasion; (2) Congestive heart failure and/or respiratory failure; (3) Severe comorbid illness, such as end-stage renal disease, persistent poorly-controlled diabetes mellitus or hemoglobin A1C ≥ 8.5, uncontrolled hypertension (systolic blood pressure ≥ 180 mmHg or diastolic blood pressure ≥ 120 mmHg), with a life expectancy of < 6 mo; (4) Severe allergy or anaphylaxis/anaphylactoid to NAC; (5) Pregnancy; and (6) History of non-steroid anti-inflammatory drugs, steroids, or NAC use within 21 d of trial initiation. All enrolled patients agreed on receiving cTACE treatment and provided informed consent before participating in the study.
Based on previous results, the incidence of PES among patients with HCC after receiving cTACE was > 60%[12]. Superiority of the DEXA regimen over the control regimen was defined as a 25% decrease in PES. Intravenous DEXA was hypothesized to reduce the incidence of PES by 20%. This study used a two-tailed test that calculated the requirement of at least 44 patients in each group to obtain a P value < 0.05 with alpha and beta errors of 5% and 20%, respectively.
The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki and was approved by the Institutional Review Board of Faculty of Medicine, Vajira Hospital (COA 051/2564).
All patients were admitted at least 24 h before the prescheduled cTACE procedure and were randomly (1:1) assigned to either NAC–DEXA or placebo group. The randomization sequence was computer-generated in blocks of four and stratified according to Child-Pugh classification (class A or B). All patients were blinded to the treatment assignment. Both groups underwent therapy initiation 24 h prior to the procedure. The specific dosage of the NAC-DEXA protocol was based on a previously reported recommended dosage[11,17]. The NAC-DEXA group received intravenous infusion of 5% dextrose with NAC, with an initial loading dose of 150 mg/kg/h over 1 h followed by 12.5 mg/kg/h for 4 h and 10 mg of intravenous DEXA every 12 h; this was followed by continuous intravenous infusion of 6.25 mg/kg/h NAC and 4 mg of intravenous DEXA every 12 h for the remaining 48 h post-TACE. The placebo group received 5% glucose in normal saline for 48 h at an infusion rate of 60 mL/h post-TACE (as shown in Supplementary Figure 1). If mild-to-moderate allergic symptoms developed (e.g., urticarial rash or bronchospasm), treatment was temporarily stopped for 1 h and intravenous antihistamine was immediately administered; treatment was resumed after the symptoms subsided. If severe allergic or anaphylactoid reaction occurred, treatment was permanently stopped, and the patient was treated according to standard protocol for severe allergic reaction. cTACE was performed by two interventional radiologists (Tanasoontrarat W and Claimon T) who were blinded to the randomization assignment. Pre-procedure single intravenous dose of ceftriaxone (1 g) or amoxicillin-clavulanic acid (1.2 g) along with single intravenous dose of ondansetron (8 mg) was administered to all patients. The femoral artery was catheterized under local anesthesia. A thorough angiographic examination was performed to locate all of the tumor-feeding arteries. An emulsion of lipiodol (2.5-15 mL) and chemotherapeutic agent (mitomycin, 5-20 mg) was infused into the feeders at an optimal dose determined by the interventional radiologist to be sufficient for tumor control. Thereafter, gelatin sponge particles were injected through the tumor-feeding branch. Selective cTACE was defined as occlusion of the segmental or subsegmental arterial feeder.
After completion of the procedure, all patients were admitted in the hospital for at least 72 h. During hospitalization period, the following parameters were recorded: Symptoms (nausea, vomiting, fever, abdominal pain, and anorexia), vital signs, and other adverse events. Laboratory parameters, including liver function test, erythrocyte sedimentation rate, and C-reactive protein, were assessed at 24 and 48 h post-procedure. Hemoculture, urinalysis, complete blood count, and chest X-ray were performed if body temperature was > 38 °C.
The primary outcome was the development of PES after TACE within 48 h. In the present study, PES was identified using three different definitions: (1) SWOG toxicity coding score characterized by fever, nausea, vomiting, and/or abdominal pain within 48 h post-procedure, defined as calculated sum score more than two point (Supplementary Table 1)[7]; (2) Criteria defined by Ogasawara et al[11] in a randomized double-blind control study of DEXA, based on Common Terminology Criteria for Adverse Events (CTCAE) [version 5 (Supplementary Table 2) with symptoms other than those of grade I]; and (3) Criteria defined by Siramolpiwat et al[17] in a randomized controlled trial of single NAC dose (temperature ≥ 38.5 °C and 3-fold higher alanine transaminase level from baseline within 48 h post-procedure). The secondary outcome was the development of post-TACE liver decompensation, defined as an increase in Child-Pugh score of more than two points or newly developed decompensating events, such as ascites, hepatic encephalopathy, or serum total bilirubin > 2 mg/dL. Other cumulative adverse events (classified by CTCAE) and length of hospital stay were compared between the two groups. In patients with suspected infection or > 38 °C body temperature, laboratory testing and septic work-up were performed; moreover, these patients were treated with empirical antibiotic therapy until fever subsided or hemoculture was negative. All patients were followed up after 7 d to evaluate other post-procedure events.
Continuous data are presented as mean with standard deviation (SD) or median with range. Student’s t test or the Mann-Whitney U test was performed to compare between two groups. For categorical data, Chi-square test or Fisher’s exact test was applied. Statistical significance was set at P < 0.05. For analysis of factors that impact PES development, a logistic regression analysis was performed. Data are reported as odds ratio (OR) with 95% confidence interval (CI). All serious adverse events were reported to the Institutional Review Board of the Faculty of Medicine, Vajira Hospital, Navamindradhiraj University.
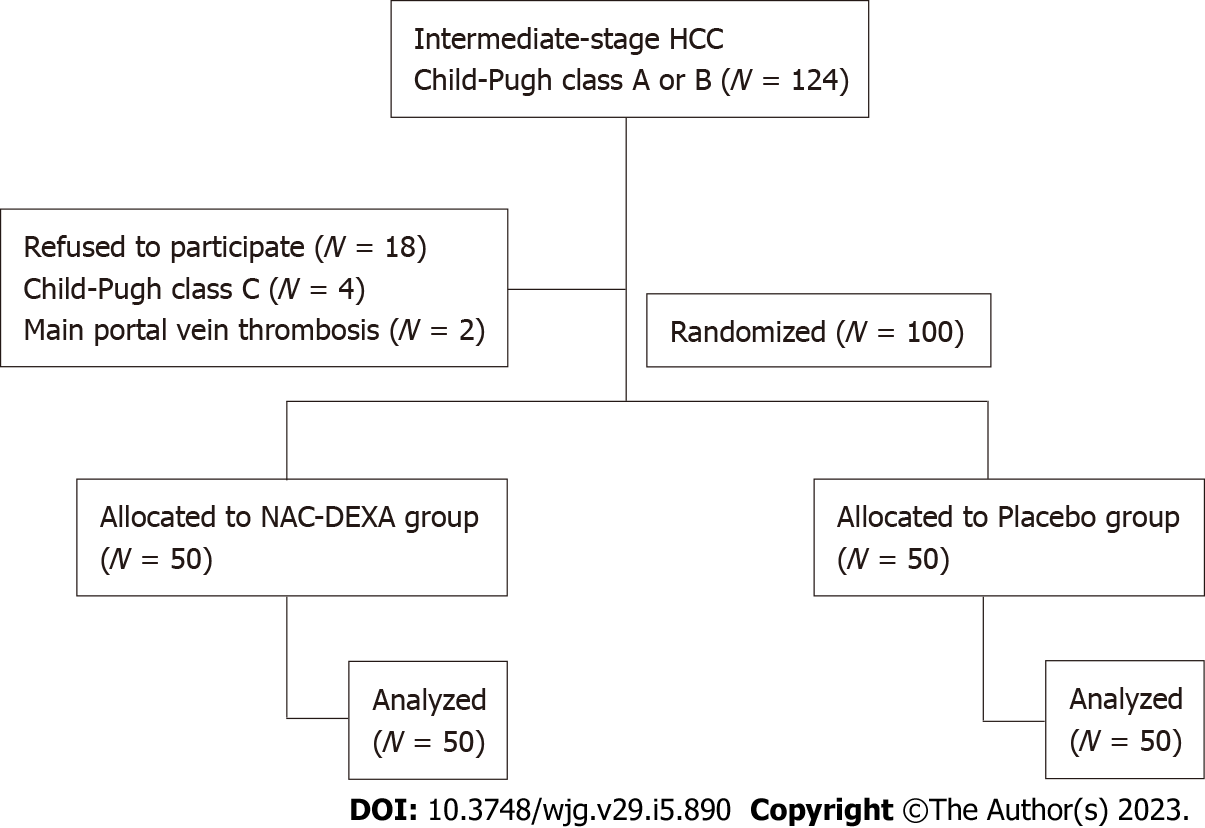
A total of 124 patients with HCC were screened from November 2020 to January 2022. Eighteen patients who refused to participate in this study, four patients who progressed to Child-Pugh class C, and two patients with main portal vein thrombosis were excluded. The remaining 100 patients who underwent TACE randomly received NAC-DEXA (n = 50) or placebo (n = 50) treatment. Figure 1 illustrates a flow chart of patients enrolled in this study. The mean age of patients was 60.6 years, with a male predominance (89%). The prevalence of comorbid diabetes was not different between the NAC-DEXA and placebo groups (34% vs 32%, P = 0.83). All patients exhibited cirrhosis, the majority of which with an etiology of chronic hepatitis B and alcoholic cirrhosis, followed by chronic hepatitis C, and a minority showing non-alcoholic steatohepatitis etiology. Most patients (83%) were classified as Child-Pugh class A, with no difference in mean Child-Pugh scores between the two groups (5.5 ± 0.8 in NAC-DEXA vs 5.5 ± 0.9 in placebo). Almost all patients (91%) were in BCLC stage B. Nine patient in BCLC stage A were justified for TACE as it would serve as a bridging therapy before curative treatment. Nearly half of the patients exhibited alpha fetoprotein (AFP) level > 200; however, the distribution of AFP level was not statically significant in both groups. Per tumor characteristics, no difference in the median tumor diameter between the two groups (NAC-DEXA, 5.5 cm; range 1.4-19 cm vs placebo, 7.95 cm; range 1.4-17.2 cm; P = 0.39) was observed. More than 50% of patients in both groups had multiple nodules (Table 1). Approximately 34% of the patients underwent their first TACE session. The type of chemotherapy and the volume of lipiodol did not differ between the two groups. Level of embolization was selected based on tumor position. In the same TACE episode, more than one-third (36%) of patients in the NAC-DEXA group had embolization of more than two branches.
| N-acetylcysteine + dexamethasone | Placebo | P value | |
| Sex | |||
| Male | 44 (88%) | 45 (90%) | 0.75 |
| Age (mean ± SD) | 60.8 ± 10.52 | 60.46 ± 11.27 | 0.88 |
| Underlying disease | |||
| Diabetic mellitus | 17 (34%) | 16 (32%) | 0.83 |
| Hypertension | 22 (14%) | 28 (56%) | 0.23 |
| Dyslipidemia | 14 (28%) | 18 (36%) | 0.40 |
| CKD | 1 (2%) | 2 (4%) | 0.56 |
| HIV | 1 (2%) | 1 (2%) | 1.00 |
| Tumor characteristic | |||
| Size | |||
| Median (range) | 5.5 (1.4-19) | 7.95 (1.4-17.2) | 0.39 |
| > 3 cm | 40 (80%) | 42 (84%) | 0.65 |
| Number | |||
| Median (range) | 2 (1-10) | 2 (1-10) | 0.21 |
| 1 | 23 (46%) | 17 (34%) | 0.56 |
| ≥ 2 | 27 (54%) | 33(66%) | |
| Etiology | |||
| Hepatitis B | 23 (46%) | 28 (56%) | 0.31 |
| Hepatitis C | 15 (30%) | 12 (24%) | 0.50 |
| Alcoholic | 22 (44%) | 20 (40%) | 0.67 |
| Non-alcoholic steatohepatitis | 3 (6%) | 4 (8%) | 0.70 |
| Staging | |||
| BCLC-A | 6 (12%) | 3 (6%) | 0.23 |
| BCLC-B | 44 (88%) | 47 (94%) | |
| Child-Pugh Score | |||
| A (5-6) | 40 (80%) | 43 (86%) | 0.42 |
| B (7-8) | 10 (20%) | 7 (14%) | |
| ALBI score (mean ± SD) | -2.61 ± 0.58 | -2.54 ± 0.53 | 0.54 |
| Alpha fetoprotein (ng/mL) | |||
| < 20 | 18 (36%) | 19 (38%) | 0.50 |
| 20-200 | 14 (28%) | 9 (18%) | |
| 201-1000 | 10 (20%) | 9 (18%) | |
| > 1000 | 8 (16%) | 13 (26%) | |
| Episode of TACE | |||
| 1 | 16 (32%) | 18 (36%) | 0.67 |
| 2-5 | 34 (68%) | 32 (64%) | |
| Embolization agents | |||
| Mitomycin (mg) | |||
| 5-10 | 1 (2%) | 3 (6%) | 0.07 |
| 10.1-15 | 20 (40%) | 10 (20%) | |
| 15.1-20 | 29 (58%) | 37 (74%) | |
| Lipiodol (mL) | |||
| < 5 | 1 (2%) | 1 (2%) | 0.47 |
| 5-10 | 13 (26%) | 8 (16%) | |
| > 10 | 36 (72%) | 41 (82%) | |
| Level of embolization | |||
| Left hepatic artery | 17 (34%) | 14 (28%) | 0.51 |
| Right hepatic artery | 46 (92%) | 44 (88%) | 0.50 |
| Middle hepatic artery | 5 (10%) | 3 (6%) | 0.46 |
| ≥ 2 major branches | 18 (36%) | 8 (16%) | 0.02 |
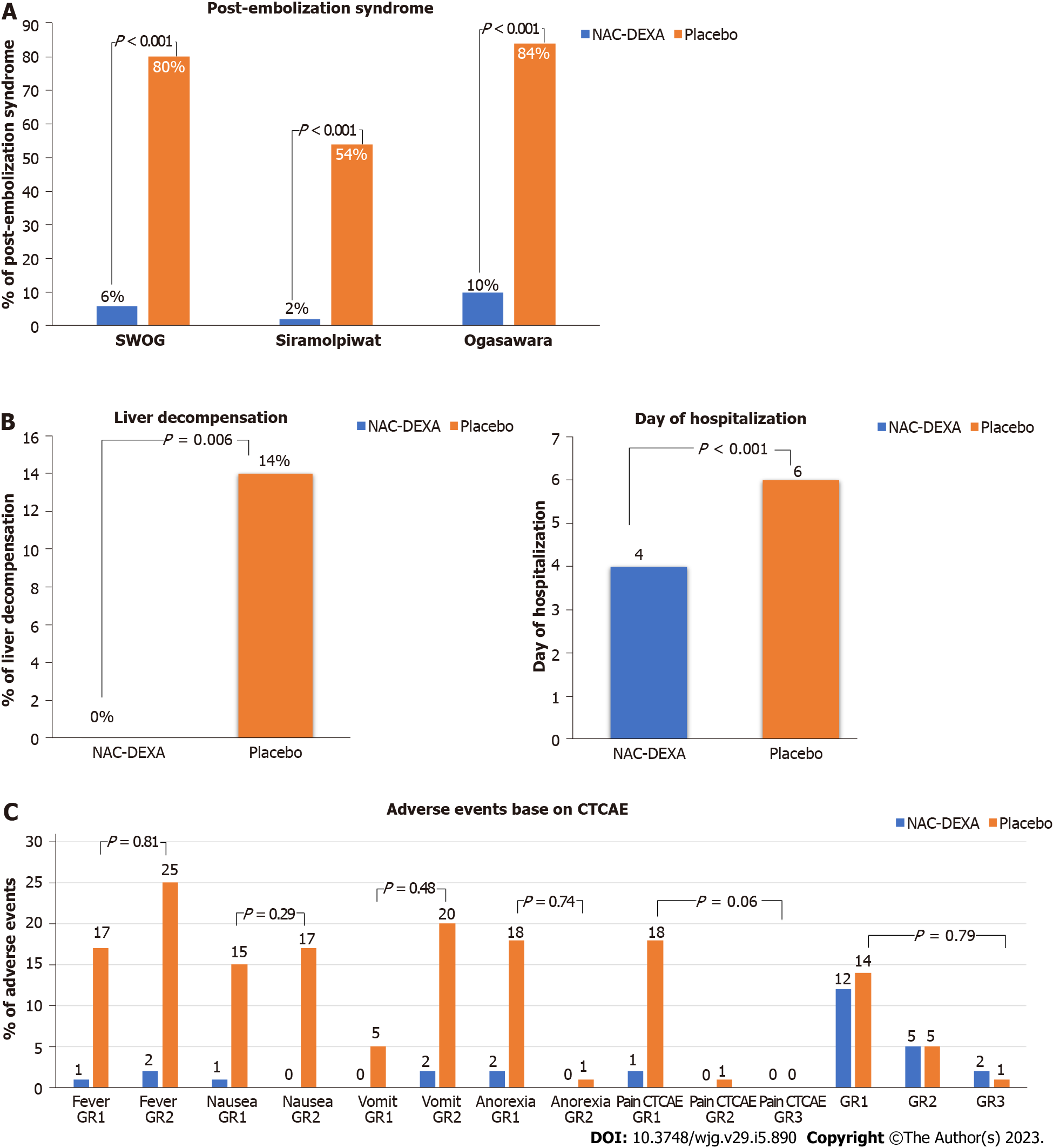
According to the various pre-defined criteria mention above, PES was detected in 43% of the patients. Most patients with PES had fever (93.0%) and nausea (72.1%), while only five (11.6%) patients had abdominal pain. The occurrence of PES after TACE was significantly lower in the NAC-DEXA group than in the placebo group in all PES-defining criteria (SWOG score more than 2 point, 6% vs 80%; Siramolpiwat et al[17] criteria, 2% vs 54%; and Ogasawara et al[11] criteria, 10 % vs 84 %; all P < 0.001) as shown in Figure 2A. The NAC-DEXA group had a lower mean SWOG PES score than the placebo group (0.38 ± 1.1 vs 4.04 ± 2.2; P < 0.001). Baseline characteristic comparison of patients with or without PES after TACE is shown in Table 2. The PES group had a higher proportion of patients with large tumor size (> 5 cm) (67.4% vs 47.4%; P = 0.045) and massive tumor burden (up to 12 criteria; 48.8% vs 26.3%; P = 0.02). Neither volume of embolizing agents nor level of vessels embolization influenced PES occurrence. Nevertheless, most (86%) patients in the PES group underwent TACE with single vessel embolization technique.
| PES after cTACE | P value | Liver decompensation after TACE | P value | |||
| Yes (n = 43) | No (n = 57) | Yes (n = 7) | No (n = 93) | |||
| Male | 40 (93%) | 49 (86%) | 0.26 | 7 (100%) | 82 (88.2%) | 0.34 |
| Age (mean ± SD) | 60.72 ± 11.11 | 60.56 ± 10.74 | 0.26 | 63.57 ± 7.91 | 60.41 ± 11.04 | 0.35 |
| Child-Pugh score | 5.35 ± 0.69 | 5.61 ± 0.9 | 0.09 | 5.86 ± 0.9 | 5.47 ± 0.82 | 0.24 |
| A (5-6) | 40 (93%) | 43 (75.4%) | 0.02 | 5 (71.4%) | 78 (83.9%) | 0.4 |
| B (7-8) | 3 (7%) | 14 (24.6%) | 0.02 | 2 (28.6%) | 15 (16.1%) | 0.4 |
| ALBI (median; IQR) | -2.72 (-3.05, -2.29) | -2.65 (-2.93, -2.2) | 0.47 | -1.95 (-2.74, -1.77) | -2.69 (-3.04, -2.29) | 0.09 |
| MELD (mean ± SD) | 11.67 ± 3.54 | 12.05 ± 3.78 | 0.61 | 12.71 ± 3.86 | 11.83 ± 3.66 | 0.54 |
| Staging | ||||||
| BCLC-A | 3 (7%) | 6 (10.5%) | 0.54 | 1 (14.3%) | 8 (8.6%) | 0.61 |
| BCLC-B | 40 (93%) | 51 (89.5%) | 0.54 | 6 (85.7%) | 85 (91.4%) | 0.61 |
| Tumor characteristics | ||||||
| AFP ≥ 200 ng/mL | 17 (39.5%) | 27 (47.4%) | 0.44 | 3 (42.9%) | 28 (30.1%) | 0.48 |
| Median (range) | 9.6 (4,13.2) | 5 (3.2,10) | 0.05 | 5.7 (3.2,15) | 7 (3.2,13) | 0.8 |
| Large tumor ≥ 5 cm | 29 (67.4%) | 27 (47.4%) | 0.045 | 4 (57.1%) | 52 (55.9%) | 0.95 |
| Number | ||||||
| 1 | 18 (41.9%) | 22 (38.6%) | 0.74 | 4 (57.1%) | 36 (38.7%) | 0.34 |
| ≥ 2 | 25 (58.1%) | 35 (61.4%) | 3 (42.9%) | 57 (61.3%) | ||
| Size plus number | ||||||
| Up to 7 | 32 (74.4%) | 37 (64.9%) | 0.31 | 4 (57.1%) | 65 (69.9%) | 0.48 |
| Up to 12 | 21 (48.8%) | 15 (26.3%) | 0.02 | 3 (42.9%) | 33 (35.5%) | 0.7 |
| cTACE episode | ||||||
| 1 | 15 (34.9%) | 19 (33.3%) | 0.87 | 4 (57.1%) | 30 (32.3%) | 0.18 |
| ≥ 2 (2-5) | 28 (65.1%) | 38 (66.7%) | 3 (42.9%) | 63 (67.7%) | ||
| Embolization agent | ||||||
| Mitomycin (mg) | ||||||
| < 10 | 2 (4.7%) | 2 (3.5%) | 0.44 | 0 (0%) | 4 (4.3%) | 0.51 |
| 10-15 | 10 (23.3%) | 20 (35.1%) | 1 (14.3%) | 29 (31.2%) | ||
| 15.1-20 | 31 (72.1%) | 35 (61.4%) | 6 (85.7%) | 60 (64.5%) | ||
| Lipiodol (mL) | ||||||
| < 5 | 1 (2.3%) | 1 (1.8%) | 0.32 | 0 (0%) | 2 (2.2%) | 0.82 |
| 5-10 | 6 (14%) | 15 (26.3%) | 1 (14.3%) | 20 (21.5%) | ||
| > 10 | 36 (83.7%) | 41 (71.9%) | 6 (85.7%) | 71 (76.3%) | ||
| Level of embolization | ||||||
| Left hepatic artery | 10 (23.3%) | 18 (31.6%) | 0.36 | 3 (42.9%) | 28 (30.1%) | 0.32 |
| Right hepatic artery | 38 (88.4%) | 44 (77.2%) | 0.15 | 7 (100%) | 83 (89.2%) | 0.37 |
| Middle hepatic artery | 1 (2.3%) | 7 (12.3%) | 0.07 | 0 (0%) | 8 (8.6%) | 0.43 |
| 1 vessel | 37 (86%) | 34 (59.6%) | 0.004 | 3 (42.9%) | 67 (72%) | 0.25 |
| ≥ 2 vessels | 6 (14%) | 23 (40.4%) | 0.007 | 4 (57.1%) | 26(28%) | 0.02 |
| Baseline laboratory (median; IQR) | ||||||
| Hct (%) | 36.8 (32.3, 40.3) | 35 (32.6, 38.9) | 0.33 | 39.8 (36.6, 40.6) | 35.2 (32.2, 39) | 0.04 |
| WBC (× 109/L) | 5.83 (4.50, 7.18) | 5.48 (4.13, 6.97) | 0.18 | 5.49 (4.14, 6.08) | 5.58 (4.29, 7.07) | 0.57 |
| Platelet (× 109/L) | 186 (125, 241) | 144 (103, 204) | 0.09 | 105 (65, 226) | 171 (117, 230) | 0.39 |
| PTT (s) | 12.9 (12.3, 13.8) | 13.5 (12.7, 14.7) | 0.02 | 13 (12.1, 13.2) | 13.1 (12.4, 14.2) | 0.34 |
| AST (U/L) | 51 (35, 102) | 47 (37, 79) | 0.78 | 163 (102, 169) | 49 (36, 77) | 0.003 |
| ALT (U/L) | 35 (20, 80) | 33 (21, 51) | 0.29 | 106 (80, 139) | 31 (20, 51) | < 0.001 |
| ALP (U/L) | 124 (104, 290) | 120 (93, 153) | 0.211 | 290 (121, 386) | 120 (94, 169) | 0.111 |
| Albumin (g/dL) | 4 (3.6, 4.2) | 3.9 (3.5, 4.2) | 0.569 | 3.7 (3.2, 4.1) | 3.9 (3.6, 4.2) | 0.360 |
| Total bilirubin (mg/dL) | 0.59 (0.39, 1) | 0.69 (0.51, 1.3) | 0.163 | 1.59 (0.82, 2.11) | 0.59 (0.42, 1.01) | 0.005 |
| Direct bilirubin (mg/dL) | 0.35 (0.24, 0.65) | 0.39 (0.26, 0.78) | 0.415 | 0.91 (0.49, 1.76) | 0.35 (0.24, 0.65) | 0.004 |
| Presence of PES, n (%) | - | - | - | 7 (100%) | 36 (38.7%) | 0.002 |
| Fever | 40 (93.0%) | 5 (8.8%) | < 0.001 | 7 (100%) | 38 (40.9%) | 0.002 |
| Vomit | 25 (58.1%) | 2 (3.5%) | < 0.001 | 3 (42.9%) | 24 (25.8%) | 0.327 |
| Pain | 5 (11.6%) | 0 (0.0%) | 0.008 | 0 (0.0%) | 5 (5.37%) | 0.529 |
| Anorexia | 21 (48.8%) | 2 (3.5%) | < 0.001 | 4 (57.1%) | 17 (18.3%) | 0.015 |
Interestingly, post-TACE liver decompensation occurred only in the placebo group (14% vs 0%; P < 0.006). All cases were accompanied by PES. A higher proportion of patients with baseline Child-Pugh class B was observed in the post-TACE liver decompensation group, but no statistical significance was found (28.6% vs 16.1%, P = 0.39). A higher proportion of patients with abnormal liver function test, except albumin levels, was observed in the liver decompensation group. Neither tumor burden nor number of cTACE episodes influenced the occurrence of post-TACE liver decompensation. Multiple vessel embolization was performed for more patients in the post-TACE liver decompensation group compared with the group without liver decompensation (57.1% vs 28%, P = 0.02). A shorter median duration of hospital stay was observed in the NAC-DEXA group (4 vs 6 d; P < 0.001) as seen in Figure 2B. Most patients with PES were febrile, requiring empirical antibiotics therapy that was provided until negative hemoculture was obtained; this was the main reason for increased duration of hospitalization. Acute kidney injury was observed in three patients with baseline chronic kidney disease. All of them showed improved creatinine level and glomerular infiltration rate and received standard intravenous fluids at a rate of 60 mL/h from 24 h pre-TACE till 48 h post-TACE.
In univariate analysis of tumor volume, calculated increase in Albumin-Bilirubin (ALBI) score of more than 0.5 and a dynamic change in liver function (more than 20-fold and 1.5-fold increase from baseline in transaminase and bilirubin levels, respectively) within 48 h post-TACE were associated with the development of PES. Multivariate analysis showed that only intravenous NAC-DEXA pre-procedure could reduce the incidence of PES (OR = 0.04; 95%CI: 0.01-0.2; P < 0.001) (Table 3).
| Parameter | PES after cTACE | Liver decompensation after cTACE | ||||||||||
| Crude OR | 95%CI | P value | aOR | 95%CI | P value | Crude OR | 95%CI | P value | aOR | 95%CI | P value | |
| NAC + DEXA | 0.02 | 0-0.06 | < 0.01 | 0.04 | 0.01-0.2 | < 0.01 | 0 | 0-1 | 1.00 | 0 | 0-1 | 1.00 |
| Size + number up to 12 | 1.09 | 1-1.19 | 0.04 | 1.08 | 0.92-1.27 | 0.33 | 0.99 | 0.84-1.16 | 0.88 | - | - | - |
| AST rise > 20 folds in 48 h | 1.51 | 1.09-2.09 | 0.01 | 1.35 | 0.72-2.52 | 0.35 | 1.7 | 0.95-3.03 | 0.07 | 1.3 | 0.56-3.02 | 0.54 |
| ALBI change > 0.5 | 7.58 | 1.96-29.36 | 0.003 | 3.03 | 0.39-23.67 | 0.29 | 122.52 | 5.6-2681 | 0.002 | 42.77 | 1.01-1810 | 0.049 |
| Total bilirubin rising > 1.5 folds | 3.83 | 1.8-8.14 | < 0.01 | 1.43 | 0.42-4.87 | 0.57 | 2.79 | 1.23-6.31 | 0.01 | 1.46 | 0.52-4.1 | 0.48 |
| South west oncology grading > 4 | 1 | 0-1 | 0.99 | - | - | - | 1.43 | 1.04-1.96 | 0.03 | 0.94 | 0.51-1.76 | 0.86 |
In univariate analysis, an occurrence of PES after TACE with an SWOG score > 4, more than 0.5 point increase in ALBI score, and a 1.5-fold increase from baseline in bilirubin level were associated with the development of liver decompensation (Table 3). In multivariate analysis, only a dynamic change in ALBI score > 0.5 point was considered an important risk for occurrence of liver decompensation with an OR of 42.77 (95%CI: 1.01-1810; P = 0.049).
Only two patients in the NAC-DEXA group developed a minor allergic skin reaction; in which, we disrupted treatment immediately. Six hours after drug discontinuation and administration of anti-allergic medication, the symptoms resolved. Subsequently, both patients completed the NAC-DEXA protocol with a lower infusion rate. No serious adverse event was reported in the NAC-DEXA group (Figure 2C). One patient in the placebo group died within 90 d post-procedure due to severe sepsis with liver decompensation. Although most patients were febrile post-TACE procedure, none contracted intrahospital secondary bacterial infection. The incidence of hyperglycemia did not differ between the NAC-DEXA and placebo groups (34% vs 32%, P = 0.83). Only three patients experienced grade 3 CTCAE hyperglycemia that was managed with antidiabetic therapy. According to changes in the liver function test at 48 h post-TACE, the 3-fold increase in total bilirubin level from baseline, but not transaminase, was more pronounced in the placebo group compared with that in the NAC-DEXA group (58 % vs 18 %, P = 0.006; Supplementary Table 3).
Pathogenesis of PES is conceivably related to multiple factors, such as direct toxic effects of chemotherapeutic agents and release of inflammatory cytokines related to tumor cell necrosis or ischemic hypoxic injury of normal hepatocytes[13,17,20]. The incidence of PES after TACE, per previous reports, ranges widely (45%-83%). Studies that did not provide a strategic prophylaxis for PES reported an incidence of up to 80%. The incidence of PES in the present study was 43%. PES was verified by the occurrence of symptoms such as fever, nausea, vomiting, and abdominal pain, using SWOG toxicity code score of more than two point[7]. Fever is the most common symptom exhibiting a heterogeneity of prevalence (11.6%-74%), followed by nausea (11.6%-80.6%) and vomiting (16.2%-58.9%)[6,9,12,13,21]; in this study, the reported prevalence is 90%, 66%, and 54% for fever, nausea, and vomiting, respectively. Amelioration of nausea and vomiting by ondansetron premedication (8 mg) and selection of the less adverse chemotherapeutic events-causing agent, mitomycin C, may explain the lower incidence of nausea/vomiting in the present study than that in previous studies. Despite the application of super-selective single vessel embolization technique by radio-intervention at our center, a large tumor burden and a trend of higher mitomycin dose (> 10 mg) was observed and may have affected both tumor and normal liver cell necrosis, resulting in a higher PES occurrence.
NAC not only lowers free-radical levels, attributed to its antioxidant properties, but also acts as an indirect antioxidant by increasing the glutathione level and anti-inflammatory effect[22]. Therefore, many gastrointestinal guidelines recommend NAC for the treatment of alcoholic hepatitis[23], acetaminophen overdose[24], and non-acetaminophen acute liver failure[25]. Other favorable effects could be linked to its hepatoprotective activity. Interestingly, Siramolpiwat et al[17] demonstrated this protective effect; intravenous NAC minimized PES compared with the placebo group even though criteria for PES diagnosis was defined as only occurrence of fever and elevated serum alanine transaminase, without reference to clinical symptoms.
The beneficial effects of DEXA were presumably attributed to its antiemetic and inflammation dampening properties. Kogut et al[26] demonstrated that patients receiving prophylactic DEXA tended to require lower doses of antiemetic agents than those who do not. Ogasawara et al[11] reported that intravenous administration of a combination of DEXA and antiemetics (total 36 mg) for 3 d ameliorated PES by 52.5%. Recently, a randomized controlled trial by Sainamthip et al[12] demonstrated that a single dose of intravenous DEXA (8 mg) can prevent PES, achieving a negative PES rate of 63.3%.
We decided to maximize the protective effect of DEXA and NAC in prevention of PES by combining the two, with intravenous administration of DEXA (cumulative dose of 36 mg) and NAC 24 h before and continuous infusion until 48 h after cTACE. Interestingly, our study found that pre-TACE therapy with NAC-DEXA regimen led to a lower PES occurrence of less than 10%, which is the lowest incidence compared with those reported in previous publications (24.6% in a NAC study[17] and 37%-47% in DEXA studies[11,12]. The synergistic effect of NAC and DEXA can diminish systemic inflammatory response and ischemic hepatitis[27-29]. Hence, this study emphasizes the advantage of NAC-DEXA combination due to its synergistic PES-reducing effect.
Post-TACE liver decompensation is one of the most important complications and is associated with significant morbidity and mortality[21,30]. It is characterized by an increase in Child-Pugh score of more than 2 points, ≥ 2 mg/dL rise in serum total bilirubin level, newly developed ascites, or hepatic encephalopathy within two weeks post-procedure. In the present study, seven patients developed post-TACE liver decompensation. Previous studies reported that portal vein thrombosis, poor baseline liver status, high serum AFP, and PES were associated with this complication[17,27,28]. Correspondingly, all patients with liver decompensation in our study had concurrent PES. In patients who underwent post-TACE without any prophylaxis treatment, we observed a lower incidence (14%) of liver decompen
ALBI score is the only objective parameter to stratify patients into different grades and is used to predict the prognosis of all HCC stages. This new model outperforms the Child-Pugh score for evaluation of liver function reserve. In a previous study, evaluation of baseline ALBI score in HCC patients who underwent cTACE not only predicted survival but also estimated liver decompensation and liver failure[31]. In our study, the mean pre-treatment ALBI score was not significantly different between the NAC-DEXA and placebo groups. Our findings indicate that the proportion of preserved liver, defined by ALBI grade 1, was comparable between the two groups. Moreover, all patients who developed post-TACE liver decompensation had a baseline ALBI grade 1 or 2 with a mean pre-treatment ALBI score of -2.57. The increase in ALBI score was hypothesized to be a novel non-invasive tool for earlier prediction of post-TACE liver decompensation than Child-Pugh score. Our study demonstrated that a dynamic increase in ALBI score of more than 0.5 point had a marked impact on liver decompensation. Thus, the application of dynamic increase in ALBI score, but not albumin level, for a prediction of early post-TACE liver decompensation requires further research.
Our study had some limitations. First, we did not perform dose optimization for DEXA and NAC in order to minimize PES. Second, this dual treatment was not compared with single DEXA. The dynamic changes in cytokine levels due to this dual treatment is an interesting topic for further study.
A combination of DEXA and NAC can not only maximize the reduction in PES incidence but also shorten hospitalization period in HCC patients undergoing cTACE procedure. Dynamic alterations in ALBI score, but not CPS, may predict liver decompensation.
Transarterial chemoembolization (TACE) is the current standard treatment for intermediate-stage hepatocellular carcinoma (HCC). Post-embolization syndrome (PES) is a complex clinical syndrome which may occur after conventional TACE (cTACE). Either N-acetylcysteine (NAC) or dexamethasone (DEXA) is used to prevent PES.
The synergistic effect of the combined therapy for preventing PES and liver decompensation has not been determined.
The aim of this study was to evaluate the efficacy of NAC and DEXA combination in preventing PES and liver decompensation after cTACE.
A single-center randomized controlled clinical trial.
Our study provides clinical evidence that intravenous NAC plus DEXA administration ameliorates the occurrence of post-TACE PES in patients with intermediate-stage HCC. Interestingly, we found that a dynamic change in Albumin-Bilirubin (ALBI) score was a risk factor for post-TACE liver decompensation.
A combination of NAC and DEXA ameliorated the occurrence of PES after cTACE in patients with intermediate-stage HCC.
The application of dynamic increase in ALBI score for a prediction of early post-TACE liver decompensation requires further research.
We thank Dr Chadakarn Phaloprakarn for the scientific advice and Miss Kanokwan Sansuk for the reference format.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Thailand
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Spiliopoulos S, Greece; Xu X, China S-Editor: Wang JJ L-Editor: A P-Editor: Wang JJ
| 1. | Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet. 2018;391:1301-1314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2800] [Cited by in RCA: 4108] [Article Influence: 586.9] [Reference Citation Analysis (6)] |
| 2. | Somboon K, Siramolpiwat S, Vilaichone RK. Epidemiology and survival of hepatocellular carcinoma in the central region of Thailand. Asian Pac J Cancer Prev. 2014;15:3567-3570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 39] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 3. | Wigmore SJ, Redhead DN, Thomson BN, Currie EJ, Parks RW, Madhavan KK, Garden OJ. Postchemoembolisation syndrome--tumour necrosis or hepatocyte injury? Br J Cancer. 2003;89:1423-1427. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 71] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 4. | Tasneem AA, Abbas Z, Luck NH, Hassan SM, Faiq SM. Adverse events following transarterial chemoembolization for hepatocellular carcinoma and factors predicting such events. J Pak Med Assoc. 2013;63:239-244. [PubMed] |
| 5. | Blackburn H, West S. Management of Postembolization Syndrome Following Hepatic Transarterial Chemoembolization for Primary or Metastatic Liver Cancer. Cancer Nurs. 2016;39:E1-E18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 65] [Article Influence: 8.1] [Reference Citation Analysis (1)] |
| 6. | Li CP, Chao Y, Chen LT, Lee RC, Lee WP, Yuan JN, Yen SH, Lee SD. Fever after transcatheter arterial chemoembolization for hepatocellular carcinoma: incidence and risk factor analysis. Scand J Gastroenterol. 2008;43:992-999. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 7. | Green S, Weiss GR. Southwest Oncology Group standard response criteria, endpoint definitions and toxicity criteria. Invest New Drugs. 1992;10:239-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 468] [Cited by in RCA: 470] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 8. | Dalal R, McGee RG, Riordan SM, Webster AC. Probiotics for people with hepatic encephalopathy. Cochrane Database Syst Rev. 2017;2:CD008716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 56] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 9. | He JJ, Yin XX, Wang T, Chen MY, Li XL, Yang XJ, Shao HY. Factors influencing postembolization syndrome in patients with hepatocellular carcinoma undergoing first transcatheter arterial chemoembolization. J Cancer Res Ther. 2021;17:777-783. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 10. | Hesketh PJ, Kris MG, Basch E, Bohlke K, Barbour SY, Clark-Snow RA, Danso MA, Dennis K, Dupuis LL, Dusetzina SB, Eng C, Feyer PC, Jordan K, Noonan K, Sparacio D, Somerfield MR, Lyman GH. Antiemetics: American Society of Clinical Oncology Clinical Practice Guideline Update. J Clin Oncol. 2017;35:3240-3261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 350] [Cited by in RCA: 369] [Article Influence: 46.1] [Reference Citation Analysis (0)] |
| 11. | Ogasawara S, Chiba T, Ooka Y, Kanogawa N, Motoyama T, Suzuki E, Tawada A, Nagai K, Nakagawa T, Sugawara T, Hanaoka H, Kanai F, Yokosuka O. A randomized placebo-controlled trial of prophylactic dexamethasone for transcatheter arterial chemoembolization. Hepatology. 2018;67:575-585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 60] [Article Influence: 8.6] [Reference Citation Analysis (1)] |
| 12. | Sainamthip P, Kongphanich C, Prasongsook N, Chirapongsathorn S. Single dose dexamethasone prophylaxis of postembolisation syndrome after chemoembolisation in hepatocellular carcinoma patient: A randomised, double-blind, placebo-controlled study. World J Clin Cases. 2021;9:9059-9069. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 6] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 13. | Yang H, Seon J, Sung PS, Oh JS, Lee HL, Jang B, Chun HJ, Jang JW, Bae SH, Choi JY, Yoon SK. Dexamethasone Prophylaxis to Alleviate Postembolization Syndrome after Transarterial Chemoembolization for Hepatocellular Carcinoma: A Randomized, Double-Blinded, Placebo-Controlled Study. J Vasc Interv Radiol. 2017;28:1503-1511.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 41] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 14. | Sun Y, Pu LY, Lu L, Wang XH, Zhang F, Rao JH. N-acetylcysteine attenuates reactive-oxygen-species-mediated endoplasmic reticulum stress during liver ischemia-reperfusion injury. World J Gastroenterol. 2014;20:15289-15298. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 61] [Cited by in RCA: 76] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 15. | Wang C, Chen K, Xia Y, Dai W, Wang F, Shen M, Cheng P, Wang J, Lu J, Zhang Y, Yang J, Zhu R, Zhang H, Li J, Zheng Y, Zhou Y, Guo C. N-acetylcysteine attenuates ischemia-reperfusion-induced apoptosis and autophagy in mouse liver via regulation of the ROS/JNK/Bcl-2 pathway. PLoS One. 2014;9:e108855. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 81] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 16. | Hsieh CC, Hsieh SC, Chiu JH, Wu YL. Protective Effects of N-acetylcysteine and a Prostaglandin E1 Analog, Alprostadil, Against Hepatic Ischemia: Reperfusion Injury in Rats. J Tradit Complement Med. 2014;4:64-71. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 17. | Siramolpiwat S, Punjachaipornpon T, Pornthisarn B, Vilaichone RK, Chonprasertsuk S, Tangaroonsanti A, Bhanthumkomol P, Phumyen A, Yasiri A, Kaewmanee M. N-Acetylcysteine Prevents Post-embolization Syndrome in Patients with Hepatocellular Carcinoma Following Transarterial Chemoembolization. Dig Dis Sci. 2019;64:3337-3345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 18. | Marrero JA, Kulik LM, Sirlin CB, Zhu AX, Finn RS, Abecassis MM, Roberts LR, Heimbach JK. Diagnosis, Staging, and Management of Hepatocellular Carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology. 2018;68:723-750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2121] [Cited by in RCA: 3241] [Article Influence: 463.0] [Reference Citation Analysis (1)] |
| 19. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol. 2018;69:182-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5593] [Cited by in RCA: 6060] [Article Influence: 865.7] [Reference Citation Analysis (3)] |
| 20. | Chan AO, Yuen MF, Hui CK, Tso WK, Lai CL. A prospective study regarding the complications of transcatheter intraarterial lipiodol chemoembolization in patients with hepatocellular carcinoma. Cancer. 2002;94:1747-1752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 183] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 21. | Leung DA, Goin JE, Sickles C, Raskay BJ, Soulen MC. Determinants of postembolization syndrome after hepatic chemoembolization. J Vasc Interv Radiol. 2001;12:321-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 177] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 22. | Tenório MCDS, Graciliano NG, Moura FA, Oliveira ACM, Goulart MOF. N-Acetylcysteine (NAC): Impacts on Human Health. Antioxidants (Basel). 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 105] [Cited by in RCA: 243] [Article Influence: 60.8] [Reference Citation Analysis (0)] |
| 23. | Nguyen-Khac E, Thevenot T, Piquet MA, Benferhat S, Goria O, Chatelain D, Tramier B, Dewaele F, Ghrib S, Rudler M, Carbonell N, Tossou H, Bental A, Bernard-Chabert B, Dupas JL; AAH-NAC Study Group. Glucocorticoids plus N-acetylcysteine in severe alcoholic hepatitis. N Engl J Med. 2011;365:1781-1789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 262] [Cited by in RCA: 295] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 24. | Prescott LF, Critchley JA. The treatment of acetaminophen poisoning. Annu Rev Pharmacol Toxicol. 1983;23:87-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 116] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 25. | Lee WM, Hynan LS, Rossaro L, Fontana RJ, Stravitz RT, Larson AM, Davern TJ 2nd, Murray NG, McCashland T, Reisch JS, Robuck PR; Acute Liver Failure Study Group. Intravenous N-acetylcysteine improves transplant-free survival in early stage non-acetaminophen acute liver failure. Gastroenterology. 2009;137:856-864, 864.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 452] [Cited by in RCA: 429] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 26. | Kogut MJ, Chewning RH, Harris WP, Hippe DS, Padia SA. Postembolization syndrome after hepatic transarterial chemoembolization: effect of prophylactic steroids on postprocedure medication requirements. J Vasc Interv Radiol. 2013;24:326-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 27. | Garwood ER, Fidelman N, Hoch SE, Kerlan RK Jr, Yao FY. Morbidity and mortality following transarterial liver chemoembolization in patients with hepatocellular carcinoma and synthetic hepatic dysfunction. Liver Transpl. 2013;19:164-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 61] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 28. | Huang YS, Chiang JH, Wu JC, Chang FY, Lee SD. Risk of hepatic failure after transcatheter arterial chemoembolization for hepatocellular carcinoma: predictive value of the monoethylglycinexylidide test. Am J Gastroenterol. 2002;97:1223-1227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 60] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 29. | Hsin IF, Hsu CY, Huang HC, Huang YH, Lin HC, Lee RC, Chiang JH, Lee FY, Huo TI, Lee SD. Liver failure after transarterial chemoembolization for patients with hepatocellular carcinoma and ascites: incidence, risk factors, and prognostic prediction. J Clin Gastroenterol. 2011;45:556-562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 50] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 30. | Min YW, Kim J, Kim S, Sung YK, Lee JH, Gwak GY, Paik YH, Choi MS, Koh KC, Paik SW, Yoo BC. Risk factors and a predictive model for acute hepatic failure after transcatheter arterial chemoembolization in patients with hepatocellular carcinoma. Liver Int. 2013;33:197-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 31. | Hansmann J, Evers MJ, Bui JT, Lokken RP, Lipnik AJ, Gaba RC, Ray CE Jr. Albumin-Bilirubin and Platelet-Albumin-Bilirubin Grades Accurately Predict Overall Survival in High-Risk Patients Undergoing Conventional Transarterial Chemoembolization for Hepatocellular Carcinoma. J Vasc Interv Radiol. 2017;28:1224-1231.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 53] [Article Influence: 6.6] [Reference Citation Analysis (0)] |