Published online Feb 7, 2023. doi: 10.3748/wjg.v29.i5.851
Peer-review started: September 25, 2022
First decision: November 5, 2022
Revised: November 16, 2022
Accepted: December 13, 2022
Article in press: December 13, 2022
Published online: February 7, 2023
Processing time: 134 Days and 1.4 Hours
Postoperative recurrence (POR) after ileocecal resection (ICR) affects most Crohn's disease patients within 3-5 years after surgery. Adherent-invasive Escherichia coli (AIEC) typified by the LF82 strain are pathobionts that are frequently detected in POR of Crohn's disease and have a potential role in the early stages of the disease pathogenesis. Saccharomyces cerevisiae CNCM I-3856 is a probiotic yeast reported to inhibit AIEC adhesion to intestinal epithelial cells and to favor their elimination from the gut.
To evaluate the efficacy of CNCM I-3856 in preventing POR induced by LF82 in an HLA-B27 transgenic (TgB27) rat model.
Sixty-four rats [strain F344, 38 TgB27, 26 control non-Tg (nTg)] underwent an ICR at the 12th wk (W12) of life and were sacrificed at the 18th wk (W18) of life. TgB27 rats were challenged daily with oral administration of LF82 (109 colony forming units (CFUs)/day (d), n = 8), PBS (n = 5), CNCM I-3856 (109 CFUs/d, n = 7) or a combination of LF82 and CNCM I-3856 (n = 18). nTg rats receiving LF82 (n = 5), PBS (n = 5), CNCM I-3856 (n = 7) or CNCM I-3856 and LF82 (n = 9) under the same conditions were used as controls. POR was analyzed using macroscopic (from 0 to 4) and histologic (from 0 to 6) scores. Luminal LF82 quantifications were performed weekly for each animal. Adherent LF82 and inflammatory/regulatory cytokines were quantified in biopsies at W12 and W18. Data are expressed as the median with the interquartile range.
nTg animals did not develop POR. A total of 7/8 (87%) of the TgB27 rats receiving LF82 alone had POR (macroscopic score ≥ 2), which was significantly prevented by CNCM I-3856 administration [6/18 (33%) TgB27 rats, P = 0.01]. Macroscopic lesions were located 2 cm above the anastomosis in the TgB27 rats receiving LF82 alone and consisted of ulcerations with a score of 3.5 (2 - 4). Seven out of 18 TgB27 rats (39%) receiving CNCM I-3856 and LF82 had no macroscopic lesions. Compared to untreated TgB27 animals receiving LF82 alone, coadministration of CNCM I-3856 and LF82 significantly reduced the macroscopic [3.5 (2 - 4) vs 1 (0 - 3), P = 0.002] and histological lesions by more than 50% [4.5 (3.3 - 5.8) vs 2 (1.3 - 3), P = 0.003]. The levels of adherent LF82 were correlated with anastomotic macroscopic scores in TgB27 rats (r = 0.49, P = 0.006), with a higher risk of POR in animals having high levels of luminal LF82 (71.4% vs 25%, P = 0.02). Administration of CNCM I-3856 significantly reduced the levels of luminal and adherent LF82, increased the production of interleukin (IL)-10 and decreased the production of IL-23 and IL-17 in TgB27 rats.
In a reliable model of POR induced by LF82 in TgB27 rats, CNCM I-3856 prevents macroscopic POR by decreasing LF82 infection and gut inflammation.
Core Tip: Gut dysbiosis plays a main role in the postoperative recurrence (POR) of Crohn's disease (CD). CD dysbiosis is characterized by a lower microbiota diversity with an increase in pathogenic species. Among them, adherent-invasive Escherichia coli (AIEC) has been linked to POR. Saccharomyces cerevisiae (S. cerevisiae) CNCM I-3856 is a probiotic yeast that specifically targets AIEC by preventing the bacterial adhesion process and inhibiting its persistence within the bowel. This study confirmed the capacity of S. cerevisiae CNCM I-3856 to prevent AIEC-induced POR by decreasing the infection in a transgenic HLA-B27 rat model of POR after ileocecal resection.
- Citation: Valibouze C, Speca S, Dubuquoy C, Mourey F, M'Ba L, Schneider L, Titecat M, Foligné B, Genin M, Neut C, Zerbib P, Desreumaux P. Saccharomyces cerevisiae prevents postoperative recurrence of Crohn's disease modeled by ileocecal resection in HLA-B27 transgenic rats. World J Gastroenterol 2023; 29(5): 851-866
- URL: https://www.wjgnet.com/1007-9327/full/v29/i5/851.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i5.851
Crohn's disease (CD) is a complex chronic inflammatory bowel disease that requires surgical resection of macroscopic lesions in approximately 30%-50% of patients in their lifetime[1]. Unfortunately, surgery is not curative, and endoscopic recurrence at the anastomotic site occurs in up to 70% of patients in the first year after surgery, followed by clinical recurrence a few years later[2]. Postoperative management of these patients is crucial to identify those at highest risk of recurrence to begin rapid prophylactic treatments targeting mainly tumor necrosis factor α (TNFα)[3], interleukins 12/23 and α4β7 integrins on leukocytes[4]. Given the high rate of recurrence after intestinal resection for CD and the cost and potential adverse effects of biologic therapies used in prophylaxis, there is a clear need to identify the mechanisms leading to postoperative recurrence (POR), to develop noninvasive methods predicting recurrence and to propose new evidence-based therapeutic strategies.
The physiopathology sustaining POR of CD remains partially unknown. Abnormal interactions between the mucosal/mesenteric immune system and the intestinal microbiota favored by surgical techniques and environmental factors are pivotal hallmarks in POR dynamics[5]. Recently, ileal transcriptome analyses of CD patients found a gene signature of POR characterized by an upregulation of the interleukin (IL)-23 and IL-17 pathways together with abnormal JAK/STAT activation[6]. Numerous changes in the microbial composition and a reduction in species diversity have been observed in the intestinal flora of CD patients[7], and a few studies have identified an intestinal microbial signature associated with POR. Recolonization of the neoterminal ileum by Escherichia coli (E. coli), Bacteroides, and Fusobacteriaceae and the depletion of Streptococcaceae, Actinomycineae and Faecalibacterium are associated with endoscopic recurrence of CD[8]. Among these microorganisms, adherent-invasive E. coli (AIEC) isolated more than 20 years ago by Darfeuille-Michaud et al[9] from the ileal mucosa of a patient with CD[10] remains one of the most prominent and influential strains associated with CD. AIEC are pathobionts found in approximately 30% of CD patients and in 10% of healthy controls[11]. They are not strictly pathogenic bacteria, and their influence on CD physiopathology remains incompletely understood. However, AIEC is associated with the early stages of CD and is predictive of endoscopic POR at 6 mo[12], reinforcing the need for interventional studies targeting these bacteria to better understand their direct impact on mucosal inflammation and to find new opportunities to treat CD patients.
Several therapeutic strategies, including the use of antibiotics[13], pre/probiotics[14] and fecal microbiota transplantation[15], have been proposed to target the intestinal flora in CD. Due to side effects or limited efficacy, their routine utilization cannot be recommended[16,17]. Other strategies to inhibit adhesion or to specifically erase AIEC using FimH blockers[18,19] or specific bacteriophages[20,21] are ongoing and seem more promising in preclinical studies. In this context, Saccharomyces cerevisiae (S. cerevisiae) CNCM I-3856 is a probiotic yeast with good tolerance and beneficial effects on gastrointestinal symptoms[22,23] that has been shown to agglutinate the LF82 AIEC strain and to prevent its adhesion to intestinal epithelial cells in vitro, favoring LF82 elimination from the gut of mice[24]. Among the thousands of strains belonging to the AIEC family and identified from European and USA isolates, LF82 remains the most studied reference strain that can both adhere to and invade epithelial cells and, moreover, survive and replicate within macrophages without inducing cellular death[25,26].
In the present study, we developed a new animal model of POR of CD occurring 6 wk after ileocecal resection (ICR) in HLA-B27 transgenic (Tg) rats[27,28] infected by the LF82 AIEC strain[29] to better evaluate the causal role of LF82 on the early steps of CD lesions and the effectiveness of a rationally selected S. cerevisiae CNCM I-3856 probiotic to prevent recurrence of the disease.
HLA-B27 transgenic (Tg) and nontransgenic (nTg) control Fisher rats (strain F344) were provided by Professor M. Breban (Cochin Institute, INSERM U1016, Paris, France). Sixty-four rats were maintained in a specific pathogen-free facility at the Institut Pasteur (Lille, France) and were fed a standard diet with free access to water. Animals were maintained at a constant temperature with a 12-hour light/dark cycle. Intragastric gavage administration was carried out with conscious animals using straight gavage needles appropriate for the animal size. Surgery was performed under general anesthesia, and postoperative analgesia by opioid treatment was provided. All animals were euthanized by cervical dislocation under general anesthesia. Experiments were realized according to the European directive 2016/63/UE enforced by the decree n°2013-118 and authorized by the departmental ethics committee (No. CEEA 01292-01).
The streptomycin-kanamycin-resistant AIEC strain LF82 isolated from an ileal biopsy of a patient with CD was provided by Professor Nicolas Barnich (Clermont-Auvergne University, France) and used as an AIEC reference strain[30]. Bacteria were routinely grown at 37 °C in Brain-Heart broth or on Drigalski agar plates. The dry S. cerevisiae CNCM I-3856 yeast strain was provided by Lesaffre International (Marcq-en-Baroeul, France). The LF82 and S. cerevisiae CNCM I-3856 strains were rehydrated at room temperature in PBS (pH = 7.2, 2 × 109 colony forming units (CFUs)/mL) before gavage.
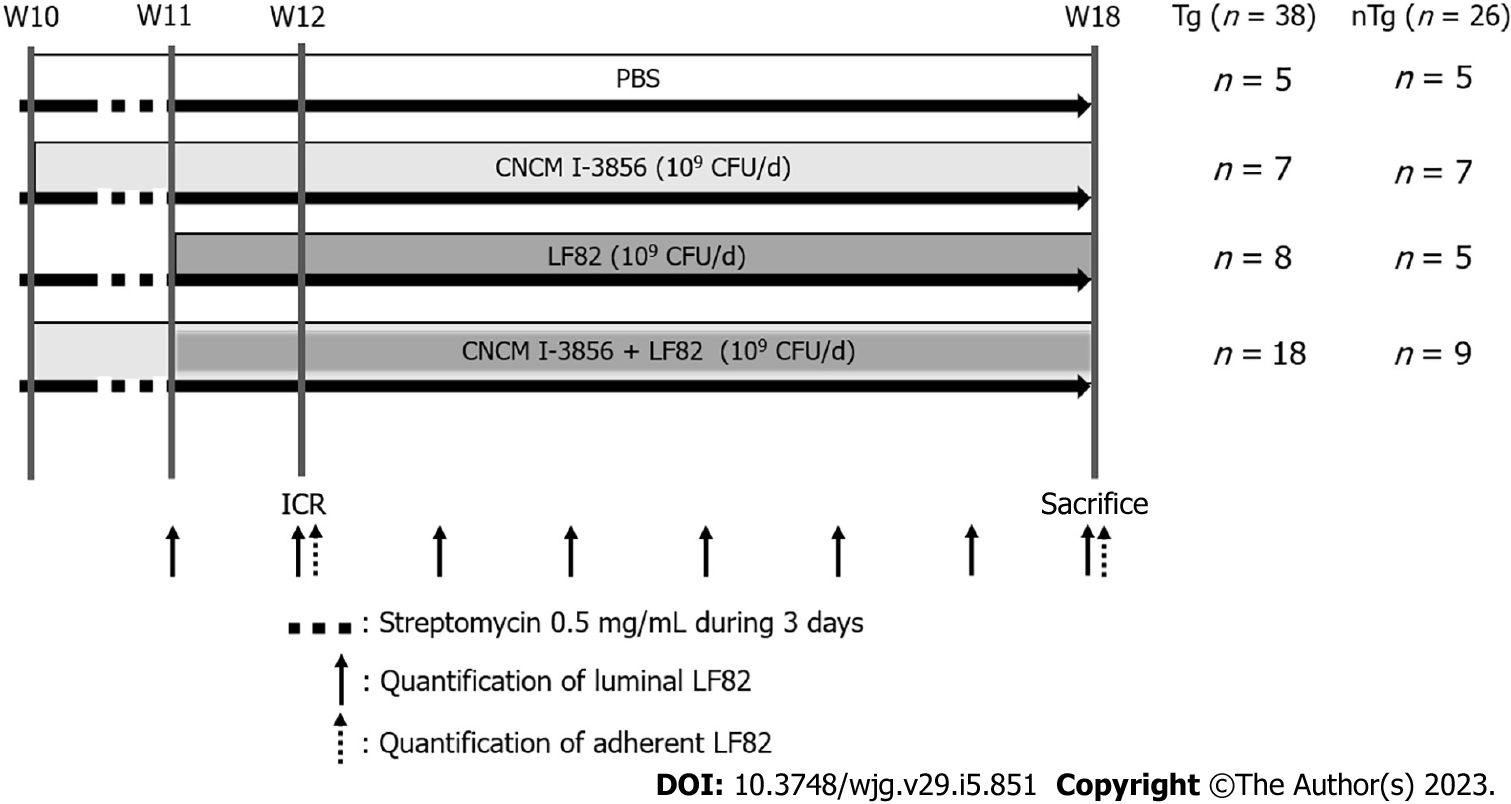
ICR with end-to-end anastomosis was performed at 12 wk (W) of life (W12) in 64 rats (38 Tg, 26 nTg) (Figure 1). ICR was performed blindly by two operators (Caroline Dubuquoy and Caroline Valibouze) in Tg and nTg animals. Tg rats were challenged daily by oral gavage in the morning with PBS (n = 5), S. cerevisiae CNCM I-3856 alone (109 CFUs/day (d)) (n = 7), LF82 alone (109 CFUs/d) in the afternoon (n = 8), or the combination of S. cerevisiae CNCM I-3856 (109 CFUs/d) and LF82 (109 CFUs/d) (n = 18) given in the morning and in the afternoon, respectively. Age-matched nTg rats receiving PBS (n = 5), S. cerevisiae CNCMI-3856 alone (n = 7), LF82 alone (n = 5), or the combination of S. cerevisiae CNCM I-3856 and LF82 (n = 9) under the same conditions were used as controls. LF82 was administered from W11 to W18, and S. cerevisiae CNCM I-3856 was similarly administered from W10 to W18 in Tg and nTg rats. Streptomycin was given in drinking water at 0.5 mg/mL for the last 3 d of W10 in Tg and control animals. The rats were followed during the eight-week procedure for weight changes (% of change compared to initial body weight at W11), diarrhea and the presence of macroscopic bloody stools and were killed at W18.
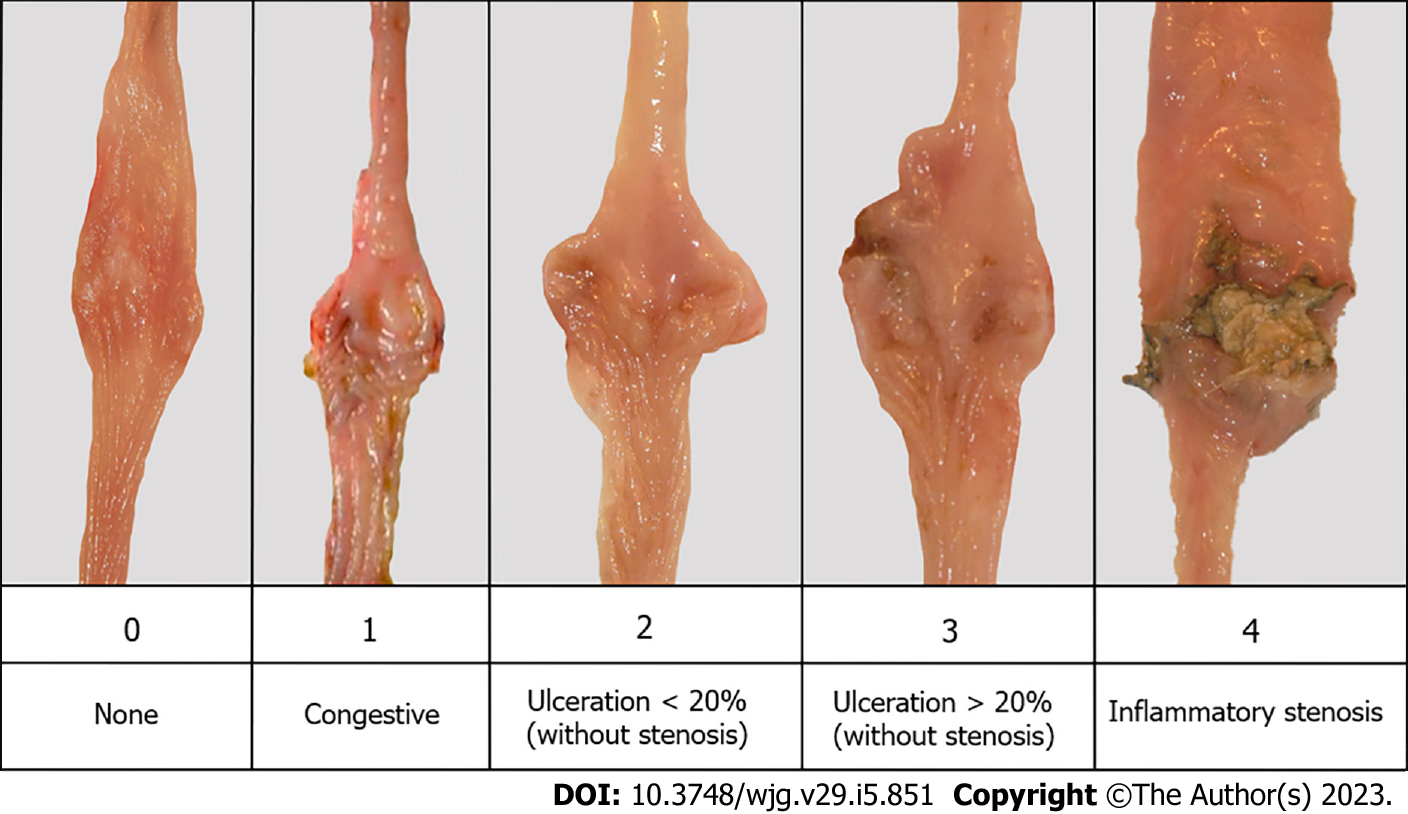
At W18, the whole intestine was excised and photographed. Anastomotic macroscopic lesions (± 2 cm above anastomosis) were assessed blindly using a macroscopic grading scale adapted from the Rutgeerts score ranging from 0 to 4 (Figure 2)[2]. By analogy with endoscopic recurrence after surgery in patients with CD (25), POR was defined by a macroscopic score of ≥ 2 corresponding to the presence of ulcerations ± stenosis. The results were expressed as the median with the interquartile range (IQR).
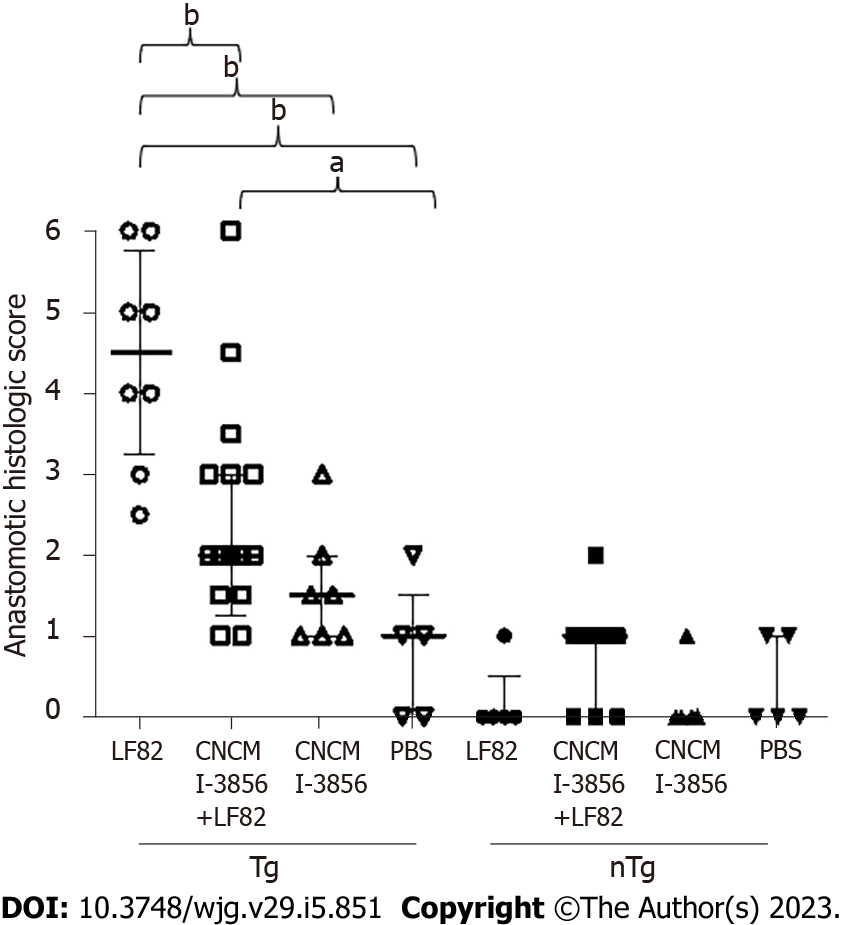
Transparietal biopsies of anastomotic areas were collected during surgery at W12 and W18. Tissues were fixed in 4% buffered formaldehyde, embedded in paraffin and stained by May-Grunwald Giemsa for scoring (from 0 to 6) using the adapted score of Geboes (Table 1)[31]. Identical areas of each section of the different biopsy specimens were examined at 10× magnification by two blinded observers familiar with the scoring system (Caroline Dubuquoy and Caroline Valibouze). Anastomotic histologic scores were expressed as the median score with IQR when an interobserver coefficient of variation < 15% was obtained.
| Score | Histologic lesions |
| 0 | None |
| 1 | Inflammatory infiltrate and mucosal erosions < 30% of the section |
| 2 | 30% < inflammatory infiltrate and mucosal erosions < 70% of the section |
| 3 | Inflammatory infiltrate and mucosal erosions > 70% of the section |
| 4 | Mucosal ulceration < 30% of the section |
| 5 | 30% < mucosal ulceration < 70% of the section |
| 6 | Mucosal ulceration > 70% of the section |
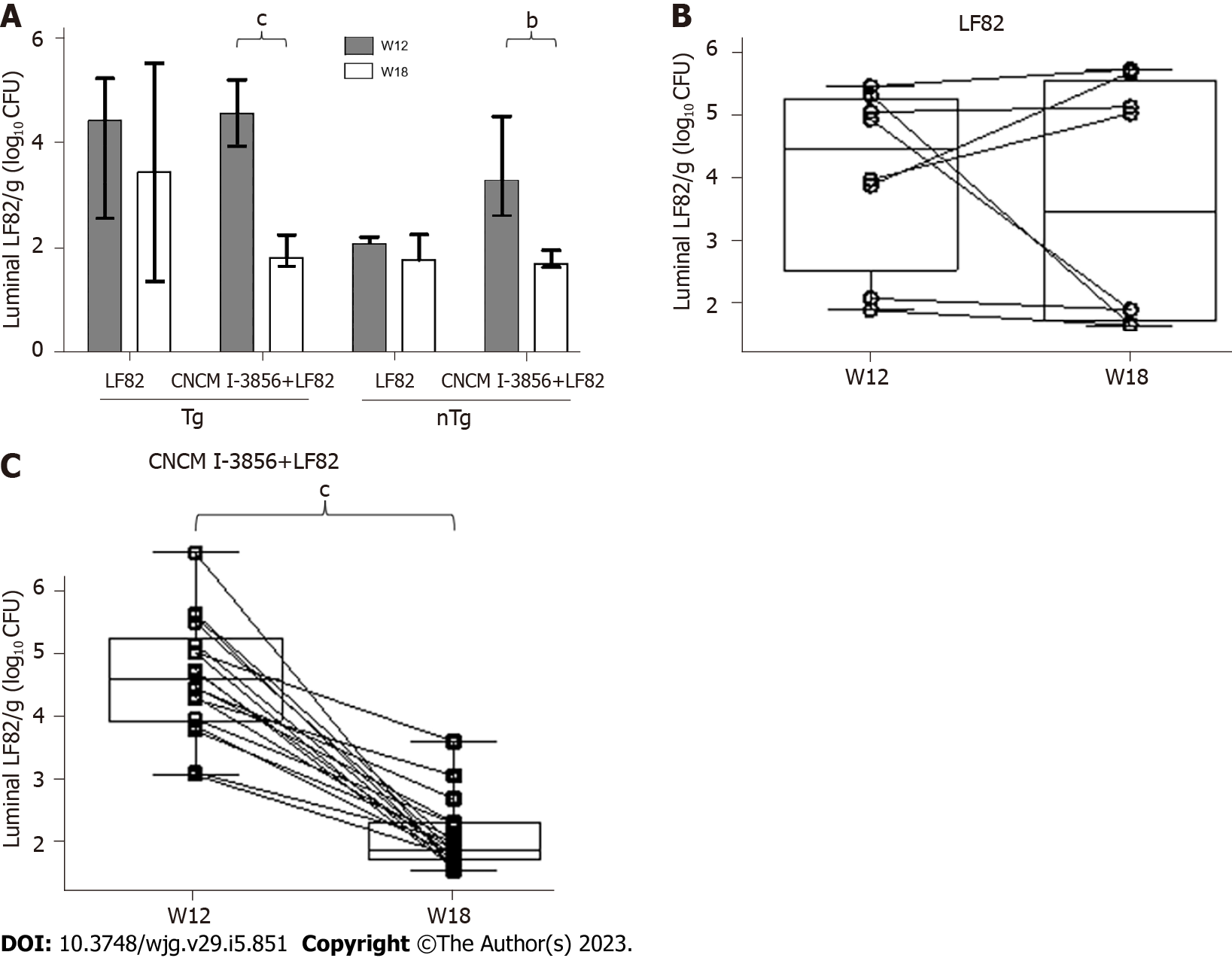
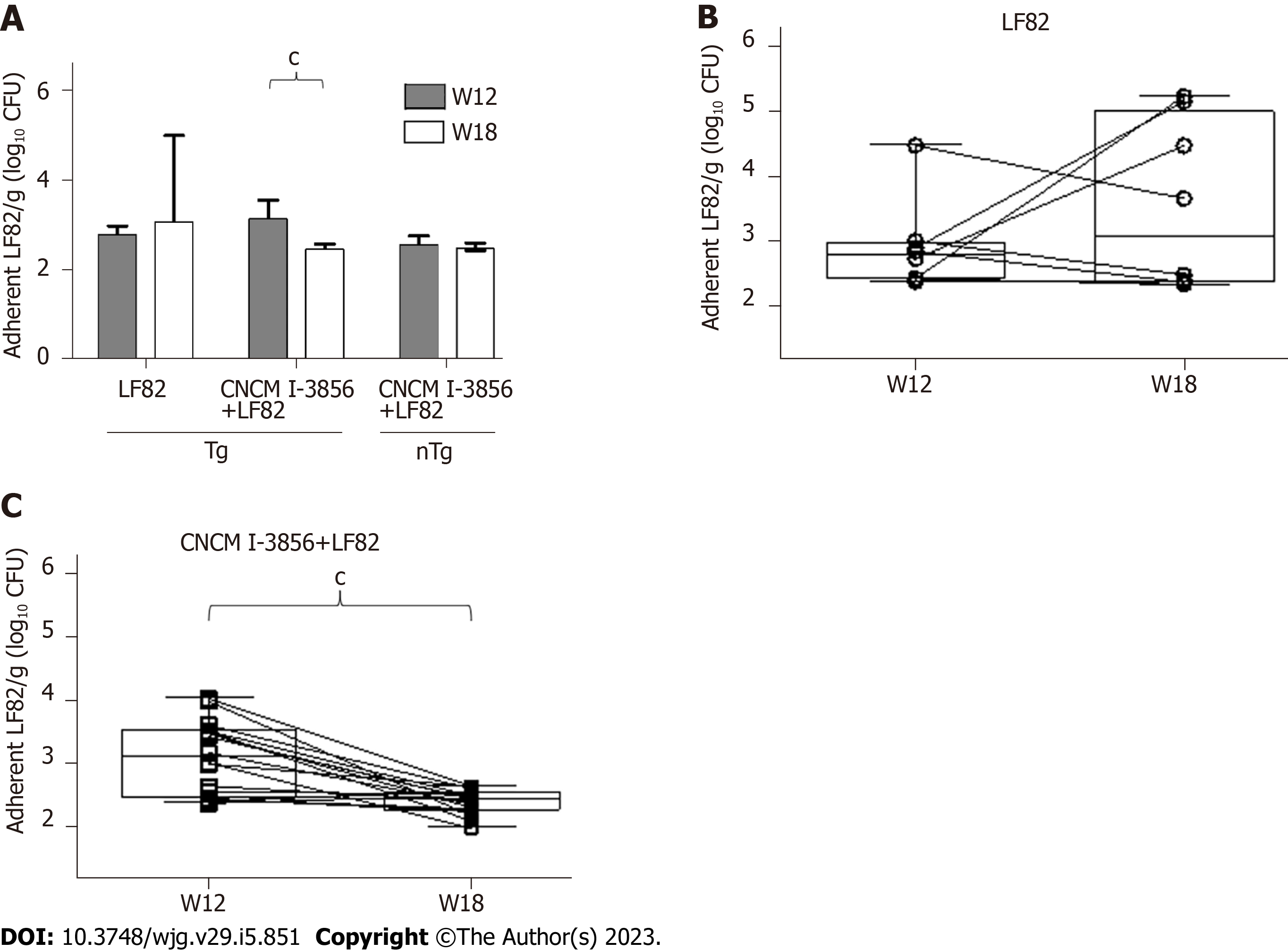
Feces (10 - 600 mg) were collected weekly from W11 to W18 for each animal after abdominal massage for the quantification of luminal LF82. Mucosal anastomotic swabs (10 - 100 mg) were performed at W12 during surgery and at sacrifice (W18) in all animals for the quantification of anastomotic adherent LF82. Fresh feces and swabs were collected in 1.5 mL of sterile cysteinated Ringer’s solution. After serial dilutions, samples were incubated for 24 - 48 h at 37 °C in Drigalski agar containing 100 µg/mL streptomycin to select and quantify LF82 expressed as log10 CFUs per gram of feces. The results are expressed as the median with the IQR.
Anastomotic biopsies were frozen at -80 °C, and total RNA was extracted using a Nucleospin RNA kit (Macherey Nagel). After RNAse inactivation, genomic DNA was suppressed from the samples by DNAse treatment, and total RNA was extracted in RNAse-free water. The RNA content was measured using a NanoDrop Spectrophotometer (Thermo Fisher Scientific, Waltham, Massachusetts, USA). Retro-transcription of total RNA was achieved using a High-Capacity cDNA Reverse Transcription Kit (Thermo Fisher Scientific). Random primers, RT buffer and reverse transcriptase were added to 1 µg of total RNA, and the samples were incubated for 10 min at 25 °C, then 2 h at 37 °C and finally 5 min at 85 °C in the Gene AmpPCR System 9700 automaton (Thermos Fisher Scientific, Waltham, Massachusetts, USA). All kits were used according to the manufacturers’ protocols. IL-1β, IL-6, TNFα, interferon (IFN)γ, IL-17, IL-23 and IL-10 were quantified by quantitative polymerase chain reaction (PCR) in real time for 40 cycles in the StepOnePlus™ Real Time PCR system (Thermo Fisher Scientific, Waltham, Massachusetts, USA) using SYBR Green PCR Master Mix (Thermo Fisher Scientific). qPCR signal quantification was expressed relative to the expression of β-actin as the reference gene. The results are expressed as the median with IQR.
Data are expressed as the median with IQR. Comparisons were performed using the nonparametric Mann-Whitney test for unmatched data and the Wilcoxon signed-rank test for matched data. Pearson’s chi-square test was used for contingency analysis. The correlation between macroscopic scores and the number of LF82 was tested using Spearman’s test. To classify animals with low or high quantities of LF82, a cutoff value was determined using the receiver operating characteristic (ROC) curve. The risk of recurrence for low and high producers was compared using Pearson’s chi-square test. All statistical tests were two-tailed and considered statistically significant if P < 0.05. Statistical analyses were conducted using the GraphPad Prism 5.00 (GraphPad Software, San Diego, CA) software package for PCR and Xlstat 2020.1 version for the ROC curve.
No mortality, diarrhea or bloody stools were observed in any animals receiving PBS, LF82 alone, S. cerevisiae CNCM I-3856 alone or S. cerevisiae CNCM I-3856 and LF82 during the 8-wk observation study.
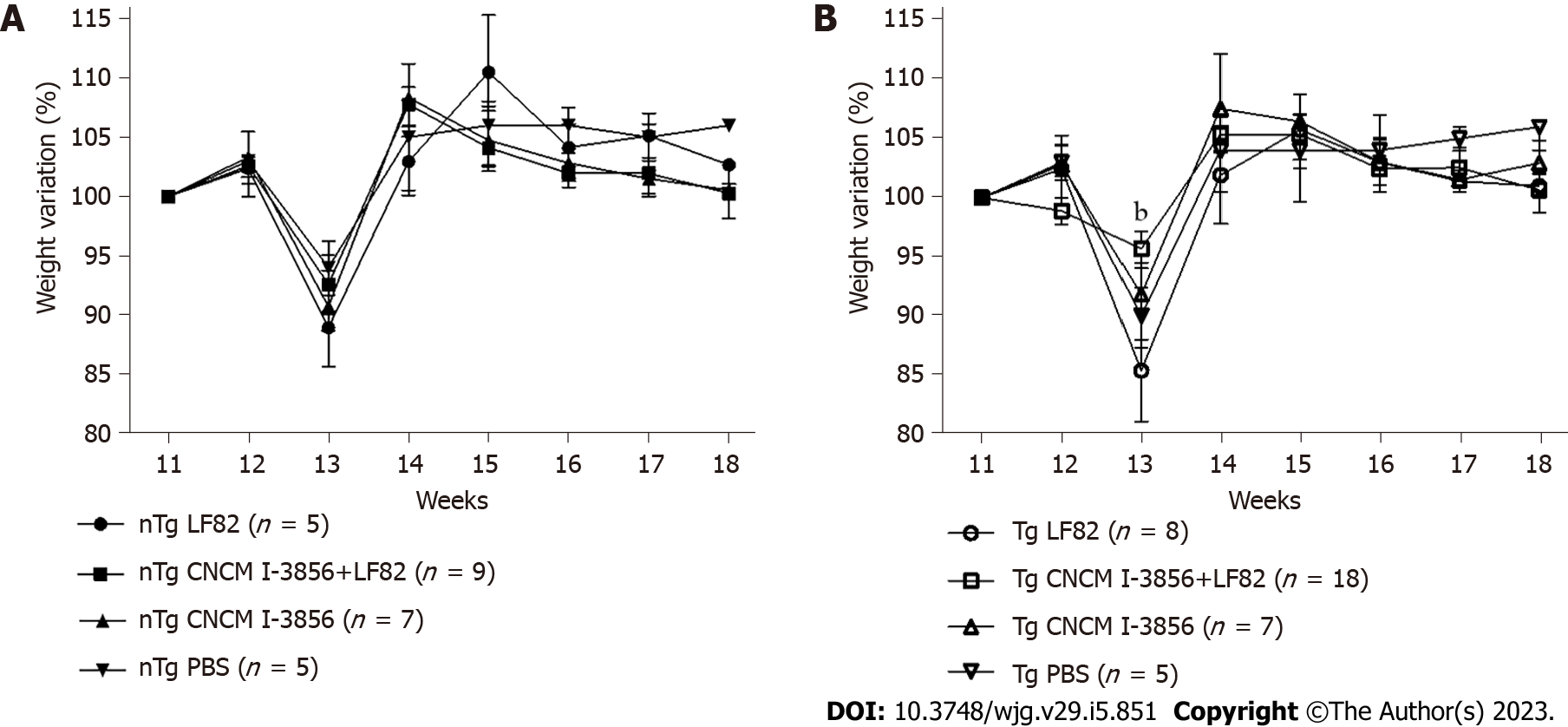
A similar pattern of weight evolution was observed in nTg (Figure 3A) and Tg animals (Figure 3B), with significant weight loss occurring one week after surgery followed by a weight recovery phase. More important weight loss was transiently observed at W13 in Tg rats receiving LF82 vs S. cerevisiae CNCM I-3856 and LF82 (95.7, IQR: 92 - 97 vs 85.4, IQR: 81 - 94, P = 0.007). The global weight changes assessed by the relative difference in weight variation between W11 and W18 were similar in the 4 groups of Tg and nTg animals.
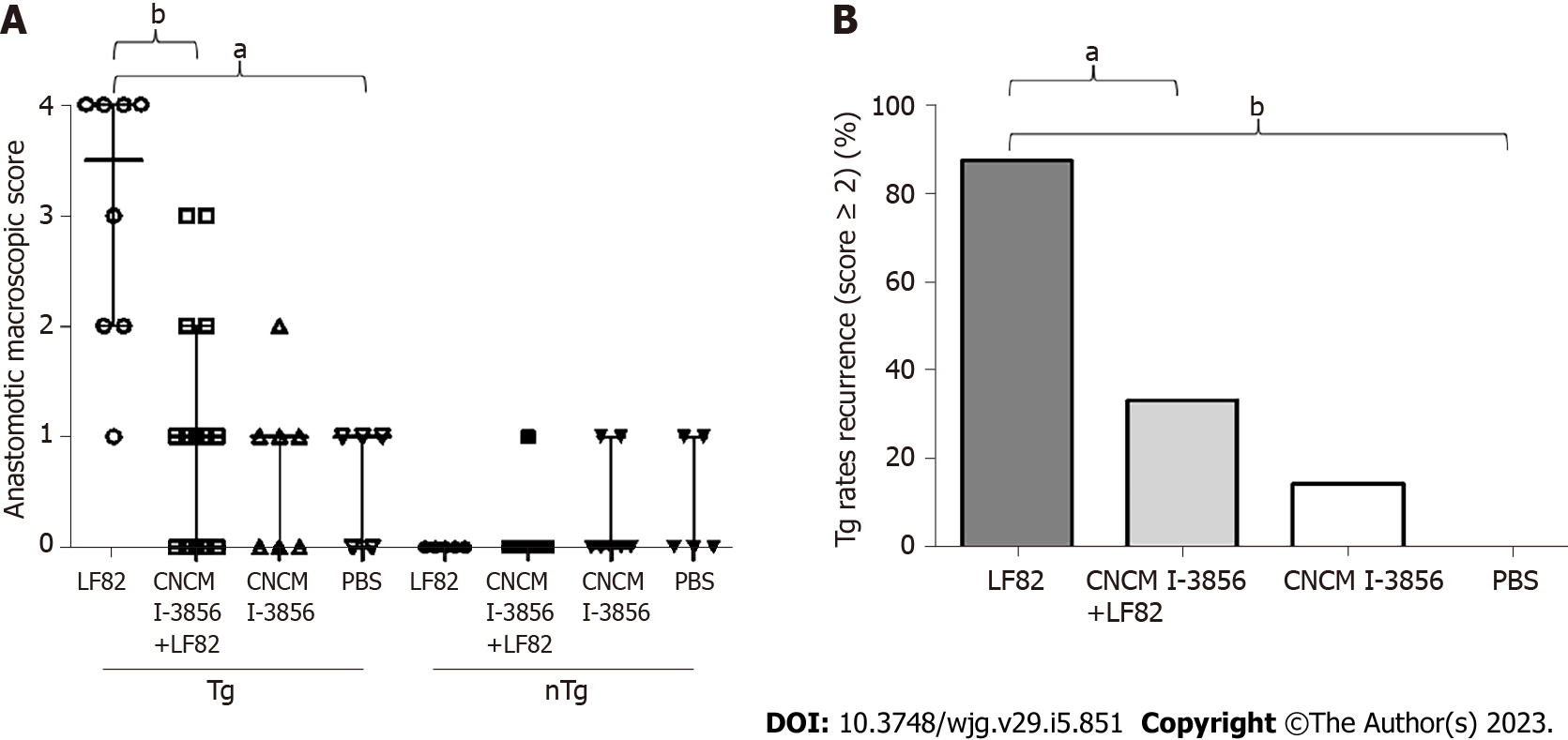
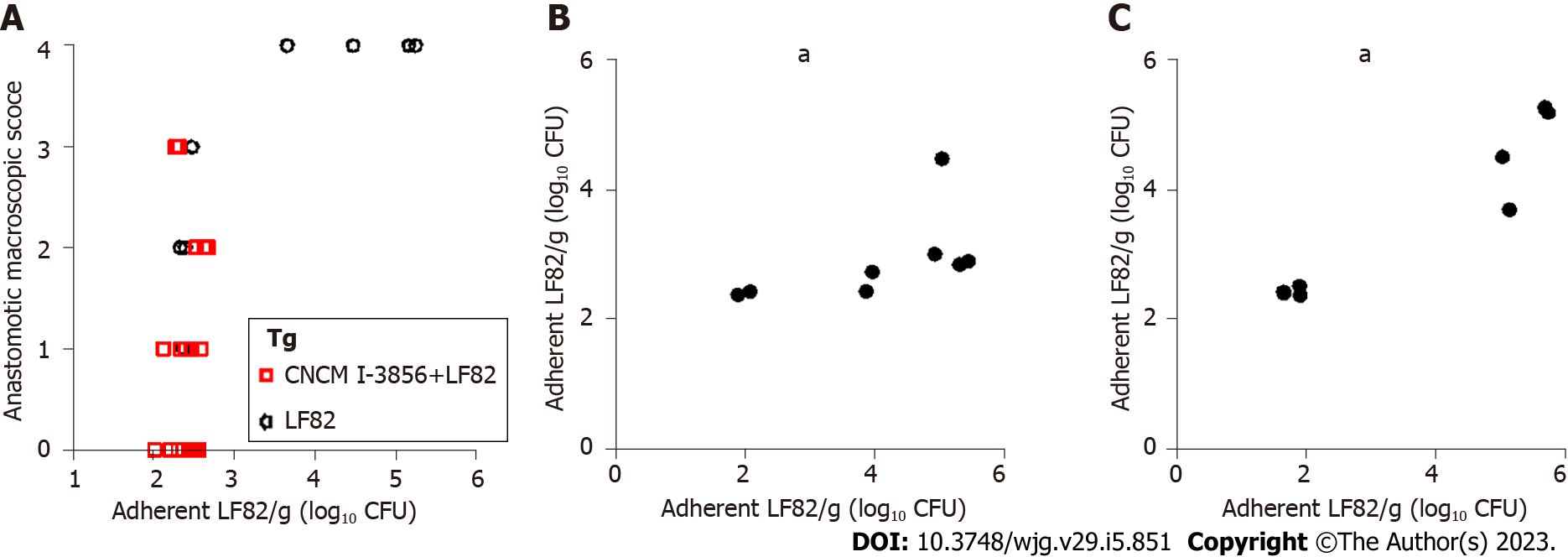
No intestinal lesions were present at W12 in any animal. No macroscopic lesions (or therefore POR) were observed at W18 in control nTg animals receiving PBS, S. cerevisiae CNCM I-3856 alone, LF82 alone, or S. cerevisiae CNCM I-3856 and LF82 (Figure 4A). In contrast, anastomotic macroscopic lesions corresponding mainly to edema and ulcerations on more than 20% of the anastomotic area without stenosis were observed in Tg rats receiving LF82 (3.5, IQR: 2 - 4), leading to 87.5% POR in this group of animals (Figure 4A and B). Compared to untreated Tg rats receiving LF82 (3.5, IQR: 2 - 4), coadministration of S. cerevisiae CNCM I-3856 and LF82 significantly reduced the macroscopic score (1, IQR: 0 - 2, P = 0.002) and POR (87.5% vs 33.3%, P = 0.01) by more than 60% (Figure 4A and B). Anastomotic macroscopic lesions were similar in Tg rats receiving PBS or S. cerevisiae CNCM I-3856 alone or S. cerevisiae CNCM I-3856 and LF82, without a difference compared to those of control nTg animals (Figure 4A).
No histologic lesions were present at W12 in any animal (data not shown). At W18, no significant and only mild histologic lesions characterized by neutrophil infiltration not exceeding 30% of lamina propria cells were observed in control nTg animals receiving either PBS, LF82 alone, S. cerevisiae CNCM I-3856 alone or S. cerevisiae CNCM I-3856 and LF82 (Figure 5). In contrast, erosions and mucosal ulcerations associated with moderate neutrophil infiltration were observed at W18 in Tg rats receiving LF82 (4.5, IQR: 3.3 - 5.8) (Figure 5). Compared to untreated Tg animals receiving LF82, coadministration of S. cerevisiae CNCM I-3856 and LF82 significantly reduced the histological lesions by more than 50% (4.5, IQR: 3.3 - 5.8 vs 2, IQR: 1.3-3, P = 0.003) (Figure 5). No significant lesions were observed in Tg rats receiving PBS or S. cerevisiae CNCM I-3856 alone, which was not different from the findings in control nTg animals (Figure 5).
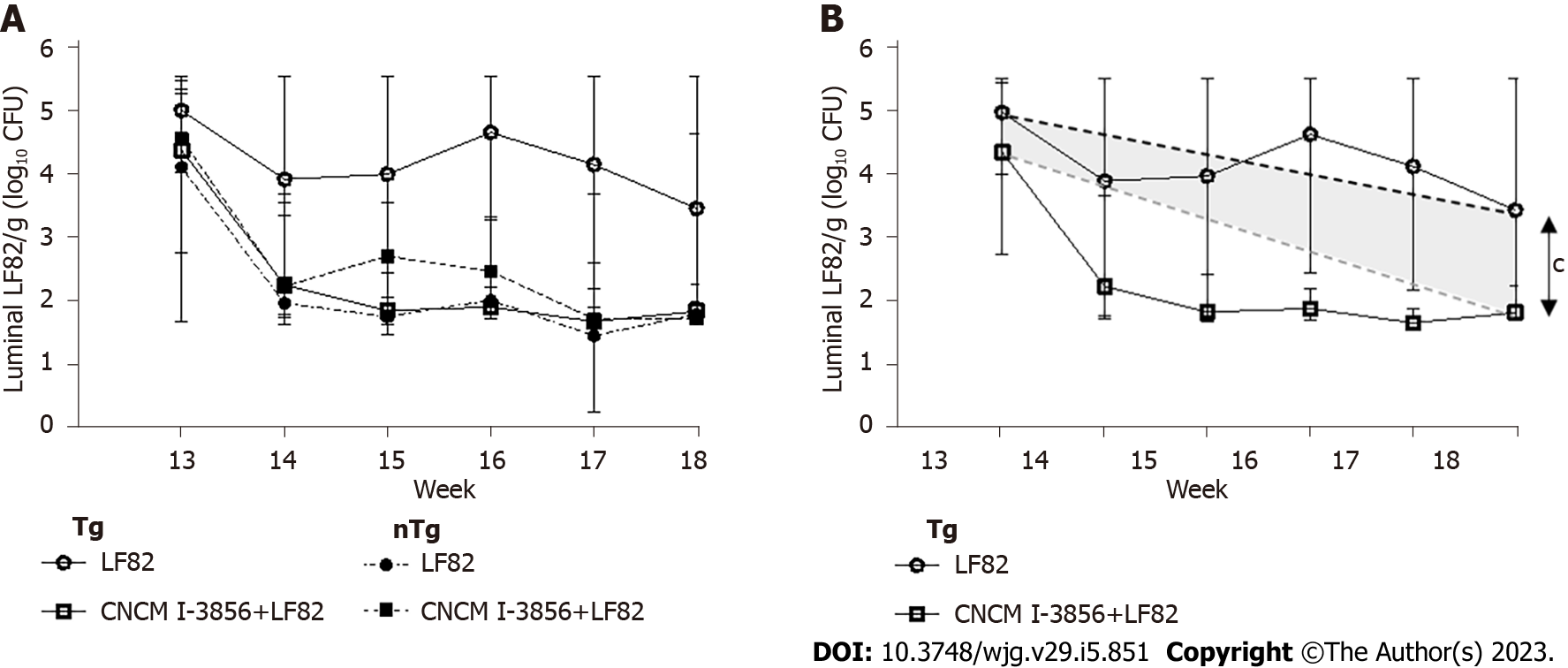
At W12, i.e., one week after the beginning of LF82 administration (109 CFUs/d), the quantities of luminal (Figure 6A) and adherent (Figure 7A) LF82 were similar in Tg and nTg rats receiving LF82 alone or S. cerevisiae CNCM I-3856 and LF82. The levels of luminal (4.4, IQR: 2.5 - 5.2 vs 3.4, IQR: 1.7 - 5.5) and adherent (2.7, IQR: 2.4 - 3 vs 3.1, IQR: 2.3 - 5) LF82 remained similar between W12 and W18 in Tg rats receiving LF82 alone (Figures 6 and 7), while a significant decrease in luminal (4.6, IQR: 3.5 - 5.2 vs 1.8, IQR: 1.7 - 2.3, P = 0.0002) and adherent (3.1, IQR: 2.5 - 3.6 vs 2.5, IQR: 2.3 - 2.6, P = 0.0005) LF82 was observed between W12 and W18 in paired Tg animals receiving S. cerevisiae CNCM I-3856 and LF82 (Figures 6 and 7).
In addition, the global persistence of viable luminal LF82 after surgery and during the last 5 wk of the study was significantly higher in the stools of Tg rats receiving LF82 alone (0.22, IQR: 2.071e-008 - 0.7) compared to Tg rats receiving S. cerevisiae CNCM I-3856 and LF82 (-0.6, IQR: -0.7 - 0.3, P = 0.0004) (Figure 8).
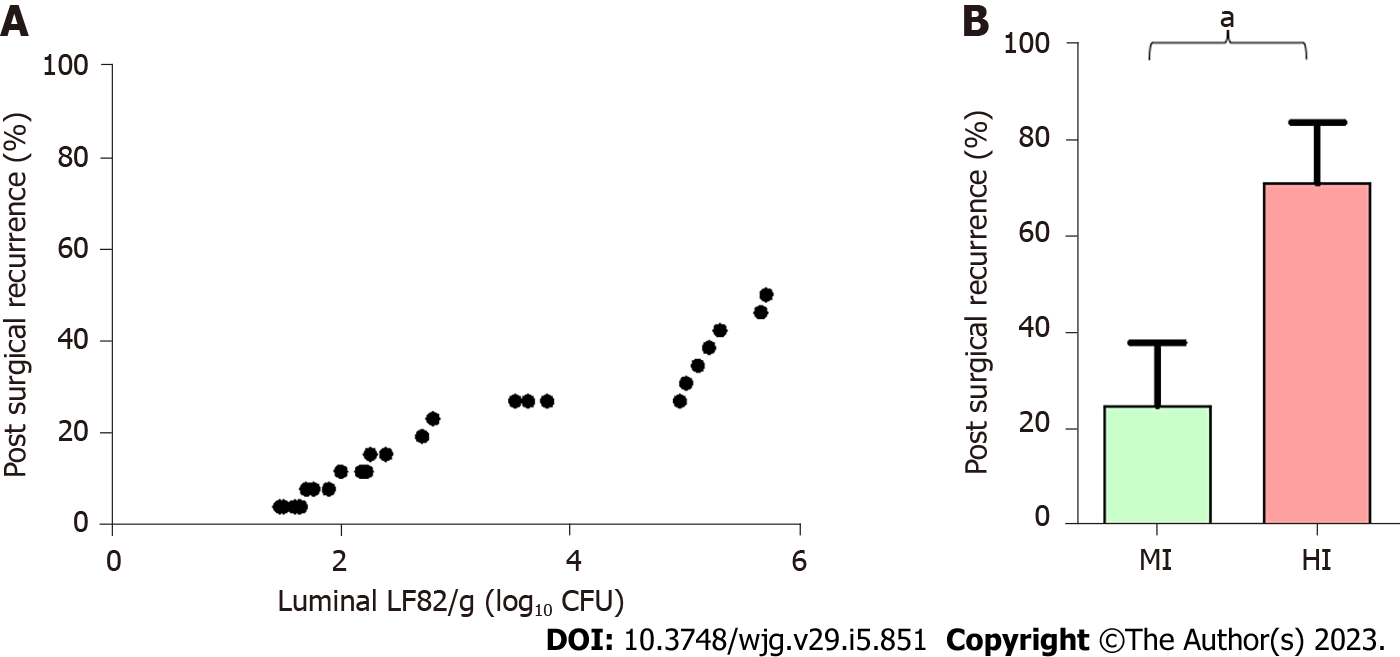
A correlation was found between the levels of adherent LF82 and the scores of anastomotic macroscopic lesions observed at W18 in Tg animals receiving LF82 alone or in combination with S. cerevisiae CNCM I-3856 (r = 0.49, P = 0.006) (Figure 9A). These levels of anastomotic adherent LF82 were correlated at W12 (r = 0.81, P = 0.02) and W18 (r = 0.79, P = 0.03) with the levels of luminal LF82 in paired Tg animals receiving LF82 alone (Figure 9B and C). Next, we analyzed whether luminal LF82 Levels at W14 may be predictive of POR in the 26 Tg rats receiving LF82 alone (n = 8) or in combination with S. cerevisiae CNCM I-3856 (n = 18). Using a cutoff value of 2.262 Log10 CFUs of luminal LF82 per gram of stool determined by the ROC curve, 14 animals at W14 were classified as highly infected by LF82, and 12 were classified as mildly infected (Figure 10A). POR was significantly more frequent in the highly infected Tg rats than in the mildly infected Tg rats (71.4% vs 25%, P = 0.02) (Figure 10B). A value of 2.262 Log10 CFUs luminal LF82 per gram of stool at W14 had an 80% sensitivity, 69.2% specificity, 71.4% positive predictive value and 75% negative predictive value for POR.
The levels of IL-1β, IL-6, TNFα and IFNγ mRNA were variable and similar in all Tg and nTg animals at W12 and W18, regardless of the presence of POR, LF82 administration or treatment with S. cerevisiae CNCM I-3856 (data not shown).
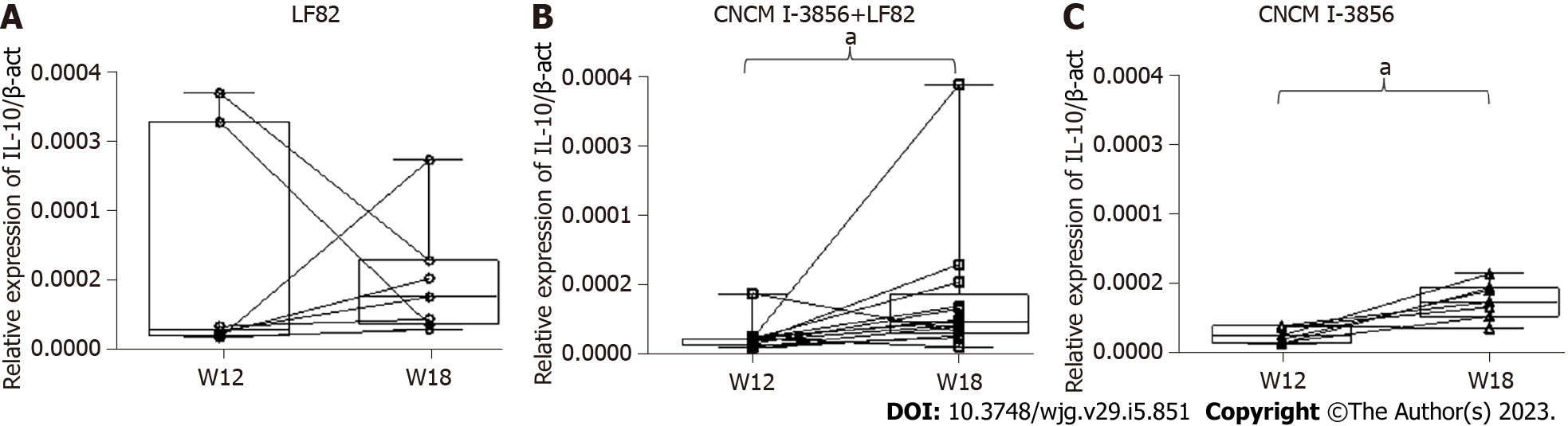
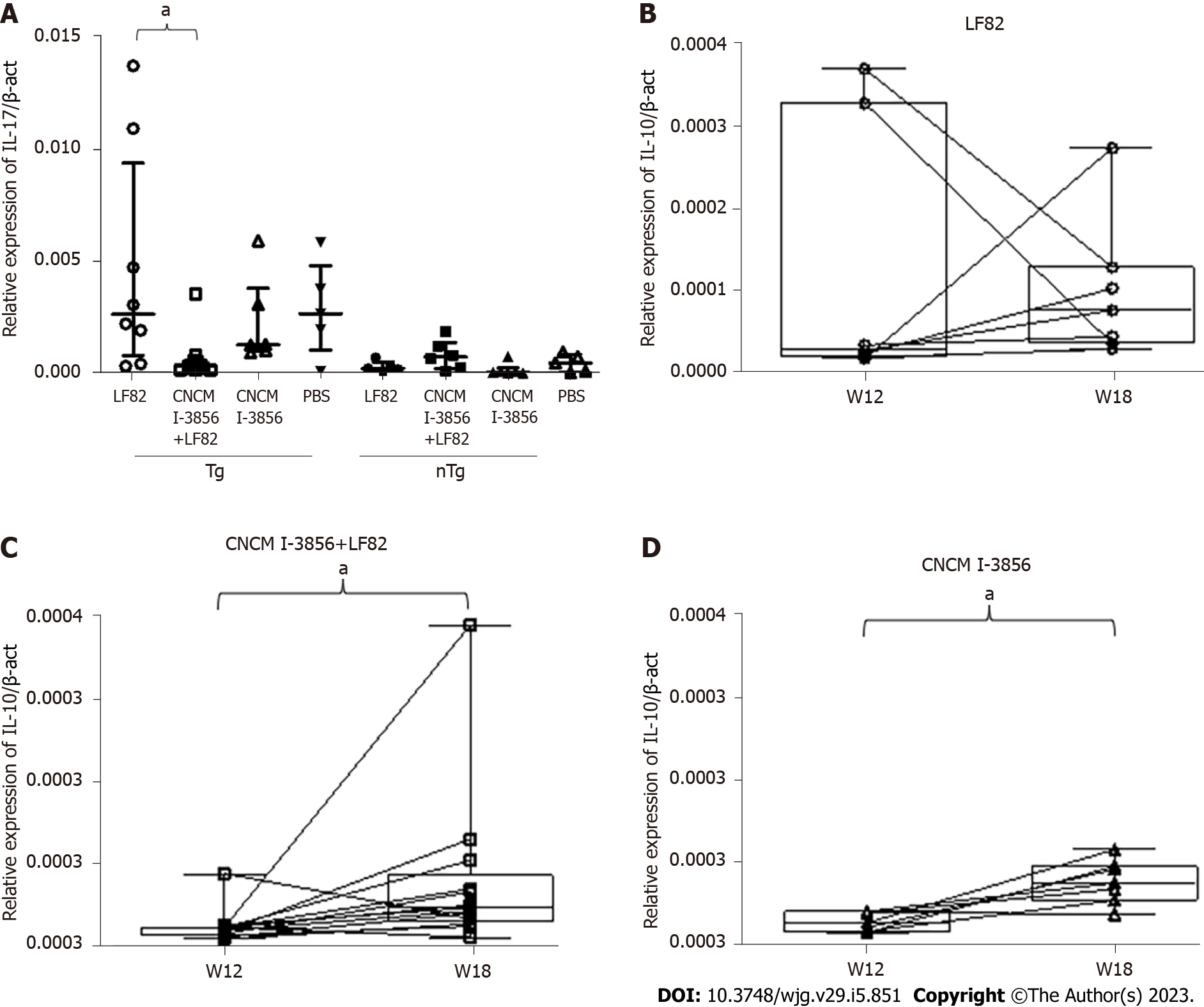
Concerning IL-10 mRNA levels, the only significant difference found by paired analysis in the different groups of animals revealed higher levels of IL-10 mRNA at W18 compared to W12 in animals receiving S. cerevisiae CNCM I-3856. In the Tg groups, administration of S. cerevisiae CNCM I-3856 with or without the coadministration of LF82 induced a significant increase in IL-10 production between surgery and sacrifice (2.5 × 105, IQR: 1.7 × 105 - 2.6 × 105vs 4.9 × 105, IQR: 3.3 × 105 - 9 × 105, P = 0.017 and 2.6 × 105, IQR: 1.5 × 105 - 3.9 × 105vs 7.4 × 105, IQR: 5.3 × 105 - 0.4 × 105, P = 0.031, respectively), while similar IL-10 Levels were found in animals receiving LF82 alone (Figure 11A-C).
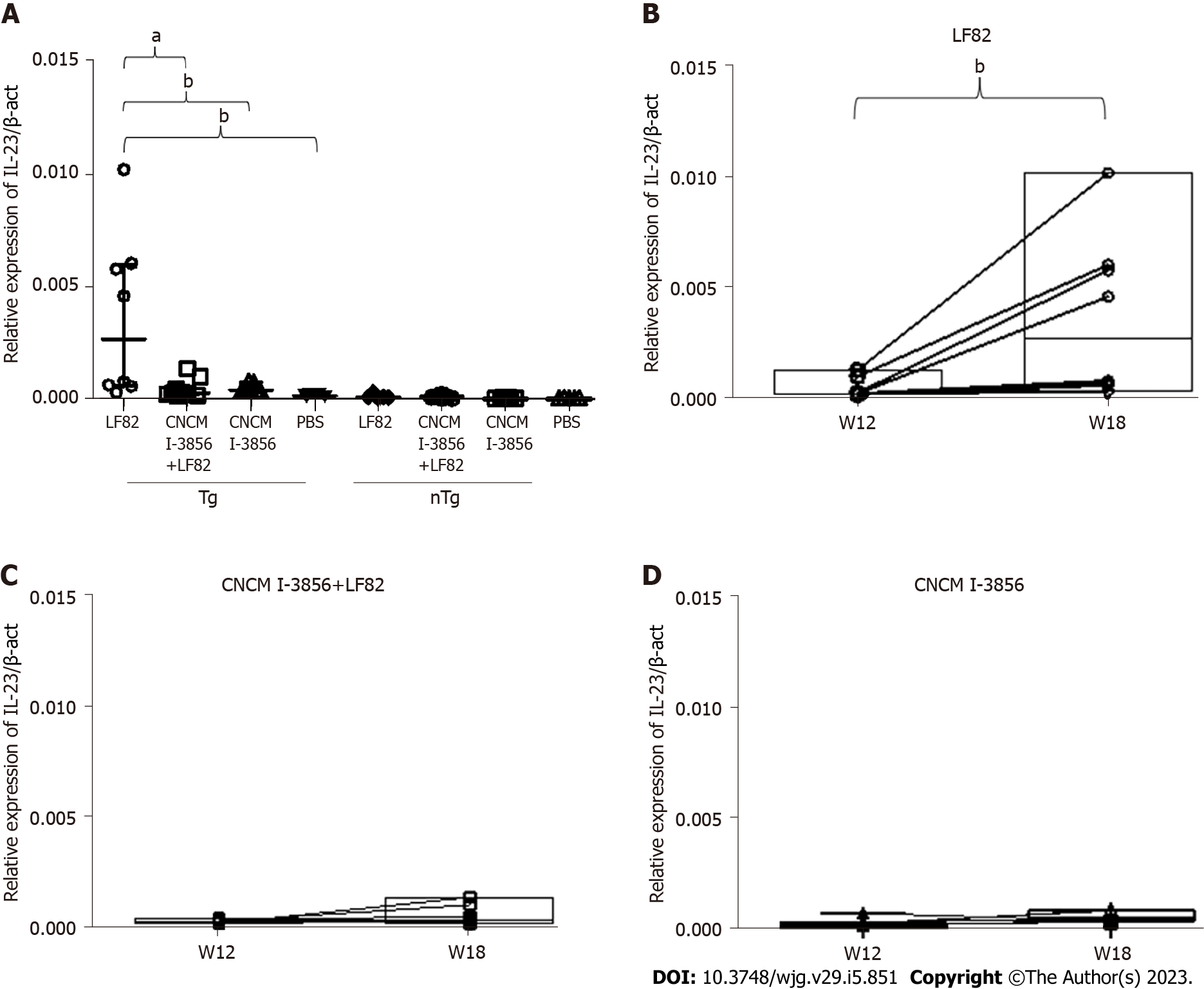
Concerning IL-23 mRNA levels, a significant increase was observed at W18 in Tg animals receiving LF82 alone in comparison to the groups of rats treated with S. cerevisiae CNCM I-3856 with or without administration of LF82 (P = 0.04 and P = 0.006, respectively) (Figure 12A). Additionally, using a paired t test, a significant increase in inflammatory IL-23 production was observed between surgery and sacrifice in the Tg group receiving LF82 alone (2.2 × 104, IQR: 1.8 × 104 - 8 × 104vs 26.9 × 104, IQR: 6.1 × 104 - 6 × 104, P = 0.008), while no significant difference was observed in the Tg groups treated with S. cerevisiae CNCM I-3856 with or without administration of LF82 (Figure 12B-D).
Analysis of IL-17 mRNA levels found significantly higher rates at W18 in Tg rats receiving LF82 in comparison with the Tg group receiving S. cerevisiae CNCM I-3856 and LF82 (2.7 × 104, IQR: 0.8 × 104 - 9, 5 × 104vs 0.4 × 104, IQR: 0.2 × 104 - 0.6 × 104, P = 0.015) (Figure 13).
The role of the intestinal microbiota composition and diversity in POR of CD is important. Among intestinal microorganisms potentially involved in POR, many studies support the roles of AIEC in early ileal lesions of CD and particularly in endoscopic POR occurring 6 mo after CD-related ileocolonic resection[12]. In the present study, we show that the probiotic S. cerevisiae CNCM I–3856 prevents LF82-induced POR occurring 6 wk after ICR in susceptible HLA-B27 Tg rats. In our model, oral administration of the LF82 AIEC strain induced POR in 85% of HLA-B27 Tg rats raised in a controlled pathogen-free facility. The lesions developed in a concentration-dependent manner to the amount of adherent LF82; moreover, they shared many similarities with CD lesions, including erosions and ulcers that could lead to stenosis, transparietal neutrophil infiltration, and a shift in cytokine profiles toward the IL-23/IL-17 axis. The goal of the postoperative management of CD is to identify patients at highest risk of recurrence to begin prophylactic treatment with biotherapies[8]. In our study, a high fecal concentration of LF82 had a 70% positive predictive value for POR occurring 4 wk later. The utility of this noninvasive diagnostic biomarker for predicting POR should be considered in future clinical studies evaluating the postoperative management of CD patients.
S. cerevisiae CNCM I-3856[22] is a probiotic yeast that has already been evaluated in large-scale clinical studies showing the safety and efficacy of this strain for abdominal pain management in patients with irritable bowel syndrome[22,32-34]. In the present study, daily oral administration of S. cerevisiae CNCM I-3856 at 109 CFU/d was perfectly tolerated and reduced the severity and frequency of POR by more than 60% in HLA-B27 Tg rats. Moreover, an absence of LF82-induced POR without any macroscopic lesions was observed in 40% of transgenic animals treated preventively with S. cerevisiae CNCM I-3856. To our knowledge, this is the first time that a probiotic treatment showed such efficacy in preventing POR in a rodent preclinical model of POR of CD.
Different mechanisms of action may be involved in the therapeutic preventive effect of S. cerevisiae CNCM I-3856 against POR. Specific fractions of β6-glucan and α4-glucan expressed by S. cerevisiae CNCM I-3856 represent the strongest anti-adhesive yeast cell wall components against AIEC adhesion[24,35]. In our study, prevention of LF82-induced POR by S. cerevisiae CNCM I-3856 was associated with a significant decrease in adherent LF82 in the intestinal mucosa of animals together with a decrease in the persistence of luminal LF82, demonstrating the ability of S. cerevisiae CNCM I-3856 to decolonize AIEC from the gut of rats. Additional preclinical studies will be performed in our model using specific soluble glucan fractions of S. cerevisiae CNCM I-3856 to avoid the constraints of a live probiotic and to optimize the therapeutic efficacy. Another possible mechanism by which S. cerevisiae CNCM I-3856 prevents POR resides in its immunomodulatory and anti-inflammatory capacities[36]. We observed that the administration of S. cerevisiae CNCM I-3856 significantly increased IL-10 production in the intestine of rats and restored the local upregulation of IL-17 and IL-23 associated with LF82-induced POR in transgenic animals. The capacity of S. cerevisiae to induce IL-10 production has already been highlighted in vitro in bone-marrow dendritic cells and in porcine jejunal epithelial cells[36,37]. In the gut, IL-10 is produced by leukocytes and intestinal epithelial cells and plays important roles in maintaining gut homeostasis and harmonizing the interaction between host immunity and luminal microorganisms[38]. In a previous study of 79 patients with CD undergoing a first ileocolectomy and ileocolonic anastomosis, we reported that a low ileal IL-10 mRNA concentration was predictive of endoscopic recurrence occurring 3 mo later[39]. Thus, the ability of S. cerevisiae CNCM I-3856 to induce the intestinal production of IL-10 could be a key factor in preventing POR in our model.
In conclusion, our results identified S. cerevisiae CNCM I-3856 as a new and original candidate for the prevention of POR in selected AIEC-infected CD patients. In a reliable model of ICR in HLA-B27 Tg rats mimicking POR of CD, S. cerevisiae CNCM I-3856 was found to prevent macroscopic and histologic POR through a pathobiont AIEC-targeted mechanism and through its ability to induce intestinal IL-10 production. Given that the majority of patients with CD wish to have safe, natural, nonchemotherapeutic treatment, the S. cerevisiae CNCM I-3856 probiotic, which is already an alternative solution for the management of patients with irritable bowel syndrome because of its ability to alleviate abdominal pain and to improve quality of life, should represent a promising therapeutic solution in the management of postoperative CD.
The presence of adherent-invasive Escherichia coli (AIEC) in intestinal flora is associated with postoperative recurrence (POR) of crohn's disease (CD). Saccharomyces cerevisiae (S. cerevisiae) CNCM I-3856 is a safe and effective probiotic yeast that has already been evaluated in randomized placebo-controlled studies in patients with irritable bowel syndrome. Preclinical studies demonstrate the capacity of S. cerevisiae CNCM I-3856 to agglutinate invasive Escherichia coli strains and to prevent their adhesion to intestinal epithelial cells, favoring AIEC elimination from the gut of mice.
To demonstrate that S. cerevisiae CNCM I-3856 should be considered as a postoperative prophylactic medical therapy in CD patients harboring AIEC bacteria.
To evaluate the beneficial effect of S. cerevisiae CNCM I-3856 and its mechanisms of action in preventing AIEC-induced POR in an HLA-B27 transgenic (TgB27) rat model of CD.
TgB27 and control rats underwent an ileocecal resection at the 12th wk of life and sacrificed 6 wk later to assess POR using macroscopic and histological scores and quantification of mucosal inflammatory/ regulatory cytokines. Animals were challenged daily with an oral administration of AIEC and were treated orally with S. cerevisiae CNCM I-3856 (109 colony forming units/day). Luminal and adherent AIEC were regularly quantified throughout the duration of the study.
Eighty-seven percent of TgB27 rats developed POR characterized by anastomotic macroscopic ulcerations, transparietal neutrophil infiltration and a shift in the cytokine profile toward the interleukin (IL)-17/IL-23 axis. Oral administration of S. cerevisiae CNCM I-3856 reduced this POR by more than 60%, increased AIEC elimination from the gut, induced intestinal IL-10 production and restored the local upregulation of IL-17/IL-23. A high concentration of AIEC quantified in the stool of rats after surgery had a 70% positive predictive value for POR occurring 4 wk later.
Ileocecal resection in TgB27 rats is a novel, useful, reliable model mimicking POR of CD and aided the discovery of new therapeutic targets. Oral administration of S. cerevisiae CNCM I-3856 safely prevented POR of CD through AIEC decolonization and immunomodulatory/anti-inflammatory capacities.
The probiotic S. cerevisiae CNCM I-3856, which is already an alternative solution for the management of patients with irritable bowel syndrome to improve abdominal pain and quality of life, should represent a promising prophylactic natural nonchemotherapeutic solution in the management of postoperative CD. Monitoring AIEC levels in stool after surgery for CD should be considered as a companion test to identify patients at high risk of POR and to monitor treatment response.
We thank the Foundation DigestScience for its help in the breeding of the HLA-B27 transgenic animals and Lesaffre Company for the provision of S. cerevisiae CNCM I-3856.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: France
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ding JX, China; Fabbri N, Italy S-Editor: Liu GL L-Editor: A P-Editor: Yu HG
| 1. | Bouguen G, Peyrin-Biroulet L. Surgery for adult Crohn's disease: what is the actual risk? Gut. 2011;60:1178-1181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 172] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 2. | Rutgeerts P, Geboes K, Vantrappen G, Beyls J, Kerremans R, Hiele M. Predictability of the postoperative course of Crohn's disease. Gastroenterology. 1990;99:956-963. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1226] [Cited by in RCA: 1221] [Article Influence: 34.9] [Reference Citation Analysis (0)] |
| 3. | Shah RS, Click BH. Medical therapies for postoperative Crohn's disease. Therap Adv Gastroenterol. 2021;14:1756284821993581. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 4. | Mañosa M, Fernández-Clotet A, Nos P, Martín-Arranz MD, Manceñido N, Carbajo A, Hinojosa E, Hernández-Camba A, Muñoz-Pérez R, Boscá-Watts M, Calvo M, Sierra-Ausín M, Sánchez-Rodríguez E, Barreiro-de Acosta M, Núñez-Alonso A, Zabana Y, Márquez L, Gisbert JP, Guardiola J, Sáinz E, Delgado-Guillena P, Busquets D, van Domselaar M, Girona E, Lorente R, Casas-Deza D, Huguet JM, Maestro S, Cabello MJ, Castro J, Iborra M, Cañete F, Calafat M, Domènech E; ENEIDA registry by GETECCU. Ustekinumab and vedolizumab for the prevention of postoperative recurrence of Crohn's disease: Results from the ENEIDA registry. Dig Liver Dis. 2022;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 5. | Chaim FHM, Negreiros LMV, Steigleder KM, Siqueira NSN, Genaro LM, Oliveira PSP, Martinez CAR, Ayrizono MLS, Fagundes JJ, Leal RF. Aspects Towards the Anastomotic Healing in Crohn's Disease: Clinical Approach and Current Gaps in Research. Front Surg. 2022;9:882625. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 6. | Ngollo M, Perez K, Hammoudi N, Gorelik Y, Delord M, Auzolle C, Bottois H, Cazals-Hatem D, Bezault M, Nancey S, Nachury M, Treton X, Fumery M, Buisson A, Barnich N, Seksik P; REMIND Study Group Investigators , Shen-Orr SS, Le Bourhis L, Allez M. Identification of Gene Expression Profiles Associated with an Increased Risk of Post-Operative Recurrence in Crohn's Disease. J Crohns Colitis. 2022;16:1269-1280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 19] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 7. | Caparrós E, Wiest R, Scharl M, Rogler G, Gutiérrez Casbas A, Yilmaz B, Wawrzyniak M, Francés R. Dysbiotic microbiota interactions in Crohn's disease. Gut Microbes. 2021;13:1949096. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 58] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 8. | Battat R, Sandborn WJ. Advances in the Comprehensive Management of Postoperative Crohn's Disease. Clin Gastroenterol Hepatol. 2022;20:1436-1449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 9. | Darfeuille-Michaud A, Boudeau J, Bulois P, Neut C, Glasser AL, Barnich N, Bringer MA, Swidsinski A, Beaugerie L, Colombel JF. High prevalence of adherent-invasive Escherichia coli associated with ileal mucosa in Crohn's disease. Gastroenterology. 2004;127:412-421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1075] [Cited by in RCA: 1123] [Article Influence: 53.5] [Reference Citation Analysis (0)] |
| 10. | Nadalian B, Yadegar A, Houri H, Olfatifar M, Shahrokh S, Asadzadeh Aghdaei H, Suzuki H, Zali MR. Prevalence of the pathobiont adherent-invasive Escherichia coli and inflammatory bowel disease: a systematic review and meta-analysis. J Gastroenterol Hepatol. 2021;36:852-863. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 50] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 11. | Kamali Dolatabadi R, Feizi A, Halaji M, Fazeli H, Adibi P. The Prevalence of Adherent-Invasive Escherichia coli and Its Association With Inflammatory Bowel Diseases: A Systematic Review and Meta-Analysis. Front Med (Lausanne). 2021;8:730243. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 12. | Buisson A, Sokol H, Hammoudi N, Nancey S, Treton X, Nachury M, Fumery M, Hébuterne X, Rodrigues M, Hugot JP, Boschetti G, Stefanescu C, Wils P, Seksik P, Le Bourhis L, Bezault M, Sauvanet P, Pereira B, Allez M, Barnich N; Remind study group. Role of adherent and invasive Escherichia coli in Crohn's disease: lessons from the postoperative recurrence model. Gut. 2022;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 33] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 13. | Elhag DA, Kumar M, Saadaoui M, Akobeng AK, Al-Mudahka F, Elawad M, Al Khodor S. Inflammatory Bowel Disease Treatments and Predictive Biomarkers of Therapeutic Response. Int J Mol Sci. 2022;23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 63] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 14. | Mishra J, Stubbs M, Kuang L, Vara N, Kumar P, Kumar N. Inflammatory Bowel Disease Therapeutics: A Focus on Probiotic Engineering. Mediators Inflamm. 2022;2022:9621668. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 33] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 15. | Borody TJ, Dolai S, Gunaratne AW, Clancy RL. Targeting the microbiome in Crohn's disease. Expert Rev Clin Immunol. 2022;18:873-877. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 16. | Di Sario A, Sassaroli P, Daretti L, Annulli G, Schiada L, Falcioni G, Bendia E, Antuono S, Benedetti A. Postoperative Recurrence of Crohn's Disease: Pathophysiology, Diagnosis and Treatment. Curr Pharm Biotechnol. 2017;18:979-988. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 17. | Bourreille A, Cadiot G, Le Dreau G, Laharie D, Beaugerie L, Dupas JL, Marteau P, Rampal P, Moyse D, Saleh A, Le Guern ME, Galmiche JP; FLORABEST Study Group. Saccharomyces boulardii does not prevent relapse of Crohn's disease. Clin Gastroenterol Hepatol. 2013;11:982-987. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 121] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 18. | Chevalier G, Laveissière A, Desachy G, Barnich N, Sivignon A, Maresca M, Nicoletti C, Di Pasquale E, Martinez-Medina M, Simpson KW, Yajnik V, Sokol H; MOBIDIC Study Investigators, Plassais J, Strozzi F, Cervino A, Morra R, Bonny C. Blockage of bacterial FimH prevents mucosal inflammation associated with Crohn's disease. Microbiome. 2021;9:176. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 32] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 19. | Reinisch W, Hébuterne X, Buisson A, Schreiber S, Desreumaux P, Primas C, Paillarse JM, Chevalier G, Bonny C. Safety, pharmacokinetic, and pharmacodynamic study of sibofimloc, a novel FimH blocker in patients with active Crohn's disease. J Gastroenterol Hepatol. 2022;37:832-840. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 15] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 20. | Galtier M, De Sordi L, Sivignon A, de Vallée A, Maura D, Neut C, Rahmouni O, Wannerberger K, Darfeuille-Michaud A, Desreumaux P, Barnich N, Debarbieux L. Bacteriophages Targeting Adherent Invasive Escherichia coli Strains as a Promising New Treatment for Crohn's Disease. J Crohns Colitis. 2017;11:840-847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 81] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 21. | Titécat M, Rousseaux C, Dubuquoy C, Foligné B, Rahmouni O, Mahieux S, Desreumaux P, Woolston J, Sulakvelidze A, Wannerberger K, Neut C. Safety and Efficacy of an AIEC-targeted Bacteriophage Cocktail in a Mice Colitis Model. J Crohns Colitis. 2022;16:1617-1627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 27] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 22. | Mourey F, Decherf A, Jeanne JF, Clément-Ziza M, Grisoni ML, Machuron F, Legrain-Raspaud S, Bourreille A, Desreumaux P. Saccharomyces cerevisiae I-3856 in irritable bowel syndrome with predominant constipation. World J Gastroenterol. 2022;28:2509-2522. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 5] [Cited by in RCA: 17] [Article Influence: 5.7] [Reference Citation Analysis (4)] |
| 23. | Cayzeele-Decherf A, Pélerin F, Leuillet S, Douillard B, Housez B, Cazaubiel M, Jacobson GK, Jüsten P, Desreumaux P. Saccharomyces cerevisiae CNCM I-3856 in irritable bowel syndrome: An individual subject meta-analysis. World J Gastroenterol. 2017;23:336-344. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 41] [Cited by in RCA: 39] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 24. | Sivignon A, de Vallée A, Barnich N, Denizot J, Darcha C, Pignède G, Vandekerckove P, Darfeuille-Michaud A. Saccharomyces cerevisiae CNCM I-3856 prevents colitis induced by AIEC bacteria in the transgenic mouse model mimicking Crohn's disease. Inflamm Bowel Dis. 2015;21:276-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 68] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 25. | Martinez-Medina M, Aldeguer X, Lopez-Siles M, González-Huix F, López-Oliu C, Dahbi G, Blanco JE, Blanco J, Garcia-Gil LJ, Darfeuille-Michaud A. Molecular diversity of Escherichia coli in the human gut: new ecological evidence supporting the role of adherent-invasive E. coli (AIEC) in Crohn's disease. Inflamm Bowel Dis. 2009;15:872-882. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 317] [Cited by in RCA: 305] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 26. | Darfeuille-Michaud A, Neut C, Barnich N, Lederman E, Di Martino P, Desreumaux P, Gambiez L, Joly B, Cortot A, Colombel JF. Presence of adherent Escherichia coli strains in ileal mucosa of patients with Crohn's disease. Gastroenterology. 1998;115:1405-1413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 609] [Cited by in RCA: 638] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 27. | Blondeaux A, Speca S, Valibouze C, Lambin T, Dubuquoy C, Titecat M, Blanquart H, Neut C, Genin M, Zerbib P, Foligne B, Desreumaux P. Sa1539: Tofacitinib treatment prevents post-operative recurrence of crohn's disease modeled by ileocecal resection in HLA-B27 transgenic rats. Gastroenterology. 2022;162:S-406. [DOI] [Full Text] |
| 28. | Chau A, Lucil S, Chater C, Speca S, Djouina M, Dubuquoy C, Koriche D, Dubuquoy L, Neut C, Pruvot FR, Desreumaux P, Zerbib P, Pariente B. 1053 - Hla B27 Transgenic Rat: A New Animal Model of Ileitis Post Surgery Reproducing Inflammatory Disease. Gastroenterology. 2018;154:S-199. [DOI] [Full Text] |
| 29. | Valibouze C, Speca S, Lambin T, Dubuquoy C, Dubuquoy L, M’Ba L, Schneider L, Rousseaux C, Ballet N, Decherf A, Titecat M, Foligne B, Desreumaux P, Neut C, Zerbib P. Su1807 – Post-Operative Recurrence After Ileo-Caecal Resection for Crohn's Disease: Towards an Anti-Adherent Invasive Escherichia Coli (AIEC) Strategy with Rationaly Selected Saccharomyces Cerevisiae Probiotic. Gastroenterology. 2019;156:S-620. [DOI] [Full Text] |
| 30. | Boudeau J, Glasser AL, Masseret E, Joly B, Darfeuille-Michaud A. Invasive ability of an Escherichia coli strain isolated from the ileal mucosa of a patient with Crohn's disease. Infect Immun. 1999;67:4499-4509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 332] [Cited by in RCA: 360] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 31. | D'Haens GR, Geboes K, Peeters M, Baert F, Penninckx F, Rutgeerts P. Early lesions of recurrent Crohn's disease caused by infusion of intestinal contents in excluded ileum. Gastroenterology. 1998;114:262-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 614] [Cited by in RCA: 605] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 32. | Pineton de Chambrun G, Neut C, Chau A, Cazaubiel M, Pelerin F, Justen P, Desreumaux P. A randomized clinical trial of Saccharomyces cerevisiae versus placebo in the irritable bowel syndrome. Dig Liver Dis. 2015;47:119-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 79] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 33. | Spiller R, Pélerin F, Cayzeele Decherf A, Maudet C, Housez B, Cazaubiel M, Jüsten P. Randomized double blind placebo-controlled trial of Saccharomyces cerevisiae CNCM I-3856 in irritable bowel syndrome: improvement in abdominal pain and bloating in those with predominant constipation. United European Gastroenterol J. 2016;4:353-362. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 65] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 34. | Gayathri R, Aruna T, Malar S, Shilpa B, Dhanasekar KR. Efficacy of Saccharomyces cerevisiae CNCM I-3856 as an add-on therapy for irritable bowel syndrome. Int J Colorectal Dis. 2020;35:139-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 35. | Sivignon A, Yu SY, Ballet N, Vandekerckove P, Barnich N, Guerardel Y. Heteropolysaccharides from S. cerevisiae show anti-adhesive properties against E. coli associated with Crohn's disease. Carbohydr Polym. 2021;271:118415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 36. | Liu Y, Chang J, Wang P, Yin QQ, Huang WW, Liu CQ, Bai XX, Zhu Q, Gao TZ, Zhou P. Effects of Saccharomyces cerevisiae on alleviating cytotoxicity of porcine jejunal epithelia cells induced by deoxynivalenol. AMB Express. 2019;9:137. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 37. | Sokol H, Leducq V, Aschard H, Pham HP, Jegou S, Landman C, Cohen D, Liguori G, Bourrier A, Nion-Larmurier I, Cosnes J, Seksik P, Langella P, Skurnik D, Richard ML, Beaugerie L. Fungal microbiota dysbiosis in IBD. Gut. 2017;66:1039-1048. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 658] [Cited by in RCA: 897] [Article Influence: 112.1] [Reference Citation Analysis (0)] |
| 38. | Nguyen HD, Aljamaei HM, Stadnyk AW. The Production and Function of Endogenous Interleukin-10 in Intestinal Epithelial Cells and Gut Homeostasis. Cell Mol Gastroenterol Hepatol. 2021;12:1343-1352. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 44] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 39. | Meresse B, Rutgeerts P, Malchow H, Dubucquoi S, Dessaint JP, Cohard M, Colombel JF, Desreumaux P. Low ileal interleukin 10 concentrations are predictive of endoscopic recurrence in patients with Crohn's disease. Gut. 2002;50:25-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 41] [Article Influence: 1.8] [Reference Citation Analysis (0)] |