Published online Feb 7, 2023. doi: 10.3748/wjg.v29.i5.800
Peer-review started: November 22, 2022
First decision: December 10, 2022
Revised: December 12, 2022
Accepted: January 18, 2023
Article in press: January 18, 2023
Published online: February 7, 2023
Processing time: 75 Days and 21.7 Hours
Since the first identification in December of 2019 and the fast spread of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, it has represented a dramatic global public health concern. Though affecting mainly the respiratory system, SARS-CoV-2 disease, defined as coronavirus disease 2019 (COVID-19), may have a systemic involvement leading to multiple organ dysfunction. Experimental evidence about the SARS-CoV-2 tropism for the liver and the increasing of hepatic cytolysis enzymes during infection support the presence of a pathophysiological relationship between liver and SARS-CoV-2. On the other side, patients with chronic liver disease have been demonstrated to have a poor prognosis with COVID-19. In particular, patients with liver cirrhosis appear extremely vulnerable to infection. Moreover, the etiology of liver disease and the vaccination status could affect the COVID-19 outcomes. This review analyzes the impact of the disease stage and the related causes on morbidity and mortality, clinical outcomes during SARS-CoV-2 infection, as well as the efficacy of vaccination in patients with chronic liver disease.
Core Tip: It has been observed, since the early months of the pandemic, that pre-existing liver disease was associated with a worsening of clinical outcomes in severe acute respiratory syndrome coronavirus 2 infection. A correlation exists between severity of liver disease and coronavirus disease 2019-related adverse outcomes. The etiology of liver disease could significantly affect mortality rates, as well as vaccination status.
- Citation: Nevola R, Criscuolo L, Beccia D, Delle Femine A, Ruocco R, Imbriani S, Alfano M, Villani A, Russo A, Perillo P, Marfella R, Adinolfi LE, Sasso FC, Marrone A, Rinaldi L. Impact of chronic liver disease on SARS-CoV-2 infection outcomes: Roles of stage, etiology and vaccination. World J Gastroenterol 2023; 29(5): 800-814
- URL: https://www.wjgnet.com/1007-9327/full/v29/i5/800.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i5.800
From December 2019, the fast spread of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), a new virus belonging to the Coronavirus family of respiratory pathogens, has represented a major public health problem worldwide, leading to the declaration of a pandemic status in March 2020 by the World Health Organization (WHO)[1]. Despite the enormous efforts by health personnel and organizations, coronavirus disease 2019 (COVID-19) has caused more than 6.5 million deaths worldwide to date[2]. While the new virus variants show a milder clinical picture with predominant involvement of the upper respiratory tract, the most severe form of SARS-CoV-2 infection characterized by acute respiratory distress syndrome (ARDS) still represents an important cause of morbidity and mortality[3-5]. As observed since the first pandemic phases, organ involvement in COVID-19 is not limited to the respiratory tract, but can result in systemic disease with cardiovascular, renal, neurological and, last but not least, hepatic involvement. In particular, increases in the indices of hepatic cytolysis or cholestasis [mean as an increase of aspartate aminotransferase (AST), alanine aminotransferase (ALT), total bilirubin (TBIL), gamma-glutamyl transferase (GGT) and alkaline phosphatase (ALP) upper range value] or a more severe acute liver injury (ALI) [defined as ALT and/or AST over 3 × upper limit of normal (ULN) or ALP, GGT, and/or TBIL over 2 × ULN] can be found in 14%-53% of SARS-CoV-2 infection[6]. The mechanism of COVID-19-associated ALI is probably multifactorial, given the combination of direct viral cytopathic effect, cytokine-induced inflammatory damage, hypoxic and drug induced liver injury[7]. ALI has been demonstrated to be a predictor of unfavorable SARS-CoV-2 infection course[8,9]. High levels of AST and TBIL at hospital admission are associated with an increased risk of COVID-19 progression[10]. The coexistence of previous liver disease worsens the outcomes of COVID-19. In fact, if on one hand SARS-CoV-2 infection can determine liver injury[6], on the other hand chronic liver diseases (CLDs) are associated to immune system and hemostasis alterations[11] that are able to accelerate some pathogenetic mechanisms of SARS-CoV-2, as cytokine storm and prothrombotic state, affecting the outcomes significantly[12,13]. In particular, patients with liver cirrhosis are at a high risk of an unfavorable SARS-CoV-2 infection course, with significantly higher mortality rates than the general population[14,15]. The occurrence of ALI during COVID-19 in this population is an additional predictor of all-cause mortality[16]. The risk of adverse outcomes in patients with chronic hepatitis is still poorly understood. Moreover, the etiology of CLD, the disease stage, potential concomitant therapies (e.g., immunosuppressive) and vaccination status could significantly impact the outcomes of COVID-19.
The aim of this review is to describe the role of liver disease during COVID-19, analyzing if and how much the stage of the disease and the related etiology can affect the SARS-CoV-2 infection outcomes and examine what is known on the clinical efficacy of vaccination in patients with CLD to date.
Despite the prevalence of a pre-existing liver disease in COVID-19 patients appearing low (between 0.6% and 3.4%)[17-19] and not significantly related to the risk of contracting SARS-CoV-2 infection[20], the first studies showed that the presence of CLD was associated with an increase in both hospitalization rate and overall mortality[14]. In this regard, Singh et al[14] reported a relative risk (RR) for death in patients with pre-existing liver disease of 2.8 (95%CI: 1.9-4.0). Similarly, also Williamson et al[19] and Galiero et al[21] found a significant association between liver disease and COVID-19 mortality. A meta-analysis including about 25000 patients confirmed that CLDs were significantly associated with more severe COVID-19 course [odds ratio (OR): 1.48; 95%CI: 1.17-1.87] and overall mortality (OR: 1.78; 95%CI: 1.09-2.93)[20]. Other studies instead did not show this association[22,23]. For example, Simon et al[22] reported that patients with CLD show an increased risk of hospitalization for COVID-19 but not an increased mortality. Furthermore, in a recent retrospective case-control study, patients with CLD did not show more need for invasive mechanical ventilation, as well as admission to intensive care unit (ICU) and overall mortality, compared to patients without liver disease[23].
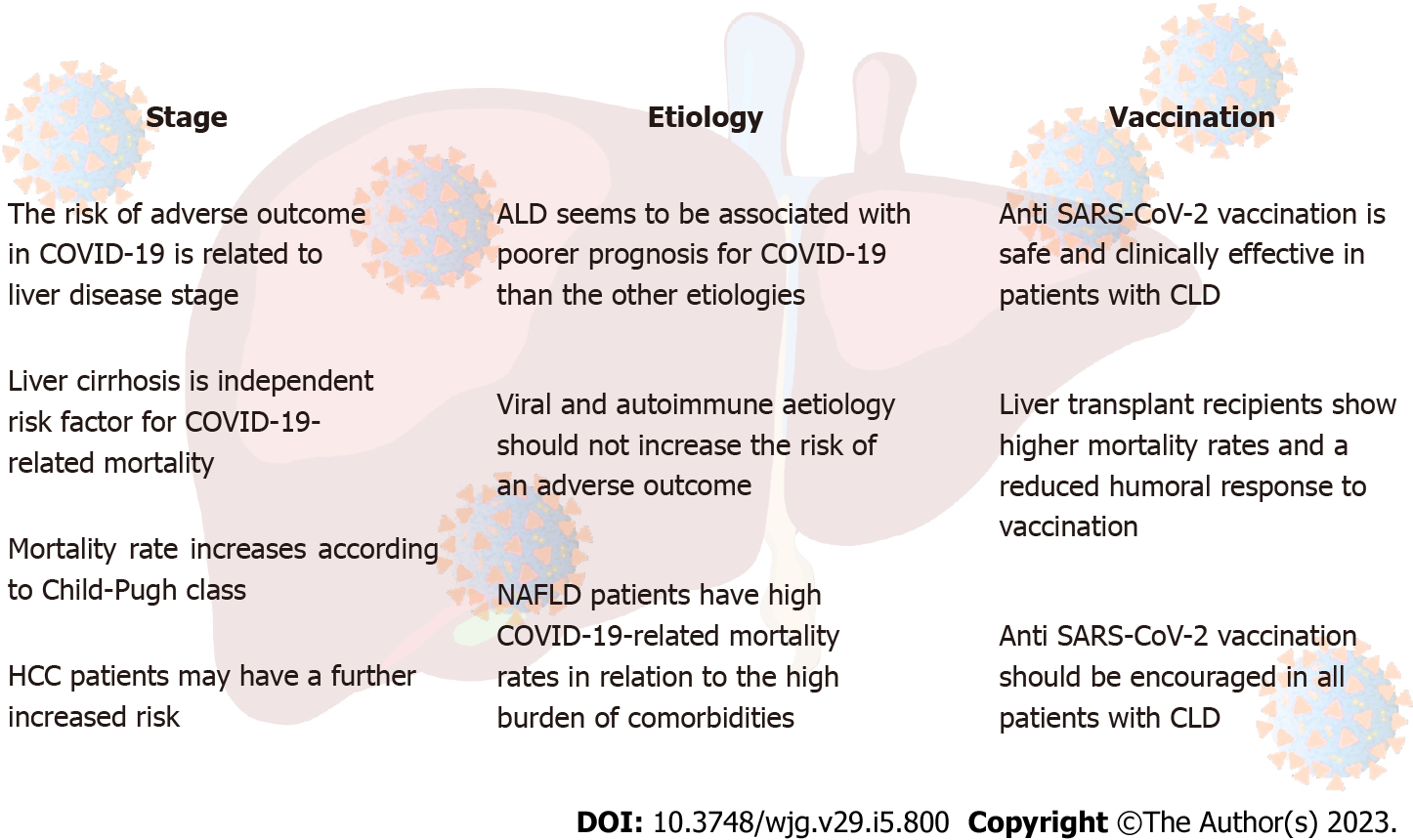
Therefore, data on the association between liver disease and increase of mortality rates related to COVID-19 are conflicting. However, the spectrum of CLDs is very heterogeneous both for etiology and for stage disease. In fact, the risk appears directly related to the latter. Studies that evaluated all liver disease stages could have been affected by different mortality rates in patients with CLD, greater in cirrhosis stage, particularly if in decompensation. In this regard, Mallet et al[15] more recently reported, on a large French cohort, a significant increase in the need for mechanical ventilation (OR: 1.54; 95%CI: 1.44-1.64) and mortality rate (OR: 1.79; 95%CI: 1.71-1.87) in patients with CLD. However, when stratified by disease severity, authors observed that patients with decompensated liver cirrhosis showed a significantly higher mortality rate whereas patients with mild liver disease or compensated cirrhosis were not at increased risk of COVID-19-related mortality[15]. These data have been confirmed by meta-analysis of Middleton et al[24], in which liver cirrhosis has been shown to be associated to an increased risk of all-cause mortality in COVID-19 compared to non-cirrhotic patients. Current evidences on the role of disease stage, etiology, and vaccination on COVID-19 outcomes are summarized in Figure 1.
Regardless of the etiology, the stage of liver cirrhosis is characterized by a high degree of patient frailty. The impairment of immune system, the concurrent increase of both thrombotic and hemorrhagic risk and the protein-calorie malnutrition make the patient suffering from cirrhosis vulnerable to various kinds of injuries. The frailty of the cirrhotic patient in the setting of COVID-19 is expressed by an excess of mortality and hospitalization rate compared to patients without CLD[14,15,19]. Extracting data of the subgroup of cirrhotic patients, Singh et al[14] had already reported that the risk of death in these patients was a 4.6-fold increase compared to ones without liver disease. As already mentioned, the mortality rate appears higher in patients with cirrhosis not only compared to patients without CLD but also when compared to patients with CLD but without cirrhosis (32% vs 8%, respectively; P < 0.001)[25]. In particular, compared to cirrhotic/SARS-CoV-2 negative patients and to non-cirrhotic/SARS-CoV-2 positive patients, those with liver cirrhosis and COVID-19 had a 2.38- and 3.31-times adjusted hazard ratio of 30-d death, respectively[26]. Similar results were found by Ioannou et al[27]. Overall, the 30-d COVID-19-related mortality rate in patients with cirrhosis is very high, ranging from 32% to 47% of cases[25,28,29]. In fact, liver cirrhosis has been proven to be an independent risk factor for COVID-19 related mortality (OR: 3.1)[29]. However, no updated mortality data are available for the new viral variants, with an apparently lower lethality rate than the wild type. Although most studies consider liver cirrhosis as an independent predictor of the risk of COVID-19-related death[15,24,26,29,30], some data would indicate that the high mortality rates in patients with cirrhosis and COVID-19 result from cirrhosis-associated comorbidities and extrahepatic organ failure rather than the liver disease itself[31]. After propensity score matching for age, sex, and extra-hepatic comorbidities, mortality rate during COVID-19 appears to be similar between patients with and without cirrhosis (28.8% vs 26.1%, respectively; P = 0.644). These results still could have been affected by the typology of matching and the methodology of data collection through registers. Among patients with liver cirrhosis, the coexistence of obesity and diabetes would further worsen the outcomes[16].
Similarly, to what was recently reported for kidney failure[32], COVID-19-related mortality risk was strongly associated with the stage of liver impairment. Overall, the 30-d mortality risk is significantly increased in patients with decompensated liver cirrhosis hospitalized for COVID-19[15,30]. Mortality rates increased according to Child-Pugh (CP) class, raising from 19% of class A (OR: 1.90), to 35% of class B (OR: 4.14) up to 51% of class C (OR: 9.32)[25]. In particular a CP score ≥ 9 at hospital admission would be predictive of high mortality[16]. Compared to patients without liver disease, a CP class B and C cirrhosis would bring an additional mortality rate of +20% and +38.1%, respectively[25]. In this regard, it seems that the Chronic Liver Failure Consortium had better performance in predicting 28-d mortality than CP score and model for end-stage liver disease-Na in patients with cirrhosis and COVID-19[29]. Moreover, in cirrhotic patients, an increasing trend of bilirubin and AST/ALT ratio[16] or the occurrence of liver injury[10,21] would be predictive of mortality.
The most frequent cause of mortality in patients with liver cirrhosis and COVID-19 remains the respiratory failure (71%)[25]. However, acute liver decompensation occurred in up to 46% of patients, even without respiratory symptoms. Liver related complications increased according to stage of liver disease[16]. It is known that infections (bacterial more than viral) may lead to liver decompensation, hepatorenal syndrome and portosystemic encephalopathy, and are one of the most frequent causes of acute-on-chronic liver failure (ACLF) and death in patients with cirrhosis. Conclusive data on the comparison between the SARS-CoV-2 infection outcomes and those of other infectious precipitants are currently not available. However, some data indicate that in-hospital mortality rates would be significantly higher in cirrhotic patients with COVID-19 than in those with other bacterial infections[28]. Overall, about 45% of patients with CLD develop ACLF[16]. Higher rates are reported for patients with liver cirrhosis. Moreover, cirrhotic patients with diabetes or obesity had higher ACLF rates than non-diabetic or normal weight patients (OR: 2.1 and 8.9, respectively)[16]. Similarly, to other viral infections, ACLF during COVID-19 could result from an immune-mediated response to viral antigens in the context of a cytokine storm[33], as well as a direct cytopathic effect or iatrogenic injury. Multi-organ damage caused by SARS-CoV-2 is significantly more frequent in immunocompromised patients[34]. The impairment of the immune system in the case of liver cirrhosis due to bone marrow suppression, lower protein synthesis and cirrhosis associated immune dysfunction syndrome, could explain the high rates of ACLF and the severe course in cirrhotic patients.
If in-hospital, COVID-19-related mortality was significantly increased in patients with liver cirrhosis and little is known about post-acute outcomes. Recently, Vaishnav et al[35] analyzed the post-discharge mortality of cirrhotic patients with SARS-CoV-2 infection. The data indicate that mortality rates within 2 mo of discharge among COVID-19 survivors are comparable between patients with liver cirrhosis and those without.
Little data is currently available on SARS-CoV-2 infection clinical course and outcomes in patients with hepatocellular carcinoma (HCC). Indeed, in studies performed during the first pandemic phase on COVID-19-related outcomes in cancer patients, those with HCC were underrepresented[36,37].
Although data are not univocal[25], several studies include the presence of HCC among the independent predictors of COVID-19-related mortality[15,30,38]. Among patients with CLD and COVID-19, HCC patients had 3.31 times the hazard of death for all causes, regardless of the presence of liver cirrhosis[30]. Beyond the association with mortality, according to Mallet et al[15] the presence of HCC is also predictive of a severe course of COVID-19 and a greater need for mechanical ventilation. Muñoz-Martínez et al[38] evaluated the SARS-CoV-2 infection course in 250 patients with primary liver cancer (218 with HCC and 32 with intrahepatic cholangiocarcinoma). In patients with HCC, a 30-d mortality rate of 18.4% was observed, with a statistically significant trend according to the stage of cirrhosis (assessed by CP) and tumor [assessed by Barcelona Clinic Liver Cancer (BCLC)]. In particular, the mortality rates increased from 6.10% for BCLC-0/A, to 11.76% for BCLC-B, to 20.69% for BCLC-C and 34.52% for BCLC-D[38].
The high COVID-19 related mortality in patients with HCC could result from the link between viral infection and the impairment of the immune-system secondary to active neoplasm, antineoplastic therapy and the frequent coexistence of liver cirrhosis.
In the analysis of the correlation between SARS-CoV-2 infection and CLD, patients who have undergone liver transplant (LT) represent a separate group due to the effects of chronic immunosuppressive therapy. The hypothesis that this therapy could increase the susceptibility to SARS-CoV-2 has been suggested from some population studies[39-41]. Observational studies on western populations have reported a mortality of 16%-22% in hepatotransplant patients with SARS-CoV-2 infection[42,43] in liver-transplant patients with SARS-CoV-2 infection, finding an increased survival in the short-term LT recipients (< 2 years), usually treated with full doses of immunosuppressants. This data support the hypothesis that, more than the immunosuppressive effect itself, the main cause of death in these patients is represented by the long-term cardio-metabolic effects induced by immunosuppressive drugs[44].
The study of the correlation between the type of immunosuppressive drug and COVID-19 outcomes in patients who have undergone LT has led to non-univocal results. During the first pandemic wave, Colmenero et al[39] reported that mycophenolate mofetil therapy in liver-transplant patients with SARS-CoV-2 infection was associated with an increased risk of a severe course of COVID-19 (RR 3.94, P = 0.003)[39]. Therapies with calcineurin inhibitors or everolimus, instead, have been shown to not be associated with an increased likelihood of adverse outcome. Furthermore, discontinuation of immunosuppressive therapy did not show benefits[39]. On the contrary, Webb et al[45] did not find any correlation between the type of immunosuppressant and mortality rate in patient with previous LT and SARS-CoV-2 infection. They highlighted that LT seems to not significantly increase the COVID-19-related mortality rate. These data are supported by the results of a meta-analysis including the main studies performed on LT patients with SARS-CoV-2 infection[46]. In these patients the 30-d mortality was comparable to the mortality rate found in the general population (OR: 0.90, 95%CI: 0.55-1.47). In light of unavailability of univocal data, the European Association for the Study of the Liver suggests to personalize immunosuppressive therapy changes based on patient's medical history, disease severity and the type of ongoing immunosuppressive therapy[47].
As mentioned above, if liver cirrhosis is associated with high rates of COVID-19-related mortality, several data indicate that patients with chronic hepatitis do not show an increased risk[15]. However, in addition to the stage of the disease, the different etiology could also affect the COVID-19 outcomes (Figure 1).
During the pandemic, the relationship between alcohol and SARS-CoV-2 infection has been shown to be bidirectional. On one hand, the isolation and socio-economic uncertainties resulting from the COVID-19 pandemic have led to an increase in alcohol consumption[48], already on the rise in the last 20 years[49]. On the other hand, several studies reported that alcohol-related liver disease (ALD) seems to be associated with a poorer prognosis for COVID-19 than the other etiologies[15,25,30,50]. In this regard, Marjot et al[25] showed ALD to be an independent risk factor for death from COVID-19 (OR: 1.79; 95%CI: 1.03-3.13). Similar results have been obtained from Mallet et al[15] and Kim et al[30]. Recently Bailey et al[50] confirmed that alcohol use disorders (AUDs) worsen the COVID-19 course and are associated with an increased hospitalization rate and all-cause mortality compared to patients with SARS-CoV-2 infection but without AUDs.
AUDs are already known as a risk factor for ARDS and ARDS-related multiorgan failure[51]. In fact, chronic alcohol consumption has been demonstrated to cause significative alterations in epithelial and endothelial cell function, surfactant synthesis and secretion, lung matrix composition and alveolar-capillary barrier function. Such alterations could increase susceptibility to respiratory pathogens, like SARS-CoV-2, leading to higher ARDS rates and adverse outcomes compared to patients without AUDs. Furthermore, ethanol exposure could stimulate the activity of key inflammatory mediators with a pro-inflammatory response further exacerbated by SARS-CoV-2 infection, resulting in a more severe course of COVID-19[52].
Several studies analyzed the mutual interaction between chronic hepatitis B virus (HBV) and SARS-CoV-2 infection, investigating whether the underlying viral disease could determine a worse prognosis during the COVID-19 course. Numerous data suggest that patients with chronic HBV infection have similar characteristics to HBV-negative patients in prevalence of laboratory abnormalities (changes in cytolysis and cholestasis liver markers), severity of the COVID-19 course and mortality[53-55]. The absence of a significant correlation between COVID-19-related outcomes and chronic viral hepatitis is confirmed by meta-analysis of Sarkar et al[56], in which the Authors found no significant impact on overall mortality during SARS-CoV-2 infection. Neither the degree of HBV replicative activity seems to affect the SARS-CoV-2 infection outcomes; inactive carriers or patients with previous infection have ALI and mortality rates comparable to patients with active hepatitis[57,58]. As further demonstration of the absence of correlation between HBV replicative activity and COVID-19-related outcomes, antiviral therapy for HBV is not able to determine a significant impact on mortality, need for admission to the ICU and hospitalization length[59]. Apparently, conflicting with these data, Yang et al[60] showed that the HBeAg-positive chronic HBV hepatitis are associated with a higher rate of hospitalization in ICU and mortality. However, the Authors did not stratify the study cohort in relation to disease stage and the impact of the presence of liver cirrhosis and organ failure on these results is unknown. Finally, the role and safety of immunosuppressive therapies (e.g., corticosteroids, IL-6 pathway inhibitors such as tocilizumab) used in cases of SARS-CoV-2-related ARDS were evaluated for the risk of HBV reactivation in patients with biohumoral signs of previous infection (HBsAg-negative, HBcAb-positive). In these patients, the risk of HBV reactivation following immunosuppressive treatment for COVID-19 appears negligible and not influenced by any antiviral prophylaxis[61].
Little data are available to date on the association between the severity of COVID-19 course and chronic hepatitis C virus (HCV) infection. Some studies report an increase of mortality for patients with chronic HCV infection[62]. However, also in this case, the proportion of patients with liver cirrhosis and the related impact on outcomes is unknown. Butt et al[63] showed similar COVID-19-related mortality rates among HCV-positive patients compared to HCV-negatives, despite a higher hospitalization rate. However, in the study population, HCV patients show a higher prevalence of liver cirrhosis than those not with HCV (8.1% vs 1.4%, respectively; P < 0.0001). Lastly, Cerbu et al[64] investigated the differences in outcomes between patients with active HCV infection and those who were under treatment or achieved sustained virological response. They found that patients with active infection showed an overall worse prognosis in terms of hospitalization, severe COVID-19 course, ICU admission and all-cause mortality compared to non-viremic patients. Regarding this, the early treatment with sofosbuvir/velpatasvir combination (used for HCV infection) in patients with COVID-19 has been shown to be effective in speeding up the clearance of SARS-CoV-2 and preventing disease progression[65].
Non-alcoholic fatty liver disease (NAFLD) is currently the most frequent etiology of liver disease worldwide, affecting approximately 32.5% of the global population[66]. It is closely associated to metabolic comorbidities such as obesity, diabetes mellitus, arterial hypertension and chronic kidney failure[67]. Such comorbidities related to NAFLD have been shown to play a predictive role for adverse outcomes in COVID-19, being associated with higher rates of hospitalization, mechanical ventilation and mortality[19,68]. For these reasons, great attention has been paid to determine whether NAFLD itself could represent an independent prognostic factor in COVID-19. However, studies in this setting are affected by the variability in the definition of NALFD patients, using for this purpose clinical-anamnestic or radiological (by ultrasound or computed tomography) criteria or score [hepatic steatosis index (HSI)] in different ways. Data currently available are not univocal. In one of the very first reports, Ji et al[69] showed that, net of comorbidities, NAFLD (diagnosed by ultrasound or by a value > 36 of the sums of HSI and body mass index) was an independent predictor of COVID-19 progression (OR: 6.4; 95%CI: 1.5-31.2). Furthermore, NAFLD was associated with higher prevalence of ALI during hospital stay and a slower viral clearance compared to the control group without NAFLD. Mahamid et al[70] later confirmed these data, despite the small cohort size. Conversely, in a recent case control study, NAFLD was not found to be associated with higher in-hospital mortality rates, need for ventilatory support, ICU admission, or overall length of hospital stay[71]. Similar results have been obtained by Marjot et al[25] and Kim et al[30]. Also, in the study by Vrsaljko et al[72], NAFLD is not shown to be independently correlated to a severe course of COVID-19 and to mortality rates in the multivariate analysis, while it appears significantly related to the hospitalization length and the incidence of pulmonary thrombosis.
Moreover, the nomenclature of NALFD recently has been changed to metabolic associated fatty liver disease (MAFLD)[73]. At the same time, the diagnostic criteria have been redefined and the results do not overlap with the previous ones. These new criteria have also been recently applied in the setting of patients with SARS-CoV-2 infection, showing conflicting data in this case as well. In this regard, Vázquez-Medina et al[74] reported that patients with MAFLD, but not those with NAFLD, have higher mortality rates (55.0% vs 38.3%; P = 0.02) than the control group not MAFLD/not NAFLD, whereas both MAFLD and NAFLD are associated with a higher rate of orotracheal intubation. Gao et al[75] confirmed that MAFLD increases by 4 times the risk of a severe course of COVID-19 and the association remains even after adjusting for age, sex, and comorbidities. Surprisingly, some preliminary data would indicate that the correlation between MAFLD and severity of COVID-19 course is more significant in patients under 60[76]. In contrast to the above-mentioned studies, Campos-Murguía et al[77] observed that fibrosis rather than MAFLD is associated with a severe course of COVID-19 (increased need for mechanical ventilation, increased incidence of acute kidney injury), and higher mortality. However, in most of these studies enrolled patients were not evaluated for the possible presence of liver cirrhosis. This could represent a significant bias with a potential impact on the results. As expected, the presence of intermediate or advanced liver fibrosis in patients with MAFLD is indeed associated with a higher risk of severe COVID-19[78]. In this regard, advanced fibrosis has been shown to determine a significant increase in mortality risk both in patients diagnosed with MAFLD and in those diagnosed with NAFLD compared to patients without CLD[74] and patients with mild or moderate liver fibrosis[79].
Recently, a large-scale 2-sample Mendelian randomization analysis had been carried out in order to provide possible conclusive data on the association between NAFLD and the COVID-19 course[80]. Although NAFLD is associated with a severe course of SARS-CoV-2 infection in univariate analysis, this association disappears after adjustment for age, sex, and comorbidities. Therefore, the Authors conclude that there is no evidence supporting that NAFLD is a causal risk factor for severe COVID-19. The results favoring this association are probably attributable to the correlation between NAFLD and obesity.
The incidence of COVID-19 in patients with autoimmune hepatitis (AIH) is comparable to that of the general population[81,82]. Despite that available data are limited, the main studies agree that patients with AIH do not present significant differences in hospitalization rates, disease severity and mortality during SARS-CoV-2 infection compared to patients with non-AIH CLD and the general population[83,84]. However, steroid treatment during COVID-19, when not indicated, can cause a more severe course of the disease. While steroids represent a cornerstone in the therapy of SARS-CoV-2-related ARDS[85,86], particularly when associated to antiviral drugs[87], their use is not recommended in the absence of respiratory failure due to lack of benefit and potential worsening of outcomes[85,86]. In this regard, Efe et al[88]recently highlighted that the use of corticosteroids or azathioprine for AIH therapy is associated with a significant increase in the risk of severe form of COVID-19 (OR: 4.73; 95%CI: 1.12-25.89), even after adjusting for demographic characteristics, comorbidities and presence of liver cirrhosis. However, in the absence of conclusive data, any remodulation of immunosuppressive therapy during SARS-CoV-2 infection should be personalized after a careful assessment of the risks and benefits[47].
The global availability of anti-SARS-CoV-2 vaccines in the last months of 2020 has resulted in a reduction of hospitalization and mortality rates from COVID-19 despite the succession of different viral variants[89]. Given their vulnerability profile in cases of SARS-CoV-2 infection, such vaccines have been strictly recommended in LT recipients and patients with CLD, with priority to cirrhotic patients and those with HCC or ALD (Figure 1)[90-92]. In this setting, vaccines have found to be generally safe[93-95], although sporadic cases of post-vaccinal ALI are reported, with predominantly hepatocellular and immune-mediated injury due to a probable aberrant response of the immune system after vaccine stimulation[96].
Despite the strong indication, patients with CLD (particularly those with liver cirrhosis) appear underrepresented in phase III trials of anti-SARS-CoV-2 vaccines, both for mRNA and viral vector ones[97-99]. In fact, CLD and in particular the presence of significant liver fibrosis could negatively affect the production of innate immunity proteins and the count and performance of B and T lymphocytes[100]. For this reason, in the last 2 years, a growing number of clinical studies have investigated the efficacy of anti-SARS-CoV-2 vaccines in patients with CLD. Anti-SARS-CoV-2 vaccines are able to determine both a T-cell and a humoral response, stimulating the production of antibodies directed against the Spike protein (seroconversion) and neutralizing antibodies[101,102]. Prospective studies compared the immunological response induced by a full course of mRNA vaccines and/or viral vector vaccines in patients with liver cirrhosis and controls[93-95]. Almost all patients with liver cirrhosis showed production of anti-Spike antibodies and a neutralizing antibody activity, similar to patients without liver cirrhosis[93]. A recent meta-analysis confirmed comparable seroconversion rates between cirrhotic patients and patients with CLD without cirrhosis[100]. Despite comparable seroconversion rates, Iavarone et al[95] reported a statistically significant difference in the antibody titer developed by patients with liver cirrhosis after vaccination compared to controls (1751 U/mL vs 4523 U/mL; P = 0.012). In particular, CP classes B and C and the presence of HCC would appear to be independently associated with low levels of antibody titer. We hypothesize that this suboptimal vaccine response could potentially indicate partial protection against SARS-CoV-2 infection and a reduction in its duration, particularly in patients with decompensated cirrhosis. Furthermore, with regard to the cellular response induced by the vaccine, the production of interferon-gamma after spike-specific stimulation of T lymphocytes is detectable only in 65.4% of patients with liver cirrhosis, against 100% of controls[94].
Beyond humoral response, little data are available on clinical efficacy of anti-SARS-CoV-2 vaccines in patients with CLD. The most important knowledge on this issue have been acquired from Veterans Outcomes and Costs Associated with Liver Disease cohort[103] and National COVID Cohort Collaborative registers[104]. In the first study, John et al[103] compared overall and COVID-19-related mortality 60 d after SARS-CoV-2 infection in cirrhotic patients receiving mRNA vaccine and cirrhotic patients not previously vaccinated. Unequivocally, postvaccination COVID-19 was associated with reduced mortality rates (HR: 0.21; 95%CI: 0.11-0.42) compared to unvaccinated patients with liver cirrhosis, with an overall reduction in the risk of death of approximately 80%. The benefit of vaccination is also statistically significant in patients with decompensated cirrhosis and in those who have not completed the vaccination course[103]. Similar results have been reported also by Ge et al[104], whose study is available only in “pre-print” version to date. This study, carried out during SARS-CoV-2 alpha and delta variants predominance, describes a 66% reduction in 30-d mortality in vaccinated cirrhotic patients compared to unvaccinated patients with liver cirrhosis. Furthermore, the administration of the third dose of mRNA vaccine in cirrhotic patients seems to lead to an 80% reduction in cases of SARS-CoV-2 infection (symptomatic or asymptomatic) and a 100% reduction in the severe forms of COVID-19 and related death compared to the administration of two doses, overcoming the hyporesponsiveness to vaccines in these patients[105]. The impact of the third vaccine dose appears stronger among patients with compensated rather than decompensated cirrhosis.
If patients with liver cirrhosis show overall suboptimal but effective seroconversion rates on protection against risk of death and a severe COVID-19 course, data obtained from LT recipients appear less encouraging. In fact, LT recipients show lower vaccine response[94,100,106]. Seroconversion rates in these patients range from 47.5% and 65%, significantly lower than controls[94,107]. Overall, 28% of patients undergoing LT did neither develop a T-cell nor a humoral response after vaccination[94]. An optimal humoral response is developed in only one third of liver transplant patients[107]. Among the factors associated with vaccine response rate in this setting, studies agree in identifying advanced age, alcoholic etiology of liver disease, and immunosuppressive therapy as independent predictors of reduced antibody response[93,106,108]. Conclusive data on the correlation with the specific immunosuppressive regimen are not available to date. Some studies would indicate that the reduced antibody response is particularly significant for patients treated with mycophenolate mofetil[106,108] or high doses of steroids[106]. Other manuscripts reported instead a negative correlation with calcineurin inhibitors compared to other immunosuppressive drugs[94]. Thuluvath et al[107] highlights that the use of ≥ 2 immunosuppression drugs is associated with poor immune response.
Finally, at the moment, few data are available about the influence of CLD etiology on vaccine-induced immune responses. Among the various etiologies, despite the high seroconversion rates, AIH patients show a significantly lower antibody titer than both patients with autoimmune cholestatic liver disease and controls, independently from the presence of liver cirrhosis and the ongoing immunosuppressive therapy[109]. Despite this, the clinical benefit of vaccination appears consistently in AIH patients, showing a significative reduction in hospitalization, severe course and COVID-19-related mortality rates (adjusted OR: 0.20)[110].
Patients with CLD are more vulnerable to SARS-CoV-2 infection. In particular, patients with liver cirrhosis show higher hospitalization rates, severe COVID-19 course and mortality than the general population. Mortality rates increase according to stage of cirrhosis. Among etiologies, ALD is the most frequently associated to adverse outcomes. Patients with NAFLD have high mortality rates and severe COVID-19 course in relation to the high burden of comorbidities. Anti-SARS-CoV-2 vaccination is safe and effective in patients with CLD: in particular, patients with liver cirrhosis benefit from a complete vaccination course. Patients who have undergone liver transplant show higher mortality rates and a reduced humoral response to vaccination. In any case, vaccination should be encouraged in all patients with CLD, particularly for those at higher risk due to disease stage and related etiology.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Barve P, United States; Velikova TV, Bulgaria S-Editor: Zhang H L-Editor: Filipodia P-Editor: Zhang H
| 1. | Cucinotta D, Vanelli M. WHO Declares COVID-19 a Pandemic. Acta Biomed. 2020;91:157-160. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2621] [Reference Citation Analysis (0)] |
| 2. | WHO Coronavirus (COVID-19) Dashboard. [cited 22 November 2022]. Available from: https://covid19.who.int. |
| 3. | Fan Y, Li X, Zhang L, Wan S, Zhou F. SARS-CoV-2 Omicron variant: recent progress and future perspectives. Signal Transduct Target Ther. 2022;7:141. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 362] [Article Influence: 120.7] [Reference Citation Analysis (0)] |
| 4. | Le TTB, Vasanthakumaran T, Thi Hien HN, Hung IC, Luu MN, Khan ZA, An NT, Tran VP, Lee WJ, Abdul Aziz JM, Ali T, Dumre SP, Huy NT. SARS-CoV-2 Omicron and its current known unknowns: A narrative review. Rev Med Virol. 2022;e2398. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 21] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 5. | Lewnard JA, Hong VX, Patel MM, Kahn R, Lipsitch M, Tartof SY. Clinical outcomes associated with SARS-CoV-2 Omicron (B.1.1.529) variant and BA.1/BA.1.1 or BA.2 subvariant infection in Southern California. Nat Med. 2022;28:1933-1943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 218] [Article Influence: 72.7] [Reference Citation Analysis (0)] |
| 6. | Jothimani D, Venugopal R, Abedin MF, Kaliamoorthy I, Rela M. COVID-19 and the liver. J Hepatol. 2020;73:1231-1240. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 364] [Cited by in RCA: 358] [Article Influence: 71.6] [Reference Citation Analysis (1)] |
| 7. | Yu D, Du Q, Yan S, Guo XG, He Y, Zhu G, Zhao K, Ouyang S. Liver injury in COVID-19: clinical features and treatment management. Virol J. 2021;18:121. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 74] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 8. | Sharma A, Jaiswal P, Kerakhan Y, Saravanan L, Murtaza Z, Zergham A, Honganur NS, Akbar A, Deol A, Francis B, Patel S, Mehta D, Jaiswal R, Singh J, Patel U, Malik P. Liver disease and outcomes among COVID-19 hospitalized patients - A systematic review and meta-analysis. Ann Hepatol. 2021;21:100273. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 81] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 9. | Yadav DK, Singh A, Zhang Q, Bai X, Zhang W, Yadav RK, Zhiwei L, Adhikari VP, Liang T. Involvement of liver in COVID-19: systematic review and meta-analysis. Gut. 2021;70:807-809. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 82] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 10. | Russo A, Pisaturo M, Palladino R, Maggi P, Numis FG, Gentile I, Sangiovanni V, Esposito V, Punzi R, Calabria G, Rescigno C, Salomone Megna A, Masullo A, Manzillo E, Russo G, Parrella R, Dell'Aquila G, Gambardella M, Ponticiello A, Coppola N; On Behalf Of CoviCam Group. Prognostic Value of Transaminases and Bilirubin Levels at Admission to Hospital on Disease Progression and Mortality in Patients with COVID-19-An Observational Retrospective Study. Pathogens. 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 11. | Albillos A, Lario M, Álvarez-Mon M. Cirrhosis-associated immune dysfunction: distinctive features and clinical relevance. J Hepatol. 2014;61:1385-1396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 691] [Cited by in RCA: 847] [Article Influence: 77.0] [Reference Citation Analysis (1)] |
| 12. | Zhang C, Shi L, Wang FS. Liver injury in COVID-19: management and challenges. Lancet Gastroenterol Hepatol. 2020;5:428-430. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1348] [Cited by in RCA: 1295] [Article Influence: 259.0] [Reference Citation Analysis (4)] |
| 13. | Sonzogni A, Previtali G, Seghezzi M, Grazia Alessio M, Gianatti A, Licini L, Morotti D, Zerbi P, Carsana L, Rossi R, Lauri E, Pellegrinelli A, Nebuloni M. Liver histopathology in severe COVID 19 respiratory failure is suggestive of vascular alterations. Liver Int. 2020;40:2110-2116. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 162] [Cited by in RCA: 218] [Article Influence: 43.6] [Reference Citation Analysis (0)] |
| 14. | Singh S, Khan A. Clinical Characteristics and Outcomes of Coronavirus Disease 2019 Among Patients With Preexisting Liver Disease in the United States: A Multicenter Research Network Study. Gastroenterology. 2020;159:768-771.e3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 205] [Cited by in RCA: 270] [Article Influence: 54.0] [Reference Citation Analysis (0)] |
| 15. | Mallet V, Beeker N, Bouam S, Sogni P, Pol S; Demosthenes research group. Prognosis of French COVID-19 patients with chronic liver disease: A national retrospective cohort study for 2020. J Hepatol. 2021;75:848-855. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 49] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 16. | Sarin SK, Choudhury A, Lau GK, Zheng MH, Ji D, Abd-Elsalam S, Hwang J, Qi X, Cua IH, Suh JI, Park JG, Putcharoen O, Kaewdech A, Piratvisuth T, Treeprasertsuk S, Park S, Wejnaruemarn S, Payawal DA, Baatarkhuu O, Ahn SH, Yeo CD, Alonzo UR, Chinbayar T, Loho IM, Yokosuka O, Jafri W, Tan S, Soo LI, Tanwandee T, Gani R, Anand L, Esmail ES, Khalaf M, Alam S, Lin CY, Chuang WL, Soin AS, Garg HK, Kalista K, Batsukh B, Purnomo HD, Dara VP, Rathi P, Al Mahtab M, Shukla A, Sharma MK, Omata M; APASL COVID Task Force, APASL COVID Liver Injury Spectrum Study (APCOLIS Study-NCT 04345640). Pre-existing liver disease is associated with poor outcome in patients with SARS CoV2 infection; The APCOLIS Study (APASL COVID-19 Liver Injury Spectrum Study). Hepatol Int. 2020;14:690-700. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 217] [Cited by in RCA: 216] [Article Influence: 43.2] [Reference Citation Analysis (1)] |
| 17. | Mantovani A, Beatrice G, Dalbeni A. Coronavirus disease 2019 and prevalence of chronic liver disease: A meta-analysis. Liver Int. 2020;40:1316-1320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 120] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 18. | Docherty AB, Harrison EM, Green CA, Hardwick HE, Pius R, Norman L, Holden KA, Read JM, Dondelinger F, Carson G, Merson L, Lee J, Plotkin D, Sigfrid L, Halpin S, Jackson C, Gamble C, Horby PW, Nguyen-Van-Tam JS, Ho A, Russell CD, Dunning J, Openshaw PJ, Baillie JK, Semple MG; ISARIC4C investigators. Features of 20 133 UK patients in hospital with covid-19 using the ISARIC WHO Clinical Characterisation Protocol: prospective observational cohort study. BMJ. 2020;369:m1985. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2061] [Cited by in RCA: 2063] [Article Influence: 412.6] [Reference Citation Analysis (0)] |
| 19. | Williamson EJ, Walker AJ, Bhaskaran K, Bacon S, Bates C, Morton CE, Curtis HJ, Mehrkar A, Evans D, Inglesby P, Cockburn J, McDonald HI, MacKenna B, Tomlinson L, Douglas IJ, Rentsch CT, Mathur R, Wong AYS, Grieve R, Harrison D, Forbes H, Schultze A, Croker R, Parry J, Hester F, Harper S, Perera R, Evans SJW, Smeeth L, Goldacre B. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584:430-436. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4343] [Cited by in RCA: 4205] [Article Influence: 841.0] [Reference Citation Analysis (0)] |
| 20. | Kovalic AJ, Satapathy SK, Thuluvath PJ. Prevalence of chronic liver disease in patients with COVID-19 and their clinical outcomes: a systematic review and meta-analysis. Hepatol Int. 2020;14:612-620. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 96] [Cited by in RCA: 99] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 21. | Galiero R, Pafundi PC, Simeon V, Rinaldi L, Perrella A, Vetrano E, Caturano A, Alfano M, Beccia D, Nevola R, Marfella R, Sardu C, Coppola C, Scarano F, Maggi P, De Lucia Sposito P, Vocciante L, Rescigno C, Sbreglia C, Fraganza F, Parrella R, Romano A, Calabria G, Polverino B, Pagano A, Bologna C, Amitrano M, Esposito V, Coppola N, Maturo N, Adinolfi LE, Chiodini P, Sasso FC; COVOCA Study Group. Impact of chronic liver disease upon admission on COVID-19 in-hospital mortality: Findings from COVOCA study. PLoS One. 2020;15:e0243700. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 41] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 22. | Simon TG, Hagström H, Sharma R, Söderling J, Roelstraete B, Larsson E, Ludvigsson JF. Risk of severe COVID-19 and mortality in patients with established chronic liver disease: a nationwide matched cohort study. BMC Gastroenterol. 2021;21:439. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 23. | Ferreira AI, Sarmento MH, Cotter J. Predictors of clinical outcomes of hospitalized patients with Covid-19: focusing on pre-existing liver disease. Intern Emerg Med. 2022;17:2209-2217. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 24. | Middleton P, Hsu C, Lythgoe MP. Clinical outcomes in COVID-19 and cirrhosis: a systematic review and meta-analysis of observational studies. BMJ Open Gastroenterol. 2021;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 27] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 25. | Marjot T, Moon AM, Cook JA, Abd-Elsalam S, Aloman C, Armstrong MJ, Pose E, Brenner EJ, Cargill T, Catana MA, Dhanasekaran R, Eshraghian A, García-Juárez I, Gill US, Jones PD, Kennedy J, Marshall A, Matthews C, Mells G, Mercer C, Perumalswami PV, Avitabile E, Qi X, Su F, Ufere NN, Wong YJ, Zheng MH, Barnes E, Barritt AS 4th, Webb GJ. Outcomes following SARS-CoV-2 infection in patients with chronic liver disease: An international registry study. J Hepatol. 2021;74:567-577. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 399] [Cited by in RCA: 384] [Article Influence: 96.0] [Reference Citation Analysis (0)] |
| 26. | Ge J, Pletcher MJ, Lai JC; N3C Consortium. Outcomes of SARS-CoV-2 Infection in Patients With Chronic Liver Disease and Cirrhosis: A National COVID Cohort Collaborative Study. Gastroenterology. 2021;161:1487-1501.e5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 80] [Cited by in RCA: 86] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 27. | Ioannou GN, Liang PS, Locke E, Green P, Berry K, O'Hare AM, Shah JA, Crothers K, Eastment MC, Fan VS, Dominitz JA. Cirrhosis and Severe Acute Respiratory Syndrome Coronavirus 2 Infection in US Veterans: Risk of Infection, Hospitalization, Ventilation, and Mortality. Hepatology. 2021;74:322-335. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 74] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 28. | Iavarone M, D'Ambrosio R, Soria A, Triolo M, Pugliese N, Del Poggio P, Perricone G, Massironi S, Spinetti A, Buscarini E, Viganò M, Carriero C, Fagiuoli S, Aghemo A, Belli LS, Lucà M, Pedaci M, Rimondi A, Rumi MG, Invernizzi P, Bonfanti P, Lampertico P. High rates of 30-day mortality in patients with cirrhosis and COVID-19. J Hepatol. 2020;73:1063-1071. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 279] [Cited by in RCA: 276] [Article Influence: 55.2] [Reference Citation Analysis (2)] |
| 29. | Mendizabal M, Ridruejo E, Piñero F, Anders M, Padilla M, Toro LG, Torre A, Montes P, Urzúa A, Gonzalez Ballerga E, Silveyra MD, Michelato D, Díaz J, Peralta M, Pages J, García SR, Gutierrez Lozano I, Macias Y, Cocozzella D, Chavez-Tapia N, Tagle M, Dominguez A, Varón A, Vera Pozo E, Higuera-de la Tijera F, Bustios C, Conte D, Escajadillo N, Gómez AJ, Tenorio L, Castillo Barradas M, Schinoni MI, Bessone F, Contreras F, Nazal L, Sanchez A, García M, Brutti J, Cabrera MC, Miranda-Zazueta G, Rojas G, Cattaneo M, Castro-Narro G, Rubinstein F, Silva MO. Comparison of different prognostic scores for patients with cirrhosis hospitalized with SARS-CoV-2 infection. Ann Hepatol. 2021;25:100350. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 30. | Kim D, Adeniji N, Latt N, Kumar S, Bloom PP, Aby ES, Perumalswami P, Roytman M, Li M, Vogel AS, Catana AM, Wegermann K, Carr RM, Aloman C, Chen VL, Rabiee A, Sadowski B, Nguyen V, Dunn W, Chavin KD, Zhou K, Lizaola-Mayo B, Moghe A, Debes J, Lee TH, Branch AD, Viveiros K, Chan W, Chascsa DM, Kwo P, Dhanasekaran R. Predictors of Outcomes of COVID-19 in Patients With Chronic Liver Disease: US Multi-center Study. Clin Gastroenterol Hepatol. 2021;19:1469-1479.e19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 176] [Cited by in RCA: 184] [Article Influence: 46.0] [Reference Citation Analysis (0)] |
| 31. | Brozat JF, Hanses F, Haelberger M, Stecher M, Dreher M, Tometten L, Ruethrich MM, Vehreschild JJ, Trautwein C, Borgmann S, Vehreschild MJGT, Jakob CEM, Stallmach A, Wille K, Hellwig K, Isberner N, Reuken PA, Geisler F, Nattermann J, Bruns T; LEOSS study group. COVID-19 mortality in cirrhosis is determined by cirrhosis-associated comorbidities and extrahepatic organ failure: Results from the multinational LEOSS registry. United European Gastroenterol J. 2022;10:409-424. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 32. | Galiero R, Simeon V, Loffredo G, Caturano A, Rinaldi L, Vetrano E, Medicamento G, Alfano M, Beccia D, Brin C, Colantuoni S, Di Salvo J, Epifani R, Nevola R, Marfella R, Sardu C, Coppola C, Scarano F, Maggi P, Calabrese C, De Lucia Sposito P, Rescigno C, Sbreglia C, Fraganza F, Parrella R, Romano A, Calabria G, Polverino B, Pagano A, Numis FG, Bologna C, Nunziata M, Esposito V, Coppola N, Maturo N, Nasti R, Di Micco P, Perrella A, Lettieri M, Adinolfi LE, Chiodini P, Sasso FC; On Behalf Of Covoca Study Group. Association between Renal Function at Admission and COVID-19 in-Hospital Mortality in Southern Italy: Findings from the Prospective Multicenter Italian COVOCA Study. J Clin Med. 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (1)] |
| 33. | Adams DH, Hubscher SG. Systemic viral infections and collateral damage in the liver. Am J Pathol. 2006;168:1057-1059. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 96] [Cited by in RCA: 117] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 34. | D'Antiga L. Coronaviruses and Immunosuppressed Patients: The Facts During the Third Epidemic. Liver Transpl. 2020;26:832-834. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 479] [Cited by in RCA: 492] [Article Influence: 98.4] [Reference Citation Analysis (0)] |
| 35. | Vaishnav M, Elhence A, Biswas S, Pathak P, Anand A, Sheikh S, Singh V, Maitra S, Goel A, Shalimar. The Outcome in Cirrhosis after Hospital Discharge is Not Worsened with COVID-19 Infection: A Propensity Score-matched Analysis. J Clin Exp Hepatol. 2022;12:830-840. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 36. | Zhang L, Zhu F, Xie L, Wang C, Wang J, Chen R, Jia P, Guan HQ, Peng L, Chen Y, Peng P, Zhang P, Chu Q, Shen Q, Wang Y, Xu SY, Zhao JP, Zhou M. Clinical characteristics of COVID-19-infected cancer patients: a retrospective case study in three hospitals within Wuhan, China. Ann Oncol. 2020;31:894-901. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 819] [Cited by in RCA: 1016] [Article Influence: 203.2] [Reference Citation Analysis (0)] |
| 37. | Mehta V, Goel S, Kabarriti R, Cole D, Goldfinger M, Acuna-Villaorduna A, Pradhan K, Thota R, Reissman S, Sparano JA, Gartrell BA, Smith RV, Ohri N, Garg M, Racine AD, Kalnicki S, Perez-Soler R, Halmos B, Verma A. Case Fatality Rate of Cancer Patients with COVID-19 in a New York Hospital System. Cancer Discov. 2020;10:935-941. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 493] [Cited by in RCA: 576] [Article Influence: 115.2] [Reference Citation Analysis (0)] |
| 38. | Muñoz-Martínez S, Sapena V, Forner A, Bruix J, Sanduzzi-Zamparelli M, Ríos J, Bouattour M, El-Kassas M, Leal CRG, Mocan T, Nault JC, Alves RCP, Reeves HL, da Fonseca L, García-Juárez I, Pinato DJ, Varela M, Alqahtani SA, Alvares-da-Silva MR, Bandi JC, Rimassa L, Lozano M, González Santiago JM, Tacke F, Sala M, Anders M, Lachenmayer A, Piñero F, França A, Guarino M, Elvevi A, Cabibbo G, Peck-Radosavljevic M, Rojas Á, Vergara M, Braconi C, Pascual S, Perelló C, Mello V, Rodríguez-Lope C, Acevedo J, Villani R, Hollande C, Vilgrain V, Tawheed A, Ferguson Theodoro C, Sparchez Z, Blaise L, Viera-Alves DE, Watson R, Carrilho FJ, Moctezuma-Velázquez C, D'Alessio A, Iavarone M, Reig M. Outcome of liver cancer patients with SARS-CoV-2 infection: An International, Multicentre, Cohort Study. Liver Int. 2022;42:1891-1901. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 12] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 39. | Colmenero J, Rodríguez-Perálvarez M, Salcedo M, Arias-Milla A, Muñoz-Serrano A, Graus J, Nuño J, Gastaca M, Bustamante-Schneider J, Cachero A, Lladó L, Caballero A, Fernández-Yunquera A, Loinaz C, Fernández I, Fondevila C, Navasa M, Iñarrairaegui M, Castells L, Pascual S, Ramírez P, Vinaixa C, González-Dieguez ML, González-Grande R, Hierro L, Nogueras F, Otero A, Álamo JM, Blanco-Fernández G, Fábrega E, García-Pajares F, Montero JL, Tomé S, De la Rosa G, Pons JA. Epidemiological pattern, incidence, and outcomes of COVID-19 in liver transplant patients. J Hepatol. 2021;74:148-155. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 270] [Cited by in RCA: 270] [Article Influence: 67.5] [Reference Citation Analysis (0)] |
| 40. | Ravanan R, Callaghan CJ, Mumford L, Ushiro-Lumb I, Thorburn D, Casey J, Friend P, Parameshwar J, Currie I, Burnapp L, Baker R, Dudley J, Oniscu GC, Berman M, Asher J, Harvey D, Manara A, Manas D, Gardiner D, Forsythe JLR. SARS-CoV-2 infection and early mortality of waitlisted and solid organ transplant recipients in England: A national cohort study. Am J Transplant. 2020;20:3008-3018. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 126] [Cited by in RCA: 120] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 41. | Trapani S, Masiero L, Puoti F, Rota MC, Del Manso M, Lombardini L, Riccardo F, Amoroso A, Pezzotti P, Grossi PA, Brusaferro S, Cardillo M; Italian Network of Regional Transplant Coordinating Centers Collaborating group; Italian Surveillance System of Covid-19, Italian Society for Organ Transplantation (SITO), The Italian Board of Experts in Liver Transplantation (I-BELT) Study Group, Italian Association for the Study of the Liver (AISF), Italian Society of Nephrology (SIN), SIN-SITO Study Group. Incidence and outcome of SARS-CoV-2 infection on solid organ transplantation recipients: A nationwide population-based study. Am J Transplant. 2021;21:2509-2521. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 71] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 42. | Belli LS, Duvoux C, Karam V, Adam R, Cuervas-Mons V, Pasulo L, Loinaz C, Invernizzi F, Patrono D, Bhoori S, Ciccarelli O, Morelli MC, Castells L, Lopez-Lopez V, Conti S, Fondevila C, Polak W. COVID-19 in liver transplant recipients: preliminary data from the ELITA/ELTR registry. Lancet Gastroenterol Hepatol. 2020;5:724-725. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 65] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 43. | Rabiee A, Sadowski B, Adeniji N, Perumalswami PV, Nguyen V, Moghe A, Latt NL, Kumar S, Aloman C, Catana AM, Bloom PP, Chavin KD, Carr RM, Dunn W, Chen VL, Aby ES, Debes JD, Dhanasekaran R; COLD Consortium. Liver Injury in Liver Transplant Recipients With Coronavirus Disease 2019 (COVID-19): U.S. Multicenter Experience. Hepatology. 2020;72:1900-1911. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 67] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 44. | Bhoori S, Rossi RE, Citterio D, Mazzaferro V. COVID-19 in long-term liver transplant patients: preliminary experience from an Italian transplant centre in Lombardy. Lancet Gastroenterol Hepatol. 2020;5:532-533. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 198] [Cited by in RCA: 205] [Article Influence: 41.0] [Reference Citation Analysis (0)] |
| 45. | Webb GJ, Marjot T, Cook JA, Aloman C, Armstrong MJ, Brenner EJ, Catana MA, Cargill T, Dhanasekaran R, García-Juárez I, Hagström H, Kennedy JM, Marshall A, Masson S, Mercer CJ, Perumalswami PV, Ruiz I, Thaker S, Ufere NN, Barnes E, Barritt AS 4th, Moon AM. Outcomes following SARS-CoV-2 infection in liver transplant recipients: an international registry study. Lancet Gastroenterol Hepatol. 2020;5:1008-1016. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 197] [Article Influence: 39.4] [Reference Citation Analysis (0)] |
| 46. | Gatti M, Rinaldi M, Bussini L, Bonazzetti C, Pascale R, Pasquini Z, Faní F, Pinho Guedes MN, Azzini AM, Carrara E, Palacios-Baena ZR, Caponcello G, Reyna-Villasmil E, Tacconelli E, Rodríguez-Baño J, Viale P, Giannella M; ORCHESTRA study group; Infectious Diseases Unit; Department of Integrated Management of Infectious Risk; IRCCS Policlinico Sant’Orsola; Department of Medical and Surgical Sciences; University of Bologna in Bologna, Italy; Division of Infectious Diseases; Department of Diagnostics and Public Health, University of Verona in Verona, Italy; Infectious Diseases and Microbiology Unit; Hospital Universitario Virgen Macarena; Department of Medicine, University of Sevilla/Biomedicines Institute of Sevilla in Sevilla, Spain. Clinical outcome in solid organ transplant recipients affected by COVID-19 compared to general population: a systematic review and meta-analysis. Clin Microbiol Infect. 2022;28:1057-1065. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 46] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 47. | Marjot T, Eberhardt CS, Boettler T, Belli LS, Berenguer M, Buti M, Jalan R, Mondelli MU, Moreau R, Shouval D, Berg T, Cornberg M. Impact of COVID-19 on the liver and on the care of patients with chronic liver disease, hepatobiliary cancer, and liver transplantation: An updated EASL position paper. J Hepatol. 2022;77:1161-1197. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 58] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 48. | Lee BP, Dodge JL, Leventhal A, Terrault NA. Retail Alcohol and Tobacco Sales During COVID-19. Ann Intern Med. 2021;174:1027-1029. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 76] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 49. | Grant BF, Chou SP, Saha TD, Pickering RP, Kerridge BT, Ruan WJ, Huang B, Jung J, Zhang H, Fan A, Hasin DS. Prevalence of 12-Month Alcohol Use, High-Risk Drinking, and DSM-IV Alcohol Use Disorder in the United States, 2001-2002 to 2012-2013: Results From the National Epidemiologic Survey on Alcohol and Related Conditions. JAMA Psychiatry. 2017;74:911-923. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 761] [Cited by in RCA: 1019] [Article Influence: 127.4] [Reference Citation Analysis (0)] |
| 50. | Bailey KL, Sayles H, Campbell J, Khalid N, Anglim M, Ponce J, Wyatt TA, McClay JC, Burnham EL, Anzalone A, Hanson C. COVID-19 patients with documented alcohol use disorder or alcohol-related complications are more likely to be hospitalized and have higher all-cause mortality. Alcohol Clin Exp Res. 2022;46:1023-1035. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 51. | Moss M, Burnham EL. Chronic alcohol abuse, acute respiratory distress syndrome, and multiple organ dysfunction. Crit Care Med. 2003;31:S207-S212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 96] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 52. | Huang W, Zhou H, Hodgkinson C, Montero A, Goldman D, Chang SL. Network Meta-Analysis on the Mechanisms Underlying Alcohol Augmentation of COVID-19 Pathologies. Alcohol Clin Exp Res. 2021;45:675-688. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 30] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 53. | Liu J, Wang T, Cai Q, Sun L, Huang D, Zhou G, He Q, Wang FS, Liu L, Chen J. Longitudinal changes of liver function and hepatitis B reactivation in COVID-19 patients with pre-existing chronic hepatitis B virus infection. Hepatol Res. 2020;50:1211-1221. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 63] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 54. | Ding ZY, Li GX, Chen L, Shu C, Song J, Wang W, Wang YW, Chen Q, Jin GN, Liu TT, Liang JN, Zhu P, Zhu W, Li Y, Zhang BH, Feng H, Zhang WG, Yin ZY, Yu WK, Yang Y, Zhang HQ, Tang ZP, Wang H, Hu JB, Liu JH, Yin P, Chen XP, Zhang B; Tongji Multidisciplinary Team for Treating COVID-19 (TTTC). Association of liver abnormalities with in-hospital mortality in patients with COVID-19. J Hepatol. 2021;74:1295-1302. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 114] [Cited by in RCA: 109] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 55. | Zhang B, Huang W, Zhang S. Clinical Features and Outcomes of Coronavirus Disease 2019 (COVID-19) Patients With Chronic Hepatitis B Virus Infection. Clin Gastroenterol Hepatol. 2020;18:2633-2637. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 56. | Sarkar S, Khanna P, Singh AK. Impact of COVID-19 in patients with concurrent co-infections: A systematic review and meta-analyses. J Med Virol. 2021;93:2385-2395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 69] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 57. | Chen L, Huang S, Yang J, Cheng X, Shang Z, Lu H, Cheng J. Clinical characteristics in patients with SARS-CoV-2/HBV co-infection. J Viral Hepat. 2020;27:1504-1507. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 55] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 58. | Yip TC, Wong VW, Lui GC, Chow VC, Tse YK, Hui VW, Liang LY, Chan HL, Hui DS, Wong GL. Current and Past Infections of HBV Do Not Increase Mortality in Patients With COVID-19. Hepatology. 2021;74:1750-1765. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 45] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 59. | Choe JW, Jung YK, Yim HJ, Seo GH. Clinical Effect of Hepatitis B Virus on COVID-19 Infected Patients: A Nationwide Population-Based Study Using the Health Insurance Review & Assessment Service Database. J Korean Med Sci. 2022;37:e29. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 20] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 60. | Yang S, Wang S, Du M, Liu M, Liu Y, He Y. Patients with COVID-19 and HBV Coinfection are at Risk of Poor Prognosis. Infect Dis Ther. 2022;11:1229-1242. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 23] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 61. | Boettler T, Marjot T, Newsome PN, Mondelli MU, Maticic M, Cordero E, Jalan R, Moreau R, Cornberg M, Berg T. Impact of COVID-19 on the care of patients with liver disease: EASL-ESCMID position paper after 6 months of the pandemic. JHEP Rep. 2020;2:100169. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 103] [Cited by in RCA: 121] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 62. | Ronderos D, Omar AMS, Abbas H, Makker J, Baiomi A, Sun H, Mantri N, Choi Y, Fortuzi K, Shin D, Patel H, Chilimuri S. Chronic hepatitis-C infection in COVID-19 patients is associated with in-hospital mortality. World J Clin Cases. 2021;9:8749-8762. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 21] [Cited by in RCA: 28] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 63. | Butt AA, Yan P, Chotani RA, Shaikh OS. Mortality is not increased in SARS-CoV-2 infected persons with hepatitis C virus infection. Liver Int. 2021;41:1824-1831. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 32] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 64. | Cerbu B, Pantea S, Bratosin F, Vidican I, Turaiche M, Frent S, Borsi E, Marincu I. Liver Impairment and Hematological Changes in Patients with Chronic Hepatitis C and COVID-19: A Retrospective Study after One Year of Pandemic. Medicina (Kaunas). 2021;57. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 65. | Messina V, Nevola R, Izzi A, De Lucia Sposito P, Marrone A, Rega R, Fusco R, Lumino P, Rinaldi L, Gaglione P, Simeone F, Sasso FC, Maggi P, Adinolfi LE. Efficacy and safety of the sofosbuvir/velpatasvir combination for the treatment of patients with early mild to moderate COVID-19. Sci Rep. 2022;12:5771. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 66. | Riazi K, Azhari H, Charette JH, Underwood FE, King JA, Afshar EE, Swain MG, Congly SE, Kaplan GG, Shaheen AA. The prevalence and incidence of NAFLD worldwide: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2022;7:851-861. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 1174] [Article Influence: 391.3] [Reference Citation Analysis (1)] |
| 67. | Acierno C, Caturano A, Pafundi PC, Nevola R, Adinolfi LE, Sasso FC. Nonalcoholic fatty liver disease and type 2 diabetes: pathophysiological mechanisms shared between the two faces of the same coin. Explor Med. 2020;1:287-306. [RCA] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 68. | Nevola R, Marrone A, Cozzolino D, Cuomo G, Romano CP, Rinaldi L, Aprea C, Padula A, Ranieri R, Gjeloshi K, Ricozzi C, Ruosi C, Imbriani S, Meo LA, Sellitto A, Cinone F, Carusone C, Abitabile M, Nappo F, Signoriello G, Adinolfi LE. Predictors of in-hospital mortality of COVID-19 patients and the role of telemetry in an internal medicine ward during the third phase of the pandemic. Eur Rev Med Pharmacol Sci. 2022;26:1777-1785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 69. | Ji D, Qin E, Xu J, Zhang D, Cheng G, Wang Y, Lau G. Non-alcoholic fatty liver diseases in patients with COVID-19: A retrospective study. J Hepatol. 2020;73:451-453. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 336] [Cited by in RCA: 408] [Article Influence: 81.6] [Reference Citation Analysis (2)] |
| 70. | Mahamid M, Nseir W, Khoury T, Mahamid B, Nubania A, Sub-Laban K, Schifter J, Mari A, Sbeit W, Goldin E. Nonalcoholic fatty liver disease is associated with COVID-19 severity independently of metabolic syndrome: a retrospective case-control study. Eur J Gastroenterol Hepatol. 2021;33:1578-1581. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 68] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 71. | Madan K, Rastogi R, Bhargava R, Dagar V, Singla V, Sahu A, Singh P, Garg P, Aggarwal B, Singh RK. Is Fatty Liver Associated with Increased Mortality and Morbidity in Coronavirus Disease 2019 (COVID-19) Pneumonia? J Clin Exp Hepatol. 2022;12:1320-1327. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 12] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 72. | Vrsaljko N, Samadan L, Viskovic K, Mehmedović A, Budimir J, Vince A, Papic N. Association of Nonalcoholic Fatty Liver Disease With COVID-19 Severity and Pulmonary Thrombosis: CovidFAT, a Prospective, Observational Cohort Study. Open Forum Infect Dis. 2022;9:ofac073. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 22] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 73. | Eslam M, Newsome PN, Sarin SK, Anstee QM, Targher G, Romero-Gomez M, Zelber-Sagi S, Wai-Sun Wong V, Dufour JF, Schattenberg JM, Kawaguchi T, Arrese M, Valenti L, Shiha G, Tiribelli C, Yki-Järvinen H, Fan JG, Grønbæk H, Yilmaz Y, Cortez-Pinto H, Oliveira CP, Bedossa P, Adams LA, Zheng MH, Fouad Y, Chan WK, Mendez-Sanchez N, Ahn SH, Castera L, Bugianesi E, Ratziu V, George J. A new definition for metabolic dysfunction-associated fatty liver disease: An international expert consensus statement. J Hepatol. 2020;73:202-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2883] [Cited by in RCA: 2827] [Article Influence: 565.4] [Reference Citation Analysis (1)] |
| 74. | Vázquez-Medina MU, Cerda-Reyes E, Galeana-Pavón A, López-Luna CE, Ramírez-Portillo PM, Ibañez-Cervantes G, Torres-Vázquez J, Vargas-De-León C. Interaction of metabolic dysfunction-associated fatty liver disease and nonalcoholic fatty liver disease with advanced fibrosis in the death and intubation of patients hospitalized with coronavirus disease 2019. Hepatol Commun. 2022;6:2000-2010. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 11] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 75. | Gao F, Zheng KI, Wang XB, Yan HD, Sun QF, Pan KH, Wang TY, Chen YP, George J, Zheng MH. Metabolic associated fatty liver disease increases coronavirus disease 2019 disease severity in nondiabetic patients. J Gastroenterol Hepatol. 2021;36:204-207. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 80] [Cited by in RCA: 85] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 76. | Zhou YJ, Zheng KI, Wang XB, Yan HD, Sun QF, Pan KH, Wang TY, Ma HL, Chen YP, George J, Zheng MH. Younger patients with MAFLD are at increased risk of severe COVID-19 illness: A multicenter preliminary analysis. J Hepatol. 2020;73:719-721. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 104] [Cited by in RCA: 106] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 77. | Campos-Murguía A, Román-Calleja BM, Toledo-Coronado IV, González-Regueiro JA, Solís-Ortega AA, Kúsulas-Delint D, Cruz-Contreras M, Cruz-Yedra N, Cubero FJ, Nevzorova YA, Martínez-Cabrera CF, Moreno-Guillén P, Lozano-Cruz OA, Chapa-Ibargüengoitia M, Gulías-Herrero A, Aguilar-Salinas CA, Ruiz-Margáin A, Macías-Rodríguez RU. Liver fibrosis in patients with metabolic associated fatty liver disease is a risk factor for adverse outcomes in COVID-19. Dig Liver Dis. 2021;53:525-533. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 26] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 78. | Targher G, Mantovani A, Byrne CD, Wang XB, Yan HD, Sun QF, Pan KH, Zheng KI, Chen YP, Eslam M, George J, Zheng MH. Risk of severe illness from COVID-19 in patients with metabolic dysfunction-associated fatty liver disease and increased fibrosis scores. Gut. 2020;69:1545-1547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 161] [Article Influence: 32.2] [Reference Citation Analysis (0)] |
| 79. | Elfeki MA, Robles J, Akhtar Z, Ullah F, Ganapathiraju I, Tran C, Inman C, Collin SM, Rosa R. Impact of Fibrosis-4 Index Prior to COVID-19 on Outcomes in Patients at Risk of Non-alcoholic Fatty Liver Disease. Dig Dis Sci. 2022;67:3333-3339. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 12] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 80. | Li J, Tian A, Zhu H, Chen L, Wen J, Liu W, Chen P. Mendelian Randomization Analysis Reveals No Causal Relationship Between Nonalcoholic Fatty Liver Disease and Severe COVID-19. Clin Gastroenterol Hepatol. 2022;20:1553-1560.e78. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 40] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 81. | Di Giorgio A, Nicastro E, Speziani C, De Giorgio M, Pasulo L, Magro B, Fagiuoli S, D' Antiga L. Health status of patients with autoimmune liver disease during SARS-CoV-2 outbreak in northern Italy. J Hepatol. 2020;73:702-705. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 46] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 82. | Zecher BF, Buescher G, Willemse J, Walmsley M, Taylor A, Leburgue A, Schramm C, Lohse AW, Sebode M. Prevalence of COVID-19 in patients with autoimmune liver disease in Europe: A patient-oriented online survey. United European Gastroenterol J. 2021;9:797-808. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 83. | Marjot T, Buescher G, Sebode M, Barnes E, Barritt AS 4th, Armstrong MJ, Baldelli L, Kennedy J, Mercer C, Ozga AK, Casar C, Schramm C; contributing Members and Collaborators of ERN RARE-LIVER/COVID-Hep/SECURE-Cirrhosis, Moon AM, Webb GJ, Lohse AW. SARS-CoV-2 infection in patients with autoimmune hepatitis. J Hepatol. 2021;74:1335-1343. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 89] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 84. | Efe C, Dhanasekaran R, Lammert C, Ebik B, Higuera-de la Tijera F, Aloman C, Rıza Calışkan A, Peralta M, Gerussi A, Massoumi H, Catana AM, Torgutalp M, Purnak T, Rigamonti C, Gomez Aldana AJ, Khakoo N, Kacmaz H, Nazal L, Frager S, Demir N, Irak K, Ellik ZM, Balaban Y, Atay K, Eren F, Cristoferi L, Batıbay E, Urzua Á, Snijders R, Kıyıcı M, Akyıldız M, Ekin N, Carr RM, Harputluoğlu M, Hatemi I, Mendizabal M, Silva M, Idilman R, Silveira M, Drenth JPH, Assis DN, Björnsson E, Boyer JL, Invernizzi P, Levy C, Schiano TD, Ridruejo E, Wahlin S. Outcome of COVID-19 in Patients With Autoimmune Hepatitis: An International Multicenter Study. Hepatology. 2021;73:2099-2109. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 55] [Article Influence: 13.8] [Reference Citation Analysis (1)] |
| 85. | Bassetti M, Giacobbe DR, Bruzzi P, Barisione E, Centanni S, Castaldo N, Corcione S, De Rosa FG, Di Marco F, Gori A, Gramegna A, Granata G, Gratarola A, Maraolo AE, Mikulska M, Lombardi A, Pea F, Petrosillo N, Radovanovic D, Santus P, Signori A, Sozio E, Tagliabue E, Tascini C, Vancheri C, Vena A, Viale P, Blasi F; Italian Society of Anti-infective Therapy (SITA) and the Italian Society of Pulmonology (SIP). Clinical Management of Adult Patients with COVID-19 Outside Intensive Care Units: Guidelines from the Italian Society of Anti-Infective Therapy (SITA) and the Italian Society of Pulmonology (SIP). Infect Dis Ther. 2021;10:1837-1885. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 29] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 86. | COVID-19 Treatment Guidelines Panel. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. [cited 22 November 2022]. In: National Institutes of Health. Available at https://www.covid19treatmentguidelines.nih.gov/. |
| 87. | Marrone A, Nevola R, Sellitto A, Cozzolino D, Romano C, Cuomo G, Aprea C, Schwartzbaum MXP, Ricozzi C, Imbriani S, Rinaldi L, Gjeloshi K, Padula A, Ranieri R, Ruosi C, Meo LA, Abitabile M, Cinone F, Carusone C, Adinolfi LE. Remdesivir Plus Dexamethasone Versus Dexamethasone Alone for the Treatment of Coronavirus Disease 2019 (COVID-19) Patients Requiring Supplemental O2 Therapy: A Prospective Controlled Nonrandomized Study. Clin Infect Dis. 2022;75:e403-e409. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 47] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 88. | Efe C, Lammert C, Taşçılar K, Dhanasekaran R, Ebik B, Higuera-de la Tijera F, Calışkan AR, Peralta M, Gerussi A, Massoumi H, Catana AM, Purnak T, Rigamonti C, Aldana AJG, Khakoo N, Nazal L, Frager S, Demir N, Irak K, Melekoğlu-Ellik Z, Kacmaz H, Balaban Y, Atay K, Eren F, Alvares-da-Silva MR, Cristoferi L, Urzua Á, Eşkazan T, Magro B, Snijders R, Barutçu S, Lytvyak E, Zazueta GM, Demirezer-Bolat A, Aydın M, Heurgue-Berlot A, De Martin E, Ekin N, Yıldırım S, Yavuz A, Bıyık M, Narro GC, Kıyıcı M, Akyıldız M, Kahramanoğlu-Aksoy E, Vincent M, Carr RM, Günşar F, Reyes EC, Harputluoğlu M, Aloman C, Gatselis NK, Üstündağ Y, Brahm J, Vargas NCE, Güzelbulut F, Garcia SR, Aguirre J, Anders M, Ratusnu N, Hatemi I, Mendizabal M, Floreani A, Fagiuoli S, Silva M, Idilman R, Satapathy SK, Silveira M, Drenth JPH, Dalekos GN, N Assis D, Björnsson E, Boyer JL, Yoshida EM, Invernizzi P, Levy C, Montano-Loza AJ, Schiano TD, Ridruejo E, Wahlin S. Effects of immunosuppressive drugs on COVID-19 severity in patients with autoimmune hepatitis. Liver Int. 2022;42:607-614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 27] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 89. | Zheng C, Shao W, Chen X, Zhang B, Wang G, Zhang W. Real-world effectiveness of COVID-19 vaccines: a literature review and meta-analysis. Int J Infect Dis. 2022;114:252-260. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 141] [Cited by in RCA: 413] [Article Influence: 137.7] [Reference Citation Analysis (0)] |
| 90. | Fix OK, Blumberg EA, Chang KM, Chu J, Chung RT, Goacher EK, Hameed B, Kaul DR, Kulik LM, Kwok RM, McGuire BM, Mulligan DC, Price JC, Reau NS, Reddy KR, Reynolds A, Rosen HR, Russo MW, Schilsky ML, Verna EC, Ward JW, Fontana RJ; AASLD COVID-19 Vaccine Working Group. American Association for the Study of Liver Diseases Expert Panel Consensus Statement: Vaccines to Prevent Coronavirus Disease 2019 Infection in Patients With Liver Disease. Hepatology. 2021;74:1049-1064. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 138] [Article Influence: 34.5] [Reference Citation Analysis (0)] |
| 91. | Cornberg M, Buti M, Eberhardt CS, Grossi PA, Shouval D. EASL position paper on the use of COVID-19 vaccines in patients with chronic liver diseases, hepatobiliary cancer and liver transplant recipients. J Hepatol. 2021;74:944-951. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 169] [Cited by in RCA: 174] [Article Influence: 43.5] [Reference Citation Analysis (0)] |
| 92. | Russo FP, Piano S, Bruno R, Burra P, Puoti M, Masarone M, Montagnese S, Ponziani FR, Petta S, Aghemo A; Italian Association for the Study of the Liver. Italian association for the study of the liver position statement on SARS-CoV2 vaccination. Dig Liver Dis. 2021;53:677-681. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 93. | Bakasis AD, Bitzogli K, Mouziouras D, Pouliakis A, Roumpoutsou M, Goules AV, Androutsakos T. Antibody Responses after SARS-CoV-2 Vaccination in Patients with Liver Diseases. Viruses. 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 31] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 94. | Ruether DF, Schaub GM, Duengelhoef PM, Haag F, Brehm TT, Fathi A, Wehmeyer M, Jahnke-Triankowski J, Mayer L, Hoffmann A, Fischer L, Addo MM, Lütgehetmann M, Lohse AW, Schulze Zur Wiesch J, Sterneck M. SARS-CoV2-specific Humoral and T-cell Immune Response After Second Vaccination in Liver Cirrhosis and Transplant Patients. Clin Gastroenterol Hepatol. 2022;20:162-172.e9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 116] [Article Influence: 38.7] [Reference Citation Analysis (0)] |
| 95. | Iavarone M, Tosetti G, Facchetti F, Topa M, Er JM, Hang SK, Licari D, Lombardi A, D'Ambrosio R, Degasperi E, Loglio A, Oggioni C, Perbellini R, Caccia R, Bandera A, Gori A, Ceriotti F, Scudeller L, Bertoletti A, Lampertico P. Spike-specific humoral and cellular immune responses after COVID-19 mRNA vaccination in patients with cirrhosis: A prospective single center study. Dig Liver Dis. 2022;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 16] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 96. | Shroff H, Satapathy SK, Crawford JM, Todd NJ, VanWagner LB. Liver injury following SARS-CoV-2 vaccination: A multicenter case series. J Hepatol. 2022;76:211-214. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 68] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 97. | Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, Diemert D, Spector SA, Rouphael N, Creech CB, McGettigan J, Khetan S, Segall N, Solis J, Brosz A, Fierro C, Schwartz H, Neuzil K, Corey L, Gilbert P, Janes H, Follmann D, Marovich M, Mascola J, Polakowski L, Ledgerwood J, Graham BS, Bennett H, Pajon R, Knightly C, Leav B, Deng W, Zhou H, Han S, Ivarsson M, Miller J, Zaks T; COVE Study Group. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N Engl J Med. 2021;384:403-416. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7073] [Cited by in RCA: 7564] [Article Influence: 1891.0] [Reference Citation Analysis (1)] |
| 98. | Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, Perez JL, Pérez Marc G, Moreira ED, Zerbini C, Bailey R, Swanson KA, Roychoudhury S, Koury K, Li P, Kalina WV, Cooper D, Frenck RW Jr, Hammitt LL, Türeci Ö, Nell H, Schaefer A, Ünal S, Tresnan DB, Mather S, Dormitzer PR, Şahin U, Jansen KU, Gruber WC; C4591001 Clinical Trial Group. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N Engl J Med. 2020;383:2603-2615. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10556] [Cited by in RCA: 10695] [Article Influence: 2139.0] [Reference Citation Analysis (1)] |
| 99. | Falsey AR, Sobieszczyk ME, Hirsch I, Sproule S, Robb ML, Corey L, Neuzil KM, Hahn W, Hunt J, Mulligan MJ, McEvoy C, DeJesus E, Hassman M, Little SJ, Pahud BA, Durbin A, Pickrell P, Daar ES, Bush L, Solis J, Carr QO, Oyedele T, Buchbinder S, Cowden J, Vargas SL, Guerreros Benavides A, Call R, Keefer MC, Kirkpatrick BD, Pullman J, Tong T, Brewinski Isaacs M, Benkeser D, Janes HE, Nason MC, Green JA, Kelly EJ, Maaske J, Mueller N, Shoemaker K, Takas T, Marshall RP, Pangalos MN, Villafana T, Gonzalez-Lopez A; AstraZeneca AZD1222 Clinical Study Group. Phase 3 Safety and Efficacy of AZD1222 (ChAdOx1 nCoV-19) Covid-19 Vaccine. N Engl J Med. 2021;385:2348-2360. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 495] [Cited by in RCA: 493] [Article Influence: 123.3] [Reference Citation Analysis (0)] |
| 100. | Cheung KS, Mok CH, Mao X, Zhang R, Hung IF, Seto WK, Yuen MF. COVID-19 vaccine immunogenicity among chronic liver disease patients and liver transplant recipients: A meta-analysis. Clin Mol Hepatol. 2022;28:890-911. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 33] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 101. | Koch T, Mellinghoff SC, Shamsrizi P, Addo MM, Dahlke C. Correlates of Vaccine-Induced Protection against SARS-CoV-2. Vaccines (Basel). 2021;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 42] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 102. | Skelly DT, Harding AC, Gilbert-Jaramillo J, Knight ML, Longet S, Brown A, Adele S, Adland E, Brown H; Medawar Laboratory Team, Tipton T, Stafford L, Mentzer AJ, Johnson SA, Amini A; OPTIC (Oxford Protective T cell Immunology for COVID-19) Clinical Group, Tan TK, Schimanski L, Huang KA, Rijal P; PITCH (Protective Immunity T cells in Health Care Worker) Study Group; C-MORE/PHOSP-C Group, Frater J, Goulder P, Conlon CP, Jeffery K, Dold C, Pollard AJ, Sigal A, de Oliveira T, Townsend AR, Klenerman P, Dunachie SJ, Barnes E, Carroll MW, James WS. Two doses of SARS-CoV-2 vaccination induce robust immune responses to emerging SARS-CoV-2 variants of concern. Nat Commun. 2021;12:5061. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 129] [Cited by in RCA: 131] [Article Influence: 32.8] [Reference Citation Analysis (0)] |
| 103. | John BV, Deng Y, Schwartz KB, Taddei TH, Kaplan DE, Martin P, Chao HH, Dahman B. Postvaccination COVID-19 infection is associated with reduced mortality in patients with cirrhosis. Hepatology. 2022;76:126-138. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 51] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 104. | Ge J, Digitale JC, Pletcher MJ, Lai JC; N3C Consortium. Breakthrough SARS-CoV-2 Infection Outcomes in Vaccinated Patients with Chronic Liver Disease and Cirrhosis: A National COVID Cohort Collaborative Study. medRxiv. 2022;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 105. | John BV, Ferreira RD, Doshi A, Kaplan DE, Taddei TH, Spector SA, Paulus E, Deng Y, Bastaich D, Dahman B. Third dose of COVID-19 mRNA vaccine appears to overcome vaccine hyporesponsiveness in patients with cirrhosis. J Hepatol. 2022;77:1349-1358. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 28] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 106. | Rabinowich L, Grupper A, Baruch R, Ben-Yehoyada M, Halperin T, Turner D, Katchman E, Levi S, Houri I, Lubezky N, Shibolet O, Katchman H. Low immunogenicity to SARS-CoV-2 vaccination among liver transplant recipients. J Hepatol. 2021;75:435-438. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 281] [Cited by in RCA: 272] [Article Influence: 68.0] [Reference Citation Analysis (0)] |
| 107. | Thuluvath PJ, Robarts P, Chauhan M. Analysis of antibody responses after COVID-19 vaccination in liver transplant recipients and those with chronic liver diseases. J Hepatol. 2021;75:1434-1439. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 130] [Article Influence: 32.5] [Reference Citation Analysis (0)] |
| 108. | Timmermann L, Globke B, Lurje G, Schmelzle M, Schöning W, Öllinger R, Pratschke J, Eberspächer B, Drosten C, Hofmann J, Eurich D. Humoral Immune Response following SARS-CoV-2 Vaccination in Liver Transplant Recipients. Vaccines (Basel). 2021;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 28] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 109. | Duengelhoef P, Hartl J, Rüther D, Steinmann S, Brehm TT, Weltzsch JP, Glaser F, Schaub GM, Sterneck M, Sebode M, Weiler-Normann C, Addo MM, Lütgehetmann M, Haag F, Schramm C, Schulze Zur Wiesch J, Lohse AW. SARS-CoV-2 vaccination response in patients with autoimmune hepatitis and autoimmune cholestatic liver disease. United European Gastroenterol J. 2022;10:319-329. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 26] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 110. | Efe C, Taşçılar K, Gerussi A, Bolis F, Lammert C, Ebik B, Stättermayer AF, Cengiz M, Gökçe DT, Cristoferi L, Peralta M, Massoumi H, Montes P, Cerda E, Rigamonti C, Yapalı S, Adali G, Çalışkan AR, Balaban Y, Eren F, Eşkazan T, Barutçu S, Lytvyak E, Zazueta GM, Kayhan MA, Heurgue-Berlot A, De Martin E, Yavuz A, Bıyık M, Narro GC, Duman S, Hernandez N, Gatselis NK, Aguirre J, Idilman R, Silva M, Mendizabal M, Atay K, Güzelbulut F, Dhanasekaran R, Montano-Loza AJ, Dalekos GN, Ridruejo E, Invernizzi P, Wahlin S. SARS-CoV-2 vaccination and risk of severe COVID-19 outcomes in patients with autoimmune hepatitis. J Autoimmun. 2022;132:102906. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |