Published online Feb 7, 2023. doi: 10.3748/wjg.v29.i5.766
Peer-review started: September 7, 2022
First decision: October 19, 2022
Revised: October 25, 2022
Accepted: January 17, 2023
Article in press: January 17, 2023
Published online: February 7, 2023
Processing time: 152 Days and 3.7 Hours
Many mechanisms have been proposed to explain the hypothetical state of hepatic tolerance, which is described by eventual imbalances or deregulation in the balance of cytokines, mediators, effectors, and regulatory cells in the complex milieu of the liver. In this section, we will comment on the importance of donor-specific anti-human leukocyte antigen (HLA) antibodies (DSA) as well as the compatibility and pairings of HLA and killer-cell immunoglobulin-like receptor (KIR) genotypes in the evolution of liver transplantation. Thus, HLA compatibility, viral infections, and HLA-C/KIR combinations have all been linked to liver transplant rejection and survival. There have been reports of increased risk of acute and chronic rejection with ductopenia, faster graft fibrosis, biliary problems, poorer survival, and even de novo autoimmune hepatitis when DSAs are present in the recipient. Higher mean fluorescence intensity (MFI) values of the DSAs and smaller graft size were associated with poorer patient outcomes, implying that high-risk patients with preformed DSAs should be considered for selecting the graft placed and desensitization methods, according to the investigators. Similarly, in a combined kidney-liver transplant, a pretransplant with a visible expression of several DSAs revealed that these antibodies were resistant to treatment. The renal graft was lost owing to antibody-mediated rejection (AMR). The HLA antigens expressed by the transplanted liver graft influenced antibody elimination. Pathologists are increasingly diagnosing AMR in liver transplants, and desensitization therapy has even been employed in situations of AMR, particularly in patients with DSAs in kidney-hepatic transplants and high-class II MFI due to Luminex. In conclusion, after revealing the negative impacts of DSAs with high MFI, pretransplant virtual crossmatch techniques may be appropriate to improve evolution; however, they may extend cold ischemia periods by requiring the donor to be typed.
Core Tip: This editorial aimed to raise realities, doubts, and ambiguities in the fundamental role of alloantibodies and the compatibility and association of the proteins encoded by the human leukocyte antigen and killer-cell immunoglobulin-like receptor genes in liver transplantation.
- Citation: Muro M, Legaz I. Importance of human leukocyte antigen antibodies and leukocyte antigen/killer-cell immunoglobulin-like receptor genes in liver transplantation. World J Gastroenterol 2023; 29(5): 766-772
- URL: https://www.wjgnet.com/1007-9327/full/v29/i5/766.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i5.766
In the vast majority of transplants performed today, there is a clear demonstration of the role played by the best human leukocyte antigen (HLA) compatibility and the absence of donor-specific anti-HLA antibodies (DSA) in its positive evolution, and it is an increasingly important role. The significance of the role of compatibility and killer-cell immunoglobulin-like receptor (KIR) genotypes (especially in hematopoietic stem cell transplantation) has not been demonstrated in the case of liver transplantation. This suggests that the classic concept of the liver is different and may be an “immunologically privileged” organ. Transplant (even if there is a positive pretransplant crossmatch and DSAs are known) without accounting for donor and recipient typing can lead to antibody-mediated rejection (AMR)[1]. However, there are articles where this has been re-evaluated, and new essential effects of antibodies and compatibility in acute rejection, chronic rejection (CR), fibrosis, and liver transplant survival appear.
The hypothetical state of tolerance of the liver has been explained by many causes (profusely explained in an article of its own), and is explained by eventual imbalances or deregulations in the balance of cytokines, mediator, effectors, and regulatory cells in the complex microenvironment of the liver, including increased or decreased expression of costimulatory or soluble molecules, specific genetic profiles, or even a protective role of Kupffer cells[2-9].
Here we focused on commenting on the role of DSA antibodies and the compatibility and pairings of HLA and KIR genotypes with the evolution of liver transplantation. Thus, HLA compatibility, viral infections, and HLA-C/KIR combinations have been classically related to liver transplant rejection and survival[10-13].
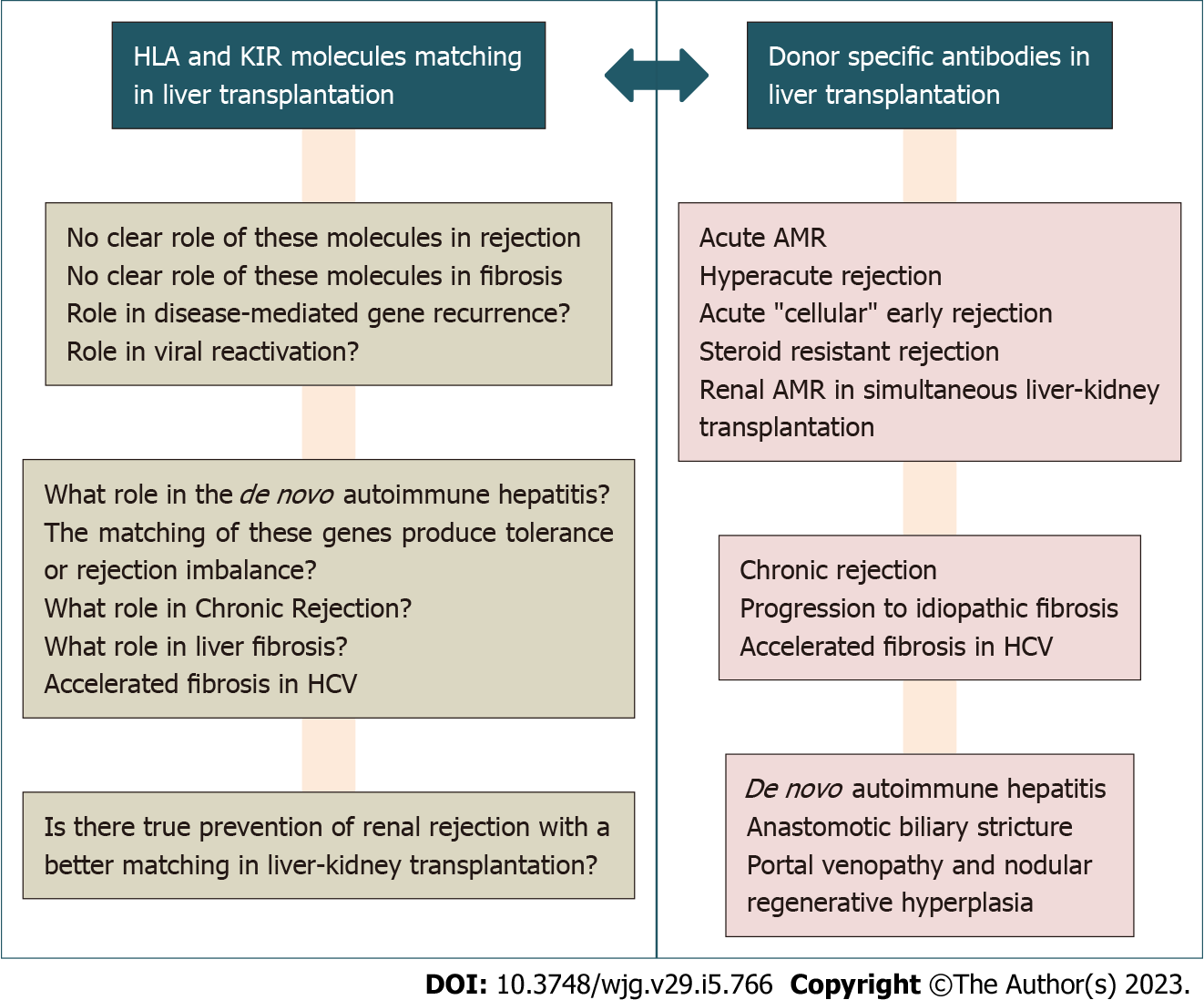
Regarding the existence of DSA antibodies present in the recipient, there are reports of increased risk of acute rejection and CR with ductopenia, accelerated graft fibrosis, biliary complications, worse survival, and even de novo autoimmune hepatitis[2,14]. However, some series and research groups reported different results and disparate causes (Figure 1). However, the literature on the role of DSAs and AMR is limited to clinical cases and small series[15].
Regarding preformed antibodies in the recipient before implantation, there is literature that reveals that patients with preformed DSA presented a worse graft evolution in living donor transplantation[16]. Higher mean fluorescence intensity (MFI) values of the DSAs and small graft size were associated with worse patient outcomes, suggesting to the authors that high-risk patients with preformed DSAs should be considered for selecting the graft implanted and desensitization protocols. Likewise, a pretransplant with the tangible expression of multiple DSAs[17] in a combined kidney-liver transplant showed that these antibodies were refractory to treatment, and the renal graft was lost due to AMR. The elimination of the antibodies depended on the HLA antigens expressed by the implanted liver graft.
In this sense, pathologists diagnose AMR in liver transplants with increasing frequency, and desensitization therapy has even been used in AMR cases, especially in patients with DSAs in kidney-hepatic transplants with high-class II MFI due to Luminex[18-20].
Although it is daring to assert categorically that the presence of DSAs contraindicates transplantation due to the same scientific literature, which is disparate between series, authors, and transplant centers, it is not well-defined over time (studies of positive, negative, and neutral papers) and the best methods of antibody diagnosis, evaluation of biopsies, and anti-rejection treatments[15]. In this way, regular DSA-post-transplant monitoring cannot as yet be recommended in routine practice but may be helpful in selected cases.
In the case of combined kidney transplants, there is also controversy and disparity between studies and groups. Thus, pretransplant DSAs increase the risk of AMR in the kidney and liver and worsen survival[12], with no data on the case of heart and lung combined with the liver. It has also been observed that the pretransplantation presence of anti-HLA class II antibodies and especially with positive complement fixation C1q or C3d have a risk of early AMR and a worse evolution of the transplant due to association of the graft with deposits of C4d in sinusoidal endothelial cells, increased fibrosis, CR, cirrhosis, and centrilobular fibrosis[2,13,16].
Regarding the development of de novo DSA (dnDSA), it has been estimated that immunosuppression may also play a role in the development of dnDSA. Thus, the coefficient of tacrolimus variation and mean tacrolimus levels have been reported to be associated with no dnDSA generation[21].
Other authors found that patients with an immunosuppressive regimen without withdrawal calcineurin inhibitors (mTOR inhibitors and/or maintenance with mycophenolic acid) have a higher prevalence of developing dnDSA post-transplant than patients with a standard regimen[22]. However, dnDSAs with calcineurin-free immunosuppression were associated with normal graft histology. The use of rituximab induction among DSA recipients has also been considered[23]. A dose of rituximab > 300 mg/m2 was well tolerated and achieved a lower incidence of AMR.
In addition, everolimus combined with tacrolimus was associated with negative HLA and DSA antibody status[24]. Viral etiology of liver disease, hepatocellular carcinoma, and higher degrees of graft steatosis were associated with a lower rate of HLA antibodies. The impact of HLA and DSA antibodies was associated with higher levels of transaminases and bilirubin. In addition, a significant association was detected between higher degrees of inflammation and the presence of HLA and DSA antibodies. Thus, DSA would be associated with histological and biochemical inflammation of the graft after liver transplantation, while fibrosis seems unaffected.
There are also cases in the literature of living donor liver transplants who developed acute AMR after desensitization to perform DSA and were successfully treated with bortezomib and everolimus therapy[25]. In this regard, in sensitized combined liver-kidney transplant recipients, the “delayed” kidney transplant approach was associated with a significant reduction in total and class I DSAs after liver transplantation before kidney transplantation[26], allowing therapeutic interventions such as plasmapheresis, providing optimal results similar to those of crossmatched recipients.
Finally, regarding single or triple-therapy monotherapy, it has been reported that the development of class II DSA occurs more often with immunosuppressive monotherapy and may ultimately result in chronic rejection and graft fibrosis[27].
On the other hand, Shin et al[21] found that patients without T-cell rejection in pediatric liver transplantation were more likely to have dnDSAs for HLA-DQ7 and less likely to have these DSAs for HLA-DQ2. Therefore, they deduced that a load of mismatched epitopes predicted the non-generation of these DSAs. At the same time, the specificity of de novo DSAs could determine alloimmunity.
Also, references for the location and the importance of the correct detection of these DSAs would corroborate that the existence of intragraft DSA and intragraft union reaction of C3d (using a fluorescent analysis technique of capture of immunocomplexes) harms the outcome of the transplant, unlike DSA present in serum, with no impact[28].
Finally, it has been reported that the incidence of DSA after liver transplantation is higher in children than in adults, that DSAs directed against HLA class II molecules, mainly DQ, occur more often, and that the presence of such anti-class II DSA (DQ/DR), especially of the complement-binding IgG3 subclass, may be associated with endothelial injury, T-cell-mediated rejection (TCMR), inflammation, and fibrosis[29-31].
Regarding the positive, negative, or neutral role of the compatibility of the HLA and/or KIR genes, it is a subject of almost as much debate as the subject of antibodies. Historical studies have commented on any of the possibilities[10-12,32], and at the moment, there is no consistency in all the studies reviewed in this editorial. Regarding the role of HLA incompatibility and the evolution of the liver allograft, it is not separate from promoting the development of DSAs, with the logical criterion that the more incompatibilities, the more possibilities exist to develop antibodies DSAs de novo. Thus, the new molecular HLA incompatibility (MM) improves the prediction of the evolution of the transplant. Thus, in a study by Ono et al[33] on liver transplantation from a living donor, the risk of TCMR and the development of dnDSA were evaluated using eplets. MM in HLA-DQB1 eplets was associated with TCMR. The predicted indirectly recognizable HLA epitopes II (PIRCHE-II) score for the HLA-DQB1 gene was also significantly higher in patients with TCMR. Moreover, DQB1-EpMMs ≥ 9 and DQB1-predicted indirectly recognizable HLA epitopes II score ≥ 3 were predictors of dnDSA formation. Thus, MM analysis may be applied toward tailored immunosuppression based on individual risks.
In this sense, a very recent article[34] on living donor transplants found that the more HLA incompatibilities there are, the worse the patient’s survival was (for A + B + DR, A + B + C, DR + DQ, and A + B + C + DR + DQ). For HLA-B + DR mismatches, the risk of a TCMR was more pronounced in adults but not in children. It has also been reported in 1042 liver transplants and 9.38 years of follow-up that HLA-A mismatch was strongly associated with graft failure and mortality, especially with two mismatches[35].
However, other groups commented that incompatibility was not associated with acute rejection, early allograft dysfunction, or survival in living donor liver transplants[36]. The impact of HLA-A and HLA-DR incompatibility on cytomegalovirus reactivation and sepsis were significant but with very low significance and were not conclusive.
There is very little published and consistent literature on KIR compatibility, particularly in liver transplantation[10,16,32,37-41]. From more recent authorship, we know that the incidence of acute rejection does not correlate with HLA compatibility nor with KIR alleles or genotypes of the recipient, but the frequency of C2+ donors did increase in the rejection group and was more frequent when the recipient expressed KIR2DS4[39].
In another study, grafts from donors without HLA-C2 alleles produced more rejection than in recipients from donors with at least one HLA-C2 allele[42], consistent with a previous study of ours[32], which showed that HLA-C2 homozygotes receiving HLA-C1/C2 grafts had a higher risk of rejection than HLA-C1 homozygotes. Other groups, however, did not find this association in their series[38], so the issue is still under open debate.
In conclusion, after demonstrating the adverse effects of DSAs with high MFI, perhaps pretransplant virtual cross match protocols could be appropriate to improve evolution, although they could increase cold ischemia times by having to type the donor. Although today, there is no particular problem as the times of typing results have been shortened, which also allows the optimization of compatibility and HLA and KIR genotypes[15,43].
In our modest opinion, monitoring of dnDSAs should also be universally adopted in all transplant centers to avoid possible post-transplant complications as much as possible. More extensive cohort studies, including the MFI intensity of each DSA in the donor, the role of the different HLA and KIR compatibility, and particular combinations between donor and recipient, are needed to clarify their actual role in the post-transplant period.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Transplantation
Country/Territory of origin: Spain
Peer-review report's scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Gupta R, India; Sugawara Y, Japan S-Editor: Zhang H L-Editor: Filipodia P-Editor: Zhang H
| 1. | Donaldson PT, Thomson LJ, Heads A, Underhill JA, Vaughan RW, Rolando N, Williams R. IgG donor-specific crossmatches are not associated with graft rejection or poor graft survival after liver transplantation. An assessment by cytotoxicity and flow cytometry. Transplantation. 1995;60:1016-1023. [PubMed] |
| 2. | Muro M, Moya-Quiles MR, Mrowiec A. Humoral Response in Liver Allograft Transplantation: A Review of the Role of Anti-Human Leukocyte Antigen (HLA) Antibodies. Curr Protein Pept Sci. 2016;17:776-784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 3. | Boix F, Mrowiec A, Muro M. Cytokine Expression Profile as Predictive Surrogate Biomarkers for Clinical Events in the Field of Solid Organ Transplantation. Curr Protein Pept Sci. 2017;18:240-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 4. | Millán O, Rafael-Valdivia L, San Segundo D, Boix F, Castro-Panete MJ, López-Hoyos M, Muro M, Valero-Hervás D, Rimola A, Navasa M, Muñoz P, Miras M, Andrés A, Guirado L, Pascual J, Brunet M. Should IFN-γ, IL-17 and IL-2 be considered predictive biomarkers of acute rejection in liver and kidney transplant? Clin Immunol. 2014;154:141-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 55] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 5. | Boix-Giner F, Millan O, San Segundo D, Muñoz-Cacho P, Mancebo E, Llorente S, Rafael-Valdivia L, Rimola A, Fábrega E, Mrowiec A, Allende L, Minguela A, Bolarín JM, Paz-Artal E, López-Hoyos M, Brunet M, Muro M. High frequency of central memory regulatory T cells allows detection of liver recipients at risk of early acute rejection within the first month after transplantation. Int Immunol. 2016;28:55-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 6. | Boix F, Bolarín JM, Mrowiec A, Eguía J, Gonzalez-Martinez G, de la Peña J, Galian JA, Alfaro R, Moya-Quiles MR, Legaz I, Campillo JA, Ramírez P, García-Alonso A, Pons JA, Sánchez-Bueno F, Minguela A, Llorente S, Muro M. CD28 biomarker quantification and expression level profiles in CD4+ T-lymphocytes in solid organ transplantation. Transpl Immunol. 2017;42:9-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 7. | Boix F, Millan O, San Segundo D, Mancebo E, Rimola A, Fabrega E, Fortuna V, Mrowiec A, Castro-Panete MJ, Peña Jde L, Llorente S, Minguela A, Bolarin JM, Paz-Artal E, Lopez-Hoyos M, Brunet M, Muro M. High expression of CD38, CD69, CD95 and CD154 biomarkers in cultured peripheral T lymphocytes correlates with an increased risk of acute rejection in liver allograft recipients. Immunobiology. 2016;221:595-603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 8. | Boix F, Trujillo C, Muro M. Cell-Mediated Immunity (CMI) as the Instrument to Assess the Response Against the Allograft: Present and Future. Curr Protein Pept Sci. 2018;19:1092-1106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 9. | Marín LA, Muro M, Moya-Quiles MR, Miras M, Minguela A, Bermejo J, Sanchez-Bueno F, Parrilla P, Alvarez-López MR. Study of Fas (CD95) and FasL (CD178) polymorphisms in liver transplant recipients. Tissue Antigens. 2006;67:117-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 10. | Moya-Quiles MR, Muro M, Torío A, Sánchez-Bueno F, Miras M, Marín L, García-Alonso AM, Parrilla P, Dausset J, Alvarez-López MR. Human leukocyte antigen-C in short- and long-term liver graft acceptance. Liver Transpl. 2003;9:218-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 11. | Legaz I, López-Álvarez MR, Campillo JA, Moya-Quiles MR, Bolarín JM, de la Peña J, Salgado G, Gimeno L, García-Alonso AM, Muro M, Miras M, Alonso C, Álvarez-López MR, Minguela A. KIR gene mismatching and KIR/C ligands in liver transplantation: consequences for short-term liver allograft injury. Transplantation. 2013;95:1037-1044. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 34] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 12. | Tait BD, Süsal C, Gebel HM, Nickerson PW, Zachary AA, Claas FH, Reed EF, Bray RA, Campbell P, Chapman JR, Coates PT, Colvin RB, Cozzi E, Doxiadis II, Fuggle SV, Gill J, Glotz D, Lachmann N, Mohanakumar T, Suciu-Foca N, Sumitran-Holgersson S, Tanabe K, Taylor CJ, Tyan DB, Webster A, Zeevi A, Opelz G. Consensus guidelines on the testing and clinical management issues associated with HLA and non-HLA antibodies in transplantation. Transplantation. 2013;95:19-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 576] [Cited by in RCA: 621] [Article Influence: 51.8] [Reference Citation Analysis (0)] |
| 13. | Tambur AR, Herrera ND, Haarberg KM, Cusick MF, Gordon RA, Leventhal JR, Friedewald JJ, Glotz D. Assessing Antibody Strength: Comparison of MFI, C1q, and Titer Information. Am J Transplant. 2015;15:2421-2430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 213] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 14. | Wesson RN, Etchill EW, Garonzik-Wang J. Application and interpretation of histocompatibility data in liver transplantation. Curr Opin Organ Transplant. 2017;22:499-504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 15. | Legaz I, Boix F, López M, Alfaro R, Galián JA, Llorente S, Campillo JA, Botella C, Ramírez P, Sánchez-Bueno F, Pons JA, Moya-Quiles MR, Minguela A, Muro M. Influence of Preformed Antibodies in Liver Transplantation. J Clin Med. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 16. | Goto R, Ito M, Kawamura N, Watanabe M, Ganchiku Y, Kamiyama T, Shimamura T, Taketomi A. The impact of preformed donor-specific antibodies in living donor liver transplantation according to graft volume. Immun Inflamm Dis. 2022;10:e586. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 17. | Ramon DS, Troop DM, Kinard TN, Jadlowiec CC, Ryan MS, Hewitt WR Jr, Olsen LG, Jaramillo A, Taner T, Heilman RL. Alloantibodies after simultaneous liver-kidney transplant: A story of primary nonfunction, retransplantation, and antibody-mediated rejection. Am J Transplant. 2022;22:977-985. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 18. | Cuadrado A, San Segundo D, López-Hoyos M, Crespo J, Fábrega E. Clinical significance of donor-specific human leukocyte antigen antibodies in liver transplantation. World J Gastroenterol. 2015;21:11016-11026. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 30] [Cited by in RCA: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 19. | Kozlowski T, Rubinas T, Nickeleit V, Woosley J, Schmitz J, Collins D, Hayashi P, Passannante A, Andreoni K. Liver allograft antibody-mediated rejection with demonstration of sinusoidal C4d staining and circulating donor-specific antibodies. Liver Transpl. 2011;17:357-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 117] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 20. | Tambur AR, Campbell P, Claas FH, Feng S, Gebel HM, Jackson AM, Mannon RB, Reed EF, Tinckam K, Askar M, Chandraker A, Chang PP, Colvin M, Demetris AJ, Diamond JM, Dipchand AI, Fairchild RL, Ford ML, Friedewald J, Gill RG, Glotz D, Goldberg H, Hachem R, Knechtle S, Kobashigawa J, Levine DJ, Levitsky J, Mengel M, Milford E, Newell KA, O'Leary JG, Palmer S, Randhawa P, Smith J, Snyder L, Starling RC, Sweet S, Taner T, Taylor CJ, Woodle S, Zeevi A, Nickerson P. Sensitization in Transplantation: Assessment of Risk (STAR) 2017 Working Group Meeting Report. Am J Transplant. 2018;18:1604-1614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 224] [Article Influence: 32.0] [Reference Citation Analysis (0)] |
| 21. | Shin S, Lee M, Dente E, Yazigi N, Khan KM, Kaufman SS, Ahn J, Timofeeva OA, Ekong UD. Mismatch epitope load predicts de novo-DSA-free survival in pediatric liver transplantation. Pediatr Transplant. 2022;26:e14251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 22. | Meszaros M, Dubois V, Congy-Jolivet N, Hamada S, Thevenin C, Faure S, Boillot O, Kamar N, Pageaux GP, Del Bello A, Dumortier J. Impact of calcineurin inhibitor-free immunosuppression on de novo donor-specific antibody formation in liver transplant recipients. Liver Int. 2022;42:1132-1143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 23. | Akamatsu N, Hasegawa K, Sakamoto S, Ohdan H, Nakagawa K, Egawa H. Rituximab Desensitization in Liver Transplant Recipients With Preformed Donor-specific HLA Antibodies: A Japanese Nationwide Survey. Transplant Direct. 2021;7:e729. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 24. | Gül-Klein S, Hegermann H, Röhle R, Schmelzle M, Tacke F, Schöning W, Öllinger R, Dziodzio T, Maier P, Plewe JM, Horst D, Sauer IM, Pratschke J, Lachmann N, Eurich D. Donor-Specific Antibodies Against Donor Human Leukocyte Antigen are Associated with Graft Inflammation but Not with Fibrosis Long-Term After Liver Transplantation: An Analysis of Protocol Biopsies. J Inflamm Res. 2021;14:2697-2712. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 25. | Komagome M, Maki A, Nagata R, Masuda W, Kogure R, Mitsui T, Ninomiya R, Akamatsu N, Hasegawa K, Beck Y. Refractory Acute Antibody Mediated Rejection in Liver Transplant After Desensitization of Preformed Donor Specific Antibody-Validity of Bortezomib and Everolimus: A Case Report. Transplant Proc. 2022;54:147-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Reference Citation Analysis (0)] |
| 26. | Goggins WC, Ekser B, Rokop Z, Lutz AJ, Mihaylov P, Mangus RS, Fridell JA, Powelson JA, Kubal CA. Combined liver-kidney transplantation with positive crossmatch: Role of delayed kidney transplantation. Surgery. 2021;170:1240-1247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 27. | Sultani B, Marget M, Briem-Richter A, Herrmann J, Meisner S, Grabhorn EF, Ozga AK, Weidemann S, Herden U, Fischer L, Sterneck M. Presence of donor specific HLA class 2 antibodies (DSA class 2) is associated with development of graft fibrosis more than 10 years after liver transplantation-a retrospective single center study. Clin Transplant. 2021;35:e14336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 28. | Nakamura T, Shirouzu T, Sugimoto R, Harada S, Yoshikawa M, Nobori S, Ushigome H, Kawai S. Intra-Liver Allograft C3d-Binding Donor-Specific Anti-HLA Antibodies Predict Rejection After Liver Transplantation. Transplant Proc. 2022;54:450-453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 29. | Vionnet J, Sempoux C, Pascual M, Sánchez-Fueyo A, Colmenero J. Donor-specific antibodies in liver transplantation. Gastroenterol Hepatol. 2020;43:34-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 30. | Liu W, Wang K, Xiao YL, Liu C, Gao W, Li DH. Clinical relevance of donor-specific human leukocyte antigen antibodies after pediatric liver transplantation. Exp Ther Med. 2021;22:867. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 31. | Götz JK, Kiene H, Goldschmidt I, Junge N, Pfister ED, Leiskau C, Brown RM, Immenschuh S, Baumann U. Current Evidence on the Clinical Relevance of Donor-specific Antibodies in Paediatric Liver Transplantation. J Pediatr Gastroenterol Nutr. 2021;72:788-793. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 32. | López-Alvarez MR, Moya-Quiles MR, Minguela A, Gil J, Miras M, Campillo JA, Díaz-Alderete MA, García-Alonso AM, Sánchez-Bueno F, Vicario JL, Muro M, Alvarez-López MR. HLA-C matching and liver transplants: donor-recipient genotypes influence early outcome and CD8+KIR2D+ T-cells recuperation. Transplantation. 2009;88:S54-S61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 33. | Ono K, Ide K, Tanaka Y, Ohira M, Tahara H, Tanimine N, Yamane H, Ohdan H. Molecular Mismatch Predicts T Cell-Mediated Rejection and De Novo Donor-Specific Antibody Formation After Living Donor Liver Transplantation. Liver Transpl. 2021;27:1592-1602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 34. | Tajima T, Hata K, Kusakabe J, Miyauchi H, Yurugi K, Hishida R, Ogawa E, Okamoto T, Sonoda M, Kageyama S, Zhao X, Ito T, Seo S, Okajima H, Nagao M, Haga H, Uemoto S, Hatano E. The impact of human leukocyte antigen mismatch on recipient outcomes in living-donor liver transplantation. Liver Transpl. 2022;28:1588-1602. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 35. | Bricogne C, Halliday N, Fernando R, Tsochatzis EA, Davidson BR, Harber M, Westbrook RH. Donor-recipient human leukocyte antigen A mismatching is associated with hepatic artery thrombosis, sepsis, graft loss, and reduced survival after liver transplant. Liver Transpl. 2022;28:1306-1320. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 36. | Mittal S, Sinha P, Sarin S, Rastogi A, Gupta E, Bajpai M, Pamecha V, Trehanpati N. Impact of human leukocyte antigen compatibility on outcomes of living donor liver transplantation: Experience from a tertiary care center. Transpl Infect Dis. 2021;23:e13644. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 37. | Zamir MR, Shahi A, Salehi S, Amirzargar A. Natural killer cells and killer cell immunoglobulin-like receptors in solid organ transplantation: Protectors or opponents? Transplant Rev (Orlando). 2022;36:100723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 38. | Moroso V, van der Meer A, Tilanus HW, Kazemier G, van der Laan LJ, Metselaar HJ, Joosten I, Kwekkeboom J. Donor and recipient HLA/KIR genotypes do not predict liver transplantation outcome. Transpl Int. 2011;24:932-942. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 39. | Chen R, Yi H, Zhen J, Fan M, Xiao L, Yu Q, Yang Z, Ning L, Deng Z, Chen G. Donor with HLA-C2 is associated with acute rejection following liver transplantation in Southern Chinese. HLA. 2022;100:133-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 40. | López-Alvarez MR, Gómez-Mateo J, Ruiz-Merino G, Campillo JA, Miras M, García-Alonso AM, Sánchez-Bueno F, Parrilla P, Alvarez-López MR, Minguela A. Analysis of KIR2D receptors on peripheral blood lymphocytes from liver graft recipients. Transpl Immunol. 2006;17:51-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 41. | Legaz I, Navarro-Noguera E, Bolarín JM, García-Alonso AM, Luna Maldonado A, Mrowiec A, Campillo JA, Gimeno L, Moya-Quiles R, Álvarez-López Mdel R, Minguela Puras A, Miras M, Sánchez-Bueno F, Muro M. Epidemiology, Evolution, and Long-Term Survival of Alcoholic Cirrhosis Patients Submitted to Liver Transplantation in Southeastern Spain. Alcohol Clin Exp Res. 2016;40:794-805. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 42. | Lee H, Park KH, Park HS, Ryu JH, Lim J, Kim Y, Na GH, Kim DG, Oh EJ. Human Leukocyte Antigen-C Genotype and Killer Immunoglobulin-like Receptor-Ligand Matching in Korean Living Donor Liver Transplantation. Ann Lab Med. 2017;37:45-52. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 43. | Muro M. The endless history or search for the true role of alloantibodies in liver transplantation. Clin Res Hepatol Gastroenterol. 2021;45:101544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |