Published online Dec 28, 2023. doi: 10.3748/wjg.v29.i48.6222
Peer-review started: September 22, 2023
First decision: November 20, 2023
Revised: November 27, 2023
Accepted: December 14, 2023
Article in press: December 14, 2023
Published online: December 28, 2023
Processing time: 95 Days and 16.4 Hours
Ulcerative colitis (UC) is a chronic gastrointestinal disorder characterized by inflammation and ulceration, representing a significant predisposition to colorectal cancer. Recent advances in single-cell RNA sequencing (scRNA-seq) technology offer a promising avenue for dissecting the complex cellular inter-actions and molecular signatures driving UC pathology.
To utilize scRNA-seq technology to dissect the complex cellular interactions and molecular signatures that underlie UC pathology.
In this research, we integrated and analyzed the scRNA-seq data from UC patients. Moreover, we conducted mRNA and protein level assays as well as pathology-related staining tests on clinical patient samples.
In this study, we identified the sustained upregulation of inflammatory response pathways during UC progression, characterized the features of damaged endo-thelial cells in colitis. Furthermore, we uncovered the downregulation of phospholysine phosphohistidine inorganic pyrophosphate phosphatase (LHPP) has a negative correlation with signal transducer and activator of transcription 3. Significant downregulation of LHPP in UC patient tissues and plasma suggests that LHPP may serve as a potential therapeutic target for UC. This paper highlights the importance of LHPP as a potential key target in UC and unveils its potential role in inflammation regulation.
The findings suggest that LHPP may serve as a potential therapeutic target for UC, emphasizing its importance as a potential key target in UC and unveiling its role in inflammation regulation.
Core Tip: Ulcerative colitis (UC), a chronic inflammatory bowel disease linked to colorectal cancer, was investigated using single-cell RNA sequencing technology. The study unveiled sustained upregulation of inflammatory response pathways and characterized damaged endothelial cells during UC progression. Notably, the downregulation of phospholysine phosphohistidine inorganic pyrophosphate phosphatase (LHPP) exhibited a negative correlation with signal transducer and activator of transcription 3. LHPP's significant downregulation in UC patient tissues and plasma suggests its potential as a therapeutic target. The findings highlight LHPP as a key target in UC and emphasize its role in inflammation regulation, offering insights for potential therapeutic interventions.
- Citation: Wang YF, He RY, Xu C, Li XL, Cao YF. Single-cell analysis identifies phospholysine phosphohistidine inorganic pyrophosphate phosphatase as a target in ulcerative colitis. World J Gastroenterol 2023; 29(48): 6222-6234
- URL: https://www.wjgnet.com/1007-9327/full/v29/i48/6222.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i48.6222
Ulcerative colitis (UC) is a chronic inflammatory bowel disease (IBD) characterized by mucosal inflammation and ulceration primarily affecting the colon and rectum[1]. It is a multifactorial[2,3]. Individuals with UC often experience debilitating symptoms such as abdominal pain, bloody diarrhea, diarrhea, rectal bleeding, and an increased risk of colorectal cancer (CRC), severely impacting their quality of life. Extensive research over the years has yielded valuable insights into the pathogenesis of UC, highlighting the importance of aberrant immune responses and the gut microbiome in disease development and progression[4-6]. However, a comprehensive understanding of the cellular and molecular mechanisms underlying UC development and progression remains elusive, and there is a growing need for more precise and targeted therapeutic interventions[7].
Recent advances in single-cell RNA sequencing (scRNA-seq) technology have revolutionized our ability to dissect the heterogeneity of cell populations within complex tissues[8-10]. This revolutionary technique empowers researchers to discern distinct cell subsets, unveil novel cellular pathways, and delve into the dynamics of immune responses with unparalleled resolution[11,12]. In the context of UC, scRNA-seq offers a unique opportunity to decipher the intricate cellular interactions and molecular signatures driving disease pathology[13-17].
Phospholysine phosphohistidine inorganic pyrophosphate phosphatase (LHPP), a histidine phosphatase protein, has been implicated in diverse biological processes, including tumor suppression in hepatocellular carcinoma[18], increasing the expression of cleaved-poly (ADP-ribose) polymerase and cleaved-Casp3 protein to promote apoptosis[19], and inflammation regulation and immune response modulation. Recent studies have suggested its potential correlation with survival of CRC patients[20]. Another study reports that the loss of LHPP in intestinal epithelial cells correlate with colitis in mice, suggests the involvement of LHPP in IBD[21,22]. However, the precise involvement and impact of LHPP in the context of UC remain an unexplored territory, offering a promising avenue for further investigation.
In this study, we integrated the current single-cell sequencing data of UC[15,16], shedding light on the specific cell types, transcriptional profiles, and immune signaling pathways that play pivotal roles in UC pathogenesis. By interrogating the single-cell landscape of UC-affected colonic tissues, we identified sustained upregulation of inflammatory response pathways during the progression of UC and characterized the features of damaged endothelial cells (EC) in colitis. Through integrated analysis of UC disease databases utilizing single-cell data, we uncovered that LHPP exhibits sustained downregulation in UC, displaying a negative correlation with signal transducer and activator of transcription 3 (STAT3). Notably, our experimental results in UC patients corroborated that when STAT3 expression is upregulated, LHPP expression is downregulated, suggesting a potential role for STAT3 in transcriptionally inhibiting LHPP expression. Furthermore, LHPP not only exhibited decreased expression in the intestinal tissue of UC patients but also displayed reduced expression in their plasma samples. This observation suggests that LHPP may serve as a critical factor in the pathogenesis of UC.
The raw data of healthy and UC human clonal tissues were from GSE125527 in GEO database. The R package Seurat (version 4.0.2) was used for construction of cell atlas of human clonal tissues. In brief, the function “CreateSeuratObject” was used to load gene expression matrix of each sample. The function ‘SCTransfrom’ was used for finding high variable genes, normalization and scaling of the gene expression matrix for each sample, the ‘PrepSCTIntegration’ and ‘FindIntegrationAnchors’ functions were used for selecting the anchors for integration all samples. The function “IntegrateData” was used for the following integration. After integration, the function “ScaleData” was used to scale the integrated expression matrix, then the principle component analysis and Dimensionality reduction of dataset were performed by the functions “RunPCA” and “RunUMAP”, the functions “FindNeighbors” and “FindClusters” were used to cell clustering and identification. The function ‘FindAllMarkers’ (|avg_log2FC| ≥ 0.5 and p_val_adj ≤ 0.05) was used to calculate marker genes for each cell type.
The ‘FindMarkers’ function in Seurat was used to identify differentially expressed genes in diseased vs healthy group (diseased/healthy) of each cell type, which were based on normalized data and the Wilcoxon test. The screening criteria for significantly differentially expressed genes were selected by BH-adjusted P value < 0.05 and |log2FC| > 0.25.
To characterize the mobilization and of EC during UC, the R package Monocle2 was used to perform pseudotime trajectory inference for EC of healthy and UC tissues. The top 3000 high variable genes were used to calculate the pseudotime. The functions “plot_pseudotime_heatmap” and “plot_genes_in_pseudotime” were used to perform time-related gene analysis.
The gene sets were downloaded from MSigDB (https://www.gsea-msigdb.org/gsea) and the ‘AddModuleScore’ function of Seurat was used to calculate gene set scores.
The biopsy samples were collected from 12 heathy control subjects and 15 UC patients from the Department of Gastroenterology, The Third Affiliated Hospital of Zhejiang Chinese Medical University (Hangzhou, China, ZSLL-KY-2023-031-01). The protocol was approved by the Institutional Ethics Committee of The Third Affiliated Hospital of Zhejiang Chinese Medical University and was in concordance with the Helsinki Declaration. Biopsy specimens were collected and washed twice with phosphate-buffered saline, then frozen by liquid nitrogen.
Colonic samples were subjected to physical homogenization and total RNA extracted by TRIzol Reagent (Invitrogen). Then cDNA was synthesized using the high-capacity reverse transcription kit (Thermo Fisher) following the manufacturer’s instructions. The qPCR reactions were performed using TaqMan gene expression and assays on ABI QuantStudio 5 (Applied Biosystems, Thermo-Fisher).
Tissues were lysed using SDS lysis buffer (containing 100 mmol/L Tris-HCl (pH = 7.0), 1% SDS and 2% 2-mercaptoethanol, supplemented with 1 x protease inhibitors from Roche) and incubated at 105 ℃ for 10 min. Total protein was extracted and quantified using the BCA Kit (Abcam). Equal amounts proteins were subjected to SDS-PAGE electrophoresis and subsequently transferred onto PVDF membranes (Millipore). After blocking with 5% skim milk, the membranes were treated overnight at 4 ℃ with the following antibodies: Mouse monoclonal antibody against GAPDH (1: 2000 dilution; 0411; sc47724); Rabbit monoclonal antibody against STAT3 (1: 1000 dilution; abcam; ab68153). Rabbit monoclonal antibody against STAT3 (1: 500 dilution; abcam; ab254788). Following incubation with HRP-conjugated secondary antibodies, the PVDF membrane was visualized using an enhanced chemiluminescence (ECL) kit (Thermo).
The protein levels of IL6 (Biolegend) and LHPP (Abbexa) in the blood were determined using ELISA according to the manufacturer’s instructions. Following incubation with the detection kit, the plate was analyzed at 450 nm using a chemiluminescence immunoassay system (Dxl800Access, Beckman, United States).
All data were statistical analyzed using GraphPad Prism software version 8.0. The data were presented as the mean ± SEM. Comparisons were conducted using the two-tailed student's t test or one-way ANOVA. P values < 0.05 were considered statistically significant.
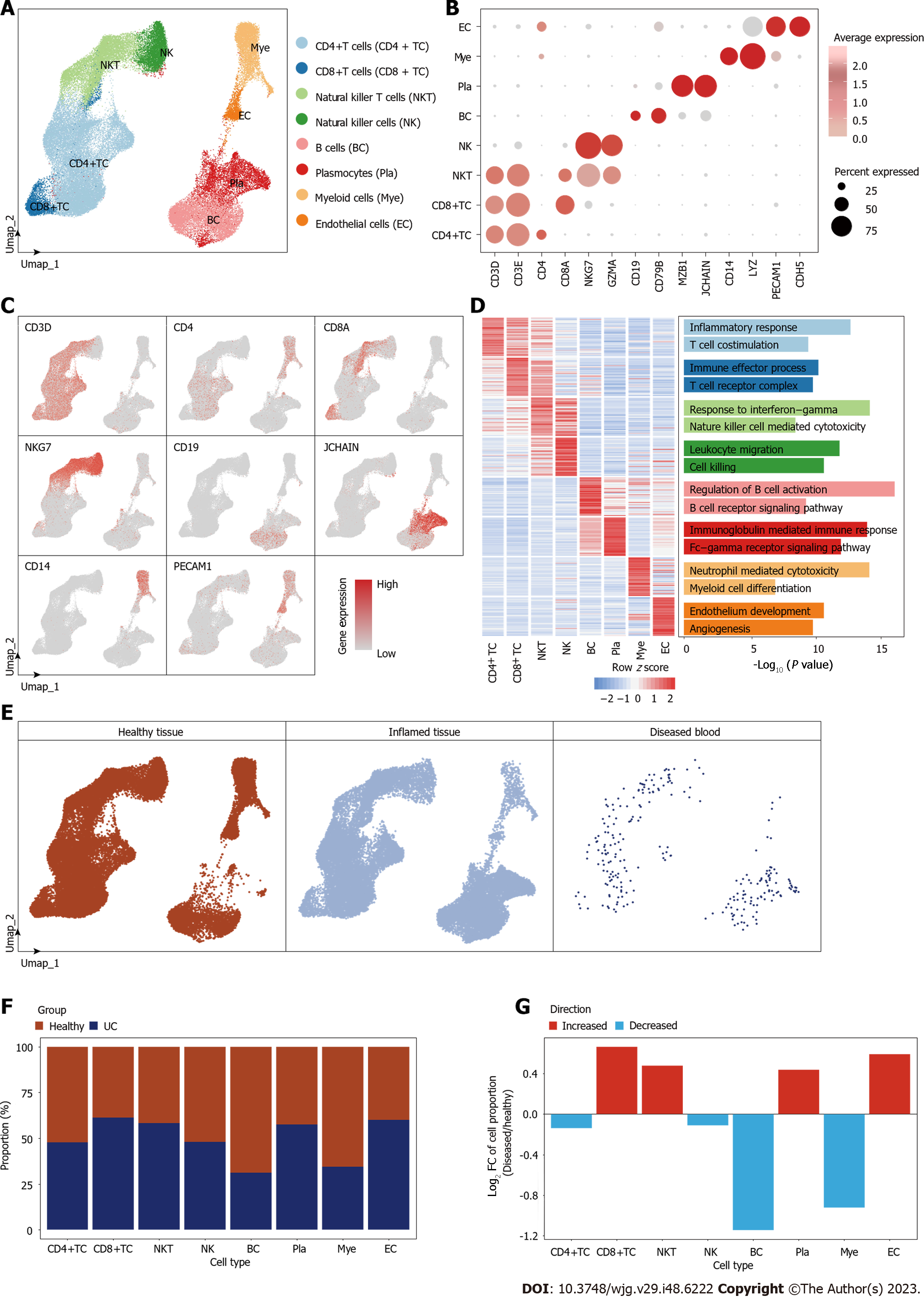
To systematically elucidate the microenvironmental changes occurring during the development of UC, we conducted an analysis utilizing publicly available single-cell datasets from healthy individuals and UC patients. Following quality control (Supplementary Figures 1 and 2), a total of 64643 high quality cells were filtered from healthy (n = 8) and UC (n = 8) individuals, we identified eight major cell types, which were CD4+ T cells (37.44%), CD8+ T cells (4.70%), natural killer T cells (NKT) (15.00%), natural killer cells (7.98%), B cells (13.81%), myeloid immune cells (8.80%), and EC (2.45%) (Figure 1A, Supplementary Figure 1). The accuracy of cell identification was confirmed by the expression of canonical marker genes and top marker gene annotation of each cell type (Figure 1B-D). The distribution between healthy and inflamed tissues indicated that there is no specific cell types generated during the inflammatory process (Figure 1E, Supplementary Figure 2).
Regarding alterations in cellular composition, we observed that during UC development, the proportions of CD8+ T cells and NKT cells were increased (Figure 1F and G). This suggests a pronounced activation response by the host immune system in the face of inflammation, mobilizing these immune cell populations to target and eliminate diseased cells. The heightened presence of CD8+ T cells, renowned for their cytotoxic capabilities, signifies a concerted effort by the immune system to target and eliminate inflamed UC tissues. NKT cells, with their unique properties bridging the innate and adaptive immune responses, may also play a crucial role in anti-inflammatory. This bolstering of cytotoxic immune cells within the colon microenvironment is an encouraging sign, suggesting that the immune system is actively engaged in combating the UC. However, the observed decline in the proportions of CD4+ T cells and B cells is a matter of concern (Figure 1F and G). CD4+ T cells play a vital role in mediating diverse immune responses, including antigen presentation to cytotoxic CD8+ T cells and regulation of immune tolerance. A reduction in their presence could suggest a potential weakening of the immune system's ability to recognize inflammatory cells. Similarly, B cells, which are instrumental in antibody production and antigen presentation, also exhibited diminishing proportions. This decline might impede the cytotoxic ability against UC of CD8+ T and NKT cells. The reduction in CD4+ T cells and B cells might be indicative of an evolving immune evasion strategy employed by UC cells, where the colon microenvironment becomes less conducive to an effective immune response and gains a tendency of the chronic, long-term inflammation.
Concurrently, we observed a progressive increase in the proportion of ECs within the colon microenvironment (Figure 1F and G). This phenomenon underscored the significant role of angiogenesis in the UC’s progression. The increasing angiogenesis has been proved a strong link with inflammation in various inflammatory diseases. Angiogenesis and inflammation cooperative with each other, and hypoxia acts as a common stimulus for both[23]. Furthermore, a robust vascular network can facilitate the intravasation and extravasation of inflammation factors, enabling metastasis to distant sites within the body.
These findings offer valuable insights into the alterations taking place within the microenvironment during the progression of UC. The body responds to inflammation through a series of immune cell activities. The augmented angiogenesis underscores the significance of vascularization in the inflammation microenvironment.
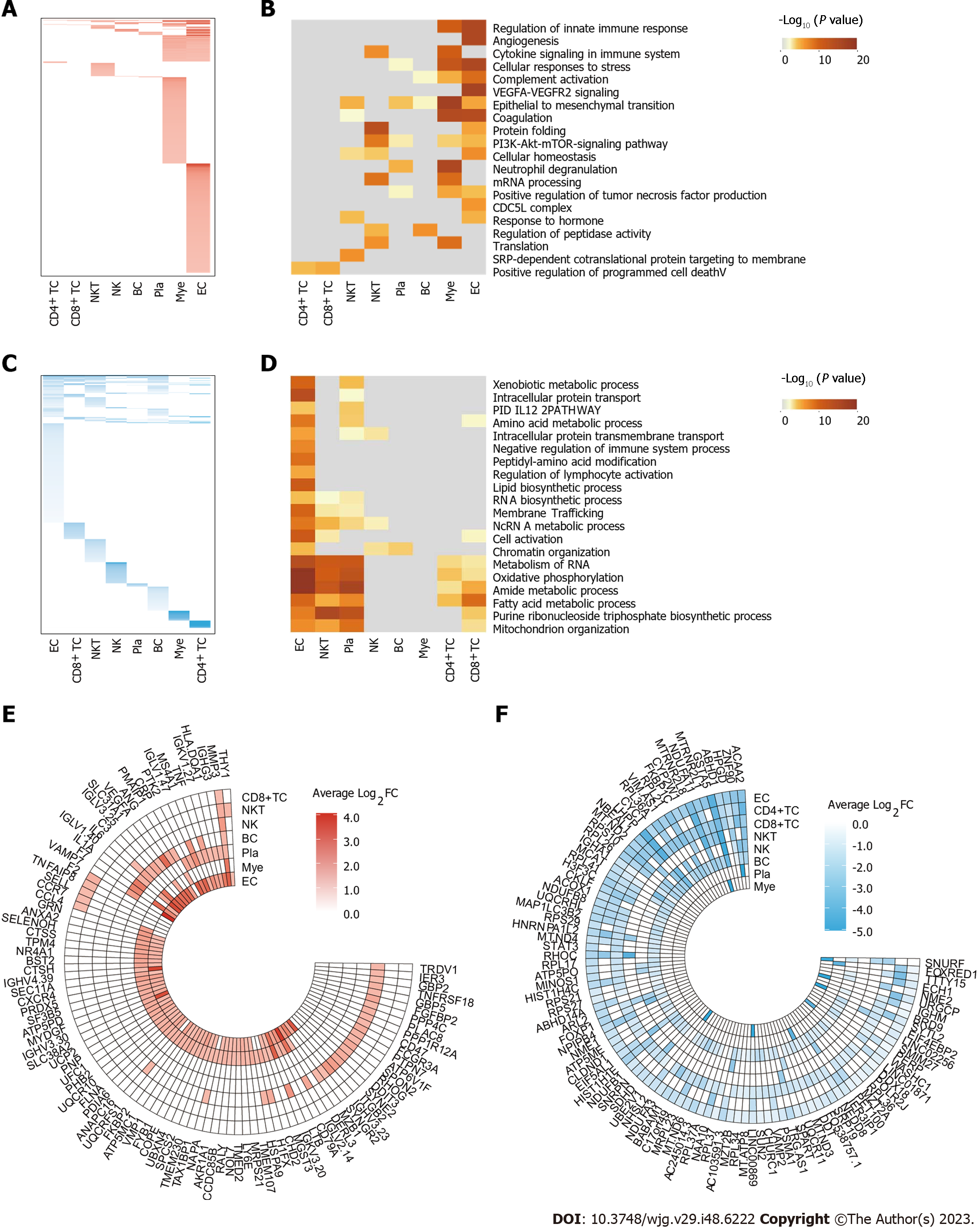
We systematically investigated the differential gene expression profiles across all cell types during the process of UC development. Overall, we identified 706 upregulated and 1471 downregulated differential genes. Notably, ECs exhibited the highest number of both upregulated and downregulated DEGs. These trends in differential gene expression to some extent reflect the inflammatory response of these cells during the progression of UC (Figure 2A-D).
Upon examining the upregulated differential genes within each cell type, we identified a substantial association with the regulation of the innate immune response, such as "regulation of innate immune response", "cytokine Signaling in the Immune system", and "neutrophil degranulation". These findings suggested an elevated immune response and immune cell infiltration within the inflamed tissue. During the UC, the body recruited immune cells to the inflamed tissue for the purpose of clearing infections, necrotic cells, or other pathological cells. Notably, the upregulation of the "epithelial to mesenchymal transition" pathway was also observed, hinting at the possibility that sustained inflammation may induce tissue fibrosis. Additionally, the upregulation of the "Angiogenesis" pathway signifies the presence of neovascularization within the inflamed tissue, which aligns with our previous observation of an increased proportion of ECs. These findings underscored the dynamic response of the body to inflammation, involving the recruitment of immune cells to the site of inflammation, concurrent local tissue fibrosis, and angiogenesis.
In the realm of downregulated genes, a notable decrease in the expression of genes associated with various metabolic processes, including "xenobiotic metabolic process", "oxidative phosphorylation", and "fatty acid metabolic process". These findings indicated that inflammation may lead to a functional decline in affected tissues. Moreover, the downregulation of genes related to "chromatin organization", "cell activation" and "intracellular protein transmembrane transport" indicated alterations in cellular states within the inflamed tissue. These observations collectively imply that inflammation induced substantial changes in cellular function and identity.
These findings of differential gene expression profiles across diverse cell types during UC process has unveiled intriguing insights into the dynamic molecular changes occurring within the inflammation microenvironment, corresponding with the high frequency DEGs across all cell types (Figure 2E and F). The activation of inflammation-related pathways, immune cell recruitment, tissue fibrosis, and angiogenesis collectively reflect the body's response to inflammation. Concurrently, the downregulation of metabolic pathways and changes in cellular state suggest a functional decline within the affected tissues. These differential gene transcription profiles provide a valuable landscape for assisting in the identification of potential therapeutic targets for UC.
Prior research has emphasized the regulatory role of ECs in immune responses, encompassing functions like filtration, endocytosis, antigen presentation, and leukocyte recruitment[24,25]. Aberrant crosstalk between ECs and immune cells were established in injury tissues[26,27]. Furthermore, ECs undergo significant alterations themselves, contributing to inflammation[28]. These findings not only underscore the intricate interplay between ECs and the inflammatory microenvironment but also accentuate the potential of targeting EC-specific mechanisms as a promising avenue for UC therapy.
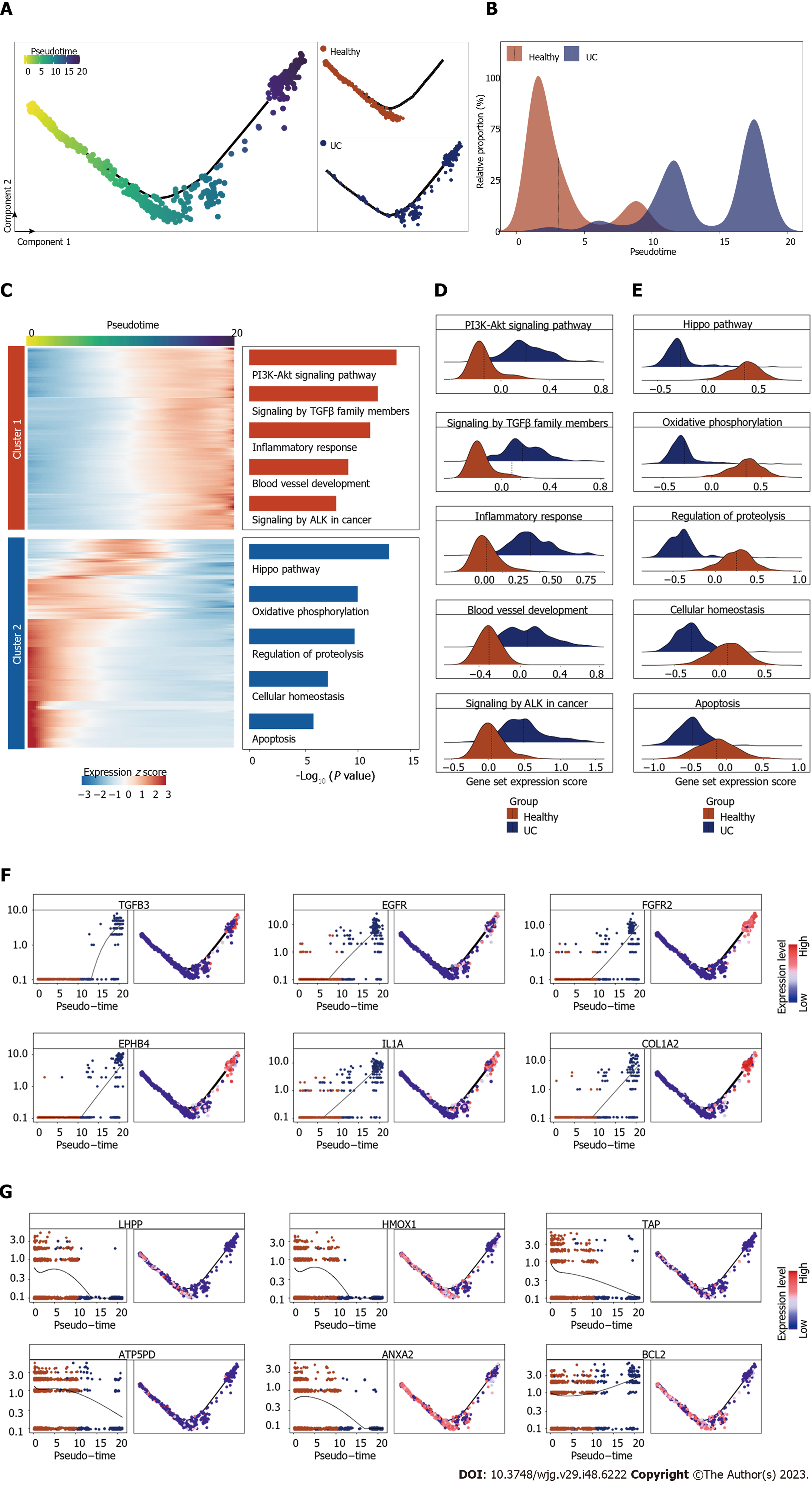
To gain a deeper understanding of the specific cellular changes that ECs undergo during UC, we conducted pseudotime analysis to elucidate the progressive changes in EC states. Notably, we identified a distinct differentiation trajectory from healthy to UC-associated ECs. Healthy ECs were predominantly situated at the front end of the differentiation trajectory, while UC-associated subpopulations were concentrated toward the middle and end, providing compelling evidence that ECs undergo a series of alterations in their cellular states during UC (Figure 3A and B).
We then focused on the transcriptomic profiles alterations during UC by conducting gene expression patterns that gradually evolved along the pseudotime trajectory. These genes were then categorized into two distinct groups based on their expression trends: the cluster 1 displayed increasing expression as pseudotime advanced, while the cluster 2 exhibited decreasing expression (Figure 3C).
The upregulated genes offer insights into the transcriptional alterations occurring in ECs during the inflammatory process. Notably, they were primarily associated with critical signaling pathways, including the phosphatidylinositol-3-kinase-protein kinase B signaling pathway, known as a key regulator in multiple inflammations. the TGF-beta pathway, known for its role in cellular proliferation and differentiation, as well as tumor genesis. The blood vessel development pathway, indicated ECs' active engagement in inflammation-related angiogenesis processes. Additionally, the anaplastic lymphoma kinase pathway in cancer pathway, suggested that ECs possibly implicating them in a potential predisposition towards carcinogenesis (Figure 3C and D). Intriguingly, these observations underscored the active involvement of ECs in angiogenesis and their responsiveness to various signals driving inflammation. Moreover, it hints at a proclivity of ECs themselves towards malignant transformation. Conversely, the downregulated genes were predominantly linked to pathways such as the Hippo signaling pathway, which plays a pivotal role in regulating cell proliferation and organ size. The apoptosis pathway, responsible for programmed cell death and damaged cell clearance. Furthermore, cellular homeostasis pathways, indicated the disorder of cell state (Figure 3C and E). Collectively, the suppression of these pathways suggests that ECs might undergo functional changes, contributing to their altered roles during inflammation progression.
Among genes whose expression exhibited the most significant changes along the pseudotime trajectory, TGFB3, a member of the transforming growth factor-beta family, is well-known for its role in regulating cell growth, differentiation, and immune responses. Its upregulation in ECs implies its potential involvement in the activation of signaling pathways critical for inflammation-related angiogenesis and microenvironment remodeling. Similarly, the upregulation of EGFR and FGFR2 suggests the heightened responsiveness of ECs to growth factor signaling, possibly fueling their angiogenic activities and interaction with inflammation microenvironment. The increased expression of EPHB4, a receptor involved in cell-cell communication and tissue patterning, hints at its role in EC differentiation and response to inflammation. Moreover, the upregulation of ILA and COL1A2 highlights the active participation of ECs in immune responses and extracellular matrix remodeling within the UC microenvironment (Figure 3F). Conversely, we observed a downregulation of LHPP, a phosphatase implicated in cell cycle regulation, may suggest a reduced control over EC proliferation and differentiation during UC progression. HMOX1, a stress-responsive enzyme, exhibited decreased expression, possibly indicating altered oxidative stress responses in ECs within the UC microenvironment. The decreased expression of ATP5PD may implicate alterations in energy metabolism within ECs during inflammation. The downregulation of ANXA2, a protein involved in cellular processes such as membrane trafficking and cell adhesion, suggests potential changes in cell-cell interactions. The downregulation of BCL2 may indicate alterations in apoptosis regulation in ECs within the UC pathological process (Figure 3G).
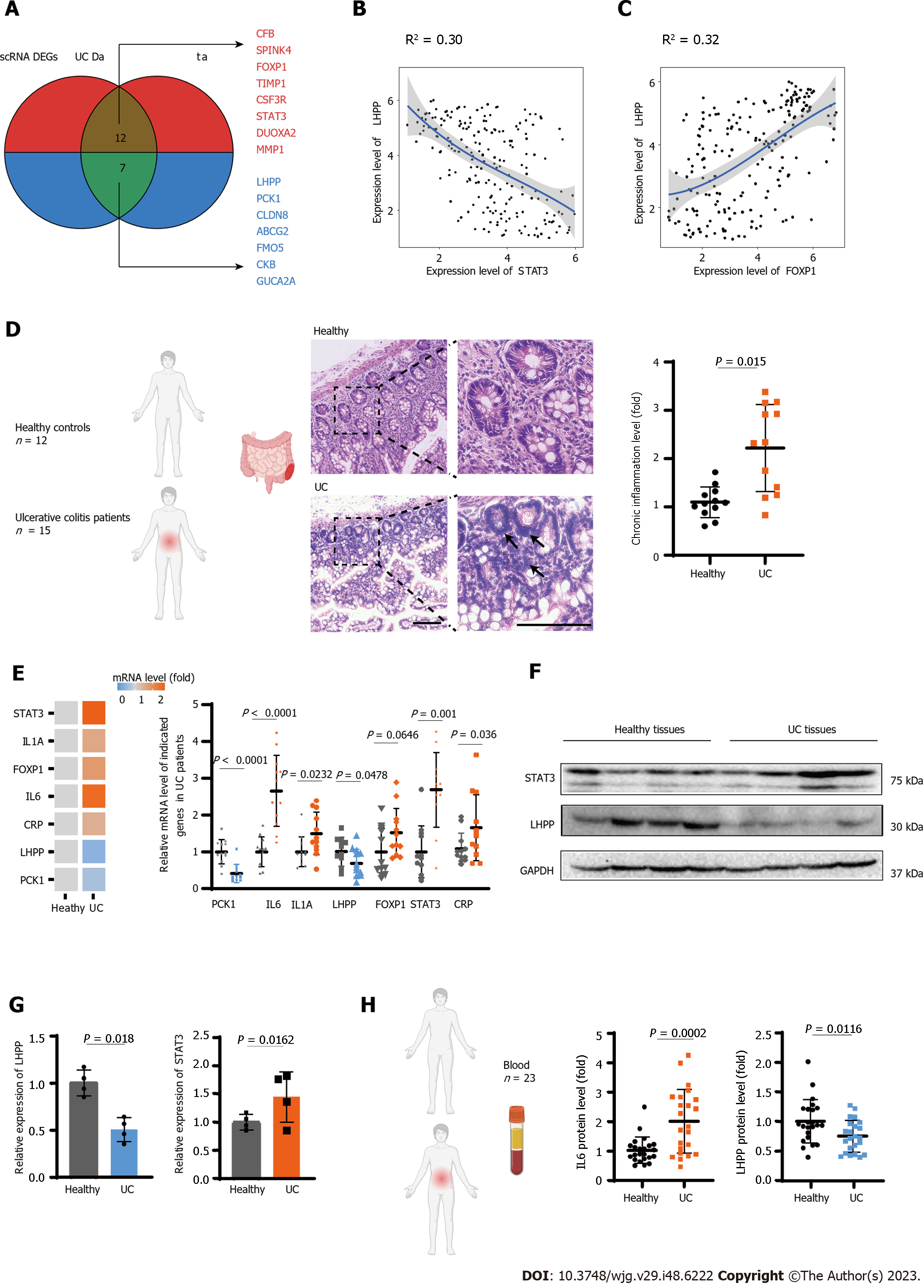
To deepen our insight of the correlation among dysregulated genes in the UC transcriptome profile and uncover key regulator into the molecular mechanisms underlying UC pathogenesis, we conducted an integrated analysis of UC by combining data from disease databases and scRNA-seq DEGs. We identified 13 commonly upregulated genes, including CFB, SPINK4, FOXP1, TIMP1, CSF3R, STAT3, DUOXA2 and MMP1, and 7 downregulated genes (LHPPP, CK1, CLDN8, ABCG2, FMO5, CKB, GUCA2A) across these datasets (Figure 4A). Notably, apart from pathways associated with inflammation, we observed the upregulation of pivotal transcription factors such as STAT3 and FOXP1. Correlation analysis of common regulatory factors revealed a negative association between LHPP and the expression of STAT3 and FOXP1. LHPP, known for its role in cell proliferation and tumor suppression, displayed a significant negative correlation with STAT3 and a less significant correlation with FOXP1 (Figure 4B and C).
To further substantiate our findings, we recruited a cohort of 15 UC patients and 12 healthy controls, from whom we obtained intestinal tissue samples. Histological assessment (HE staining) of these tissue specimens indicated a significantly elevated presence of chronic inflammation in UC patients (P < 0.05; Figure 4D). Subsequent qPCR analysis of these tissue specimens demonstrated elevated mRNA expression levels of inflammation-related genes, including IL6, IL1A, CRP, consistent with our preceding analyses (Figures 2A and C). Notably, in comparison to the healthy control group, we observed a significant reduction in LHPP mRNA expression and an increase in STAT3 expression within the UC patient group (P < 0.05), although the increase in FOXP1 expression did not reach statistical significances (Figure 4E). Furthermore, western blot analysis of the enrolled individuals provided further confirmation, showing a substantial decrease in LHPP expression and an increase in STAT3 expression among UC patients (P < 0.05; Figure 4F and G). These findings suggest a potential transcriptional inhibitory role of STAT3 on LHPP expression.
Finally, we conducted ELISA testing on blood samples collected from 23 matched pairs of healthy individuals and UC patients. Our analysis revealed a noteworthy elevation in IL6 levels among UC patients, underscoring the systemic inflammatory effects associated with UC (P < 0.01; Figure 4H). Furthermore, the diminished levels of plasma LHPP in UC patients hint at its potential as a predictive biomarker for UC (P < 0.05; Figure 4H). These findings emphasize the systemic impact of UC-related inflammation and point towards the promising prospect of utilizing LHPP as a potential predictive marker for this condition.
UC is a complex and chronic IBD that predominantly affects the colon and rectum. Despite significant progress in understanding the pathogenesis of UC, there remains a need for more precise and targeted therapeutic interventions. Our study leveraged scRNA-seq technology to delve into the intricate cellular and molecular landscape of UC. We observed a notable upregulation of inflammatory response pathways during UC progression, indicating an active immune response against inflamed tissues. The increased presence of cytotoxic CD8+ T cells and NKT cells suggests an encouraging antitumor response, but the declining proportions of CD4+ T cells and B cells raise concerns about potential immune evasion strategies employed by UC cells.
A particularly intriguing finding in our study was the sustained downregulation of LHPP in UC, coupled with its negative correlation with STAT3. Our experimental data supported the notion that STAT3 may transcriptionally inhibit LHPP expression, both in intestinal tissue and plasma samples of UC patients. LHPP's multifaceted roles in tumor suppression, apoptosis regulation, and immune response modulation make it a promising candidate for further investigation in the context of UC pathogenesis.
Moreover, the heightened angiogenesis observed in UC underscores the importance of vascularization in disease progression. Enhanced vascularization provides UC cells with access to vital nutrients and oxygen, facilitating their survival and proliferation. This finding may open avenues for targeted therapies aimed at disrupting angiogenic processes in UC.
In summary, our study sheds light on the cellular and molecular mechanisms driving UC pathogenesis. These insights provide a foundation for future research into potential therapeutic interventions, with LHPP emerging as a key player in UC regulation and the immune response. Ultimately, unraveling the complexities of UC at the single-cell level holds promise for more effective treatments and improved outcomes for patients with this challenging condition.
This study suggests that LHPP may serve as a potential therapeutic target for UC, emphasizing its importance as a potential key target in UC and unveiling its role in inflammation regulation.
Ulcerative colitis (UC) is a chronic intestinal condition characterized by inflammation and ulceration, and it is a significant risk factor for colorectal cancer. Recent advances in single-cell RNA sequencing (scRNA-seq) technology offer a promising avenue for dissecting the complex cellular inter-actions and molecular signatures driving UC pathology.
A comprehensive understanding of the cellular and molecular mechanisms underlying UC development and progression remains elusive, and there is a growing need for more precise and targeted therapeutic interventions.
The object of this study is to utilize scRNA-seq technology to dissect the complex cellular interactions and molecular signatures that underlie UC pathology.
We integrated and analyzed the scRNA-seq data from UC patients. Moreover, we conducted mRNA and protein level assays as well as pathology-related staining tests on clinical patient samples.
We identified the sustained upregulation of inflammatory response pathways during UC progression, characterized the features of damaged endothelial cells in colitis. Furthermore, we uncovered the downregulation of phospholysine phosphohistidine inorganic pyrophosphate phosphatase (LHPP) has a negative correlation with signal transducer and activator of transcription 3. Significant downregulation of LHPP in UC patient tissues and plasma suggests that LHPP may serve as a potential therapeutic target for UC. This paper highlights the importance of LHPP as a potential key target in UC and unveils its potential role in inflammation regulation.
This study suggests that LHPP may serve as a potential therapeutic target for UC, emphasizing its importance as a potential key target in UC and unveiling its role in inflammation regulation.
This study sheds light on the cellular and molecular mechanisms driving UC pathogenesis. These insights provide a foundation for future research into potential therapeutic interventions, with LHPP emerging as a key player in UC regulation and the immune response. Ultimately, unraveling the complexities of UC at the single-cell level holds promise for more effective treatments and improved outcomes for patients with this challenging condition.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A, A
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Sheibani M, Iran; Sipos F, Hungary; Tanaka T, Japan S-Editor: Qu XL L-Editor: A P-Editor: Chen YX
| 1. | de Souza HSP, Fiocchi C, Iliopoulos D. The IBD interactome: an integrated view of aetiology, pathogenesis and therapy. Nat Rev Gastroenterol Hepatol. 2017;14:739-749. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 335] [Cited by in RCA: 316] [Article Influence: 39.5] [Reference Citation Analysis (0)] |
| 2. | Ungaro R, Mehandru S, Allen PB, Peyrin-Biroulet L, Colombel JF. Ulcerative colitis. Lancet. 2017;389:1756-1770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2199] [Cited by in RCA: 2484] [Article Influence: 310.5] [Reference Citation Analysis (2)] |
| 3. | Rubin DT, Ananthakrishnan AN, Siegel CA, Sauer BG, Long MD. ACG Clinical Guideline: Ulcerative Colitis in Adults. Am J Gastroenterol. 2019;114:384-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 870] [Cited by in RCA: 1042] [Article Influence: 173.7] [Reference Citation Analysis (0)] |
| 4. | Cioffi M, Rosa AD, Serao R, Picone I, Vietri MT. Laboratory markers in ulcerative colitis: Current insights and future advances. World J Gastrointest Pathophysiol. 2015;6:13-22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 67] [Cited by in RCA: 73] [Article Influence: 7.3] [Reference Citation Analysis (3)] |
| 5. | Lasa JS, Olivera PA, Danese S, Peyrin-Biroulet L. Efficacy and safety of biologics and small molecule drugs for patients with moderate-to-severe ulcerative colitis: a systematic review and network meta-analysis. Lancet Gastroenterol Hepatol. 2022;7:161-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 267] [Cited by in RCA: 227] [Article Influence: 75.7] [Reference Citation Analysis (0)] |
| 6. | Farid A, Sheibani M, Shojaii A, Noori M, Motevalian M. Evaluation of anti-inflammatory effects of leaf and seed extracts of Plantago major on acetic acid-induced ulcerative colitis in rats. J Ethnopharmacol. 2022;298:115595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 17] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 7. | Parikh K, Antanaviciute A, Fawkner-Corbett D, Jagielowicz M, Aulicino A, Lagerholm C, Davis S, Kinchen J, Chen HH, Alham NK, Ashley N, Johnson E, Hublitz P, Bao L, Lukomska J, Andev RS, Björklund E, Kessler BM, Fischer R, Goldin R, Koohy H, Simmons A. Colonic epithelial cell diversity in health and inflammatory bowel disease. Nature. 2019;567:49-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 289] [Cited by in RCA: 560] [Article Influence: 93.3] [Reference Citation Analysis (0)] |
| 8. | Regev A, Teichmann SA, Lander ES, Amit I, Benoist C, Birney E, Bodenmiller B, Campbell P, Carninci P, Clatworthy M, Clevers H, Deplancke B, Dunham I, Eberwine J, Eils R, Enard W, Farmer A, Fugger L, Göttgens B, Hacohen N, Haniffa M, Hemberg M, Kim S, Klenerman P, Kriegstein A, Lein E, Linnarsson S, Lundberg E, Lundeberg J, Majumder P, Marioni JC, Merad M, Mhlanga M, Nawijn M, Netea M, Nolan G, Pe'er D, Phillipakis A, Ponting CP, Quake S, Reik W, Rozenblatt-Rosen O, Sanes J, Satija R, Schumacher TN, Shalek A, Shapiro E, Sharma P, Shin JW, Stegle O, Stratton M, Stubbington MJT, Theis FJ, Uhlen M, van Oudenaarden A, Wagner A, Watt F, Weissman J, Wold B, Xavier R, Yosef N; Human Cell Atlas Meeting Participants. The Human Cell Atlas. Elife. 2017;6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1702] [Cited by in RCA: 1437] [Article Influence: 179.6] [Reference Citation Analysis (0)] |
| 9. | Tanay A, Regev A. Scaling single-cell genomics from phenomenology to mechanism. Nature. 2017;541:331-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 490] [Cited by in RCA: 531] [Article Influence: 66.4] [Reference Citation Analysis (0)] |
| 10. | Domínguez Conde C, Xu C, Jarvis LB, Rainbow DB, Wells SB, Gomes T, Howlett SK, Suchanek O, Polanski K, King HW, Mamanova L, Huang N, Szabo PA, Richardson L, Bolt L, Fasouli ES, Mahbubani KT, Prete M, Tuck L, Richoz N, Tuong ZK, Campos L, Mousa HS, Needham EJ, Pritchard S, Li T, Elmentaite R, Park J, Rahmani E, Chen D, Menon DK, Bayraktar OA, James LK, Meyer KB, Yosef N, Clatworthy MR, Sims PA, Farber DL, Saeb-Parsy K, Jones JL, Teichmann SA. Cross-tissue immune cell analysis reveals tissue-specific features in humans. Science. 2022;376:eabl5197. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 231] [Cited by in RCA: 495] [Article Influence: 165.0] [Reference Citation Analysis (0)] |
| 11. | Stuart T, Satija R. Integrative single-cell analysis. Nat Rev Genet. 2019;20:257-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 604] [Cited by in RCA: 856] [Article Influence: 142.7] [Reference Citation Analysis (0)] |
| 12. | Cui A, Li B, Wallace MS, Gonye ALK, Oetheimer C, Patel H, Tonnerre P, Holmes JA, Lieb D, Yao BS, Ma A, Roberts K, Damasio M, Chen JH, Piou D, Carlton-Smith C, Brown J, Mylvaganam R, Hon Fung JM, Sade-Feldman M, Aneja J, Gustafson J, Epstein ET, Salloum S, Brisac C, Thabet A, Kim AY, Lauer GM, Hacohen N, Chung RT, Alatrakchi N. Single-cell atlas of the liver myeloid compartment before and after cure of chronic viral hepatitis. J Hepatol. 2023;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 22] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 13. | Uniken Venema WT, Voskuil MD, Vila AV, van der Vries G, Jansen BH, Jabri B, Faber KN, Dijkstra G, Xavier RJ, Wijmenga C, Graham DB, Weersma RK, Festen EA. Single-Cell RNA Sequencing of Blood and Ileal T Cells From Patients With Crohn's Disease Reveals Tissue-Specific Characteristics and Drug Targets. Gastroenterology. 2019;156:812-815.e22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 44] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 14. | Huang B, Chen Z, Geng L, Wang J, Liang H, Cao Y, Chen H, Huang W, Su M, Wang H, Xu Y, Liu Y, Lu B, Xian H, Li H, Ren L, Xie J, Ye L, Zhao J, Chen P, Zhang L, Zhao S, Zhang T, Xu B, Che D, Si W, Gu X, Zeng L, Wang Y, Li D, Zhan Y, Delfouneso D, Lew AM, Cui J, Tang WH, Zhang Y, Gong S, Bai F, Yang M. Mucosal Profiling of Pediatric-Onset Colitis and IBD Reveals Common Pathogenics and Therapeutic Pathways. Cell. 2019;179:1160-1176.e24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 203] [Article Influence: 40.6] [Reference Citation Analysis (0)] |
| 15. | Li G, Zhang B, Hao J, Chu X, Wiestler M, Cornberg M, Xu CJ, Liu X, Li Y. Identification of Novel Population-Specific Cell Subsets in Chinese Ulcerative Colitis Patients Using Single-Cell RNA Sequencing. Cell Mol Gastroenterol Hepatol. 2021;12:99-117. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 16. | Corridoni D, Antanaviciute A, Gupta T, Fawkner-Corbett D, Aulicino A, Jagielowicz M, Parikh K, Repapi E, Taylor S, Ishikawa D, Hatano R, Yamada T, Xin W, Slawinski H, Bowden R, Napolitani G, Brain O, Morimoto C, Koohy H, Simmons A. Single-cell atlas of colonic CD8(+) T cells in ulcerative colitis. Nat Med. 2020;26:1480-1490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 147] [Article Influence: 29.4] [Reference Citation Analysis (0)] |
| 17. | Boland BS, He Z, Tsai MS, Olvera JG, Omilusik KD, Duong HG, Kim ES, Limary AE, Jin W, Milner JJ, Yu B, Patel SA, Louis TL, Tysl T, Kurd NS, Bortnick A, Quezada LK, Kanbar JN, Miralles A, Huylebroeck D, Valasek MA, Dulai PS, Singh S, Lu LF, Bui JD, Murre C, Sandborn WJ, Goldrath AW, Yeo GW, Chang JT. Heterogeneity and clonal relationships of adaptive immune cells in ulcerative colitis revealed by single-cell analyses. Sci Immunol. 2020;5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 170] [Article Influence: 34.0] [Reference Citation Analysis (0)] |
| 18. | Hindupur SK, Colombi M, Fuhs SR, Matter MS, Guri Y, Adam K, Cornu M, Piscuoglio S, Ng CKY, Betz C, Liko D, Quagliata L, Moes S, Jenoe P, Terracciano LM, Heim MH, Hunter T, Hall MN. The protein histidine phosphatase LHPP is a tumour suppressor. Nature. 2018;555:678-682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 165] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 19. | Zheng J, Dai X, Chen H, Fang C, Chen J, Sun L. Down-regulation of LHPP in cervical cancer influences cell proliferation, metastasis and apoptosis by modulating AKT. Biochem Biophys Res Commun. 2018;503:1108-1114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 55] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 20. | Hou B, Li W, Li J, Ma J, Xia P, Liu Z, Zeng Q, Zhang X, Chang D. Tumor suppressor LHPP regulates the proliferation of colorectal cancer cells via the PI3K/AKT pathway. Oncol Rep. 2020;43:536-548. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 21. | Linder M, Liko D, Kancherla V, Piscuoglio S, Hall MN. Colitis Is Associated with Loss of the Histidine Phosphatase LHPP and Upregulation of Histidine Phosphorylation in Intestinal Epithelial Cells. Biomedicines. 2023;11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 22. | Choi CR, Bakir IA, Hart AL, Graham TA. Clonal evolution of colorectal cancer in IBD. Nat Rev Gastroenterol Hepatol. 2017;14:218-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 124] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 23. | Jeong JH, Ojha U, Lee YM. Pathological angiogenesis and inflammation in tissues. Arch Pharm Res. 2021;44:1-15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 104] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 24. | Neiva KG, Warner KA, Campos MS, Zhang Z, Moren J, Danciu TE, Nör JE. Endothelial cell-derived interleukin-6 regulates tumor growth. BMC Cancer. 2014;14:99. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 25. | Eelen G, de Zeeuw P, Treps L, Harjes U, Wong BW, Carmeliet P. Endothelial Cell Metabolism. Physiol Rev. 2018;98:3-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 365] [Cited by in RCA: 393] [Article Influence: 56.1] [Reference Citation Analysis (0)] |
| 26. | Ebeling S, Kowalczyk A, Perez-Vazquez D, Mattiola I. Regulation of tumor angiogenesis by the crosstalk between innate immunity and endothelial cells. Front Oncol. 2023;13:1171794. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 27. | Gomez-Salinero JM, Rafii S. Endothelial cell adaptation in regeneration. Science. 2018;362:1116-1117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 47] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 28. | Gao J, Zhang X, Jiang L, Li Y, Zheng Q. Tumor endothelial cell-derived extracellular vesicles contribute to tumor microenvironment remodeling. Cell Commun Signal. 2022;20:97. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 30] [Article Influence: 10.0] [Reference Citation Analysis (0)] |