Published online Dec 28, 2023. doi: 10.3748/wjg.v29.i48.6208
Peer-review started: October 10, 2023
First decision: November 6, 2023
Revised: November 25, 2023
Accepted: December 12, 2023
Article in press: December 12, 2023
Published online: December 28, 2023
Processing time: 77 Days and 14.9 Hours
Endoscopic evaluation in diagnosing and managing ulcerative colitis (UC) is becoming increasingly important. Several endoscopic scoring systems have been established, including the Ulcerative Colitis Endoscopic Index of Severity (UCEIS) score and Mayo Endoscopic Subscore (MES). Furthermore, the Toronto Inflammatory Bowel Disease Global Endoscopic Reporting (TIGER) score for UC has recently been proposed; however, its clinical value remains unclear.
To investigate the clinical value of the TIGER score in UC by comparing it with the UCEIS score and MES.
This retrospective study included 166 patients with UC who underwent total colonoscopy between January 2017 and March 2023 at the Affiliated Hospital of Qingdao University (Qingdao, China). We retrospectively analysed endoscopic scores, laboratory and clinical data, treatment, and readmissions within 1 year. Spearman’s rank correlation coefficient, receiver operating characteristic curve, and univariate and multivariable logistic regression analyses were performed using IBM SPSS Statistics for Windows, version 26.0 (IBM Corp., Armonk, NY, United States) and GraphPad Prism version 9.0.0 for Windows (GraphPad Software, Boston, Massachusetts, United States).
The TIGER score significantly correlated with the UCEIS score and MES (r = 0.721, 0.626, both P < 0.001), showed good differentiating values for clinical severity among mild, moderate, and severe UC [8 (4–112.75) vs 210 (109–219) vs 328 (219–426), all P < 0.001], and exhibited predictive value in diagnosing patients with severe UC [area under the curve (AUC) = 0.897, P < 0.001]. Additionally, the TIGER (r = 0.639, 0,551, 0.488, 0.376, all P < 0.001) and UCEIS scores (r = 0.622, 0,540, 0.494, and 0.375, all P < 0.001) showed stronger correlations with laboratory and clinical parameters, including C-reactive protein, erythrocyte sedimentation rate, length of hospitalisation, and hospitalisation costs, than MES (r = 0.509, 0,351, 0.339, and 0.270, all P < 0.001). The TIGER score showed the best predictability for patients' recent advanced treatment, including systemic corticosteroids, biologics, or immunomodulators (AUC = 0.848, P < 0.001) and 1-year readmission (AUC = 0.700, P < 0.001) compared with the UCEIS score (AUC = 0.762, P < 0.001; 0.627, P < 0.05) and MES (AUC = 0.684, P < 0.001; 0.578, P = 0.132). Furthermore, a TIGER score of ≥ 317 was identified as an independent risk factor for advanced UC treatment (P = 0.011).
The TIGER score may be superior to the UCIES score and MES in improving the accuracy of clinical disease severity assessment, guiding therapeutic decision-making, and predicting short-term prognosis.
Core Tip: The manuscript introduces the clinical value of the Toronto Inflammatory Bowel Disease Global Endoscopic Reporting (TIGER) score for ulcerative colitis (UC). Our study, for the first time, validated that the TIGER score accurately reflects disease activity and is significantly correlated with laboratory parameters in patients with UC. We also defined TIGER score thresholds for upgraded treatment and 1-year readmission, providing treatment strategies and personalised disease management for patients with UC.
- Citation: Liu XY, Tian ZB, Zhang LJ, Liu AL, Zhang XF, Wu J, Ding XL. Clinical value of the Toronto inflammatory bowel disease global endoscopic reporting score in ulcerative colitis. World J Gastroenterol 2023; 29(48): 6208-6221
- URL: https://www.wjgnet.com/1007-9327/full/v29/i48/6208.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i48.6208
Ulcerative colitis (UC) is a multifactorial disease that is characterised by continuous mucosal inflammation of the colon and rectum with an increasing incidence, resulting in a high-cost burden worldwide[1,2]. Endoscopy is the principal technique for visualising lesions in the intestinal mucosa and is regarded as the gold standard for evaluating mucosal inflammation[3]. It can reflect endoscopic disease activity and plays a vital role in assessing therapeutic effects, colorectal cancer surveillance, and UC management[4-6]. These data indicate that the endoscopic score, as a concretisation of endoscopic evaluation, may be a prognostic indicator in UC[7,8]. Several endoscopic scoring systems have been developed, including the Ulcerative Colitis Endoscopic Index of Severity (UCEIS) score and Mayo Endoscopic Subscore (MES), which are commonly used in clinics and trials[9,10]. The UCEIS score, proposed by Travis et al[9] in 2012, ranges between 0 and 8, with higher scores indicating increased endoscopic severity, whereas the MES, created by Schroeder et al[10] in 1987, categorises severity into a 4-point scoring grade where patients with normal or inactive, mild, moderate, or severe disease are given scores of 0, 1, 2, or 3, respectively. Recently, the extent of mucosal inflammation has been emphasised, and the controversy on the accuracy of the UCEIS score and MES has arisen since both tools provide final scores aimed only at the most severely inflamed segment without highlighting the number of segments exhibiting moderate-to-severe inflammation[11,12]. Endoscopic scores focusing only on the severity during the medical treatment may be flawed because of the presence of segmental remission[13,14]. Therefore, attempts have been made in the past 10 years to assess disease extent and score the entire colonic mucosa[15,16]. In 2022, Zittan et al[17] proposed a reliable and useful endoscopic score, the Toronto Inflammatory Bowel Disease Global Endoscopic Reporting (TIGER) score. The TIGER score, constructed for both UC and Crohn’s disease, can accurately and comprehensively evaluate the disease severity in all colonic segments and optimise treatment strategies in patients with UC[17].
However, whether the TIGER score has better clinical value than the UCEIS score and MES remains unclear. Therefore, this study aimed to analyse the clinical value of the TIGER score in UC by comparing its relationship with disease severity, predictive potential of treatment options, and prognosis with those of the UCEIS score and MES.
This retrospective study included 166 patients aged 18–75 years with a confirmed diagnosis of UC who were initially admitted to the Affiliated Hospital of Qingdao University (Qingdao, China) between January 2017 and March 2023. The following were the exclusion criteria: (1) Patients who did not undergo a colonoscopy, those with inadequate bowel preparation, or those with difficulty in undergoing a full colonoscopy, including the terminal ileum; and (2) presence of comorbidities, such as gastrointestinal neoplasia, infectious bowel disease, previous colorectal surgery, severe cardiac or pulmonary disease, and haemopathy. Figure 1 presents the selection process of the patients.
This study was conducted in accordance with the principle of the Declaration of Helsinki (World Medical Association, 2013) and was approved by the Ethics Committee of the Affiliated Hospital of Qingdao University (Approval number: QYFY WZLL 28085). Additionally, the Ethics Committee of the Affiliated Hospital of Qingdao University waived the requirement for written informed consent due to this study’s retrospective design.
Demographic and clinical data, including age, sex, body mass index (BMI), symptoms (abdominal pain, diarrhoea, bloody stool, and fever), disease duration, medical history, assessment of disease severity, treatment programs, length of hospitalisation, hospitalisation costs, and baseline endoscopy results, were collected. Laboratory data, including C-reactive protein (CRP) level, erythrocyte sedimentation rate (ESR), white blood cell (WBC) count, neutrophil (NE) count, lymphocyte (LYM) count, platelet (PLT) count, haemoglobin (Hb) level, albumin (Alb) level, urea nitrogen (BUN) level, uric acid level, and creatinine (Cr) level, were also collected. Based on the Montreal classification system, the extent of UC was classified into three types as follows: Proctitis, left-sided colitis, and extensive colitis defined as E1, E2, and E3, respectively[18]. Furthermore, disease severity in patients with UC was assessed using the Truelove and Witts Severity Index[19].
Colonoscopy images were read independently by two experienced gastroenterologists (Ding XL and Liu AL) who were blinded to the clinical information of the patients, and the TIGER score, UCEIS score, and MES were calculated. When the results were inconsistent, a third senior physician (Tian ZB) confirmed the diagnosis and made the final decision. The TIGER score of each segment was evaluated by summing the following five items: General mucosal appearance, ulcer/erosion size, percentage of ulcer/erosion surface area, percentage of affected surface area per segment, and degree of narrowing[17]. Segments with a TIGER score of ≥ 5, which represent moderate-to-severe endoscopic disease activity, received an additional 100 points[17,20]. The total TIGER score was the sum of the five segmental scores (terminal ileum, ascending, transverse, descending colon, and rectum), and the first digit implied the number of moderate-to-severe endoscopic active segments[17]. The UCEIS score was determined using the following three descriptors with a total score ranging from 0 to 8: Erosion and ulcers, bleeding, and vascular pattern[9]. Furthermore, the MES was scored between 0 and 3 according to the following parameters: Erythema, vascular pattern, bleeding, friability, and ulcerations[10].
Statistical analyses and the construction of charts were performed using IBM SPSS Statistics for Windows, version 26.0 (IBM Corp., Armonk, NY, United States) and GraphPad Prism version 9.0.0 for Windows (GraphPad Software, Boston, Massachusetts, United States), respectively. Quantitative variables with a normal distribution were presented as mean (± SD), while those with a non-normal distribution were presented as median [interquartile range (IQR)]. Qualitative variables were presented as numbers (percentages). The correlations between the TIGER score and the UCEIS score, MES, and laboratory and clinical parameters were tested using Spearman’s rank correlation coefficient (r). For continuous variables, the two-sample t-tests or Mann–Whitney U test was performed to compare two groups, and the ANOVA or Kruskal–Wallis H test was used to compare multiple groups, as appropriate. For categorical variables, the chi-square test was used for the univariate analysis. Multivariable logistic regression analysis was used to identify independent risk factors. Furthermore, we used the area under the curve (AUC) of the receiver operating characteristic (ROC) curve to compare the discrimination abilities across different scoring systems. Two-sided hypothesis tests were considered, and statistical significance was set at P < 0.05.
Overall, 166 patients were selected in this study. Notably, 98 (59%) males and 68 (41%) females were included, with a mean age of 42.29 ± 12.05 years. The patients with UC were categorised into mildly, moderately, and severely active groups comprising 44 (26.5%), 51 (30.7%), and 71 (42.8%) patients, respectively, according to Truelove and Witts Severity Index[19]. The median TIGER score, UCEIS score, and MES were 214, 5, and 3, respectively. Furthermore, 161 (97.0%), 40 (24.1%),18 (10.8%), 3 (1.8%), 2 (1.2%), 2 (1.2%), and 1 (0.6%) patients received 5-aminosalicylates (5-ASAs) orally or rectally, used systemic corticosteroids, were treated with biologics (including infliximab or vedolizumab), used immunomodulators, received tofacitinib, used thalidomide, and underwent colorectal surgery, respectively (Table 1).
| Characteristics | Values (n = 166) |
| Gender, n (%) | |
| Male | 98 (59.0) |
| Female | 68 (41.0) |
| Age (yr) | 42.29 ± 12.05 |
| BMI (kg/m2) | 22.49 ± 3.47 |
| Duration (mo) | 36.0 (12.0, 96.0) |
| Montreal classification, n (%) | |
| E1 = proctitis | 21 (12.7) |
| E2 = left-sided colitis | 47 (28.3) |
| E3 = extensive colitis | 98 (59.0) |
| Truelove and Witts Severity Index, n (%) | |
| Mild | 44 (26.5) |
| Moderate | 51 (30.7) |
| Severe | 71 (42.8) |
| Total TIGER score | 214 (109, 324) |
| UCEIS score | 5 (3, 6) |
| MES | 3 (2, 3) |
| Treatment, n (%) | |
| 5-ASAs | 161 (97.0) |
| Systemic corticosteroids | 40 (24.1) |
| IFX | 8 (4.8) |
| VDZ | 10 (6) |
| Immunomodulators | 3 (1.8) |
| Tofacitinib | 2 (1.2) |
| Thalidomide | 2 (1.2) |
| Colectomy | 1 (0.6) |
| Length of hospitalization (d) | 7.00 (4.75-10.00) |
| Hospitalisation costs (CNY) | 9088.11 (6788.40-12500.72) |
| Readmission within 1 yr, n (%) | 48 (33.8) |
| CRP (mg/L) | 3.58 (1.44-9.96) |
| ESR (mm/60 min) | 10 (6-18) |
| WBC (× 109/L) | 6.95 (5.53-8.62) |
| NE (× 109/L) | 4.29 (3.03-5.59) |
| LYM (× 109/L) | 1.81 (1.45-2.27) |
| PLT (× 109/L) | 273.50 (216.75-365.50) |
| Hb (g/L) | 127.00 (104.75-143.00) |
| Alb (g/L) | 42.65 (37.50-54.10) |
| BUN (mmol/L) | 3.96 (3.01-5.02) |
| UA (µmol/L) | 296.39 ± 103.49 |
| Cr (µmol/L) | 57.50 (49.00-69.00) |
The TIGER score was strongly correlated with the UCEIS score (r = 0.721, P < 0.001) and moderately correlated with MES (r = 0.626, P < 0.001). Additionally, a moderate correlation was observed between the UCEIS score and MES (r = 0.681, P < 0.001).
Using the Truelove and Witts Severity Index[19], the performance of the three endoscopic scoring systems in patients with different clinical severities was evaluated. Significant differences were observed in the three endoscopic scoring systems among patients with different disease severities (all P < 0.001). Comparison within each group revealed that both the TIGER and UCEIS scores showed significant distinctions from each other [median (IQR): 8 (4–112.75) vs 210 (109–219) vs 328 (219–426), all P < 0.001; 3 (2–4) vs 4 (4–5) vs 6 (5–6), all P < 0.001], although no significant difference was found in the MES between moderate and severe disease severities (P > 0.05) (Figure 2).
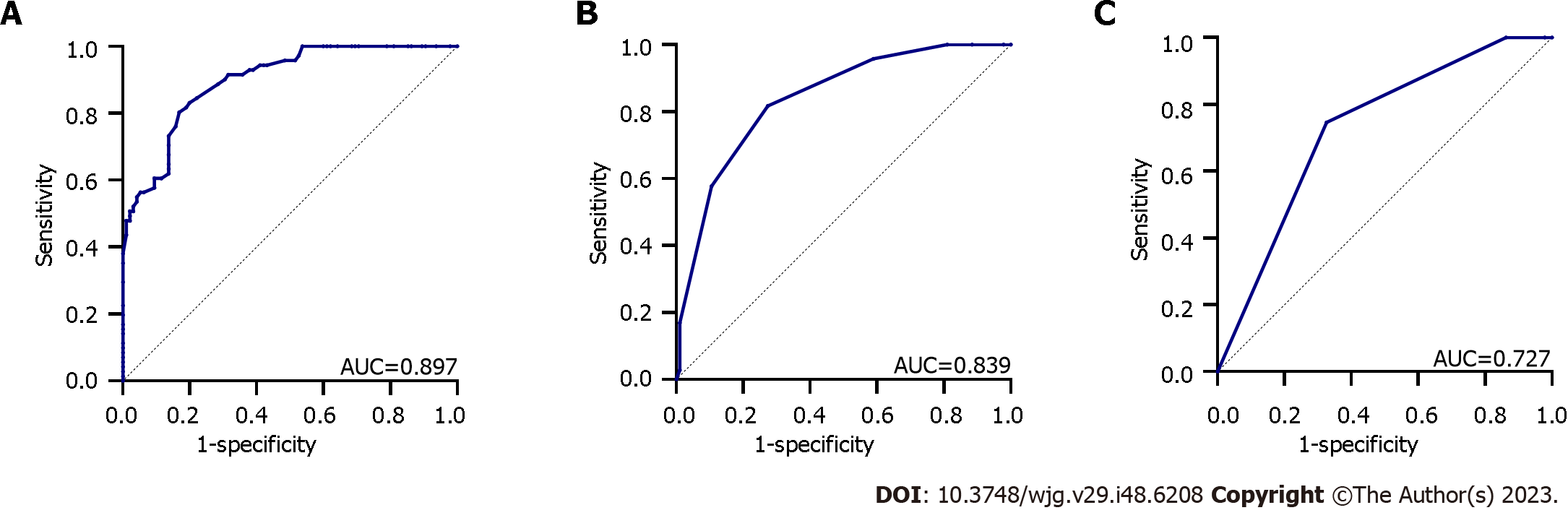
To evaluate the diagnostic performance of the three endoscopic scoring systems for severe UC, assessed using the Truelove and Witts Severity Index[19], the patients were further categorised into mild-to-moderate (n = 95) and severe (n = 71) groups, and the diagnostic value was analysed using the ROC curve. The results revealed that the TIGER score exhibited superior diagnostic performance, with an AUC, sensitivity, specificity, and cut-off value of 0.897, 80.3%, 83.2%, and 217.5, respectively (P < 0.001), demonstrating a good diagnostic capability for severe UC. The UCEIS score ranked second, with an AUC, sensitivity, specificity, and cut-off value of 0.839, 81.7%, 72.6%, and 4.5, respectively (P < 0.001), whereas the MES exhibited relatively poor accuracy for diagnosing severe patients with UC, showing an AUC, sensitivity, specificity, and cut-off value of 0.727, 74.6%, 67.4%, and 2.5, respectively (P < 0.001) (Figure 3).
The three scoring systems were positively correlated with CRP level, ESR, WBC count, NE count, PLT count, length of hospitalisation, and hospitalisation costs (all P < 0.001) but were negatively correlated with Hb, Alb, and BUN levels (all P ≤ 0.001). Furthermore, the correlations between the TIGER score and CRP level, ESR, WBC count, NE count, PLT count, Alb level, and BUN level (r = 0.639, 0.551, 0.387, 0.458, 0.429, -0.422, and -0.320, all P < 0.001) were higher than those between MES and the respective parameters (r = 0.509, 0.351, 0.268, 0.310, 0.248, -0.278, and -0.251, all P ≤ 0.001). However, the correlation between the TIGER score and Hb level was lower than that of the UCEIS score but higher than the MES (all P ≤ 0.001). The correlations between the TIGER score and CRP level, ESR, length of hospitalisation, and hospitalisation costs were similar to those of the UCEIS score (all P < 0.001) and higher than those of the MES (all P < 0.001). No correlations were observed among BMI, disease duration, LYM count, or Cr level in any of the three scoring systems (all P > 0.05) (Table 2).
| Parameters | TIGER score | UCEIS score | MES | |||
| Spearman coefficient (r) | P value | Spearman coefficient (r) | P value | Spearman coefficient (r) | P value | |
| BMI | - | 0.146 | - | 0.035 | - | 0.191 |
| Duration | - | 0.660 | - | 0.604 | - | 0.814 |
| CRP | 0.639 | < 0.001 | 0.622 | < 0.001 | 0.509 | < 0.001 |
| ESR | 0.551 | < 0.001 | 0.540 | < 0.001 | 0.351 | < 0.001 |
| WBC | 0.387 | < 0.001 | 0.319 | < 0.001 | 0.268 | < 0.001 |
| NE | 0.458 | < 0.001 | 0.369 | < 0.001 | 0.310 | < 0.001 |
| LYM | - | 0.349 | - | 0.823 | - | 0.790 |
| PLT | 0.429 | < 0.001 | 0.395 | < 0.001 | 0.248 | < 0.001 |
| Hb | -0.353 | < 0.001 | -0.388 | < 0.001 | -0.245 | 0.001 |
| Alb | -0.422 | < 0.001 | -0.306 | < 0.001 | -0.278 | < 0.001 |
| BUN | -0.320 | < 0.001 | -0.295 | < 0.001 | -0.251 | 0.001 |
| UA | - | 0.233 | -0.164 | 0.035 | - | 0.510 |
| Cr | - | 0.305 | - | 0.399 | - | 0.144 |
| Stay | 0.488 | < 0.001 | 0.494 | < 0.001 | 0.339 | < 0.001 |
| Costs | 0.376 | < 0.001 | 0.375 | < 0.001 | 0.270 | < 0.001 |
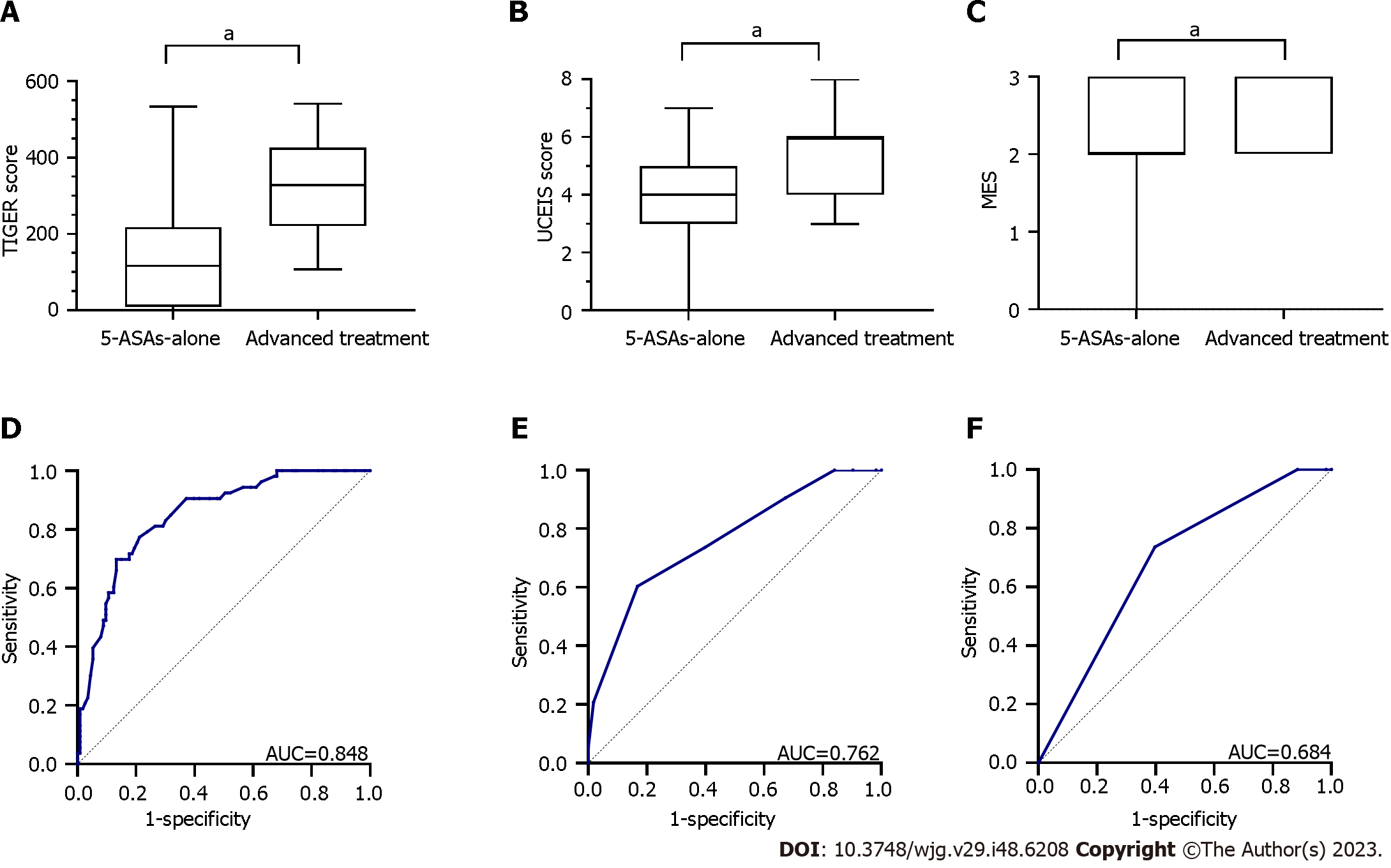
Overall, 113 (68.1%) and 53 (31.9%) patients received 5-ASAs alone and advanced treatment during this admission or within 1 month of discharge, respectively, classifying them into the 5-ASAs-alone and advanced treatment groups, respectively. Advanced treatments included systemic corticosteroids, biologics, immunomodulators, thalidomide, and surgery. Notably, patients in the advanced treatment group demonstrated significantly higher TIGER score [median (IQR): 328 (220.5–426) vs 116 (8.5–218); Z = -7.210, P < 0.0001], UCEIS score [median (IQR): 6 (4–6) vs 4 (3–5); Z = -5.543, P < 0.0001], and MES [median (IQR): 3 (2–3) vs 2 (2–3); Z = -4.272, P < 0.0001], as shown in Figure 4A-C.
ROC analysis was performed in patients with UC to compare the predictive capability for advanced treatment. The TIGER score had an AUC of 0.848 with a sensitivity, specificity, and cut-off value of 69.8%, 86.7%, and 317, respectively (P < 0.001), showing the best predictive potential for treatment escalation. The UCEIS score had an AUC of 0.762, with a sensitivity, specificity, and cut-off value of 60.4%, 83.2%, and 5.5, respectively (P < 0.001), indicating a moderate predictive capability. Furthermore, the AUC of the MES was 0.684 with a sensitivity, specificity, and cut-off value of 73.6%, 60.2%, and 2.5, respectively (P < 0.001), indicating lower predictive potential than the TIGER and UCEIS scores for advanced therapies (Figure 4D-F).
Based on the ROC curves (Figure 4D-F), we selected the TIGER score, UCEIS score, and MES of 317, 5.5, and 2.5, respectively, as the cut-off values and patients were classified into the low- and high-score groups. The TIGER score, UCEIS score, MES, extent of UC, CRP level, Hb level, and Truelove and Witts Index exhibited significant differences between the 5-ASAs-alone and advanced treatment groups (all P < 0.001; Table 3). Furthermore, the TIGER score of ≥ 317 [odds ratio (OR): 3.891; 95% confidence interval (95%CI): 1.360–11.136; P = 0.011) and extent of E3 (OR: 6.488; 95%CI: 1.617–26.027; P = 0.008) were the significant risk factors for treatment escalation (Table 4).
| Variables | 5-ASAs-alone group (n = 113) | Advanced treatment group (n = 53) | P value |
| Age | 0.373 | ||
| < 40 yr | 45 (39.8) | 25 (47.2) | |
| ≥ 40 yr | 68 (60.2) | 28 (52.8) | |
| Sex | 0.054 | ||
| Male | 61 (54.0) | 37 (69.8) | |
| Female | 52 (46.0) | 16 (30.2) | |
| Extent | < 0.001 | ||
| E1 or E2 | 65 (57.5) | 3 (5.7) | |
| E3 | 48 (42.5) | 50 (94.3) | |
| CRP | < 0.001 | ||
| < 5 mg/L | 85 (75.2) | 14 (26.4) | |
| ≥ 5 mg/L | 28 (24.8) | 39 (73.6) | |
| Hb | < 0.001 | ||
| ≥ 110 g/L | 94 (83.2) | 26 (49.1) | |
| < 110 g/L | 19 (16.8) | 27 (50.9) | |
| Truelove and Witts index | < 0.001 | ||
| Mild or moderate | 84 (74.3) | 11 (20.8) | |
| Severe | 29 (25.7) | 42 (79.2) | |
| TIGER score | < 0.001 | ||
| < 317 | 98 (86.7) | 16 (30.2) | |
| ≥ 317 | 15 (13.3) | 37 (69.8) | |
| UCEIS score | < 0.001 | ||
| < 6 | 94 (83.2) | 21 (39.6) | |
| ≥ 6 | 19 (16.8) | 32 (60.4) | |
| MES | < 0.001 | ||
| ≤ 2 | 68 (60.2) | 14 (26.4) | |
| > 2 | 45 (39.8) | 39 (73.6) |
| Variables | Odds ratio | 95%CI | P value |
| Extent, E3 | 6.488 | 1.617-26.027 | 0.008 |
| CRP ≥ 5 mg/L | 2.554 | 0.956-6.819 | 0.061 |
| Hb < 110 g/L | 1.736 | 0.615-4.899 | 0.297 |
| Truelove and Witts Index, Severe | 1.390 | 0.452-4.278 | 0.566 |
| TIGER score ≥ 317 | 3.891 | 1.360-11.136 | 0.011 |
| UCEIS score ≥ 6 | 2.171 | 0.736-6.403 | 0.160 |
| MES > 2 | 0.945 | 0.335-2.667 | 0.914 |
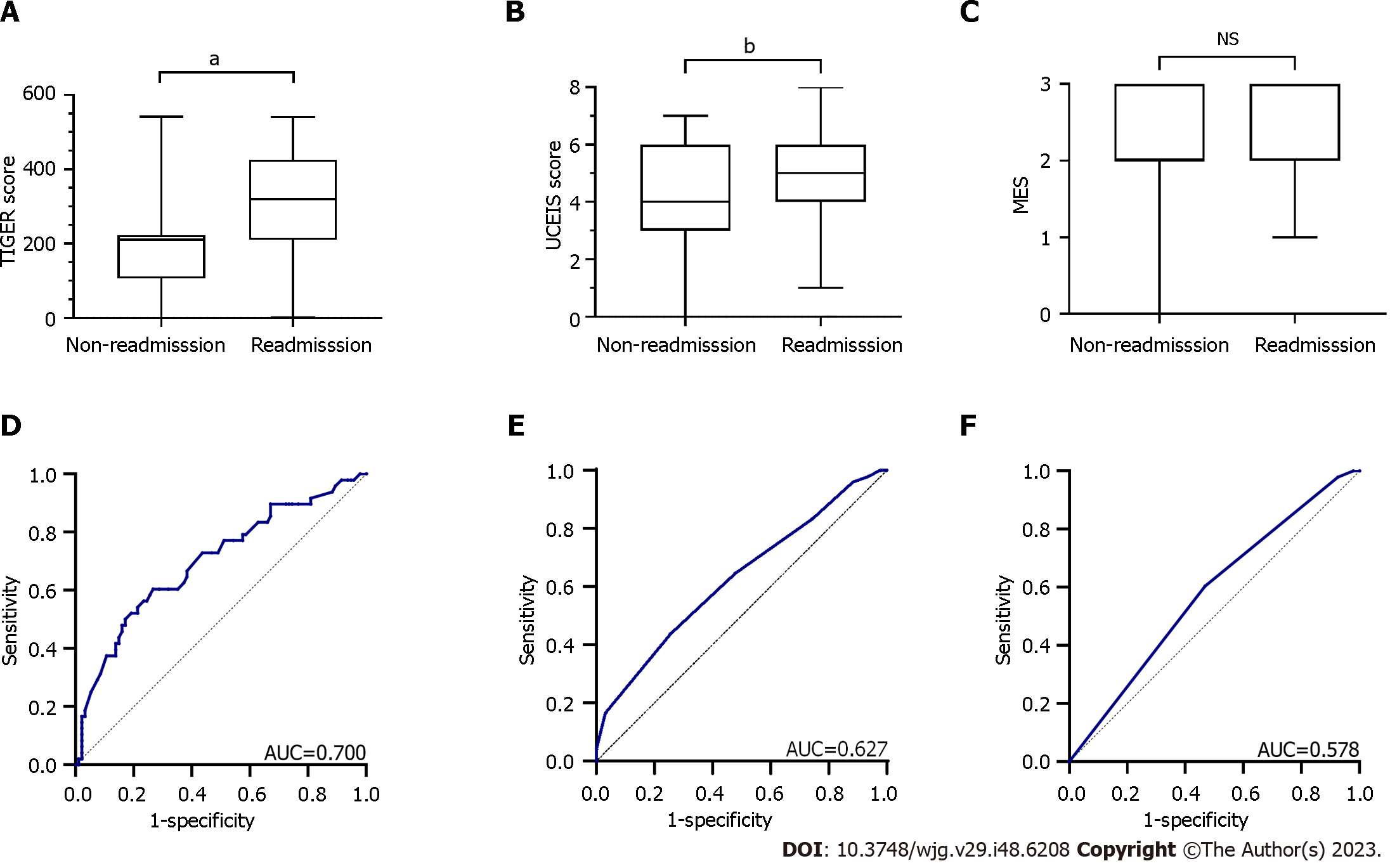
A 1-year follow-up was conducted on 142 patients to investigate the relationship between the endoscopic scores and 1-year readmission (Figure 1). Notably, 142 patients were classified into the non-readmission (n = 94) and readmission (n = 48) groups to compare the predictive value of the assessed endoscopic scoring systems for readmission within 1 year. Mann–Whitney test results revealed that the readmission group had a higher TIGER [median (IQR): 319.5 (210–425.75) vs 210 (106–222.5); Z = -3.889, P < 0.001] and UCEIS [median (IQR): 5 (4–6) vs 4 (3–6); Z = -2.529, P = 0.011] scores than the non-readmission group, whereas no significant difference was observed in the MES [median (IQR): 3 (2–3) vs 2(2–3); Z = -1.701, P = 0.089] between the two groups (Figure 5A-C).
ROC curves were drawn to compare the predictive value of the scoring systems for readmission within 1 year (Figure 5D-F). The AUC of the TIGER score was 0.700, indicating a sensitivity, specificity, and cut-off value of 60.4%, 73.4%, and 220.5, respectively (P < 0.001), demonstrating a predictive capability for readmission. The AUC of the UCEIS score was 0.627, with a sensitivity, specificity, and cut-off value of 43.8%, 74.5%, and 5.5, respectively (P < 0.05), exhibiting inferior predictive ability for readmission. Conversely, MES showed no significant predictive value compared with the two endoscopic scores mentioned above, with an AUC of 0.578 (P = 0.132).
In this study, we compared the clinical utility of the TIGER score, UCEIS score, and MES and identified their roles in predicting disease burden and short-term clinical outcomes.
The three indices consistently reflected endoscopic findings, and the TIGER score strongly and moderately correlated with the UCEIS score and MES, respectively. Xu et al[20] indicated that the TIGER score correlated with the UCEIS score and MES, with correlation coefficients of 0.6193 and 0.4527, respectively, similar to our results. These findings demonstrate a better correlation between the TIGER and UCEIS scores, which we attribute to the better definition and grading of the descriptors in the two scoring systems, such as more detailed scoring criteria for erosions and ulcers[21].
Furthermore, using the Truelove and Witts Severity Index[19], we discovered that the TIGER and UCEIS scores could distinguish between the different UC severities. Notably, the TIGER score demonstrated optimal diagnostic performance for severe UC. This superior performance may be because the TIGER score assesses total bowel segments and considers both inflammation and the extent of UC, whereas the UCEIS score and MES exclude the extent of UC. Interestingly, the extent is one of the dimensions used to evaluate endoscopic severity and can influence the overall severity of UC[22]. Osada et al[23] demonstrated that total colonoscopy could provide complete information on patients with UC and improve the accuracy of clinical disease assessment, which is consistent with our conclusion. Nevertheless, because of factors including discomfort and complications, total colonoscopic studies included fewer acute severe cases, which might have resulted in different results. Gomes et al[24] revealed a poor correlation between total colonoscopic findings and clinical manifestations. Moreover, a finer categorization and larger scale of the scoring system may be more advantageous and accurate in reflecting inflammatory burden and treatment response[22,25]. The UCEIS score, ranging from 0 to 8, provides a larger scale and finer gradings of ulcers and bleeding than the MES. Song et al[26] also demonstrated that the UCEIS score was superior to MES in diagnosing UC severity. Therefore, we infer that the TIGER score can provide a detailed description of the ulcers (size and percentage of surface) and localised inflammation in relation to the bowel segment and a wide range of scores between 0 and 560, resulting in optimal performance when reflecting the overall severity[17].
In this study, we observed that the TIGER score was significantly correlated with the clinical parameters of active inflammation, particularly CRP levels, and the burden parameters including length of stay and cost, whereas the MES exhibited disadvantages in evaluating the clinical activity of UC compared to the TIGER and UCEIS scores. Previous studies have demonstrated that objective blood markers, including CRP, ESR, WBC, PLT, Alb, and Hb, are relevant to UC endoscopic severity and disease activity[27,28]. Additionally, the association between endoscopic scores and inflammatory burden has been confirmed in other studies[29,30]. Recent studies have shown that CRP, a typical acute-phase protein, reflects the inflammatory state of the entire colon, which aligns with our findings that demonstrate the existence of a correlation between the TIGER score and CRP levels[31]. Additionally, Zittan et al[17] reported that the TIGER score was positively correlated with faecal calprotectin levels and the inflammatory bowel disease (IBD) Disk score, indicating that the disease condition and burden of UC may be observed using the TIGER score. Inflammatory biomarkers can exacerbate damage to the epithelial barrier and imbalance of the intestinal mucosal immune system and influence the synthesis of related protein synthesis in UC[32,33], which may be manifested in endoscopic mucosal inflammation and reflected by the TIGER score since it contains a clear description of mucosal appearance and ulcer conditions and could precisely describe and assess the entire intestine.
Five-ASAs are still recommended as the standard treatment and maintenance strategies for patients with mild-to-moderate UC, whereas systemic corticosteroids, thiopurines, biologics, immunomodulators, and tofacitinib are considered upgraded treatment options for those with moderate-to-severe disease activity or those with 5-ASAs failure or intolerance[34,35]. In this study, we observed that the TIGER score had a superior predictive potential for advanced treatment in patients with UC and demonstrated that a TIGER score of ≥ 317 was an independent risk factor for indicating that patients with three or more segments involved in moderate-to-severe endoscopic activity were more likely to upgrade their treatment programs. This could be a useful indication of escalating treatment. Severe endoscopic activity and extensive colitis may represent a severe degree of disease, leading to therapy escalation and poor prognosis[36-38], which could explain why patients with higher TIGER scores were at a higher risk of advanced treatment in this study. Bálint et al[39] suggested that a scoring system should provide additional information on the localization and extent of the disease and argued that this could guide treatment choices, which is consistent with the above mentioned results.
Meanwhile, a high readmission rate indicates increased disease severity and poor prognosis in UC[40]. Therefore, the prediction of readmissions could help clinicians develop healthcare plans and manage patients. We observed that the TIGER score in the readmission group was higher than that in the non-readmission group and it displayed the best predictive capability for readmission within 1 year. These results indicate that the TIGER score could help predict short-term prognosis in patients with UC. Higher TIGER scores indicate more severe UC, which may also be associated with a higher readmission rate[40]. Notably, the UCEIS score was observed to be an independent risk factor for the 1-year readmission, demonstrating that endoscopic scores might be associated with early readmission[41].
The TIGER score requires further validation in clinical practice before its broad adoption. Five descriptors in each of the five segments were evaluated during the endoscopic examination to assess the mucosal inflammation of the entire intestine, resulting in 25 items that required scoring. For segments with a TIGER score of ≥ 5, 100 bonus points were counted, and the segmental scores were summed to determine the final total TIGER score. With the recent development of high-performance computers, advanced optical technologies, molecular imaging, and artificial intelligence algorithms, a computer-aided diagnostic system for patients with IBD to improve endoscopic assessment has become possible[42-45]. Although the TIGER score requires calculation and total colonoscopy, it can be intelligently calculated currently and may be automatically scored in the future by analysing the entire colonic mucosa.
Furthermore, we validated, for the first time, that the TIGER score could accurately reflect disease activity and significantly correlate with laboratory parameters in patients with UC. Moreover, we also defined TIGER score thresholds for upgraded treatment and 1-year readmission, providing treatment strategies and personalised disease management for patients with UC. Additionally, this study included a larger cohort of patients with UC than a previous study[17].
However, this study had some limitations. First, this was a single-centre retrospective study. Second, some patients with acute severe UC could not undergo total colonoscopy and were excluded from the study. Therefore, multicenter prospective studies are warranted.
The TIGER score is a useful scoring method that provides an overall intestinal evaluation of endoscopic activity and demonstrates a significant correlation with the UCEIS score, MES, and laboratory indices, particularly CRP levels. Furthermore, the TIGER score may be superior to the UCEIS and MES scoring systems in improving the accuracy of clinical disease severity assessment, guiding therapeutic decision-making to some extent, and predicting short-term clinical outcomes.
Endoscopy is crucial in the diagnosis, assessment, and management of ulcerative colitis (UC). Several endoscopic scoring systems have been established to make endoscopic evaluation quantified and objective, including the Ulcerative Colitis Endoscopic Index of Severity (UCEIS) score and Mayo Endoscopic Subscore (MES). The Toronto Inflammatory Bowel Disease Global Endoscopic Reporting (TIGER) score for UC, which considers the extent of UC involvement and reflects the number of segments with moderate-to-severe inflammation, was proposed in 2022.
Although the TIGER score is a novel and reliable tool for reflecting complete endoscopic inflammation, its clinical value remains unclear.
To assess the clinical value of the TIGER score by comparing it with the UCEIS score and MES.
We performed a retrospective study that included 166 patients with UC who underwent total colonoscopy. Spearman's rank correlation coefficient was used to estimate the linear associations of three scores and laboratory/clinical parameters. The receiver-operating characteristic curve was performed to compare the predictive potentials of the three scores for predicting severe UC, patients’ recent advanced treatment, and 1-year readmission. Univariate and multivariable logistic regression analyses were performed to investigate the independent risk factors for treatment escalation.
The TIGER score showed a significant correlation with the UCEIS score, MES, and laboratory indices, particularly C-reactive protein levels. Additionally, the TIGER score exhibited the best predictive capability for diagnosing patients with severe UC, upgrading treatment options, and 1-year readmission and a TIGER score of ≥ 317 was found to be an independent risk factor for treatment escalation in UC.
The TIGER score exhibits an advantage in assessing the disease severity of UC, guiding treatment decisions, and predicting short-term prognosis compared to the UCEIS score and MES.
The TIGER score may have significant clinical utility in evaluating, treating, and managing patients with UC, although multicenter prospective studies are required to promote its use.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Emran TB, Bangladesh; Poullis A, United Kingdom S-Editor: Lin C L-Editor: A P-Editor: Chen YX
| 1. | Ungaro R, Mehandru S, Allen PB, Peyrin-Biroulet L, Colombel JF. Ulcerative colitis. Lancet. 2017;389:1756-1770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2199] [Cited by in RCA: 2487] [Article Influence: 310.9] [Reference Citation Analysis (2)] |
| 2. | Zhao M, Gönczi L, Lakatos PL, Burisch J. The Burden of Inflammatory Bowel Disease in Europe in 2020. J Crohns Colitis. 2021;15:1573-1587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 270] [Cited by in RCA: 256] [Article Influence: 64.0] [Reference Citation Analysis (0)] |
| 3. | Turner D, Ricciuto A, Lewis A, D'Amico F, Dhaliwal J, Griffiths AM, Bettenworth D, Sandborn WJ, Sands BE, Reinisch W, Schölmerich J, Bemelman W, Danese S, Mary JY, Rubin D, Colombel JF, Peyrin-Biroulet L, Dotan I, Abreu MT, Dignass A; International Organization for the Study of IBD. STRIDE-II: An Update on the Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE) Initiative of the International Organization for the Study of IBD (IOIBD): Determining Therapeutic Goals for Treat-to-Target strategies in IBD. Gastroenterology. 2021;160:1570-1583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 473] [Cited by in RCA: 1643] [Article Influence: 410.8] [Reference Citation Analysis (1)] |
| 4. | Ruscio MD, Cedola M, Mangone M, Brighi S. How to assess endoscopic disease activity in ulcerative colitis in 2022. Ann Gastroenterol. 2022;35:462-470. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 5. | Hong SM, Baek DH. A Review of Colonoscopy in Intestinal Diseases. Diagnostics (Basel). 2023;13:1262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 6. | Leighton JA, Shen B, Baron TH, Adler DG, Davila R, Egan JV, Faigel DO, Gan SI, Hirota WK, Lichtenstein D, Qureshi WA, Rajan E, Zuckerman MJ, VanGuilder T, Fanelli RD; Standards of Practice Committee, American Society for Gastrointestinal Endoscopy. ASGE guideline: endoscopy in the diagnosis and treatment of inflammatory bowel disease. Gastrointest Endosc. 2006;63:558-565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 161] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 7. | Chen L, Yang J, Fang L, Wu W, Feng B, Shi Y, Sun M, Sun X, Liu Z. The Degree of Ulcerative Colitis Burden of Luminal Inflammation score is superior to predicting medium- to long-term prognosis in patients with active ulcerative colitis. Therap Adv Gastroenterol. 2020;13:1756284820981210. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 8. | Arai M, Naganuma M, Sugimoto S, Kiyohara H, Ono K, Mori K, Saigusa K, Nanki K, Mutaguchi M, Mizuno S, Bessho R, Nakazato Y, Hosoe N, Matsuoka K, Inoue N, Ogata H, Iwao Y, Kanai T. The Ulcerative Colitis Endoscopic Index of Severity is Useful to Predict Medium- to Long-Term Prognosis in Ulcerative Colitis Patients with Clinical Remission. J Crohns Colitis. 2016;10:1303-1309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 60] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 9. | Travis SP, Schnell D, Krzeski P, Abreu MT, Altman DG, Colombel JF, Feagan BG, Hanauer SB, Lémann M, Lichtenstein GR, Marteau PR, Reinisch W, Sands BE, Yacyshyn BR, Bernhardt CA, Mary JY, Sandborn WJ. Developing an instrument to assess the endoscopic severity of ulcerative colitis: the Ulcerative Colitis Endoscopic Index of Severity (UCEIS). Gut. 2012;61:535-542. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 353] [Cited by in RCA: 452] [Article Influence: 34.8] [Reference Citation Analysis (0)] |
| 10. | Schroeder KW, Tremaine WJ, Ilstrup DM. Coated oral 5-aminosalicylic acid therapy for mildly to moderately active ulcerative colitis. A randomized study. N Engl J Med. 1987;317:1625-1629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1958] [Cited by in RCA: 2252] [Article Influence: 59.3] [Reference Citation Analysis (0)] |
| 11. | Scarozza P, Marafini I, Laudisi F, Troncone E, Schmitt H, Lenti MV, Costa S, Rocchetti I, De Cristofaro E, Salvatori S, Frezzati L, Di Sabatino A, Atreya R, Neurath MF, Calabrese E, Monteleone G. Extent of Mucosal Inflammation in Ulcerative Colitis Influences the Clinical Remission Induced by Vedolizumab. J Clin Med. 2020;9:385. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 12. | Pagnini C, Menasci F, Desideri F, Corleto VD, Delle Fave G, Di Giulio E. Endoscopic scores for inflammatory bowel disease in the era of 'mucosal healing': Old problem, new perspectives. Dig Liver Dis. 2016;48:703-708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 13. | Sharara AI, Malaeb M, Lenfant M, Ferrante M. Assessment of Endoscopic Disease Activity in Ulcerative Colitis: Is Simplicity the Ultimate Sophistication? Inflamm Intest Dis. 2022;7:7-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 16] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 14. | Lenfant M, Verstockt B, Sabino J, Vermeire S, Ferrante M. The assessment of segmental healing by the Modified Mayo Endoscopic Score (MMES) complements the prediction of long-term clinical outcomes in patients with ulcerative colitis. Aliment Pharmacol Ther. 2024;59:64-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 15. | Samuel S, Bruining DH, Loftus EV Jr, Thia KT, Schroeder KW, Tremaine WJ, Faubion WA, Kane SV, Pardi DS, de Groen PC, Harmsen WS, Zinsmeister AR, Sandborn WJ. Validation of the ulcerative colitis colonoscopic index of severity and its correlation with disease activity measures. Clin Gastroenterol Hepatol. 2013;11:49-54.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 106] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 16. | Lobatón T, Bessissow T, De Hertogh G, Lemmens B, Maedler C, Van Assche G, Vermeire S, Bisschops R, Rutgeerts P, Bitton A, Afif W, Marcus V, Ferrante M. The Modified Mayo Endoscopic Score (MMES): A New Index for the Assessment of Extension and Severity of Endoscopic Activity in Ulcerative Colitis Patients. J Crohns Colitis. 2015;9:846-852. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 119] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 17. | Zittan E, Steinhart AH, Aran H, Milgrom R, Gralnek IM, Zelber-Sagi S, Silverberg MS. The Toronto IBD Global Endoscopic Reporting [TIGER] Score: A Single, Easy to Use Endoscopic Score for Both Crohn's Disease and Ulcerative Colitis Patients. J Crohns Colitis. 2022;16:544-553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.7] [Reference Citation Analysis (1)] |
| 18. | Silverberg MS, Satsangi J, Ahmad T, Arnott ID, Bernstein CN, Brant SR, Caprilli R, Colombel JF, Gasche C, Geboes K, Jewell DP, Karban A, Loftus EV Jr, Peña AS, Riddell RH, Sachar DB, Schreiber S, Steinhart AH, Targan SR, Vermeire S, Warren BF. Toward an integrated clinical, molecular and serological classification of inflammatory bowel disease: report of a Working Party of the 2005 Montreal World Congress of Gastroenterology. Can J Gastroenterol. 2005;19 Suppl A:5A-36A. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2148] [Cited by in RCA: 2369] [Article Influence: 215.4] [Reference Citation Analysis (0)] |
| 19. | TRUELOVE SC, WITTS LJ. Cortisone in ulcerative colitis; final report on a therapeutic trial. Br Med J. 1955;2:1041-1048. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1832] [Cited by in RCA: 1868] [Article Influence: 26.7] [Reference Citation Analysis (1)] |
| 20. | Xu W, Liu F, Hua Z, Gu Y, Lian L, Cui L, Ding Z, Du P. Comparison of The Toronto IBD Global Endoscopic Reporting (TIGER) score, Mayo endoscopic score (MES), and ulcerative colitis endoscopic index of severity (UCEIS) in predicting the need for ileal pouch-anal anastomosis in patients with ulcerative colitis. Int J Colorectal Dis. 2023;38:53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 21. | Ikeya K, Hanai H, Sugimoto K, Osawa S, Kawasaki S, Iida T, Maruyama Y, Watanabe F. The Ulcerative Colitis Endoscopic Index of Severity More Accurately Reflects Clinical Outcomes and Long-term Prognosis than the Mayo Endoscopic Score. J Crohns Colitis. 2016;10:286-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 133] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 22. | Ket SN, Palmer R, Travis S. Endoscopic Disease Activity in Inflammatory Bowel Disease. Curr Gastroenterol Rep. 2015;17:50. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 23. | Osada T, Ohkusa T, Okayasu I, Yoshida T, Hirai S, Beppu K, Shibuya T, Sakamoto N, Kobayashi O, Nagahara A, Terai T, Watanabe S. Correlations among total colonoscopic findings, clinical symptoms, and laboratory markers in ulcerative colitis. J Gastroenterol Hepatol. 2008;23 Suppl 2:S262-S267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 68] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 24. | Gomes P, du Boulay C, Smith CL, Holdstock G. Relationship between disease activity indices and colonoscopic findings in patients with colonic inflammatory bowel disease. Gut. 1986;27:92-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 253] [Cited by in RCA: 257] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 25. | Takabayashi K, Kobayashi T, Matsuoka K, Levesque BG, Kawamura T, Tanaka K, Kadota T, Bise R, Uchida S, Kanai T, Ogata H. Artificial intelligence quantifying endoscopic severity of ulcerative colitis in gradation scale. Dig Endosc. 2023;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 16] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 26. | Song Z, Zhang M, Ren Y, Iang B. Improved Mayo Endoscopic Score has a higher value for evaluating clinical severity of ulcerative colitis. Nan Fang Yi Ke Da Xue Xue Bao. 2022;42:997-1005. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 27. | Cui J, Li X, Zhang Z, Gao H, Li J. Common laboratory blood test immune panel markers are useful for grading ulcerative colitis endoscopic severity. BMC Gastroenterol. 2022;22:540. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 28. | Schoepfer AM, Beglinger C, Straumann A, Trummler M, Renzulli P, Seibold F. Ulcerative colitis: correlation of the Rachmilewitz endoscopic activity index with fecal calprotectin, clinical activity, C-reactive protein, and blood leukocytes. Inflamm Bowel Dis. 2009;15:1851-1858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 246] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 29. | de Jong DC, Löwenberg M, Koumoutsos I, Ray S, Mawdsley J, Anderson S, Sanderson JD, Gecse K, Ponsioen CY, D'Haens GR, Irving PM, Samaan MA. Validation and Investigation of the Operating Characteristics of the Ulcerative Colitis Endoscopic Index of Severity. Inflamm Bowel Dis. 2019;25:937-944. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 30. | Zhang XF, Li P, Ding XL, Chen H, Wang SJ, Jin SB, Guo J, Tian ZB. Comparing the clinical application values of the Degree of Ulcerative Colitis Burden of Luminal Inflammation (DUBLIN) score and Ulcerative Colitis Endoscopic Index of Severity (UCEIS) in patients with ulcerative colitis. Gastroenterol Rep (Oxf). 2021;9:533-542. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 31. | Ishida N, Higuchi T, Miyazu T, Tamura S, Tani S, Yamade M, Iwaizumi M, Hamaya Y, Osawa S, Furuta T, Sugimoto K. C-reactive protein is superior to fecal biomarkers for evaluating colon-wide active inflammation in ulcerative colitis. Sci Rep. 2021;11:12431. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 32. | Zhang MH, Wang H, Wang HG, Wen X, Yang XZ. Effective immune-inflammation index for ulcerative colitis and activity assessments. World J Clin Cases. 2021;9:334-343. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 12] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 33. | Li X, Tang Z, Liu Y, Zhu X, Liu F. Risk prediction model based on blood biomarkers for predicting moderate to severe endoscopic activity in patients with ulcerative colitis. Front Med (Lausanne). 2023;10:1101237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 34. | Segal JP, LeBlanc JF, Hart AL. Ulcerative colitis: an update. Clin Med (Lond). 2021;21:135-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 182] [Article Influence: 45.5] [Reference Citation Analysis (0)] |
| 35. | Lamb CA, Kennedy NA, Raine T, Hendy PA, Smith PJ, Limdi JK, Hayee B, Lomer MCE, Parkes GC, Selinger C, Barrett KJ, Davies RJ, Bennett C, Gittens S, Dunlop MG, Faiz O, Fraser A, Garrick V, Johnston PD, Parkes M, Sanderson J, Terry H; IBD guidelines eDelphi consensus group, Gaya DR, Iqbal TH, Taylor SA, Smith M, Brookes M, Hansen R, Hawthorne AB. British Society of Gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults. Gut. 2019;68:s1-s106. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1402] [Cited by in RCA: 1567] [Article Influence: 261.2] [Reference Citation Analysis (0)] |
| 36. | Martí-Aguado D, Ballester MP, Mínguez M. Risk factors and management strategies associated with non-response to aminosalicylates as a maintenance treatment in ulcerative colitis. Rev Esp Enferm Dig. 2021;113:447-453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 37. | Marti-Aguado D, Ballester MP, Tosca J, Bosca-Watts MM, Navarro P, Anton R, Pascual I, Mora F, Minguez M. Long-term follow-up of patients treated with aminosalicylates for ulcerative colitis: Predictive factors of response: An observational case-control study. United European Gastroenterol J. 2019;7:1042-1050. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 38. | Mocciaro F, Renna S, Orlando A, Rizzuto G, Sinagra E, Orlando E, Cottone M. Cyclosporine or infliximab as rescue therapy in severe refractory ulcerative colitis: early and long-term data from a retrospective observational study. J Crohns Colitis. 2012;6:681-686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 49] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 39. | Bálint A, Farkas K, Szepes Z, Nagy F, Szűcs M, Tiszlavicz L, Bor R, Milassin Á, Rutka M, Fábián A, Molnár T. How disease extent can be included in the endoscopic activity index of ulcerative colitis: the panMayo score, a promising scoring system. BMC Gastroenterol. 2018;18:7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 40. | Kruger AJ, Hinton A, Afzali A. Index Severity Score and Early Readmission Predicts Increased Mortality in Ulcerative Colitis Patients. Inflamm Bowel Dis. 2019;25:894-901. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 41. | Xiang Y, Yuan Y, Liu J, Xu X, Wang Z, Hassan S, Wu Y, Sun Q, Shen Y, Wang L, Yang H, Sun J, Xu G, Huang Q. A nomogram based on clinical factors to predict calendar year readmission in patients with ulcerative colitis. Therap Adv Gastroenterol. 2023;16:17562848231189124. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 42. | Alfarone L, Parigi TL, Gabbiadini R, Dal Buono A, Spinelli A, Hassan C, Iacucci M, Repici A, Armuzzi A. Technological advances in inflammatory bowel disease endoscopy and histology. Front Med (Lausanne). 2022;9:1058875. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 43. | Solitano V, D'Amico F, Allocca M, Fiorino G, Zilli A, Loy L, Gilardi D, Radice S, Correale C, Danese S, Peyrin-Biroulet L, Furfaro F. Rediscovering histology: what is new in endoscopy for inflammatory bowel disease? Therap Adv Gastroenterol. 2021;14:17562848211005692. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 44. | van der Laan JJH, van der Waaij AM, Gabriëls RY, Festen EAM, Dijkstra G, Nagengast WB. Endoscopic imaging in inflammatory bowel disease: current developments and emerging strategies. Expert Rev Gastroenterol Hepatol. 2021;15:115-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 45. | Yao H, Najarian K, Gryak J, Bishu S, Rice MD, Waljee AK, Wilkins HJ, Stidham RW. Fully automated endoscopic disease activity assessment in ulcerative colitis. Gastrointest Endosc. 2021;93:728-736.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 83] [Article Influence: 20.8] [Reference Citation Analysis (0)] |