Published online Dec 28, 2023. doi: 10.3748/wjg.v29.i48.6179
Peer-review started: September 24, 2023
First decision: October 29, 2023
Revised: November 2, 2023
Accepted: December 18, 2023
Article in press: December 18, 2023
Published online: December 28, 2023
Processing time: 93 Days and 22.1 Hours
Follicular lymphoma (FL) is the most common indolent B-cell lymphoma (BCL) globally. Recently, its incidence has increased in Europe, the United States, and Asia, with the number of gastrointestinal FL cases expected to increase. Genetic abnormalities related to t(14;18) translocation, BCL2 overexpression, NF-κB pathway-related factors, histone acetylases, and histone methyltransferases cause FL and enhance its proliferation. Meanwhile, microRNAs are commonly used in diagnosing FL and predicting patient prognosis. Many clinical trials on novel therapeutics targeting these genetic abnormalities and immunomodulatory mechanisms have been conducted, resulting in a marked improvement in therapeutic outcomes for FL. Although developing these innovative therapeutic agents targeting specific genetic mutations and immune pathways has provided hope for curative options, FL treatment has become more complex, requiring combinatorial therapeutic regimens. However, optimal treatment combinations have not yet been achieved, highlighting the importance of a complete under-standing regarding the pathogenesis of gastrointestinal FL. Accordingly, this article reviews key research on the molecular pathogenesis of nodal FL and novel therapies targeting the causative genetic mutations. Moreover, the results of clinical trials are summarized, with a particular focus on treating nodal and gastrointestinal FLs.
Core Tip: Recently, the incidence of follicular lymphoma (FL) has increased in Asia, Europe, and the United States, with the number of gastrointestinal FL cases expected to increase. This article reviews the recent literature on the molecular origins of, innovative treatments for, and clinical trial findings on FL, focusing on gastrointestinal FL. Genetic factors [e.g., t(14;18) translocation and B-cell lymphoma 2 overexpression] drive FL growth, while microRNAs aid in its diagnosis and prognosis. Although recent trials have reported enhanced treatment outcomes, curative therapies remain elusive, and combinatorial regimens have not been optimized. Hence, gastroenterologists require a more comprehensive understanding of gastrointestinal FL pathogenesis to facilitate improved therapeutic outcomes.
- Citation: Watanabe T. Gene targeted and immune therapies for nodal and gastrointestinal follicular lymphomas. World J Gastroenterol 2023; 29(48): 6179-6197
- URL: https://www.wjgnet.com/1007-9327/full/v29/i48/6179.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i48.6179
Follicular lymphoma (FL) is the most common form of indolent B-cell lymphoma (BCL)[1]. In recent years, FL incidence has rapidly increased in Europe, the United States, and Asia[2], and the number of advanced-stage gastrointestinal FL cases is expected to increase in the near future. Although it responds well to treatment, it is prone to relapse and is refractory; hence, FL is regarded as an incurable disease with a poor prognosis. The NF-κB pathway is an intracellular signaling pathway activated by cell surface receptor stimuli in B-cell receptor (BCR)- and Toll-like receptor (TLR)-expressing cells. Indeed, the constant activation of this pathway contributes to lymphoma development. Histone methylation and acetylation of gene promoters regulate gene expression; abnormalities in gene expression can influence lymphoma development and proliferation. In addition, the t(14;18) translocation, found in most FLs, causes the overexpression of BCL2, eliciting an anti-apoptotic effect that mediates cell immortalization. Recently, genome-wide association studies (GWAS) have identified several loci involved in FL development.
Approximately 150 microRNAs (miRNAs) participate in the development of FL via enhanced cell proliferation. These include the miR-17-92 cluster, the importance of which has been evaluated in FL diagnosis and prognosis.
Rituximab, a monoclonal antibody against the CD20 surface antigen expressed in more than 90% of BCL-expressing cells, was developed approximately 20 years ago, and its combination with cyclophosphamide, doxorubicin, vincristine, and prednisolone (CHOP) represents a standard chemotherapy that continues to be the mainstay of treatment. Nevertheless, the past decade has witnessed considerable advancements in developing new FL therapeutic agents targeting various oncogenes.
This article reviews the latest findings in nodal FL, particularly related to molecular genetic analysis and diagnosis while summarizing the progress and future perspectives of novel FL therapeutics targeting various genetic abnormalities. The article concludes with a personal view of gastroenterologists' role in treating advanced-stage FL of gastrointestinal origin.
FL is the leading indolent (low-grade) BCL, accounting for 10%-20% of all non-Hodgkin lymphomas[1]. Its incidence is increasing rapidly in Western and Asian countries[2]. In particular, the incidence of FL in Japan is increasing[3]. Gastrointestinal FL (GI-FL) occurs in the lymphoid tissue of the gastrointestinal tract and is derived from the FL of B-cell lymphocytes and primarily arises from the lymph follicles (lymphoid apparatus) in the submucosa of the gastrointestinal tract. Histologically, GI-FL contains a folliculocentric lymphoma cell population; FL is categorized histopathologically as grade 1, 2, 3a, or 3b, with grade 3b usually identified and treated as an aggressive medium- or high-grade lymphoma. Most patients with grade 3b FL present with enlarged lymph nodes, and approximately 70%-85% are diagnosed with an advanced-stage III/IV disease, often associated with significant bone marrow involvement. Although the incidence of GI-FL is low and relatively rare compared with that of other lymphomas, it is more common in middle-aged and older adults, with no apparent difference in incidence between men and women; however, the peak incidence in certain age groups is not reported.
The symptoms of gastrointestinal FL vary among individuals, ranging from asymptomatic with very mild symptoms (most common) to well-defined symptoms, depending on the stage of the disease, degree of disease progression, and tumor location. The symptoms of GI-FL include: Discomfort or pain in the upper abdomen, often worsening after meals or at night; gastrointestinal symptoms: Nausea, vomiting, loss of appetite, bloating, upset stomach, diarrhea, and constipation; weight loss due to anorexia and impaired nutrient absorption; anemia due to intestinal bleeding and tumor growth (anemia-induced fatigue and shortness of breath may occur); and edema due to the compression on lymphatic vessels and blood vessels, particularly in the abdomen and lower limbs.
Among the 249 GI-FL cases reported in a previous study, 150 with clinical symptoms were asymptomatic at onset (43.4%), 9.3% presented with vague gastrointestinal symptoms, including abdominal discomfort or heartburn, 28.7% had abdominal pain, 8.0% had symptoms of bowel obstruction, such as nausea and vomiting, and 6.0% had melena, such as tar-like black stools, a symptom of upper gastrointestinal bleeding[4].
GI-FLs are diagnosed using a combination of methods. The following section details those most commonly used.
Endoscopy represents the most important diagnostic test for GI-FL as it is useful for gross diagnosis and facilitates the extraction of biopsies for pathological examination and histological analysis. Gastric malignant lymphomas are divided into several gross types[5]. Colorectal malignant lymphomas often present with characteristic polyp-like endoscopic findings, termed multiple lymphomatous polyposis (MLP)[6], which are useful for diagnosis. The frequency of GI-FL detection has increased rapidly in Japan owing to the widespread and generalized use of small bowel endoscopy, advances in endoscopic equipment, improved endoscopist techniques, and increased opportunities for health screening[7].
Tissue samples obtained from biopsies are assessed pathologically using a microscope. GI-FL is diagnosed based on the presence of folliculocentric lymphoma cell populations and other histological features characteristic of FLs. Moreover, testing for specific proteins through immunostaining is performed. The following is a list of parameters used in the pathological diagnosis of FL.
Follicular-like structures: FLs are characterized by the formation of follicular-like structures within the tumor that are B-cell aggregates forming follicles within tumors. Additional abnormal follicular structures can be present in FL.
Grade: FLs are classified into three grades (1-3) by assessing the degree of formation of follicular-like structures within the tumor and the atypia (morphological abnormalities) of lymphoma cells.
Immunohistopathological examinations: Immunostaining findings showing CD20 and CD10 positivity and BCL2 overexpression are characteristic of FL and essential for the histological diagnosis of FL.
In FLs, lymphoma cells express the CD20 surface protein, a specific B-cell marker and an important indicator for diagnosing FL. CD10 is also expressed by FL cells. BCL2 is a protein rarely expressed in normal lymphoid tissues but is overexpressed in FLs. It is involved in the regulation of apoptosis (cell death) and is a characteristic immunohistochemical marker in FLs.
Chromosomal abnormalities: The t(14;18) translocation is frequently observed in FLs, causing the fusion of BCL2 with the immunoglobulin H chain (IGH) gene, leading to overexpression of BCL2. Pathologists confirm IGH reconstruction as a basis for a definitive FL diagnosis.
Molecular genetic analyses provide important information regarding the pathogenesis and prognosis of FL. This section details the molecular genetic analyses of FLs.
Detection of t(14;18) translocation: As mentioned in the section on pathological diagnosis above (section 1.3), t(14;18) translocation is a key component of FL diagnosis.
Detection of gene rearrangements using polymerase chain reaction: In FLs, there are rearrangements of IGH and other genes. These can be detected via polymerase chain reaction, allowing for the evaluation of the presence and frequency of specific gene rearrangements.
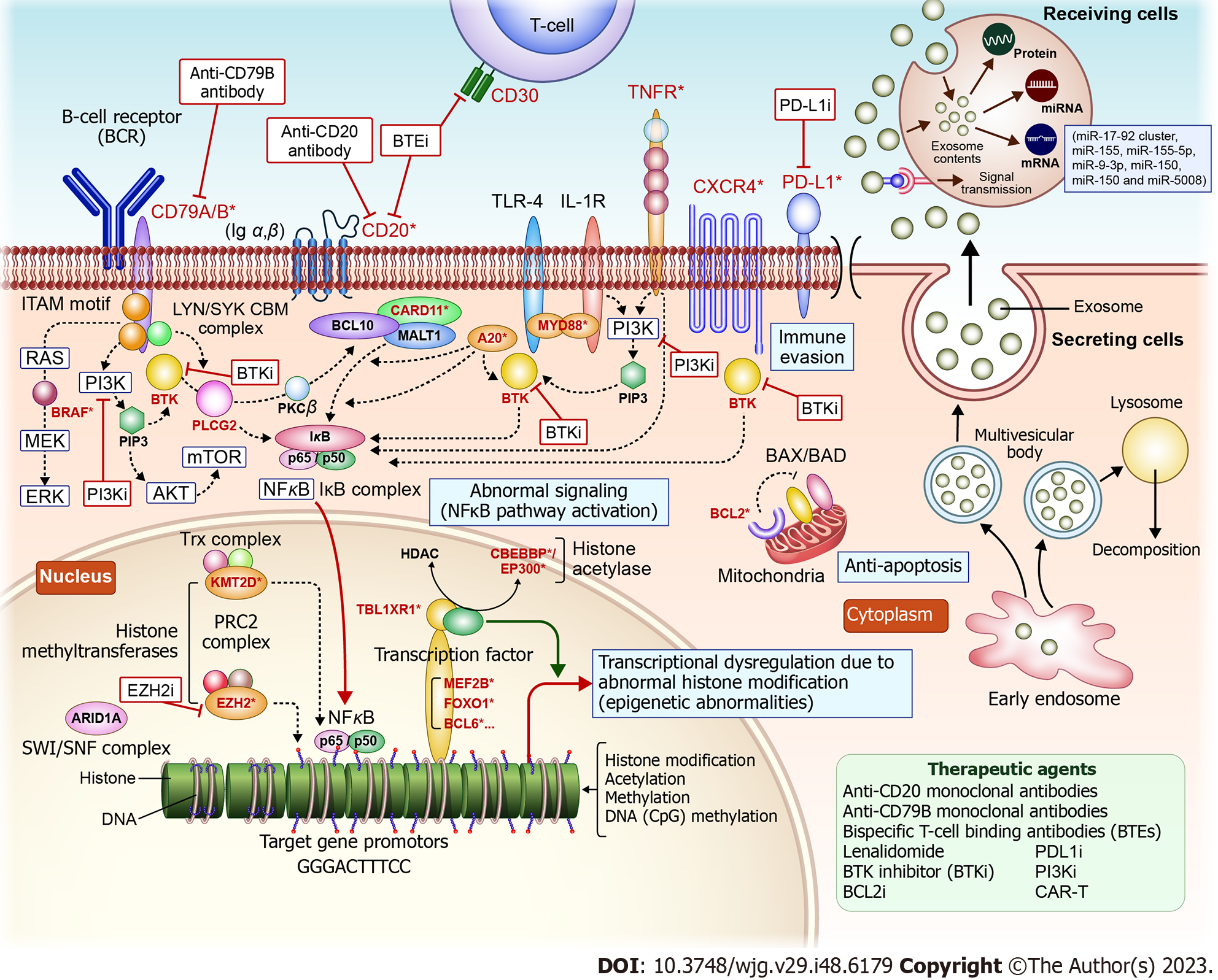
Genetic mutation analysis using next-generation sequencing: In FL, specific genetic mutations are associated with disease progression and prognosis. Next-generation sequencing (NGS) enables rapid and large-scale DNA sequencing to identify such mutations and assess their significance in FL. In particular, numerous genetic abnormalities have been characterized for BCL using NGS. These abnormalities in BCL impact proteins involved in tumorigenesis, resulting in: (1) Constant activation of the NF-κB signaling pathway; (2) Abnormal functioning of epigenome-related enzymes; (3) Abnormal expression of apoptosis-related factors; and (4) Immune system evasion (Figure 1)[8].
Constant activation of the NF-κB signaling pathway: This activation is involved in lymphoma development. Mutations in NF-κB pathway-related factors such as CD79B, MYD88, A20/tumor necrosis factor alpha-induced protein 3 (TNFAIP3), and CARD11 occur in active B-cell (ABC)-type diffuse large BCL (DLBCL).
Functional abnormalities in epigenome-related enzymes: Genetic abnormalities related to histone methyltransferases (such as KMT2D and EZH2) and histone acetylases (such as EP300 and CREBBP) occur in FL- and germinal center B-cell (GCB)-type DLBCL.
Abnormalities in apoptosis-related factors: Chromosome t(14;18) reciprocal translocations, found in most FLs and 20%-30% of DLBCLs, promote the expression of BCL with anti-apoptotic properties and are involved in cell immortalization.
Immune system evasion: Abnormalities in genes related to immune evasion, such as PDL1/L2, are detected in DCLBL-like forms, Hodgkin lymphomas, and adult T-cell lymphomas. These abnormalities possibly aid lymphoma cells in evading immune attacks. The following findings have recently been reported for each NGS-discovered BCL-related gene involved in FL development and proliferation:
Polatuzumab vedotin is a first-in-class anti-CD79B antibody-drug conjugate (ADC) that combines an anti-CD79B monoclonal antibody with a tubulin polymerization inhibitor. CD79B is specifically expressed in B-cells. Polatuzumab vedotin binds to CD79B to deliver the chemotherapeutic agents and destroy B-cells. Three major clinical trials have investigated the efficacy and safety of polatuzumab vedotin. The G029376 trial demonstrated its efficacy in relapsed/refractory (r/r) FL and DLBCL[9]. The GO29365 trial tested the efficacy of polatuzumab vedotin + bendamustine and rituximab (BR) or bendamustine and obinutuzumab therapy in patients with r/r FL or DLBCL[10]. The JO40762 (P-DRIVE) study evaluated the complete response rate in patients with r/r DLBCL treated with polatuzumab vedotin + BR therapy[11]. Comprehensive genetic analyses classified DLBCL into ABC-type DLBCL and GCB-type DLBCL. MYD88 and CD79B mutations are highly prevalent in ABC-type DLBCL and contribute to the constant activation of the NF-κB pathway, resulting in lymphoma development. In addition, ABC types with mutations in MYD88 and CD79B in BCLs have a poorer prognosis[12]. These classifications are important for predicting prognosis and treatment response.
A20/TNFAIP3 is a cytoplasmic zinc finger protein that inhibits NF-κB activity mediated by tumor necrosis factor (TNF). AIP3 deficiency is observed in ABC-type BCL, MALT lymphoma, and Hodgkin lymphoma. A20, also known as TNFAIP3, is involved in the negative regulation of the NF-κB pathway. A20 induces a suppressive inflammatory response and exhibits anti-apoptotic effects while affecting immune homeostasis. Mutations and deletions in A20 are frequent in non-GCB-typey DLBCL and DLBCL. A20 gene abnormalities are also reported in certain DLBCLs[13]. Deletions, inactivating mutations, and methylation of the A20 promoter at 6q23 inactivate A20 in various lymphomas. A20 inactivation is a key feature of lymphomas, and loss of A20 expression occurs in FLs[14]. Meanwhile, BCL10 is an essential regulator of NF-κB activation and is implicated in B-cell proliferation[15].
KMT2D is a histone lysine methyltransferase important in suppressing BCLs. Deletion of KMT2D increases the number of germinal center B-cells and promotes B-cell proliferation in mice. The overexpression of BCL2 combined with KMT2D deletion increases the incidence of lymphomas derived from germinal center B-cells. Therefore, KMT2D can function as a tumor suppressor and may be involved in reconstructing epigenetics that promote lymphoma development. Accordingly, targeting KMT2D is an effective approach for alleviating the early stages of tumorigenesis[16]. Tazemetostat is an orally administered EZH2 inhibitor that selectively inhibits a specific epigenetic-related protein, EZH2, to regulate the expression of cancer-related genes and inhibit cancer cell growth[17]. Tazemetostat was approved in Japan based on the results of clinical trials in patients with r/r B-cell non-Hodgkin lymphoma with EZH2 mutations[18,19]. Mutations in EZH2 occur in 7%-27% of FLs[20,21]; these patients may be eligible for treatment with tazemetostat. However, considering that FL is a refractory disease with frequent relapses, new treatment strategies are required. CREBBP, EZH2, MEF2B, and EP300 mutations occur in 33%, 27%, 15%, and 9% of FLs, respectively. Genome-wide analysis via NGS has shown that EZH2, CREBBP, and MLL2—histone-modifying genes—are frequently mutated in FL with essential roles in lymphomagenesis. IGH-BCL2 translocations and CREBBP mutations are early events, whereas MLL2 and TNFSFR14 mutations are late events during disease evolution. In the 2008 WHO classification, three new variants were included: (1) Pediatric FL; (2) Primary intestinal FL; and (3) In situ FL[22].
MiRNAs are non-coding RNAs involved in regulating gene expression. The aberrant expression of specific miRNAs is reported in FL. Hence, analyses of miRNAs provide essential information for predicting disease mechanisms and prognoses.
Relationship between exosomes and miRNAs: Exosomes are small vesicles released by cells containing various biomolecules (proteins, ribonucleic acids, and lipids) with myriad biological roles, including transmitting information between cells and expulsing unwanted intracellular components. MiRNAs are produced intracellularly and regulate gene expression by binding to mRNAs and regulating their stability and translation efficiency. The miRNAs produced inside cells can be wrapped in exosomes and released to the outside of the cell, facilitating the transport of miRNAs to other cells in remote areas, where they participate in information transfer and cell–cell interactions. This interaction is an important mechanism of information transfer and disease progression. The transport of miRNAs by exosomes is a form of extracellular signal transduction. When a sender cell receives a specific signal or is subjected to pathological conditions, the miRNA profile of the released exosomes changes, influencing the effect on recipient cells. Therefore, miRNA transduction by exosomes is important in regulating disease progression and cell–cell interactions (Figure 1).
MiRNAs associated with FL: Among the miRNAs related to FL are miR-17, miR-18a, miR19-a, miR-20a, miR-19b-1, and miR92a of the miR-17-92 cluster, and others are miR-155, miR-21, miR-155-5p, miR-150, miR-9-3p, miR-29 family, miR-21, miR-5008, miR-7e-5p, miR-451, miR-338-5p, miR-142, miR-376 cluster, and approximately 150 others (Table 1).
| MicroRNA(s) | Role in FL development | Sequence (5’ to 3’) | Ref. |
| miR-17 | Involved in cell proliferation and apoptosis | UAAAGUGCUUACAGUGCAGGUAG | [23-26] |
| miR-18a | Role in angiogenesis and tumorigenesis | UAAGGUGCAUCUAGUGCAGAUAG | [26] |
| miR-19a | Promotes cell survival | UGUGCAAAUCUAUGCAAAACUGA | [26] |
| miR-20a | Involved in cell cycle regulation | UAAAGUGCUCAUAGUGCAGGUAG | |
| miR-19b-1 | Promotes cell survival | UGUGCAAAUCCAUGCAAAUCUGA | |
| miR-92a | Role in angiogenesis | UAUUGCACUUGUCCCGGCCUGU | [26] |
| miR-155 | B-cell transformation in follicular lymphoma | UUAAUGCUAAUUGUGAUAGGGGU | [27-35] |
| miR-21 | Overexpressed and promotes tumor progression | UAGCUUAUCAGACUGAUGUUGA | [35] |
| miR-155-5p | Role for diagnosis of FL | UUAAUGCUAAUCGUGAUAGGGGU | [33,36] |
| miR-150 | Influences B-cell differentiation | UCUCACAUUGGUCUACAAUCU | [33,36,41] |
| miR-9-3p | Role for diagnosis of FL and in B-cell malignancies | UCUUUGGUUAUCUAGCUGUAUGA | [36-40] |
| miR-29 family | Epigenetic regulation and tumorigenesis | Varies by family member | [42] |
| miR-5008 | Regulation of endogenous mRNA levels | Sequence is not available | [43] |
| miR-7e-5p | Downregulation by c-MYC over-expression arise poor prognosis of FL | UGAGGUAGGAGGUUGUAUAGUU | [44] |
| miR-451 | Involved in cell cycle progression | AAACCGUUACCAUUACUGAGUU | [45] |
| miR-338-5p | Possible role in cellular proliferation | UAAUGACUGCACUGACCUUUGA | [45] |
| miR-142 | Role in hematopoiesis and B-cell function | UGUAGUGUUUCCUACUUUAUGGA | [46] |
| miR-376 cluster | Involvement in cellular differentiation | Varies by cluster member | [42] |
The miR-17-92 cluster is overexpressed in FLs and contains several miRNAs, including miR-17, miR-18a, miR-19a, miR-20a, miR-19b-1, and miR-92a. These are involved in cancer cell proliferation, cell cycle regulation, and apoptosis inhibition. The miRNA-17-92 cluster was first identified in malignant BCLs and has important roles in the immune system, heart and lung development, and oncogenic events[23]. Overexpression of the miR-17-92 cluster in MCL negatively regulates the expression of various BCR suppressor molecules, resulting in the overactivation of BCR signaling[24]. In a genetic analysis of 33 cases of small intestinal DLBCL and gastrointestinal MALT lymphomas, miRNA levels were reduced by more than two-thirds compared with those in normal tissues (13 species in the decreased group) and increased by more than 1.5-fold (15 species in the increased group), indicating the existence of miRNAs that were upregulated and downregulated[25], in association with FL development and tumor tissue growth. In patients with B-cell non-Hodgkin lymphoma (B-NHL), members of the miR-17-92 cluster are differentially expressed among the B-NHL subtypes, with the most prominent differences between FL and germinal center B-cell-like (GBC) subtypes[26]. MiR-18, miR-19a, and miR-92a were overexpressed in patients with B-NHL with better overall survival (OS), and high levels of miR-19a and miR-92a significantly reduced event-free survival (EFS). Meanwhile, miR-17-92 overexpression reduces the incubation period required for xenograft tumor visualization, and its knockout reduces tumor formation. MiR-17-92 expression in FL differs significantly from that in GBC, and miR-19a could have marked effects on OS and EFS in patients with B-NHL[26].
MiR-155 is a miRNA encoded by the MIR155 host gene or MIR155HG in humans. MiR-155 is involved in various physiological and pathological processes[27,28]. In vivo regulation of miR-155 expression from external molecular sources might suppress malignant tumor growth[29] and viral infections[30] while encouraging the advancement of cardiovascular diseases[31]. MIR155HG was initially identified as a gene that is transcriptionally activated via promoter insertion into a retroviral integration site commonly found in BCLs, earlier known as the B-cell integration cluster. RNA polymerase II carries out the transcription of MIR155HG. The resultant RNA is approximately 1500 nucleotides long and is capped and polyadenylated; single-stranded miR-155—a 23-nucleotide segment—is retained in exon 3 and undergoes processing from the parent RNA molecule[32]. Sequence analysis of small RNA clone libraries compared with those from other organ systems shows that miR-155-5p is one of five miRNAs (miR-142, miR-144, miR-150, miR-155, and miR-223) specific to hematopoietic cells, including B-cells, T-cells, monocytes, and granulocytes[33].
MiR-155 is transferred from leukemic cells to healthy B-cells via gap junctions and promotes their transformation into tumor-like cells. In addition, immature B-cells deficient in miR-155 evade apoptosis due to elevated BCL2 protein levels. This protein is involved in B-cell malignancy and is regulated by miR-155. Lawrie et al[34] reported that miR-155, miR-210, miR-106A, miR-149, and miR-139 are differentially regulated in non-neoplastic lymph nodes and DLBCL/FL. Meanwhile, Roehle et al[35] reported that miR-155, miR-21, and miR-221 are more highly expressed in ABC- than GCB-type cell lines. Notably, the expression levels of miR-155 and miR-21 were higher in non-malignant ABC cells than in GCB cells. Moreover, Arzuaga-Mendez et al[36] reported that miR-155 expression is a valuable biomarker for the diagnosis and differentiation of lymphoma subtypes, specifically miR-155-5p and miR-9-3p for the diagnosis of FL, and miR-150 and miR-17-92 cluster for the differential diagnosis of FL and DLBCL. Additionally, miR-9-3p is associated with tumorigenesis, functioning as an oncogene in papillary thyroid cancer[37,38] and a tumor suppressor in gastric cancer[38,39]. It also modulates breast cancer by influencing growth and drug resistance[38,40]. Lower levels of miR-150 and higher levels of its target, FOXP1, are associated with reduced OS, indicating that miR-150 might be an effective biomarker that can be quantified in formalin-fixed paraffin-embedded tissue. In addition, the MYC/miR-150/FOXP1 axis in malignant B-cells helps determine the aggressiveness and high-grade transformation of FL[41].
An array-based assay including 76 non-Hodgkin B-cell mixed lymphomas was performed to investigate the etiology of different BCLs using miRNAs. The miR-29 family and miR-17-92 cluster are regulated by MYC genes associated with MYC proteins in BCLs. A network analysis was used to demonstrate that a small set of differentially expressed miRNAs associated with MYC can either upregulate or downregulate a select group of hub proteins, including BCL2, CDK6, MYB, ZEB1, CTNNB1, BAX, and XBP1 in BCLs[42]. MiR-21 is associated with cancer progression and metastasis; it is also highly expressed in FLs and has been implicated in the regulation of cell proliferation and apoptosis.
Larrea et al[43] identified 21 genes with altered miRNA-binding sites in transformed FL (tFL), that is, FL that is transformed to have more aggressive tumor cells. More than 40% of these mutations are present only in patients with tFL. Mutations in BCL2 and EZH2 reduce the binding efficiency of miR-5008 and miR-5008, regulating endogenous mRNA levels[43].
Additionally, the downregulation of miR-7e-5p by c-MYC overexpression is associated with a poor prognosis in patients with FL[44]. Furthermore, a study reported several abnormally altered miRNAs in FL, specifically, 39 significantly downregulated and 27 significantly upregulated miRNAs. MiR-451 was the most downregulated (345-fold decrease), while miR-338-5p was the most upregulated (172-fold increase) compared with those in healthy donors[45].
Recurrent mutations in hsa-miR-142 and editing of the hsa-miR-376 cluster were observed in diffuse large B-cell and FLs. A direct physical interaction between miRNA and mRNA was observed using argonaute-2 photoactivated ribonucleoside-enhanced cross-linking and immunoprecipitation experiments. This integrated analysis revealed several regulatory pathways associated with lymphoma pathogenesis, including the Ras, PI3K-Akt, and MAPK signaling pathways affected by lymphoma mutations. The regulation of mRNAs via miRNAs is highly relevant to lymphoma pathogenesis[46]. The onset of malignant tumors, such as malignant lymphomas and other cancers, as well as the proliferation of tumor cells, is regulated quantitatively by miRNAs, not qualitatively. The intricate mechanisms regulating expression are balanced through elaborate and judicious positive and negative regulation.
MiRNAs exhibit duplicity among subgroups of malignant lymphomas. Many miRNAs regulating tumor cell growth overlap between DLBCL and FL. Aberrantly expressed miRNAs have been identified in patients with hematological malignancies, suggesting that they may be novel clinical diagnostic and prognostic biomarkers. Hershkovitz-Rokah et al[47] experimentally validated miRNA expression signatures in DLBCL and FL. The authors identified unique miRNA expression patterns for each disease, with some overlap and abnormal expression of miRNAs between them. Their analysis also led to the discovery of several relevant miRNA–mRNA pairs in each disease. Furthermore, potential regulatory pathways were highlighted via gene ontology analysis. Specific miRNAs (miR-15a, miR-16, miR-17, miR-106, miR-21, miR-155, and miR-34a-5p) were aberrantly expressed in DLBCL tumor tissues; their specific expression patterns proved useful for DLBCL diagnosis. The overexpression of these miRNAs influences FL pathogenesis. However, given the highly complex functions and interactions of miRNAs, various aspects remain to be elucidated. Understanding how these specific miRNAs are involved in the development and progression of FL is essential. In particular, genes and signaling pathways targeted by these miRNAs should be identified, as their regulation can alter cancer cell behavior[47].
MiRNAs are promising biomarkers for FL diagnosis and prognosis and are relatively stable in the blood and tissues. Hence, they can be measured using relatively simple experimental techniques. This could have potential applications for non-invasive diagnosis and prognosis of FL. Notably, the functions of individual miRNAs are complex and can each affect numerous genes and signaling pathways, requiring comprehensive and cumulative interpretation of research results to enable the development of effective treatments and new biomarkers.
Indeed, miRNAs represent potential new treatment targets for FL as they can be easily produced with simple 20-30 nucleotide sequences. Hence, if they can be administered at high concentrations to tumors via intravascular infusion, transarterial injection, or direct injection, miRNAs can elicit therapeutic effects by upregulating or downregulating relevant oncogenes.
GWAS examine the association between genetic polymorphisms (SNPs) and specific diseases. In FL, GWAS have identified genetic variants associated with the risk of disease development. For example, Skibola et al[48] conducted a large GWAS comprising 4523 cases and 13344 controls of European descent and identified five non-human leukocyte antigen (HLA) FL susceptibility loci and mutations in the HLA gene associated with FL risk: 11q23.3 near CXCR5, 11q24.3 near ETS1, 3q28 at LPP, 18q21.33 near BCL2, and 8q24.21 near PVT1. In addition, four HLA-DRβ1 multichain amino acids and independent signals of HLA class II rs17203612 and HLA class I rs3130437 were associated with FL risk. Therefore, mutations at several common loci outside the HLA region significantly contribute to FL risk[48]. Indeed, mutations at specific gene loci other than HLA have been identified for each of the five subtypes of non-Hodgkin lymphoma, namely, DLBCL, FL, chronic lymphocytic leukemia (CLL), MZL, and primary central nervous system lymphoma (PCNSL)[45]. The WEE1 Locus is a "potential therapeutic marker"[49]. Moore et al[50] estimated the odds ratios (ORs) and 95% confidence intervals (CIs) using logistic regression for the subjects' height and the risk of developing the four non-Hodgkin lymphoma subtypes. A slight significant difference was observed in an increased risk of developing CLL (OR = 1.08, 95%CI: 1.00-1.17, P = 0.049), whereas no significant association was observed with DLBCL, FL, or MZL[50].
Epidemiological studies have shown that the risk of non-Hodgkin lymphoma, including FL, is higher in patients with autoimmune disease. This association has been analyzed in a GWAS[51,52]. No association was reported between B-cell-mediated autoimmune diseases and the risk of FL or MZL[51]. Moreover, there was no association between autoimmune diseases and the risk of developing non-Hodgkin lymphoma; however, non-Hodgkin lymphoma shared more genetic etiologies with autoimmune diseases (P = 0.0041) than solid tumors[52]. In particular, genes involved in apoptosis and telomere length are associated with autoimmune diseases and non-Hodgkin lymphoma[52]. Additionally, Choi et al[53] constructed a site-specific polygenic risk score (PRS) using GWAS and identified risk variants for nine tumor types, including FL. They also estimated the hazard ratio for each tumor type in relation to the PRS in 400807 people of European descent from the United Kingdom Biobank; significant capacity reactivity was observed, with a two-fold higher risk in FL[53].
An association between other tumor types and blood lipid levels was reported by Kleinstern et al[54]. The authors analyzed the association between each non-Hodgkin lymphoma subtype and lipid traits using GWAS. High-density lipoprotein (HDL) cholesterol positively correlated with FL, DLBCL, and MZL, whereas triglyceride (TG) negatively correlated with MZL. There was no strong correlation between the OR and P value. Meanwhile, Wang et al[55] analyzed 2686 cases of FL through GWAS using SNP2HLA; the risk of developing FL increased with an increasing number of homozygous HLA class II loci. This supports the role of HLA zygosity in non-Hodgkin lymphoma cases and indicates that different immune pathways could underlie the etiology of non-Hodgkin lymphoma[55].
The association between prognostic predictors of FL and various loci has also been evaluated using a GWAS[56]. In a multicenter meta-analysis of 586 patients diagnosed with FL in Denmark, Sweden, and the United States, loci strongly associated with lymphoma-specific mortality were observed at SNPs on 17q24 (rs10491178)[56]. In addition, two high-binding SNPs in interleukin-8 (rs4073) were associated with OS[56]. Conde et al[57] showed that multiple independent loci on the X chromosome could be involved in the etiology of FL. The Xq21.1 signal was also observed in DLBCL, indicating the sharing of a susceptibility locus with FL[57]. Multiple loci involved in the development of B-cell non-Hodgkin lymphoma, including FL, have been identified. For example, several loci, beyond the five loci reported by Waller et al[58], are associated with developing non-Hodgkin lymphoma; a GWAS identified approximately 150 Loci associated with non-Hodgkin lymphoma development[58]. However, these loci have been identified in fragments; therefore, several other important loci could remain unknown. Further GWAS studies and advances in analysis methods will lead to the discovery of more new loci associated with the development of FL, elucidation of the mechanisms underlying the interactions that lead to lymphoma development, development of new therapeutic agents targeting these loci, and the era of FL cure (Figure 2).
Imaging studies are used to assess the disease stage. Common imaging modalities include chest radiography, ultrasonography, computed tomography, and magnetic resonance imaging. These assess the lymph node and visceral enlargement, presence of metastases, and location and size of the lesion.
Additional tests could be required in advanced stages or under certain circumstances. Bone marrow biopsies and cerebrospinal fluid tests help confirm the extent of the disease and the sites involved. Several diagnostic specialists (pathologists, hematologists, and radiologists) are involved in the diagnosis process to increase accuracy. Once the diagnosis is confirmed, an appropriate treatment strategy (chemotherapy, radiotherapy, immunotherapy, or surgery) is selected based on the disease stage and general health of the patient.
The following factors influence treatment selection for GI-FL[59,60]: (1) Lesion characteristics and progression: The lesions' location, size, and spread are assessed to determine the characteristics and progression of the disease. This provides a basis for determining disease risk and prognosis; (2) Establishing a pathological diagnosis: The pathological diagnosis of FL should be based on appropriate laboratory and histological evaluations. A pathological diagnosis forms the basis for choosing the most appropriate treatment[61]; and (3) Accurate staging assessment: Staging of the disease is based on accurate diagnosis and evaluation. The stage is important for assessing disease progression and developing a proper treatment plan[59]. Treatment decisions are determined by histological subtype and the extent of the disease[62], that is, stage (Lugano classification) (Table 2)[60]. Although histological grade is a good predictor of prognosis in nodal FL, this alone cannot determine the treatment. Stage, which indicates the extent and spread of the disease, determines the treatment; both stage and histological grade can be used concurrently. In primary GI-FL, personalized treatment choices are determined based on the individual circumstances of each case and the changes over time, in addition to the Lugano classification and histological grade[7].
| Stage | |
| I | Tumor confined to GI tract |
| Primary site or multiple, non-contiguous lesions | |
| II | Tumor extends into the abdomen from primary GI site |
| Nodal involvement | |
| II1 | Local: Paragastric in cases of gastric lymphoma and para-intestinal for intestinal lymphoma |
| II2 | Distant: Mesenteric in case of an intestinal primary lymphoma; otherwise paraaortic, paracaval, pelvic, or inguinal |
| IIE | Penetration of serosa involving adjacent organs or tissues: Enumerate sites of involvement, e.g., IIE (pancreas), IIE (large intestine), IIE (post-intestinal wall) |
| Where there is nodal involvement and penetration involving adjacent organs, the stage is denoted using a subscript (1 or 2) and E, e.g., II1E (pancreas) | |
| IV | Disseminated extra-nodal involvement or a GI tract lesion with supradiaphragmatic nodal involvement |
For advanced stages (III and IV), irradiation is used if the disease is confined to a small number of areas that can be irradiated; this is augmented with chemotherapy with or without immunotherapy depending on the extent of organ involvement, spread, number of sites, and presence of distant metastases. In stages III and IV, if there are one, two, or three fewer lesions and irradiation is feasible in the area, radiation plus the extent of organ-specific invasion, spread, number of sites, and presence of distant metastases may be considered. Additionally, chemotherapy, chemotherapy plus immunotherapy (or in some cases a triple therapy), and, if appropriate, for example, if the patient has symptoms of obstruction of the digestive tract, where resection would improve symptoms and patient quality of life, surgical resection can be considered. Treatment should be customized to individual cases considering the specific requirements and constraints. In GI-FL, the characteristics of the gastrointestinal tract should be considered, and treatment measures are tailored to the disease status of each patient[7]. GI-FL treatment should be personalized based on the disease stage and patient's general health status. If necessary, the treatment plan should be reviewed regularly and modified based on disease progression, treatment efficacy, and patient response.
A watch-and-wait approach may be adopted in some patients to treat GI-FL. In this approach, patients diagnosed in the early stages of the disease are not immediately administered treatment but are regularly observed and monitored. When GI-FL is detected early, patients have a good prognosis. In these cases, a good 10-year prognosis is possible with surgical resection alone or with R-CHOP treatment (a combination of chemotherapy and targeted therapy). However, "watch-and-wait" is often the mainstay of treatment owing to the side effects and reduced patient quality of life. Schmatz et al[63] compared 63 patients with stage I GI-FL in treatment and "watch-and-wait" groups; there was no difference in progression-free survival (PFS) or OS. In another study, patients with GI-FL and low tumor volume were divided into a "watch-and-wait" group (15 patients) and a rituximab combined with chemotherapy group (14 patients); no difference in prognosis was observed[64]. Given the broad distribution of GI-FL at the time of diagnosis, localized treatment options are often deemed inappropriate. However, certain pathological features, including lower tumor extension and invasiveness than those in nodal FL, suggest that “watch-and-wait” is a viable option. This approach is promising in some subtypes of GI-FL with a favorable prognosis. Iwamuro et al[65] recently reported a long-term retrospective study for localized (stage I or II) GI-FL patients managed with a watch-and-wait strategy, revealing 5-year and 10-year event-free survival rates of 91.1% and 86.9%, respectively. Hence, this strategy is reasonable for GI-FL patients in early disease stages. However, the individual patient’s condition prior to treatment, the overall effect of possible treatments, and their side effects should be considered. The “watch-and-wait” approach should be explained to the patient as an option before choosing the treatment.
Radiotherapy can be used to treat localized lesions, particularly when the lesions are localized or when used in combination with other treatment modalities. Radiotherapy uses high-energy radiation to destroy tumor cells. However, several factors have led to radiotherapy not being effectively adopted for treating nodal or gastrointestinal FL[66,67].
Wound avoidance: Radiotherapy can affect the surrounding tissues, particularly in the gastrointestinal tract, an area prone to wounding; therefore, other treatment options could be chosen to avoid wounding. Wound avoidance can reduce treatment-related complications and improve quality of life.
Dose limitation: The gastrointestinal tract is an area where radiation doses must be limited as it is sensitive to radiation damage, which could be associated with an increased risk of complications and side effects. Therefore, elective treatments may be preferred.
Advances in chemotherapy: Following the introduction of drug combinations and target-directed therapies, chemotherapy has proven more effective and selective than radiotherapy.
Risk of side effects and complications: Radiotherapy risks severe side effects and complications in some patients. Particularly in treating the gastrointestinal tract, radiotherapy can significantly impact daily activities, such as food intake and bowel movements. To minimize these risks, other treatment options are preferred.
These factors tend to reduce the use of radiotherapy in favor of more elective treatments and a multifaceted approach centered on chemotherapy. However, the indications of radiotherapy and the balance of benefits and risks must be assessed for each case. Radiotherapy could be beneficial for certain conditions to prevent FL progression.
Several novel FL agents are used primarily for treating nodal FL. This is the mainstay of treatment for advanced primary GI-FL, which is expected to show an increased incidence in the near future.
Antibodies: Monoclonal antibodies: Immunotherapy, particularly with rituximab and other anti-CD antibodies, is exceedingly effective in managing FL and constitutes a vital and superior component of modern treatment regimens. The success rate of rituximab monotherapy is 67% in untreated FL and 46% in patients with relapsed FL[68]. A German low-grade lymphoma study showed that rituximab plus CHOP was superior to CHOP alone in patients with advanced-stage FL and that OS was significantly improved[69]. In addition to rituximab, several other antibody-based agents have been developed, including tafatasitamab[70], polatuzumab vedotin[9], loncastiximab tecilin[71], maglorimab[72], and obinutumab[73]. Further, the combination of tafacitumab and lenalidomide[70] and polatuzumab vedotin[9] is effective. These therapies are promising for treating FL and other lymphomas; hence, clinical trials are ongoing. Additionally, biosimilars of rituximab have been approved, including CT-P10, which has shown efficacy and safety comparable to those of rituximab[74]. These developments are important for the treatment of FL and could contribute to reducing healthcare costs.
Bispecific T-cell-binding antibodies: Bispecific T-cell-binding antibodies (BTEs) are potent therapeutics that target specific tissues and cells by binding multiple antigens. They are commonly used to treat FL, with CD3 and CD20 being the primary targets. Mosnetuzumab and glofitumab are notable BTEs. Mosnetuzumab, a CD20 × CD3 bispecific antibody, demonstrated a high overall response rate (ORR) of 66% and a complete response rate (CRR) of 49% in a phase I study on r/r FL[75]. When combined with lenalidomide, an ORR of 92% and a CRR of 77% were achieved. Glofitamab, another BTE, showed positive results in a phase I study on r/r B-cell non-Hodgkin lymphoma, including painless lymphoma, with an ORR of 65.7% and CRR of 57.1%. Higher response rates were observed when the drug was combined with obinutuzumab[76]. The anti-CD3 and anti-CD20 BTE, epcoritamab, showed promising efficacy in phase I/II trials of r/r non-Hodgkin lymphoma and FL with a high ORR and CRR[77,78]. Similarly, odronestamab, a CD20 × CD3 bsAb, showed impressive results in phase I trials of r/r FL[79,80]. Overall, BTE is an effective immunotherapeutic strategy for FL. Further progress is expected from ongoing clinical trials.
Anti-PD-L1 antibodies: Anti-PD-L1 antibodies such as atezolizumab and pembrolizumab have shown promising efficacy in FL by enhancing T-cell function and antibody-dependent cellular cytotoxicity (ADCC) in NK cells. In a phase I study that combined atezolizumab and obinutumab, the ORR was found to be 54% (CRR: 23%) in patients with r/r FL and DLBCL, while an ORR of 17% (CRR: 4%) was observed for DLBCL alone. The PFS reached 9 mo for FL and 3 mo for DLBCL[81]. Another trial using pembrolizumab in combination with rituximab exhibited an ORR of 67% and a CRR of 50% in patients with r/r FL. The median PFS and 3-year OS rates were 12.6 mo and 97%, respectively. During the median follow-up duration of 35 mo, 23% of the patients maintained remission[82]. By enhancing T-cell function and NK cell ADCC, PD-1 blockade represents a fundamental mechanism of action that could further improve the therapeutic efficacy of anti-PD-1 antibody therapy in patients with FL.
Immunomodulatory drugs: Lenalidomide is an oral immunomodulatory drug used to treat FL and exhibits tumor-killing and immunomodulatory properties[83]. Clinical trials have shown that combining lenalidomide and rituximab (R2) has a higher ORR and a longer time to progression than lenalidomide alone[84]. The phase III AUGMENT trial showed a significant improvement in PFS with R2 compared with that with rituximab alone[85]. The phase IIIb MAGNIFY trial investigated the extension of R2 treatment and confirmed an ORR of 69% and a PFS of 40 mo[86]. The phase II GALEN trial tested a single arm of lenalidomide and obinutuzumab and reported an ORR of 95%, 2-year PFS of 65%, and OS of 87%[87]. Meanwhile, the phase III RELEVANCE trial showed no superiority of R2 over chemoimmunotherapy for frontline FL[88]. The phase II E2408 trial assessed the efficacy of three different approaches: Bendamustine/rituximab with bortezomib (BR) induction with R2 maintenance, BR induction with rituximab maintenance, and BR induction with maintenance using the proteasome inhibitor bortezomib and rituximab. The trial found that all three groups demonstrated similar and high CRRs[89]. Separately, a clinical trial utilizing lenalidomide and obinutuzumab, as the frontline therapy for patients with advanced untreated FL, reported encouraging results, with an ORR of 98%, CRR of 92%, and 2-year PFS of 96%[90]. Network meta-analyses of randomized controlled trials comparing treatment efficacy[91] were conducted to determine the optimal treatment regimens, sequences, and combination therapies. The evolution and diversification of therapeutics for FL have expanded the combinations of drugs with different mechanisms of action and treatment sequences; however, further clinical trials are needed to determine the most effective treatment regimen and sequence.
Molecular targeted therapies (small-molecule compounds): Bruton’s tyrosine kinase inhibitors: Bruton’s tyrosine kinase (BTK) plays a crucial role in regulating B-cell differentiation and activation in immune cells. For blood cancers like B-cell non-Hodgkin lymphoma and CLL, including FL, BTK inhibitors (BTKis) are currently under examination for their potential therapeutic benefits. First-generation ibrutinib, second-generation acalabrutinib and zanubrutinib, and third-generation piltobrutinib have demonstrated high effectiveness against B-cell non-Hodgkin lymphoma and CLL[92-96]. However, zanubrutinib monotherapy has limited efficacy in r/r FL[92]. Combination therapy with ibrutinib and rituximab resulted in better response rates in patients with r/r FL[95]. Acarabrutinib showed good efficacy and was well-tolerated by untreated and r/r FL patients[97]. In patients with r/r FL, the combination of zanubrutinib and obinutuzumab showed better PFS than obinutuzumab alone[98]. BTKis exhibited superior activity when used in combination therapies; hence, future studies should focus on evaluating various combinations of novel BTKis and other agents in patients with FL.
Proapoptotic pathway inhibitors (BCL2 inhibitors): Venetoclax, a potent BCL2 inhibitor, exhibits strong binding to BCL2, an anti-apoptotic protein often found in high levels in various blood cancers. By releasing apoptosis-promoting proteins, venetoclax triggers rapid and irreversible apoptosis in blood cancer cells. In a phase I trial of patients with FL, venetoclax monotherapy demonstrated an ORR of 38% and median PFS of 11 mo[99]. The phase II CONTRALTO trial compared different treatment groups, with the combination of venetoclax and rituximab presenting a CRR of 17%, venetoclax plus BR showing a CRR of 75%, and BR combined with venetoclax having a CRR of 69%[100]. Moreover, a phase I/II study reported promising results with the combination therapy of venetoclax and ibrutinib, showing an ORR of 69% and CRR of 25%[101]. BCL2 inhibitors have great potential for improving outcomes in patients with FL caused by BCL2 overexpression.
Epigenetic regulators: Drugs targeting the epigenetic regulation of gene expression, such as DNA methylation, are promising in treating blood cancers. Tazemetostat, an inhibitor of epigenetic regulator (EZH2) and histone methyltransferase, has shown positive outcomes. In a phase II study, tazemetostat treatment resulted in a higher ORR in patients with EZH2 mutations than in wild-type patients[19]. Patients with high-risk FL exhibited particularly favorable responses. The combination therapy of tazemetostat with rituximab and R2 is currently under investigation. Treatment with vorinostat, a histone deacetylase inhibitor, resulted in an ORR of 49% and median PFS of 20 mo in a phase II study of patients with r/r FL[102]. In a phase II study, vorinostat, in combination with rituximab, achieved an ORR of 50% and CRR of 41%[103]. Mocetinostat, another histone deacetylase inhibitor, showed limited efficacy, with an ORR of 12% in a phase II trial in patients with r/r FL[104].
EZH2 and histone deacetylase inhibitors hold promise as epigenetic regulators for the treatment of FL and are expected to yield improved therapeutic outcomes, particularly for tFL.
Phosphatidylinositol-3 kinase inhibitors: The BCR signaling pathway is homeostatically activated in B-cell tumors, leading to the development of inhibitors targeting this pathway. Phosphatidylinositol-3 kinase (PI3K) is a lipid kinase that mediates the phosphorylation of inositol ring 3 of inositol phospholipids—a membrane component[105]. Class I PI3Ks play an important role in signaling and are subdivided into four isoforms, α, β, ϒ, and δ[106]. Idelalisib, which selectively inhibits the δ isoform, is used in the treatment of r/r FL; it has shown the highest efficacy among PI3K inhibitors[107]. Nevertheless, most patients experience adverse events, and life-threatening adverse events limit the use of these agents[108,109]. Duvelisib, a dual inhibitor of PI3K-δ and PI3K-γ, has demonstrated impressive efficacy (70%); however, its use is also associated with a relatively high occurrence of adverse events (grade 3 or higher)[110,111]. It is the only PI3K inhibitor approved for treating r/r FL. Umbralisib is a selective PI3K-δ and casein kinase-1-ε (CK1ε) inhibitor and a fourth-generation PI3Ki with a potential therapeutic role in FL[112,113]. Parsaclisib is a potent PI3K isoform inhibitor that effectively treats r/r FL; however, it is also associated with frequent adverse events[114,115]. Zandelisib, a novel PI3-Kδ inhibitor, has shown good efficacy in the treatment of r/r indolent non-Hodgkin lymphoma with minimal adverse events[116]. However, clinical trials of these inhibitors are ongoing and require careful patient selection and implementation owing to the high frequency of adverse events.
PI3K/Akt/mTOR signaling pathway inhibitors: The PI3K/Akt/mTOR signaling pathway promotes abnormal regulation of tumor cell growth, metabolism, and survival[117]. Dual inhibitors aimed at this pathway have been developed and exhibit encouraging therapeutic effects, including high efficacy at minimal doses and low drug resistance[118]. Temsirolimus, a TOR inhibitor, when used with lenalidomide, showed synergistic effects in untreated advanced lymphomas, particularly in r/r classical Hodgkin lymphoma. However, hematological adverse events were frequent, with three grade 5 adverse events reported[119]. Further development of this novel PI3K/Akt/mTOR dual inhibitor and additional clinical trial data will enhance therapeutic outcomes and promote its use in FL treatment.
Cell-based therapies: CAR-T cell therapy is an innovative treatment for r/r hematological malignancies involving genetically engineered autologous T cells with chimeric antigen receptors (CARs) to target cancer cells. To date, CAR-T cell therapy has shown high efficacy; however, it is limited by hematological toxicity, including post-treatment cytoreduction[120,121]. In addition, several other challenges exist in the development of CAR-T therapies, including complex logistics, manufacturing constraints, toxicity concerns, and economic burdens[122]. Clinical trials have contrasted the effectiveness and safety of three cellular therapies: Autologous, allogeneic, and CAR-T cell therapies[123]. Recent clinical trials have evaluated autologous anti-CD19 CAR-T agents, such as axicabtagene ciloleucel (axi-cell), tisagenlecleucel (tisa-cell), and lisocabtagene maraleucel (liso-cell), all of which have shown remarkable efficacy against r/r DLBCL and FL. Various clinical trials have suggested that axi-cells can achieve an ORR of 94%, CRR of 79%, and sustained remission rate of 40%[124,125]. Tisa-cells have also shown substantial efficacy, achieving an ORR of 86% and CRR of 69% after a median follow-up of 17 mo[126-128]. Liso-cells, which are autologous anti-CD19 CAR-T cells, displayed high efficacy in r/r DLBCL where hematopoietic stem cell transplantation is not planned[129] and may be utilized in r/r FL in the future. The TRANSFORM and PILOT studies have also highlighted the potent efficacy of liso-cells in second-line treatment of r/r large BCL, leading to their approval as a third-line treatment option for aggressive BCL[130]. Despite these challenges, CAR-T-cell therapy could be developed as a fundamental treatment modality for FL.
Response-adapted post-induction strategy: The FOLL12 study compared standard rituximab maintenance therapy with experimental post-induction therapy for patients with FL. Experimental therapy ranged from observation of patients with the complete metabolic response (CMR) and minimal residual disease (MRD) negativity to administering four doses of rituximab to patients with CMR and MRD positivity until MRD became negative, to one dose of ibritumomab tiuxetan for non-CMR patients, followed by three doses of the standard treatment with rituximab maintenance. With a median follow-up of 53 mo, patients in the standard treatment group had significantly better PFS than those in the experimental group (3-year PFS, 86% vs 72%; P < 0.001). However, for patients with FL who responded positively to induction therapy, the standard 2-year rituximab maintenance therapy extended PFS following the remission induction[131].
Similar to that for nodal FL, the treatment strategy for GL-FL should be carefully considered based on Lugano stage and the histological grade. Furthermore, personalized treatment choices should be made based on the circumstances of individual patients and their changes over time[7]. The possibility of gastrointestinal perforation due to tumor reduction should always be considered, as this could be a lethal side effect. Moreover, the watch-and-wait strategy has been the mainstay option for GI-FL treatment. However, with the current advances in novel therapeutic agents, many advanced cases are increasingly being treated aggressively with chemotherapy, immunotherapy, or a combination of the two. Surgical resection and postoperative chemotherapy, immunotherapy, or a combination of these, could be an option in some instances, such as gastrointestinal obstruction[7]. Therefore, the therapeutic approach should be more individualized in GI-FL. Depending on the site and stage of the lesion and the patient's general health, a combination of surgery, chemotherapy, radiotherapy, and immunotherapy may be chosen. Conservative surgical or endoscopic treatment of the gastrointestinal tract may be an option depending on the tumor's specific anatomy and histological characteristics.
Molecular genetic analysis of FL has revealed overexpression of the BCL2 gene, rearrangement of the IGH gene, and genetic mutations in NF-κB pathway-related factors. To this end, genetic abnormalities related to histone methyltransferases and histone acetylases have been characterized. Approximately 150 miRNAs involved in the development and proliferation of FL, including the miR-17-92 cluster, miR-155, miR-155-5p, miR-9-3p, miR-150, miR-155, and miR-5008, have been identified. Their role as prognostic predictors has been investigated. Recently, GWAS have identified several loci involved in FL development.
There has been significant progress in the development of novel FL therapeutics targeting the genes responsible for FL pathogenesis and tumor growth, including anti-CD20, CD79 monoclonal antibodies, BTEs, anti-PD-L1 antibodies, lenalidomide, and immunomodulators such as lenalidomide, BTKis, BCL2 inhibitors, EZH2 inhibitors, PI3K inhibitors, dual inhibitors of the PI3K/Akt/mTOR signaling pathway, and CAR-T cell therapy. The efficacy of these novel agents is demonstrated in numerous clinical trials, not only as single agents but also in combination. Recent research focuses on identifying the best combination and the order of treatments in multi-drug therapy. The number of advanced cases of primary GI-FL and nodal cases is expected to increase. Gastroenterologists must be trained and offered sufficient practice in treating these patients, especially those in advanced stages.
I thank Dr. Watanabe T for his useful suggestions and comments on Figure 1, and I also thank Miss. Watanabe M for her useful comments on therapeutic agents.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology & hepatology
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Eid N, Malaysia; Shahriari M, Iran S-Editor: Yan JP L-Editor: Wang TQ P-Editor: Yan JP
| 1. | The world health organization classification of malignant lymphomas in Japan: incidence of recently recognized entities. Lymphoma Study Group of Japanese Pathologists. Pathol Int. 2000;50:696-702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 306] [Cited by in RCA: 343] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 2. | Jaffe ES, Harris NL, Stein H, Vardiman J. WHO Classification Tumors of the Hematopoietic and Lymphoid Tissues. Lyon: IARC Press, 2001. Available from: https://www.iarc.who.int/news-events/who-classification-of-tumours-of-haematopoietic-and-lymphoid-tissues-2/. |
| 3. | Chihara D, Ito H, Matsuda T, Shibata A, Katsumi A, Nakamura S, Tomotaka S, Morton LM, Weisenburger DD, Matsuo K. Differences in incidence and trends of haematological malignancies in Japan and the United States. Br J Haematol. 2014;164:536-545. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 193] [Cited by in RCA: 260] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 4. | Yamamoto S, Nakase H, Yamashita K, Matsuura M, Takada M, Kawanami C, Chiba T. Gastrointestinal follicular lymphoma: review of the literature. J Gastroenterol. 2010;45:370-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 82] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 5. | Watanabe T, Suda T, Hirono H, Hasegawa K, Soga K, Shibasaki K, Umezu H. Successful treatment of mucosa-associated lymphoid tissue lymphoma in a patient with gastric and rectal lesions with metachronous and ectopic development. Rare Tumors. 2011;3:e24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 6. | Watanabe T, Homma N, Ogata N, Saito H, Kanefuji T, Hasegawa K, Soga K, Shibasaki K, Endo T, Ajioka Y. Complete response in a patient with colonic mantle cell lymphoma with multiple lymphomatous polyposis treated with combination chemotherapy using anti-CD20 antibody and cladribine. Gut. 2007;56:449-450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 7. | Watanabe T. Recent advances in treatment of nodal and gastrointestinal follicular lymphoma. World J Gastroenterol. 2023;29:3574-3594. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 4] [Cited by in RCA: 6] [Article Influence: 3.0] [Reference Citation Analysis (11)] |
| 8. | Tomita A. Advances in molecular pathogenesis and targeted therapy of B-cell lymphoma. Gendai Igaku 2021; 68: 87-91. |
| 9. | Morschhauser F, Flinn IW, Advani R, Sehn LH, Diefenbach C, Kolibaba K, Press OW, Salles G, Tilly H, Chen AI, Assouline S, Cheson BD, Dreyling M, Hagenbeek A, Zinzani PL, Jones S, Cheng J, Lu D, Penuel E, Hirata J, Wenger M, Chu YW, Sharman J. Polatuzumab vedotin or pinatuzumab vedotin plus rituximab in patients with relapsed or refractory non-Hodgkin lymphoma: final results from a phase 2 randomised study (ROMULUS). Lancet Haematol. 2019;6:e254-e265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 188] [Article Influence: 31.3] [Reference Citation Analysis (0)] |
| 10. | Sehn LH, Herrera AF, Flowers CR, Kamdar MK, McMillan A, Hertzberg M, Assouline S, Kim TM, Kim WS, Ozcan M, Hirata J, Penuel E, Paulson JN, Cheng J, Ku G, Matasar MJ. Polatuzumab Vedotin in Relapsed or Refractory Diffuse Large B-Cell Lymphoma. J Clin Oncol. 2020;38:155-165. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 283] [Cited by in RCA: 545] [Article Influence: 90.8] [Reference Citation Analysis (0)] |
| 11. | Terui Y, Rai S, Izutsu K, Yamaguchi M, Takizawa J, Kuroda J, Ishikawa T, Kato K, Suehiro Y, Fukuhara N, Ohmine K, Goto H, Yamamoto K, Kanemura N, Ueda Y, Ishizawa K, Kumagai K, Kawasaki A, Saito T, Hashizume M, Shibayama H. A phase 2 study of polatuzumab vedotin + bendamustine + rituximab in relapsed/refractory diffuse large B-cell lymphoma. Cancer Sci. 2021;112:2845-2854. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 37] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 12. | Tsukamoto T, Tokuda Y, Nakano M, Tashiro K, Kuroda J. Expression of activated B-cell gene signature is predictive of the outcome of follicular lymphoma. Blood Adv. 2022;6:1932-1936. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 13. | Fujii M, Takata K, Chuang SS, Miyata-Takata T, Ando M, Sato Y, Yoshino T. A20 (TNFAIP3) Alterations in Primary Intestinal Diffuse Large B-cell Lymphoma. Acta Med Okayama. 2018;72:23-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 14. | Hirsch B, Grünbaum M, Wagner F, Bi Y, Lucka L, Du MQ, Stein H, Dürkop H. A novel A20 (TNFAIP3) antibody (Ber-A20) can be used to detect unmutated A20 by immunohistology. Histopathology. 2012;60:E19-E27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 15. | Xu W, Xue L, Sun Y, Henry A, Battle JM, Micault M, Morris SW. Bcl10 is an essential regulator for A20 gene expression. J Physiol Biochem. 2013;69:821-834. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 16. | Zhang J, Dominguez-Sola D, Hussein S, Lee JE, Holmes AB, Bansal M, Vlasevska S, Mo T, Tang H, Basso K, Ge K, Dalla-Favera R, Pasqualucci L. Disruption of KMT2D perturbs germinal center B cell development and promotes lymphomagenesis. Nat Med. 2015;21:1190-1198. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 278] [Cited by in RCA: 352] [Article Influence: 35.2] [Reference Citation Analysis (0)] |
| 17. | Knutson SK, Kawano S, Minoshima Y, Warholic NM, Huang KC, Xiao Y, Kadowaki T, Uesugi M, Kuznetsov G, Kumar N, Wigle TJ, Klaus CR, Allain CJ, Raimondi A, Waters NJ, Smith JJ, Porter-Scott M, Chesworth R, Moyer MP, Copeland RA, Richon VM, Uenaka T, Pollock RM, Kuntz KW, Yokoi A, Keilhack H. Selective inhibition of EZH2 by EPZ-6438 leads to potent antitumor activity in EZH2-mutant non-Hodgkin lymphoma. Mol Cancer Ther. 2014;13:842-854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 383] [Cited by in RCA: 427] [Article Influence: 38.8] [Reference Citation Analysis (0)] |
| 18. | Izutsu K, Ando K, Nishikori M, Shibayama H, Teshima T, Kuroda J, Kato K, Imaizumi Y, Nosaka K, Sakai R, Hojo S, Nakanishi T, Rai S. Phase II study of tazemetostat for relapsed or refractory B-cell non-Hodgkin lymphoma with EZH2 mutation in Japan. Cancer Sci. 2021;112:3627-3635. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 42] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 19. | Morschhauser F, Tilly H, Chaidos A, McKay P, Phillips T, Assouline S, Batlevi CL, Campbell P, Ribrag V, Damaj GL, Dickinson M, Jurczak W, Kazmierczak M, Opat S, Radford J, Schmitt A, Yang J, Whalen J, Agarwal S, Adib D, Salles G. Tazemetostat for patients with relapsed or refractory follicular lymphoma: an open-label, single-arm, multicentre, phase 2 trial. Lancet Oncol. 2020;21:1433-1442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 392] [Article Influence: 78.4] [Reference Citation Analysis (0)] |
| 20. | Bödör C, Grossmann V, Popov N, Okosun J, O'Riain C, Tan K, Marzec J, Araf S, Wang J, Lee AM, Clear A, Montoto S, Matthews J, Iqbal S, Rajnai H, Rosenwald A, Ott G, Campo E, Rimsza LM, Smeland EB, Chan WC, Braziel RM, Staudt LM, Wright G, Lister TA, Elemento O, Hills R, Gribben JG, Chelala C, Matolcsy A, Kohlmann A, Haferlach T, Gascoyne RD, Fitzgibbon J. EZH2 mutations are frequent and represent an early event in follicular lymphoma. Blood. 2013;122:3165-3168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 251] [Cited by in RCA: 261] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 21. | Morin RD, Johnson NA, Severson TM, Mungall AJ, An J, Goya R, Paul JE, Boyle M, Woolcock BW, Kuchenbauer F, Yap D, Humphries RK, Griffith OL, Shah S, Zhu H, Kimbara M, Shashkin P, Charlot JF, Tcherpakov M, Corbett R, Tam A, Varhol R, Smailus D, Moksa M, Zhao Y, Delaney A, Qian H, Birol I, Schein J, Moore R, Holt R, Horsman DE, Connors JM, Jones S, Aparicio S, Hirst M, Gascoyne RD, Marra MA. Somatic mutations altering EZH2 (Tyr641) in follicular and diffuse large B-cell lymphomas of germinal-center origin. Nat Genet. 2010;42:181-185. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1322] [Cited by in RCA: 1331] [Article Influence: 88.7] [Reference Citation Analysis (0)] |
| 22. | Takata K, Miyata-Takata T, Sato Y, Yoshino T. Pathology of follicular lymphoma. J Clin Exp Hematop. 2014;54:3-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 37] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 23. | Mogilyansky E, Rigoutsos I. The miR-17/92 cluster: a comprehensive update on its genomics, genetics, functions and increasingly important and numerous roles in health and disease. Cell Death Differ. 2013;20:1603-1614. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 570] [Cited by in RCA: 691] [Article Influence: 62.8] [Reference Citation Analysis (0)] |
| 24. | Jiang C, Bi C, Jiang X, Tian T, Huang X, Wang C, Fernandez MR, Iqbal J, Chan WC, McKeithan TW, Lewis RE, Fu K. The miR-17~92 cluster activates mTORC1 in mantle cell lymphoma by targeting multiple regulators in the STK11/AMPK/TSC/mTOR pathway. Br J Haematol. 2019;185:616-620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 25. | Nakamura S. Molecular aberrations in tentestinal B-cell lymphomas: comprehensive analyses for translocations and microRNA expression. Available from: https://kaken.nii.ac.jp/file/KAKENHI-PROJECT-25460418/25460418seika.pdf. |
| 26. | Yan S, Jia C, Quan L, Zhao L, Tian Y, Liu A. Significance of the microRNA1792 gene cluster expressed in Bcell nonHodgkin's lymphoma. Mol Med Rep. 2019;20:2459-2467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 27. | Tili E, Croce CM, Michaille JJ. miR-155: on the crosstalk between inflammation and cancer. Int Rev Immunol. 2009;28:264-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 264] [Cited by in RCA: 282] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 28. | O'Connell RM, Rao DS, Baltimore D. microRNA regulation of inflammatory responses. Annu Rev Immunol. 2012;30:295-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 634] [Cited by in RCA: 711] [Article Influence: 54.7] [Reference Citation Analysis (0)] |
| 29. | Babar IA, Cheng CJ, Booth CJ, Liang X, Weidhaas JB, Saltzman WM, Slack FJ. Nanoparticle-based therapy in an in vivo microRNA-155 (miR-155)-dependent mouse model of lymphoma. Proc Natl Acad Sci U S A. 2012;109:E1695-E1704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 373] [Cited by in RCA: 393] [Article Influence: 30.2] [Reference Citation Analysis (0)] |
| 30. | Wang L, Toomey NL, Diaz LA, Walker G, Ramos JC, Barber GN, Ning S. Oncogenic IRFs provide a survival advantage for Epstein-Barr virus- or human T-cell leukemia virus type 1-transformed cells through induction of BIC expression. J Virol. 2011;85:8328-8337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 41] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 31. | Corsten MF, Papageorgiou A, Verhesen W, Carai P, Lindow M, Obad S, Summer G, Coort SL, Hazebroek M, van Leeuwen R, Gijbels MJ, Wijnands E, Biessen EA, De Winther MP, Stassen FR, Carmeliet P, Kauppinen S, Schroen B, Heymans S. MicroRNA profiling identifies microRNA-155 as an adverse mediator of cardiac injury and dysfunction during acute viral myocarditis. Circ Res. 2012;111:415-425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 163] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 32. | Kim VN, Han J, Siomi MC. Biogenesis of small RNAs in animals. Nat Rev Mol Cell Biol. 2009;10:126-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2362] [Cited by in RCA: 2458] [Article Influence: 153.6] [Reference Citation Analysis (0)] |
| 33. | Landgraf P, Rusu M, Sheridan R, Sewer A, Iovino N, Aravin A, Pfeffer S, Rice A, Kamphorst AO, Landthaler M, Lin C, Socci ND, Hermida L, Fulci V, Chiaretti S, Foà R, Schliwka J, Fuchs U, Novosel A, Müller RU, Schermer B, Bissels U, Inman J, Phan Q, Chien M, Weir DB, Choksi R, De Vita G, Frezzetti D, Trompeter HI, Hornung V, Teng G, Hartmann G, Palkovits M, Di Lauro R, Wernet P, Macino G, Rogler CE, Nagle JW, Ju J, Papavasiliou FN, Benzing T, Lichter P, Tam W, Brownstein MJ, Bosio A, Borkhardt A, Russo JJ, Sander C, Zavolan M, Tuschl T. A mammalian microRNA expression atlas based on small RNA library sequencing. Cell. 2007;129:1401-1414. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3103] [Cited by in RCA: 2974] [Article Influence: 165.2] [Reference Citation Analysis (1)] |
| 34. | Lawrie CH, Soneji S, Marafioti T, Cooper CD, Palazzo S, Paterson JC, Cattan H, Enver T, Mager R, Boultwood J, Wainscoat JS, Hatton CS. MicroRNA expression distinguishes between germinal center B cell-like and activated B cell-like subtypes of diffuse large B cell lymphoma. Int J Cancer. 2007;121:1156-1161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 314] [Cited by in RCA: 293] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 35. | Roehle A, Hoefig KP, Repsilber D, Thorns C, Ziepert M, Wesche KO, Thiere M, Loeffler M, Klapper W, Pfreundschuh M, Matolcsy A, Bernd HW, Reiniger L, Merz H, Feller AC. MicroRNA signatures characterize diffuse large B-cell lymphomas and follicular lymphomas. Br J Haematol. 2008;142:732-744. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 136] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 36. | Arzuaga-Mendez J, Lopez-Santillan M, Garcia-Ruiz JC, Lopez-Lopez E, Martin-Guerrero I. Systematic review of the potential of MicroRNAs in the management of patients with follicular lymphoma. Crit Rev Oncol Hematol. 2021;159:103247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 37. | Zhang J, Liu Y, Liu Z, Wang XM, Yin DT, Zheng LL, Zhang DY, Lu XB. Differential expression profiling and functional analysis of microRNAs through stage I-III papillary thyroid carcinoma. Int J Med Sci. 2013;10:585-592. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 38. | Wang Y, Dong L, Wan F, Chen F, Liu D, Chen D, Long J. MiR-9-3p regulates the biological functions and drug resistance of gemcitabine-treated breast cancer cells and affects tumor growth through targeting MTDH. Cell Death Dis. 2021;12:861. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 39. | Meng Q, Xiang L, Fu J, Chu X, Wang C, Yan B. Transcriptome profiling reveals miR-9-3p as a novel tumor suppressor in gastric cancer. Oncotarget. 2017;8:37321-37331. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 40. | Barbano R, Pasculli B, Rendina M, Fontana A, Fusilli C, Copetti M, Castellana S, Valori VM, Morritti M, Graziano P, Luigi C, Coco M, Picardo F, Mazza T, Evron E, Murgo R, Maiello E, Esteller M, Fazio VM, Parrella P. Stepwise analysis of MIR9 loci identifies miR-9-5p to be involved in Oestrogen regulated pathways in breast cancer patients. Sci Rep. 2017;7:45283. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 42] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 41. | Musilova K, Devan J, Cerna K, Seda V, Pavlasova G, Sharma S, Oppelt J, Pytlik R, Prochazka V, Prouzova Z, Trbusek M, Zlamalikova L, Liskova K, Kruzova L, Jarosova M, Mareckova A, Kornauth C, Simonitsch-Klupp I, Schiefer AI, Merkel O, Mocikova H, Burda P, Machova Polakova K, Kren L, Mayer J, Zent CS, Trneny M, Evans AG, Janikova A, Mraz M. miR-150 downregulation contributes to the high-grade transformation of follicular lymphoma by upregulating FOXP1 levels. Blood. 2018;132:2389-2400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 41] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 42. | Malpeli G, Barbi S, Tosadori G, Greco C, Zupo S, Pedron S, Brunelli M, Bertolaso A, Scupoli MT, Krampera M, Kamga PT, Croce CM, Calin GA, Scarpa A, Zamò A. MYC-related microRNAs signatures in non-Hodgkin B-cell lymphomas and their relationships with core cellular pathways. Oncotarget. 2018;9:29753-29771. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 43. | Larrea E, Fernandez-Mercado M, Guerra-Assunção JA, Wang J, Goicoechea I, Gaafar A, Ceberio I, Lobo C, Okosun J, Enright AJ, Fitzgibbon J, Lawrie CH. Identification of Recurrent Mutations in the microRNA-Binding Sites of B-Cell Lymphoma-Associated Genes in Follicular Lymphoma. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 44. | Lou X, Fu J, Zhao X, Zhuansun X, Rong C, Sun M, Niu H, Wu L, Zhang Y, An L, Guo L, Wan S, Wang S. MiR-7e-5p downregulation promotes transformation of low-grade follicular lymphoma to aggressive lymphoma by modulating an immunosuppressive stroma through the upregulation of FasL in M1 macrophages. J Exp Clin Cancer Res. 2020;39:237. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 45. | Takei Y, Ohnishi N, Kisaka M, Mihara K. Determination of abnormally expressed microRNAs in bone marrow smears from patients with follicular lymphomas. Springerplus. 2014;3:288. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 46. | Hezaveh K, Kloetgen A, Bernhart SH, Mahapatra KD, Lenze D, Richter J, Haake A, Bergmann AK, Brors B, Burkhardt B, Claviez A, Drexler HG, Eils R, Haas S, Hoffmann S, Karsch D, Klapper W, Kleinheinz K, Korbel J, Kretzmer H, Kreuz M, Küppers R, Lawerenz C, Leich E, Loeffler M, Mantovani-Loeffler L, López C, McHardy AC, Möller P, Rohde M, Rosenstiel P, Rosenwald A, Schilhabel M, Schlesner M, Scholz I, Stadler PF, Stilgenbauer S, Sungalee S, Szczepanowski M, Trümper L, Weniger MA, Siebert R, Borkhardt A, Hummel M, Hoell JI; ICGC MMML-Seq Project. Alterations of microRNA and microRNA-regulated messenger RNA expression in germinal center B-cell lymphomas determined by integrative sequencing analysis. Haematologica. 2016;101:1380-1389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 38] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 47. | Hershkovitz-Rokah O, Geva P, Salmon-Divon M, Shpilberg O, Liberman-Aronov S. Network analysis of microRNAs, genes and their regulation in diffuse and follicular B-cell lymphomas. Oncotarget. 2018;9:7928-7941. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 48. | Skibola CF, Berndt SI, Vijai J, Conde L, Wang Z, Yeager M, de Bakker PI, Birmann BM, Vajdic CM, Foo JN, Bracci PM, Vermeulen RC, Slager SL, de Sanjose S, Wang SS, Linet MS, Salles G, Lan Q, Severi G, Hjalgrim H, Lightfoot T, Melbye M, Gu J, Ghesquières H, Link BK, Morton LM, Holly EA, Smith A, Tinker LF, Teras LR, Kricker A, Becker N, Purdue MP, Spinelli JJ, Zhang Y, Giles GG, Vineis P, Monnereau A, Bertrand KA, Albanes D, Zeleniuch-Jacquotte A, Gabbas A, Chung CC, Burdett L, Hutchinson A, Lawrence C, Montalvan R, Liang L, Huang J, Ma B, Liu J, Adami HO, Glimelius B, Ye Y, Nowakowski GS, Dogan A, Thompson CA, Habermann TM, Novak AJ, Liebow M, Witzig TE, Weiner GJ, Schenk M, Hartge P, De Roos AJ, Cozen W, Zhi D, Akers NK, Riby J, Smith MT, Lacher M, Villano DJ, Maria A, Roman E, Kane E, Jackson RD, North KE, Diver WR, Turner J, Armstrong BK, Benavente Y, Boffetta P, Brennan P, Foretova L, Maynadie M, Staines A, McKay J, Brooks-Wilson AR, Zheng T, Holford TR, Chamosa S, Kaaks R, Kelly RS, Ohlsson B, Travis RC, Weiderpass E, Clavel J, Giovannucci E, Kraft P, Virtamo J, Mazza P, Cocco P, Ennas MG, Chiu BC, Fraumeni JF Jr, Nieters A, Offit K, Wu X, Cerhan JR, Smedby KE, Chanock SJ, Rothman N. Genome-wide association study identifies five susceptibility loci for follicular lymphoma outside the HLA region. Am J Hum Genet. 2014;95:462-471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 87] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 49. | Hernández-Verdin I, Labreche K, Benazra M, Mokhtari K, Hoang-Xuan K, Alentorn A. Tracking the Genetic Susceptibility Background of B-Cell Non-Hodgkin's Lymphomas from Genome-Wide Association Studies. Int J Mol Sci. 2020;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 50. | Moore A, Kane E, Wang Z, Panagiotou OA, Teras LR, Monnereau A, Wong Doo N, Machiela MJ, Skibola CF, Slager SL, Salles G, Camp NJ, Bracci PM, Nieters A, Vermeulen RCH, Vijai J, Smedby KE, Zhang Y, Vajdic CM, Cozen W, Spinelli JJ, Hjalgrim H, Giles GG, Link BK, Clavel J, Arslan AA, Purdue MP, Tinker LF, Albanes D, Ferri GM, Habermann TM, Adami HO, Becker N, Benavente Y, Bisanzi S, Boffetta P, Brennan P, Brooks-Wilson AR, Canzian F, Conde L, Cox DG, Curtin K, Foretova L, Gapstur SM, Ghesquières H, Glenn M, Glimelius B, Jackson RD, Lan Q, Liebow M, Maynadie M, McKay J, Melbye M, Miligi L, Milne RL, Molina TJ, Morton LM, North KE, Offit K, Padoan M, Patel AV, Piro S, Ravichandran V, Riboli E, de Sanjose S, Severson RK, Southey MC, Staines A, Stewart C, Travis RC, Weiderpass E, Weinstein S, Zheng T, Chanock SJ, Chatterjee N, Rothman N, Birmann BM, Cerhan JR, Berndt SI. Genetically Determined Height and Risk of Non-hodgkin Lymphoma. Front Oncol. 2019;9:1539. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 51. | Wang SS, Vajdic CM, Linet MS, Slager SL, Voutsinas J, Nieters A, Casabonne D, Cerhan JR, Cozen W, Alarcón G, Martínez-Maza O, Brown EE, Bracci PM, Turner J, Hjalgrim H, Bhatti P, Zhang Y, Birmann BM, Flowers CR, Paltiel O, Holly EA, Kane E, Weisenburger DD, Maynadié M, Cocco P, Foretova L, Breen EC, Lan Q, Brooks-Wilson A, De Roos AJ, Smith MT, Roman E, Boffetta P, Kricker A, Zheng T, Skibola CF, Clavel J, Monnereau A, Chanock SJ, Rothman N, Benavente Y, Hartge P, Smedby KE. B-Cell NHL Subtype Risk Associated with Autoimmune Conditions and PRS. Cancer Epidemiol Biomarkers Prev. 2022;31:1103-1110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 52. | Din L, Sheikh M, Kosaraju N, Smedby KE, Bernatsky S, Berndt SI, Skibola CF, Nieters A, Wang S, McKay JD, Cocco P, Maynadié M, Foretová L, Staines A, Mack TM, de Sanjosé S, Vyse TJ, Padyukov L, Monnereau A, Arslan AA, Moore A, Brooks-Wilson AR, Novak AJ, Glimelius B, Birmann BM, Link BK, Stewart C, Vajdic CM, Haioun C, Magnani C, Conti DV, Cox DG, Casabonne D, Albanes D, Kane E, Roman E, Muzi G, Salles G, Giles GG, Adami HO, Ghesquières H, De Vivo I, Clavel J, Cerhan JR, Spinelli JJ, Hofmann J, Vijai J, Curtin K, Costenbader KH, Onel K, Offit K, Teras LR, Morton L, Conde L, Miligi L, Melbye M, Ennas MG, Liebow M, Purdue MP, Glenn M, Southey MC, Din M, Rothman N, Camp NJ, Wong Doo N, Becker N, Pradhan N, Bracci PM, Boffetta P, Vineis P, Brennan P, Kraft P, Lan Q, Severson RK, Vermeulen RCH, Milne RL, Kaaks R, Travis RC, Weinstein SJ, Chanock SJ, Ansell SM, Slager SL, Zheng T, Zhang Y, Benavente Y, Taub Z, Madireddy L, Gourraud PA, Oksenberg JR, Cozen W, Hjalgrim H, Khankhanian P. Genetic overlap between autoimmune diseases and non-Hodgkin lymphoma subtypes. Genet Epidemiol. 2019;43:844-863. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 53. | Choi J, Jia G, Wen W, Long J, Zheng W. Evaluating polygenic risk scores in assessing risk of nine solid and hematologic cancers in European descendants. Int J Cancer. 2020;147:3416-3423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 54. | Kleinstern G, Camp NJ, Berndt SI, Birmann BM, Nieters A, Bracci PM, McKay JD, Ghesquières H, Lan Q, Hjalgrim H, Benavente Y, Monnereau A, Wang SS, Zhang Y, Purdue MP, Zeleniuch-Jacquotte A, Giles GG, Vermeulen R, Cocco P, Albanes D, Teras LR, Brooks-Wilson AR, Vajdic CM, Kane E, Caporaso NE, Smedby KE, Salles G, Vijai J, Chanock SJ, Skibola CF, Rothman N, Slager SL, Cerhan JR. Lipid Trait Variants and the Risk of Non-Hodgkin Lymphoma Subtypes: A Mendelian Randomization Study. Cancer Epidemiol Biomarkers Prev. 2020;29:1074-1078. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 55. | Wang SS, Carrington M, Berndt SI, Slager SL, Bracci PM, Voutsinas J, Cerhan JR, Smedby KE, Hjalgrim H, Vijai J, Morton LM, Vermeulen R, Paltiel O, Vajdic CM, Linet MS, Nieters A, de Sanjose S, Cozen W, Brown EE, Turner J, Spinelli JJ, Zheng T, Birmann BM, Flowers CR, Becker N, Holly EA, Kane E, Weisenburger D, Maynadie M, Cocco P, Albanes D, Weinstein SJ, Teras LR, Diver WR, Lax SJ, Travis RC, Kaaks R, Riboli E, Benavente Y, Brennan P, McKay J, Delfau-Larue MH, Link BK, Magnani C, Ennas MG, Latte G, Feldman AL, Doo NW, Giles GG, Southey MC, Milne RL, Offit K, Musinsky J, Arslan AA, Purdue MP, Adami HO, Melbye M, Glimelius B, Conde L, Camp NJ, Glenn M, Curtin K, Clavel J, Monnereau A, Cox DG, Ghesquières H, Salles G, Bofetta P, Foretova L, Staines A, Davis S, Severson RK, Lan Q, Brooks-Wilson A, Smith MT, Roman E, Kricker A, Zhang Y, Kraft P, Chanock SJ, Rothman N, Hartge P, Skibola CF. HLA Class I and II Diversity Contributes to the Etiologic Heterogeneity of Non-Hodgkin Lymphoma Subtypes. Cancer Res. 2018;78:4086-4096. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 56. | Baecklund F, Foo JN, Bracci P, Darabi H, Karlsson R, Hjalgrim H, Rosenquist R, Adami HO, Glimelius B, Melbye M, Conde L, Liu J, Humphreys K, Skibola CF, Smedby KE. A comprehensive evaluation of the role of genetic variation in follicular lymphoma survival. BMC Med Genet. 2014;15:113. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 57. | Conde L, Foo JN, Riby J, Liu J, Darabi H, Hjalgrim H, Bracci PM, Smedby KE, Skibola CF. X chromosome-wide association study of follicular lymphoma. Br J Haematol. 2013;162:858-862. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 58. | Waller RG, Klein RJ, Vijai J, McKay JD, Clay-Gilmour A, Wei X, Madsen MJ, Sborov DW, Curtin K, Slager SL, Offit K, Vachon CM, Lipkin SM, Dumontet C, Camp NJ. Sequencing at lymphoid neoplasm susceptibility loci maps six myeloma risk genes. Hum Mol Genet. 2021;30:1142-1153. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 59. | ESMO. ESMO Clinical Practice Guidelines: Haematological Malignancies. Available from: https://www.esmo.org/guidelines/haematological-malignancies/primary-extranodal-lymphoma. |
| 60. | NCCN. NCCN Guidelines: B-cell Lymphomas. Available from: https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1457. |
| 61. | Watanabe T. Treatment strategies for nodal and gastrointestinal follicular lymphoma: current status and future development. World J Gastroenterol. 2010;16:5543-5554. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 11] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 62. | Rohatiner A, d'Amore F, Coiffier B, Crowther D, Gospodarowicz M, Isaacson P, Lister TA, Norton A, Salem P, Shipp M. Report on a workshop convened to discuss the pathological and staging classifications of gastrointestinal tract lymphoma. Ann Oncol. 1994;5:397-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 363] [Cited by in RCA: 350] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 63. | Schmatz AI, Streubel B, Kretschmer-Chott E, Püspök A, Jäger U, Mannhalter C, Tiemann M, Ott G, Fischbach W, Herzog P, Seitz G, Stolte M, Raderer M, Chott A. Primary follicular lymphoma of the duodenum is a distinct mucosal/submucosal variant of follicular lymphoma: a retrospective study of 63 cases. J Clin Oncol. 2011;29:1445-1451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 151] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 64. | Tari A, Kitadai Y, Mouri R, Takigawa H, Asaoku H, Mihara K, Takata K, Fujihara M, Yoshino T, Koga T, Fujimori S, Tanaka S, Chayama K. Watch-and-wait policy versus rituximab-combined chemotherapy in Japanese patients with intestinal follicular lymphoma. J Gastroenterol Hepatol. 2018;33:1461-1468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 65. | Iwamuro M, Tanaka T, Ennishi D, Matsueda K, Yoshioka M, Miyahara K, Sakaguchi C, Nishimura M, Nagahara T, Mannami T, Takenaka R, Oka S, Inoue M, Takimoto H, Inaba T, Kobayashi S, Toyokawa T, Tsugeno H, Suzuki S, Sawada S, Tanaka S, Tsuzuki T, Okada H. Long-term outcomes of patients with primary intestinal follicular lymphoma managed with watch-and-wait strategy. Sci Rep. 2023;13:5858. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 7] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 66. | Ghielmini M, Vitolo U, Kimby E, Montoto S, Walewski J, Pfreundschuh M, Federico M, Hoskin P, McNamara C, Caligaris-Cappio F, Stilgenbauer S, Marcus R, Trneny M, Dreger P, Montserrat E, Dreyling M; Panel Members of the 1st ESMO Consensus Conference on Malignant Lymphoma. ESMO Guidelines consensus conference on malignant lymphoma 2011 part 1: diffuse large B-cell lymphoma (DLBCL), follicular lymphoma (FL) and chronic lymphocytic leukemia (CLL). Ann Oncol. 2013;24:561-576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 174] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 67. | Dreyling M, Ghielmini M, Rule S, Salles G, Vitolo U, Ladetto M; ESMO Guidelines Committee. Newly diagnosed and relapsed follicular lymphoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2016;27:v83-v90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 163] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 68. | Ghielmini M, Schmitz SF, Cogliatti SB, Pichert G, Hummerjohann J, Waltzer U, Fey MF, Betticher DC, Martinelli G, Peccatori F, Hess U, Zucca E, Stupp R, Kovacsovics T, Helg C, Lohri A, Bargetzi M, Vorobiof D, Cerny T. Prolonged treatment with rituximab in patients with follicular lymphoma significantly increases event-free survival and response duration compared with the standard weekly x 4 schedule. Blood. 2004;103:4416-4423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 448] [Cited by in RCA: 421] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 69. | Hiddemann W, Kneba M, Dreyling M, Schmitz N, Lengfelder E, Schmits R, Reiser M, Metzner B, Harder H, Hegewisch-Becker S, Fischer T, Kropff M, Reis HE, Freund M, Wörmann B, Fuchs R, Planker M, Schimke J, Eimermacher H, Trümper L, Aldaoud A, Parwaresch R, Unterhalt M. Frontline therapy with rituximab added to the combination of cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) significantly improves the outcome for patients with advanced-stage follicular lymphoma compared with therapy with CHOP alone: results of a prospective randomized study of the German Low-Grade Lymphoma Study Group. Blood. 2005;106:3725-3732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1004] [Cited by in RCA: 1020] [Article Influence: 51.0] [Reference Citation Analysis (0)] |
| 70. | Duell J, Maddocks KJ, González-Barca E, Jurczak W, Liberati AM, De Vos S, Nagy Z, Obr A, Gaidano G, Abrisqueta P, Kalakonda N, André M, Dreyling M, Menne T, Tournilhac O, Augustin M, Rosenwald A, Dirnberger-Hertweck M, Weirather J, Ambarkhane S, Salles G. Long-term outcomes from the Phase II L-MIND study of tafasitamab (MOR208) plus lenalidomide in patients with relapsed or refractory diffuse large B-cell lymphoma. Haematologica. 2021;106:2417-2426. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 93] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 71. | Hamadani M, Radford J, Carlo-Stella C, Caimi PF, Reid E, O'Connor OA, Feingold JM, Ardeshna KM, Townsend W, Solh M, Heffner LT, Ungar D, Wang L, Boni J, Havenith K, Qin Y, Kahl BS. Final results of a phase 1 study of loncastuximab tesirine in relapsed/refractory B-cell non-Hodgkin lymphoma. Blood. 2021;137:2634-2645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 131] [Article Influence: 32.8] [Reference Citation Analysis (0)] |
| 72. | Advani R, Flinn I, Popplewell L, Forero A, Bartlett NL, Ghosh N, Kline J, Roschewski M, LaCasce A, Collins GP, Tran T, Lynn J, Chen JY, Volkmer JP, Agoram B, Huang J, Majeti R, Weissman IL, Takimoto CH, Chao MP, Smith SM. CD47 Blockade by Hu5F9-G4 and Rituximab in Non-Hodgkin's Lymphoma. N Engl J Med. 2018;379:1711-1721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 599] [Cited by in RCA: 857] [Article Influence: 122.4] [Reference Citation Analysis (0)] |
| 73. | Davies A, Kater AP, Sharman JP, Stilgenbauer S, Vitolo U, Klein C, Parreira J, Salles G. Obinutuzumab in the treatment of B-cell malignancies: a comprehensive review. Future Oncol. 2022;18:2943-2966. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 74. | Gmoshinskiĭ IV, Mazo VK, Shaternikov VA. [Breakdown of the soluble soybean antigen in the digestive tract of adult rats]. Vopr Pitan. 1986;43-46. [PubMed] |
| 75. | Budde LE, Assouline S, Sehn LH, Schuster SJ, Yoon SS, Yoon DH, Matasar MJ, Bosch F, Kim WS, Nastoupil LJ, Flinn IW, Shadman M, Diefenbach C, O'Hear C, Huang H, Kwan A, Li CC, Piccione EC, Wei MC, Yin S, Bartlett NL. Single-Agent Mosunetuzumab Shows Durable Complete Responses in Patients With Relapsed or Refractory B-Cell Lymphomas: Phase I Dose-Escalation Study. J Clin Oncol. 2022;40:481-491. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 228] [Article Influence: 76.0] [Reference Citation Analysis (0)] |
| 76. | Hutchings M, Morschhauser F, Iacoboni G, Carlo-Stella C, Offner FC, Sureda A, Salles G, Martínez-Lopez J, Crump M, Thomas DN, Morcos PN, Ferlini C, Bröske AE, Belousov A, Bacac M, Dimier N, Carlile DJ, Lundberg L, Perez-Callejo D, Umaña P, Moore T, Weisser M, Dickinson MJ. Glofitamab, a Novel, Bivalent CD20-Targeting T-Cell-Engaging Bispecific Antibody, Induces Durable Complete Remissions in Relapsed or Refractory B-Cell Lymphoma: A Phase I Trial. J Clin Oncol. 2021;39:1959-1970. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 259] [Cited by in RCA: 311] [Article Influence: 77.8] [Reference Citation Analysis (0)] |
| 77. | Hutchings M, Mous R, Clausen MR, Johnson P, Linton KM, Chamuleau MED, Lewis DJ, Sureda Balari A, Cunningham D, Oliveri RS, Elliott B, DeMarco D, Azaryan A, Chiu C, Li T, Chen KM, Ahmadi T, Lugtenburg PJ. Dose escalation of subcutaneous epcoritamab in patients with relapsed or refractory B-cell non-Hodgkin lymphoma: an open-label, phase 1/2 study. Lancet. 2021;398:1157-1169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 219] [Article Influence: 54.8] [Reference Citation Analysis (0)] |
| 78. | Falchi L, Lepp¨ AS, Wahlin BE, Nijland M, Christensen JH, De Vos S, Holte H, Linton KM, Abbas A, Wang LW, Dinh M, Elliott B, Belada D. Subcutaneous epcoritamab with rituximab + lenalidomide (R2) in patients (pts) with relapsed or refractory (R/R) follicular lymphoma (FL): Update from a phase 1/2 trial. J Clin Oncol. 2022;40 Suppl 16:7524. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 79. | Bannerji R, Arnason JE, Advani RH, Brown JR, Allan JN, Ansell SM, Barnes JA, O'Brien SM, Chávez JC, Duell J, Rosenwald A, Crombie JL, Ufkin M, Li J, Zhu M, Ambati SR, Chaudhry A, Lowy I, Topp MS. Odronextamab, a human CD20×CD3 bispecific antibody in patients with CD20-positive B-cell malignancies (ELM-1): results from the relapsed or refractory non-Hodgkin lymphoma cohort in a single-arm, multicentre, phase 1 trial. Lancet Haematol. 2022;9:e327-e339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 179] [Article Influence: 59.7] [Reference Citation Analysis (0)] |
| 80. | Dickinson M. Challenges in the development of bispecific antibodies for non-Hodgkin lymphoma. Lancet Haematol. 2022;9:e314-e315. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 81. | Palomba ML, Till BG, Park SI, Morschhauser F, Cartron G, Marks R, Shivhare M, Hong WJ, Raval A, Chang AC, Penuel E, Popplewell LL. Combination of Atezolizumab and Obinutuzumab in Patients with Relapsed/Refractory Follicular Lymphoma and Diffuse Large B-Cell Lymphoma: Results from a Phase 1b Study. Clin Lymphoma Myeloma Leuk. 2022;22:e443-e451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 14] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 82. | Nastoupil LJ, Chin CK, Westin JR, Fowler NH, Samaniego F, Cheng X, Ma MCJ, Wang Z, Chu F, Dsouza L, Obi C, Mims J, Feng L, Zhou S, Green M, Davis RE, Neelapu SS. Safety and activity of pembrolizumab in combination with rituximab in relapsed or refractory follicular lymphoma. Blood Adv. 2022;6:1143-1151. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 34] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 83. | Gandhi AK, Kang J, Havens CG, Conklin T, Ning Y, Wu L, Ito T, Ando H, Waldman MF, Thakurta A, Klippel A, Handa H, Daniel TO, Schafer PH, Chopra R. Immunomodulatory agents lenalidomide and pomalidomide co-stimulate T cells by inducing degradation of T cell repressors Ikaros and Aiolos via modulation of the E3 ubiquitin ligase complex CRL4(CRBN.). Br J Haematol. 2014;164:811-821. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 379] [Cited by in RCA: 510] [Article Influence: 42.5] [Reference Citation Analysis (0)] |
| 84. | Leonard JP, Jung SH, Johnson J, Pitcher BN, Bartlett NL, Blum KA, Czuczman M, Giguere JK, Cheson BD. Randomized Trial of Lenalidomide Alone Versus Lenalidomide Plus Rituximab in Patients With Recurrent Follicular Lymphoma: CALGB 50401 (Alliance). J Clin Oncol. 2015;33:3635-3640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 154] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 85. | Leonard JP, Trneny M, Izutsu K, Fowler NH, Hong X, Zhu J, Zhang H, Offner F, Scheliga A, Nowakowski GS, Pinto A, Re F, Fogliatto LM, Scheinberg P, Flinn IW, Moreira C, Cabeçadas J, Liu D, Kalambakas S, Fustier P, Wu C, Gribben JG; AUGMENT Trial Investigators. AUGMENT: A Phase III Study of Lenalidomide Plus Rituximab Versus Placebo Plus Rituximab in Relapsed or Refractory Indolent Lymphoma. J Clin Oncol. 2019;37:1188-1199. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 179] [Cited by in RCA: 294] [Article Influence: 49.0] [Reference Citation Analysis (0)] |
| 86. | Coleman M, Andorsky DJ, Yacoub A, Melear JM, Fanning SR, Kolibaba KS, Jason M, Lansigan F, Reynolds CM, Nowakowski GS, Gharibo M, Ahn E, Li J, Rummel MJ, Sharman JP. Patients with relapsed/refractory marginal zone lymphoma in the MAGNIFY phase IIIb interim analysis of induction R2 followed by maintenance. Blood. 2020;136 Suppl 1:24-25. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 87. | Morschhauser F, Le Gouill S, Feugier P, Bailly S, Nicolas-Virelizier E, Bijou F, Salles GA, Tilly H, Fruchart C, Van Eygen K, Snauwaert S, Bonnet C, Haioun C, Thieblemont C, Bouabdallah R, Wu KL, Canioni D, Meignin V, Cartron G, Houot R. Obinutuzumab combined with lenalidomide for relapsed or refractory follicular B-cell lymphoma (GALEN): a multicentre, single-arm, phase 2 study. Lancet Haematol. 2019;6:e429-e437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 67] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 88. | Morschhauser F, Fowler NH, Feugier P, Bouabdallah R, Tilly H, Palomba ML, Fruchart C, Libby EN, Casasnovas RO, Flinn IW, Haioun C, Maisonneuve H, Ysebaert L, Bartlett NL, Bouabdallah K, Brice P, Ribrag V, Daguindau N, Le Gouill S, Pica GM, Martin Garcia-Sancho A, López-Guillermo A, Larouche JF, Ando K, Gomes da Silva M, André M, Zachée P, Sehn LH, Tobinai K, Cartron G, Liu D, Wang J, Xerri L, Salles GA; RELEVANCE Trial Investigators. Rituximab plus Lenalidomide in Advanced Untreated Follicular Lymphoma. N Engl J Med. 2018;379:934-947. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 248] [Cited by in RCA: 267] [Article Influence: 38.1] [Reference Citation Analysis (0)] |
| 89. | Evens AM, Hong F, Habermann TM, Advani RH, Gascoyne RD, Witzig TE, Quon A, Ranheim EA, Ansell SM, Cheema PS, Dy PA, O'Brien TE, Winter JN, Cescon TP, Chang JE, Kahl BS. A Three-Arm Randomized Phase II Study of Bendamustine/Rituximab with Bortezomib Induction or Lenalidomide Continuation in Untreated Follicular Lymphoma: ECOG-ACRIN E2408. Clin Cancer Res. 2020;26:4468-4477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 90. | Nastoupil LJ, Westin JR, Hagemeister FB, Lee HJ, Fayad L, Samaniego F, Ahmed S, Claret L, Steiner RE, Nair R, Parmar S, Rodriguez MA, Wang ML, Green MR, Neelapu SS, Fowler NH. Results of a phase II study of obinutuzumab in combination with lenalidomide in previously untreated, high tumor burden follicular lymphoma (FL). Blood. 2019;134 Suppl 1:125. [RCA] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 91. | Wang Y, Zhou S, Qi X, Yang F, Maurer MJ, Habermann TM, Witzig TE, Wang ML, Nowakowski GS. Efficacy of front-line immunochemotherapy for follicular lymphoma: a network meta-analysis of randomized controlled trials. Blood Cancer J. 2022;12:1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 92. | Phillips T, Chan H, Tam CS, Tedeschi A, Johnston P, Oh SY, Opat S, Eom HS, Allewelt H, Stern JC, Tan Z, Novotny W, Huang J, Trotman J. Zanubrutinib monotherapy in relapsed/refractory indolent non-Hodgkin lymphoma. Blood Adv. 2022;6:3472-3479. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 28] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 93. | Song Y, Zhou K, Zou D, Zhou J, Hu J, Yang H, Zhang H, Ji J, Xu W, Jin J, Lv F, Feng R, Gao S, Guo H, Zhou L, Huang J, Novotny W, Kim P, Yu Y, Wu B, Zhu J. Zanubrutinib in relapsed/refractory mantle cell lymphoma: long-term efficacy and safety results from a phase 2 study. Blood. 2022;139:3148-3158. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 82] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 94. | Byrd JC, Brown JR, O'Brien S, Barrientos JC, Kay NE, Reddy NM, Coutre S, Tam CS, Mulligan SP, Jaeger U, Devereux S, Barr PM, Furman RR, Kipps TJ, Cymbalista F, Pocock C, Thornton P, Caligaris-Cappio F, Robak T, Delgado J, Schuster SJ, Montillo M, Schuh A, de Vos S, Gill D, Bloor A, Dearden C, Moreno C, Jones JJ, Chu AD, Fardis M, McGreivy J, Clow F, James DF, Hillmen P; RESONATE Investigators. Ibrutinib versus ofatumumab in previously treated chronic lymphoid leukemia. N Engl J Med. 2014;371:213-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1166] [Cited by in RCA: 1289] [Article Influence: 117.2] [Reference Citation Analysis (0)] |
| 95. | Sharman JP, Egyed M, Jurczak W, Skarbnik A, Pagel JM, Flinn IW, Kamdar M, Munir T, Walewska R, Corbett G, Fogliatto LM, Herishanu Y, Banerji V, Coutre S, Follows G, Walker P, Karlsson K, Ghia P, Janssens A, Cymbalista F, Woyach JA, Salles G, Wierda WG, Izumi R, Munugalavadla V, Patel P, Wang MH, Wong S, Byrd JC. Acalabrutinib with or without obinutuzumab versus chlorambucil and obinutuzmab for treatment-naive chronic lymphocytic leukaemia (ELEVATE TN): a randomised, controlled, phase 3 trial. Lancet. 2020;395:1278-1291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 283] [Cited by in RCA: 451] [Article Influence: 90.2] [Reference Citation Analysis (0)] |
| 96. | Mato AR, Shah NN, Jurczak W, Cheah CY, Pagel JM, Woyach JA, Fakhri B, Eyre TA, Lamanna N, Patel MR, Alencar A, Lech-Maranda E, Wierda WG, Coombs CC, Gerson JN, Ghia P, Le Gouill S, Lewis DJ, Sundaram S, Cohen JB, Flinn IW, Tam CS, Barve MA, Kuss B, Taylor J, Abdel-Wahab O, Schuster SJ, Palomba ML, Lewis KL, Roeker LE, Davids MS, Tan XN, Fenske TS, Wallin J, Tsai DE, Ku NC, Zhu E, Chen J, Yin M, Nair B, Ebata K, Marella N, Brown JR, Wang M. Pirtobrutinib in relapsed or refractory B-cell malignancies (BRUIN): a phase 1/2 study. Lancet. 2021;397:892-901. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 313] [Cited by in RCA: 310] [Article Influence: 77.5] [Reference Citation Analysis (0)] |
| 97. | Fowler NH, Coleman M, Stevens DA, Smith SM, Venugopal P, Martin P, Phillips TJ, Agajanian R, Stephens DM, Izumi R, Cheung J, Slatter JG, Yin M, Hiremath M, Hunder NNH. Acalabrutinib alone or in combination with rituximab (R) in follicular lymphoma (FL). J Clin Oncol. 2018;36 Suppl 15:7549. |
| 98. | Zinzani PL, Mayer J, Auer R, Bijou F, de Oliverira AC, Flowers C, Merli M, Bouabdallah K, Ganly PS, Johnson R, Yuen S, Kingsley E, Tumyan G, Assouline SE, Ivanova E, Kim P, Huang J, Delarue R, Trotman J. Zanubrutinib plus obinutuzumab (ZO) vs obinutuzumab (O) monotherapy in patients (pts) with relapsed or refractory (R/R) follicular lymphoma (FL): Primary analysis of the phase 2 randomized ROSEWOOD trial. J Clin Oncol. 2022;40 Suppl 16:7510. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 99. | Parikh A, Gopalakrishnan S, Freise KJ, Verdugo ME, Menon RM, Mensing S, Salem AH. Exposure-response evaluations of venetoclax efficacy and safety in patients with non-Hodgkin lymphoma. Leuk Lymphoma. 2018;59:871-879. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 100. | Zinzani PL, Flinn IW, Yuen SLS, Topp MS, Rusconi C, Fleury I, Le Dû K, Arthur C, Pro B, Gritti G, Crump M, Petrich A, Samineni D, Sinha A, Punnoose EA, Szafer-Glusman E, Spielewoy N, Mobasher M, Humphrey K, Kornacker M, Hiddemann W. Venetoclax-rituximab with or without bendamustine vs bendamustine-rituximab in relapsed/refractory follicular lymphoma. Blood. 2020;136:2628-2637. [PubMed] |
| 101. | Ujjani CS, Lai C, Leslie LA, Ramzi P, Tan M, Wang S, Wang HK, Shim E, Swanson N, Broome CM, Gopal AK, Smith SD, Warren EH, Blue K, Kdiry S, Till BG, Lynch RC, Shadman M, Johnson M, Coye H, Shelby M, Tseng YD, Shustov A, Maloney DG, Cheson BD. Ibrutinib and venetoclax in relapsed and refractory follicular lymphoma. Blood. 2020;136 Suppl 1:46-47. [RCA] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 102. | Ogura M, Ando K, Suzuki T, Ishizawa K, Oh SY, Itoh K, Yamamoto K, Au WY, Tien HF, Matsuno Y, Terauchi T, Mori M, Tanaka Y, Shimamoto T, Tobinai K, Kim WS. A multicentre phase II study of vorinostat in patients with relapsed or refractory indolent B-cell non-Hodgkin lymphoma and mantle cell lymphoma. Br J Haematol. 2014;165:768-776. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 93] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 103. | Chen R, Frankel P, Popplewell L, Siddiqi T, Ruel N, Rotter A, Thomas SH, Mott M, Nathwani N, Htut M, Nademanee A, Forman SJ, Kirschbaum M. A phase II study of vorinostat and rituximab for treatment of newly diagnosed and relapsed/refractory indolent non-Hodgkin lymphoma. Haematologica. 2015;100:357-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 60] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 104. | Batlevi CL, Crump M, Andreadis C, Rizzieri D, Assouline SE, Fox S, van der Jagt RHC, Copeland A, Potvin D, Chao R, Younes A. A phase 2 study of mocetinostat, a histone deacetylase inhibitor, in relapsed or refractory lymphoma. Br J Haematol. 2017;178:434-441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 82] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 105. | Vanhaesebroeck B, Leevers SJ, Ahmadi K, Timms J, Katso R, Driscoll PC, Woscholski R, Parker PJ, Waterfield MD. Synthesis and function of 3-phosphorylated inositol lipids. Annu Rev Biochem. 2001;70:535-602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1214] [Cited by in RCA: 1221] [Article Influence: 53.1] [Reference Citation Analysis (0)] |
| 106. | Bi L, Okabe I, Bernard DJ, Nussbaum RL. Early embryonic lethality in mice deficient in the p110beta catalytic subunit of PI 3-kinase. Mamm Genome. 2002;13:169-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 128] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 107. | Isidori A, Loscocco F, Visani G, Paolasini S, Scalzulli P, Musto P, Perrone T, Guarini A, Pastore D, Mazza P, Tonialini L, Pavone V, De Santis G, Tarantini G. Real-life efficacy and safety of idelalisib in 55 double-refractory follicular lymphoma patients. Br J Haematol. 2022;199:339-343. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 108. | Gordon MJ, Huang J, Chan RJ, Bhargava P, Danilov AV. Medical comorbidities in patients with chronic lymphocytic leukaemia treated with idelalisib: analysis of two large randomised clinical trials. Br J Haematol. 2021;192:720-728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 109. | Coutré SE, Barrientos JC, Brown JR, de Vos S, Furman RR, Keating MJ, Li D, O'Brien SM, Pagel JM, Poleski MH, Sharman JP, Yao NS, Zelenetz AD. Management of adverse events associated with idelalisib treatment: expert panel opinion. Leuk Lymphoma. 2015;56:2779-2786. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 250] [Cited by in RCA: 253] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 110. | Sasaki T, Irie-Sasaki J, Jones RG, Oliveira-dos-Santos AJ, Stanford WL, Bolon B, Wakeham A, Itie A, Bouchard D, Kozieradzki I, Joza N, Mak TW, Ohashi PS, Suzuki A, Penninger JM. Function of PI3Kgamma in thymocyte development, T cell activation, and neutrophil migration. Science. 2000;287:1040-1046. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 848] [Cited by in RCA: 869] [Article Influence: 34.8] [Reference Citation Analysis (0)] |
| 111. | Wang Z, Zhou H, Xu J, Wang J, Niu T. Safety and efficacy of dual PI3K-δ, γ inhibitor, duvelisib in patients with relapsed or refractory lymphoid neoplasms: A systematic review and meta-analysis of prospective clinical trials. Front Immunol. 2022;13:1070660. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 112. | Maharaj K, Powers JJ, Achille A, Mediavilla-Varela M, Gamal W, Burger KL, Fonseca R, Jiang K, Miskin HP, Maryanski D, Monastyrskyi A, Duckett DR, Roush WR, Cleveland JL, Sahakian E, Pinilla-Ibarz J. The dual PI3Kδ/CK1ε inhibitor umbralisib exhibits unique immunomodulatory effects on CLL T cells. Blood Adv. 2020;4:3072-3084. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 53] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 113. | Schweitzer J, Hoffman M, Graf SA. The evidence to date on umbralisib for the treatment of refractory marginal zone lymphoma and follicular lymphoma. Expert Opin Pharmacother. 2022;23:535-541. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 114. | Fukuhara N, Suehiro Y, Kato H, Kusumoto S, Coronado C, Rappold E, Zhao W, Li J, Gilmartin A, Izutsu K. Parsaclisib in Japanese patients with relapsed or refractory B-cell lymphoma (CITADEL-111): A phase Ib study. Cancer Sci. 2022;113:1702-1711. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Reference Citation Analysis (0)] |
| 115. | Forero-Torres A, Ramchandren R, Yacoub A, Wertheim MS, Edenfield WJ, Caimi P, Gutierrez M, Akard L, Escobar C, Call J, Persky D, Iyer S, DeMarini DJ, Zhou L, Chen X, Dawkins F, Phillips TJ. Parsaclisib, a potent and highly selective PI3Kδ inhibitor, in patients with relapsed or refractory B-cell malignancies. Blood. 2019;133:1742-1752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 86] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 116. | Goto H, Izutsu K, Ennishi D, Mishima Y, Makita S, Kato K, Hanaya M, Hirano S, Narushima K, Teshima T, Nagai H, Ishizawa K. Zandelisib (ME-401) in Japanese patients with relapsed or refractory indolent non-Hodgkin's lymphoma: an open-label, multicenter, dose-escalation phase 1 study. Int J Hematol. 2022;116:911-921. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 117. | Wu X, Xu Y, Liang Q, Yang X, Huang J, Wang J, Zhang H, Shi J. Recent Advances in Dual PI3K/mTOR Inhibitors for Tumour Treatment. Front Pharmacol. 2022;13:875372. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 56] [Reference Citation Analysis (0)] |
| 118. | McCurdy A, Visram A. The Role of Belantamab Mafodotin, Selinexor, and Melflufen in Multiple Myeloma. Curr Hematol Malig Rep. 2022;17:306-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 119. | Major A, Kline J, Karrison TG, Fishkin PAS, Kimball AS, Petrich AM, Nattam S, Rao K, Sleckman BG, Cohen K, Besien KV, Rapoport AP, Smith SM. Phase I/II clinical trial of temsirolimus and lenalidomide in patients with relapsed and refractory lymphomas. Haematologica. 2022;107:1608-1618. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 23] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 120. | Sharma N, Reagan PM, Liesveld JL. Cytopenia after CAR-T Cell Therapy-A Brief Review of a Complex Problem. Cancers (Basel). 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 78] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 121. | Yassine F, Murthy H, Ghabashi E, Kharfan-Dabaja MA, Iqbal M. Understanding the Etiology of Pancytopenias in the CAR T-Cell Therapy Setting: What We Know and What We Don't? Hematol Oncol Stem Cell Ther. 2022;15:122-130. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 122. | Gajra A, Zalenski A, Sannareddy A, Jeune-Smith Y, Kapinos K, Kansagra A. Barriers to Chimeric Antigen Receptor T-Cell (CAR-T) Therapies in Clinical Practice. Pharmaceut Med. 2022;36:163-171. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 56] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 123. | Goldsmith SR, Ghobadi A, Dipersio JF, Hill B, Shadman M, Jain T. Chimeric Antigen Receptor T Cell Therapy versus Hematopoietic Stem Cell Transplantation: An Evolving Perspective. Transplant Cell Ther. 2022;28:727-736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 124. | Jacobson CA, Chavez JC, Sehgal AR, William BM, Munoz J, Salles G, Munshi PN, Casulo C, Maloney DG, de Vos S, Reshef R, Leslie LA, Yakoub-Agha I, Oluwole OO, Fung HCH, Rosenblatt J, Rossi JM, Goyal L, Plaks V, Yang Y, Vezan R, Avanzi MP, Neelapu SS. Axicabtagene ciloleucel in relapsed or refractory indolent non-Hodgkin lymphoma (ZUMA-5): a single-arm, multicentre, phase 2 trial. Lancet Oncol. 2022;23:91-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 392] [Article Influence: 98.0] [Reference Citation Analysis (0)] |
| 125. | Mohty R, Kharfan-Dabaja MA. CAR T-cell therapy for follicular lymphoma and mantle cell lymphoma. Ther Adv Hematol. 2022;13:20406207221142133. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 126. | Jacobson CA, Chavez JC, Sehgal A, William BM, Munoz J, Salles GA. Outcomes in ZUMA-5 with axicabtagene ciloleucel (axi-cel) in patients (pts) Outcomes in ZUMA-5 with axicabtagene ciloleucel (axi-cel) in patients (pts) with relapsed/refractory (R/R) indolent non-Hodgkin lymphoma (iNHL) who had the high-risk feature of progression within 24 mo from initiation of first anti-CD20-containing chemoimmunotherapy (POD24). J Clin Oncol. 2021;39 Suppl 15:7515. [RCA] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 127. | Fowler NH, Dickinson M, Dreyling M, Martinez-Lopez J, Kolstad A, Butler J, Ghosh M, Popplewell L, Chavez JC, Bachy E, Kato K, Harigae H, Kersten MJ, Andreadis C, Riedell PA, Ho PJ, Pérez-Simón JA, Chen AI, Nastoupil LJ, von Tresckow B, Ferreri AJM, Teshima T, Patten PEM, McGuirk JP, Petzer AL, Offner F, Viardot A, Zinzani PL, Malladi R, Zia A, Awasthi R, Masood A, Anak O, Schuster SJ, Thieblemont C. Tisagenlecleucel in adult relapsed or refractory follicular lymphoma: the phase 2 ELARA trial. Nat Med. 2022;28:325-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 318] [Article Influence: 106.0] [Reference Citation Analysis (0)] |
| 128. | Salles G, Schuster SJ, Dreyling M, Fischer L, Kuruvilla J, Patten PEM, von Tresckow B, Smith SM, Jiménez-Ubieto A, Davis KL, Anjos C, Chu J, Zhang J, Lobetti Bodoni C, Thieblemont C, Fowler NH, Dickinson M, Martínez-López J, Wang Y, Link BK. Efficacy comparison of tisagenlecleucel vs usual care in patients with relapsed or refractory follicular lymphoma. Blood Adv. 2022;6:5835-5843. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 27] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 129. | Sehgal A, Hoda D, Riedell PA, Ghosh N, Hamadani M, Hildebrandt GC, Godwin JE, Reagan PM, Wagner-Johnston N, Essell J, Nath R, Solomon SR, Champion R, Licitra E, Fanning S, Gupta N, Dubowy R, D'Andrea A, Wang L, Ogasawara K, Thorpe J, Gordon LI. Lisocabtagene maraleucel as second-line therapy in adults with relapsed or refractory large B-cell lymphoma who were not intended for haematopoietic stem cell transplantation (PILOT): an open-label, phase 2 study. Lancet Oncol. 2022;23:1066-1077. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 136] [Article Influence: 45.3] [Reference Citation Analysis (0)] |
| 130. | St-Pierre F, Gordon LI. Lisocabtagene maraleucel in the treatment of relapsed/refractory large B-cell lymphoma. Future Oncol. 2023;19:19-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 131. | Luminari S, Manni M, Galimberti S, Versari A, Tucci A, Boccomini C, Farina L, Olivieri J, Marcheselli L, Guerra L, Ferrero S, Arcaini L, Cavallo F, Kovalchuk S, Skrypets T, Del Giudice I, Chauvie S, Patti C, Stelitano C, Ricci F, Pinto A, Margiotta Casaluci G, Zilioli VR, Merli A, Ladetto M, Bolis S, Pavone V, Chiarenza A, Arcari A, Anastasia A, Dondi A, Mannina D, Federico M; Fondazione Italiana Linfomi. Response-Adapted Postinduction Strategy in Patients With Advanced-Stage Follicular Lymphoma: The FOLL12 Study. J Clin Oncol. 2022;40:729-739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 62] [Article Influence: 15.5] [Reference Citation Analysis (0)] |