Published online Dec 21, 2023. doi: 10.3748/wjg.v29.i47.6122
Peer-review started: August 28, 2023
First decision: September 23, 2023
Revised: October 23, 2023
Accepted: December 1, 2023
Article in press: December 1, 2023
Published online: December 21, 2023
Processing time: 112 Days and 17.9 Hours
Patients with Barcelona clinic liver cancer (BCLC) stage B hepatocellular carcinoma (HCC) are considerably heterogeneous in terms of tumor burden, liver function, and performance status. To improve the poor survival outcomes of these patients, treatment approaches other than transarterial chemoembolization (TACE), which is recommended by HCC guidelines, have been adopted in real-world clinical practice. We hypothesize that this non-adherence to treatment guidelines, particularly with respect to the use of liver resection, improves survival in patients with stage B HCC.
To assess guideline adherence in South Korean patients with stage B HCC and study its impact on survival.
A retrospective analysis was conducted using data from 2008 to 2016 obtained from the Korea Central Cancer Registry. Patients with stage B HCC were cate
In South Korea, over the study period from 2008 to 2016, a notable trend was observed in adherence to HCC guidelines. Adherence to the EASL guidelines started relatively high, ranging from 77% to 80% between 2008 and 2012, but it gradually declined to 58.8% to 71.6% from 2013 to 2016. Adherence to the AASLD guidelines began at 71.7% to 75.9% from 2008 to 2010, and then it fluctuated between 49.2% and 73.8% from 2011 to 2016. In contrast, adherence to the APASL guidelines remained consistently high, staying within the range of 90.14% to 94.5% throughout the entire study period. Upward treatment, for example with liver resection, liver transplantation, or radiofrequency ablation, significantly improved the survival of patients with BCLC stage B HCC compared to that of patients treated in adherence to the guidelines (for patients analyzed according to the 2000 EASL guidelines, the 5-year survival rates were 63.4% vs 27.2%, P < 0.001), although results varied depending on the guidelines. Progression-free survival rates were also significantly improved upon the use of upward treatments in certain groups. Patients receiving upward treatments were typically < 70 years old, had platelet counts > 105/μL, and serum albumin levels ≥ 3.5 g/dL.
Adherence to guidelines significantly influences survival in South Korean stage B HCC patients. Curative treatments outperform TACE, but liver resection should be selected with caution due to disease heterogeneity.
Core Tip: The current hepatocellular carcinoma (HCC) guidelines do not recommend curative treatments, except liver transplantation, for patients with Barcelona clinic liver cancer stage B HCC. Our study suggests survival benefits for selected patients aged < 70 years, with platelet counts > 105/μL and albumin levels ≥ 3.5 g/dL, even if the liver function corresponds to Child-Pugh score B7, beyond the Milan criteria and outside the up-to-7 criteria. As for the B2 group of the Kinki criteria, which presents a highly diverse population of patients with stage B HCC, curative strategies should be considered with caution through a multidisciplinary approach.
- Citation: Han JE, Cho HJ, Cheong JY, Lim SG, Yang MJ, Noh CK, Lee GH, Kim SS. Impact of guideline adherence on the prognosis of Barcelona clinic liver cancer stage B hepatocellular carcinoma. World J Gastroenterol 2023; 29(47): 6122-6137
- URL: https://www.wjgnet.com/1007-9327/full/v29/i47/6122.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i47.6122
Hepatocellular carcinoma (HCC), the most common type of primary liver cancer and the second leading cause of cancer mortality, is a significant worldwide public health issue. In 2020, liver cancer was the second most common cause of premature death from cancer among persons aged 30 to 69 years, even in high-income countries[1]. In South Korea, HCC has the second highest mortality rate across all age groups and places a heavy burden on the working-age population, with considerable economic consequences[2].
To ensure effective management and treatment of HCC, various international guidelines have been drawn up, including those from the Asian Pacific Association for the Study of the Liver (APASL), the European Association for the Study of the Liver (EASL), and the American Association for the Study of Liver Diseases (AASLD)[3-7]. The AASLD and EASL guidelines are based on the Barcelona Clinic Liver Cancer (BCLC) staging system, which considers factors such as tumor characteristics (number, size, vascular invasion, and extrahepatic localization), liver function [Child-Pugh score (CPS)], and performance status (PS) as defined by the Eastern Cooperative Oncology Group scale to determine appropriate treatment options and predict patient prognosis. Patients with stage B, which typically includes those with multinodular tumors, a CPS of A or B, a PS of 0, and no vascular invasion or extrahepatic spread exhibits extreme heterogeneity with tumor size and number, liver function and PS. It encompasses patients with single tumors larger than 5 cm and those with multiple tumors, leading to differences in tumor burden. Varying degree of impairment in liver function and PS, and preference introduce additional diversity in treatment approaches.
The BCLC staging system strongly recommends transarterial chemoembolization (TACE) for patients with stage B HCC. However, in East-Asia there is a notable deviation from this recommendation, with liver resection being considered a viable treatment option for patients with stage B HCC. Nonrandomized controlled trials performed in East Asian populations have revealed that around half of patients with stage B HCC undergo TACE, while an equal proportion receive liver resection. After sensitivity analysis, liver resection demonstrated superior survival outcomes to TACE for patients with stage B HCC[8]. This reveals the potential benefits of adopting non-adherent treatment modalities to improve the prognosis of patients with stage B HCC. Consequently, HCC guidelines have continuously evolved in response to global clinical evidences[9-13].
The Korea Central Cancer Registry (KCCR), established in 1980 by the Ministry of Health and Welfare, is a hospital-based nationwide cancer registry. Its primary goal is to accurately record cancer incidence in South Korea, facilitating cancer research and treatment planning through the development of a comprehensive cancer database. Each year, all newly diagnosed cancer patients are registered within this system[14].
This study aims to evaluate the adherence to each set of HCC guidelines (EASL, AASLD, and APASL) in South Korea between 2008 and 2016, using data from the KCCR. Additionally, we aim to assess the impact of non-adherence to guidelines on the survival outcomes of patients with stage B HCC. By identifying specific patient subgroups that benefit from treatment that deviates from the guidelines, this study could significantly contribute to the refinement of guidelines to allow improved real-world management of patients with stage B HCC.
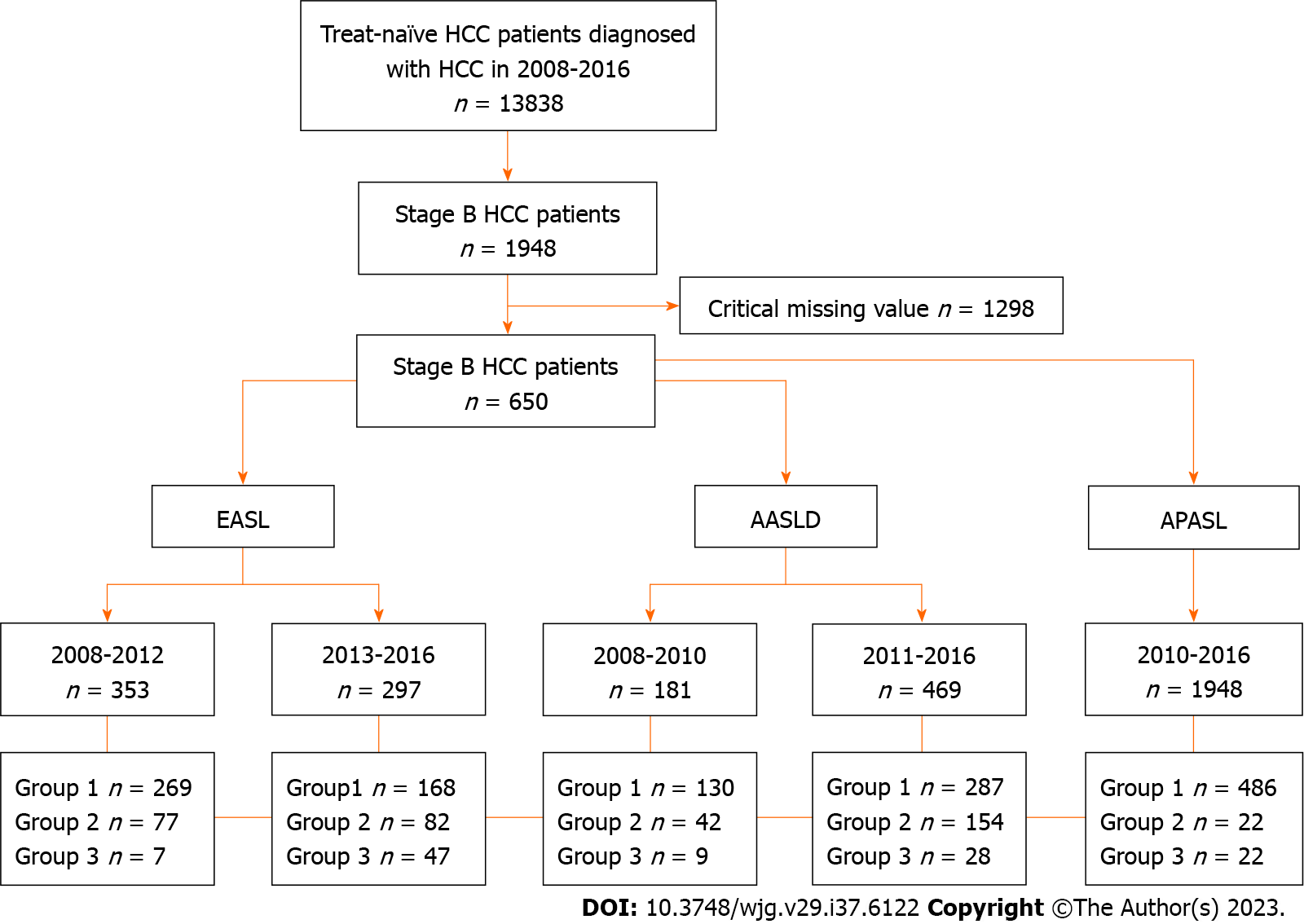
This was a retrospective multicenter cohort study that included 13838 treatment-naïve patients with HCC registered in the KCCR from 2008 to 2016 and followed up until December 2019. The diagnosis of HCC was made based on pathological findings of surgical specimens or liver biopsies, or radiologic findings of liver dynamic computed tomography or magnetic resonance imaging. Stage B HCC was defined as multinodular tumors with a CPS of A or B, PS of 0, and no vascular invasion or cancer-related symptoms, in accordance with the BCLC staging system. A total of 650 patients with BCLC stage B HCC were selected and divided into three groups based on compliance with the EASL (2000, 2012), AASLD (2005, 2010), and APASL (2010) guidelines[3-7], guideline-adherent, upward, and downward treatment, excluding 1298 patients with critical missing value (Figure 1).
The primary endpoint was HCC-related death, and the secondary endpoint was tumor recurrence after the initial HCC treatment. HCC-related survival was measured from the date of the first treatment until HCC-related death or the last follow-up. Progression-free survival (PFS) was measured from the date of the first treatment to the date of the second treatment. Tumor recurrence was determined when the period between consecutive treatments was longer than one month.
Guideline adherence was defined differently for each guideline based on the grades of evidence and recommendations (Supplementary Table 1)[3-7,15]. Among non-adherent treatments, upward treatment referred to more aggressive or curative treatments than those recommended in the BCLC staging system or updated treatments with proven efficacy. Downward treatment referred to moving from left to right in the BCLC staging system or treatments under clinical trials with no proven efficacy. All guidelines recommended TACE as standard therapy for unresectable, large, or multifocal stage B HCC. The APASL guidelines state that liver resection can be considered if HCC is confined to the liver, anatomically resectable, and satisfactory liver function reserve is present.
Data on the anthropometric parameters age, sex, body mass index (BMI, kg/m2), etiology (hepatitis B or C, alcohol consumption), presence of diabetes mellitus and hypertension, ascites, CPS, and Mayo End-Stage Liver Disease (MELD) score were collected. Levels of serum creatinine, sodium, and alanine aminotransferase (ALT), platelet counts, serum albumin levels, total bilirubin levels, and international normalized ratio (INR) were recorded as laboratory parameters. Data on tumor number, maximum tumor diameter, and alpha-fetoprotein (AFP) levels were collected as tumor factors. All laboratory parameters were measured using a conventional automated analyzer.
All statistical analyses were performed using SAS software version 9.4 (SAS Institute Inc., Cary, NC, United States). Continuous variables with normal distribution (age, BMI, CPS, MELD score, serum creatinine, sodium, ALT, platelets, serum albumin, total bilirubin, INR, tumor number, maximum tumor diameter, and AFP) are expressed as mean ± SD. The χ2-test with Fisher’s exact test was used to compare categorical variables (sex, etiology, and ascites). HCC-related survival and PFS was compared using the Kaplan-Meier method with the log-rank test. Univariate Cox regression analysis was performed and multivariate Cox regression analysis was conducted using selected variables sorted through stepwise selection to identify reliable predictors of survival in patients with stage B HCC. The modified Bolondi or Kinki subclassification system was used to categorize patients based on liver function and tumor status as follows: B1 (CPS of 5-7 and within up-to-7), B2 (CPS of 5-7 and beyond up-to-7), and B3 (CPS of 8, 9, and any tumor status) (Table 1)[16,17]. Propensity score matching (PSM) analysis was performed for variables, such as age, etiology, platelet count, serum albumin level, tumor burden, and MELD score to balance differences of baseline characteristics between patients who underwent liver resection and TACE during the subgroup analysis based on Kinki criteria. The results are presented as hazard ratios (HRs) with 95% confidence intervals (95%CIs). Statistical significance was set at P < 0.05.
| Subclassification | B1 | B2 | B3 | |
| Child-Pugh score | 5-7 | 5-7 | 8, 9 | |
| ‘Beyond Milan’ and within ‘up to 7 criteria’ | In | Out | Any | |
| In | Out | |||
| Concept of treatment strategy | Curative | Non-curative | Curative intent if within up-to-7 criteria | Palliative, no treatment |
| Treatment option | Resection | TACE with DC beads | Transplantation | HAIC |
| RFA | HAIC | RFA | Superselective TACE with DC beads | |
| Superselective cTACE | Sorafenib | Superselective cTACE | ||
The baseline characteristics of the patients treated in accordance with the EASL, AASLD, and APASL guidelines, are detailed in Tables 2 and 3, and Supplementary Table 2. Of the 353 patients analyzed according to the 2000 EASL guidelines, 76.2% received guideline-compliant treatment, and 21.8% received upward treatment. Of the patients analyzed according to the 2012 EASL guidelines, 27.6% received upward treatment; the seven patients who received downward treatment were excluded from the analysis due to low sample size. Patients in the upward treatment group, compared to guideline-adherent patients, had a younger average age (57.5 vs 60.7 years) and lower rates of diabetes (13.0% vs 29.4%). They also had lower ALT levels, CPS, MELD scores, and tumor numbers, as well as higher sodium levels, platelet counts, and serum albumin levels. Of the patients assessed according to the 2005 AASLD guidelines, nine downward treated patients were excluded due to the low sample size, and the 26.1% of patients who received upward treatment had fewer tumors compared with guideline-adherent patients. Of the patients analyzed according to the 2010 AASLD guidelines, 32.8% received upward treatment; these patients were younger (average age: 59.6 vs 62.6 years) and had lower rates of diabetes (21.4% vs 30.3%) and fewer tumors than guideline-adherent patients. In contrast, of the patients assessed according to the 2010 APASL guidelines, only 4.2% received upward treatment, with the vast majority (91.7%) treated in line with the guidelines. The upward treatment group had higher BMI and serum sodium levels than the treatment-adherent group (Supplementary Table 2).
| Variables | 2000 EASL guidelines (HCC patients, 2008-2012) | 2012 EASL guidelines (HCC patients, 2013-2016) | |||||
| Guideline-adherent | Upward treatment | P value | Guideline-adherent | Upward treatment | Downward treatment | P value | |
| No. of patients | 269.0 | 77.0 | 168.0 | 82.0 | 47.0 | ||
| Age (yr) | 60.7 ± 10.4 | 57.5 ± 10.2 | 0.017 | 62.6 ± 10.5 | 60.9 ± 9.7 | 64.8 ± 9.0 | 0.100 |
| Male sex (n, %) | 226 (84.0) | 68 (88.3) | 0.469 | 146 (86.9) | 70 (85.4) | 40 (85.1) | 0.921 |
| BMI (kg/m2) | 24.5 ± 4.3 | 24.6 ± 3.1 | 0.919 | 23.8 ± 3.3 | 23.6 ± 4.2 | 23.2 ± 3.4 | 0.626 |
| DM (n, %) | 79 (29.4) | 10 (13.0) | 0.004 | 51 (30.4) | 24 (29.3) | 14 (29.8) | 0.984 |
| Hypertension (n, %) | 98 (36.4) | 28 (38.4) | 0.991 | 58 (34.5) | 40 (48.8) | 19 (40.4) | 0.095 |
| Etiology | |||||||
| Hepatitis B (n, %) | 166 (61.7) | 51 (66.2) | 0.469 | 99 (58.9) | 51 (62.2) | 27 (57.4) | 0.729 |
| Hepatitis C (n, %) | 36 (13.4) | 8 (10.4) | 0.487 | 26 (15.5) | 5 (6.1) | 4 (8.5) | 0.014 |
| Alcohol (n, %) | 98 (36.4) | 30 (39.0) | 0.685 | 72 (42.9) | 41 (50.0) | 21 (44.7) | 0.566 |
| Ascites (n, %) | 23 (8.6) | 1 (1.3) | 0.085 | 19 (11.3) | 9 (11.0) | 7 (14.9) | 0.522 |
| Creatinine (mg/dL) | 1.0 ± 0.6 | 0.9 ± 0.2 | 0.160 | 0.9 ± 0.5 | 1.0 ± 0.6 | 0.9 ± 0.3 | 0.624 |
| Sodium (mmol/L) | 139.4 ± 3.2 | 140.3 ± 2.9 | 0.021 | 138.7 ± 2.9 | 139.1 ± 3.8 | 137.8 ± 2.7 | 0.074 |
| Alanine aminotransferase (IU/L) | 49.2 ± 35.8 | 43.5 ± 32.8 | 0.206 | 50.7 ± 44.0 | 43.7 ± 26.3 | 43.9 ± 30.9 | 0.297 |
| Platelet count (109/L) | 143.8 ± 71.5 | 172.8 ± 68.1 | 0.002 | 146.4 ± 69.6 | 175.8 ± 74.4 | 173.6 ± 91.6 | 0.005 |
| Serum albumin (g/dL) | 3.8 ± 0.5 | 4.1 ± 0.6 | < 0.001 | 3.8 ± 0.5 | 4.1 ± 0.5 | 3.6 ± 0.6 | < 0.001 |
| Total bilirubin (mg/dL) | 1.1 ± 1.2 | 1.0 ± 0.6 | 0.475 | 1.0 ± 0.9 | 0.9 ± 0.8 | 1.1 ± 0.7 | 0.173 |
| INR | 1.1 ± 0.1 | 1.1 ± 0.2 | 0.017 | 1.1 ± 0.2 | 1.1 ± 0.1 | 1.1 ± 0.1 | 0.064 |
| Child-Pugh score | 5.5 ± 0.7 | 5.2 ± 0.5 | 0.025 | 5.5 ± 0.9 | 5.3 ± 0.7 | 5.8 ± 1.0 | 0.021 |
| MELD score | 8.8 ± 2.5 | 7.8 ± 2.2 | 0.002 | 8.7 ± 2.7 | 8.0 ± 2.5 | 8.8 ± 2.4 | 0.164 |
| Alpha-fetoprotein (ng/mL) | 1772.6 ± 7218.6 | 1826.7 ± 5996.8 | 0.952 | 3757.3 ± 19911.7 | 3186.6 ± 20583.6 | 2553.0 ± 9928.3 | 0.918 |
| Numbers of tumor | 3.7 ± 1.3 | 2.8 ± 1.2 | < 0.001 | 3.7 ± 1.3 | 3.0 ± 1.3 | 4.0 ± 1.4 | < 0.001 |
| Maximum tumor diameter (cm) | 4.8 ± 3.0 | 4.8 ± 2.2 | 0.989 | 5.1 ± 3.1 | 5.9 ± 3.4 | 6.0 ± 3.7 | 0.070 |
| Variables | 2005 AASLD guidelines (HCC patients, 2008-2010) | 2010 AASLD guidelines (HCC patients, 2011-2016) | ||||
| Guideline-adherent | Upward treatment | P value | Guideline-adherent | Upward treatment | P value | |
| No. of patients | 130 | 46 | 287 | 154 | ||
| Age (yr) | 60.8 ± 8.9 | 58.3 ± 10.2 | 0.286 | 62.6 ± 10.8 | 59.6 ± 10.0 | 0.005 |
| Male sex (n, %) | 106 (81.5) | 40 (87) | 0.498 | 252 (87.8) | 130 (84.4) | 0.319 |
| BMI (kg/m2) | 24.6 ± 4.5 | 24.2 ± 2.6 | 0.533 | 24.1 ± 3.4 | 24.0 ± 3.9 | 0.811 |
| DM (n, %) | 32.3 | 19.6 | 0.131 | 87 (30.3) | 33 (21.4) | 0.046 |
| Hypertension (n, %) | 40.8 | 30.4 | 0.289 | 107 (37.3) | 63 (40.9) | 0.456 |
| Etiology | ||||||
| Hepatitis B (n, %) | 86 (66.2) | 30 (34.8) | 0.908 | 164 (57.1) | 93 (60.4) | 0.656 |
| Hepatitis C (n, %) | 14 (10.8) | 5 (10.9) | 0.985 | 43 (15.0) | 16 (10.4) | 0.087 |
| Alcohol (n, %) | 47 (36.2) | 19 (41.3) | 0.596 | 117 (40.8) | 68 (44.2) | 0.492 |
| Ascites (n, %) | 4 (3.1) | 4 (9.5) | 0.156 | 14 (4.9) | 36 (23.4) | < 0.001 |
| Creatinine (mg/dL) | 1.0 ± 0.7 | 0.9 ± 0.2 | 0.345 | 0.9 ± 0.4 | 0.9 ± 0.7 | 0.786 |
| Sodium (mmol/L) | 140.0 ± 3.0 | 139.8 ± 4.0 | 0.736 | 139.1 ± 2.8 | 138.6 ± 3.6 | 0.092 |
| Alanine aminotransferase (IU/L) | 51.3 ± 36.6 | 42.6 ± 25.3 | 0.138 | 48.5 ± 40.7 | 42.7 ± 27.0 | 0.112 |
| Platelet count (109/L) | 146.1 ± 70.3 | 151.3 ± 74.1 | 0.669 | 151.2 ± 67.8 | 158.3 ± 79.1 | 0.326 |
| Serum albumin (g/dL) | 3.9 ± 0.4 | 3.8 ± 0.7 | 0.063 | 3.9 ± 0.5 | 3.8 ± 0.7 | 0.146 |
| Total bilirubin (mg/dL) | 0.9 ± 0.4 | 1.6 ± 2.5 | 0.005 | 0.9 ± 0.4 | 1.2 ± 1.2 | < 0.001 |
| INR | 1.1 ± 0.1 | 1.2 ± 0.2 | 0.074 | 1.1 ± 0.1 | 1.1 ± 0.2 | < 0.001 |
| Child-Pugh score | 5.3 ± 0.5 | 5.8 ± 1.2 | < 0.001 | 5.3 ± 0.5 | 5.8 ± 1.1 | < 0.001 |
| MELD score | 8.6 ± 2.0 | 9.2 ± 3.5 | 0.186 | 8.2 ± 2.1 | 9.1 ± 3.3 | < 0.001 |
| Alpha-fetoprotein (ng/mL) | 1516.5 ± 5731.8 | 440.0 ± 1635.9 | 0.212 | 2442.3 ± 10522.8 | 3862.8 ± 23036.1 | 0.379 |
| Numbers of tumor | 3.7 ± 1.3 | 3.0 ± 1.3 | 0.001 | 3.7 ± 1.3 | 3.1 ± 1.3 | < 0.001 |
| Maximum tumor diameter (cm) | 4.3 ± 1.9 | 4.1 ± 1.9 | 0.579 | 5.1 ± 3.0 | 5.5 ± 3.5 | 0.169 |
With respect to treatment strategies, among the 155 patients who received treatment upward of the EASL guidelines, 72.9% underwent liver resection, 9.7% received a liver transplant, and 8.4% had radiofrequency ablation (RFA). According to the AASLD guidelines, 200 patients received upward treatment, with 56.5% of these patients undergoing liver resection, 7.5% receiving a liver transplant, and 7% undergoing RFA. Additionally, 58 patients were classified as undergoing upward treatment due to CPS B liver function while receiving transcatheter chemotherapy (TACE, drug-eluting bead TACE, transarterial radioembolization). Under APASL guidelines, most patients with stage B HCC (486 of 530) adhered to guidelines, with 94 of the guideline-adherent patients undergoing liver resections. Among the 22 patients receiving upward treatment, 50% received a liver transplant and 50% underwent RFA (Table 4). These findings underscore the diverse treatment approaches available for stage B HCC and the need for personalized management strategies.
| Treatment strategy | 2000 EASL | 2012 EASL | 2005 AASLD | 2010 AASLD | 2010 APASL | |||||||
| Adherence | Upward | Adherence | Upward | Downward | Adherence | Upward | Adherence | Upward | Adherence | Upward | Downward | |
| Total, n (%) | 269 (76.2) | 77 (21.8) | 168 (56.6) | 82 (27.6) | 47 (15.8) | 130 (71.8) | 46 (15.5) | 287 (61.2) | 154 (32.8) | 486 (91.7) | 22 (4.7) | 22 (4.7) |
| Liver resection | 0 | 56 (72.7) | 0 | 57 (63.4) | 0 | 0 | 26 (56.5) | 0 | 87 (56.5) | 94 (19.3) | 0 | 0 |
| Liver transplantation | 0 | 11 (14.3) | 0 | 4 (4.9) | 0 | 0 | 6 (13.0) | 0 | 9 (5.8) | 0 | 11 (50) | 0 |
| Radiofrequency ablation | 0 | 6 (7.8) | 0 | 7 (8.5) | 0 | 0 | 4 (8.7) | 0 | 10 (6.5) | 0 | 11 (50) | 0 |
| TACE | 261 (97) | 0 | 168 (100) | 0 | 0 | 130 (100) | 10 (21.7) | 269 (93.7) | 45 (28.2) | 6 | 0 | 0 |
| TACE with drug-eluting beads | 0 | 4 (5.2) | 0 | 10 (12.2) | 0 | 0 | 0 | 12 (4.2) | 2 (1.3) | 14 (2.9) | 0 | 0 |
| Radioembolization (Yttrium-90) | 0 | 0 | 0 | 4 (4.9) | 0 | 0 | 0 | 0 | 1 (0.6) | 4 (0.8) | 0 | 0 |
| Chemotherapy | 8 (1.9) | 0 | 0 | 0 | 13 (27.7) | 0 | 0 | 3 (0.9) | 0 | 0 | 0 | 20 (90.7) |
| Radiation therapy | 0 | 0 | 0 | 0 | 2 (4.3) | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| No treatment | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 (9.1) |
Over the study period (2008-2016), there was a discernible trend in adherence rates to HCC guidelines. Adherence to the EASL guidelines initially ranged from 77% to 80% (2008-2012) but showed a downward tendency to 58.8% to 71.6% (2013-2016). Similarly, adherence to the AASLD guidelines started at 71.7% to 75.9% (2008-2010) and subsequently varied between 49.2% and 73.8% (2011-2016). In contrast, adherence to the APASL guidelines was consistently high, at 90.1% to 94.5% throughout the study period (Figure 2).
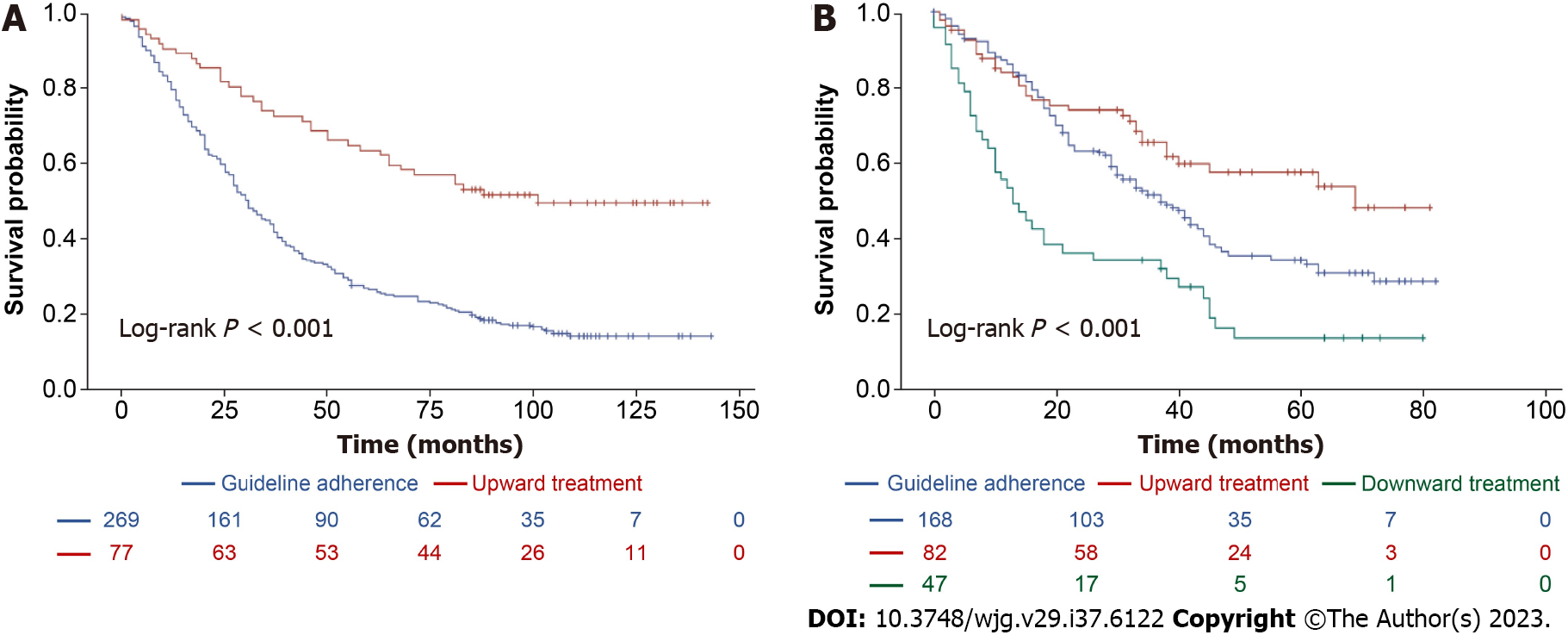
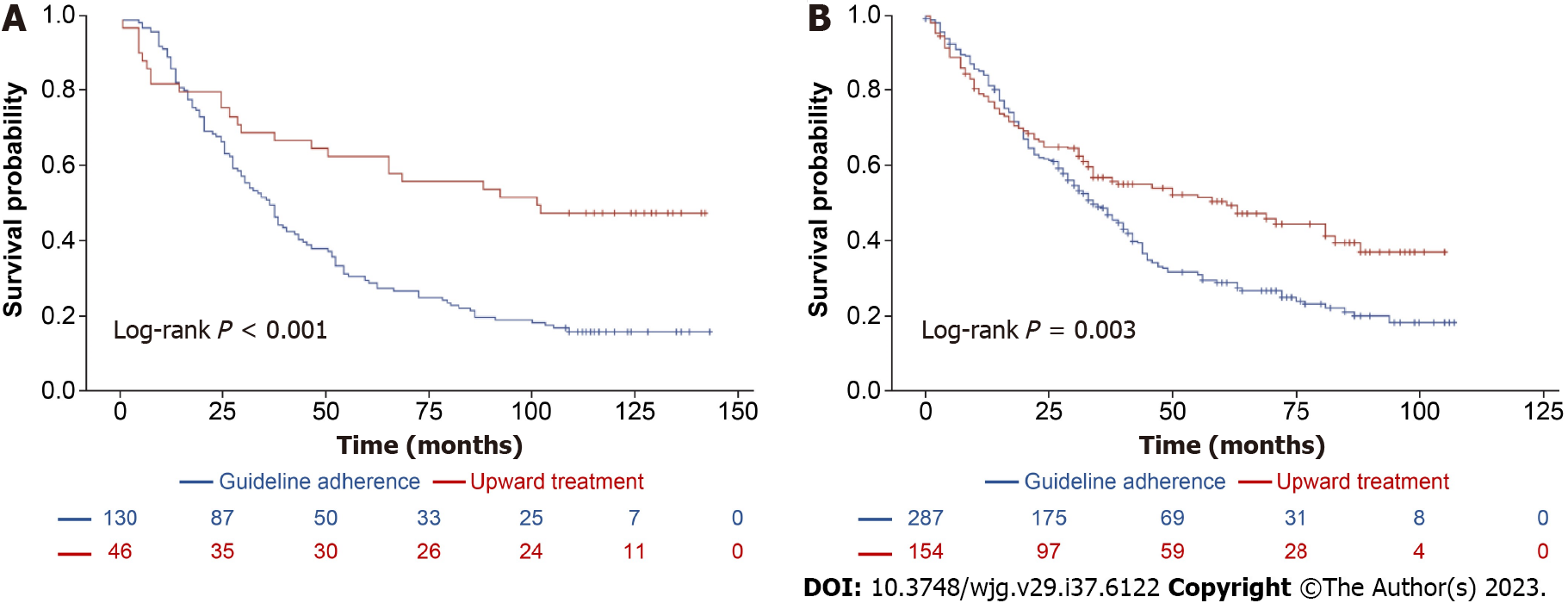
According to the 2000 EASL guidelines, Patients who underwent upward treatment had significantly better 5-year survival rates than those who received guideline-adherent treatment (63.4% vs 27.2%, log-rank P < 0.001, Figure 3A). Risk factors for HCC-related deaths included > 4 tumors and a maximum tumor diameter > 10 cm. Upward treatment (HR 0.448, 95%CI: 0.310-0.647, P < 0.001) and a higher platelet count (> 105/μL; HR 0.672, 95%CI: 0.507-0.890, P = 0.006) were associated with significantly improved HCC-related survival (Table 5). According to 2012 EASL Guidelines, upward treatment demonstrated the best survival outcome of all the treatment groups (5-year survival rates: 57.3% vs 35.2%, log-rank P < 0.001, Figure 3B). Risk factors for HCC-related deaths included > 70 years of age, male sex, total bilirubin level > 1.2 mg/dL, AFP > 200 ng/mL, > 4 tumors, maximum tumor diameter > 5 cm, and downward treatment. However, upward treatment (HR 0.720, 95%CI: 0.478-1.086, P = 0.117) did not significantly improve HCC-related survival (Table 5). With respect to the 2005 AASLD Guidelines, patients who underwent upward treatment had significantly better 5-year survival rates than those who received guideline-adherent treatment (63% vs 30%, log-rank P < 0.001, Figure 4A). Risk factors for HCC-related death included > 4 tumors and a maximum tumor diameter > 5 cm. Upward treatment (HR 0.465, 95%CI: 0.322-0.670, P < 0.001) and a platelet count > 105/μL (HR 0.684, 95%CI: 0.518-0.904, P = 0.008) significantly improved HCC-related survival outcomes in patients with HCC between 2008 and 2010. For patients assessed under the 2010 AASLD guidelines, patients who underwent upward treatment demonstrated better 5-year survival rates than those who received guideline-adherent treatment (50% vs 29.3%, log-rank P < 0.001, Figure 4B). Factors associated with HCC-related deaths included > 70 years of age, CPS > 7, > 4 tumors, and a maximum tumor diameter > 5 cm. Upward treatment (HR 0.478, 95%CI: 0.333-0.685, P < 0.001) and serum albumin levels > 3.5 g/dL (HR 0.596, 95%CI: 0.416-0.855, P = 0.005) were associated with improved HCC-related survival (Table 6). With respect to the 2010 APASL guidelines, patients who received guideline-adherent treatment showed the highest survival rates among all the groups (1-year survival rates: 84.1%, 77.3%, and 36.4%, in the guideline-adherent, upward, and downward treatment groups, respectively, log-rank P < 0.001, Supplementary Figure 1). Risk factors for HCC-related death included > 70 years of age, INR > 1.2, total bilirubin level > 1.2 mg/dL, > 4 tumors, a maximum tumor diameter > 5 cm, and downward treatment. Upward treatment (HR 0.704, 95%CI: 0.372-1.333, P =0.281) was not associated with better survival outcomes (Supplementary Table 3), which may be attributed to the relatively limited number of patients in the upward treatment group compared with the guide-adherent group.
| Variables | 2000 EASL guidelines (HCC patients, 2008-2012) | 2012 EASL guidelines (HCC patients, 2013-2016) | ||||||
| Univariate analysis | Multivariate analysis | Univariate analysis | Multivariate analysis | |||||
| HR (95%CI) | P value | HR (95%CI) | P value | HR (95%CI) | P value | HR (95%CI) | P value | |
| Age (≥ 70 yr) | 1.339 (1.004-1.783) | 0.046 | 1.300 (0.961-1.758) | 0.089 | 1.838 (1.336-2.529) | < 0.001 | 1.765 (1.269-2.687) | 0.001 |
| Male sex | 1.239 (0.875-1.754) | 0.227 | 0.592 (0.401-0.874) | 0.008 | 0.610 (0.409-0.910) | 0.153 | ||
| BMI (≥ 25kg/m2) | 0.890 (0.695-1.140) | 0.356 | 0.829 (0.606-1.135) | 0.242 | ||||
| DM | 1.153 (0.880-1.510) | 0.301 | 1.027 (0.740-1.424) | 0.875 | ||||
| Hypertension | 1.072 (0.836-1.375) | 0.583 | 1.026 (0.757-1.391) | 0.867 | ||||
| Etiology | ||||||||
| Hepatitis B | 0.902 (0.704-1.156) | 0.415 | 0.852 (0.624-1.165) | 0.316 | ||||
| Hepatitis C | 1.056 (0.743-1.501) | 0.763 | 1.270 (0.822-1.962) | 0.282 | ||||
| Alcohol | 1.109 (0.866-1.420) | 0.393 | 0.960 (0.711-1.295) | 0.787 | ||||
| Ascites | ||||||||
| Mild | 2.499 (1.543-4.048) | < 0.001 | 1.552 (0.962-2.503) | 0.072 | ||||
| Moderate to severe | 1.302 (0.486-3.509) | 0.597 | 1.736 (0.767-3.929) | 0.186 | ||||
| Creatinine (> 1 mg/dL) | 1.283 (0.975-1.689) | 0.076 | 1.123 (0.786-1.605) | 0.524 | ||||
| Sodium (> 135 mmol/L) | 0.620 (0.411-0.937) | 0.024 | 0.615 (0.408-0.929) | 0.021 | ||||
| Alanine aminotransferase (> 80 IU/L) | 1.166 (0.813-1.672) | 0.403 | 0.886 (0.556-1.412) | 0.610 | ||||
| Platelet count (> 105/μL) | 0.685 (0.529-0.886) | 0.004 | 0.672 (0.507-0.890) | 0.006 | 0.735 (0.526-1.025) | 0.07 | ||
| Serum albumin (≥ 3.5 g/dL) | 0.694 (0.523-0.921) | 0.011 | 0.605 (0.428-0.855) | 0.004 | ||||
| Total bilirubin (> 1.2 mg/dL) | 1.529 (1.153-2.027) | 0.003 | 1.510 (1.096-2.081) | 0.012 | 1.391 (0.998-1.938) | 0.051 | ||
| INR (> 1.2) | 1.303 (0.982-1.729) | 0.067 | 1.333 (0.904-1.967) | 0.147 | ||||
| Child-Pugh score (≥ 7) | 1.808 (1.187-2.754) | 0.006 | 1.586 (0.994-2.530) | 0.053 | ||||
| MELD score (> 9) | 1.463 (1.112-1.926) | 0.007 | 1.438 (1.035-1.998) | 0.030 | ||||
| Alpha-fetoprotein (≥ 200 ng/mL) | 1.287 (0.993-1.668) | 0.056 | 1.626 (1.196-2.211) | 0.002 | 1.392 (1.001-1.936) | 0.049 | ||
| Numbers of tumor (> 3) | 1.810 (1.419-2.309) | < 0.001 | 1.685 (1.293-2.196) | < 0.001 | 1.654 (1.219-2.244) | 0.001 | 1.673 (1.178-2.275) | 0.003 |
| Maximum tumor diameter (cm) | ||||||||
| < 2 | Ref | Ref | ||||||
| 2-5 | 0.642 (0.409-1.008) | 0.054 | 0.969 (0.608-1.545) | 0.894 | 1.489 (0.807-2.750) | 0.203 | 1.725 (0.908-3.276) | 0.096 |
| 5-10 | 1.061 (0.666-1.689) | 0.803 | 1.862 (1.136-3.055) | 0.0138 | 1.694 (0.912-3.148) | 0.095 | 2.378 (1.228-4.603) | 0.010 |
| > 10 | 2.511 (1.328-4.748) | 0.005 | 4.377 (2.268-8.448) | < 0.001 | 4.023 (2.051-7.892) | < 0.001 | 4.358 (2.120-8.956) | < 0.001 |
| Treatment | ||||||||
| Guideline adherence | Ref | Ref | ||||||
| Upward | 0.372 (0.263-0.525) | < 0.001 | 0.448 (0.310-0.647) | < 0.001 | 0.631 (0.426-0.935) | 0.022 | 0.720 (0.478-1.086) | 0.117 |
| Downward | 1.974 (1.362-2.859) | < 0.001 | 1.838 (1.257-2.687) | 0.002 | ||||
| Variables | 2005 AASLD guidelines (HCC patients, 2008-2010) | 2010 AASLD guidelines (HCC patients, 2011-2016) | ||||||
| Univariate analysis | Multivariate analysis | Univariate analysis | Multivariate analysis | |||||
| HR (95%CI) | P value | HR (95%CI) | P value | HR (95%CI) | P value | HR (95%CI) | P value | |
| Age (≥ 70 yr) | 1.229 (0.809-1.865) | 0.334 | 1.210 (0.896-1.635) | 0.214 | 1.775 (1.376-2.289) | < 0.001 | 1.587 (1.206-2.089) | 0.001 |
| Male sex | 1.237 (0.776-1.972) | 0.371 | 0.832 (0.602-1.152) | 0.268 | ||||
| BMI (≥ 25 kg/m2) | 1.164 (0.819-1.654) | 0.397 | 0.827 (0.650-1.053) | 0.123 | ||||
| DM | 0.990 (0.681-1.438) | 0.958 | 1.236 (0.954-1.600) | 0.109 | ||||
| Hypertension | 1.129 (0.798-1.596) | 0.493 | 1.101 (0.868-1.398) | 0.428 | ||||
| Etiology | ||||||||
| Hepatitis B | 0.867 (0.608-1.235) | 0.429 | 0.828 (0.652-1.051) | 0.121 | ||||
| Hepatitis C | 1.068 (0.633-1.801) | 0.807 | 1.225 (0.884-1.698) | 0.223 | ||||
| Alcohol | 1.155 (0.815-1.637) | 0.417 | 0.994 (0.785-1.259) | 0.961 | ||||
| Ascites | ||||||||
| Mild | 1.984 (0.872-4.516) | 0.102 | 1.907 (1.303-2.791) | 0.001 | ||||
| Creatinine (> 1 mg/dL) | 1.322 (0.901-1.940) | 0.153 | 1.206 (0.919-1.582) | 0.176 | ||||
| Sodium (≥ 135 mmol/L) | 0.876 (0.445-1.724) | 0.702 | 0.573 (0.407-0.807) | 0.002 | ||||
| Alanine aminotransferase (> 80 IU/L) | 1.021 (0.614-1.699) | 0.937 | 0.968 (0.670-1.400) | 0.865 | ||||
| Platelet count (> 105/μL) | 0.590 (0.414-0.839) | 0.003 | 0.684 (0.518-0.904) | 0.008 | 0.736 (0.568-0.952) | 0.020 | ||
| Serum albumin (≥ 3.5 g/dL) | 0.865 (0.573-1.306) | 0.491 | 0.533 (0.408-0.696) | < 0.001 | 0.596 (0.416-0.855) | 0.005 | ||
| Total bilirubin (> 1.2 mg/dL) | 1.619 (1.102-2.378) | 0.014 | 1.509 (1.162-1.959) | 0.002 | ||||
| INR (> 1.2) | 1.229 (0.838-1.802) | 0.291 | 1.421 (1.054-1.915) | 0.021 | ||||
| Child-Pugh score (≥ 7) | 1.430 (0.667-3.065) | 0.358 | 1.822 (1.288-2.577) | 0.001 | 2.429 (1.434-4.114) | 0.001 | ||
| MELD score (> 9) | 1.465 (1.018-2.108) | 0.040 | 1.521 (1.163-1.988) | 0.002 | ||||
| Alpha-fetoprotein (≥ 200 ng/mL) | 1.436 (1.004-2.055) | 0.048 | 1.319 (1.029-1.692) | 0.029 | ||||
| Numbers of tumor (> 3) | 1.458 (1.036-2.051) | 0.030 | 1.570 (1.208-2.040) | 0.001 | 1.830 (1.443-2.320) | < 0.001 | 1.870 (1.426-2.452) | < 0.001 |
| Maximum tumor diameter | ||||||||
| < 2 | Ref | Ref | ||||||
| 2-5 | 0.767 (0.408-1.442) | 0.411 | 0.967 (0.606-1.543) | 0.889 | 0.974 (0.623-1.522) | 0.908 | 1.294 (0.794-2.107) | 0.310 |
| 5-10 | 0.925 (0.475-1.800) | 0.818 | 1.792 (1.093-2.939) | 0.021 | 1.459 (0.929-2.291) | 0.101 | 2.210 (1.338-3.650) | 0.002 |
| > 10 | 4.267 (0.918-19.839) | 0.064 | 3.437 (1.784-6.623) | < 0.001 | 2.692 (1.578-4.594) | < 0.001 | 3.261 (1.834-5.797) | < 0.001 |
| Treatment | ||||||||
| Guideline adherence | Ref | Ref | ||||||
| Upward | 0.442 (0.283-0.691) | < 0.001 | 0.465 (0.322-0.670) | < 0.001 | 0.678 (0.524-0.879) | 0.003 | 0.478 (0.333-0.685) | < 0.001 |
These findings suggest that adherence to different guidelines and specific treatment choices played a crucial role in the prognosis of patients with HCC, with common risk factors, including tumor characteristics, patient age, and liver function influencing survival outcomes.
For patients analyzed according to the 2000 EASL guidelines, there was no significant difference in PFS between the guideline-adherent and upward treatment groups (Supplementary Figure 2A). However, with respect to the 2012 EASL guidelines, the guideline-adherent group had markedly improved 1-year PFS compared with the upward treatment group (60.5% vs 39.8%, log-rank P < 0.001, Supplementary Figure 2B). Between 2013 and 2016, upward treatment (HR 0.648, 95%CI: 0.461-0.909, P = 0.012) and serum albumin levels ≥ 3.5 g/dL (HR 0.74, 95%CI: 0.568-0.964, P = 0.026) were associated with improved PFS (Supplementary Table 5).
For patients assessed under the 2005 AASLD guidelines, no significant difference in PFS was observed between guideline-adherent and upward treatment groups (Supplementary Figure 3A). However, with respect to the 2010 AASLD guidelines, upward treatment was associated with superior 1-year PFS than guideline adherence (58.6% vs 38.9%, log-rank P < 0.001, Supplementary Figure 3B). Between 2011 and 2016, upward treatment (HR 0.556, 95%CI: 0.426-0.726, P < 0.001), and serum albumin levels ≥ 3.5 g/dL (HR 0.689, 95%CI: 0.511-0.928, P = 0.014) were associated with improved PFS (Supplementary Table 5).
With respect to the 2010 APASL guidelines, the upward treatment group exhibited the highest 1-year PFS rate (75%, 44.8%, and 31.3% in upward, guideline-adherent and downward treatment groups, respectively, log-rank P = 0.028, Supplementary Figure 4). Risk factors for tumor progression included > 70 years of age, > 4 tumors, a maximum tumor diameter > 5 cm, and downward treatment. Compared to guideline adherence, between 2010 and 2016, upward treatment (HR 0.561, 95%CI: 0.313-1.004, P = 0.052) and a platelet count > 105/μL (HR 0.740, 95%CI: 0.587-0.932, P = 0.011) were associated with a significant improvement in PFS (Supplementary Table 6).
In summary, regardless of the specific guidelines followed, factors such as adherence to guidelines, treatment choice (especially upward treatment), serum albumin levels, and platelet count consistently played pivotal roles in determining the prognosis of patients with HCC, particularly in terms of PFS.
Participants were categorized into BCLC stage B1 (40.6%, n = 263), B2 (55.1%, n = 357), and B3 (4.3%, n = 28). Among B1 and B2 patients (96.7% of the total), a significant portion received upward treatment (66.7% and 70%, respectively, Supplementary Tables 7 and 10).
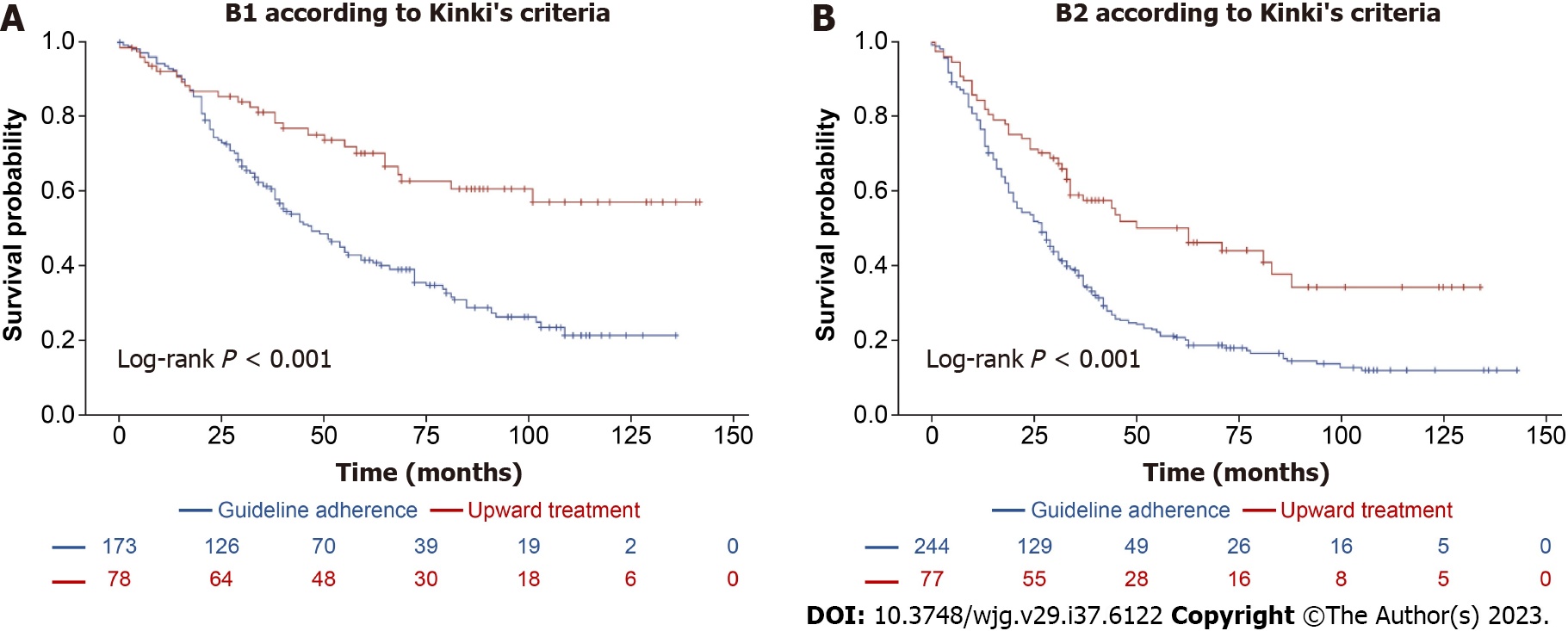
In the B1 group, patients who received upward treatment had a significantly higher 5-year survival rate compared with those whose treatment adhered to guidelines (71.1% vs 41.4%, log-rank P < 0.001, Figure 5A). Upward treatment was associated with a significant improvement in survival outcomes (HR 0.470, 95%CI: 0.288-0.766, P = 0.002), even after PSM at a 1:1 ratio for variables, such as platelet count, serum albumin level, MELD score, number of tumors, and maximum tumor diameter (Supplementary Tables 8 and 9, Supplementary Figure 5). In the B2 group, a similar trend was observed, with a higher 5-year survival rate in patients receiving upward treatment compared with those whose treatment adhered to guidelines (51.2% vs 21.6%, log-rank P < 0.001, Figure 5B). Upward treatment remained a robust indicator of improved survival (HR 0.553, 95%CI: 0.317-0.965, P = 0.037, Supplementary Tables 11 and 12, Supplementary Figure 6) after 1:1 PSM for variables, such as age, etiology, sodium level, platelet count, serum albumin level, MELD score, number of tumors, and maximum tumor diameter.
Interestingly, despite the Kinki criteria recommending TACE, hepatic arterial infusion chemotherapy, and systemic chemotherapy as treatment options for patients with B2 HCC, liver resection, liver transplantation, or RFA resulted in superior outcomes for over 70% of patients with B2 HCC compared with following the guidelines. These findings highlight the potential benefits of individualized treatment approaches beyond guideline recommendations for certain BCLC subgroups.
This large-scale, longitudinal study examined real-world data from patients with stage B HCC in South Korea over an 8-year period. As this was a nationwide multicenter study using data from the KCCR, random and representative selection of patients with HCC was performed. The adherence rate to guidelines for the treatment of stage B HCC has not increased over time, highlighting a gap between official recommendations and clinical practice. This study examines the implications of treatment decisions for patients with stage B HCC.
Notably, the present study revealed that liver resection is a common treatment option for stage B HCC in South Korea, deviating from EASL and AASLD guidelines. This reflects the tendency of Asian countries to adopt more aggressive HCC treatment strategies than Western countries[18-20]. Furthermore, curative treatments, including liver resection, yield better survival outcomes than TACE in certain patients. Prognostic factors for patients with stage B HCC after curative treatment included age, tumor number, maximum tumor diameter, and underlying liver function, aligning with prior large-scale studies[22-24]. Overall, these findings suggest that curative treatments may significantly improve the prognosis of patients with stage B HCC, even after accounting for potential selection bias.
Achieving significant increases in EASL and AASLD guideline adherence rates over time remains elusive in East Asian countries. One plausible explanation for this lies in the complex and multifaceted nature of HCC, which often requires tailored treatment strategies that may not always align with the standard guidelines. Moreover, the preference for curative or aggressive treatments for stage B HCC in East Asian countries may be attributed to the higher incidence of HCC in these countries compared with that in Western countries, largely due to a higher prevalence of chronic hepatitis B. This has necessitated the development of specialized treatment approaches. The establishment of specialized liver centers and multidisciplinary teams has resulted in the cultivation of expertise in various treatment modalities. Over time, the tradition of aggressive HCC treatment, including liver resection and transplantation, has become ingrained based on continuous research and clinical trials, leading to innovative strategies. Moreover, variations in healthcare infrastructure, clinical practice, demographics and differences in treatment preferences could all fundamentally make the differences for guideline non-adherence across regions in different countries.
In 2022, the BCLC group updated their recommendations for HCC treatment, sub-classifying stage B HCC patients into three groups based on tumor characteristics and potential treatment responses; those eligible for extended liver transplantation criteria despite multiple HCCs, those suitable for TACE due to well-defined HCC nodules and preserved portal flow, and those with diffuse, infiltrative, and extensive HCC that may benefit from systemic therapy[25]. However, the updated BCLC staging system still does not recommend liver resection as a feasible therapy for stage B HCC due to the lack of prospective studies. Notably, a Chinese randomized controlled trial and a South Korean retrospective cohort study have demonstrated potential survival benefits of liver resection over TACE in selected patients with multiple HCCs[26]. In a South Korean retrospective cohort study, two periods (2003-2005 and 2008-2010) were compared to assess changing treatment trends. The results indicated that patients with stage 0-C HCC who underwent curative treatments in the later cohort achieved superior 5-year survival outcomes to those who received non-curative therapy[27]. The potential survival benefits of liver resection over TACE in selected patients with stage B HCC have been verified through systematic reviews and meta-analyses[28,30-33]. Considering real-world scenarios [21-24,29] that demonstrate superior outcomes with liver resection can provide robust evidence for the adoption of curative treatments in patients with more advanced HCC. However, careful patient selection is required, considering individual patient characteristics and institutional expertise, to maximize the survival benefit.
Patients with chronic liver disease are at an increased risk of post-hepatectomy liver failure; however, advances in preoperative assessments such as portal hypertension evaluation, future liver remnant volume or function prediction, portal vein embolization, surgical techniques, and postoperative management have expanded the possibilities of liver resection even in more advanced stages of HCC. As a result, portal hypertension, multifocal HCCs, and portal vein thrombosis are now recognized as manageable challenges in HCC treatment. Overall, the importance of multidisciplinary evaluation and meticulous planning in the selection of treatment strategies for stage B HCC cannot be overstated; where technically feasible, surgical resection remains a vital option.
Our study has several limitations that warrant consideration when interpreting the results. First, given its retrospective nature, there is a possibility that treatment strategies were influenced by physician or patient preferences, introducing inherent bias. To establish the safety and effectiveness of curative treatments for stage B HCC, well-designed prospective studies are essential. Second, our study excluded certain patients with stage B HCC who may benefit from alternative treatments or systemic therapy according to the 2022 BCLC staging system, due to the limited number of participants. This exclusion could impact the generalizability of our findings. Third, we were unable to account for potential confounding factors such as tumor location, pathology, degree of differentiation, and imaging characteristics, as these data were not available in the KCCR. These factors can influence treatment choices and prognosis, potentially affecting our results. Lastly, due to the small sample size, we did not conduct a survival analysis comparing the B3 group with the B1 and B2 groups. While our study offers valuable insights into stage B HCC treatment and prognosis, well-designed prospective studies that overcome these limitations are necessary for a more comprehensive understanding of HCC and its management.
We propose that the eligibility criteria for liver resection be expanded to patients with stage B HCC in selected patients aged < 70 years, with platelet counts > 105/μL, and serum albumin levels ≥ 3.5 g/dL, even in cases where the liver function corresponds to CPS B7 or the HCC status is beyond the Milan criteria and outside the up-to-7 criteria. However, careful patient selection by considering liver function, tumor location, and tumor burden is crucial.
The present study verified the discrepancy between guideline recommendations and real-world clinical practice in the treatment of stage B HCC, with liver resection often chosen against guideline recommendations, resulting in improved survival for selected patients. Multidisciplinary evaluation is crucial for the selection of appropriate curative treatments in patients with stage B HCC, considering patient characteristics and institutional expertise. Prospective studies are required to further assess the clinical implications of curative treatments in stage B HCC.
Hepatocellular carcinoma (HCC) is a major global health concern, and the second leading cause of cancer mortality worldwide. Treatment guidelines are based on the Barcelona Clinic Liver Cancer staging system, but in East Asian countries, liver resection is often preferred to transarterial chemoembolization for stage B HCC due to better survival outcomes.
The need for regional adaptations in HCC treatment guidelines to improve the prognosis of patients with stage B HCC.
This study aims to evaluate adherence to international HCC guidelines in South Korea using data from 2008-2016, investigate the treatment strategies for stage B HCC, analyze the impact of guideline non-adherence on survival, and identify patient subgroups who may benefit from guideline deviation to improve real-world management.
In this retrospective analysis, data from the Korea Central Cancer Registry from 2008 to 2016 were utilized. Patients with stage B HCC were categorized into groups based on treatment adherence to HCC guidelines from Asian Pacific, European, and American associations for the study of liver diseases. The primary outcome was HCC-related deaths, with tumor recurrence as a secondary outcome; statistical analysis was performed using Kaplan-Meier curves with log-rank tests and multivariable Cox regression analysis to analyze survival outcomes and predictors.
The adherence to European Association for the Study of the Liver and American Association for the Study of Liver Diseases HCC treatment guidelines exhibit a declining trend over time in South Korea. Curative treatments, which were a deviation from guideline recommendations, led to significantly improved survival rates. Patients receiving upward treatments were < 70 years of age, and had platelet counts > 105/μL and serum albumin levels ≥ 3.5 g/dL.
This study, based on real-world data in South Korea, revealed a persistent gap between treatment guideline recommendations and real clinical practice for patients with stage B HCC; liver resection, which was often chosen against guideline recommendations, resulted in improved survival for selected patients.
These findings suggest that expanding the eligibility criteria for liver resection in specific patient groups may be beneficial. The study also highlights the need for careful patient selection through a multidisciplinary approach when considering curative treatments for stage B HCC. However, prospective studies are needed to further evaluate the clinical implications of curative treatments in stage B HCC.
The database used in this study was provided by the Korean Central Cancer Registry, Ministry of Health and Welfare, South Korea, and the Korean Liver Cancer Association.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: South Korea
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Soldera J, Brazil; Wang YJ, China S-Editor: Lin C L-Editor: A P-Editor: Yuan YY
| 1. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 64442] [Article Influence: 16110.5] [Reference Citation Analysis (176)] |
| 2. | Statistics Korea. [cited 3 August 2022]. Available from: https://kostat.go.kr/portal/korea/kor_nw/1/1/index.board?bmode=read&aSeq=403046)3. |
| 3. | 3 European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol. 2018;69:182-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5593] [Cited by in RCA: 6038] [Article Influence: 862.6] [Reference Citation Analysis (3)] |
| 4. | Bruix J, Sherman M; Practice Guidelines Committee, American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma. Hepatology. 2005;42:1208-1236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4333] [Cited by in RCA: 4507] [Article Influence: 225.4] [Reference Citation Analysis (0)] |
| 5. | Bruix J, Sherman M; American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020-1022. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5972] [Cited by in RCA: 6569] [Article Influence: 469.2] [Reference Citation Analysis (1)] |
| 6. | Omata M, Lesmana LA, Tateishi R, Chen PJ, Lin SM, Yoshida H, Kudo M, Lee JM, Choi BI, Poon RT, Shiina S, Cheng AL, Jia JD, Obi S, Han KH, Jafri W, Chow P, Lim SG, Chawla YK, Budihusodo U, Gani RA, Lesmana CR, Putranto TA, Liaw YF, Sarin SK. Asian Pacific Association for the Study of the Liver consensus recommendations on hepatocellular carcinoma. Hepatol Int. 2010;4:439-474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 797] [Cited by in RCA: 841] [Article Influence: 56.1] [Reference Citation Analysis (0)] |
| 7. | Bruix J, Sherman M, Llovet JM, Beaugrand M, Lencioni R, Burroughs AK, Christensen E, Pagliaro L, Colombo M, Rodés J; EASL Panel of Experts on HCC. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J Hepatol. 2001;35:421-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3252] [Cited by in RCA: 3242] [Article Influence: 135.1] [Reference Citation Analysis (0)] |
| 8. | Torimura T, Iwamoto H. Treatment and the prognosis of hepatocellular carcinoma in Asia. Liver Int. 2022;42:2042-2054. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 75] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 9. | Torzilli G, Belghiti J, Kokudo N, Takayama T, Capussotti L, Nuzzo G, Vauthey JN, Choti MA, De Santibanes E, Donadon M, Morenghi E, Makuuchi M. A snapshot of the effective indications and results of surgery for hepatocellular carcinoma in tertiary referral centers: is it adherent to the EASL/AASLD recommendations?: an observational study of the HCC East-West study group. Ann Surg. 2013;257:929-937. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 333] [Cited by in RCA: 416] [Article Influence: 34.7] [Reference Citation Analysis (0)] |
| 10. | Kim SE, Lee HC, Kim KM, Lim YS, Chung YH, Lee YS, Suh DJ. Applicability of the BCLC staging system to patients with hepatocellular carcinoma in Korea: analysis at a single center with a liver transplant center. Korean J Hepatol. 2011;17:113-119. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 11. | Kudo M. A Paradigm Change in the Treatment Strategy for Hepatocellular Carcinoma. Liver Cancer. 2020;9:367-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 12. | Alkhatib A, Gomaa A, Allam N, Rewisha E, Waked I. Real Life Treatment of Hepatocellular Carcinoma: Impact of Deviation from Guidelines for Recommended Therapy. Asian Pac J Cancer Prev. 2015;16:6929-6934. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 13. | Radu P, Groza I, Iancu C, Al Hajjar N, Andreica V, Sparchez Z. Treatment of hepatocellular carcinoma in a tertiary Romanian center. Deviations from BCLC recommendations and influence on survival rate. J Gastrointestin Liver Dis. 2013;22:291-297. [PubMed] [DOI] [Full Text] |
| 14. | National Cancer Center Korea. National Cancer Control Programs. [cited 3 August 2022]. Available from: https://ncc.re.kr/main.ncc?uri=english/sub04_ControlPrograms. |
| 15. | Omata M, Cheng AL, Kokudo N, Kudo M, Lee JM, Jia J, Tateishi R, Han KH, Chawla YK, Shiina S, Jafri W, Payawal DA, Ohki T, Ogasawara S, Chen PJ, Lesmana CRA, Lesmana LA, Gani RA, Obi S, Dokmeci AK, Sarin SK. Asia-Pacific clinical practice guidelines on the management of hepatocellular carcinoma: a 2017 update. Hepatol Int. 2017;11:317-370. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1628] [Cited by in RCA: 1637] [Article Influence: 204.6] [Reference Citation Analysis (0)] |
| 16. | Arizumi T, Ueshima K, Iwanishi M, Minami T, Chishina H, Kono M, Takita M, Kitai S, Inoue T, Yada N, Hagiwara S, Ida H, Minami Y, Sakurai T, Kitano M, Nishida N, Kudo M. Validation of a Modified Substaging System (Kinki Criteria) for Patients with Intermediate-Stage Hepatocellular Carcinoma. Oncology. 2015;89 Suppl 2:47-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 17. | Kudo M, Arizumi T, Ueshima K, Sakurai T, Kitano M, Nishida N. Subclassification of BCLC B Stage Hepatocellular Carcinoma and Treatment Strategies: Proposal of Modified Bolondi's Subclassification (Kinki Criteria). Dig Dis. 2015;33:751-758. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 171] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 18. | Foerster F, Galle PR. Comparison of the current international guidelines on the management of HCC. JHEP Rep. 2019;1:114-119. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 19. | Clinical Practice Guidelines for Hepatocellular Carcinoma Differ between Japan, United States, and Europe. Liver Cancer. 2015;4:85-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 53] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 20. | Choo SP, Tan WL, Goh BKP, Tai WM, Zhu AX. Comparison of hepatocellular carcinoma in Eastern versus Western populations. Cancer. 2016;122:3430-3446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 214] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 21. | Yamamoto M, Kobayashi T, Hashimoto M, Kuroda S, Kawaoka T, Aikata H, Chayama K, Ohdan H. Significance of liver resection for intermediate stage hepatocellular carcinoma according to subclassification. BMC Cancer. 2021;21:668. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 22. | Tsilimigras DI, Moris D, Hyer JM, Bagante F, Sahara K, Moro A, Paredes AZ, Mehta R, Ratti F, Marques HP, Silva S, Soubrane O, Lam V, Poultsides GA, Popescu I, Alexandrescu S, Martel G, Workneh A, Guglielmi A, Hugh T, Aldrighetti L, Endo I, Sasaki K, Rodarte AI, Aucejo FN, Pawlik TM. Hepatocellular carcinoma tumour burden score to stratify prognosis after resection. Br J Surg. 2020;107:854-864. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 109] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 23. | Li ZL, Yu JJ, Guo JW, Sui CJ, Dai BH, Zhang WG, Chen TH, Li C, Gu WM, Zhou YH, Wang H, Zhang YM, Mao XH, Pawlik TM, Wang MD, Liang L, Wu H, Lau WY, Wu MC, Shen F, Yang T. Liver resection is justified for multinodular hepatocellular carcinoma in selected patients with cirrhosis: A multicenter analysis of 1,066 patients. Eur J Surg Oncol. 2019;45:800-807. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 24. | Kim H, Ahn SW, Hong SK, Yoon KC, Kim HS, Choi YR, Lee HW, Yi NJ, Lee KW, Suh KS; Korean Liver Cancer Association. Survival benefit of liver resection for Barcelona Clinic Liver Cancer stage B hepatocellular carcinoma. Br J Surg. 2017;104:1045-1052. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 50] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 25. | Reig M, Forner A, Rimola J, Ferrer-Fàbrega J, Burrel M, Garcia-Criado Á, Kelley RK, Galle PR, Mazzaferro V, Salem R, Sangro B, Singal AG, Vogel A, Fuster J, Ayuso C, Bruix J. BCLC strategy for prognosis prediction and treatment recommendation: The 2022 update. J Hepatol. 2022;76:681-693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1904] [Cited by in RCA: 2578] [Article Influence: 859.3] [Reference Citation Analysis (59)] |
| 26. | Yin L, Li H, Li AJ, Lau WY, Pan ZY, Lai EC, Wu MC, Zhou WP. Partial hepatectomy vs. transcatheter arterial chemoembolization for resectable multiple hepatocellular carcinoma beyond Milan Criteria: a RCT. J Hepatol. 2014;61:82-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 270] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 27. | Reig M, Forner A, Rimola J, Ferrer-Fàbrega J, Burrel M, Garcia-Criado Á, Kelley RK, Galle PR, Mazzaferro V, Salem R, Sangro B, Singal AG, Vogel A, Fuster J, Ayuso C, Bruix J. BCLC strategy for prognosis prediction and treatment recommendation: The 2022 update. J Hepatol. 2022;76:681-693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1904] [Cited by in RCA: 2578] [Article Influence: 859.3] [Reference Citation Analysis (59)] |
| 28. | Glantzounis GK, Paliouras A, Stylianidi MC, Milionis H, Tzimas P, Roukos D, Pentheroudakis G, Felekouras E. The role of liver resection in the management of intermediate and advanced stage hepatocellular carcinoma. A systematic review. Eur J Surg Oncol. 2018;44:195-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 62] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 29. | Ciria R, López-Cillero P, Gallardo AB, Cabrera J, Pleguezuelo M, Ayllón MD, Luque A, Zurera L, Espejo JJ, Rodríguez-Perálvarez M, Montero JL, de la Mata M, Briceño J. Optimizing the management of patients with BCLC stage-B hepatocellular carcinoma: Modern surgical resection as a feasible alternative to transarterial chemoemolization. Eur J Surg Oncol. 2015;41:1153-1161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 67] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 30. | Tada T, Kumada T, Toyoda H, Tsuji K, Hiraoka A, Itobayashi E, Nouso K, Kariyama K, Ishikawa T, Hirooka M, Hiasa Y. Role of hepatic resection in patients with intermediate-stage hepatocellular carcinoma: A multicenter study from Japan. Cancer Sci. 2017;108:1414-1420. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 40] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 31. | Luo J, Peng ZW, Guo RP, Zhang YQ, Li JQ, Chen MS, Shi M. Hepatic resection versus transarterial lipiodol chemoembolization as the initial treatment for large, multiple, and resectable hepatocellular carcinomas: a prospective nonrandomized analysis. Radiology. 2011;259:286-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 56] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 32. | Hyun MH, Lee YS, Kim JH, Lee CU, Jung YK, Seo YS, Yim HJ, Yeon JE, Byun KS. Hepatic resection compared to chemoembolization in intermediate- to advanced-stage hepatocellular carcinoma: A meta-analysis of high-quality studies. Hepatology. 2018;68:977-993. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 159] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 33. | Qi X, Wang D, Su C, Li H, Guo X. Hepatic resection versus transarterial chemoembolization for the initial treatment of hepatocellular carcinoma: A systematic review and meta-analysis. Oncotarget. 2015;6:18715-18733. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 58] [Article Influence: 5.8] [Reference Citation Analysis (0)] |