Published online Dec 21, 2023. doi: 10.3748/wjg.v29.i47.6111
Peer-review started: June 10, 2023
First decision: August 8, 2023
Revised: August 24, 2023
Accepted: November 29, 2023
Article in press: November 29, 2023
Published online: December 21, 2023
Processing time: 188 Days and 16.3 Hours
Although the usefulness of endoscopic scores, such as the Mayo Endoscopic Subscore (MES), Ulcerative Colitis Endoscopic Index of Severity (UCEIS), and Ulcerative Colitis Colonoscopic Index of Severity (UCCIS), and biomarkers such as fecal calprotectin (FC) for predicting relapse in ulcerative colitis (UC) has been reported, few studies have included endoscopic scores for evaluating the entire colon.
To compare the usefulness of FC value and MES, UCEIS, and UCCIS for pre
In total, 75 patients with UC in clinical and endoscopic remission who visited our institution between February 2019 and March 2022 were enrolled. The diagnosis of UC was confirmed based on the clinical presentation, endoscopic findings, and histology, according to the current established criteria for UC. Fecal samples were collected the day before or after the colonoscopy for measurement of FC. Endo
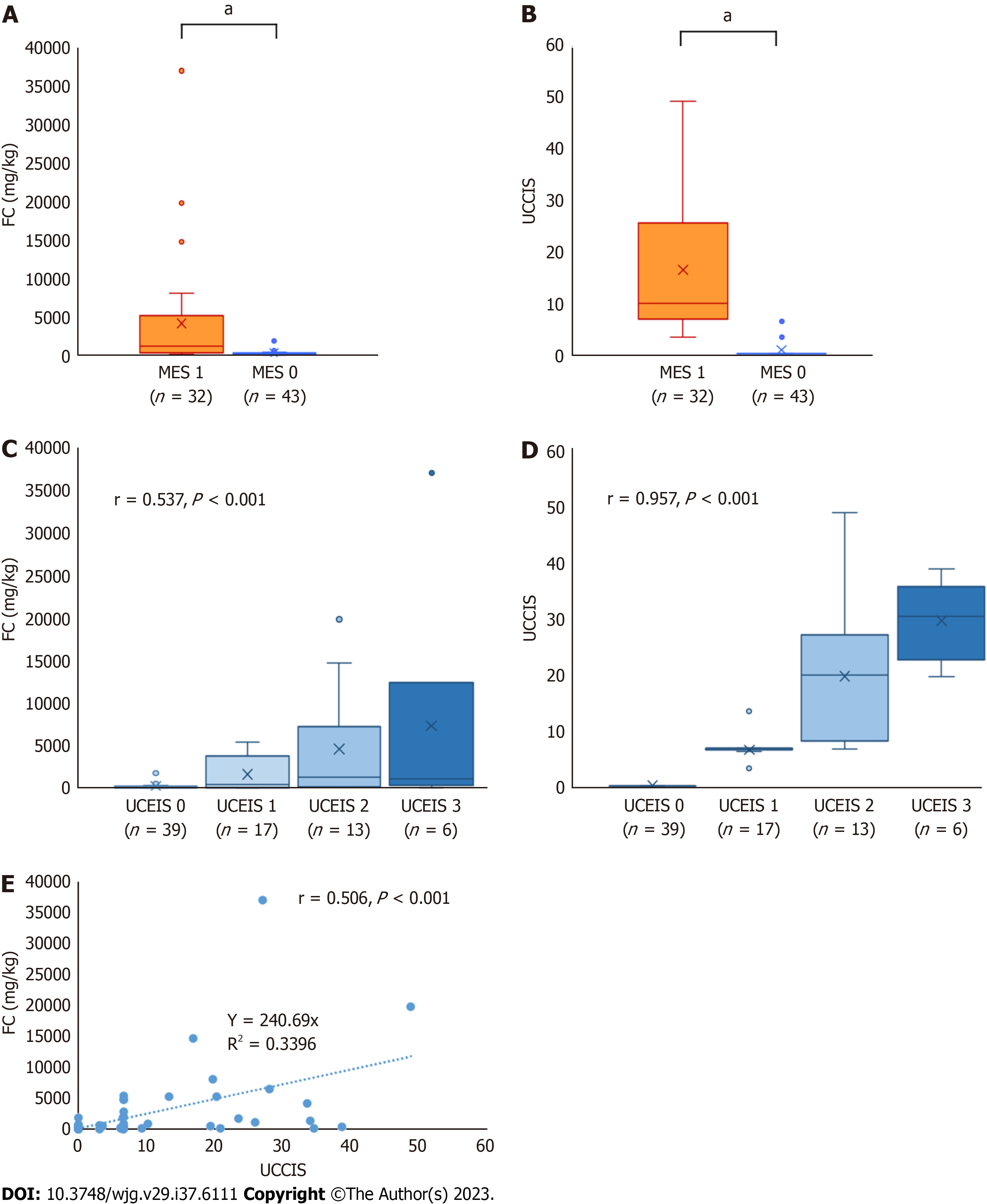
FC and UCCIS showed a significant correlation with UCEIS (r = 0.537, P < 0.001 and r = 0.957, P < 0.001, respec
The three endoscopic scores and FC may predict UC relapse during clinical remission. Among these scores, UCEIS may be the most useful in terms of ease of evaluation and accuracy.
Core Tip: We evaluated the usefulness of fecal calprotectin and endoscopic scores, including the Mayo Endoscopic Subscore, Ulcerative Colitis Endoscopic Index of Severity (UCEIS), and Ulcerative Colitis Colonoscopic Index of Severity, in patients with ulcerative colitis (UC) in remission. All three endoscopic scores and fecal calprotectin are useful for predicting relapse in UC. The UCEIS is easy to evaluate and appears to be highly accurate in predicting relapse.
- Citation: Ishida N, Ito T, Takahashi K, Asai Y, Miyazu T, Higuchi T, Tamura S, Tani S, Yamade M, Iwaizumi M, Hamaya Y, Osawa S, Sugimoto K. Comparison of fecal calprotectin levels and endoscopic scores for predicting relapse in patients with ulcerative colitis in remission. World J Gastroenterol 2023; 29(47): 6111-6121
- URL: https://www.wjgnet.com/1007-9327/full/v29/i47/6111.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i47.6111
With the advances in treatment options for ulcerative colitis (UC), achieving mucosal healing has become a key the
The endoscopic score can predict the prognosis of UC, with higher scores indicating higher rates of subsequent hospitalizations and surgeries[16-18]. A previous report on patients with UC with mucosal healing showing an MES of 1 or less showed that the subsequent relapse rate was significantly higher in the MES 1 group than in the MES 0 group[19]. Thus, while the endoscopic score has been shown to contribute to the prediction of subsequent relapse, biomarkers have also been identified as effective predictors[20-25]. Particularly, there are many reports on the prediction of relapse in UC using FC[20-24].
As previously mentioned, biomarkers reflect the endoscopic scores and contribute to the subsequent prediction of prognosis. In this study, we analyzed the relative efficacy of endoscopic scores against that of biomarkers in predicting relapse. Considering the possibility that this analysis may require a more detailed endoscopic score than just MES and UCEIS, we also incorporated the Ulcerative Colitis Colonoscopic Index of Severity (UCCIS), which provides a comprehensive assessment of the overall colorectal score[26,27].
In total, 75 patients with UC in clinical remission who visited the Hamamatsu University School of Medicine between February 2019 and March 2022 were enrolled. These patients were diagnosed with UC based on their clinical presen
In this study, the clinical activity of UC was evaluated using the clinical activity index (CAI) according to Rachmilewitz[29]. Endoscopic scores for UC were assessed using MES, UCEIS, and UCCIS[2,3,26]. MES was evaluated according to the following criteria: 0, normal or inactive disease; 1, mild disease with erythema, decreased vascular pattern, and mild friability; 2, moderate disease with marked erythema, absence of vascular patterns, friability, and erosions; and 3, severe disease with spontaneous bleeding and ulceration[2]. The UCEIS score was evaluated by calculating the sum of three descriptors: vascular pattern (score 0-2), erosions and ulcers (score 0-3), and bleeding (score 0-3)[3]. The UCCIS score was assessed using the following descriptors in the five segments of the ascending colon, transverse colon, descending colon, sigmoid colon, and rectum: vascular pattern (score 0-2), granularity (score 0-2), erosions and ulcers (score 0-4), and bleeding/friability (score 0-2). These descriptor scores were then applied to the following formula: UCCIS = 3.1 × sum (vascular pattern across five segments) + 3.6 × sum (granularity across five segments) + 3.5 × sum (ulceration across five segments) + 2.5 × sum (bleeding/friability across five segments)[26]. Clinical remission was defined as CAI 4 or less, and mucosal healing was defined as MES 0 or MES 1. Patients who met these criteria were included in this study.
Fecal samples were collected in plastic tubes for FC measurement and stored at -20 ℃ until shipment to the laboratory (SRL Inc., Tokyo, Japan). The measurements were performed using a Phadia 250 Immunoassay Analyzer (HITACHI Ltd., Tokyo, Japan) and Elia A Calprotectin 2 reagent (Phadia GmbH, Freiburg, Germany) using fluorescence enzyme immunoassay principles. As colonoscopic preparation could influence the results of FC, fecal samples were collected the day before or after the colonoscopy.
This retrospective, single-center observational study aimed to evaluate whether MES, UCEIS, UCCIS, and FC serve as predictors of clinical relapse. The primary outcome measure was the assessment of the association between relapse within 12 mo and MES, UCEIS, UCCIS, and FC. The secondary outcome was the comparison between endoscopic scores and biomarkers in the enrolled patients with UC with mucosal healing.
Patients enrolled in this study made outpatient visits at intervals of 3 or more months. These patients were outpatients for more than 12 mo or until relapse. Clinical relapse was defined as an increase in CAI above baseline due to the worsening of diarrhea and abdominal pain or frequent or bloody stools requiring modification or addition of treatment. Changes in treatment were made at the discretion of each attending physician.
Statistical analyses were performed using IBM SPSS Statistics for Windows, version 24 (IBM Corp., Armonk, N.Y., United States) and EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan)[30]. Differences were assessed using the Mann-Whitney U test or Student’s t-test. Correlations were analyzed using Spearman's correlation coefficient. Receiver-operating characteristic (ROC) analysis was performed for endoscopy score and relapse prediction. The cumulative non-failure rate was evaluated using Kaplan-Meier analysis with the log-rank test. P < 0.05 was considered statistically significant.
The study protocol was reviewed and approved by the ethics committee of Hamamatsu University School of Medicine (No. 20-322). This study was conducted in accordance with the Good Clinical Practice principles in adherence to the Declaration of Helsinki.
In total, 75 patients with UC were enrolled in this study. The baseline patient characteristics are shown in Table 1. The median patient age and disease duration were 49 years and 8 years, respectively. A total of 43 patients had an MES of 0, and 32 had an MES of 1. UCEIS scores ranged from 0 to 3, and the median UCCIS and FC values were 0 and 174 mg/kg, respectively.
| Characteristic | All, n = 75 |
| Age in yr, median [IQR] | 49 [36, 62] |
| Male/Female, n (%) | 45 (60.0)/30 (40.0) |
| Disease duration in yr, median [IQR] | 8 [5, 13] |
| Disease extent, n (%) | |
| Extensive colitis | 45 (60.0) |
| Left-sided colitis | 24 (32.0) |
| Proctitis | 6 (8.0) |
| CAI by the Rachmilewitz index, median [IQR] | 0 [0, 1] |
| MES, n (%) | |
| MES 0 | 43 (57.3) |
| MES 1 | 32 (42.7) |
| UCEIS, n (%) | |
| UCEIS 0 | 39 (52.0) |
| UCEIS 1 | 17 (22.7) |
| UCEIS 2 | 13 (17.3) |
| UCEIS 3 | 6 (8.0) |
| UCCIS, median [IQR] | 0 [0, 6.7] |
| FC in mg/kg, median [IQR] | 174 [43, 810] |
| Medication used during the study, n (%) | |
| Oral 5-ASA | 48 (64.0) |
| Suppository steroids | 2 (2.7) |
| Systemic steroids | 9 (12.0) |
| Immunomodulators | 23 (30.7) |
| Biologics | 30 (40.0) |
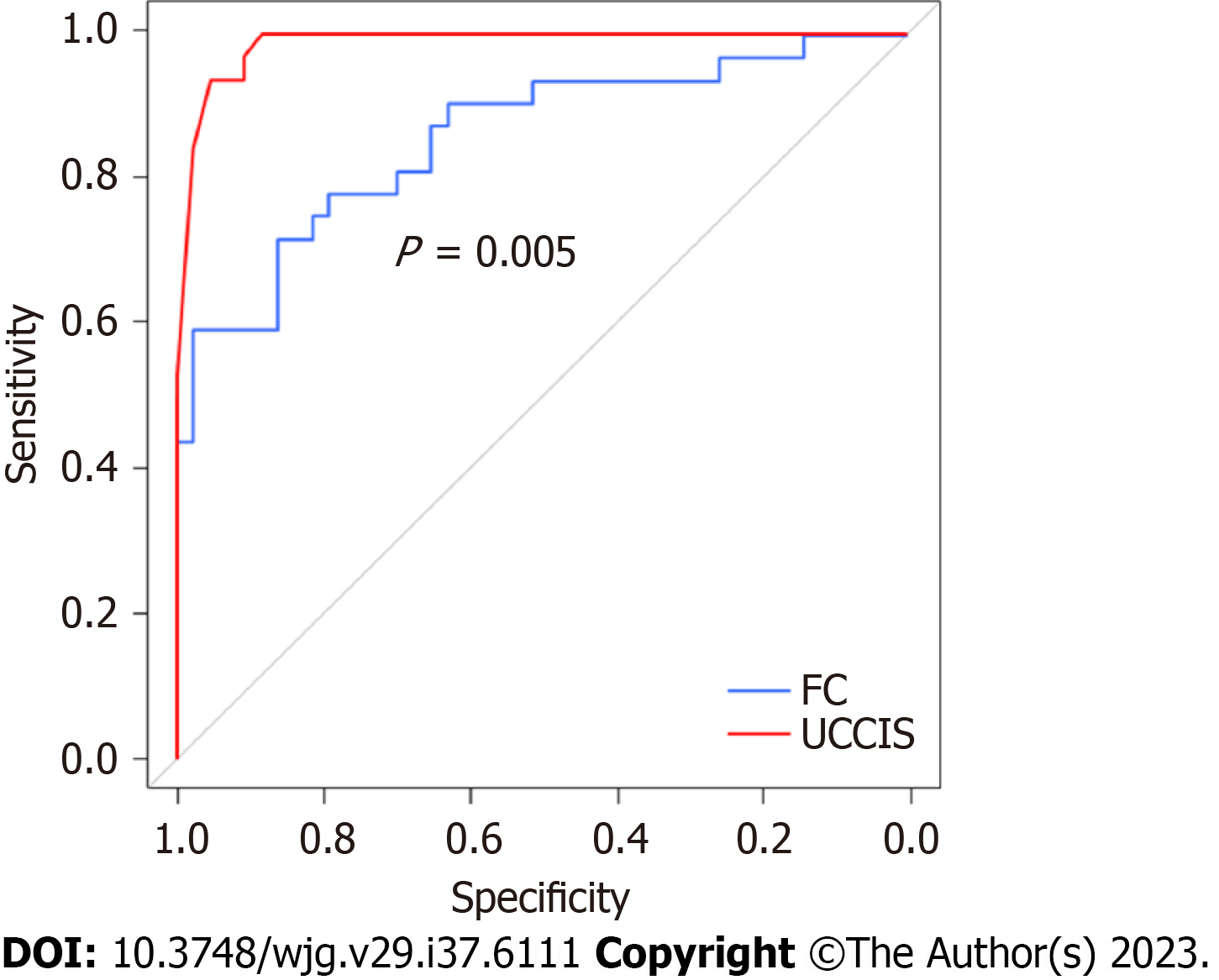
First, the association between endoscopic score and FC was assessed in enrolled patients with UC with MES 0 and 1. FC and UCCIS were significantly higher in the MES 1 group than in the MES 0 group (P < 0.001 and P < 0.001, respectively; Figure 1A and B). Both FC and UCCIS showed a significant correlation with UCEIS (r = 0.537, P < 0.001 and r = 0.957, P < 0.001, respectively; Figure 1C and D). A significant correlation was also observed between FC and UCCIS (r = 0.506, P < 0.001; Figure 1E). ROC analysis to predict MES 0 showed cut-off values of FC 385 mg/kg and UCCIS 6.6, with an area under the curve (AUC) of 0.858 [95% confidence interval (CI): 0.770-0.946] and 0.987 (95%CI: 0.969-1.000; Table 2). The AUC of UCCIS was significantly higher than that of FC (P < 0.001; Figure 2).
| Factor | FC | UCCIS |
| Cut-off value | 385 | 6.6 |
| AUC (95%CI) | 0.858 (0.770-0.946) | 0.987 (0.969-1.000) |
| PPV | 0.793 | 0.992 |
| NPV | 0.804 | 0.994 |
| Sensitivity | 0.719 | 0.992 |
| Specificity | 0.804 | 0.985 |
| Accuracy | 0.800 | 0.947 |
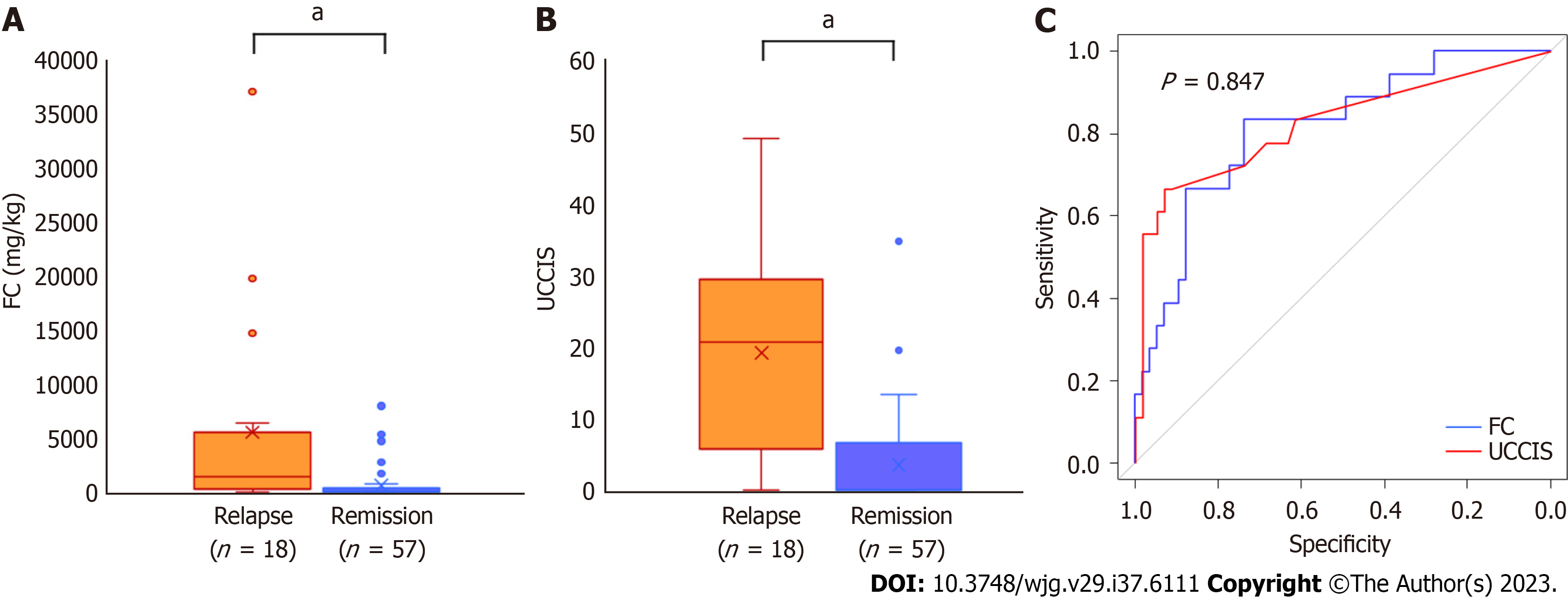
In total, 18 (24.0%) patients experienced clinical relapse during the 1-year follow-up period. The baseline FC and UCCIS values were significantly higher in the relapse group than in the remission group (P < 0.001 and P < 0.001, respectively; Figure 3A and B). In the ROC analysis for predicting clinical relapse, the cut-off value for FC was 323 mg/kg, and the AUC was 0.813 (95%CI: 0.698-0.927; Figure 3C). The cut-off value for UCCIS was 10.2, and the AUC was 0.823 (95%CI: 0.697-0.949), with no significant difference (Figure 3C).
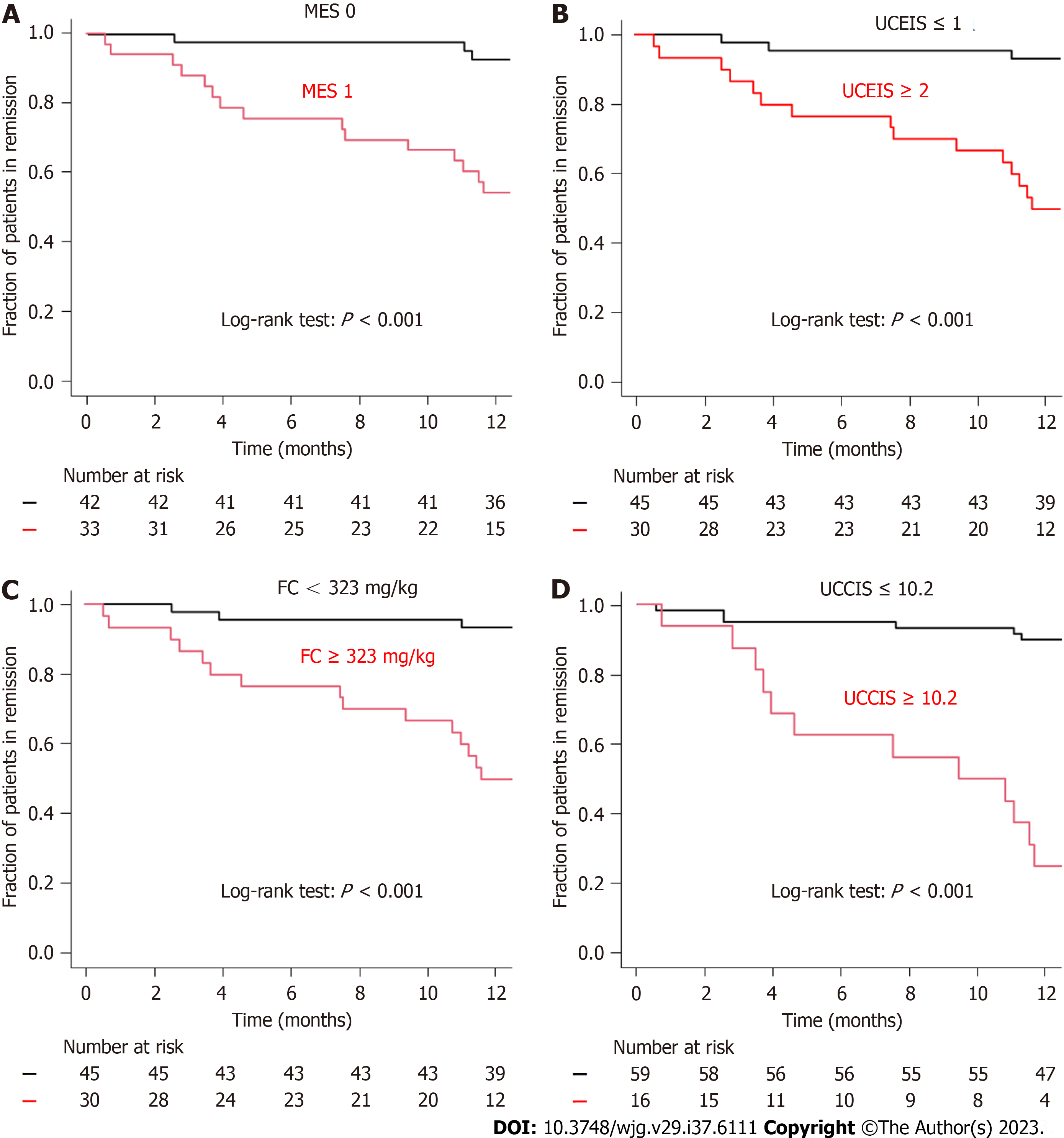
Kaplan-Meier analysis was used to assess the remission maintenance rate by grouping by each endoscopic score and cut-off value. When the endoscopic score was grouped by MES 0 and 1 and UCEIS ≤ 1 and ≥ 2, a significant difference was observed in the log-rank test (P < 0.001 and P < 0.001, respectively; Figure 4A and B). The analysis also revealed significant differences between the FC < 323 and FC ≥ 323 groups and UCCIS < 10.2 and UCCIS ≥ 10.2 groups using the log-rank test (P < 0.001 and P < 0.001, respectively; Figure 4C and D). Regarding the accuracy of relapse prediction, UCCIS had the highest accuracy at 86.7%, followed by UCEIS at 85.3% (Table 3). The accuracies of FC and MES were 76.0% and 73.3%, respectively.
| Factor | Sensitivity | Specificity | PPV | NPV | Accuracy |
| FC ≥ 323 | 0.500 | 0.933 | 0.833 | 0.737 | 0.760 |
| UCCIS ≥ 10.2 | 0.750 | 0.898 | 0.667 | 0.930 | 0.867 |
| MES 1 | 0.833 | 0.702 | 0.469 | 0.930 | 0.733 |
| UCEIS ≥ 2 | 0.722 | 0.895 | 0.684 | 0.911 | 0.853 |
This study showed that FC, MES, UCEIS, and UCCIS are useful for predicting relapse in patients with UC in clinical remission. Endoscopic and biomarker assessment must be used in current clinical practice for UC, in which achievement of mucosal healing is the goal of treatment because endoscopic scores and biomarkers have been reported to contribute to subsequent prognosis in patients with UC[16-25]. MES, a simple endoscopic score, is often used in large-scale clinical trials and real-world clinical practice. Although the simplicity of MES makes it easy to use, it cannot be used for detailed scoring[2]. On the other hand, UCEIS, which evaluates vascular, bleeding, and erosion/ulcer patterns, is capable of providing a more detailed evaluation compared to MES[3]. However, the assessment of MES and UCEIS is performed on the most active lesions, located in the sigmoid colon or rectum, thus only assessing localized areas. There are several reports on endoscopic scores that evaluate the activity of the entire colon. UCCIS, like UCEIS, is calculated by scoring each item and substituting those scores into the formula[26]. Although UCCIS evaluates the entire colon, its complexity of scoring poses considerable challenges.
Biomarkers quantify activity and enable detailed evaluation of inflammation[21]. In Japan, endoscopic examination and biomarker measurements cannot be performed in the same month. As previously mentioned, each endoscopic score and biomarker has its own advantages and disadvantages. To the best of our knowledge, no studies have yet compared the abilities of MES, UCEIS, UCCIS, and FC, a representative biomarker, to predict relapse.
In this study, we investigated the prediction of relapse and evaluated the relationship between FC, UCEIS, and UCCIS in patients with a mucosal healing score of MES 1 or less. A few reports on biomarkers have evaluated the association between biomarkers and endoscopic scores in the entire severity range of MES, from 0 to 3. Guardiola et al[31] reported that FC is useful for evaluating UC activity, including histological evaluation, in patients with UC who are in clinical and endoscopic remission. Previously, we reported a significant correlation between FC and UCCIS in UC with an MES ≤ 1 (r = 0.653, P < 0.001)[32]. In that study, FC showed a significant correlation with UCEIS and UCCIS, indicating that FC is a sensitive biomarker that reflects endoscopic activity even among patients who have achieved mucosal healing.
Regarding the prediction of relapse, which is the main purpose of this study, it was found that FC, MES, UCEIS, and UCCIS are all useful for predicting relapse within 1 year. Several reports on the prediction of recurrence using endoscopic scores have shown that MES 1 was associated with a significantly higher risk of relapse compared to MES 0 and MES 1[19]. We have also previously shown the usefulness of MES for relapse prediction in the analysis that examined the relapse prediction ability of fecal occult blood test[33]. Conversely, Yamamoto et al[34] reported that a similar analysis did not show a significant difference in predicting 1-year relapse, suggesting that the relapse prediction ability of MES is controversial. Arai et al[35] examined relapse prediction using UCEIS and reported that UCEIS is useful in mid- to long-term relapse prediction. We previously reported recurrence prediction using UCCIS, and the analysis was performed over a long-term observation period of 2 years and 5 years[36].
The cut-off of UCEIS in this study was set at 2, and the analysis was performed accordingly. This was because other UCEIS scores were also grouped and analyzed; however, the analysis grouped by scores of 2 or more and 1 or less showed the most accurate results. Arai et al[35] also reported that grouping based on a UCEIS cut-off of 2 or higher and 1 or lower was useful, and the cut-off value of UCEIS 2 was considered to be valid. Moreover, we did not perform multivariate analysis because UCEIS and UCCIS have a strong correlation close to 1, and including both these variables would have rendered the statistical analysis inconsequential. Instead, we examined the sensitivity, specificity, positive predictive values, negative predictive values, and accuracy. Regarding accuracy, both UCEIS and UCCIS exhibited an accuracy of 80% or more and were considered to be useful scores for predicting relapse. However, the UCCIS is an extremely complicated scoring system in which four items are evaluated across five colonic segments, and the scores are substituted into a formula. Therefore, it is not realistic to use this score in clinical practice. Hence, the UCEIS emerges as a preferable endoscopic scoring system in predicting relapse, owing to its accuracy and ease of use in clinical practice. Intensifying treatment based on the UCEIS score in real-world clinical practice could help prevent relapse; hence, further prospective studies in this regard are desired.
The strength of this study is that endoscopic examination and biomarker measurements were performed simultaneously. However, currently, biomarkers and endoscopic measurements cannot be performed together in clinical practice. Nevertheless, several limitations to this study must be acknowledged. First, it was a single-center retrospective analysis conducted in a small number of patients. Second, our results were not compared with other biomarkers, such as leucine-rich alpha-2 glycoprotein; histological findings were also not considered. Third, biomarker and endoscopic evaluations were not performed at the time of relapse.
In conclusion, MES, UCEIS, UCCIS, and FC were useful for predicting relapse in patients with UC in clinical remission. Among the three endoscopic scores evaluated, UCEIS may be the most useful in terms of ease of evaluation and predictive accuracy.
The goal of ulcerative colitis (UC) treatment is to achieve mucosal healing, for which endoscopic evaluation is recom
To evaluate whether FC and MES, UCEIS, and UCCIS are useful for predicting relapse in patients with UC in clinical remission.
Overall, 75 patients with UC in clinical remission, with a clinical activity index (CAI) according to Rachmilewitz score was ≤ 4, underwent colonoscopic examination and FC measurements.
We assessed whether the enrolled patients experienced UC relapse within 12 mo after endoscopic examination and FC measurement. Clinical relapse was defined as an increase in CAI above baseline due to worsening of diarrhea and abdominal pain or frequent or bloody stools, requiring modification or addition of treatment. We also evaluated the association between endoscopic scores and FC.
Cut-off values and areas under the curve (AUC) for FC and UCCIS in the receiver-operating characteristic analysis to predict clinical relapse were 323 mg/kg, 0.813 [95% confidence interval (CI)]: 0.698-0.927], and 10.2, for FC, AUC, and UCCIS, respectively.
The AUC was 0.823 (95%CI: 0.697-0.949). Univariate analysis was performed using these cut-off values (FC < 323 mg/kg vs ≥ 323 mg/kg; UCCIS < 10.2 vs ≥ 10.2; MES 0 vs 1; and UCEIS ≤ 1 vs ≥ 2). The accuracy of relapse prediction was the highest with UCCIS, followed by UCIES, FC, and MES.
MES, UCEIS, UCCIS, and FC were useful for predicting relapse in patients with UC in clinical remission.
UCCIS comprehensively evaluates the endoscopic activity of UC, helping to predict its relapse. However, its complexity poses a challenge. Among the three endoscopic scores, UCEIS may be the most useful in terms of ease of evaluation and accuracy.
We would like to express our appreciation to the staff of the gastroenterology ward, outpatient clinic, and the de
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Rocha R, Brazil; Teramoto-Matsubara OT, Mexico S-Editor: Qu XL L-Editor: Filipodia P-Editor: Xu ZH
| 1. | Peyrin-Biroulet L, Sandborn W, Sands BE, Reinisch W, Bemelman W, Bryant RV, D'Haens G, Dotan I, Dubinsky M, Feagan B, Fiorino G, Gearry R, Krishnareddy S, Lakatos PL, Loftus EV Jr, Marteau P, Munkholm P, Murdoch TB, Ordás I, Panaccione R, Riddell RH, Ruel J, Rubin DT, Samaan M, Siegel CA, Silverberg MS, Stoker J, Schreiber S, Travis S, Van Assche G, Danese S, Panes J, Bouguen G, O'Donnell S, Pariente B, Winer S, Hanauer S, Colombel JF. Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE): Determining Therapeutic Goals for Treat-to-Target. Am J Gastroenterol. 2015;110:1324-1338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1487] [Cited by in RCA: 1407] [Article Influence: 140.7] [Reference Citation Analysis (115)] |
| 2. | Schroeder KW, Tremaine WJ, Ilstrup DM. Coated oral 5-aminosalicylic acid therapy for mildly to moderately active ulcerative colitis. A randomized study. N Engl J Med. 1987;317:1625-1629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1958] [Cited by in RCA: 2248] [Article Influence: 59.2] [Reference Citation Analysis (0)] |
| 3. | D'Haens G, Sandborn WJ, Feagan BG, Geboes K, Hanauer SB, Irvine EJ, Lémann M, Marteau P, Rutgeerts P, Schölmerich J, Sutherland LR. A review of activity indices and efficacy end points for clinical trials of medical therapy in adults with ulcerative colitis. Gastroenterology. 2007;132:763-786. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 722] [Cited by in RCA: 794] [Article Influence: 44.1] [Reference Citation Analysis (1)] |
| 4. | Sands BE. Biomarkers of Inflammation in Inflammatory Bowel Disease. Gastroenterology. 2015;149:1275-1285.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 287] [Article Influence: 28.7] [Reference Citation Analysis (0)] |
| 5. | Schoepfer AM, Beglinger C, Straumann A, Trummler M, Renzulli P, Seibold F. Ulcerative colitis: correlation of the Rachmilewitz endoscopic activity index with fecal calprotectin, clinical activity, C-reactive protein, and blood leukocytes. Inflamm Bowel Dis. 2009;15:1851-1858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 246] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 6. | Lin WC, Wong JM, Tung CC, Lin CP, Chou JW, Wang HY, Shieh MJ, Chang CH, Liu HH, Wei SC; Taiwan Society of Inflammatory Bowel Disease Multicenter Study. Fecal calprotectin correlated with endoscopic remission for Asian inflammatory bowel disease patients. World J Gastroenterol. 2015;21:13566-13573. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 33] [Cited by in RCA: 36] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 7. | Nakarai A, Kato J, Hiraoka S, Kuriyama M, Akita M, Hirakawa T, Okada H, Yamamoto K. Evaluation of mucosal healing of ulcerative colitis by a quantitative fecal immunochemical test. Am J Gastroenterol. 2013;108:83-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 76] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 8. | Nakarai A, Kato J, Hiraoka S, Takashima S, Takei D, Inokuchi T, Sugihara Y, Takahara M, Harada K, Okada H. Ulcerative colitis patients in clinical remission demonstrate correlations between fecal immunochemical test results, mucosal healing, and risk of relapse. World J Gastroenterol. 2016;22:5079-5087. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 18] [Cited by in RCA: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 9. | Takashima S, Kato J, Hiraoka S, Nakarai A, Takei D, Inokuchi T, Sugihara Y, Takahara M, Harada K, Okada H, Tanaka T, Yamamoto K. Evaluation of Mucosal Healing in Ulcerative Colitis by Fecal Calprotectin Vs. Fecal Immunochemical Test. Am J Gastroenterol. 2015;110:873-880. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 90] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 10. | Shinzaki S, Matsuoka K, Iijima H, Mizuno S, Serada S, Fujimoto M, Arai N, Koyama N, Morii E, Watanabe M, Hibi T, Kanai T, Takehara T, Naka T. Leucine-rich Alpha-2 Glycoprotein is a Serum Biomarker of Mucosal Healing in Ulcerative Colitis. J Crohns Colitis. 2017;11:84-91. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 114] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 11. | Yasutomi E, Inokuchi T, Hiraoka S, Takei K, Igawa S, Yamamoto S, Ohmori M, Oka S, Yamasaki Y, Kinugasa H, Takahara M, Harada K, Furukawa M, Itoshima K, Okada K, Otsuka F, Tanaka T, Mitsuhashi T, Kato J, Okada H. Leucine-rich alpha-2 glycoprotein as a marker of mucosal healing in inflammatory bowel disease. Sci Rep. 2021;11:11086. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 61] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 12. | Shimoyama T, Yamamoto T, Yoshiyama S, Nishikawa R, Umegae S. Leucine-Rich Alpha-2 Glycoprotein Is a Reliable Serum Biomarker for Evaluating Clinical and Endoscopic Disease Activity in Inflammatory Bowel Disease. Inflamm Bowel Dis. 2023;29:1399-1408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 27] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 13. | Reinisch W, Bressler B, Curtis R, Parikh A, Yang H, Rosario M, Røseth A, Danese S, Feagan B, Sands BE, Ginsburg P, Dassopoulos T, Lewis J, Xu J, Wyant T. Fecal Calprotectin Responses Following Induction Therapy With Vedolizumab in Moderate to Severe Ulcerative Colitis: A Post Hoc Analysis of GEMINI 1. Inflamm Bowel Dis. 2019;25:803-810. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 14. | Danese S, Sands BE, Abreu MT, O'Brien CD, Bravatà I, Nazar M, Miao Y, Wang Y, Rowbotham D, Leong RWL, Arasaradnam RP, Afif W, Marano C. Early Symptomatic Improvement After Ustekinumab Therapy in Patients With Ulcerative Colitis: 16-Week Data From the UNIFI Trial. Clin Gastroenterol Hepatol. 2022;20:2858-2867.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 11] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 15. | Loftus EV Jr, Colombel JF, Takeuchi K, Gao X, Panaccione R, Danese S, Dubinsky M, Schreiber S, Ilo D, Finney-Hayward T, Zhou W, Phillips C, Gonzalez YS, Shu L, Yao X, Zhou Q, Vermeire S. Upadacitinib Therapy Reduces Ulcerative Colitis Symptoms as Early as Day 1 of Induction Treatment. Clin Gastroenterol Hepatol. 2023;21:2347-2358.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 53] [Reference Citation Analysis (0)] |
| 16. | Colombel JF, Rutgeerts P, Reinisch W, Esser D, Wang Y, Lang Y, Marano CW, Strauss R, Oddens BJ, Feagan BG, Hanauer SB, Lichtenstein GR, Present D, Sands BE, Sandborn WJ. Early mucosal healing with infliximab is associated with improved long-term clinical outcomes in ulcerative colitis. Gastroenterology. 2011;141:1194-1201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 643] [Cited by in RCA: 727] [Article Influence: 51.9] [Reference Citation Analysis (0)] |
| 17. | Xie T, Zhang T, Ding C, Dai X, Li Y, Guo Z, Wei Y, Gong J, Zhu W, Li J. Ulcerative Colitis Endoscopic Index of Severity (UCEIS) vs Mayo Endoscopic Score (MES) in guiding the need for colectomy in patients with acute severe colitis. Gastroenterol Rep (Oxf). 2018;6:38-44. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 91] [Cited by in RCA: 67] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 18. | Corte C, Fernandopulle N, Catuneanu AM, Burger D, Cesarini M, White L, Keshav S, Travis S. Association between the ulcerative colitis endoscopic index of severity (UCEIS) and outcomes in acute severe ulcerative colitis. J Crohns Colitis. 2015;9:376-381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 105] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 19. | Barreiro-de Acosta M, Vallejo N, de la Iglesia D, Uribarri L, Bastón I, Ferreiro-Iglesias R, Lorenzo A, Domínguez-Muñoz JE. Evaluation of the Risk of Relapse in Ulcerative Colitis According to the Degree of Mucosal Healing (Mayo 0 vs 1): A Longitudinal Cohort Study. J Crohns Colitis. 2016;10:13-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 178] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 20. | Fiorino G, Danese S, Peyrin-Biroulet L, Sans M, Bonelli F, Calleri M, Zierold C, Pollastro R, Moretti F, Malesci A. LIAISON(®) Calprotectin for the prediction of relapse in quiescent ulcerative colitis: The EuReCa study. United European Gastroenterol J. 2022;10:836-843. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 21. | Urushikubo J, Yanai S, Nakamura S, Kawasaki K, Akasaka R, Sato K, Toya Y, Asakura K, Gonai T, Sugai T, Matsumoto T. Practical fecal calprotectin cut-off value for Japanese patients with ulcerative colitis. World J Gastroenterol. 2018;24:4384-4392. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 20] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 22. | Scaioli E, Digby RJ, Belluzzi A. Different Cutoff Levels of Fecal Calprotectin to Predict Clinical Relapse in Ulcerative Colitis. Inflamm Bowel Dis. 2016;22:E26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 23. | Theede K, Holck S, Ibsen P, Kallemose T, Nordgaard-Lassen I, Nielsen AM. Fecal Calprotectin Predicts Relapse and Histological Mucosal Healing in Ulcerative Colitis. Inflamm Bowel Dis. 2016;22:1042-1048. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 115] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 24. | Nakarai A, Hiraoka S, Takahashi S, Inaba T, Higashi R, Mizuno M, Takashima S, Inokuchi T, Sugihara Y, Takahara M, Harada K, Kato J, Okada H. Simultaneous Measurements of Faecal Calprotectin and the Faecal Immunochemical Test in Quiescent Ulcerative Colitis Patients Can Stratify Risk of Relapse. J Crohns Colitis. 2018;12:71-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 25. | Hiraoka S, Kato J, Nakarai A, Takashima S, Inokuchi T, Takei D, Sugihara Y, Takahara M, Harada K, Okada H. Consecutive Measurements by Faecal Immunochemical Test in Quiescent Ulcerative Colitis Patients Can Detect Clinical Relapse. J Crohns Colitis. 2016;10:687-694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 26. | Neumann H, Neurath MF. Ulcerative colitis: UCCIS--a reproducible tool to assess mucosal healing. Nat Rev Gastroenterol Hepatol. 2012;9:692-694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 27. | Samuel S, Bruining DH, Loftus EV Jr, Thia KT, Schroeder KW, Tremaine WJ, Faubion WA, Kane SV, Pardi DS, de Groen PC, Harmsen WS, Zinsmeister AR, Sandborn WJ. Validation of the ulcerative colitis colonoscopic index of severity and its correlation with disease activity measures. Clin Gastroenterol Hepatol. 2013;11:49-54.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 106] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 28. | Magro F, Gionchetti P, Eliakim R, Ardizzone S, Armuzzi A, Barreiro-de Acosta M, Burisch J, Gecse KB, Hart AL, Hindryckx P, Langner C, Limdi JK, Pellino G, Zagórowicz E, Raine T, Harbord M, Rieder F; European Crohn’s and Colitis Organisation [ECCO]. Third European Evidence-based Consensus on Diagnosis and Management of Ulcerative Colitis. Part 1: Definitions, Diagnosis, Extra-intestinal Manifestations, Pregnancy, Cancer Surveillance, Surgery, and Ileo-anal Pouch Disorders. J Crohns Colitis. 2017;11:649-670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1446] [Cited by in RCA: 1292] [Article Influence: 161.5] [Reference Citation Analysis (0)] |
| 29. | Rachmilewitz D. Coated mesalazine (5-aminosalicylic acid) vs sulphasalazine in the treatment of active ulcerative colitis: a randomised trial. BMJ. 1989;298:82-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 771] [Cited by in RCA: 805] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 30. | Kanda Y. Investigation of the freely available easy-to-use software 'EZR' for medical statistics. Bone Marrow Transplant. 2013;48:452-458. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9275] [Cited by in RCA: 13252] [Article Influence: 1104.3] [Reference Citation Analysis (0)] |
| 31. | Guardiola J, Lobatón T, Rodríguez-Alonso L, Ruiz-Cerulla A, Arajol C, Loayza C, Sanjuan X, Sánchez E, Rodríguez-Moranta F. Fecal level of calprotectin identifies histologic inflammation in patients with ulcerative colitis in clinical and endoscopic remission. Clin Gastroenterol Hepatol. 2014;12:1865-1870. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 107] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 32. | Ishida N, Higuchi T, Miyazu T, Tamura S, Tani S, Yamade M, Iwaizumi M, Hamaya Y, Osawa S, Furuta T, Sugimoto K. C-reactive protein is superior to fecal biomarkers for evaluating colon-wide active inflammation in ulcerative colitis. Sci Rep. 2021;11:12431. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 33. | Ishida N, Matsuura T, Asai Y, Miyazu T, Tamura S, Tani S, Yamade M, Iwaizumi M, Hamaya Y, Osawa S, Furuta T, Sugimoto K. Predicting Ulcerative Colitis Relapse in Clinical Remission With Fecal Immunochemical Occult Blood Test or Prostaglandin E-Major Urinary Metabolite. Clin Transl Gastroenterol. 2022;13:e00501. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Reference Citation Analysis (0)] |
| 34. | Yamamoto T, Shimoyama T, Umegae S, Matsumoto K. Endoscopic score vs. fecal biomarkers for predicting relapse in patients with ulcerative colitis after clinical remission and mucosal healing. Clin Transl Gastroenterol. 2018;9:136. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 41] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 35. | Arai M, Naganuma M, Sugimoto S, Kiyohara H, Ono K, Mori K, Saigusa K, Nanki K, Mutaguchi M, Mizuno S, Bessho R, Nakazato Y, Hosoe N, Matsuoka K, Inoue N, Ogata H, Iwao Y, Kanai T. The Ulcerative Colitis Endoscopic Index of Severity is Useful to Predict Medium- to Long-Term Prognosis in Ulcerative Colitis Patients with Clinical Remission. J Crohns Colitis. 2016;10:1303-1309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 59] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 36. | Ishida N, Onoue S, Miyazu T, Tamura S, Tani S, Yamade M, Iwaizumi M, Hamaya Y, Osawa S, Furuta T, Sugimoto K. Further research on the clinical relevance of the ulcerative colitis colonoscopic index of severity for predicting 5-year relapse. Int J Colorectal Dis. 2021;36:2661-2670. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |