Published online Dec 14, 2023. doi: 10.3748/wjg.v29.i46.6049
Peer-review started: August 3, 2023
First decision: October 9, 2023
Revised: October 25, 2023
Accepted: November 27, 2023
Article in press: November 27, 2023
Published online: December 14, 2023
Processing time: 131 Days and 16.8 Hours
Pancreatic transplantation is considered by the American Diabetes Association and the European Association for the Study of Diabetes an acceptable surgical procedure in patients with type 1 diabetes also undergoing kidney transplantation in pre-final or end-stage renal disease if no contraindications are present. Pancreatic transplantation, however, is a complex surgical procedure and may lead to a range of postoperative complications that can significantly impact graft function and patient outcomes. Postoperative computed tomography (CT) is often adopted to evaluate perfusion of the transplanted pancreas, identify complications and as a guide for interventional radiology procedures. CT assessment after pancreatic transplantation should start with the evaluation of the arterial Y-graft, the venous anastomosis and the duodenojejunostomy. With regard to complications, CT allows for the identification of vascular complications, such as throm
Core Tip: Pancreatic transplantation is a complex surgical procedure and, similarly to any major surgical intervention, may lead to a range of postoperative complications that can significantly impact graft function and patient outcomes. Computed tomography offers non-invasive and accurate visualization of the transplanted pancreas and surrounding structures, providing detailed anatomical information and aiding in the detection of complications.
- Citation: D'Alessandro C, Todisco M, Di Bella C, Crimì F, Furian L, Quaia E, Vernuccio F. Surgical complications after pancreatic transplantation: A computed tomography imaging pictorial review. World J Gastroenterol 2023; 29(46): 6049-6059
- URL: https://www.wjgnet.com/1007-9327/full/v29/i46/6049.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i46.6049
Pancreatic transplantation is considered by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) an acceptable surgical procedure in patients with type 1 diabetes (T1D) also undergoing kidney transplantation [i.e. simultaneous pancreas and kidney (SPK) transplant] in pre-final or end-stage renal disease if no contraindications are present[1]. Pancreatic transplantation has been considered for years the only treatment for T1D that consistently establishes an insulin-independent, normoglycemic state, with 5-year graft survival of 83%, 55% and 70%, in case of SPK, pancreatic transplantation alone (PTA) or pancreas after a kidney transplantation (PAK), respectively[2-4]. The main indication of PTA is presence of T1D and normal or near-normal renal function in patients who suffer from hypoglycemia unawareness, which results in impaired quality of life or with difficulty adhering to the requirement of insulin injection[5]. In few selected cases, transplantation of pancreatic islet – a less invasive procedure consisting in the transplantation of islets of Langerhans in the recipient hepatic portal system – is indicated as an alternative to pancreatic transplantation by ADA and EASD[1]. However, intrahepatic islet transplantation for T1D is limited because of the need of multiple infusions and poor islet viability after transplantation, and more recently intracutaneous transplantation of islets has also been investigated[6]. In a 20-year span from 2000 to 2020, only about 4365 islet allotransplants have been performed according to a recent worldwide survey[7], while the number of pancreatic transplantations in the 10-year span from 2010 to 2019 is of about 23000 procedures[8].
Pancreatic transplantation is a complex surgical procedure and, similarly to any major surgical intervention, may lead to a range of postoperative complications that can significantly impact graft function and patient outcomes[9,10]. The occurrence of postoperative complications may vary depending on factors such as the type of transplantation (SPK, PAK or PTA) and the specific patient population. Cross-sectional imaging – i.e., Doppler ultrasound (US), contrast-enhanced US (CEUS) and computed tomography (CT) – plays a pivotal role in the evaluation of the transplanted pancreas. US is the preferred initial imaging modality for evaluating the transplanted pancreas due to its safe, non-invasive, radiation-free, simple, quick, and repeatable approach. However, US is often affected by factors such as intestinal gas interference and operator proficiency, and partial thrombosis may be easily missed[11]. CT offers non-invasive and accurate visualization of the transplanted pancreas and surrounding structures, providing detailed anatomical information and aiding in the detection of complications. Indeed, CT allows for the identification of vascular complications, such as thrombosis or stenosis of blood vessels supplying the graft as well as bleeding or pseudoaneurysms, the detection of pancreatic fluid collections, including pseudocysts, abscesses, or leaks, the assessment of bowel complications (anastomotic leaks, ileus or obstruction), while it has a limited role in the evaluation of graft rejection[12,13]. Magnetic resonance imaging (MRI) is rarely uncommonly used for examination the assessment of pancreatic graft-related complications, and it is preferred particularly in patients with declining renal function. The main adoption of CT compared to MRI is based on different reasons, including wider availability of CT compared to MRI particularly in the emergency and urgent settings, the lower acquisition time of CT exams compared to MRI which is important in imaging acutely ill and intensively monitored patients, but also to the lower spatial and temporal resolution, creating difficulties in the evaluation of the enteric anastomosis and vascular complications. Contrast medium administration in CT should not be a contraindication in kidney transplant recipients. Fananapazir et al[14] demonstrated that the incidence of acute kidney injury in patients with transplanted kidney submitted to CT scans with low-osmolality iodine-based contrast material was 7% when considering the threshold of ≥ 0.3 mg/dL for the increase in serum creatinine levels. The incidence of contrast induced nephropathy after contrast-enhanced CT was similar (6.1%) in a study by Cheungpasitporn et al[15]. However, in a study involving about 6175 patients, McDonald et al[16] demonstrated lack of significant difference in the onset if contrast induced nephropathy between patients with a solitary kidney, including kidney transplant recipients (4.1%), and those with bilateral kidneys (4.2%). The assessment of serum creatinine for calculating the estimated glomerular filtration rate is recommended before contrast medium administration within 7 d before CT in patients with an acute disease, an acute deterioration of a chronic disease or in hospitalized patients, and preventive hydration protocols need to be considered in at-risk patients as indicated by guidelines[17-19].
This pictorial review is aimed at illustrating CT findings of surgical-related complications after pancreatic trans
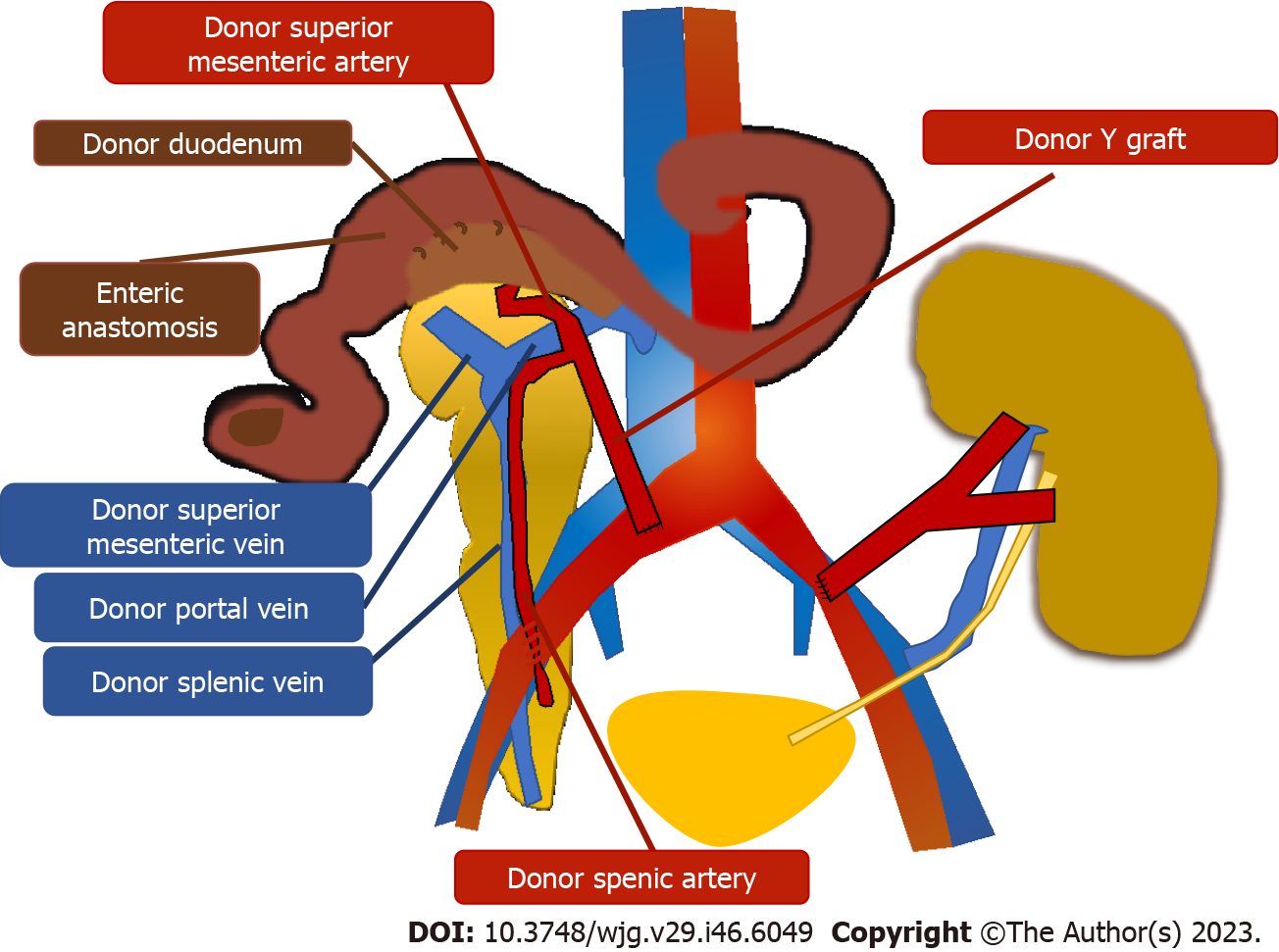
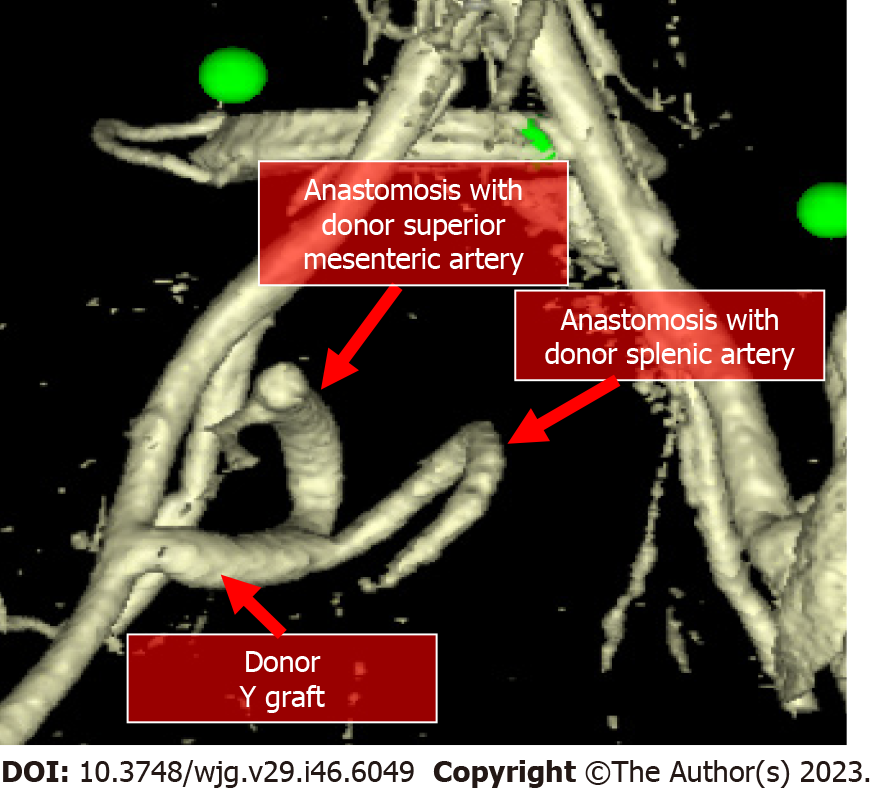
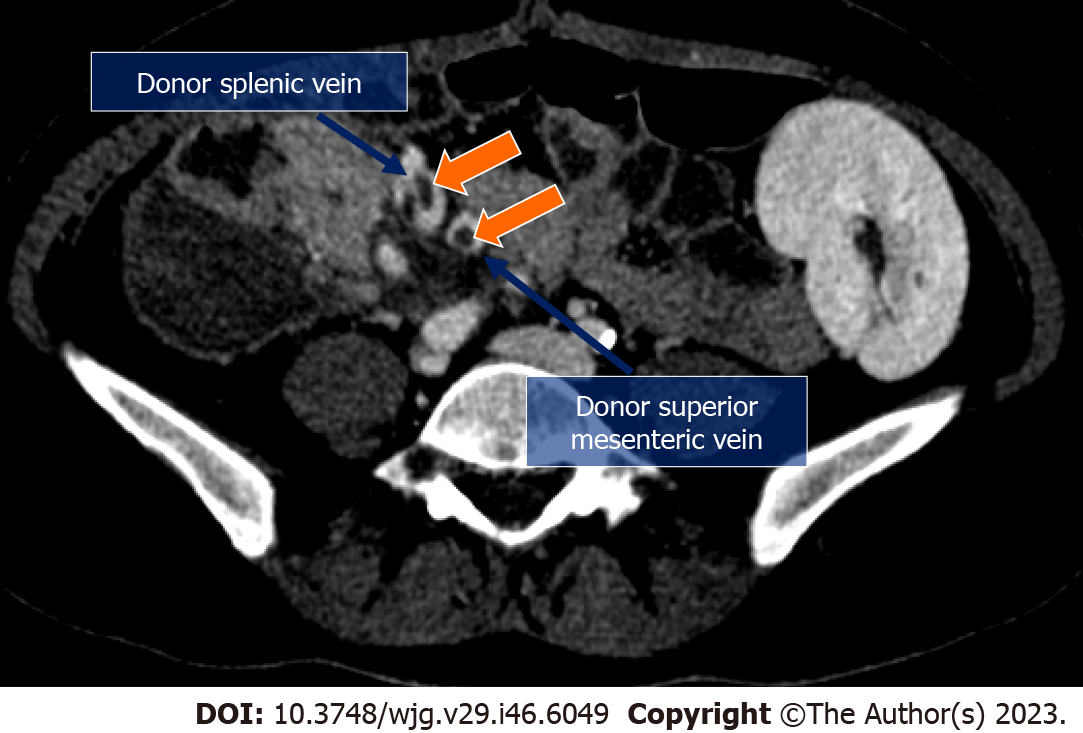
After organ procurement from a deceased donor, a meticulous graft back-table surgery is necessary before pancreatic transplantation. Main steps are: Splenectomy, removal of the excess fat surrounding the pancreas and ligation of small vessels and lymphatics along the inferior margin of the pancreatic tail, coursing anteriorly around the surface of the neck and head of the pancreas to the proximal duodenal staple line. A suture of the vessels at the root of the mesentery and inferior mesenteric vein is performed followed by an oversewing of the mesenteric staple line. A vascular preparation is necessary using a Y conduit of donor iliac artery which is anastomosed to the superior mesenteric and splenic arteries of the graft (Figures 1 and 2). Assessment of blood supply to the entire pancreas graft exclusively via cross-circulation between splenic artery and superior mesenteric artery is mandatory in order to guarantee blood supply to all the pancreas graft. By flushing the superior mesenteric artery and not looking for back-flushing through the gastroduodenal artery is possible to recognize the need for vascular reconstruction of the gastroduodenal artery to guarantee blood supply to head of the pancreas graft and duodenal segment, and possibly reduce the incidence of duodenal complications[20]. A duodenal shortening with stapler and oversewing of the staple line is then completed. At this stage, the graft is ready for implantation.
Through a midline incision, the pancreas allograft is usually placed intraperitoneally, on the right side with the head in a cranial position, and receives arterial inflow from the iliac artery (Figure 1). Venous anastomosis can be performed through systemic vein technique with the graft portal vein (PV) anastomosed to the recipient inferior vena cava or iliac vein, or through the PV technique with the graft PV anastomosed to the recipient inferior vena cava or iliac vein. In the PV technique, the graft PV is connected to the recipient superior mesenteric vein. Systemic drainage theoretically may lead to hyperinsulinemia[21], while portal drainage could allow a more physiological “first pass” effect through the liver since insulin is immediately extracted by the liver. However, the arterial anastomosis to the iliac artery tends to be more difficult using the PV technique and it requires a very long Y graft. Moreover, obesity, thickened mesentery, or an inadequate caliber of the superior mesenteric vein can make the portal drainage even harder[22]. Long-term studies comparing the two techniques have not demonstrated clear metabolic advantages with portal drainage and the use of the PV has remained marginal over the years[23]. For the exocrine pancreas drainage, anastomosis between the donor duodenum and recipient small bowel loop (i.e., jejunal or an ileal loop) is performed side-to-side with a circular stapler. After firing the stapler (trans-oral anvil delivery system EEA), the end of the donor duodenum is closed using a linear stapler. Alternatively, a bladder diversion can be performed[24]. Bladder drainage can be particularly advantageous in case of PTA, for the assessment of the concentration of urinary amylase as a marker of rejection. Disadvantages of this technique include both urologic complications such as hematuria (16%), leaks (14%), reflux pancreatitis (11%), recurrent urinary infections (10%), urethritis (3%), urethral stricture/disruption (3%), and metabolic complications due to the urinary loss of the bicarbonate-rich pancreatic juice, including hyperchloremic metabolic acidosis and dehydration[23,25]. When these complications became intractable, conversion from bladder to enteric drainage is often necessary[26]. Enteric drainage is currently the predominant technique in pancreatic transplantation[23].
Usually, recipients start receiving immunosuppression during surgery, consisting in antibody induction and a triple drug immunosuppressive therapy is started immediately postoperatively. Most of the Centers utilize a regimen consisting of tacrolimus, mycophenolate mofetil and steroid.
Heparin prophylaxis is recommended with intravenous heparin administered as a single dose during surgery after pancreas revascularization and it is continued after surgery with low molecular weight heparin or continuous infusion, at the discretion of the Transplant Centre.
Some days after transplantation, a CT scan is advisable to early recognize vascular thrombosis or any signs of vascular alterations, not yet clinically relevant.
Postoperative surgical complications of pancreatic transplantation are still common despite many improvements in surgical techniques, and may be distinguished based on time of onset[27-28]. Postoperative monitoring of the pancreatic graft by CT is requested in about 89% of cases according to a recent series of 230 pancreatic transplantations and is helpful for optimizing patient management[29].
The main reasons for requesting imaging are related to the indication per protocol even without acute clinical indication, sudden progressive hyperglycemia, persistent or abdominal tenderness[29]. However, the adoption of CT needs to be patient-tailored particularly in the context of SPK, due to the potentially nephrotoxic effect of contrast agents which is reported to be as low as 5.6% in kidney transplant recipients with the use of hypo-osmolar contrast agent[30].
Initial complications of pancreatic transplantations are largely related to technical factors, including vascular thrombosis, bleeding, enteric anastomotic leak or graft pancreatitis, and urologic complications[8]. Late complications include pseudocyst formation, post-transplant lymphoproliferative disease, pseudoaneurysms, artero-venous fistulas and rejection (Figure 3).
Graft thrombosis is the most common complication after transplant and may lead to graft failure in about 3.7%, 4.1% and 5.9% of SPK, PAK and PTA, respectively[8]. Graft loss due to infection, pancreatitis, bleeding, leaks, and other reasons occur in up to 0.5%, 0.5%, 0.5%, 0.4%, and 0.6%, respectively[8].
Graft thrombosis occurs in 7%-34% of patients after pancreas transplant, with high body mass index being a risk factor, may affect arteries or veins and can be partial or complete[10,12,29,31]. Partial graft thrombosis occurs in about 25% of cases after pancreatic transplantation, is subclinical in the majority of cases, and may resolve spontaneously with medical therapy[29]. Venous thrombosis is far more common than arterial thrombosis, but arterial thrombosis is the most dangerous leading to rapid graft loss[29,32]. Acute rejection and CT finding of pancreatitis are risk factors for graft thrombosis[12].
Contrast-enhanced CT with angiographic phase and venous phase demonstrates thrombosis as a filling defect in a vessel during the vascular phase, and allows to clearly indicate the extension of the filling defect. Arterial thrombosis (Figure 4) may lead to graft dysfunction, pancreatitis, leakage of pancreatic enzymes, sepsis, necrosis and even emphysematous transformation of the graft if it is left untreated. Pancreatic graft venous thrombi (Figure 5) can remain relatively localized possibly maintaining normal graft function, or may propagate into the PV, iliac vein, or vena cava (in case of systemic venous drainage) or superior mesenteric veins (in case of portal venous drainage). In case of vascular thrombosis, a decreased enhancement of the transplanted pancreas usually occurs dure to the organ ischemia, and the main differential diagnosis between arterial and venous thrombosis is made by direct visualization of the thrombus in the vessel.
Percutaneous thrombectomy, followed by anticoagulation, is considered a therapeutical option to remove the thrombus, with low complication rate[33].
Graft thrombosis may be graded on contrast-enhanced CT based on the system proposed by Hakeem et al[12] into:
Grade 0 – Lack of thrombosis.
Grade 1 – Peripheral thrombosis: Thrombus is located at the transected margin of the superior mesenteric vein/splenic vein or superior mesenteric artery and it is present only in a single branch.
Grade 2 – Intermediate nonocclusive thrombosis.
Venous: Thrombus extends into parenchymal vessels/main trunk of the superior mesenteric vein or splenic vein to the superior mesenteric vein/splenic vein confluence but not into the PV.
Arterial: Thrombus extends into the main trunk of the superior mesenteric artery/splenic artery to the “Y” graft but not into the Y graft.
Grade 3 – Central occlusive thrombosis.
Interestingly, despite the diagnosis of vascular thrombosis seems quite straightforward Hakeem et al[12] showed some discrepancies among radiologists in the detection with 28 new thrombosis identified on retrospective imaging review, with the grade 1 thrombosis being underestimated at initial diagnosis by the reporting radiologists.
Graft pancreatitis is amongst the most frequent complications following pancreatic transplantation, the third most common cause for re-operation following bleeding and pancreas graft thrombosis, and a common histologic feature identified in up to 61% of rejected allografts[34,35]. Graft pancreatitis is distinguished in early if it occurs within 3 mo and later after 3 mo, while physiological acute graft pancreatitis occurs within the first 72 h after reperfusion of the transplanted organ secondarily to an acute inflammatory response related to ischemic reperfusion injury[36].
Common findings in CT include focal or diffuse enlargement of the pancreatic parenchyma, with edematous changes (usually is noted a decreasement in HU value), indistinct pancreatic margins due to inflammation, and surrounding fat stranding (Figure 6). CT imaging allows to differentiate between edematous and necrotizing pancreatitis, to assess the presence of collections and to evaluate long-term evolutions including pseudocyst and walled-off necrosis[37].
Notably, graft pancreatitis and vascular thrombosis may occur simultaneously; indeed, pancreatitis can predispose to vascular thrombosis, and vascular thrombosis can also lead to graft inflammation, thus leading to some overlapping CT imaging features as shown on Figure 7.
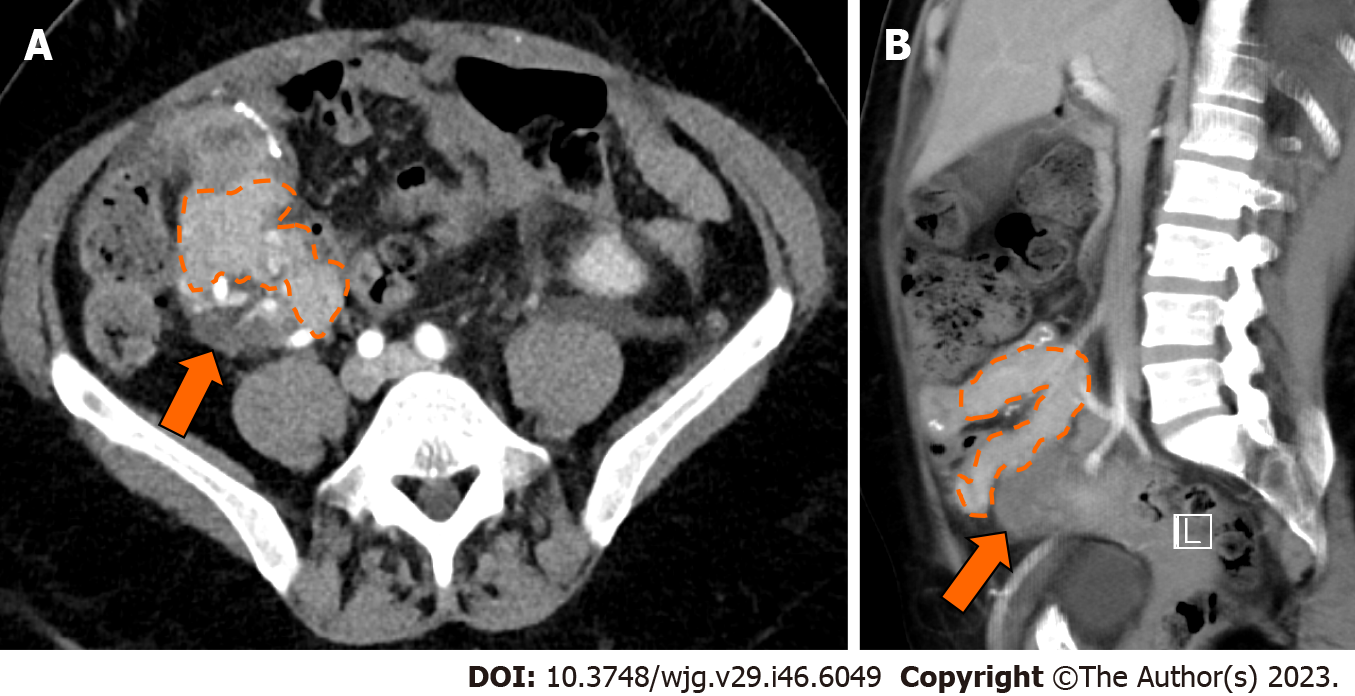
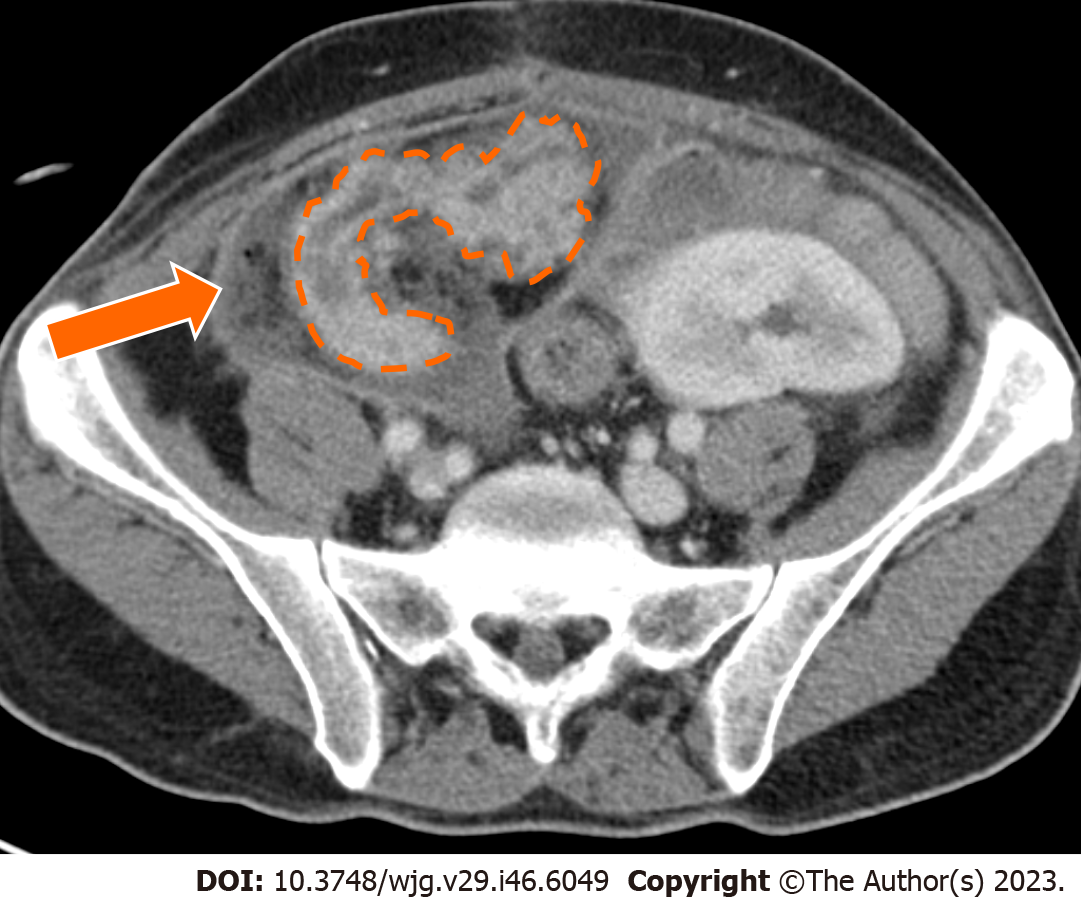
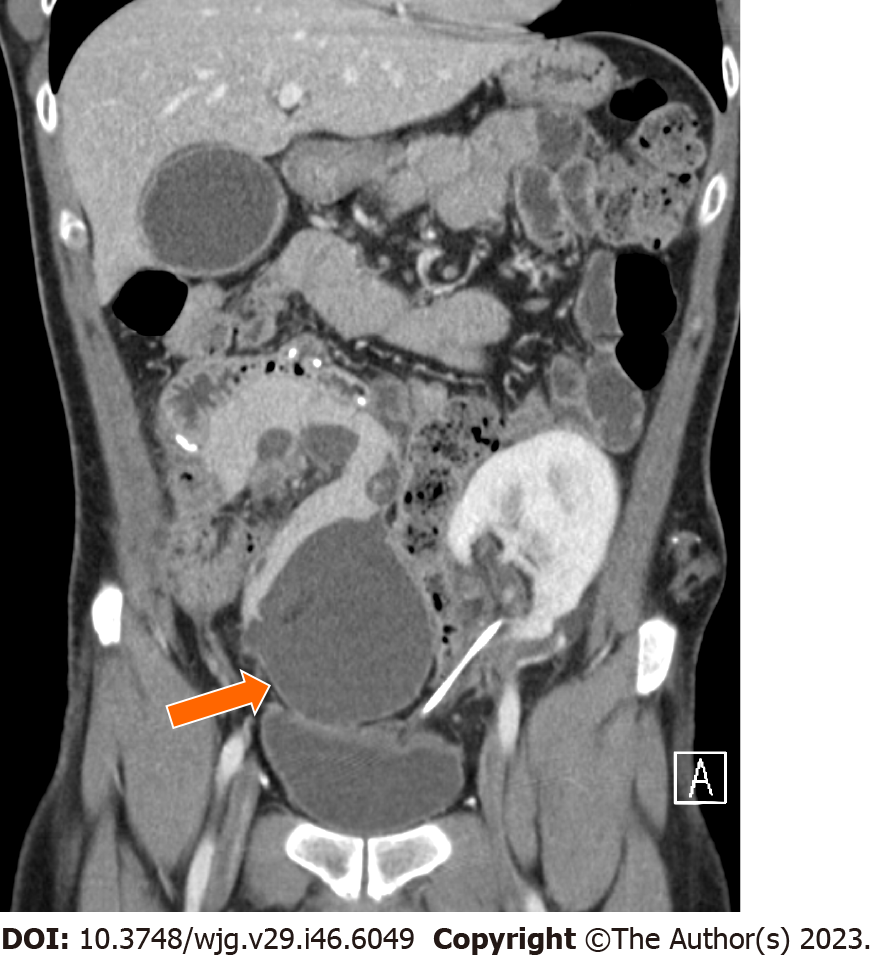
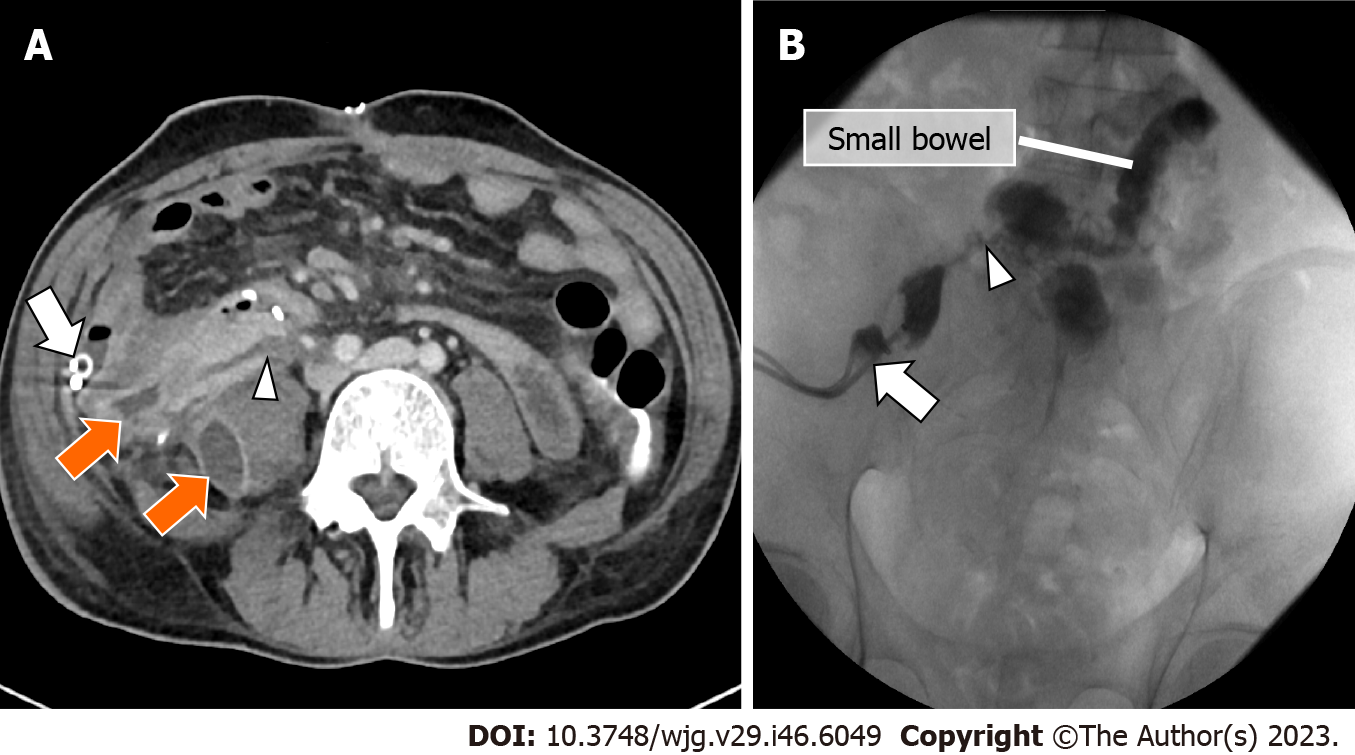
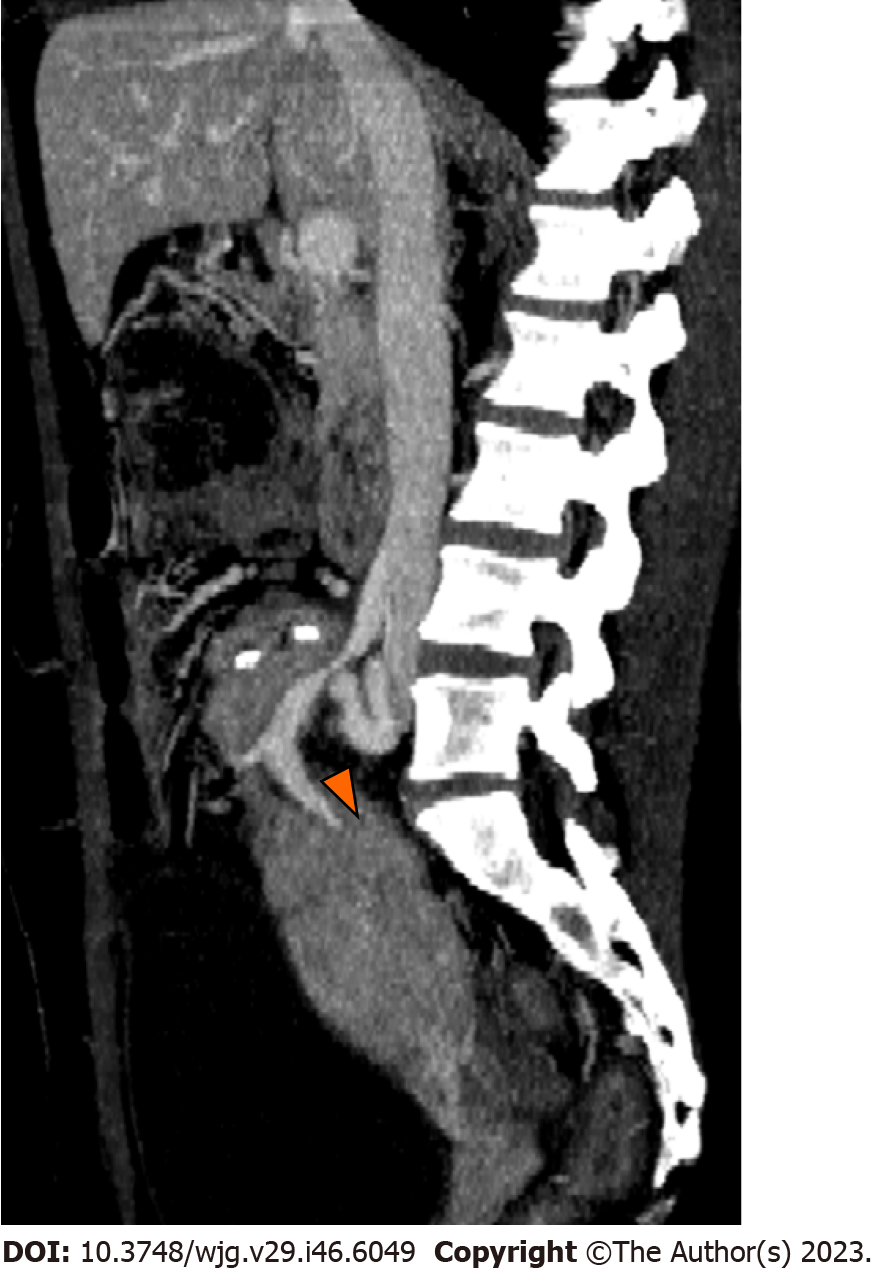
Infection may occur in the form of peripancreatic fluid collections (Figure 6), pseudocysts (Figure 8), leakage at the level of the enteric anastomosis (Figure 9), or infection of the abdominal wall surgical wound[21,38].
Fluid collections are the most common abnormality after pancreatic transplantation, may occur early or late after transplant, are clinically significant in about 16% of patients, and may lead to superinfection[38,39]. Intraabdominal collections also include seroma, hematoma, lymphocele, urinoma, or pseudocyst[11,38].
CT demonstrates an abnormal fluid collection of low attenuation with surrounding rim-enhancement and possible intralesional gas[38]. Soft tissue edema and fat stranding in the adjacent tissues may also be present. Percutaneous drainage is safe and effective for management of peripancreatic fluid collections after pancreas transplant[38].
Duodenal leaks represent about 2.5%-2.9% of complications after pancreatic transplantation[40,41] and represent the cause of re-laparotomy in about 2.1% of cases[34]. Enteric leaks after pancreatic transplantation are usually characterized by extravasation of pancreatic juice from the duodenojejunostomy site leading to focal peritonitis and collections (Figure 9), and eventually abscess formation[26]. Duodenal leaks usually occur early in the postoperative course, but may also be seen late post-transplant and increase 6-mo graft loss risk with a hazard ratio of 13.9[40-42]. Pancreatic duct fistula after focal pancreatitis or ischemia leading to duct disruption, may also result in the development of a peripancreatic collection[42].
In the early postoperative period, the occurrence of bleeding is the most common cause of reoperation[29].
The source of bleeding in the early post-operative period may be:
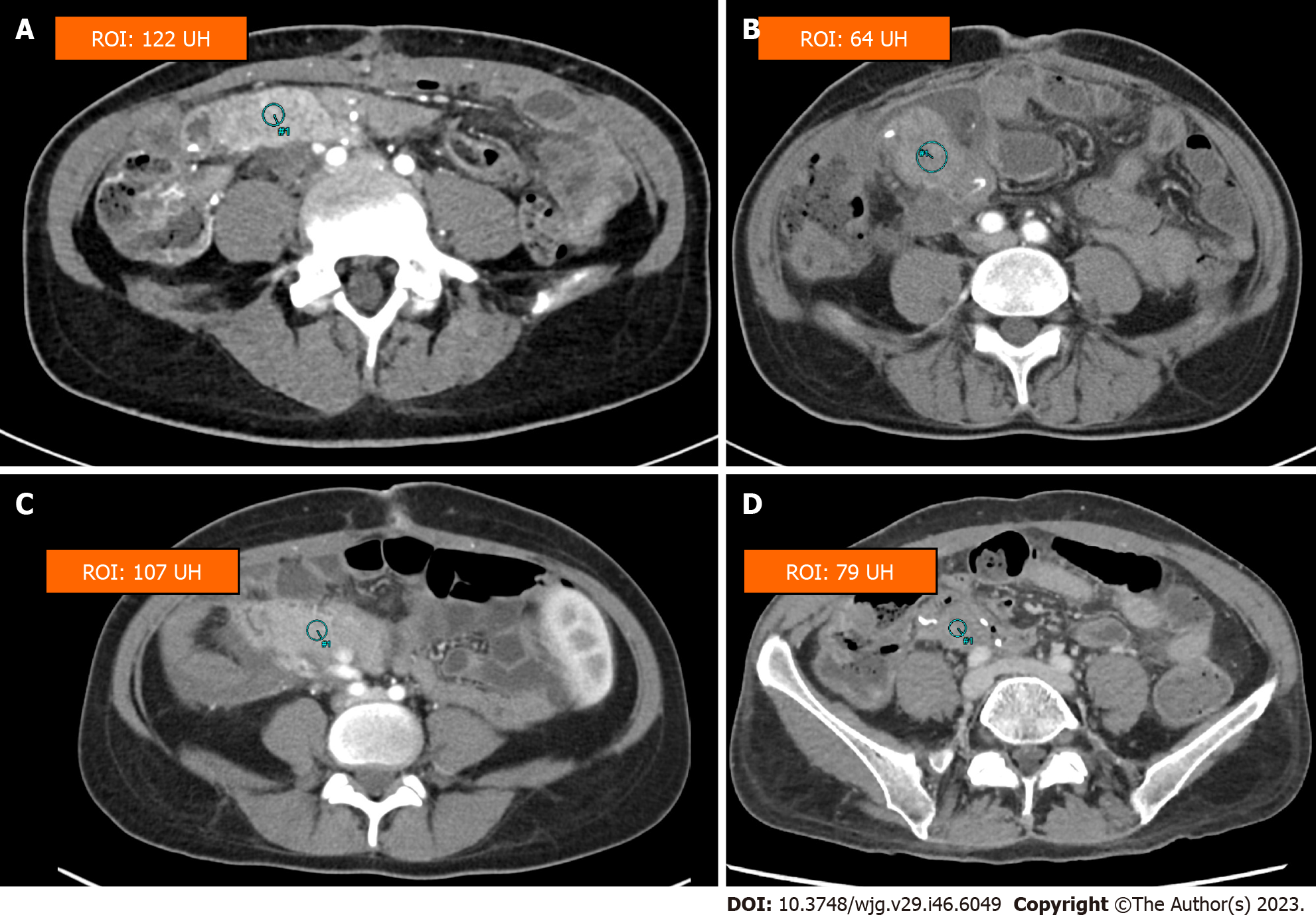
Intra-abdominal: It is usually related to damage of the peripancreatic vessels or vascular anastomosis, enhanced by the antithrombotic prophylaxis or antithrombotic therapy in patients with vascular thrombosis.
Digestive: It comes from the digestive anastomosis or the staple line of the duodenal ends. Gastrointestinal bleeding after pancreas transplant usually occur at the duodenojejunostomy due to reduced blood flow to the graft or due to ulcers at the anastomosis. Digestive bleeding may resolve after conservative measures such as correction of coagulation abnormalities, heparin withdrawal and transfusion; surgical revision may be indicated if the bleeding does not resolve.
Precontrast CT may indicate the presence of an acute hematoma as a hyperattenuating (usually above 60 UH) collection on preconstrast CT in case of intrabdominal bleeding or hyperattenuating content in the GI lumen in case of gastrointestinal bleeding. In case of active bleeding, contrast-enhanced CT acquired in the arterial and venous phases demonstrates pooling/extravasation of the contrast agent within the hematoma or within the bowel lumen and may allow to identify the culprit vessel in case of intrabdominal hemorrhage.
Bleeding due to other arterial complications (e.g. arterio-venous fistula, pseudoaneurysm, arterioenteric fistula, arteriourinary fistula) have been reported but are infrequent, with occurrence of pseudoaneurysm being more common as a late complication[32,43]. Management can be endovascular or surgical and should be individualized[43].
Pseudoaneurysm may develop particularly at the level of anastomoses as a consequence of chemical damage due to leak of pancreatic enzymes[26]. On CT imaging, pseudoaneurysm appears as a saccular enhancing outpouching from the injured artery, with enhancement similar to other arteries, but does not increase in size on delayed phases and follows the blood pool on all phases. Arterio-venous fistula is a very rare complication of pancreatic transplantation[32,43] which may result from the postoperative or post-biopsy laceration of both arterial and venous walls and may potentially lead to major bleeding or graft loss if untreated[43-45]. On CT, arterio-venous fistula will be demonstrated on arterial phase as enlarged Y-graft arteries with an early opacification of the donor draining vein.
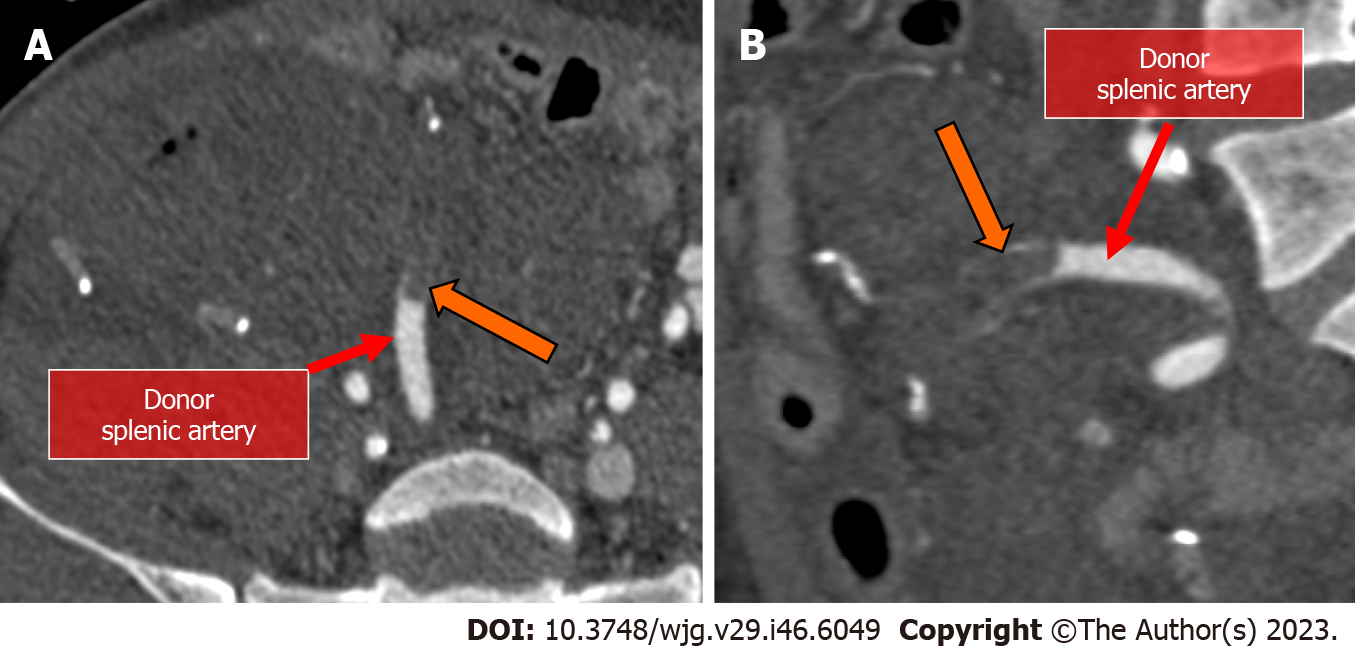
Vascular stenosis is relatively uncommon with an incidence of about 2.5% and more commonly occur at the anastomotic level[40]. Early detection of vascular stenosis is critical to avoid complications, including thrombosis, ischemia, and graft dysfunction[46]. The multiplanar reconstruction of the vascular tree provides a reliable method of visualizing the entire vascular anastomoses, detecting a focal decrease in caliber of the vessel (Figure 10).
In conclusion, pancreatic transplantation is a complex surgery with high morbidity often related to postoperative complications, which may occur during hospitalization, early in the first three months or in the late period. Cross-sectional imaging with CT plays a critical initial role in the diagnosis and management of postoperative complications in many transplanted patients who present to the emergency department for suspected transplant dysfunction. Therefore, radiologists should be aware of surgical techniques and normal imaging appearance of pancreatic transplantation and should be well-trained to recognize identify acute findings and provide key imaging information to optimize patient management.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Gong N, China; Uhlmann D, Germany S-Editor: Li L L-Editor: A P-Editor: Yuan YY
| 1. | Holt RIG, DeVries JH, Hess-Fischl A, Hirsch IB, Kirkman MS, Klupa T, Ludwig B, Nørgaard K, Pettus J, Renard E, Skyler JS, Snoek FJ, Weinstock RS, Peters AL. The management of type 1 diabetes in adults. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia. 2021;64:2609-2652. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 190] [Article Influence: 47.5] [Reference Citation Analysis (0)] |
| 2. | Gruessner AC, Sutherland DE, Gruessner RW. Long-term outcome after pancreas transplantation. Curr Opin Organ Transplant. 2012;17:100-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 60] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 3. | Sutherland DE, Gruessner RW, Gruessner AC. Pancreas transplantation for treatment of diabetes mellitus. World J Surg. 2001;25:487-496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 94] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 4. | Lipshutz GS, Wilkinson AH. Pancreas-kidney and pancreas transplantation for the treatment of diabetes mellitus. Endocrinol Metab Clin North Am. 2007;36:1015-38; x. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 41] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 5. | Al-Naseem AO, Attia A, Gonnah AR, Al-Naseem AOAS, Spiers HVM, Gruessner A, Leelarathna L, Thabit H, Augustine T. Pancreas transplantation today: quo vadis? Eur J Endocrinol. 2023;188:R73-R87. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (33)] |
| 6. | Rojas-Canales D, Walters SN, Penko D, Cultrone D, Bailey J, Chtanova T, Nitschke J, Johnston J, Kireta S, Loudovaris T, Kay TW, Kuchel TR, Hawthorne W, O'Connell PJ, Korbutt G, Greenwood JE, Grey ST, Drogemuller CJ, Coates PT. Intracutaneous Transplantation of Islets Within a Biodegradable Temporizing Matrix as an Alternative Site for Islet Transplantation. Diabetes. 2023;72:758-768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 7. | Berney T, Andres A, Bellin MD, de Koning EJP, Johnson PRV, Kay TWH, Lundgren T, Rickels MR, Scholz H, Stock PG, White S; International Islet Transplant Centers. A Worldwide Survey of Activities and Practices in Clinical Islet of Langerhans Transplantation. Transpl Int. 2022;35:10507. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 8. | Gruessner AC, Gruessner RWG. The 2022 International Pancreas Transplant Registry Report-A Review. Transplant Proc. 2022;54:1918-1943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 31] [Reference Citation Analysis (33)] |
| 9. | Gruessner AC, Gruessner RW. Long-term outcome after pancreas transplantation: a registry analysis. Curr Opin Organ Transplant. 2016;21:377-385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 86] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 10. | Troppmann C. Complications after pancreas transplantation. Curr Opin Organ Transplant. 2010;15:112-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 137] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 11. | Hameed M, Hameed S, Harvey C, Moser S, Muthusamy A. Imaging in whole organ pancreatic transplants and a multimodality review of its complications. Br J Radiol. 2021;94:20200106. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 12. | Hakeem A, Chen J, Iype S, Clatworthy MR, Watson CJE, Godfrey EM, Upponi S, Saeb-Parsy K. Pancreatic allograft thrombosis: Suggestion for a CT grading system and management algorithm. Am J Transplant. 2018;18:163-179. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 37] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 13. | Doherty DT, Khambalia HA, Summers A, Moinuddin Z, Yiannoullou P, Krishnan A, Augustine T, Naish JH, van Dellen D. Future imaging modalities for the assessment of pancreas allografts a scan of the horizon. Transplant Rev (Orlando). 2022;36:100692. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 14. | Fananapazir G, Troppmann C, Corwin MT, Nikpour AM, Naderi S, Lamba R. Incidences of acute kidney injury, dialysis, and graft loss following intravenous administration of low-osmolality iodinated contrast in patients with kidney transplants. Abdom Radiol (NY). 2016;41:2182-2186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 15. | Cheungpasitporn W, Thongprayoon C, Mao MA, Mao SA, D'Costa MR, Kittanamongkolchai W, Kashani KB. Contrast-induced acute kidney injury in kidney transplant recipients: A systematic review and meta-analysis. World J Transplant. 2017;7:81-87. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 27] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 16. | McDonald JS, Katzberg RW, McDonald RJ, Williamson EE, Kallmes DF. Is the Presence of a Solitary Kidney an Independent Risk Factor for Acute Kidney Injury after Contrast-enhanced CT? Radiology. 2016;278:74-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 17. | Isaka Y, Hayashi H, Aonuma K, Horio M, Terada Y, Doi K, Fujigaki Y, Yasuda H, Sato T, Fujikura T, Kuwatsuru R, Toei H, Murakami R, Saito Y, Hirayama A, Murohara T, Sato A, Ishii H, Takayama T, Watanabe M, Awai K, Oda S, Murakami T, Yagyu Y, Joki N, Komatsu Y, Miyauchi T, Ito Y, Miyazawa R, Kanno Y, Ogawa T, Koshi E, Kosugi T, Yasuda Y; Japanese Society of Nephrology, Japan Radiological Society, and Japanese Circulation Society Joint Working Group. Guideline on the use of iodinated contrast media in patients with kidney disease 2018. Clin Exp Nephrol. 2020;24:1-44. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 38] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 18. | van der Molen AJ, Reimer P, Dekkers IA, Bongartz G, Bellin MF, Bertolotto M, Clement O, Heinz-Peer G, Stacul F, Webb JAW, Thomsen HS. Post-contrast acute kidney injury. Part 2: risk stratification, role of hydration and other prophylactic measures, patients taking metformin and chronic dialysis patients : Recommendations for updated ESUR Contrast Medium Safety Committee guidelines. Eur Radiol. 2018;28:2856-2869. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 182] [Cited by in RCA: 196] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 19. | European Society of Urogenital Radiology. ESUR Guidelines on Contrast Agents V.10.0 Mar 2018. [cited 21 Oct 2023]. Available from: https://www.esur.org/wp-content/uploads/2022/03/ESUR-Guidelines-10_0-Final-Version.pdf. |
| 20. | Nghiem DD. Revascularization of the gastroepiploic artery in pancreas transplant. Transpl Int. 2008;21:774-777. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 21. | Diem P, Abid M, Redmon JB, Sutherland DE, Robertson RP. Systemic venous drainage of pancreas allografts as independent cause of hyperinsulinemia in type I diabetic recipients. Diabetes. 1990;39:534-540. [PubMed] [DOI] [Full Text] |
| 22. | Stratta RJ, Shokouh-Amiri MH, Egidi MF, Grewal HP, Kizilisik AT, Nezakatgoo N, Gaber LW, Gaber AO. A prospective comparison of simultaneous kidney-pancreas transplantation with systemic-enteric versus portal-enteric drainage. Ann Surg. 2001;233:740-751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 49] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 23. | Boggi U, Amorese G, Marchetti P. Surgical techniques for pancreas transplantation. Curr Opin Organ Transplant. 2010;15:102-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 33] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 24. | Stratta RJ, Gaber AO, Shokouh-Amiri MH, Reddy KS, Egidi MF, Grewal HP, Gaber LW. A prospective comparison of systemic-bladder versus portal-enteric drainage in vascularized pancreas transplantation. Surgery. 2000;127:217-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 69] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 25. | Grewal HP, Garland L, Novak K, Gaber L, Tolley EA, Gaber AO. Risk factors for postimplantation pancreatitis and pancreatic thrombosis in pancreas transplant recipients. Transplantation. 1993;56:609-612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 71] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 26. | Sindhi R, Stratta RJ, Lowell JA, Sudan D, Cushing KA, Castaldo P, Jerius JT. Experience with enteric conversion after pancreatic transplantation with bladder drainage. J Am Coll Surg. 1997;184:281-289. [PubMed] |
| 27. | Gallego Ferrero P, Crespo Del Pozo J. Imaging in pancreas transplantation complications: Temporal classification. J Med Imaging Radiat Oncol. 2018;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 28. | Ventura-Aguiar P, Cabello M, Beneyto I, Navarro Cabello D, Tabernero G, Alonso A, Ruiz JC, Llorente S; EFISPAN group. Patient and graft survival in pancreas transplant recipients: The EFISPAN study. Nefrologia (Engl Ed). 2023;43:133-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 29. | Kopp WH, van Leeuwen CAT, Lam HD, Huurman VAL, de Fijter JW, Schaapherder AF, Baranski AG, Braat AE. Retrospective study on detection, treatment, and clinical outcome of graft thrombosis following pancreas transplantation. Transpl Int. 2019;32:410-417. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 30. | Haider M, Yessayan L, Venkat KK, Goggins M, Patel A, Karthikeyan V. Incidence of contrast-induced nephropathy in kidney transplant recipients. Transplant Proc. 2015;47:379-383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 31. | Blundell J, Shahrestani S, Lendzion R, Pleass HJ, Hawthorne WJ. Risk Factors for Early Pancreatic Allograft Thrombosis Following Simultaneous Pancreas-Kidney Transplantation: A Systematic Review. Clin Appl Thromb Hemost. 2020;26:1076029620942589. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 32. | Yadav K, Young S, Finger EB, Kandaswamy R, Sutherland DER, Golzarian J, Dunn TB. Significant arterial complications after pancreas transplantation-A single-center experience and review of literature. Clin Transplant. 2017;31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 33. | Barrufet M, Burrel M, Angeles García-Criado M, Montañà X, Real MI, Ferrer J, Fernández-Cruz L, Gilabert R. Pancreas transplants venous graft thrombosis: endovascular thrombolysis for graft rescue. Cardiovasc Intervent Radiol. 2014;37:1226-1234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 34. | Cornejo-Carrasco CE, Fernández-Cruz L. Re-operation in pancreas transplantation. Transplant Proc. 2014;46:3050-3053. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 35. | van Dellen D, Summers A, Trevelyan S, Tavakoli A, Augustine T, Pararajasingam R. Incidence and Histologic Features of Transplant Graft Pancreatitis: A Single Center Experience. Exp Clin Transplant. 2015;13:449-452. [PubMed] |
| 36. | Nadalin S, Girotti P, Königsrainer A. Risk factors for and management of graft pancreatitis. Curr Opin Organ Transplant. 2013;18:89-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 33] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 37. | Thoeni RF. The revised Atlanta classification of acute pancreatitis: its importance for the radiologist and its effect on treatment. Radiology. 2012;262:751-764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 261] [Cited by in RCA: 268] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 38. | Ozturk M, Ozkan O, Laeseke P, Kleedehn MG. Peripancreatic Fluid Collections After Pancreas Transplant: Safety and Efficacy of Percutaneous Drainage. AJR Am J Roentgenol. 2021;217:404-410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 39. | Singh RP, Vrakas G, Hayek S, Anam S, Aqueel M, Olsburgh J, Calder F, Mamode N, Callaghan C, Kessaris N, Pattison J, Hilton R, Koffman G, Taylor JD, Drage MW. Clinically significant peripancreatic fluid collections after simultaneous pancreas-kidney transplantation. Transplantation. 2013;95:1263-1269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 40. | Khubutia MS, Pinchuk AV, Dmitriev IV, Balkarov AG, Storozhev RV, Anisimov YA. Surgical complications after simultaneous pancreas-kidney transplantation: A single-center experience. Asian J Surg. 2016;39:232-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 41. | Fleetwood VA, Falls C, Ohman J, Aziz A, Stalter L, Leverson G, Welch B, Kaufman DB, Al-Adra DP, Odorico JS. Post-pancreatic transplant enteric leaks: The role of the salvage operation. Am J Transplant. 2022;22:2052-2063. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 42. | Nath DS, Gruessner A, Kandaswamy R, Gruessner RW, Sutherland DE, Humar A. Late anastomotic leaks in pancreas transplant recipients - clinical characteristics and predisposing factors. Clin Transplant. 2005;19:220-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 37] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 43. | Young SJ, Bergren L, Dunn T, Shrestha P, Yadav K, Frank N, Kandaswamy R, Golzarian J. Outcomes of Endovascular Management of Late Vascular Hemorrhage After Pancreatic Transplant. AJR Am J Roentgenol. 2018;210:201-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 44. | Surowiecka-Pastewka A, Matejak-Górska M, Frączek M, Sklinda K, Walecki J, Durlik M. Endovascular Interventions in Vascular Complications After Simultaneous Pancreas and Kidney Transplantations: A Single-Center Experience. Ann Transplant. 2019;24:199-207. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 45. | Bratton CF, Hamid A, Selby JB, Baliga PK. Case report: gastrointestinal hemorrhage caused by a pancreas transplant arteriovenous fistula with large psuedoanuerysm 9 years after transplantation. Transplant Proc. 2011;43:4039-4043. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 46. | Choudhary D, Vijayvergiya R, Sharma A, Lal A, Rajan P, Kasinadhuni G, Singh S, Kenwar DB. Salvage of Graft Pancreas in a Simultaneous Pancreas-kidney Transplant Recipient With Splenic Artery Thrombosis, Infected Walled-off Necrosis, and Stenting of Y Arterial Graft Stenosis. Transplant Direct. 2022;8:e1363. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |